Effects of Two Hormonal Protocols for FTAI on the Fertility of Repeat Cows
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Methodologies Used
3.1.1. Body Condition Score Assessment
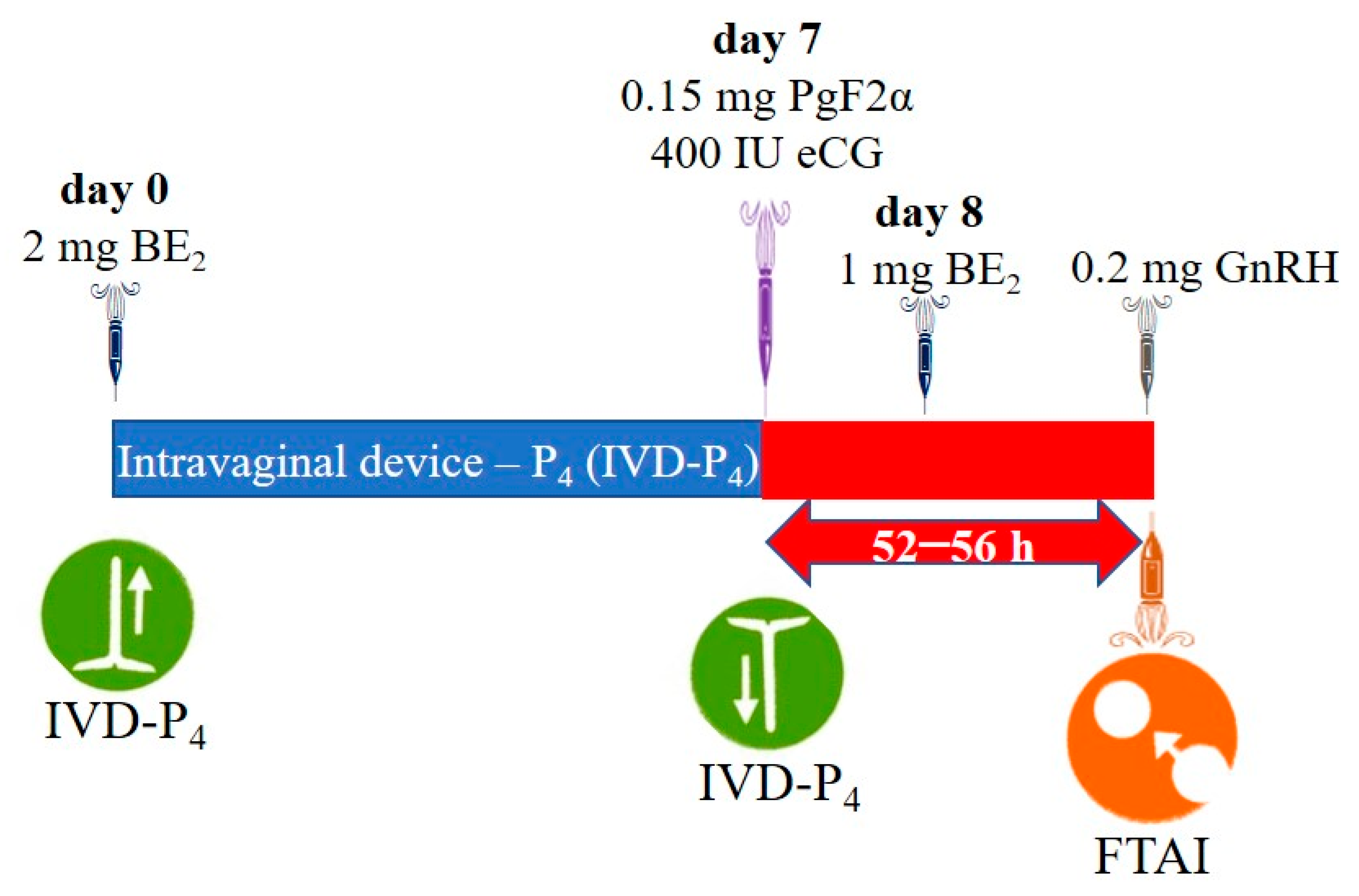
3.1.2. Hormonal Protocol Diagrams
3.1.3. Follicular Monitoring and Pregnancy Diagnosis
3.1.4. Hormonal Measurements
3.1.5. Statistical Processing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gustafsson, H.; Emanuelson, U. Characterisation of the Repeat Breeding Syndrome in Swedish Dairy Cattle. Acta Vet. Scand. 2002, 43, 115. [Google Scholar] [CrossRef] [PubMed]
- Brito, R.; Blanco, G.S.; Calderón, R.; Preval, B.; Campo, E. Patología de la Reproducción Animal, 2nd ed.; Editorial “Félix Varela”: La Habana, Cuba, 2010. [Google Scholar]
- Yusuf, M.; Nakao, T.; Ranasinghe, R.M.S.B.K.; Gautam, G.; Long, S.T.; Yoshida, C.; Koike, K.; Hayashi, A. Reproductive performance of repeat breeders in dairy herds. Theriogenology 2010, 73, 1220–1229. [Google Scholar] [CrossRef] [PubMed]
- Båge, R.; Gustafsson, H.; Larsson, B.; Forsberg, M.; Rodríguez-Martínez, H. Repeat breeding in dairy heifers: Follicular dynamics and estrous cycle characteristics in relation to sexual hormone patterns. Theriogenology 2002, 57, 2257–2269. [Google Scholar] [CrossRef] [PubMed]
- de Lima, M.A.; Morotti, F.; Bayeux, B.M.; de Rezende, R.G.; Botigelli, R.C.; De Bem, T.H.C.; Fontes, P.K.; Nogueira, M.F.G.; Meirelles, F.V.; Baruselli, P.S.; et al. Ovarian follicular dynamics, progesterone concentrations, pregnancy rates and transcriptional patterns in Bos indicus females with a high or low antral follicle count. Sci. Rep. 2020, 10, 19557. [Google Scholar] [CrossRef]
- Pérez-Marín, C.C.; Quintela, L.A. Current Insights in the Repeat Breeder Cow Syndrome. Animals 2023, 13, 2187. [Google Scholar] [CrossRef]
- Lima, R.; Hernández, M.; Rodríguez, J.L.; Betancourt, J. Behavior of dairy cows in different calf rearing systems in the period. Cuba. J. Agric. Sci. 2009, 43, 21–25. [Google Scholar]
- Yatoo, M.I.; Saxena, A.; Deepa, P.M.; Habeab, B.P.; Devi, S.; Jatav, R.S.; Dimri, U. Role of trace elements in animals: A review. Vet. World 2013, 6, 963–967. [Google Scholar] [CrossRef]
- Walsh, S.W.; Williams, E.J.; Evans, A.C.O. A review of the causes of poor fertility in high milk producing dairy cows. Anim. Reprod. Sci. 2011, 123, 127–138. [Google Scholar] [CrossRef]
- García-Díaz, J.R.; Dungula-Sapalalo, G.H.; Noval-Artíles, E.; Hernández-Barreto, M.A.; Mollineda-Trujillo, A.; Garzón-Jarrin, R. Relación entre cinquemia y fertilidad en vacas lecheras mestizas Holstein x Cebú. Arch. Zootec. 2020, 69, 96–101. [Google Scholar] [CrossRef][Green Version]
- García, J.R.; García López, R.; Cuesta, M.; Figueredo, J.M.; Quiñones, R.; Faure, R.; Pedroso, R.; Mollineda, A. Blood copper levels and their influence on reproductive indicators of cows in tropical conditions. Cuba. J. Agric. Sci. 2010, 44, 233–239. [Google Scholar]
- Sheldon, I.M.; Cronin, J.; Goetze, L.; Donofrio, G.; Schuberth, H.-J. Defining Postpartum Uterine Disease and the Mechanisms of Infection and Immunity in the Female Reproductive Tract in Cattle1. Biol. Reprod. 2009, 81, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Marín, C.; España, F. Oestrus Expression and Ovarian Function in Repeat Breeder Cows, Monitored by Ultrasonography and Progesterone Assay. Reprod. Domest. Anim. 2007, 42, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Son, D.-S.; Choe, C.-Y.; Cho, S.-R.; Choi, S.-H.; Kim, H.-J.; Hur, T.-Y.; Jung, Y.-G.; Kang, H.-G.; Kim, I.-H. A CIDR-Based Timed Embryo Transfer Protocol Increases the Pregnancy Rate of Lactating Repeat Breeder Dairy Cows. J. Reprod. Dev. 2007, 53, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- Sá Filho, M.F.; Penteado, L.; Reis, E.L.; Reis, T.A.N.P.S.; Galvão, K.N.; Baruselli, P.S. Timed artificial insemination early in the breeding season improves the reproductive performance of suckled beef cows. Theriogenology 2013, 79, 625–632. [Google Scholar] [CrossRef]
- Lima, F.S.; Risco, C.A.; Thatcher, M.J.; Benzaquen, M.E.; Archbald, L.F.; Santos, J.E.P.; Thatcher, W.W. Comparison of reproductive performance in lactating dairy cows bred by natural service or timed artificial insemination. J. Dairy Sci. 2009, 92, 5456–5466. [Google Scholar] [CrossRef]
- Bó, G.A.; de la Mata, J.J.; Baruselli, P.S.; Menchaca, A. Alternative programs for synchronizing and resynchronizing ovulation in beef cattle. Theriogenology 2016, 86, 388–396. [Google Scholar] [CrossRef]
- Lane, E.A.; Austin, E.J.; Crowe, M.A. Oestrous synchronisation in cattle—Current options following the EU regulations restricting use of oestrogenic compounds in food-producing animals: A review. Anim. Reprod. Sci. 2008, 109, 1–16. [Google Scholar] [CrossRef]
- Karamishabankareh, H.; Hajarian, H.; Shahsavari, M.; Moradinejad, R. In vivo and in vitro study of the function of the left and right bovine ovaries. Theriogenology 2015, 84, 724–731. [Google Scholar] [CrossRef]
- Kim, U.-H.; Suh, G.-H.; Nam, H.-W.; Kang, H.-G.; Kim, I.-H. Follicular wave emergence, luteal function and synchrony of ovulation following GnRH or estradiol benzoate in a CIDR-treated, lactating Holstein cows. Theriogenology 2005, 63, 260–268. [Google Scholar] [CrossRef]
- Kim, U.-H.; Suh, G.-H.; Hur, T.-Y.; Kang, S.-J.; Kang, H.-G.; Park, S.-B.; Kim, H.-S.; Kim, I.-H. Comparison of Two Types of CIDR-based Timed Artificial Insemination Protocols for Repeat Breeder Dairy Cows. J. Reprod. Dev. 2007, 53, 639–645. [Google Scholar] [CrossRef][Green Version]
- Ginther, O.J.; Baldrighi, J.M.; Siddiqui, M.A.R.; Bashir, S.T.; Rakesh, H.B. Mechanism for greater frequency of contralateral than ipsilateral relationships between corpus luteum and ovulatory follicle for wave 3 in heifers. Theriogenology 2016, 85, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Pérez, G.U.; Quispe, B.Y.; Luque, M.N.; Rojas, E.R.; Condori, C.E.; Delgado, C.A.; Pérez, D.M. Evaluación ultrasonográfica en ganado Brown Swiss sometido a un protocolo de sincronización de celo en el altiplano peruano. Rev. Investig. Vet. Perú 2019, 30, 489–494. [Google Scholar] [CrossRef]
- Alfaro-Astorima, M.I.; Ormachea-Sánchez, H.H.; Alvarado-Malca, A.E. Dinámica folicular ovárica en vacas criollas bajo condiciones de pastoreo en la zona altoandina del Perú. Sci. Agropecu. 2020, 11, 621–628. [Google Scholar] [CrossRef]
- Villagómez Amezcua Manjarrez, E.; Castillo Rojas, H.; Villa Godoy, A.; Román Ponce, H.; Vázquez Peláez, C. Influencia estacional sobre el ciclo estral y el estro en hembras cebú mantenidas en clima tropical. Rev. Mex. De Cienc. Pecu. 2012, 38, 89–103. [Google Scholar]
- Vargas Ortiz, L.M.; Barros Rodríguez, M.; Andrade Yucailla, V.C.; Aguirre Casco, C.E.; Lima Orozco, R. Acacia negra y sus potencialidades como alimento para rumiantes. Cent. Agrícola 2022, 49, 50–61. [Google Scholar]
- Narváez Bedoya, H.J.; Silva Rojas, A.V. Dinámica folicular y cuantificación de estradiol durante el cicloestral de vacas criollas de la raza Blanco Orejinegro. Rev. Investig. Vet. Perú 2020, 31, e16186. [Google Scholar] [CrossRef]
- Steel, R.G.D.; Torrie, J.H.; Dickey, D.A. Principles and Procedures of Statistics. In A Biometrical Approach; McGraw-Hill Book Company: New York, NY, USA, 1997; p. 666. [Google Scholar]
- Baruselli, P.S.; Martins, C.M.; Sales, J.N.; Ferreira, R.M. Novos avancos na superovulacao de bovinos. Acta Sci Vet. 2008, 36, s433–s448. [Google Scholar]
- Toro García, D.C.; Vega Borda, D.A.; Narváez, H.J.; Villalba Rey, D.; Alvarado, D. Comparación de inductores de la ovulación en un programa de IATF con semen sexado en novillas Bos taurus × Bos indicus. Rev. Investig. Vet. Perú 2021, 32, e19823. [Google Scholar] [CrossRef]
- De la Mata, J.; Menchaca, A.; Bó, G. Tratamientos que prolongan el proestro usando estradiol y progesterona en vaquillonas para carne. In Proceedings of the XI Simposio Internacional de Reproducción Animal, Córdoba, Argentina, 13–15 August 2015; pp. 143–157. [Google Scholar]
- Yánez-Avalos, D.O.; Barbona, I.; López-Parra, J.C.; Roberto Marini, P. Protocolo j-synch con y sin ecg en vacas brown swiss y sus cruzas con bos indicus en la amazonía ecuatoriana. Granja. Rev. Cienc. Vida 2021, 33, 8–20. [Google Scholar] [CrossRef]
- Bridges, G.A.; Ahola, J.K.; Brauner, C.; Cruppe, L.H.; Currin, J.C.; Day, M.L.; Gunn, P.J.; Jaeger, J.R.; Lake, S.L.; Lamb, G.C.; et al. Determination of the appropriate delivery of prostaglandin F2α in the five-day CO-Synch + controlled intravaginal drug release protocol in suckled beef cows1. J. Anim. Sci. 2012, 90, 4814–4822. [Google Scholar] [CrossRef]
- Bridges, G.A.; Mussard, M.L.; Helser, L.A.; Day, M.L. Comparison of follicular dynamics and hormone concentrations between the 7-day and 5-day CO-Synch + CIDR program in primiparous beef cows. Theriogenology 2014, 81, 632–638. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente Fernández, S. Regresión Logística: Estadistica Aplicada. Available online: https://tinyurl.com/yeo7o8mn (accessed on 25 January 2025).
- Levy, P.S.; Stolte, K. Statistical methods in public health and epidemiology: A look at the recent past and projections for the next decade. Stat. Methods Med. Res. 2000, 9, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, S.; Moriyoshi, M. Alteration of the Endometrial EGF Profile as a Potential Mechanism Connecting the Alterations in the Ovarian Steroid Hormone Profile to Embryonic Loss in Repeat Breeders and High-producing Cows. J. Reprod. Dev. 2013, 59, 415–420. [Google Scholar] [CrossRef]
- Yaginuma, H.; Funeshima, N.; Tanikawa, N.; Miyamura, M.; Tsuchiya, H.; Noguchi, T.; Iwata, H.; Kuwayama, T.; Shirasuna, K.; Hamano, S. Improvement of fertility in repeat breeder dairy cattle by embryo transfer following artificial insemination: Possibility of interferon tau replenishment effect. J. Reprod. Dev. 2019, 65, 223–229. [Google Scholar] [CrossRef]
- Guzmán Aguirre, F.V.; Andrade Muñoz, L.B.; Arízaga Vera, F.E.; Matute Villavicencio, R.V.; Tribulo, H.; Carcedo, J.A. Efecto del diámetro del folículo preovulatorio en el momento de la IATF y de la expresión de estro sobre la tasa de preñez en vacas Nelore con cría al pie. Dominio Cienc. 2018, 5, 733–773. [Google Scholar]
- Espinoza-Villavicencio, J.L.; Ortega-Pérez, R.; Palacios-Espinosa, A.; Valencia-Méndez, J.; Aréchiga-Flores, C.F. Crecimiento folicular ovárico en animales domésticos: Una revisión. Interciencia 2007, 32, 93–99. [Google Scholar]
- Pitaluga, P.C.S.F.; Sá Filho, M.F.; Sales, J.N.S.; Baruselli, P.S.; Vincenti, L. Manipulation of the proestrous by exogenous gonadotropin and estradiol during a timed artificial insemination protocol in suckled Bos indicus beef cows. Livest. Sci. 2013, 154, 229–234. [Google Scholar] [CrossRef]
- Balarezo-Urresta, L.; García-Díaz, J.; Noval-Artiles, E. Corporal condition and restart of the ovarian postpartum on Holstein cows in Ecuador. Rev. MVZ Cordoba 2020, 25, e1859. [Google Scholar] [CrossRef]
- Cuesta, M.; Montejo, E.; Duvergel, J. Medicina Interna Veterinaria, 1st ed.; Editorial “Félix Varela”: La Habana, Cuba, 2007; Volume Tomo I y II, p. 325. [Google Scholar]
- Parker, R. Body Condition Scoring of Dairy Casttle; Factsheet AGNES: Norwalk, CT, USA, 1989; pp. 410–420. [Google Scholar]
- Colazo, M.G.; Kastelic, J.P.; Whittaker, P.R.; Gavaga, Q.A.; Wilde, R.; Mapletoft, R.J. Fertility in beef cattle given a new or previously used CIDR insert and estradiol, with or without progesterone. Anim. Reprod. Sci. 2004, 81, 25–34. [Google Scholar] [CrossRef]
- de la Mata, J.J.; Ré, M.; Bó, G.A. 8 Combination of estrus detection and fixed-time artificial insemination in beef heifers following shortened estradiol-based protocol that provides for a lengthened proestrus. Reprod. Fertil. Dev. 2014, 27, 96–97. [Google Scholar] [CrossRef]
- Quintela, L.A.; Díaz, C.; García, P.G.; Peña, A.; Becerra, J.J. Ecografí a y Reproducción en la Vaca; Universidade de Santiago de Compostela: Santiago de Compostela, Spain, 2006; p. 92. [Google Scholar]
- Sugiura, T.; Akiyoshi, S.; Inoue, F.; Yanagawa, Y.; Moriyoshi, M.; Tajima, M.; Katagiri, S. Relationship between bovine endometrial thickness and plasma progesterone and estradiol concentrations in natural and induced estrus. J. Reprod. Dev. 2018, 64, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, H.K.; Nakao, T.; Suzuki, T.; Akita, M.; Higaki, T. Relationships between body condition score, body weight, and some nutritional parameters in plasma and resumption of ovarian cyclicity postpartum during pre-service period in high-producing dairy cows in a subtropical region in Japan. Theriogenology 2005, 64, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Hannan, M.A.; Fuenzalida, M.J.; Siddiqui, M.A.R.; Shamsuddin, M.; Beg, M.A.; Ginther, O.J. Diurnal variation in LH and temporal relationships between oscillations in LH and progesterone during the luteal phase in heifers. Theriogenology 2010, 74, 1491–1498. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. PLOS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]

| Variables | Groups | Days | ||
|---|---|---|---|---|
| 7th | 8th | 9th | ||
| Left ovarian follicles, n | 1 | 2.92 ± 0.21 a | 2.50 ± 0.21 a | 2.53 ± 0.23 a |
| 2 | 2.79 ± 0.22 a | 2.87 ± 0.23 a | 3.08 ± 0.23 a | |
| Dominant follicle diameter in the left ovary, mm | 1 | 6.88 ± 0.64 a | 8.23 ± 0.63 a | 8.70 ± 0.91 a |
| 2 | 4.87 ± 0.67 b | 8.29 ± 0.66 a | 8.54 ± 0.91 a | |
| Right ovarian follicles, n | 1 | 2.22 ± 0.21 a | 2.23 ± 0.23 a | 2.26 ± 0.26 a |
| 2 | 2.20 ± 0.22 a | 2.45 ± 0.20 a | 3.00 ± 0.26 a | |
| Dominant follicle diameter in the right ovary, mm | 1 | 9.92 ± 0.70 a | 9.42 ± 0.74 a | 11.03 ± 0.90 a |
| 2 | 7.54 ± 0.72 b | 8.79 ± 0.77 b | 9.04 ± 0.94 b | |
| Variables | Groups | |
|---|---|---|
| G1 | G2 | |
| FSH (UI) | 0.14 ± 0.005 a | 0.14 ± 0.006 a |
| Estradiol (UI) | 140.30 ± 12.45 a | 110.14 ± 9.12 b |
| LH (UI) | 0.32 ± 0.04 a | 0.20 ± 0.01 b |
| Progesterone (ng/mL) | 4.78 ± 0.41 a | 4,59 ± 0.39 a |
| Estrus rates | 0.76 a | 0.62 a |
| pregnancy rates | 0.76 a | 0.41 b |
| Factors | EC | SE | OR | χ2 | GL | p |
|---|---|---|---|---|---|---|
| Constante | −9.62 | 4.60 | - | |||
| Estradiol in blood serum | 0.01 | 0.01 | 1.51 | 5.34 | 1 | 0.021 |
| LH in blood serum | 8.56 | 4.61 | 52.31 | 6.59 | 1 | 0.010 |
| Absence of estrus at time of FTAI | −3.10 | 1.58 | 22.30 | 5.42 | 1 | 0.020 |
| P4 concentrations 15 days after FTAI | 1.37 | 0.51 | 3.95 | 16.41 | 1 | <0.001 |
| Number of inseminations per protocol | 1.56 | 1.16 | 4.80 | 2.06 | 1 | 0.150 |
| Farm | Location [North Latitude (NL) and West Longitude (WL)] | Height (m.a.s.l.) | Municipality | Province |
|---|---|---|---|---|
| El Rosario | 778,628 and 77″ NL and 987,541 WL | 3280 | Salcedo | Cotopaxi |
| Camila | 773,358 and 77″ NL and 988,390 WL | 3020 | Salcedo | Cotopaxi |
| Planchaloma | 747,202″ NL and 9,919,812 WL | 3350 | Latacunga | Cotopaxi |
| El Carbón | 771,800″ NL and 98,972,723 WL | 3200 | Pillaro | Tungurahua |
| Chañag | 775,230″ NL and 9,821,015 WL | 3228 | Riobamba | Chimborazo |
| Cubijies | 775,230″ NL and 9,821,015 WL | 3228 | Riobamba | Chimborazo |
| Statistigraph | Age (Years) | Births (n) | AIR (n) | BCS (Points) | OD (Days) | MY (L/Caw−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G1 | G2 | G1 | G2 | G1 | G2 | G1 | G2 | G1 | G2 | G1 | G2 | |
| Mean | 6.11 | 6.54 | 3.69 | 3.37 | 4.38 | 4.54 | 3.54 | 3.3 | 216.34 | 239.54 | 9.61 | 10.83 |
| SD | 1.14 | 1.06 | 0.97 | 0.82 | 1.32 | 1.41 | 0.37 | 0.23 | 54.46 | 67.13 | 2.22 | 3.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas Ortiz, L.M.; Andrade Yucailla, V.C.; García Díaz, J.R.; Acosta Lozano, N.V.; Aragadvay Yungán, R.G.; Lima Orozco, R. Effects of Two Hormonal Protocols for FTAI on the Fertility of Repeat Cows. Int. J. Mol. Sci. 2025, 26, 5499. https://doi.org/10.3390/ijms26125499
Vargas Ortiz LM, Andrade Yucailla VC, García Díaz JR, Acosta Lozano NV, Aragadvay Yungán RG, Lima Orozco R. Effects of Two Hormonal Protocols for FTAI on the Fertility of Repeat Cows. International Journal of Molecular Sciences. 2025; 26(12):5499. https://doi.org/10.3390/ijms26125499
Chicago/Turabian StyleVargas Ortiz, Luis Miguel, Verónica Cristina Andrade Yucailla, Juan Ramón García Díaz, Néstor Vicente Acosta Lozano, Ramón Gonzalo Aragadvay Yungán, and Raciel Lima Orozco. 2025. "Effects of Two Hormonal Protocols for FTAI on the Fertility of Repeat Cows" International Journal of Molecular Sciences 26, no. 12: 5499. https://doi.org/10.3390/ijms26125499
APA StyleVargas Ortiz, L. M., Andrade Yucailla, V. C., García Díaz, J. R., Acosta Lozano, N. V., Aragadvay Yungán, R. G., & Lima Orozco, R. (2025). Effects of Two Hormonal Protocols for FTAI on the Fertility of Repeat Cows. International Journal of Molecular Sciences, 26(12), 5499. https://doi.org/10.3390/ijms26125499








