Effect of the Consumption of Species from the Zingiberaceae or Berberidaceae Family on Glycemic Profile Parameters: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Eligibility Criteria
2.3. Information Sources and Search Strategy
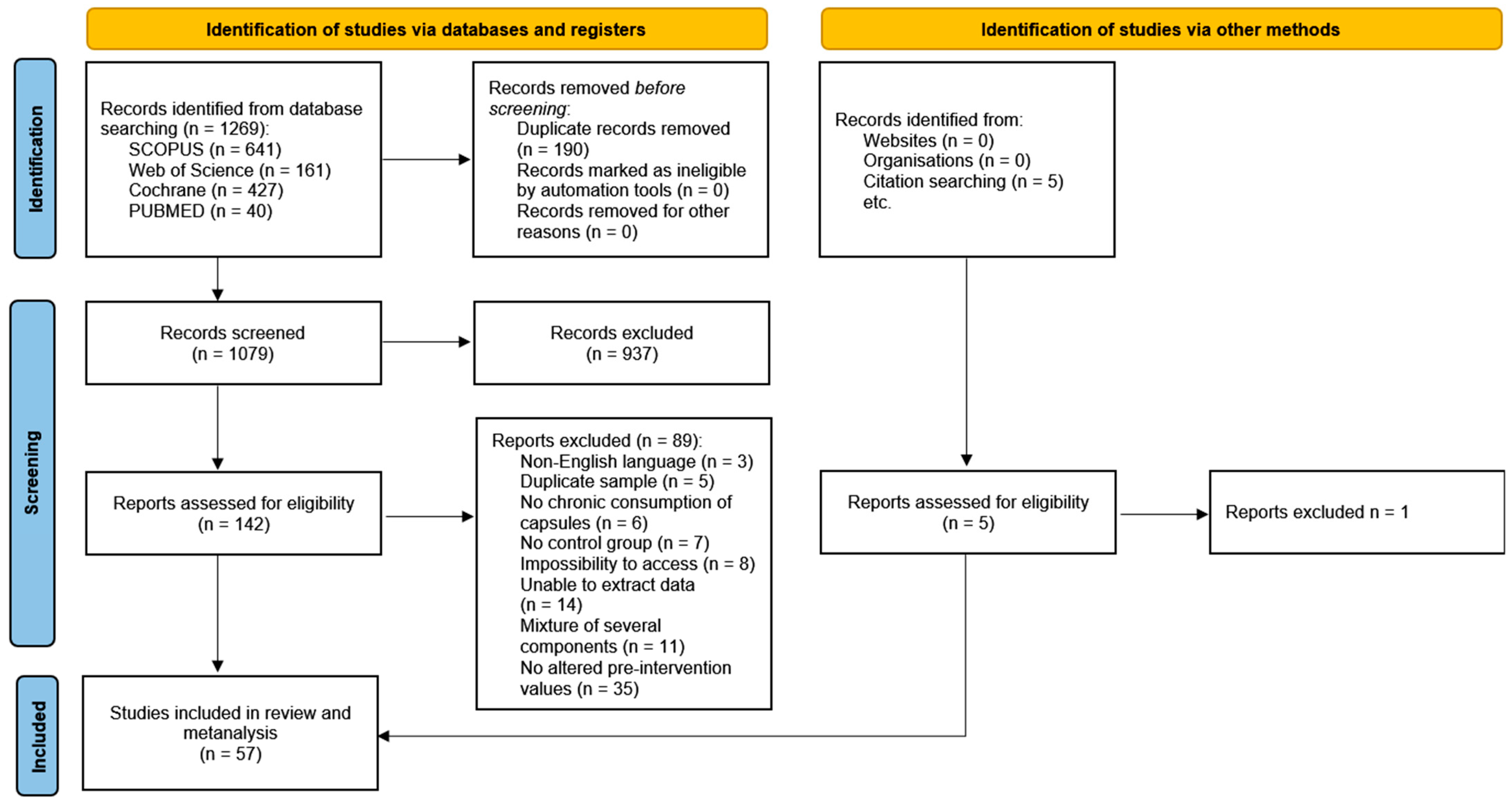
2.4. Selection Process
2.5. Data Items
2.6. Quality Assessment
2.7. Synthesis Methods
3. Results
3.1. Study Characteristics
3.2. Bioactive Compound Supplementation
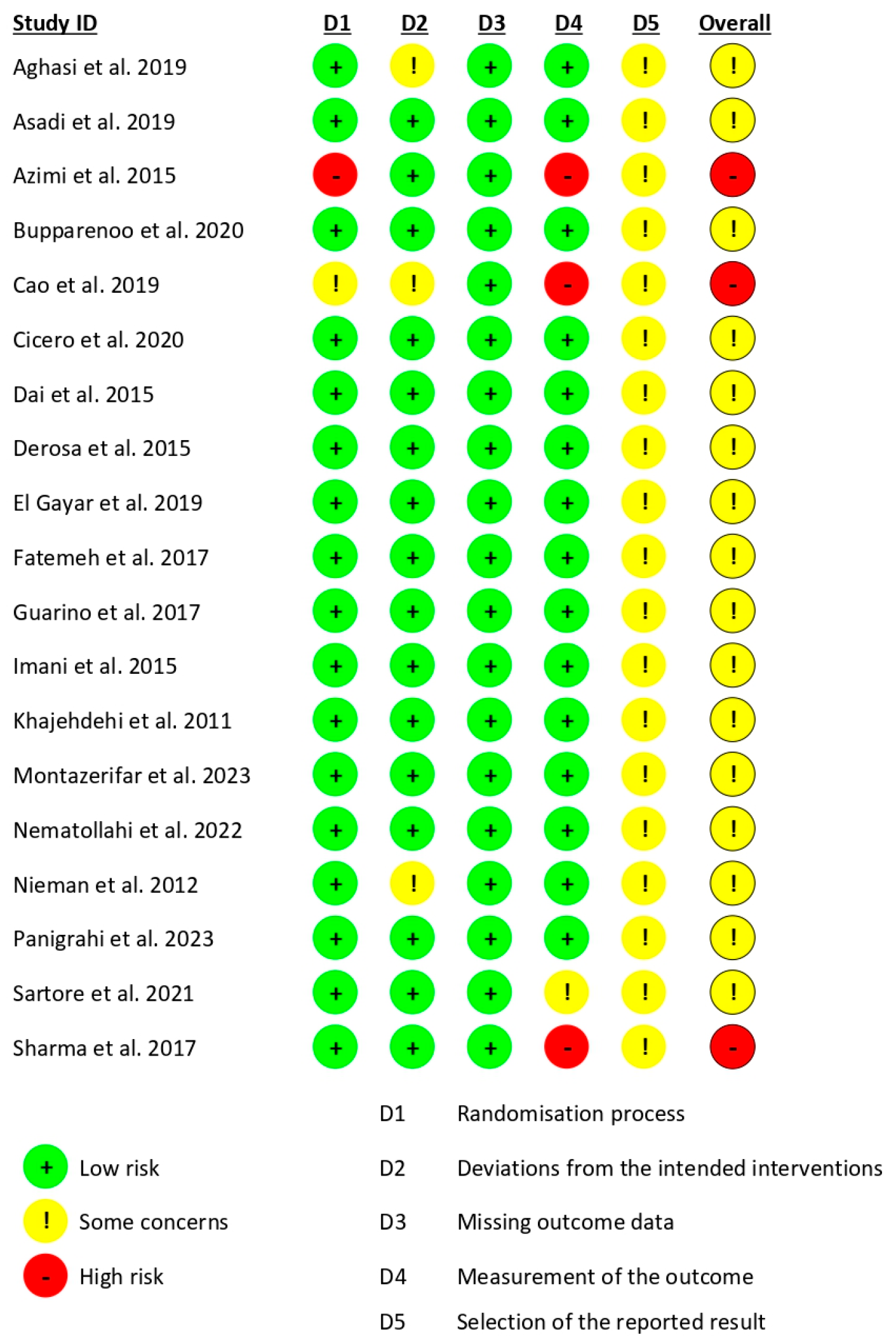
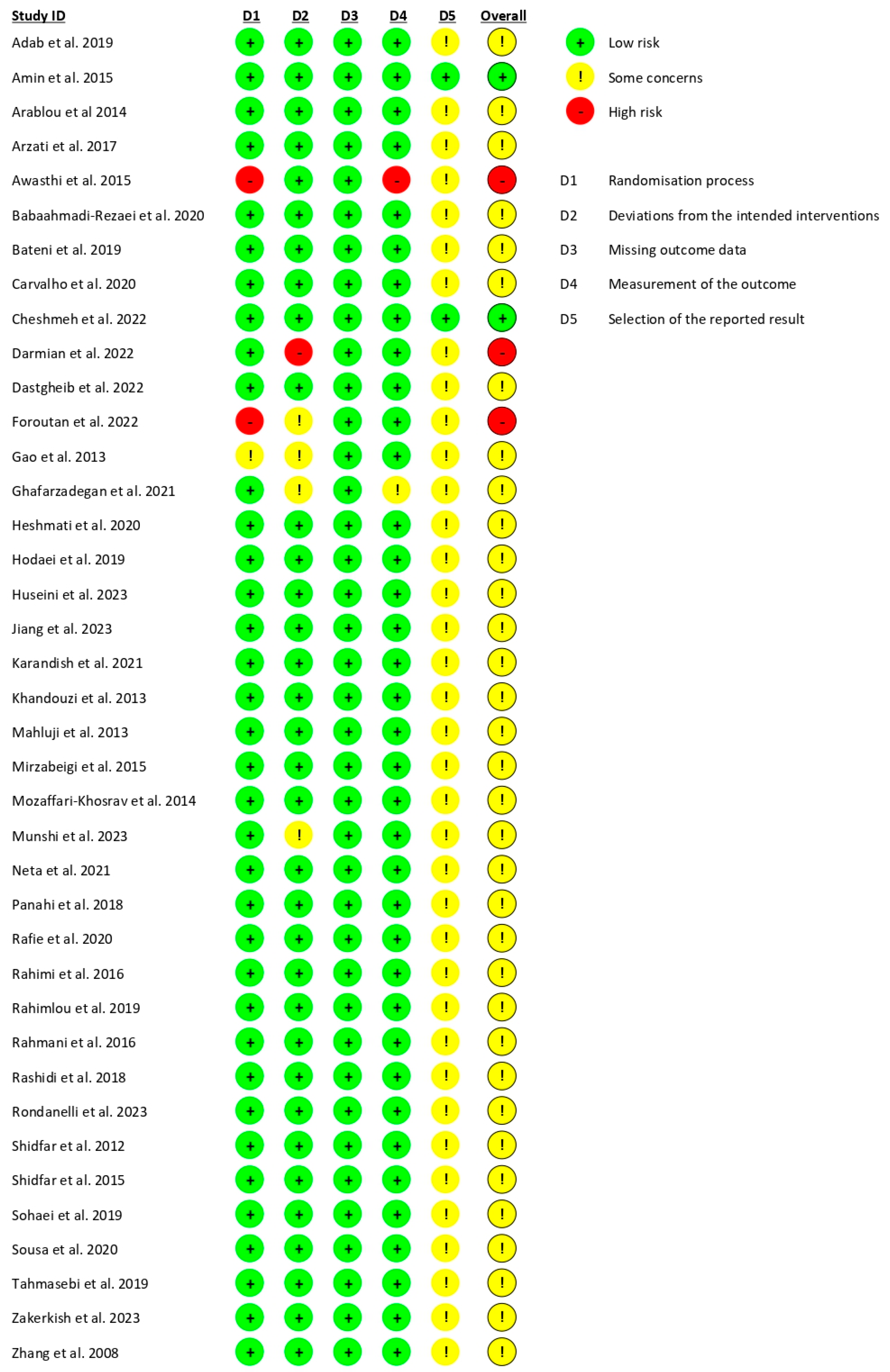
3.3. Risk of Study Bias
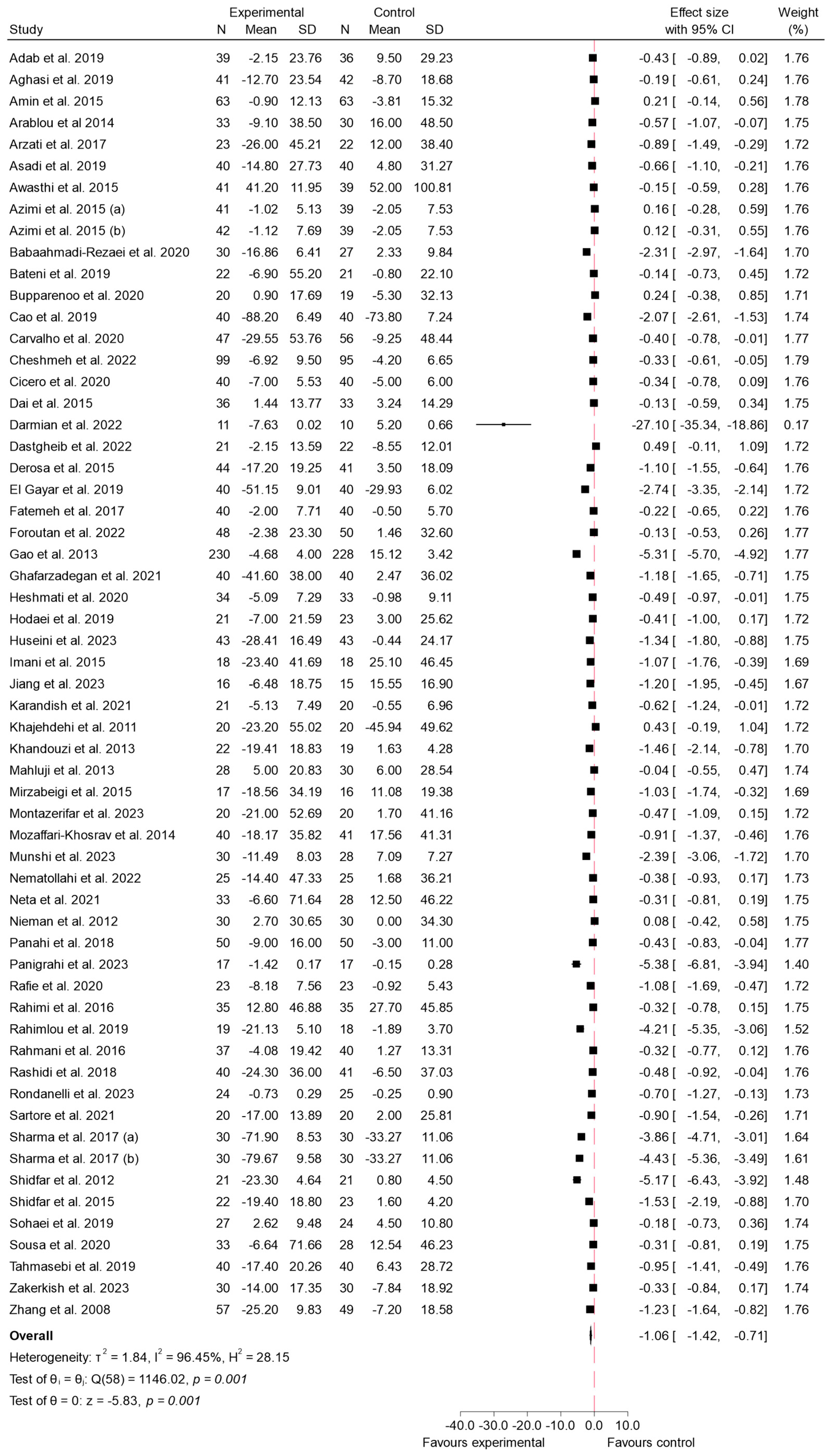
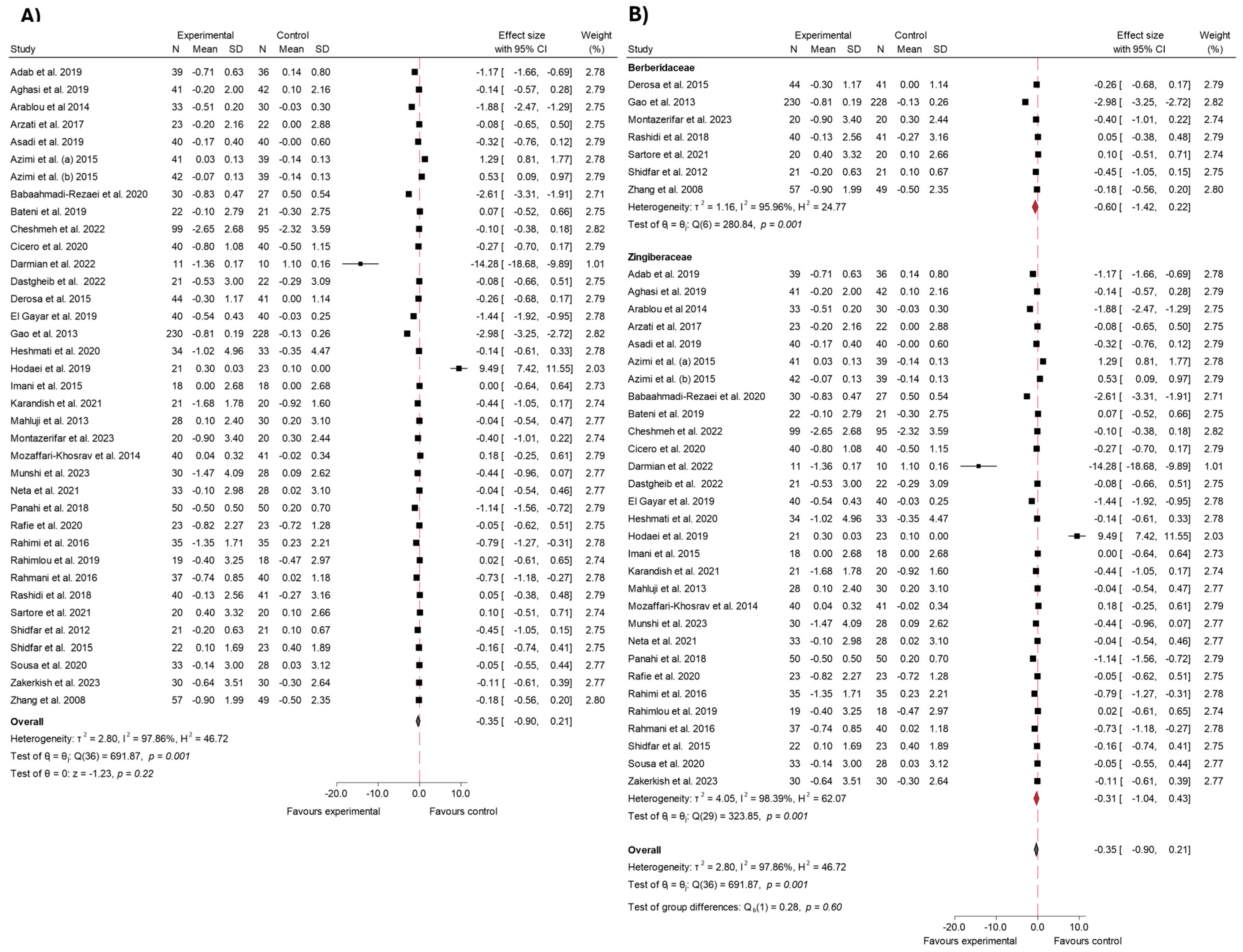
3.4. Findings from Meta-Analysis
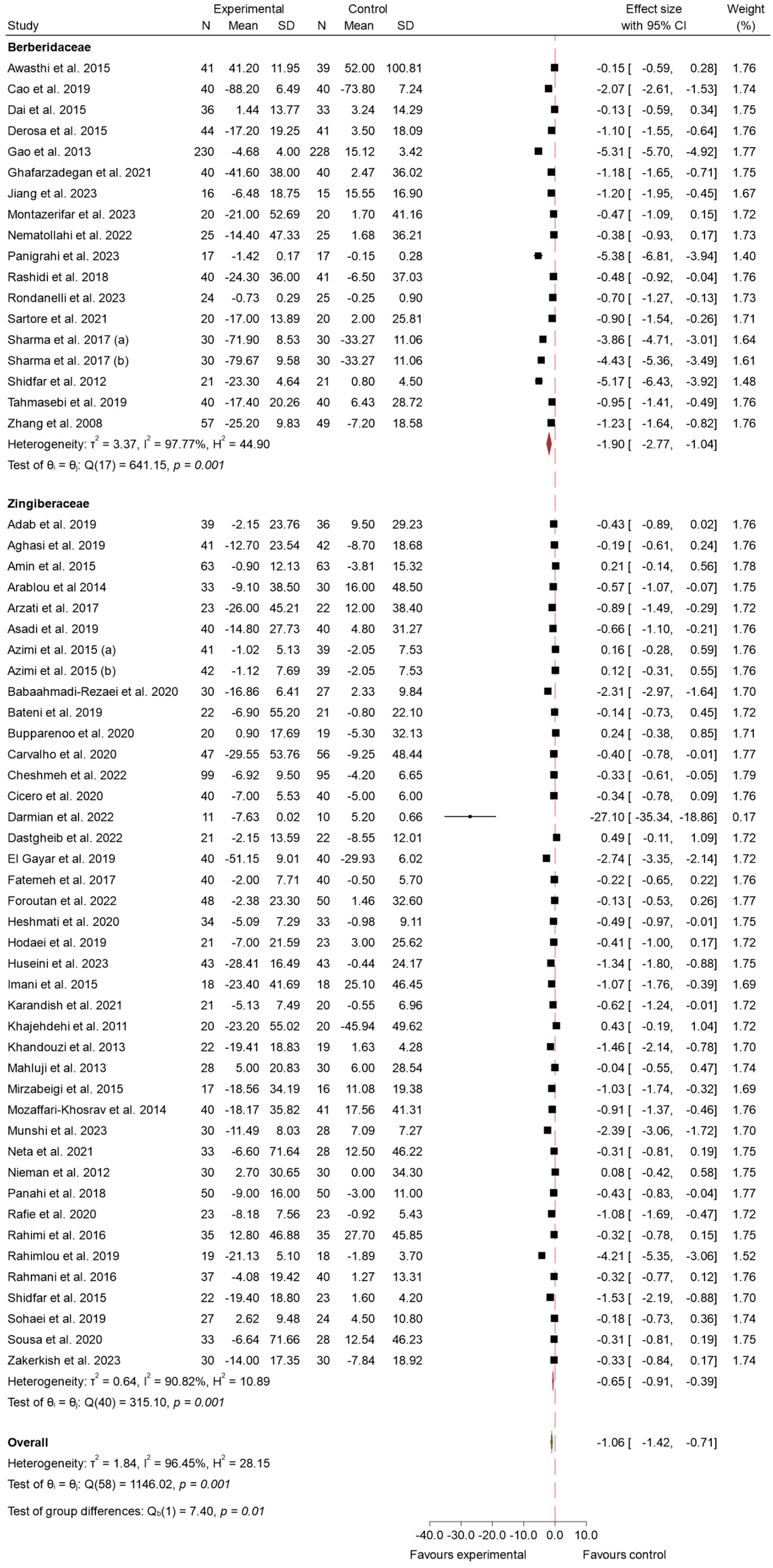
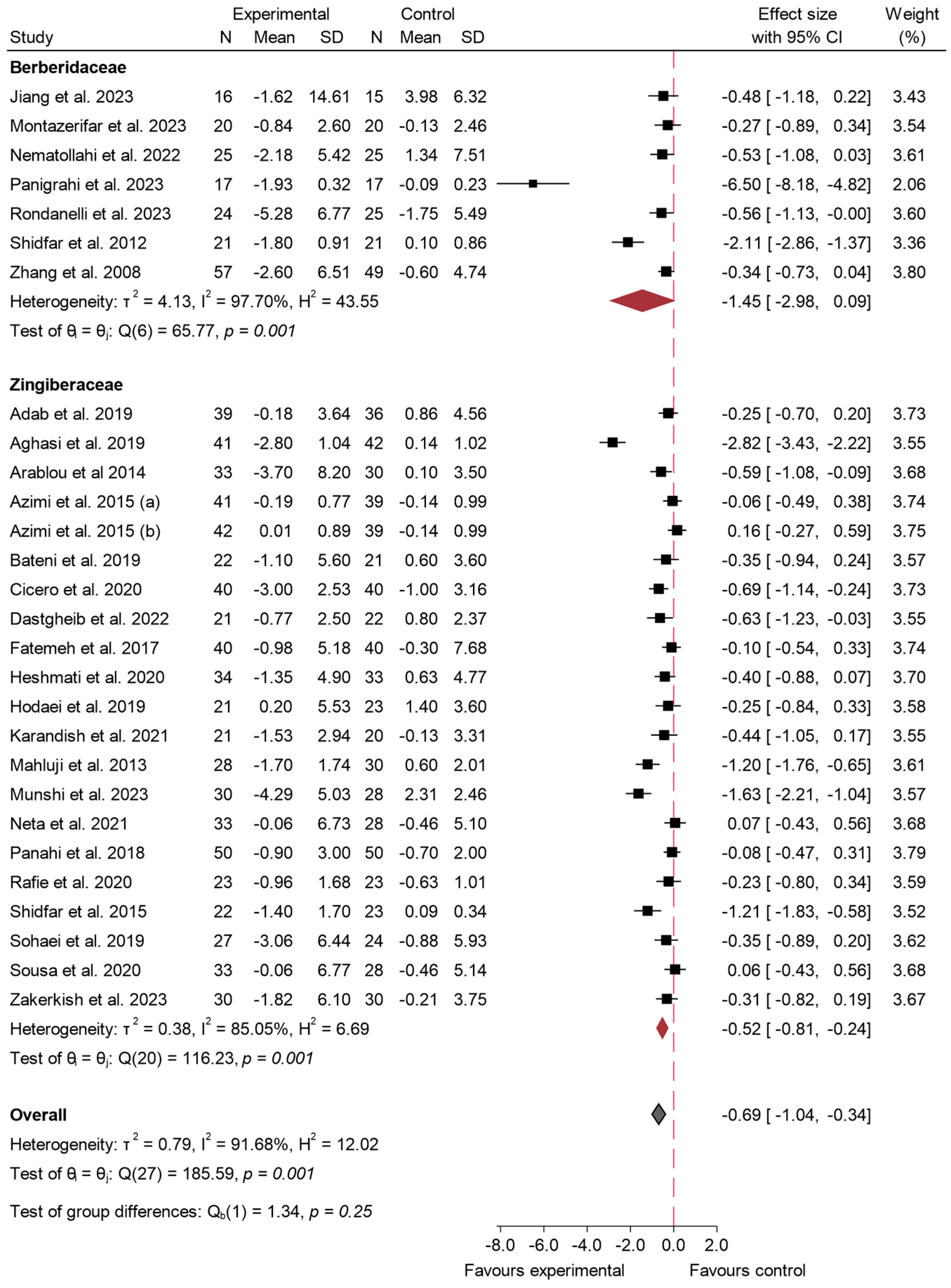
3.4.1. FBS
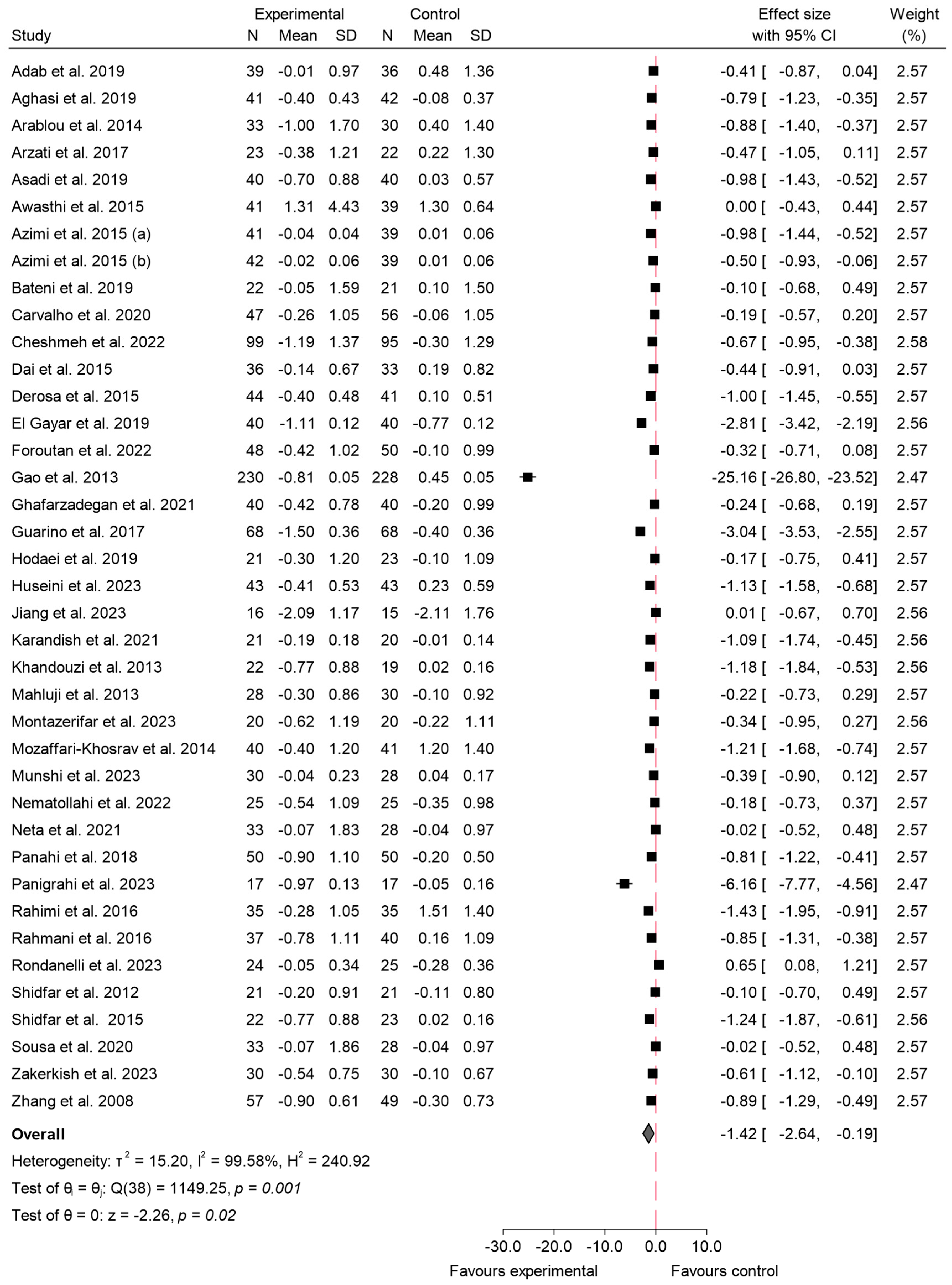
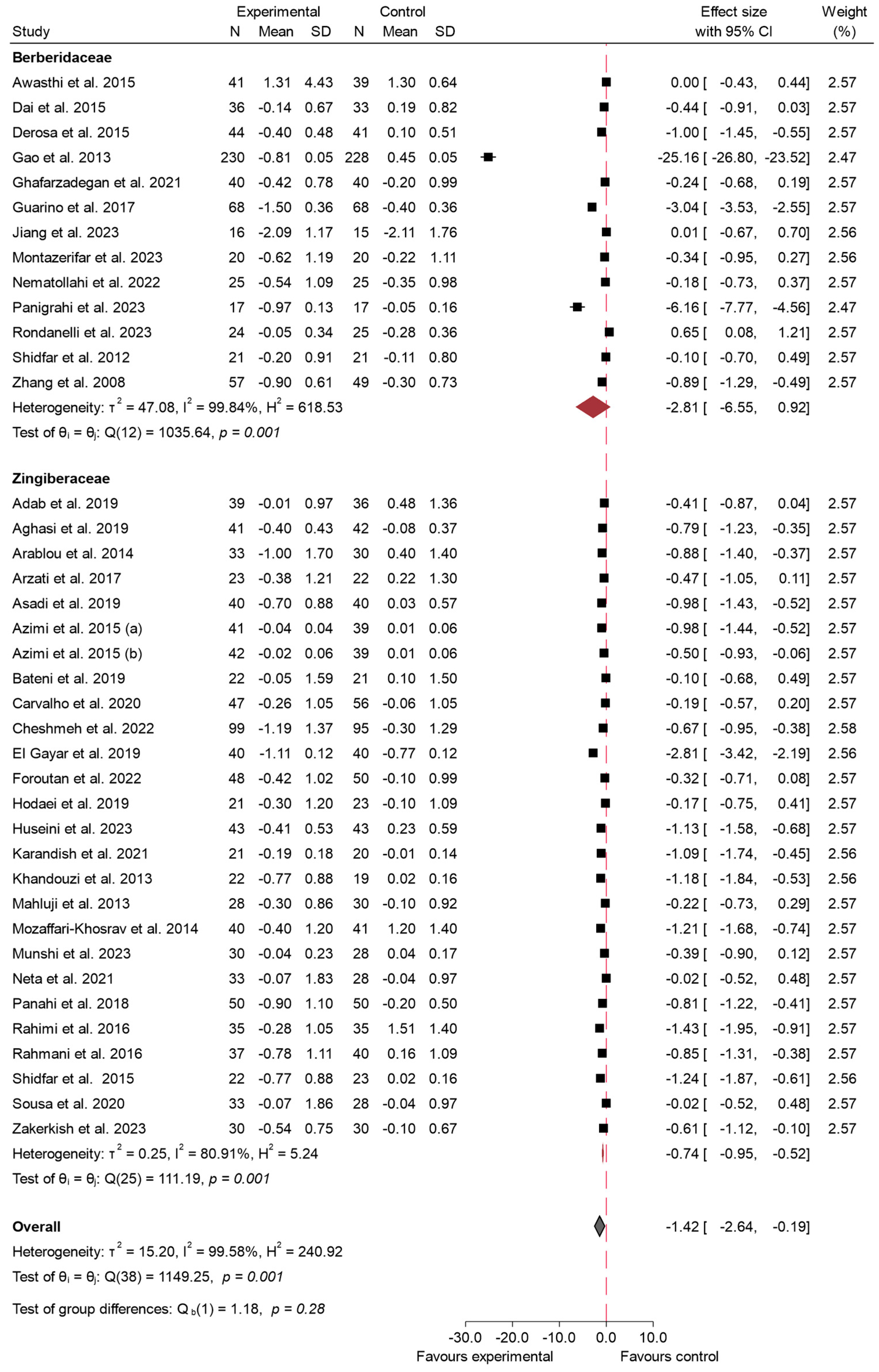
3.4.2. HbA1c
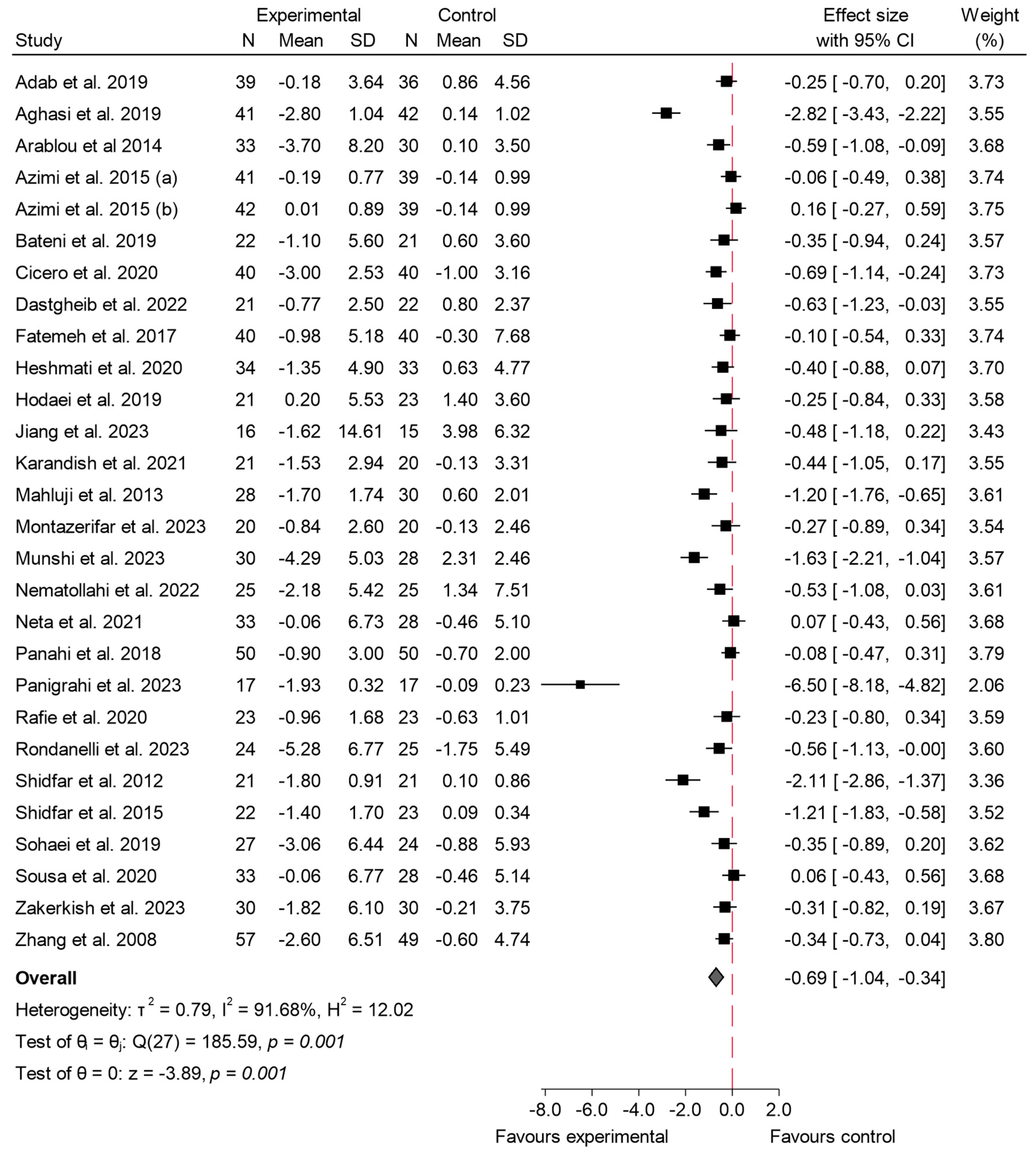
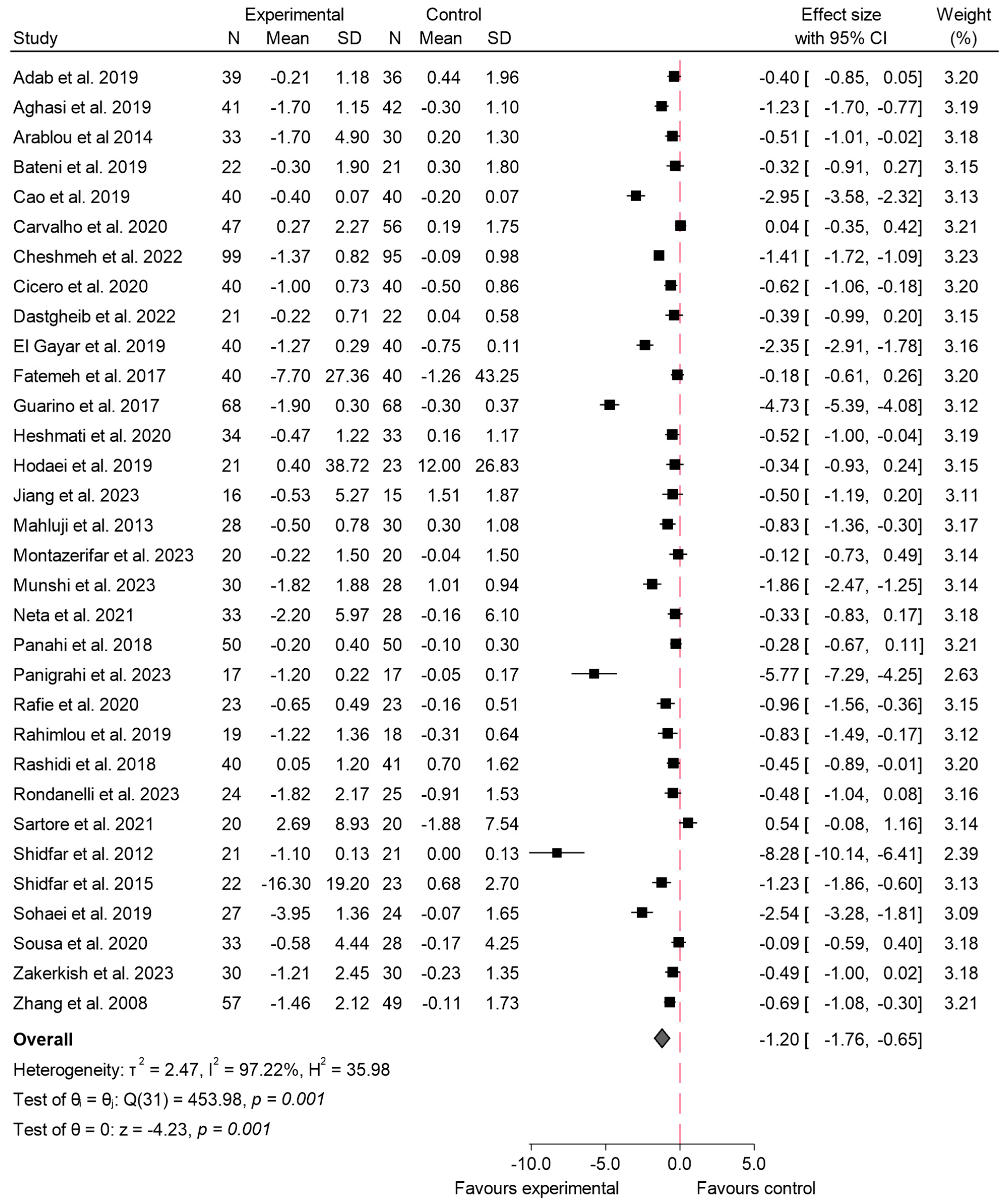
3.4.3. Insulin
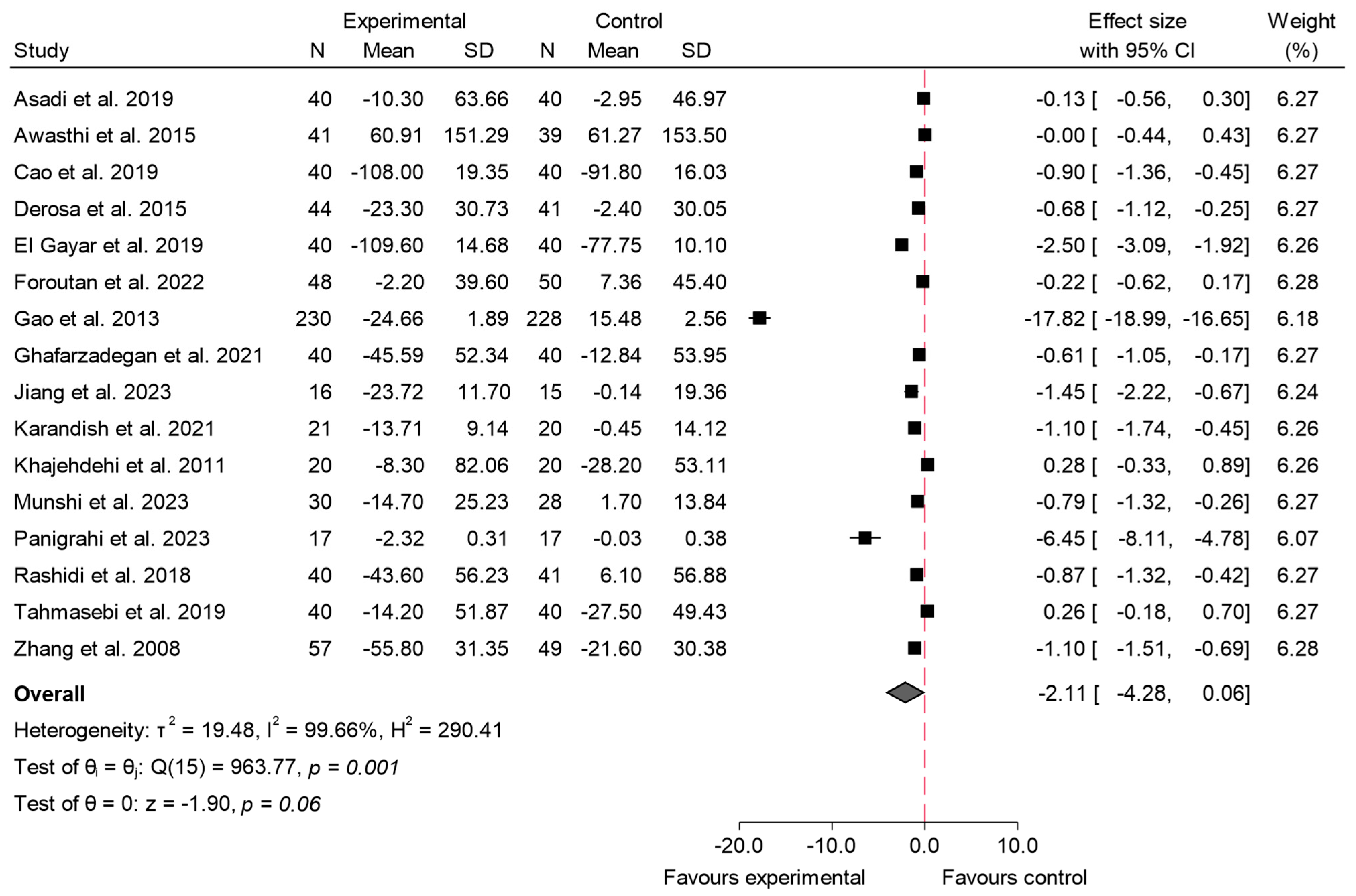
3.4.4. HOMA-IR
3.4.5. 2hPG
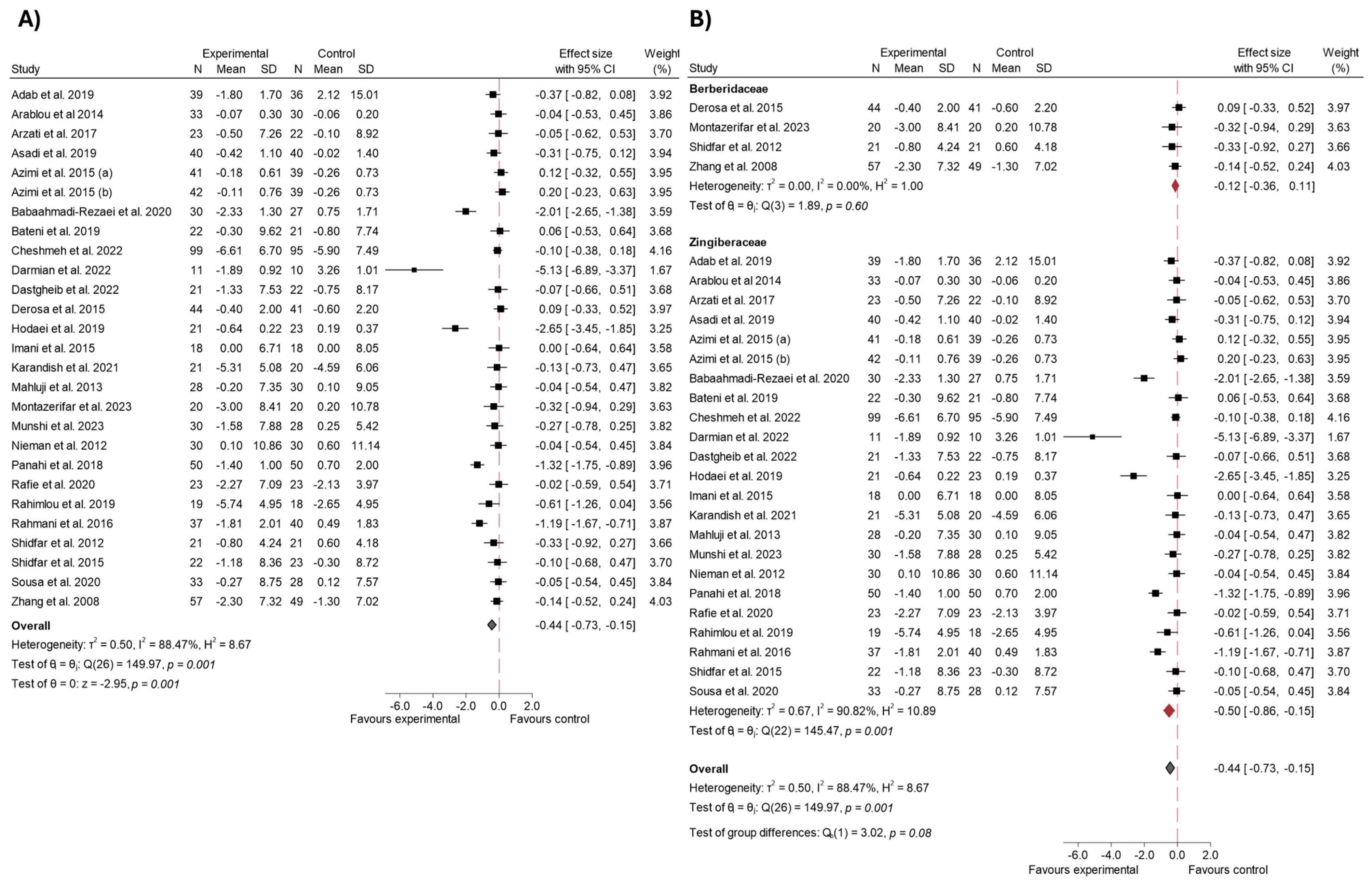
3.4.6. Body Weight and BMI
4. Discussion
4.1. Effects on Fasting Blood Glucose (FBS)
4.2. Impact on HbA1c
4.3. Impact on Insulin Levels
4.4. Impact on HOMA-IR
4.5. Impact on 2hPG
4.6. Impact on Body Weight and BMI
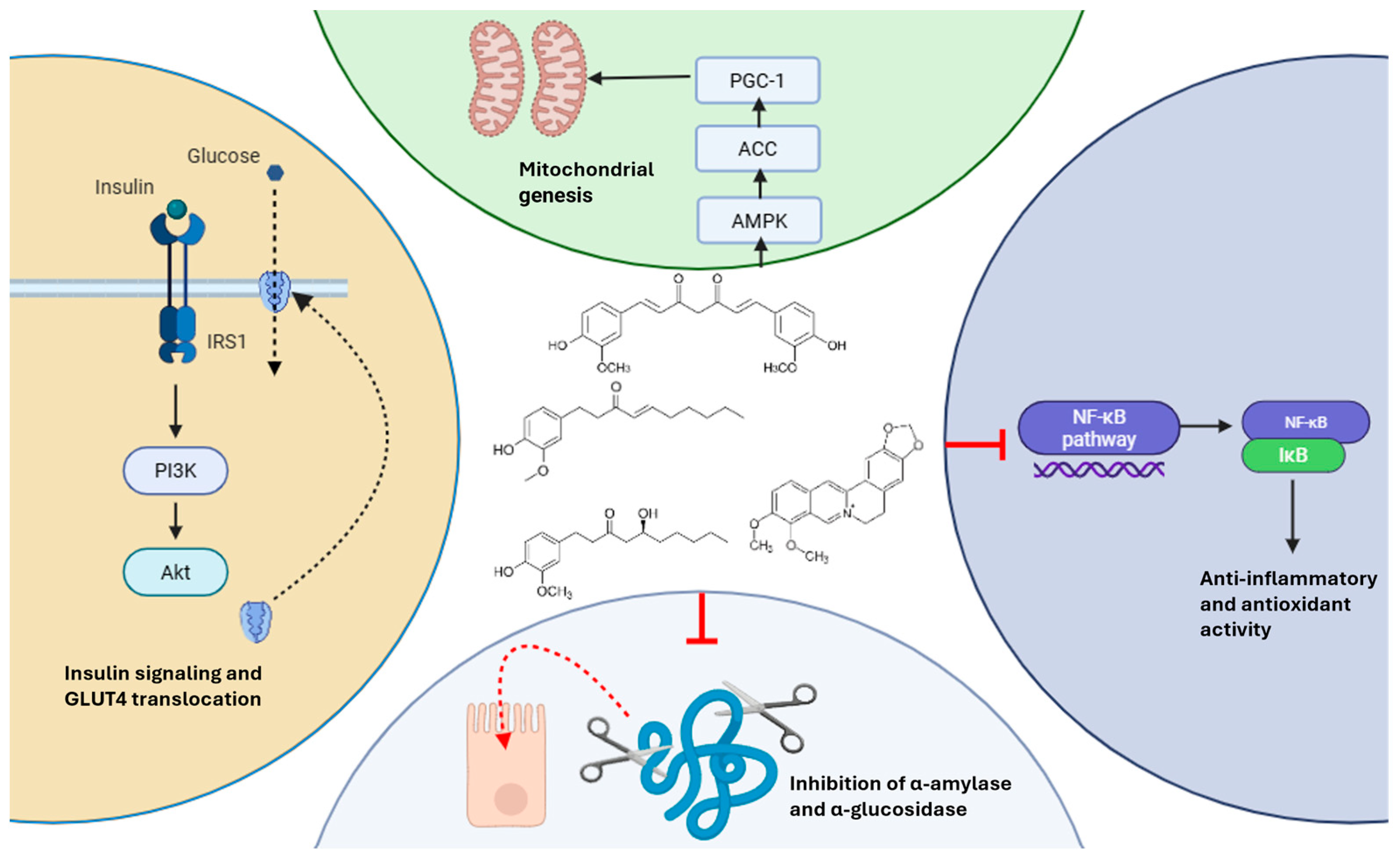
4.7. Mechanistic Summary of Glycemic Effects
4.8. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMPK | AMP-Activated Protein Kinase |
| BBR | Berberine |
| BMI | Body Mass Index |
| FBG | Fasting Blood Glucose |
| FBS | Fasting Blood Sugar |
| GLUT-4 | Glucose Transporter Type 4 |
| HbA1c | Hemoglobin A1c |
| HOMA-IR | Homeostasis Model Assessment of Insulin Resistance |
| IFG | Impaired Fasting Glucose |
| NAFLD | Non-Alcoholic Fatty Liver Disease |
| NF-κB | Nuclear Factor Kappa B |
| PCOS | Polycystic Ovary Syndrome |
| PEPCK | Phosphoenolpyruvate Carboxykinase |
| PICOS | Population, Intervention, Comparison, Outcome, Study Design |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PROSPERO | International Prospective Register of Systematic Reviews |
| RCT | Randomized Controlled Trial |
| SCFAs | Short-Chain Fatty Acids |
| T2 DM | Type 2 Diabetes Mellitus |
| WHR | Waist-to-Hip Ratio |
| 2hPG | 2-Hour Postprandial Glucose |
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Association, A.D. Standards of Medical Care in Diabetes—2022 Abridged for Primary Care Providers. Clin. Diabetes 2022, 40, 10–38. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Xu, B. Anti-Diabetic Effects and Mechanisms of Dietary Polysaccharides. Molecules 2019, 24, 2556. [Google Scholar] [CrossRef]
- Farzaei, F.; Morovati, M.R.; Farjadmand, F.; Farzaei, M.H. A Mechanistic Review on Medicinal Plants Used for Diabetes Mellitus in Traditional Persian Medicine. J. Evid. Based Complement. Altern. Med. 2017, 22, 944–955. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Zou, D.; Liu, W.; Yang, J.; Zhu, N.; Huo, L.; Wang, M.; Hong, J.; Wu, P.; et al. Treatment of Type 2 Diabetes and Dyslipidemia with the Natural Plant Alkaloid Berberine. J. Clin. Endocrinol. Metab. 2008, 93, 2559–2565. [Google Scholar] [CrossRef]
- Ye, Y.; Liu, X.; Wu, N.; Han, Y.; Wang, J.; Yu, Y.; Chen, Q. Efficacy and Safety of Berberine Alone for Several Metabolic Disorders: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Front. Pharmacol. 2021, 12, 653887. [Google Scholar] [CrossRef]
- Yin, J.; Gao, Z.; Liu, D.; Liu, Z.; Ye, J. Berberine Improves Glucose Metabolism Through Induction of Glycolysis. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E148. [Google Scholar] [CrossRef]
- Zhang, T.; He, Q.; Liu, Y.; Chen, Z.; Hu, H. Efficacy and Safety of Curcumin Supplement on Improvement of Insulin Resistance in People with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Evid. Based Complement. Alternat. Med. 2021, 2021, 4471944. Available online: https://pubmed.ncbi.nlm.nih.gov/34484389/ (accessed on 28 September 2024). [CrossRef]
- Zhang, X.; Chen, X.; Tang, Y.; Guan, X.; Deng, J.; Fan, J. Effects of medical plants from Zingiberaceae family on cardiovascular risk factors of type 2 diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. J. Food Biochem. 2022, 46, e14130. Available online: https://pubmed.ncbi.nlm.nih.gov/35332564/ (accessed on 28 September 2024). [CrossRef]
- Zhu, J.; Chen, H.; Song, Z.; Wang, X.; Sun, Z. Effects of Ginger (Zingiber officinale Roscoe) on Type 2 Diabetes Mellitus and Components of the Metabolic Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Evid. Based Complement. Alternat. Med. 2018, 2018, 5692962. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. Available online: https://www.bmj.com/content/372/bmj.n71 (accessed on 31 August 2024). [CrossRef] [PubMed]
- Eriksen, M.B.; Frandsen, T.F. The impact of patient, intervention, comparison, outcome (PICO) as a search strategy tool on literature search quality: A systematic review. J. Med. Libr. Assoc. 2018, 106, 420–431. Available online: https://pubmed.ncbi.nlm.nih.gov/30271283/ (accessed on 31 August 2024). [CrossRef] [PubMed]
- Alarcón Palacios, M.; Ojeda Gómez, R.C.; Ticse Huaricancha, I.L.; Cajachagua Hilario, K. Análisis crítico de ensayos clínicos aleatorizados: Riesgo de sesgo. Rev. Estomatol. Hered. 2015, 25, 304–308. Available online: http://www.scielo.org.pe/scielo.php?script=sci_arttext&pid=S1019-43552015000400008&lng=es&nrm=iso&tlng=es (accessed on 1 September 2024). [CrossRef]
- Cochrane Handbook for Systematic Reviews of Interventions|Cochrane Training. Available online: https://training.cochrane.org/handbook (accessed on 1 September 2024).
- Rohatgi, A. WebPlotDigitizer, Version 4.5. 2021. Available online: https://automeris.io/WebPlotDigitizer (accessed on 8 June 2025).
- Adab, Z.; Eghtesadi, S.; Vafa, M.R.; Heydari, I.; Shojaii, A.; Haqqani, H.; Arablou, T.; Eghtesadi, M. Effect of turmeric on glycemic status, lipid profile, hs-CRP, and total antioxidant capacity in hyperlipidemic type 2 diabetes mellitus patients. Phytother. Res. 2019, 33, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Aghasi, M.; Koohdani, F.; Qorbani, M.; Nasli-Esfahani, E.; Ghazi-Zahedi, S.; Khoshamal, H.; Keshavarz, A.; Sotoudeh, G. Beneficial effects of green cardamom on serum SIRT1, glycemic indices and triglyceride levels in patients with type 2 diabetes mellitus: A randomized double-blind placebo controlled clinical trial. J. Sci. Food Agric. 2019, 99, 3933–3940. [Google Scholar] [CrossRef]
- Amin, F.; Islam, N.; Anila, N.; Gilani, A.H. Clinical efficacy of the co-administration of Turmeric and Black seeds (Kalongi) in metabolic síndrome—A double blind randomized controlled trial—TAK-MetS trial. Complement. Ther. Med. 2015, 23, 165–174. Available online: https://pubmed.ncbi.nlm.nih.gov/25847554/ (accessed on 23 August 2024). [CrossRef]
- Arablou, T.; Aryaeian, N.; Valizadeh, M.; Sharifi, F.; Hosseini, A.; Djalali, M. The effect of ginger consumption on glycemic status, lipid profile and some inflammatory markers in patients with type 2 diabetes mellitus. Int. J. Food Sci. Nutr. 2014, 65, 515–520. [Google Scholar] [CrossRef]
- Arzati, M.M.; Honarvar, N.M.; Saedisomeolia, A.; Anvari, S.; Effatpanah, M.; Arzati, R.M.; Yekaninejad, M.S.; Hashemi, R.; Djalali, M. The effects of ginger on fasting blood sugar, hemoglobin A1c, and lipid profiles in patients with type 2 diabetes. Int. J. Endocrinol. Metab. 2017, 15, e57927. [Google Scholar]
- Asadi, S.; Gholami, M.S.; Siassi, F.; Qorbani, M.; Khamoshian, K.; Sotoudeh, G. Nano curcumin supplementation reduced the severity of diabetic sensorimotor polyneuropathy in patients with type 2 diabetes mellitus: A randomized double-blind placebo- controlled clinical trial. Complement. Ther. Med. 2019, 43, 253–260. [Google Scholar] [CrossRef]
- Awasthi, H.; Nath, R.; Usman, K.; Mani, D.; Khattri, S.; Nischal, A.; Singh, M.; Sawlani, K.K. Effects of a standardized Ayurvedic formulation on diabetes control in newly diagnosed Type-2 diabetics; a randomized active controlled clinical study. Complement. Ther. Med. 2015, 23, 555–561. [Google Scholar] [CrossRef]
- Azimi, P.; Ghiasvand, R.; Feizi, A.; Hariri, M.; Abbasi, B. Effects of Cinnamon, Cardamom, Saffron, and Ginger Consumption on Markers of Glycemic Control, Lipid Profile, Oxidative Stress, and Inflammation in Type 2 Diabetes Patients. Rev. Diabet. Stud. 2014, 11, 258–266. Available online: https://pubmed.ncbi.nlm.nih.gov/26177486/ (accessed on 23 August 2024). [CrossRef] [PubMed]
- Babaahmadi-Rezaei, H.; Kheirollah, A.; Hesam, S.; Ayashi, S.; Aberumand, M.; Adel, M.H.; Zamanpour, M.; Alasvand, M.; Amozgari, Z.; Noor-Behbahani, M.; et al. Decreased lipoprotein (A) and serum high-sensitivity c-reactive protein levels in male patients with atherosclerosis after supplementation with ginger: A randomized controlled trial. ARYA Atheroscler. 2020, 16, 153–160. [Google Scholar] [PubMed]
- Bateni, Z.; Rahimi, H.R.; Hedayati, M.; Afsharian, S.; Goudarzi, R.; Sohrab, G. The effects of nano-curcumin supplementation on glycemic control, blood pressure, lipid profile, and insulin resistance in patients with the metabolic syndrome: A randomized, double-blind clinical trial. Phytother. Res. 2021, 35, 3945–3953. [Google Scholar] [CrossRef] [PubMed]
- Bupparenoo, P.; Pakchotanon, R.; Narongroeknawin, P.; Asavatanabodee, P.; Chaiamnuay, S. Effect of Curcumin on Serum Urate in Asymptomatic Hyperuricemia: A Randomized Placebo-Controlled Trial. J. Diet. Suppl. 2021, 18, 248–260. [Google Scholar] [CrossRef]
- Cao, C.; Su, M. Effects of berberine on glucose-lipid metabolism, inflammatory factors and insulin resistance in patients with metabolic syndrome. Exp. Ther. Med. 2019, 17, 3009. [Google Scholar] [CrossRef]
- Carvalho, G.C.N.; Lira-Neto, J.C.G.; de Araújo, M.F.M.; de Freitas, R.W.J.F.; Zanetti, M.L.; Damasceno, M.M.C. Effectiveness of ginger in reducing metabolic levels in people with diabetes: A randomized clinical trial. Rev. Lat. Am. Enferm. 2020, 28, e3369. [Google Scholar] [CrossRef]
- Cheshmeh, S.; Elahi, N.; Ghayyem, M.; Mosaieby, E.; Moradi, S.; Pasdar, Y.; Tahmasebi, S.; Moradinazar, M. Effect of green cardamom on the expression of genes implicated in obesity and diabetes among obese women with polycystic ovary syndrome: A double blind randomized controlled trial. Genes Nutr. 2022, 17, 17. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Sahebkar, A.; Fogacci, F.; Bove, M.; Giovannini, M.; Borghi, C. Effects of phytosomal curcumin on anthropometric parameters, insulin resistance, cortisolemia and non-alcoholic fatty liver disease indices: A double-blind, placebo-controlled clinical trial. Eur. J. Nutr. 2020, 59, 477–483. [Google Scholar] [CrossRef]
- Dai, P.; Wang, J.; Lin, L.; Zhang, Y.; Wang, Z. Renoprotective effects of berberine as adjuvant therapy for hypertensive patients with type 2 diabetes mellitus: Evaluation via biochemical markers and color Doppler ultrasonography. Exp. Ther. Med. 2015, 10, 869–876. [Google Scholar] [CrossRef]
- Darmian, M.A.; Hoseini, R.; Amiri, E.; Golshani, S. Downregulated hs-CRP and MAD, upregulated GSH and TAC, and improved metabolic status following combined exercise and turmeric supplementation: A clinical trial in middle-aged women with hyperlipidemic type 2 diabetes. J. Diabetes Metab. Disord. 2022, 21, 275–283. [Google Scholar] [CrossRef]
- Dastgheib, M.; Barati-Boldaji, R.; Bahrampour, N.; Taheri, R.; Borghei, M.; Amooee, S.; Mohammadi-Sartang, M.; Wong, A.; Babajafari, S.; Mazloomi, S.M. A comparison of the effects of cinnamon, ginger, and metformin consumption on metabolic health, anthropometric indices, and sexual hormone levels in women with poly cystic ovary syndrome: A randomized double-blinded placebo-controlled clinical trial. Front. Nutr. 2022, 9, 1071515. Available online: https://pubmed.ncbi.nlm.nih.gov/36523331/ (accessed on 23 August 2024). [CrossRef]
- Derosa, G.; D’Angelo, A.; Maffioli, P. The role of a fixed Berberis aristata/Silybum marianum combination in the treatment of type 1 diabetes mellitus. Clin. Nutr. 2016, 35, 1091–1095. [Google Scholar] [CrossRef]
- El Gayar, M.H.; Aboromia, M.M.M.; Ibrahim, N.A.; Abdel Hafiz, M.H. Effects of ginger powder supplementation on glycemic status and lipid profile in newly diagnosed obese patients with type 2 diabetes mellitus. Obes. Med. 2019, 14, 100094. [Google Scholar] [CrossRef]
- Fatemeh, Y.; Siassi, F.; Rahimi, A.; Koohdani, F.; Doostan, F.; Qorbani, M.; Sotoudeh, G. The effect of cardamom supplementation on serum lipids, glycemic indices and blood pressure in overweight and obese pre-diabetic women: A randomized controlled trial. J. Diabetes Metab. Disord. 2017, 16, 40. [Google Scholar] [CrossRef]
- Foroutan, M.; Yarmohammadi, M.; Ghorbani, R.; Movahhed, H. The effect of ginger on blood sugar and urine protein in patients with type 2 diabetes mellitus; a clinical trial. J. Renal. Inj. Prev. 2020, 202, 16. Available online: https://journalrip.com/Article/jrip-26813 (accessed on 24 August 2024). [CrossRef]
- Gao, Y.; Zhou, H.; Zhao, H.; Feng, X.; Feng, J.; Li, Y.; Zhang, H.; Lu, H.; Qian, Q.; Yu, X.; et al. Clinical research of traditional chinese medical intervention on impaired glucose tolerance. Am. J. Chin. Med. 2013, 41, 21–32. [Google Scholar] [CrossRef]
- Ghafarzadegan, R.; Javaheri, J.; Asgari, M.; Golitaleb, M.; Maraki, F.; Zarei, M. The effect of combined herbal capsule on glycemic indices and lipid profile in patients with type 2 diabetes mellitus: A randomized controlled clinical trial. Jundishapur J. Nat. Pharm. Prod. 2021, 16, e109488. [Google Scholar] [CrossRef]
- Guarino, G.; Strollo, F.; Carbone, L.; Corte TDella Letizia, M.; Marino, G.; Gentile, S. Bioimpedance analysis, metabolic effects and safety of the association Berberis aristata/Bilybum marianum: A 52-week double-blind, placebo-controlled study in obese patients with type 2 diabetes. J. Biol. Regul. Homeost. Agents. 2017, 31, 495–502. [Google Scholar]
- Heshmati, J.; Moini, A.; Sepidarkish, M.; Morvaridzadeh, M.; Salehi, M.; Palmowski, A.; Mojtahedi, M.F.; Shidfar, F. Effects of curcumin supplementation on blood glucose, insulin resistance and androgens in patients with polycystic ovary syndrome: A randomized double-blind placebo-controlled clinical trial. Phytomedicine 2021, 80, 153395. [Google Scholar] [CrossRef] [PubMed]
- Hodaei, H.; Adibian, M.; Nikpayam, O.; Hedayati, M.; Sohrab, G. The effect of curcumin supplementation on anthropometric indices, insulin resistance and oxidative stress in patients with type 2 diabetes: A randomized, double-blind clinical trial. Diabetol. Metab. Syndr. 2019, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Huseini, H.F.; Mohamadzadeh, K.; Kianbakht, S.; Mohammadi, S.M.; Ahvazi, M.; Hooseini, M.S.; Khalili, N.; Foroutan, B.; Saberi, M.; Baghaei, A.; et al. Antihyperglycemic efficacy and safety of AKROPOL, a Persian medicine poly-herbal extract mixture, in the treatment of type 2 diabetic patients: A randomized, double-blind and placebo-controlled clinical trial. J. Med. Plants 2023, 22, 1–13. [Google Scholar]
- Imani, H.; Tabibi, H.; Najafi, I.; Atabak, S.; Hedayati, M.; Rahmani, L. Effects of ginger on serum glucose, advanced glycation end products, and inflammation in peritoneal dialysis patients. Nutrition 2015, 31, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Fu, Q.; Wang, S.; Zhao, J.; Chen, Y.; Li, J.; Xiao, Y.; Huang, W.; Sun, R.; Xiao, Y.; et al. Effects of Shenlian formula on microbiota and inflammatory cytokines in adults with type 2 diabetes: A double-blind randomized clinical trial. J. Tradit. Chin. Med. 2023, 43, 760. [Google Scholar] [CrossRef]
- Karandish, M.; Mozaffari-khosravi, H.; Mohammadi, S.M.; Cheraghian, B.; Azhdari, M. The effect of curcumin and zinc co-supplementation on glycemic parameters in overweight or obese prediabetic subjects: A phase 2 randomized, placebo-controlled trial with a multi-arm, parallel-group design. Phytother. Res. 2021, 35, 4377–4387. Available online: https://pubmed.ncbi.nlm.nih.gov/33893671/ (accessed on 23 August 2024). [CrossRef] [PubMed]
- Khajehdehi, P.; Pakfetrat, M.; Javidnia, K.; Azad, F.; Malekmakan, L.; Nasab, M.H.; Dehghanzadeh, G. Oral supplementation of turmeric attenuates proteinuria, transforming growth factor-β and interleukin-8 levels in patients with overt type 2 diabetic nephropathy: A randomized, double-blind and placebo-controlled study. Scand. J. Urol. Nephrol. 2011, 45, 365–370. [Google Scholar] [CrossRef]
- Khandouzi, N.; Shidfar, F.; Rajab, A.; Rahideh, T.; Hosseini, P.; Taheri, M.M. The Effects of Ginger on Fasting Blood Sugar, Hemoglobin A1c, Apolipoprotein B, Apolipoprotein A-I and Malondialdehyde in Type 2 Diabetic Patients. Iran. J. Pharm. Res. 2015, 14, 131–140. [Google Scholar]
- Lazavi, F.; Mirmiran, P.; Sohrab, G.; Nikpayam, O.; Angoorani, P.; Hedayati, M. The barberry juice effects on metabolic factors and oxidative stress in patients with type 2 diabetes: A randomized clinical trial. Complement. Ther. Clin. Pract. 2018, 31, 170–174. [Google Scholar] [CrossRef]
- Mahluji, S.; Attari, V.E.; Mobasseri, M.; Payahoo, L.; Ostadrahimi, A.; Golzari, S.E. Effects of ginger (Zingiber officinale) on plasma glucose level, HbA1c and insulin sensitivity in type 2 diabetic patients. Int. J. Food Sci. Nutr. 2013, 64, 682–686. [Google Scholar] [CrossRef]
- Mirzabeigi, P.; Hooshang Mohammadpour, A.; Salarifar, M.; Gholami, K.; Mojtahedzadeh, M.; Javadi, M.R. The Effect of Curcumin on some of Traditional and Non-traditional Cardiovascular Risk Factors: A Pilot Randomized, Double-blind, Placebo-controlled Trial. Iran. J. Pharm. Res. 2015, 14, 479–486. [Google Scholar]
- Montazerifar, F.; Shourestani, S.; Hosseini, R.; Karajibani, M.; Amanat, S.; Sedaghat, G.; Fanaei, H. Effect of Berberis vulgaris fruit powder on visfatin and metabolic profiles in type 2 diabetes mellitus patients: A randomized, double-blind, placebo-controlled trial. J. Herb. Med. 2023, 38, 100631. [Google Scholar] [CrossRef]
- Mozaffari-Khosravi, H.; Talaei, B.; Jalali, B.A.; Najarzadeh, A.; Mozayan, M.R. The effect of ginger powder supplementation on insulin resistance and glycemic indices in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Complement. Ther. Med. 2014, 22, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Munshi, R.; Karande-Patil, S.; Kumbhar, D.; Deshmukh, A.; Hingorani, L. A randomized, controlled, comparative, proof-of-concept study to evaluate the efficacy and safety of Nisha-Amalaki capsules in prediabetic patients for preventing progression to diabetes. J. Ayurveda Integr. Med. 2023, 14, 100806. [Google Scholar] [CrossRef]
- Nematollahi, S.; Pishdad, G.R.; Zakerkish, M.; Namjoyan, F.; Ahmadi Angali, K.; Borazjani, F. The effect of berberine and fenugreek seed co-supplementation on inflammatory factor, lipid and glycemic profile in patients with type 2 diabetes mellitus: A double-blind controlled randomized clinical trial. Diabetol. Metab. Syndr. 2022, 14, 120. Available online: https://pubmed.ncbi.nlm.nih.gov/35999562/ (accessed on 23 August 2024). [CrossRef]
- Neta, J.F.d.F.; Veras, V.S.; de Sousa, D.F.; Cunha, M.D.C.D.S.O.; Queiroz, M.V.O.; Neto, J.C.G.L.; Damasceno, M.M.C.; Araújo, M.F.M.; Freitas, R.W.J.F. Effectiveness of the piperine-supplemented Curcuma longa L. in metabolic control of patients with type 2 diabetes: A randomised double-blind placebo-controlled clinical trial. Int. J. Food Sci. Nutr. 2021, 72, 968–977. Available online: https://pubmed.ncbi.nlm.nih.gov/33586583/ (accessed on 23 August 2024). [CrossRef]
- Nieman, D.C.; Cialdella-Kam, L.; Knab, A.M.; Shanely, R.A. Influence of Red Pepper Spice and Turmeric on Inflammation and Oxidative Stress Biomarkers in Overweight Females: A Metabolomics Approach. Plant Foods Hum. Nutr. 2012, 67, 415–421. [Google Scholar] [CrossRef]
- Panahi, Y.; Khalili, N.; Sahebi, E.; Namazi, S.; Simental-Mendía, L.E.; Majeed, M.; Sahebkar, A.; Simental-Mendía, L. Effects of Curcuminoids Plus Piperine on Glycemic, Hepatic and Inflammatory Biomarkers in Patients with Type 2 Diabetes Mellitus: A Randomized Double-Blind Placebo-Controlled Trial. Drug Res. 2018, 68, 403–409. [Google Scholar] [CrossRef]
- Panigrahi, A.; Mohanty, S. Efficacy and safety of HIMABERB® Berberine on glycemic control in patients with prediabetes: Double-blind, placebo-controlled, and randomized pilot trial. BMC Endocr. Disord. 2023, 23, 190. Available online: https://pubmed.ncbi.nlm.nih.gov/37679692/ (accessed on 23 August 2024). [CrossRef]
- Rafie, R.; Hosseini, S.A.; Hajiani, E.; Malehi, A.S.; Mard, S.A. Effect of ginger powder supplementation in patients with non-alcoholic fatty liver disease: A randomized clinical trial. Clin. Exp. Gastroenterol. 2020, 13, 35–45. [Google Scholar] [CrossRef]
- Rahimi, H.R.; Mohammadpour, A.H.; Dastani, M.; Jaafari, M.R.; Abnous, K.; Mobarhan, M.G.; Oskuee, R.K. The effect of nano-curcumin on HbA1c, fasting blood glucose, and lipid profile in diabetic subjects: A randomized clinical trial. Avicenna J. Phytomed. 2016, 6, 567. [Google Scholar]
- Rahimlou, M.; Yari, Z.; Rayyani, E.; Keshavarz, S.A.; Hosseini, S.; Morshedzadeh, N.; Hekmatdoost, A. Effects of ginger supplementation on anthropometric, glycemic and metabolic parameters in subjects with metabolic syndrome: A randomized, double-blind, placebo-controlled study. J. Diabetes Metab. Disord. 2019, 18, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, S.; Asgary, S.; Askari, G.; Keshvari, M.; Hatamipour, M.; Feizi, A.; Sahebkar, A. Treatment of Non-alcoholic Fatty Liver Disease with Curcumin: A Randomized Placebo-controlled Trial. Phytother. Res. 2016, 30, 1540–1548. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, H.; Namjoyan, H.; Mehraban, Z.; Zakerkish, M.; Ghaderian, S.B.; Latifi, S.M. The effects of active ingredients of barberry root (Berberine) on glycemic control and insulin resistance in type 2 diabetic patients. Jundishapur J. Nat. Pharm. Prod. 2018, 13, e64180. [Google Scholar] [CrossRef]
- .Rondanelli, M.; Gasparri, C.; Petrangolini, G.; Allegrini, P.; Avenoso, D.; Fazia, T.; Bernardinelli, L.; Peroni, G.; Patelli, Z.; Mansueto, F.; et al. Berberine phospholipid exerts a positive effect on the glycemic profile of overweight subjects with impaired fasting blood glucose (IFG): A randomized double-blind placebo-controlled clinical trial. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 6718–6727. Available online: https://pubmed.ncbi.nlm.nih.gov/37522683/ (accessed on 23 August 2024). [PubMed]
- Sartore, G.; Ragazzi, E.; Antonello, G.; Cosma, C.; Lapolla, A. Effect of a new formulation of nutraceuticals as an add-on to metformin monotherapy for patients with type 2 diabetes and suboptimal glycemic control: A randomized controlled trial. Nutrients 2021, 13, 2373. [Google Scholar] [CrossRef]
- Sharma, R.K.; Sharma, B.; Jindal, M.; Gupta, A.K.; Kunwar, R.; Lata, S.; Yadav, A. Evaluation of hypolipidemic effect of stem part of Berberis aristata in type 2 diabetes mellitus patients as add on therapy. Natl. J. Physiol. Pharm. Pharmacol. 2017, 7, 1159–1169. [Google Scholar] [CrossRef]
- Shidfar, F.; Rajab, A.; Rahideh, T.; Khandouzi, N.; Hosseini, S.; Shidfar, S. The effect of ginger (Zingiber officinale) on glycemic markers in patients with type 2 diabetes. J. Complement. Integr. Med. 2015, 12, 165–170. [Google Scholar] [CrossRef]
- Shidfar, F.; Seyyed Ebrahimi, S.; Hosseini, S.; Heydari, I.; Shidfar, S.; Hajhassani, G. The Effects of Berberis vulgaris Fruit Extract on Serum Lipoproteins, apoB, apoA-I, Homocysteine, Glycemic Control and Total Antioxidant Capacity in Type 2 Diabetic Patients. Iran. J. Pharm. Res. 2012, 11, 643–652. [Google Scholar]
- Sohaei, S.; Amani, R.; Tarrahi, M.J.; Ghasemi-Tehrani, H. The effects of curcumin supplementation on glycemic status, lipid profile and hs-CRP levels in overweight/obese women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled clinical trial. Complement. Ther. Med. 2019, 47, 102201. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, D.F.; de Araújo, M.F.M.; de Mello, V.D.; Damasceno, M.M.C.; de Freitas, R.W.J.F. Cost-Effectiveness of Passion Fruit Albedo versus Turmeric in the Glycemic and Lipaemic Control of People with Type 2 Diabetes: Randomized Clinical Trial. J. Am. Coll. Nutr. 2021, 40, 679–688. Available online: https://pubmed.ncbi.nlm.nih.gov/33141635/ (accessed on 23 August 2024). [CrossRef]
- Tahmasebi, L.; Zakerkish, M.; Golfakhrabadi, F.; Namjoyan, F. Randomised clinical trial of Berberis vulgaris root extract on glycemic and lipid parameters in type 2 diabetes mellitus patients. Eur. J. Integr. Med. 2019, 32, 100998. [Google Scholar] [CrossRef]
- Zakerkish, M.; Hemmati, A.; Sebardan, F.J.; Maram, N.S. Effect of Curcumex on Serum Lipid Profile and Fasting Blood Glucose, HbA1c, and Insulin Resistance Levels in Type 2 Diabetic Patients: A Randomized, Double-Blind Clinical Trial. Jundishapur J. Nat. Pharm. Prod. 2023, 18, e136383. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Harikumar, K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. Available online: https://pubmed.ncbi.nlm.nih.gov/18662800/ (accessed on 30 May 2025). [CrossRef]
- Zhang, D.W.; Fu, M.; Gao, S.H.; Liu, J.L. Curcumin and Diabetes: A Systematic Review. Evid.-Based Complement. Altern. Med. 2013, 2013, 636053. [Google Scholar] [CrossRef] [PubMed]
- Yanai, H.; Yoshida, H. Beneficial Effects of Adiponectin on Glucose and Lipid Metabolism and Atherosclerotic Progression: Mechanisms and Perspectives. Int. J. Mol. Sci. 2019, 20, 1190. Available online: https://www.mdpi.com/1422-0067/20/5/1190/htm (accessed on 30 May 2025). [CrossRef]
- Vanucci-Bacqué, C.; Bedos-Belval, F. Anti-inflammatory activity of naturally occuring diarylheptanoids—A review. Bioorg. Med. Chem. 2021, 31, 115971. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0968089620308014 (accessed on 30 May 2025). [CrossRef] [PubMed]
- Umeno, A.; Horie, M.; Murotomi, K.; Nakajima, Y.; Yoshida, Y. Antioxidative and Antidiabetic Effects of Natural Polyphenols and Isoflavones. Molecules 2016, 21, 708. Available online: https://www.mdpi.com/1420-3049/21/6/708/htm (accessed on 30 May 2025). [CrossRef]
- He, Y.; Li, W.; Hu, G.; Sun, H.; Kong, Q. Bioactivities of EF24, a Novel Curcumin Analog: A Review. Front. Oncol. 2018, 8, 614. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC6297553/ (accessed on 30 May 2025). [CrossRef] [PubMed]
- Sudarshan, K.; Perumal, G.; Aidhen, I.S.; Doble, M. Synthesis of Unsymmetrical Linear Diarylheptanoids and their Enantiomers and Antiproliferative Activity Studies. Eur. J. Org. Chem. 2018, 2018, 6379–6387. [Google Scholar] [CrossRef]
- Sudarshan, K.; Yarlagadda, S.; Sengupta, S. Recent Advances in the Synthesis of Diarylheptanoids. Chem. Asian J. 2024, 19, e202400380. [Google Scholar] [CrossRef]
- Yin, J.; Xing, H.; Ye, J. Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism 2008, 57, 712–717. Available online: https://pubmed.ncbi.nlm.nih.gov/18442638/ (accessed on 30 May 2025). [CrossRef]
- Xie, W.; Su, F.; Wang, G.; Peng, Z.; Xu, Y.; Zhang, Y.; Xu, N.; Hou, K.; Hu, Z.; Chen, Y.; et al. Glucose-lowering effect of berberine on type 2 diabetes: A systematic review and meta-analysis. Front. Pharmacol. 2022, 13, 1015045. Available online: https://www.crd.york.ac.uk/prospero/ (accessed on 27 October 2024). [CrossRef] [PubMed]
- Zhang, X.; Zhao, Y.; Xu, J.; Xue, Z.; Zhang, M.; Pang, X.; Zhang, X.; Zhao, L. Modulation of gut microbiota by berberine and metformin during the treatment of high-fat diet-induced obesity in rats. Sci. Rep. 2015, 5, 14405. Available online: https://www.nature.com/articles/srep14405 (accessed on 30 May 2025). [CrossRef] [PubMed]
- Kong, W.J.; Wei, J.; Zuo, Z.Y.; Wang, Y.M.; Song, D.Q.; You, X.F.; Zhao, L.-X.; Pan, H.-N.; Jiang, J.-D. Combination of simvastatin with berberine improves the lipid-lowering efficacy. Metabolism 2008, 57, 1029–1037. Available online: https://pubmed.ncbi.nlm.nih.gov/18640378/ (accessed on 30 May 2025). [CrossRef]
- Utami, A.R.; Maksum, I.P.; Deawati, Y. Berberine and Its Study as an Antidiabetic Compound. Biology 2023, 12, 973–2023. Available online: https://www.mdpi.com/2079-7737/12/7/973/htm (accessed on 27 October 2024). [CrossRef]
- Servida, S.; Panzeri, E.; Tomaino, L.; Marfia, G.; Garzia, E.; Ciniglio Appiani, G.; Moroncini, G.; Colonna, V.D.G.; La Vecchia, C.; Vigna, L. Overview of Curcumin and Piperine Effects on Glucose Metabolism: The Case of an Insulinoma Patient’s Loss of Consciousness. Int. J. Mol. Sci. 2023, 24, 6621. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC10095254/ (accessed on 27 October 2024). [CrossRef] [PubMed]
- Li, A.; Liu, Q.; Li, Q.; Liu, B.; Yang, Y.; Zhang, N. Berberine Reduces Pyruvate-driven Hepatic Glucose Production by Limiting Mitochondrial Import of Pyruvate through Mitochondrial Pyruvate Carrier 1. EBioMedicine 2018, 34, 243–255. Available online: http://www.thelancet.com/article/S2352396418302858/fulltext (accessed on 27 October 2024). [CrossRef]
- Wang, Z.; Xu, D.; She, L.; Zhang, Y.; Wei, Q.; Aa, J.; Wang, G.; Liu, B.; Xie, Y. Curcumin restrains hepatic glucose production by blocking cAMP/PKA signaling and reducing acetyl CoA accumulation in high-fat diet (HFD)-fed mice. Mol. Cell. Endocrinol. 2018, 474, 127–136. Available online: https://pubmed.ncbi.nlm.nih.gov/29499209/ (accessed on 27 October 2024). [CrossRef]
- Ren, G.; Guo, J.H.; Qian, Y.Z.; Kong, W.J.; Jiang, J.D. Berberine Improves Glucose and Lipid Metabolism in HepG2 Cells Through AMPKα1 Activation. Front. Pharmacol. 2020, 11, 647. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC7225256/ (accessed on 27 October 2024). [CrossRef]
- Febriza, A.; Zahrah, A.A.; Andini, N.S.; Usman, F.; Idrus, H.H. Potential Effect of Curcumin in Lowering Blood Glucose Level in Streptozotocin-Induced Diabetic Rats. Diabetes Metab. Syndr. Obes. 2024, 17, 3305. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC11380866/ (accessed on 27 October 2024). [CrossRef]
- Ilyas, Z.; Perna, S.; Al-thawadi, S.; Alalwan, T.A.; Riva, A.; Petrangolini, G.; Gasparri, C.; Infantino, V.; Peroni, G.; Rondanelli, M. The effect of Berberine on weight loss in order to prevent obesity: A systematic review. Biomed. Pharmacother. 2020, 127, 110137. [Google Scholar] [CrossRef]
- Yang, F.; Gao, R.; Luo, X.; Liu, R.; Xiong, D. Berberine influences multiple diseases by modifying gut microbiota. Front. Nutr. 2023, 10, 1187718. [Google Scholar] [CrossRef] [PubMed]
- Roufogalis, B.D. Zingiber officinale (Ginger): A Future Outlook on Its Potential in Prevention and Treatment of Diabetes and Prediabetic States. New J. Sci. 2014, 2014, 674684. [Google Scholar] [CrossRef]
- Hussain, Y.; Khan, H.; Alotaibi, G.; Khan, F.; Alam, W.; Aschner, M.; Jeandet, P.; Saso, L. How Curcumin Targets Inflammatory Mediators in Diabetes: Therapeutic Insights and Possible Solutions. Molecules 2022, 27, 4058. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC9268477/ (accessed on 27 October 2024). [CrossRef]
- Li, Y.; Tran, V.H.; Duke, C.C.; Roufogalis, B.D. Preventive and Protective Properties of Zingiber officinale (Ginger) in Diabetes Mellitus, Diabetic Complications, and Associated Lipid and Other Metabolic Disorders: A Brief Review. Evid. Based Complement. Alternat. Med. 2012, 2012, 516870. Available online: https://pubmed.ncbi.nlm.nih.gov/23243452/ (accessed on 27 October 2024). [CrossRef]
- Chen, J.; Sun, J.; Prinz, R.A.; Li, Y.; Xu, X. Gingerenone A Sensitizes the Insulin Receptor and Increases Glucose Uptake by Inhibiting the Activity of p70 S6 Kinase. Mol. Nutr. Food Res. 2018, 62, e1800709. Available online: https://pubmed.ncbi.nlm.nih.gov/30296358/ (accessed on 27 October 2024). [CrossRef]
- Mokgalaboni, K.; Mashaba, R.G.; Phoswa, W.N.; Lebelo, S.L. Curcumin Attenuates Hyperglycemia and Inflammation in Type 2 Diabetes Mellitus: Quantitative Analysis of Randomized Controlled Trial. Nutrients 2024, 16, 4177. Available online: https://www.mdpi.com/2072-6643/16/23/4177/htm (accessed on 30 May 2025). [CrossRef] [PubMed]
- Committee, A.D.A.P.P.; ElSayed, N.A.; Aleppo, G.; Bannuru, R.R.; Bruemmer, D.; Collins, B.S.; Ekhlaspour, L.; Hilliard, M.E.; Johnson, E.L.; Khunti, K.; et al. Glycemic Goals and Hypoglycemia: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47 (Suppl. S1), S111–S125. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, X.; Yin, M.; Zhang, Y.; Huang, L.; Chen, R.; Ni, J. Effects of berberine on blood glucose in patients with type 2 diabetes mellitus: A systematic literature review and a meta-analysis. Endocr. J. 2019, 66, 51–63. Available online: https://pubmed.ncbi.nlm.nih.gov/30393248/ (accessed on 27 October 2024). [CrossRef] [PubMed]
- Ballester, P.; Cerdá, B.; Arcusa, R.; García-Muñoz, A.M.; Marhuenda, J.; Zafrilla, P. Antioxidant Activity in Extracts from Zingiberaceae Family: Cardamom, Turmeric, and Ginger. Molecules 2023, 28, 4024. Available online: https://www.mdpi.com/1420-3049/28/10/4024/htm (accessed on 1 November 2024). [CrossRef]
- Zamani, M.; Zarei, M.; Nikbaf-Shandiz, M.; Hosseini, S.; Shiraseb, F.; Asbaghi, O. The effects of berberine supplementation on cardiovascular risk factors in adults: A systematic review and dose-response meta-analysis. Front. Nutr. 2022, 9, 1013055. [Google Scholar] [CrossRef]
- Chuengsamarn, S.; Rattanamongkolgul, S.; Luechapudiporn, R.; Phisalaphong, C.; Jirawatnotai, S. Curcumin Extract for Prevention of Type 2 Diabetes. Diabetes Care 2012, 35, 2121–2127. [Google Scholar] [CrossRef] [PubMed]
- de Melo, I.S.V.; dos Santos, A.F.; Bueno, N.B. Curcumin or combined curcuminoids are effective in lowering the fasting blood glucose concentrations of individuals with dysglycemia: Systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2018, 128, 137–144. Available online: https://pubmed.ncbi.nlm.nih.gov/28928074/ (accessed on 30 May 2025). [CrossRef] [PubMed]
- Scully, T.; Ettela, A.; LeRoith, D.; Gallagher, E.J. Obesity, Type 2 Diabetes, and Cancer Risk. Front. Oncol. 2021, 10, 615375. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC7884814/ (accessed on 27 October 2024). [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e984. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC8493650/ (accessed on 27 October 2024). [CrossRef] [PubMed]
- Gumbarewicz, E.; Jarząb, A.; Stepulak, A.; Kukula-Koch, W. Zingiber officinale Rosc. in the Treatment of Metabolic Syndrome Disorders-A Review of In Vivo Studies. Int. J. Mol. Sci. 2022, 23, 15545. Available online: https://pubmed.ncbi.nlm.nih.gov/36555184/ (accessed on 27 October 2024). [CrossRef]
- Maharlouei, N.; Tabrizi, R.; Lankarani, K.B.; Rezaianzadeh, A.; Akbari, M.; Kolahdooz, F.; Rahimi, M.; Keneshlou, F.; Asemi, Z. The effects of ginger intake on weight loss and metabolic profiles among overweight and obese subjects: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2019, 59, 1753–1766. Available online: https://pubmed.ncbi.nlm.nih.gov/29393665/ (accessed on 27 October 2024). [CrossRef]
- Asbaghi, O.; Ghanbari, N.; Shekari, M.; Reiner, Ž.; Amirani, E.; Hallajzadeh, J.; Mirsafaei, L.; Asemi, Z. The effect of berberine supplementation on obesity parameters, inflammation and liver function enzymes: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. ESPEN 2020, 38, 43–49. Available online: https://pubmed.ncbi.nlm.nih.gov/32690176/ (accessed on 27 October 2024). [CrossRef]
- Ebrahimzadeh Attari, V.; Ostadrahimi, A.; Asghari Jafarabadi, M.; Mehralizadeh, S.; Mahluji, S. Changes of serum adipocytokines and body weight following Zingiber officinale supplementation in obese women: A RCT. Eur. J. Nutr. 2016, 55, 2129–2136. Available online: https://pubmed.ncbi.nlm.nih.gov/26318445/ (accessed on 27 October 2024). [CrossRef]
- Unhapipatpong, C.; Julanon, N.; Chattranukulchai Shantavasinkul, P.; Polruang, N.; Numthavaj, P.; Thakkinstian, A. An Umbrella Review of Systematic Reviews and Meta-analyses of Randomized Controlled Trials Investigating the Effect of Curcumin Supplementation on Lipid Profiles. Nutr. Rev. 2025, nuaf012. [Google Scholar] [CrossRef]
- Kasprzak-Drozd, K.; Oniszczuk, T.; Gancarz, M.; Kondracka, A.; Rusinek, R.; Oniszczuk, A. Curcumin and Weight Loss: Does It Work? Int. J. Mol. Sci. 2022, 23, 639. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC8775659/ (accessed on 27 October 2024). [CrossRef]
- Hu, X.; Zhang, Y.; Xue, Y.; Zhang, Z.; Wang, J. Berberine is a potential therapeutic agent for metabolic syndrome via brown adipose tissue activation and metabolism regulation. Am. J. Transl. Res. 2018, 10, 3322. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC6291723/ (accessed on 27 October 2024). [PubMed]


| Element | Description |
|---|---|
| P (Participants) | Individuals with alterations in glycemic profile |
| I (Intervention) | Consumption of species from the Zingiberaceae or Berberidaceae families or their extracted bioactives |
| C (Comparisons) | Control group receiving a placebo |
| O (Outcomes) | Changes in fasting plasma glucose, glycated hemoglobin (HbA1c), fasting insulin levels, HOMA-IR index, or any other parameter related to the glycemic profile |
| S (Study Design) | Randomized controlled trials |
| Reference | Year | Country | Design RCT | N | Women, n (%) | Age (Years) | BMI (kg/m2) | Family; Extract | Daily Dosage | Duration | Participants’ Health Condition | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adab et al. [16] | 2019 | Iran | Double blind, parallel. | 75 | 39 (52.0%) | 55.2 ± 7.4 | 29.4 ± 4.4 | Zingiberaceae; Turmeric | 2.1 g | 8 weeks | T2 DM | HbA1c; FBS; Insulin; HOMA-IR; Body weight; BMI |
| Aghasi et al. [17] | 2019 | Iran | Double blind, parallel. | 83 | 39 (47.0%) | 53.6 ± 6.2 | 29.1 ± 3.2 | Zingiberaceae; Green cardamom | 3 g | 10 weeks | T2 DM | HbA1c; FBS; Insulin; HOMA-IR; BMI |
| Amin et al. [18] | 2015 | Pakistan | Double blind, parallel. | 126 | 0% | 42.0 ± 13.2 | 27.8 ± 4.6 | Zingiberaceae; Turmeric | 2.4 g | 8 weeks | Prediabetes; Dyslipidaemia or pre-hypertension | FBS |
| Arablou et al. [19] | 2014 | Iran | Double blind, parallel. | 63 | 48 (76.2%) | 52.3 ± 8.6 | 26.9 ± 3.5 | Zingiberaceae; Ginger | 1.6 g | 12 weeks | T2 DM | HbA1c; FBS; Insulin; HOMA-IR; Body weight; BMI |
| Arzati et al. [20] | 2017 | Iran | Double blind, parallel. | 45 | 34 (68.0%) | 50.7 ± 8.5 | 29.6 ± 4.1 | Zingiberaceae; Ginger | 2 g | 10 weeks | T2 DM | HbA1c; FBS; Body weight; BMI |
| Asadi et al. [21] | 2019 | Iran | Double blind, parallel. | 80 | 70 (87.5%) | 54.1 ± 6.3 | 31.1 ± 4.0 | Zingiberaceae; NanoCurcumin | 80 mg | 8 weeks | T2 DM | HbA1c; FBS; Body weight; BMI; 2hPG |
| Awasthi et al. [22] | 2015 | India | Parallel, absence of blinding. | 80 | 33 (41.3%) | 45.6 ± 8.4 | 25.6 ± 2.9 | Berberidaceae; B. aristata | 500 mg | 24 weeks | T2 DM | HbA1c; FBS; 2hPG |
| Azimi et al. (a) [23] | 2015 | Iran | Single blind, parallel. | 80 | 50 (62.5%) | 54.4 ± 1.4 | 28.7 ± 0.4 | Zingiberaceae; Ginger | 3 g | 8 weeks | T2 DM | HbA1c; FBS; Insulin; Body weight; BMI |
| Azimi et al. (b) [23] | 2015 | Iran | Single blind, parallel. | 80 | 49 (60.5%) | 52.6 ± 1.7 | 28.7 ± 0.3 | Zingiberaceae; Cardamom | 3 g | 8 weeks | T2 DM | HbA1c; FBS; Insulin; Body weight; BMI |
| Babaahmadi-Rezaei et al. [24] | 2020 | Iran | Double blind, parallel. | 57 | 0 | 56.4 ± 7.7 | 27.0 ± 1.2 | Zingiberaceae; Ginger | 1.6 g | 8 weeks | Atherosclerosis | FBS; Body weight; BMI |
| Bateni et al. [25] | 2019 | Iran | Double blind, parallel. | 43 | 33 (76.7%) | 52.0 ± 8.2 | 29.7 ± 4.4 | Zingiberaceae; Curcumin | 80 mg | 12 weeks | Metabolic syndrome | HbA1c; FBS; Insulin; HOMA-IR; Body weight; BMI |
| Bupparenoo et al. [26] | 2020 | Thailand | Double blind, parallel. | 39 | 15 (38.5%) | 55.4 ± 10.9 | 27.1 ± 4.2 | Zingiberaceae; Curcumin | 1 g | 8 weeks | Asymptomatic Hyperuricemia | FBS |
| Cao et al. [27] | 2019 | China | Parallel. | 80 | 40 (50.0%) | 65.6 ± 1.8 | NR | Berberidaceae; Berberine | NR | 4 weeks | Metabolic syndrome | FBS; HOMA-IR; 2hPG |
| Carvalho et al. [28] | 2020 | Brazil | Double blind, parallel. | 103 | 71 (69.9%) | 58.6 ± 11.1 | NR | Zingiberaceae; Ginger | 1.2 g | 12 weeks | T2 DM | HbA1c; FBS; HOMA-IR |
| Cheshmeh et al. [29] | 2022 | Iran | Double blind, parallel. | 194 | 194 (100%) | 33.4 ± 5.5 | 35.0 ± 4.3 | Zingiberaceae; Green cardamom | 3.0 g | 16 weeks | Obese women with PCOS | HbA1c; FBS; Insulin; HOMA-IR; Body weight; BMI |
| Cicero et al. [30] | 2020 | Italy | Double blind, parallel. | 80 | 43 (53.8%) | 53.5 ± 4.1 | 27.0 ± 1.8 | Zingiberaceae; Curcumin | 800 mg | 8 weeks | Untreated overweight subjects with suboptimal values of FPG | FBS; Insulin; HOMA-IR; BMI |
| Dai et al. [31] | 2015 | China | Double blind, parallel. | 69 | 34 (49.3%) | 54.2 ± 11.1 | 24.3 ± 4.2 | Berberidaceae; Berberine | 300 mg | 96 weeks | Hypertension and T2 DM | HbA1c; FBS |
| Darmian et al. [32] | 2022 | Iran | Single blind, parallel. | 21 | 21 (100%) | 44.3 ± 2.2 | 29.2 ± 2.5 | Zingiberaceae; Turmeric | 2.1 g | 8 weeks | Hyperlipidemic T2 DM | FBS; Body weight; BMI |
| Dastgheib et al. [33] | 2022 | India | Double blind, parallel. | 43 | 43 (100%) | 29.2 ± 5.7 | 26.9 ± 5.0 | Zingiberaceae; Ginger | 1.5g | 8 weeks | PCOS | FBS; Insulin; HOMA-IR; Body weight; BMI |
| Derosa et al. [34] | 2015 | Italy | Double blind, parallel. | 85 | 44 (51.8%) | 30.2 ± 7.6 | 22.74 ± 1.8 | Berberidaceae; B. aristata/Silybum marianum | 1.18 g BBR + 210 mg SM | 24 weeks | T1 DM | HbA1c; FBS; Body weight; BMI; 2hPG |
| El Gayar et al. [35] | 2019 | Egypt | Single blind, parallel. | 80 | 39 (48.8%) | 46.2 ± 9.1 | 32.1 ± 1.3 | Zingiberaceae; Ginger | 1.8 g | 8 weeks | T2 DM | HbA1c; FBS; Insulin; HOMA-IR; BMI; 2hPG |
| Fatemeh et al. [36] | 2017 | Iran | Double blind, parallel. | 80 | 80 (100%) | 47.9 ± 10.3 | 29.5 ± 3.6 | Zingiberaceae; Cardamom | 3 g | 8 weeks | Overweight and obese prediabetic women | FBS; Insulin; HOMA-IR |
| Foroutan et al. [37] | 2022 | Iran | Clinical trial study. | 98 | 55 (51.9%) | 60.4 ± 9.4 | NR | Zingiberaceae; Ginger | 500 mg | 12 weeks | T2 DM | HbA1c; FBS; 2hPG |
| Gao et al. [38] | 2013 | China | Parallel. | 458 | 288 (62.9%) | 50.2 ± 1.6 | 25.2 ± 0.4 | Berberidaceae; Berberine | 10 g | 36 weeks | Impaired Glucose Tolerance | HbA1c; FBS; BMI; 2hPG |
| Ghafarzadegan et al. [39] | 2021 | Iran | Single blind, parallel. | 80 | 26 (32.5%) | 54.4 ± 7.0 | 27.3 ± 3.3 | Berberidaceae; B. vulgaris | 100 mg | 12 weeks | T2 DM | HbA1c; FBS; 2hPG |
| Guarino et al. [40] | 2017 | Italy | Double blind, parallel. | 136 | 80 (58.8%) | 55.5 ± 8.5 | 34.0 ± 4.5 | Berberidaceae; B. aristata/Silybum marianum | 1 g BBR + 210 mg SM | 52 weeks | Obese patients with T2 DM | HbA1c; HOMA-IR |
| Heshmati et al. [41] | 2020 | Iran | Double blind, parallel. | 67 | 67 (100%) | 30.9 ± 6.7 | 28.0 ± 4.9 | Zingiberaceae; Curcumin | 1,5 g | 12 weeks | PCOS | FBS; Insulin; HOMA-IR; BMI |
| Hodaei et al. [42] | 2019 | Iran | Double blind, parallel. | 44 | 22 (50.0%) | 59.4 ± 7.4 | 28.7 ± 3.2 | Zingiberaceae; Curcumin | 1.5 g | 10 weeks | T2 DM | HbA1c; FBS; Insulin; HOMA-IR; Body weight; BMI |
| Huseini et al. [43] | 2023 | Iran | Double blind, parallel. | 86 | 54 (62.7%) | 54.3 ± 6.0 | NR | Zingiberaceae; Turmeric | 1 g | 12 weeks | T2 DM | HbA1c; FBS |
| Imani et al. [44] | 2015 | Iran | Double blind, parallel. | 36 | 15 (41.7%) | 56.0 ± 8.1 | 27.0 ± 3.0 | Zingiberaceae; Ginger | 1 g | 10 weeks | Peritoneal dialysis | FBS; Body weight; BMI |
| Jiang et al. [45] | 2023 | China | Double blind, parallel. | 31 | 16 (51.6%) | 54.8 ± 10.6 | NR | Berberidaceae; Berberine | 10 g | 12 weeks | T2 DM | HbA1c; FBS; Insulin; HOMA-IR; 2hPG |
| Karandish et al. [46] | 2021 | Iran | Double blind, parallel. | 41 | 28 (68.3%) | 35.6 ± 7.2 | 30.7 ± 2.5 | Zingiberaceae; Curcumin | 400 mg | 12 weeks | Overweight or obese prediabeticsubjects | HbA1c; FBS; Insulin; Body weight; BMI; 2hPG |
| Khajehdehi et al. [47] | 2011 | Iran | Double blind, parallel. | 40 | 18 (45.0%) | 52.8 ± 9.3 | NR | Zingiberaceae; Turmeric | 1.5 g | 8 weeks | Patients with overtype 2 diabetic nephropathy | FBS; 2hPG |
| Khandouzi et al. [48] | 2013 | Iran | Double blind, parallel. | 41 | 27 (41.5%) | 46.1 ± 7.9 | NR | Zingiberaceae; Ginger | 2.0 g | 12 weeks | T2 DM | HbA1c; FBS |
| Mahluji et al. [50] | 2013 | Iran | Double blind, parallel. | 58 | 24 (41.4%) | 51.2 ± 6.9 | 29.5 ± 4.5 | Zingiberaceae; Ginger | 2 g | 8 weeks | T2 DM | HbA1c; FBS; Insulin; HOMA-IR; Body weight; BMI |
| Mirzabeigi et al. [51] | 2015 | Iran | Double blind, parallel. | 33 | 9 (27.3%) | 62.9 ± 8.5 | 28.2 ± 3.6 | Zingiberaceae; Curcumin | 2 g | 8 weeks | Patients with coronary artery disease | FBS |
| Montazerifar et al. [52] | 2023 | Iran | Double blind, parallel. | 40 | 29 (72.5%) | 55.8 ± 9.1 | 28.5 ± 4.7 | Berberidaceae; B. vulgaris | 1 g | 8 weeks | T2 DM | HbA1c; FBS; Insulin; HOMA-IR; Body weight; BMI |
| Mozaffari-Khosrav et al. [53] | 2014 | Iran | Double blind, parallel. | 81 | 50 (61.7%) | 50.5 ± 7.5 | 28.3 ± 5.1 | Zingiberaceae; Ginger | 3 g | 8 weeks | T2 DM | HbA1c; FBS; BMI |
| Munshi et al. [54] | 2023 | India | Single blind, parallel. | 58 | 36 (58.1%) | 51.5 ± 8.8 | 28.3 ± 4.4 | Zingiberaceae; C. longa | 1 g | 24 weeks | Prediabetic patients | HbA1c; FBS; Insulin; HOMA-IR; Body weight; BMI; 2hPG |
| Nematollahi et al. [55] | 2022 | Iran | Double blind, parallel. | 50 | 34 (68.0%) | 27.6 ± 3.7 | 27.6 ± 3.7 | Berberidaceae; Berberine and fenugreek | 900 mg BBR + 600 mg fenugreek | 12 weeks | T2 DM | HbA1c; FBS; Insulin |
| Neta et al. [56] | 2021 | Brazil | Double blind, parallel. | 61 | 47 (77.1%) | 62.5 ± 11.0 | 29.1 ± 4.9 | Zingiberaceae; C. longa | 500 mg | 16 weeks | T2 DM | HbA1c; FBS; Insulin; HOMA-IR; BMI |
| Nieman et al. [57] | 2012 | USA | Double blind, parallel. | 30 | 30 (100%) | 55.7 ± 7.7 | NR | Zingiberaceae; Turmeric | 2.8 g | 4 weeks | Overweight patients | FBS; Body weight |
| Panahi et al. [58] | 2018 | Iran | Double blind, parallel. | 100 | 49 (49.0%) | 42.0 ± 7.6 | 25.6 ± 2.1 | Zingiberaceae; Curcumin | 500 mg | 12 weeks | T2 DM | HbA1c; FBS; Insulin; HOMA-IR; Body weight; BMI |
| Panigrahi et al. [59] | 2023 | India | Double blind, parallel. | 34 | 26 (76.5%) | 44.2 ± 8.6 | 25.5 ± 4.2 | Berberidaceae; Berberine | 1.5 g | 12 weeks | Prediabetic patients | HbA1c; FBS; Insulin; HOMA-IR; 2hPG |
| Rafie et al. [60] | 2020 | Iran | Double blind, parallel. | 46 | 26 (56.6%) | 49.0 ± 9.7 | 31.3 ± 3.0 | Zingiberaceae; Ginger | 1.5 g | 12 weeks | Patientswith NAFLD | FBS; Insulin; HOMA-IR; Body weight; BMI |
| Rahimi et al. [61] | 2016 | Iran | Double blind, parallel. | 70 | 39 (55.7%) | 58.7 ± 11.1 | 27.1 ± 3.2 | Zingiberaceae; NanoCurcumin | 80 mg | 12 weeks | T2 DM | HbA1c; FBS; BMI |
| Rahimlou et al. [62] | 2019 | Iran | Double blind, parallel. | 37 | 16 (43.2%) | 43.9 ± 8.4 | 30.5 ± 2.7 | Zingiberaceae; Ginger | 2 g | 12 weeks | Patients with metabolicsyndrome | FBS; HOMA-IR; Body weight; BMI |
| Rahmani et al. [63] | 2016 | Iran | Double blind, parallel. | 77 | 42 (52.5%) | 47.7 ± 10.7 | 31.1 ± 5.1 | Zingiberaceae; Curcumin | 500 mg | 8 weeks | NAFLD | HbA1c; FBS; Body weight; BMI |
| Rashidi et al. [64] | 2018 | Iran | Double blind, parallel. | 81 | 50 (61.7%) | 47.6 ± 7.8 | 29.5 ± 7.3 | Berberidaceae; Berberine | 1 g | 4 weeks | T2 DM | FBS; Insulin; HOMA-IR; BMI; 2hPG |
| Rondanelli et al. [65] | 2023 | Italy | Double blind, parallel. | 49 | 28 (57.1%) | 59.5 ± 7.8 | 29.8 ± 3.3 | Berberidaceae; Berberine | 550 mg | 8 weeks | Overweight patients with an IFG sta-tus | HbA1c; FBS; Insulin; HOMA-IR |
| Sartore et al. [66] | 2021 | Italy | Single blind, parallel. | 40 | 8 (20.0%) | 67.1 ± 6.1 | 28.9 ± 4.7 | Berberidaceae; B. aristata | 250 mg | 12 weeks | T2 DM | FBS; Insulin; HOMA-IR; BMI |
| Sharma et al. (a) [67] | 2017 | India | Parallel, absence of blinding. | 60 | NR | NR | NR | Berberidaceae; B. aristata | 1.5 g | 36 weeks | T2 DM | FBS |
| Sharma et al. (b) [67] | 2017 | India | Parallel, absence of blinding. | 60 | NR | NR | NR | Berberidaceae; B. aristata | 3 g | 36 weeks | T2 DM | FBS |
| Shidfar et al. [69] | 2012 | Iran | Double blind, parallel. | 42 | NR | 52.7 ± 5.6 | 27.5 ± 1.0 | Berberidaceae; Barberry | 3 g | 12 weeks | T2 DM | HbA1c; FBS; Insulin; HOMA-IR; Body weight; BMI |
| Shidfar et al. [68] | 2015 | Iran | Double blind, parallel. | 45 | NR | 46.2 ± 8.0 | 29.4 ± 2.8 | Zingiberaceae; Ginger | 3 g | 12 weeks | T2 DM | HbA1c; FBS; Insulin; HOMA-IR; Body weight; BMI |
| Sohaei et al. [70] | 2019 | Iran | Double blind, parallel. | 51 | 51 (100%) | 29.6 ± 5.1 | 30.5 ± 4.2 | Zingiberaceae; Curcumin | 1 g | 6 weeks | PCOS | FBS; Insulin; HOMA-IR |
| Sousa et al. [71] | 2020 | Brazil | Double blind, parallel. | 61 | 47 (77.1%) | 62.6 ± 11.0 | 29.3 ± 4.9 | Zingiberaceae; Long turmeric | 500 mg | 16 weeks | T2 DM | HbA1c; FBS; Insulin; HOMA-IR; Body weight; BMI; |
| Tahmasebi et al. [72] | 2019 | Iran | Double blind, parallel. | 80 | 48 (60.0%) | 53.6 ± 7.8 | NR | Berberidaceae; B. vulgaris | 1g | 6 weeks | T2 DM | FBS; 2hPG |
| Zakerkish et al. [73] | 2023 | Iran | Double blind, parallel. | 60 | 40 (66.7%) | 55.2 ± 9.8 | 28.8 ± 4.7 | Zingiberaceae; Turmeric + Ginger + Black pepper | 948 mg | 12 weeks | T2 DM | HbA1c; FBS; Insulin; HOMA-IR; BMI |
| Zhang et al. [5] | 2008 | China | Double blind, parallel. | 106 | 49 (44.5%) | 51 ± 9.4 | 25.5 ± 3.5 | Berberidaceae; Berberine | 1 g | 12 weeks | Dyslipidemia and T2 DM | HbA1c; FBS; Insulin; HOMA-IR; Body weight; BMI; 2hPG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Victoria-Montesinos, D.; Cerdá Martínez-Pujalte, B.; Zafrilla, P.; Ballester, P.; García-Muñoz, A.M. Effect of the Consumption of Species from the Zingiberaceae or Berberidaceae Family on Glycemic Profile Parameters: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2025, 26, 5565. https://doi.org/10.3390/ijms26125565
Victoria-Montesinos D, Cerdá Martínez-Pujalte B, Zafrilla P, Ballester P, García-Muñoz AM. Effect of the Consumption of Species from the Zingiberaceae or Berberidaceae Family on Glycemic Profile Parameters: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2025; 26(12):5565. https://doi.org/10.3390/ijms26125565
Chicago/Turabian StyleVictoria-Montesinos, Desirée, Begoña Cerdá Martínez-Pujalte, Pilar Zafrilla, Pura Ballester, and Ana María García-Muñoz. 2025. "Effect of the Consumption of Species from the Zingiberaceae or Berberidaceae Family on Glycemic Profile Parameters: A Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 26, no. 12: 5565. https://doi.org/10.3390/ijms26125565
APA StyleVictoria-Montesinos, D., Cerdá Martínez-Pujalte, B., Zafrilla, P., Ballester, P., & García-Muñoz, A. M. (2025). Effect of the Consumption of Species from the Zingiberaceae or Berberidaceae Family on Glycemic Profile Parameters: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences, 26(12), 5565. https://doi.org/10.3390/ijms26125565










