Time Does Matter: The Cellular Response to Resveratrol Varies Depending on the Exposure Duration
Abstract
1. Introduction
2. Results
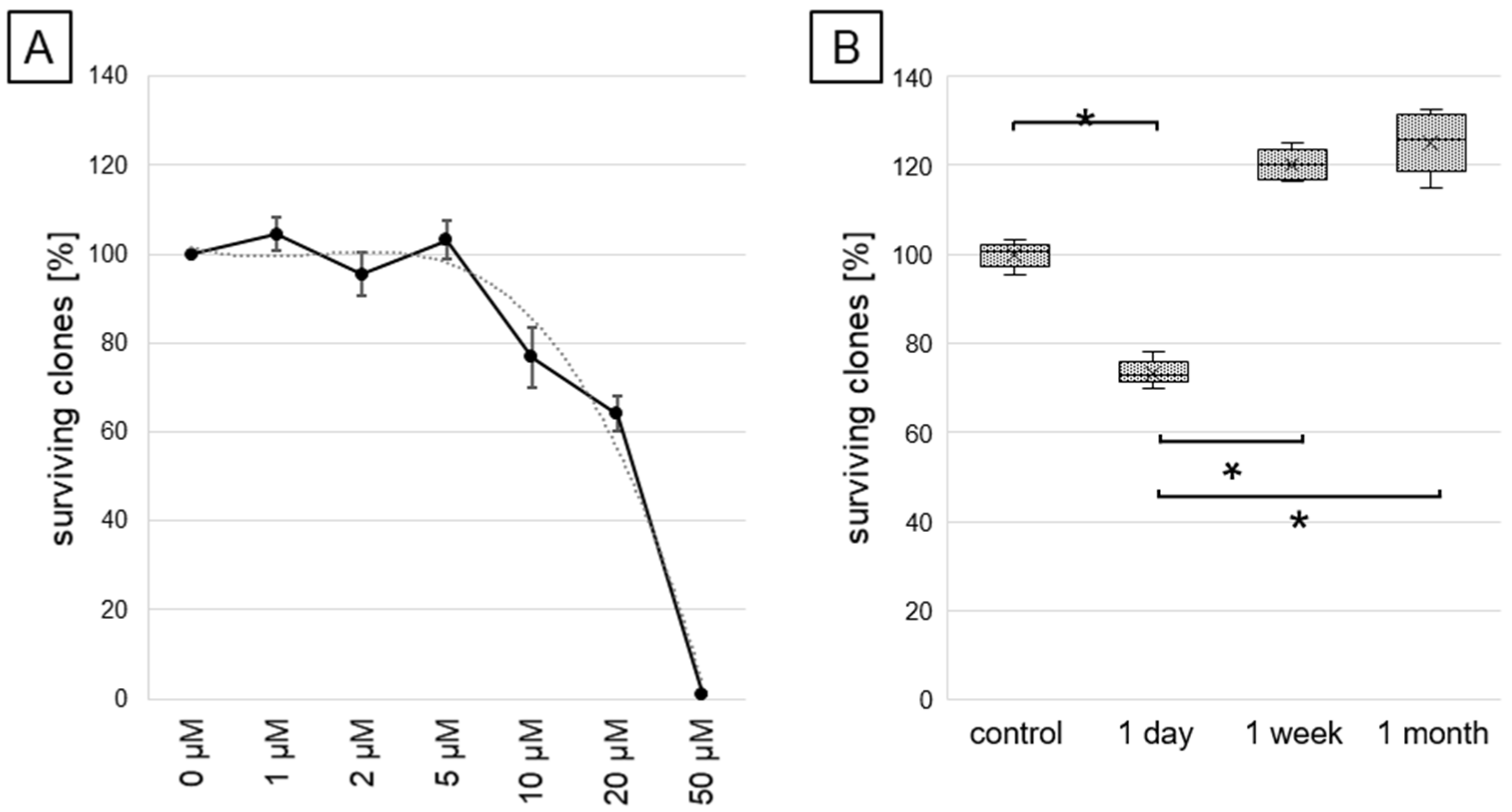
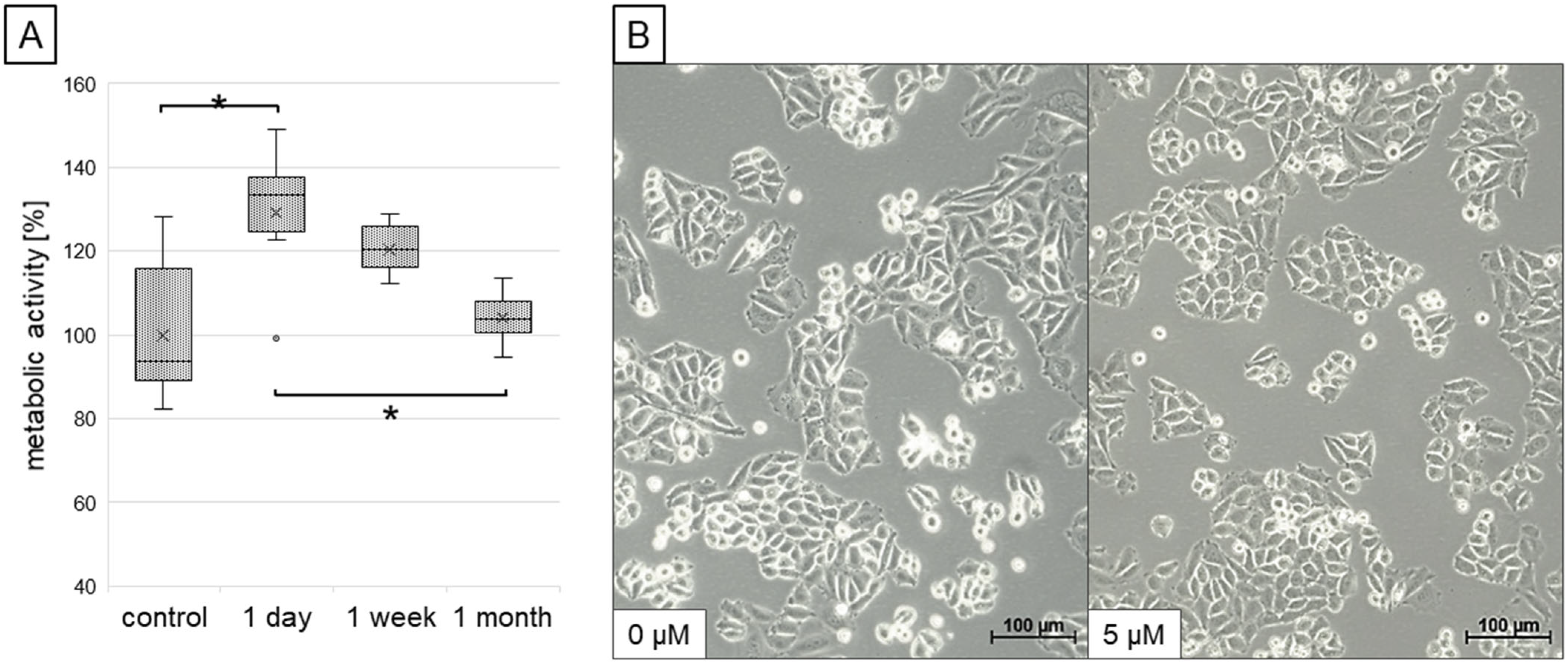
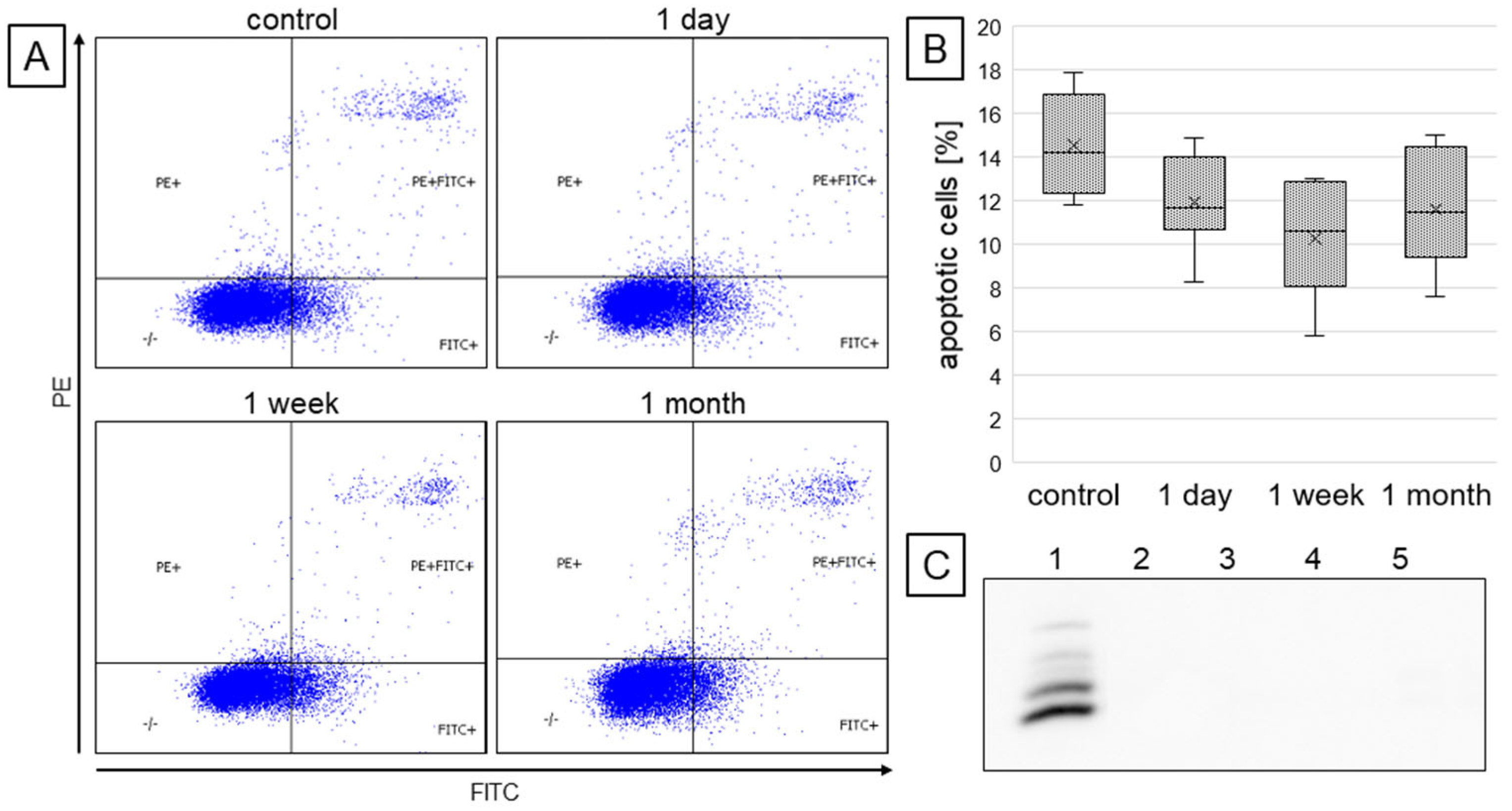
2.1. Cell Survival and Viability
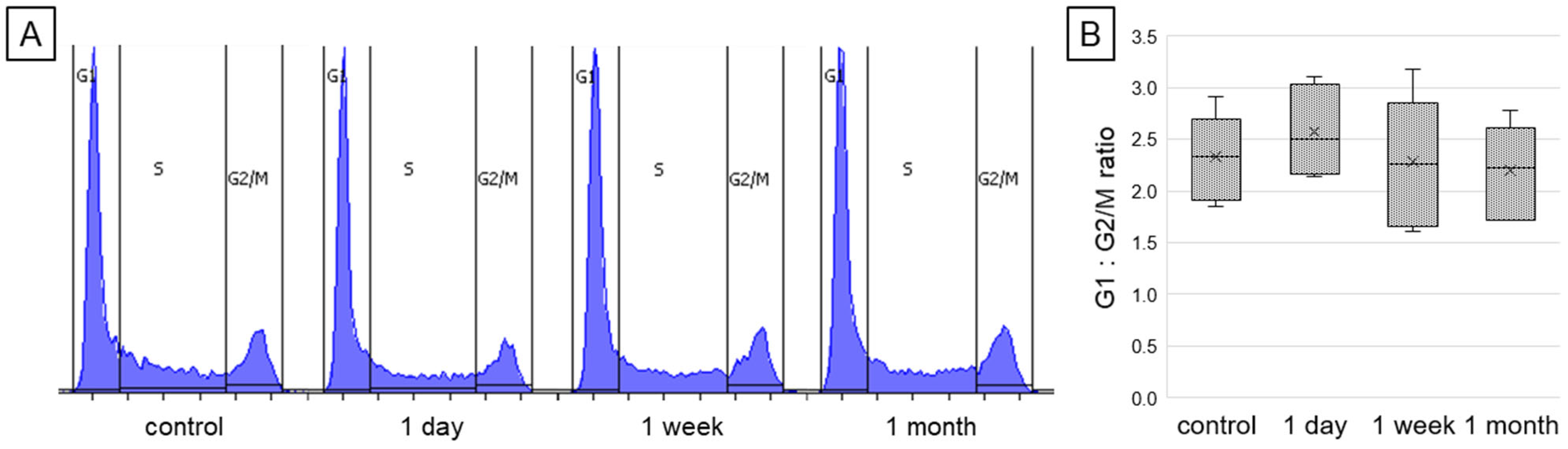
2.2. Changes in the Cell Cycle Phases Distribution
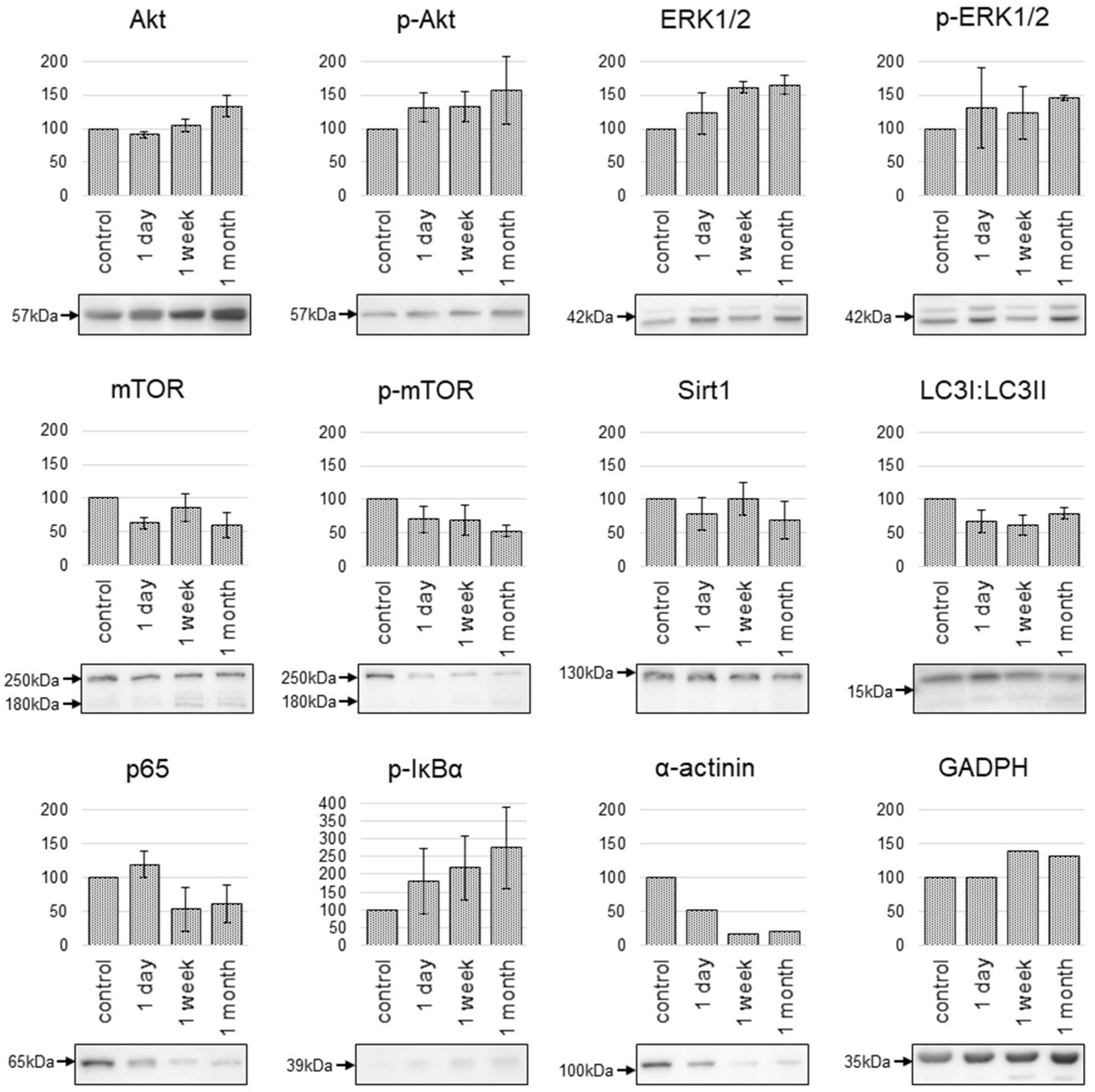
2.3. Changes in the Protein Expression
3. Discussion
4. Materials and Methods
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Akt | protein kinase B |
| AMPK | 5′ adenosine monophosphate-activated protein kinase |
| Bcl-2 | B-cell lymphoma 2 protein |
| Bcl-xL | B-cell lymphoma-extra large protein |
| DMSO | dimethyl sulfoxide |
| ERK1/2 | extracellular signal-regulated kinase 1/2 |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| IC50 | half-maximal inhibitory concentration |
| IκBα | inhibitor of nuclear factor kappa B alpha |
| LC3 | microtubule-associated protein 1A/1B-light chain 3 |
| MAPK | mitogen-activated protein kinase |
| MCL-1 | myeloid cell leukemia-1 protein |
| mTOR | mammalian target of rapamycin |
| NF-κB | nuclear factor-kappa B |
| PI | propidium iodide |
| Sirt1 | sirtuin 1 |
References
- Zhang, P.-Y. Cardioprotection by Phytochemicals via Antiplatelet Effects and Metabolism Modulations. Cell Biochem. Biophys. 2015, 73, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.; Dummi Mahadevan, G.; Anand Iyer, V.; Mukherjee, T.K.; Haribhai Patel, V.; Kumar, R.; Siddiqui, N.; Nayak, M.; Maurya, P.K.; Kumar, P. Dietary Flavonoids in Health and Diseases: A Concise Review of Their Role in Homeostasis and Therapeutics. Food Chem. 2025, 487, 144674. [Google Scholar] [CrossRef] [PubMed]
- Leifert, W.R.; Abeywardena, M.Y. Cardioprotective Actions of Grape Polyphenols. Nutr. Res. 2008, 28, 729–737. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Rana, J.N.; Mumtaz, S. Prunin: An Emerging Anticancer Flavonoid. Int. J. Mol. Sci. 2025, 26, 2678. [Google Scholar] [CrossRef] [PubMed]
- Kopec, A.; Piatkowska, E.; Leszczynska, T.; Biezanowska-Kopec, R. Prozdrowotne wlasciwosci resweratrolu. Żywn. Nauka Technol. Jakość 2011, 5, 5–15. [Google Scholar]
- Robb, E.L.; Stuart, J.A. Trans-Resveratrol as A Neuroprotectant. Molecules 2010, 15, 1196–1212. [Google Scholar] [CrossRef]
- Baarine, M.; Thandapilly, S.J.; Louis, X.L.; Mazué, F.; Yu, L.; Delmas, D.; Netticadan, T.; Lizard, G.; Latruffe, N. Pro-Apoptotic versus Anti-Apoptotic Properties of Dietary Resveratrol on Tumoral and Normal Cardiac Cells. Genes Nutr. 2011, 6, 161–169. [Google Scholar] [CrossRef]
- Rezk, Y.A.; Balulad, S.S.; Keller, R.S.; Bennett, J.A. Use of Resveratrol to Improve the Effectiveness of Cisplatin and Doxorubicin: Study in Human Gynecologic Cancer Cell Lines and in Rodent Heart. Am. J. Obstet. Gynecol. 2006, 194, e23–e26. [Google Scholar] [CrossRef]
- Patel, K.R.; Brown, V.A.; Jones, D.J.L.; Britton, R.G.; Hemingway, D.; Miller, A.S.; West, K.P.; Booth, T.D.; Perloff, M.; Crowell, J.A.; et al. Clinical Pharmacology of Resveratrol and Its Metabolites in Colorectal Cancer Patients. Cancer Res. 2010, 70, 7392–7399. [Google Scholar] [CrossRef]
- Petrovski, G.; Gurusamy, N.; Das, D.K. Resveratrol in Cardiovascular Health and Disease: Resveratrol in Heart Health. Ann. N. Y. Acad. Sci. 2011, 1215, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Vang, O.; Ahmad, N.; Baile, C.A.; Baur, J.A.; Brown, K.; Csiszar, A.; Das, D.K.; Delmas, D.; Gottfried, C.; Lin, H.-Y.; et al. What Is New for an Old Molecule? Systematic Review and Recommendations on the Use of Resveratrol. PLoS ONE 2011, 6, e19881. [Google Scholar] [CrossRef] [PubMed]
- Massimi, M.; Tomassini, A.; Sciubba, F.; Sobolev, A.P.; Devirgiliis, L.C.; Miccheli, A. Effects of Resveratrol on HepG2 Cells as Revealed by 1H-NMR Based Metabolic Profiling. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2012, 1820, 1–8. [Google Scholar] [CrossRef]
- Cottart, C.; Nivet-Antoine, V.; Beaudeux, J. Review of Recent Data on the Metabolism, Biological Effects, and Toxicity of Resveratrol in Humans. Mol. Nutr. Food Res. 2014, 58, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Mitani, T.; Ito, Y.; Harada, N.; Nakano, Y.; Inui, H.; Ashida, H.; Yamaji, R. Resveratrol Reduces the Hypoxia-Induced Resistance to Doxorubicin in Breast Cancer Cells. J. Nutr. Sci. Vitaminol. 2014, 60, 122–128. [Google Scholar] [CrossRef]
- Raj, P.; Lieben Louis, X.; Thandapilly, S.J.; Movahed, A.; Zieroth, S.; Netticadan, T. Potential of Resveratrol in the Treatment of Heart Failure. Life Sci. 2014, 95, 63–71. [Google Scholar] [CrossRef]
- Carsten, R.E.; Bachand, A.M.; Bailey, S.M.; Ullrich, R.L. Resveratrol Reduces Radiation-Induced Chromosome Aberration Frequencies in Mouse Bone Marrow Cells. Radiat. Res. 2008, 169, 633–638. [Google Scholar] [CrossRef]
- Zhang, H.; Zhai, Z.; Wang, Y.; Zhang, J.; Wu, H.; Wang, Y.; Li, C.; Li, D.; Lu, L.; Wang, X.; et al. Resveratrol Ameliorates Ionizing Irradiation-Induced Long-Term Hematopoietic Stem Cell Injury in Mice. Free Radic. Biol. Med. 2013, 54, 40–50. [Google Scholar] [CrossRef]
- Arafa, M.H.; Mohammad, N.S.; Atteia, H.H.; Abd-Elaziz, H.R. Protective Effect of Resveratrol against Doxorubicin-Induced Cardiac Toxicity and Fibrosis in Male Experimental Rats. J. Physiol. Biochem. 2014, 70, 701–711. [Google Scholar] [CrossRef]
- Basso, E.; Regazzo, G.; Fiore, M.; Palma, V.; Traversi, G.; Testa, A.; Degrassi, F.; Cozzi, R. Resveratrol Affects DNA Damage Induced by Ionizing Radiation in Human Lymphocytes in Vitro. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2016, 806, 40–46. [Google Scholar] [CrossRef]
- Piasek, A.; Bartoszek, A.; Namieśnik, J. Substancje pochodzenia roślinnego przeciwdziałające kardiotoksyczności towarzyszącej chemioterapii nowotworów. Postep. Hig. Med. Dosw. 2009, 63, 142–158. [Google Scholar]
- Gramatyka, M.; Widłak, P.; Gabryś, D.; Kulik, R.; Sokół, M. Resveratrol Administration Prevents Radiation-Related Changes in Metabolic Profiles of Hearts 20 Weeks after Irradiation of Mice with a Single 2 Gy Dose. Acta Biochim. Pol. 2020, 67, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Magyar, K.; Halmosi, R.; Palfi, A.; Feher, G.; Czopf, L.; Fulop, A.; Battyany, I.; Sumegi, B.; Toth, K.; Szabados, E. Cardioprotection by Resveratrol: A Human Clinical Trial in Patients with Stable Coronary Artery Disease. Clin. Hemorheol. Microcirc. 2012, 50, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Gramatyka, M. The Radioprotective Activity of Resveratrol—Metabolomic Point of View. Metabolites 2022, 12, 478. [Google Scholar] [CrossRef]
- Thaung Zaw, J.J.; Howe, P.R.; Wong, R.H. Long-Term Effects of Resveratrol on Cognition, Cerebrovascular Function and Cardio-Metabolic Markers in Postmenopausal Women: A 24-Month Randomised, Double-Blind, Placebo-Controlled, Crossover Study. Clin. Nutr. 2021, 40, 820–829. [Google Scholar] [CrossRef]
- Traversi, G.; Fiore, M.; Leone, S.; Basso, E.; Di Muzio, E.; Polticelli, F.; Degrassi, F.; Cozzi, R. Resveratrol and Its Methoxy-Derivatives as Modulators of DNA Damage Induced by Ionising Radiation. Mutagenesis 2016, 31, 433–441. [Google Scholar] [CrossRef]
- Scott, E.; Steward, W.P.; Gescher, A.J.; Brown, K. Resveratrol in Human Cancer Chemoprevention—Choosing the ‘Right’ Dose. Mol. Nutr. Food Res. 2012, 56, 7–13. [Google Scholar] [CrossRef]
- Wahl, D.; Bernier, M.; Simpson, S.J.; De Cabo, R.; Le Couteur, D.G. Future Directions of Resveratrol Research. Nutr. Healthy Aging 2018, 4, 287–290. [Google Scholar] [CrossRef]
- Stervbo, U.; Vang, O.; Bonnesen, C. Time- and Concentration-dependent Effects of Resveratrol in HL-60 and HepG2 Cells. Cell Prolif. 2006, 39, 479–493. [Google Scholar] [CrossRef]
- Walle, T. Bioavailability of Resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef]
- Demidenko, Z.N.; Blagosklonny, M.V. At Concentrations That Inhibit mTOR, Resveratrol Suppresses Cellular Senescence. Cell Cycle 2009, 8, 1901–1904. [Google Scholar] [CrossRef] [PubMed]
- Breuss, J.M.; Atanasov, A.G.; Uhrin, P. Resveratrol and Its Effects on the Vascular System. Int. J. Mol. Sci. 2019, 20, 1523. [Google Scholar] [CrossRef]
- Juhasz, B.; Mukherjee, S. Hormetic Response Resveratrol Against Cardioprotection. Exp. Clin. Cardiol. 2010, 15, 5. [Google Scholar]
- Denissova, N.G.; Nasello, C.M.; Yeung, P.L.; Tischfield, J.A.; Brenneman, M.A. Resveratrol Protects Mouse Embryonic Stem Cells from Ionizing Radiation by Accelerating Recovery from DNA Strand Breakage. Carcinogenesis 2012, 33, 149–155. [Google Scholar] [CrossRef]
- Ye, K.; Ji, C.-B.; Lu, X.-W.; Ni, Y.-H.; Gao, C.-L.; Chen, X.-H.; Zhao, Y.-P.; Gu, G.-X.; Guo, X.-R. Resveratrol Attenuates Radiation Damage in Caenorhabditis Elegans by Preventing Oxidative Stress. J. Radiat. Res. 2010, 51, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; van de Weijer, T.; Goossens, G.H.; Hoeks, J.; van der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie Restriction-like Effects of 30 Days of Resveratrol Supplementation on Energy Metabolism and Metabolic Profile in Obese Humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Shan, H.; Lin, L.; Zhang, M.; Diao, J.-Y.; Li, Q.; Liu, X.; Wei, J. Chronic Resveratrol Treatment Improves Cardiac Function in a Rat Model of Diabetic Cardiomyopathy via Attenuation of Mitochondrial Injury and Myocardial Apoptosis. Int. J. Clin. Exp. Med. 2016, 9, 21156–21167. [Google Scholar]
- Ghosh, H.S.; McBurney, M.; Robbins, P.D. SIRT1 Negatively Regulates the Mammalian Target of Rapamycin. PLoS ONE 2010, 5, e9199. [Google Scholar] [CrossRef]
- Mihaylova, M.M.; Shaw, R.J. The AMPK Signalling Pathway Coordinates Cell Growth, Autophagy and Metabolism. Nat. Cell Biol. 2011, 13, 1016–1023. [Google Scholar] [CrossRef]
- Edinger, A.L.; Thompson, C.B. Akt Maintains Cell Size and Survival by Increasing mTOR-Dependent Nutrient Uptake. Mol. Biol. Cell 2002, 13, 2276–2288. [Google Scholar] [CrossRef]
- Hasanvand, A. The Role of AMPK-Dependent Pathways in Cellular and Molecular Mechanisms of Metformin: A New Perspective for Treatment and Prevention of Diseases. Inflammopharmacol 2022, 30, 775–788. [Google Scholar] [CrossRef]
- Battaglioni, S.; Benjamin, D.; Wälchli, M.; Maier, T.; Hall, M.N. mTOR Substrate Phosphorylation in Growth Control. Cell 2022, 185, 1814–1836. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Zhang, H.; Zhang, X.; Zhang, S.; Zhu, S.; Wang, H. Resveratrol Protects Intestinal Epithelial Cells against Radiation-Induced Damage by Promoting Autophagy and Inhibiting Apoptosis through SIRT1 Activation. J. Radiat. Res. 2021, 62, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, R.; Satoh, R.; Takasaki, T. ERK: A Double-Edged Sword in Cancer. ERK-Dependent Apoptosis as a Potential Therapeutic Strategy for Cancer. Cells 2021, 10, 2509. [Google Scholar] [CrossRef]
- Armour, S.M.; Baur, J.A.; Hsieh, S.N.; Land-Bracha, A.; Thomas, S.M.; Sinclair, D.A. Inhibition of Mammalian S6 Kinase by Resveratrol Suppresses Autophagy. Aging 2009, 1, 515–528. [Google Scholar] [CrossRef]
- Weichhart, T. mTOR as Regulator of Lifespan, Aging, and Cellular Senescence: A Mini-Review. Gerontology 2018, 64, 127–134. [Google Scholar] [CrossRef]
- Tilstra, J.S.; Clauson, C.L.; Niedernhofer, L.J.; Robbins, P.D. NF-κB in Aging and Disease. Aging Dis. 2011, 2, 449–465. [Google Scholar]
- Fu, D.-G. Regulation of Redox Signalling and Autophagy during Cardiovascular Diseases-Role of Resveratrol. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1530–1536. [Google Scholar]
- Csiszar, A. Anti-Inflammatory Effects of Resveratrol: Possible Role in Prevention of Age-Related Cardiovascular Disease: Anti-Inflammatory Effects of Resveratrol in Aging. Ann. N. Y. Acad. Sci. 2011, 1215, 117–122. [Google Scholar] [CrossRef]
- Elmansi, A.M.; Kassem, A.; Castilla, R.M.; Miller, R.A. Downregulation of the NF-κB Protein P65 Is a Shared Phenotype among Most Anti-Aging Interventions. GeroScience 2024, 14, 2509–2723. [Google Scholar] [CrossRef]
- Fodor, K.; Tit, D.M.; Pasca, B.; Bustea, C.; Uivarosan, D.; Endres, L.; Iovan, C.; Abdel-Daim, M.M.; Bungau, S. Long-Term Resveratrol Supplementation as a Secondary Prophylaxis for Stroke. Oxidative Med. Cell. Longev. 2018, 2018, 4147320. [Google Scholar] [CrossRef]
- Price, N.L.; Gomes, A.P.; Ling, A.J.Y.; Duarte, F.V.; Martin-Montalvo, A.; North, B.J.; Agarwal, B.; Ye, L.; Ramadori, G.; Teodoro, J.S.; et al. SIRT1 Is Required for AMPK Activation and the Beneficial Effects of Resveratrol on Mitochondrial Function. Cell Metab. 2012, 15, 675–690. [Google Scholar] [CrossRef]
- Sebastià, N.; Almonacid, M.; Villaescusa, J.I.; Cervera, J.; Such, E.; Silla, M.A.; Soriano, J.M.; Montoro, A. Radioprotective Activity and Cytogenetic Effect of Resveratrol in Human Lymphocytes: An in Vitro Evaluation. Food Chem. Toxicol. 2013, 51, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Fischer, N.; Seo, E.-J.; Efferth, T. Prevention from Radiation Damage by Natural Products. Phytomedicine 2018, 47, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.-H.; Sethi, G.; Um, J.-Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The Role of Resveratrol in Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef] [PubMed]
- Balmanno, K.; Cook, S.J. Tumour Cell Survival Signalling by the ERK1/2 Pathway. Cell Death Differ. 2009, 16, 368–377. [Google Scholar] [CrossRef]
- Hara, M.R.; Cascio, M.B.; Sawa, A. GAPDH as a Sensor of NO Stress. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2006, 1762, 502–509. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, H.; Zhou, X.; Wang, H.; Yang, Y.; Zhang, J.; Wang, H. The Protective Effects of Resveratrol against Radiation-Induced Intestinal Injury. BMC Complement. Altern. Med. 2017, 17, 410. [Google Scholar] [CrossRef]
- Movahed, A.; Yu, L.; Thandapilly, S.J.; Louis, X.L.; Netticadan, T. Resveratrol Protects Adult Cardiomyocytes against Oxidative Stress Mediated Cell Injury. Arch. Biochem. Biophys. 2012, 527, 74–80. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gramatyka, M. Time Does Matter: The Cellular Response to Resveratrol Varies Depending on the Exposure Duration. Int. J. Mol. Sci. 2025, 26, 5542. https://doi.org/10.3390/ijms26125542
Gramatyka M. Time Does Matter: The Cellular Response to Resveratrol Varies Depending on the Exposure Duration. International Journal of Molecular Sciences. 2025; 26(12):5542. https://doi.org/10.3390/ijms26125542
Chicago/Turabian StyleGramatyka, Michalina. 2025. "Time Does Matter: The Cellular Response to Resveratrol Varies Depending on the Exposure Duration" International Journal of Molecular Sciences 26, no. 12: 5542. https://doi.org/10.3390/ijms26125542
APA StyleGramatyka, M. (2025). Time Does Matter: The Cellular Response to Resveratrol Varies Depending on the Exposure Duration. International Journal of Molecular Sciences, 26(12), 5542. https://doi.org/10.3390/ijms26125542





