Abstract
In vitro maturation (IVM) of oocytes retrieved from ovum pick-up (OPU) or ovarian tissue (OT) is a standard approach for patients with specific conditions where prior hormonal stimulation is contraindicated. However, the developmental competence of oocytes matured in vitro is still inferior to that of oocytes matured in vivo. Capacitation IVM (CAPA-IVM) includes an extra step of pre-maturation culture (PMC) with c-type natriuretic peptide (CNP) as a meiotic arrestor to better synchronize cytoplasmic and nuclear maturity in oocytes by allowing the cytoplasm additional time to acquire essential components critical for optimal competency. This study aims to evaluate the effect of CAPA-IVM on equine oocyte quality and developmental competence. Immature cumulus–oocyte complexes (COCs) were retrieved from slaughterhouse ovaries and matured in vitro either in CAPA-IVM (short 6 h, long 24 h pre-maturation) or standard IVM. Mature oocytes from each group were analyzed for calcium-releasing potential (n = 52) and single-oocyte proteomics (n = 44), and embryo development (n = 229) was assessed after fertilization with piezo-drilled intracytoplasmic sperm injection (ICSI). Genetic analysis of developed blastocysts (n = 41) was performed to detect chromosomal aberrations. Our findings demonstrate that CAPA-IVM of equine COCs yields significantly higher maturation rates than controls. Moreover, short CAPA-IVM with six hours pre-maturation culture showed substantially higher embryo development potential than the control group (20/69 vs. 9/63, respectively). Genetic analysis revealed a high euploidy rate in equine blastocysts regardless of the maturation conditions. Live calcium imaging of the fertilized oocytes demonstrated that the majority of oocytes displayed non-continuous calcium oscillation patterns, irrespective of maturation conditions. Single-oocyte proteomics reveals a comparable proteomic landscape between mature oocytes subjected to short CAPA-IVM and standard IVM. However, we identified four enriched gene sets with positive enrichment scores after short CAPA-IVM, related to cytoskeleton regulation, ribosomal function, and cytosolic components. Our findings indicate that CAPA-IVM holds the potential to improve oocyte quality and competence in horses. However, further fine-tuning of culture conditions would benefit the effective use of these IVM systems. Moreover, given that the mare serves as an excellent model for human reproduction, the molecular trends identified in this study could provide valuable insights for advancing human artificial reproductive technologies.
1. Introduction
In vitro maturation (IVM) of oocytes is a standard approach for patients with contraindications to conventional hormonal stimulations. This includes cases such as polycystic ovary syndrome (PCOS), ovarian hyperstimulation syndrome (OHSS), resistant ovary syndrome (ROS), and malignancies requiring immediate intervention [1]. It provides an alternative to conventional stimulation protocols where oocytes are matured in vivo before retrieval [1]. Additionally, IVM is also a routine procedure in other species, such as cattle and especially horses, where ovarian hormonal stimulation is often ineffective [2]. Furthermore, oocytes from euthanized mares or slaughterhouse ovaries also require a prior step of IVM for the successful generation of equine embryos in vitro [3], a process crucial for maximizing genetic potential and breed preservation.
IVM involves resumption of the meiosis following retrieval of immature cumulus-oocyte complexes (COCs) from ovarian tissue without or with minimal hormonal stimulation. Although these oocytes achieve nuclear maturity following IVM, their cytoplasmic maturation—which involves attaining molecular modifications such as maternal mRNA regulation, organelle redistribution, epigenetic modifications, cytoskeletal rearrangements, and calcium signaling regulation necessary for successful fertilization and embryo development [4]—remains unclear. Additionally, the developmental competence of oocytes matured in vitro is still inferior to that of oocytes matured in vivo [5,6].
Since certain modifications such as organelle redistribution, cytoskeletal remodeling, maternal mRNA accumulation, and epigenetic changes occur in vivo after the activation and progression of the follicle before the luteinizing hormone (LH) wave, the term oocyte capacitation [7] or oocyte pre-maturation [8] is used to indicate this phase of oocyte growth. However, during this phase, the follicular milieu is vital in maintaining meiotic arrest and enabling timely meiosis resumption. C-type natriuretic peptide (CNP)-derived elevation in oocyte cAMP level arrests the oocyte in the germinal vesicle (GV) phase by inhibiting maturation-promoting factor (MPF) activity through cAMP-dependent kinase A [9]. Nonetheless, a drop in oocyte and follicular cGMP levels occurs due to LH surge, which leads to upregulation of halted phosphodiesterase3 (PDE3) activity, which in turn resumes the meiosis by lowering intraoocyte cAMP [9,10,11]. Further changes in the cytoplasm involving redistribution of organelles also happen during this phase [12]. Before ovulation, the oocyte must acquire essential components required for normal fertilization and early embryo development [13].
To recapitulate these conditions in vitro, several in vitro pre-maturation strategies using meiotic arrestors have been investigated in various species, including humans, mice, goats, cattle, pigs, sheep, and horses [14,15]. The aim of this effort is to enhance the cytoplasmic maturity of oocytes, to support embryo development better. Usually, oocytes undergoing IVM are collected from follicles of varying sizes, resulting in a diverse range of GV chromatin configurations. These variations influence the timing of germinal vesicle breakdown (GVBD) [16]. Consequently, upon spontaneous resumption of meiosis, asynchronous maturation of the nucleus and cytoplasm occurs across the cohort of oocytes, complicating the precise timing required for subsequent procedures like sperm injection. Notably, the primary objective of the original biphasic IVM study was to attain synchronized meiotic progression in pig oocytes during IVM [17].
Most pre-maturation strategies in biphasic IVM mainly focus on regulating cyclic nucleotide levels in cumulus–oocyte complexes (COCs) using specific pharmacological agents. Specifically, PDE3 inhibitors, such as cilostamide, milrinone, or Org9935, have been used to modulate cyclic nucleotide levels [18,19]. Alternatively, exogenous dibutyryl cAMP (dbcAMP) supplementation [17,20] or pharmacological induction of cAMP using forskolin [21] or invasive adenylate cyclase [22] have been employed to achieve elevated cAMP levels and to better mimic the in vivo environment.
The recognition of C-type natriuretic peptide (CNP) as a physiological meiotic arrestor prompted the reassessment of the previous understanding on oocyte pre-maturation [23] and led to the basis of a CNP-mediated pre-IVM system [24]. Furthermore, assessment of CNP-based IVM systems in various species such as pigs, goats, sheep, mice, and humans also showed encouraging results [15]. As reviewed by Gilchrist et al. [15], CNP-based pre-maturation enhances oocyte quality and developmental potential by delaying premature meiotic resumption, preserving cumulus–oocyte communication, and promoting oocyte growth, metabolic activity, and chromatin remodeling. Moreover, the clinical application of CNP-based CAPA-IVM has shown promising results in human patients where prior ovarian stimulation is not feasible, such as gynecological cancer [25], and PCOS patients with variable antral follicle counts [26,27,28,29,30,31]. It can further form the basis of fertility preservation for individuals with the indication of ovariectomy, such as transgender men and oncology patients. Moreover, ongoing efforts of in vitro gametogenesis [32,33,34] can benefit from this technique to improve final oocyte quality.
In equine artificial reproductive technology (ART), the inefficacy of ovarian hormonal stimulation [2] strongly emphasizes oocyte maturation as an essential step for in vitro embryo production. Moreover, holding equine oocytes at room temperature is a common practice that facilitates the oocyte’s transportation and further convenient scheduling for subsequent procedures [35]. For the first time, Choi et al. [36,37] assessed holding equine oocytes in a holding medium containing meiotic inhibitors or not and reported comparable embryo outcomes to direct IVM. These findings highlight the resilience of equine oocytes to withstand a holding step without compromising developmental potential [38]. Since holding oocytes is a common practice, incorporating effective pre-IVM strategies could fulfill this purpose and enhance embryo outcomes. Considering insights and advancements in human CAPA-IVM, we sought to investigate the use of CNP as a meiotic arrestor to improve equine oocyte quality and developmental competence. Moreover, calcium imaging and single-oocyte proteome analysis were performed on matured oocytes with the aim of identifying key proteins affecting oocyte potential. So far, only a few studies have been published on oocyte pre-maturation culture in horses, which showed compromised [37] or improved developmental potential [36,39,40], mainly affected by the stage of immature oocytes used.
This study is the first to evaluate a CNP-based pre-maturation culture in the context of equine IVM and to apply single-oocyte proteomics to refine oocyte holding and maturation protocols in ART. The mare serves as an excellent translational model due to key physiological and developmental similarities to humans [41,42]. In addition to its value as a model species, the horse holds considerable economic, cultural, and sporting significance, motivating the ongoing development of innovative reproductive strategies to enhance genetic potential and safeguard valuable breeds. Importantly, advances in equine ARTs not only benefit the equine industry but may also provide reciprocal insights that contribute to improving human reproductive medicine.
2. Results
2.1. Long CAPA-IVM Increases Maturation Rates While Showing a Trend of Compromised Developmental Competency
In Experiment 1 (3 replicates), a total of 199 COCs were cultured in both groups. Our results show that long CAPA-IVM oocytes (n = 61) with 24 h of pre-maturation culture yielded significantly higher maturation rates (69% vs. 51%, p = 0.017) than the control group (n = 138). Oocyte survival rates were not affected by this extended maturation culture (70% vs. 60%, p = 0.163; Table 1). However, discernible features such as bigger perivitelline space and granular cytoplasm were observed in long CAPA-IVM oocytes, indicating signs of aging.

Table 1.
Survival, maturation, cleavage, and blastocyst rates in long CAPA-IVM and control group. Data is the observation of three replicates. Asterisk (*) indicates a statistically significant difference compared to the control group (p < 0.05).
To evaluate embryo development, 63 matured oocytes from the control and 34 matured oocytes from the long CAPA-IVM group were fertilized by piezo-drilled intracytoplasmic sperm injection (ICSI). Our data demonstrates a trend of compromised development in long CAPA-IVM with a lower cleavage (41% vs. 62% p = 0.050) and blastocyst rates (3% vs. 13% p = 0.114) compared to the control group. Out of 34 injected oocytes, only one embryo was able to form a blastocyst (Table 1).
2.2. Short CAPA-IVM Significantly Improved Maturation Rates and Embryo Development
In the next phase, we reduced the duration of pre-maturation culture to 6 h and assessed the effect on oocyte quality and developmental potential. For this purpose, a total of 546 oocytes (13 replicates) were cultured in both groups. Following IVM, short CAPA-IVM oocytes (n = 266) showed similar survival (63% vs. 56%, p = 0.131) yet significantly higher maturation rate (61% vs. 50%, p = 0.013) compared to the control (n = 280) (Table 2). Following IVM, mature oocytes were used to assess developmental competence and embryo genomics (seven replicates), proteomics (three replicates), and Ca oscillation patterns (three replicates).

Table 2.
Oocyte and embryo outcomes in short CAPA-IVM and control group (maturation and survival rates =13 replicates; cleavage and blastocyst rates = 7 replicates). Asterisk (*) indicates a statistically significant difference compared to the control group (p < 0.05).
After IVM, morphologically normal oocytes with an extruded polar body (69 short CAPA-IVM, 63 control; seven replicates) were fertilized by piezo-drilled ICSI to assess embryo developmental competency. A marked improvement in developmental potential was observed after short CAPA-IVM. In the short CAPA-IVM group, 56/69 (81%) injected oocytes cleaved on day 3, which was significantly higher compared to the control, i.e., 40/63 (63% p = 0.022). Similarly, a significantly higher number of embryos developed to blastocyst in short CAPA-IVM (20/69, 29%) when compared to the control group (9/63, 14% p = 0.041) (Table 2). Nonetheless, we noticed a comparable embryo growth rate in both groups, with an average day of blastocyst formation of 8.5 ± 0.246 in short CAPA-IVM and 8 ± 0.289 in control (p = 0.2633).
2.3. Equine Oocytes Demonstrate an Irregular Calcium-Releasing Pattern
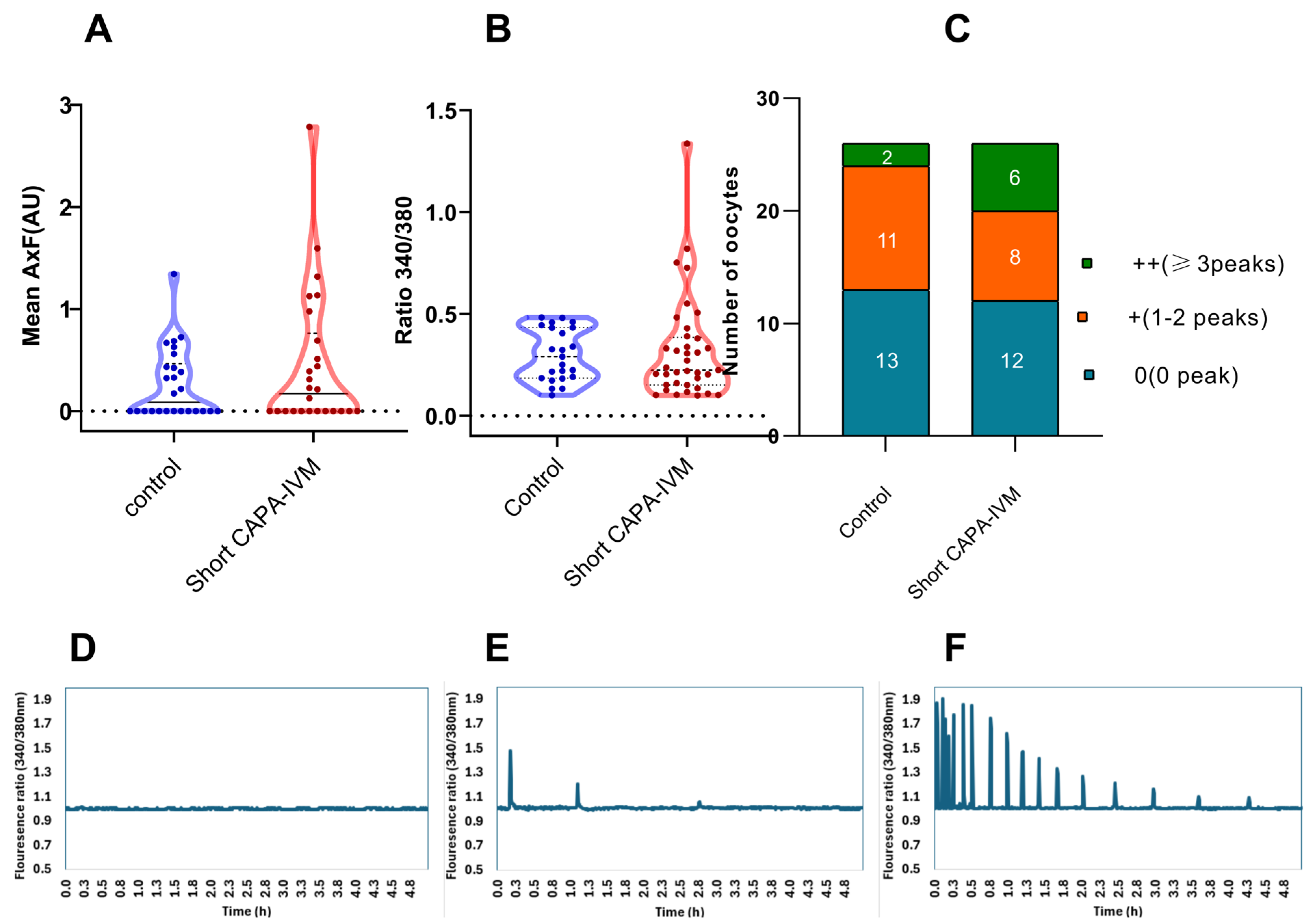
Calcium imaging was performed after fertilization with a single motile sperm to observe the activation potential of in vitro-matured oocytes. Our data reveal that a higher number of oocytes in the short CAPA-IVM group were able to produce consistent calcium release patterns (higher frequency over time) (Figure 1C). However, no significant difference in calcium release was observed in the short CAPA-IVM compared to control (0.46 ± 0.68 vs. 0.26 ± 0.34, p = 0.5565) (Figure 1A). To investigate the discrepancy between the lower number of oocytes with calcium oscillations and the higher cleavage rate, we cultured the presumed zygotes following calcium imaging. Notably, by day 3, a higher proportion of zygotes reached the cleavage stage (79% and 50%) compared to the percentage of oocytes displaying calcium peaks (50% and 46%) in the short CAPA-IVM and control groups, respectively.
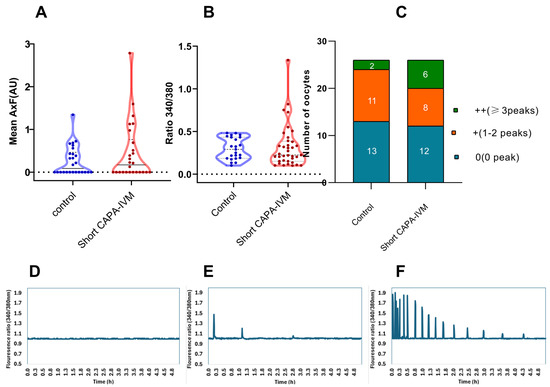
Figure 1.
Live calcium imaging of the matured oocytes from control and short CAPA-IVM groups (data from three biological replicates): (A) calcium-releasing potential represented as the product of amplitude and frequency and expressed as arbitrary units (AU) (each dot represents one oocyte), (B) mean peak amplitude (each dot represents one calcium peak) (C) number of peaks produced by oocytes in each group. (D) 0 no peak, (E) 1–2 peaks, (F) ≥3 peaks.
2.4. Genetic Analysis of Developed Embryos Shows Higher Euploidy Rates Across the Groups
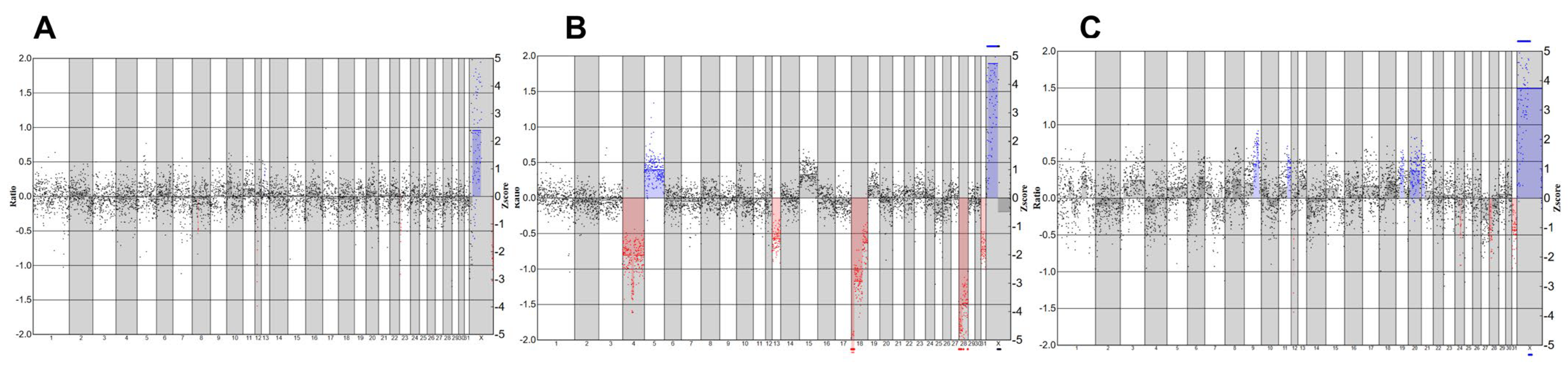
To further evaluate the effect of maturation culture on the chromosomal integrity of the embryos, we analyzed the developed blastocysts for copy number variations. Following analysis, we detected embryos with normal genetic profiles (Figure 2A), embryos with abnormal profiles (Figure 2B), and embryos with an inconclusive profile (Figure 2C). Shallow whole-genome sequencing data indicate a high euploidy rate in developed blastocysts, with 16/16 (100%) in short CAPA-IVM, 1/1 (100%) in long CAPA-IVM, and 21/23 (91%) in the control group. One blastocyst in short CAPA-IVM showed an inconclusive profile (Table 3).
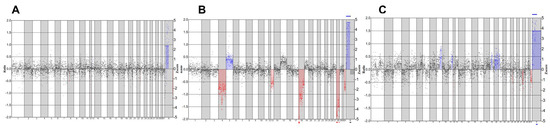
Figure 2.
Representative SWGS profiles of equine blastocysts. Blue color indicates chromosomal addition, and red color indicates chromosomal deletions (A) Normal genetic profile. (B) An abnormal genetic profile with multiple additions and deletions. (C) Inconclusive genetic profile.

Table 3.
Euploidy rate in developed blastocysts across different maturation groups.
2.5. The Proteome Is Maintained in In Vitro-Matured Oocytes Regardless of In Vitro Maturation Conditions
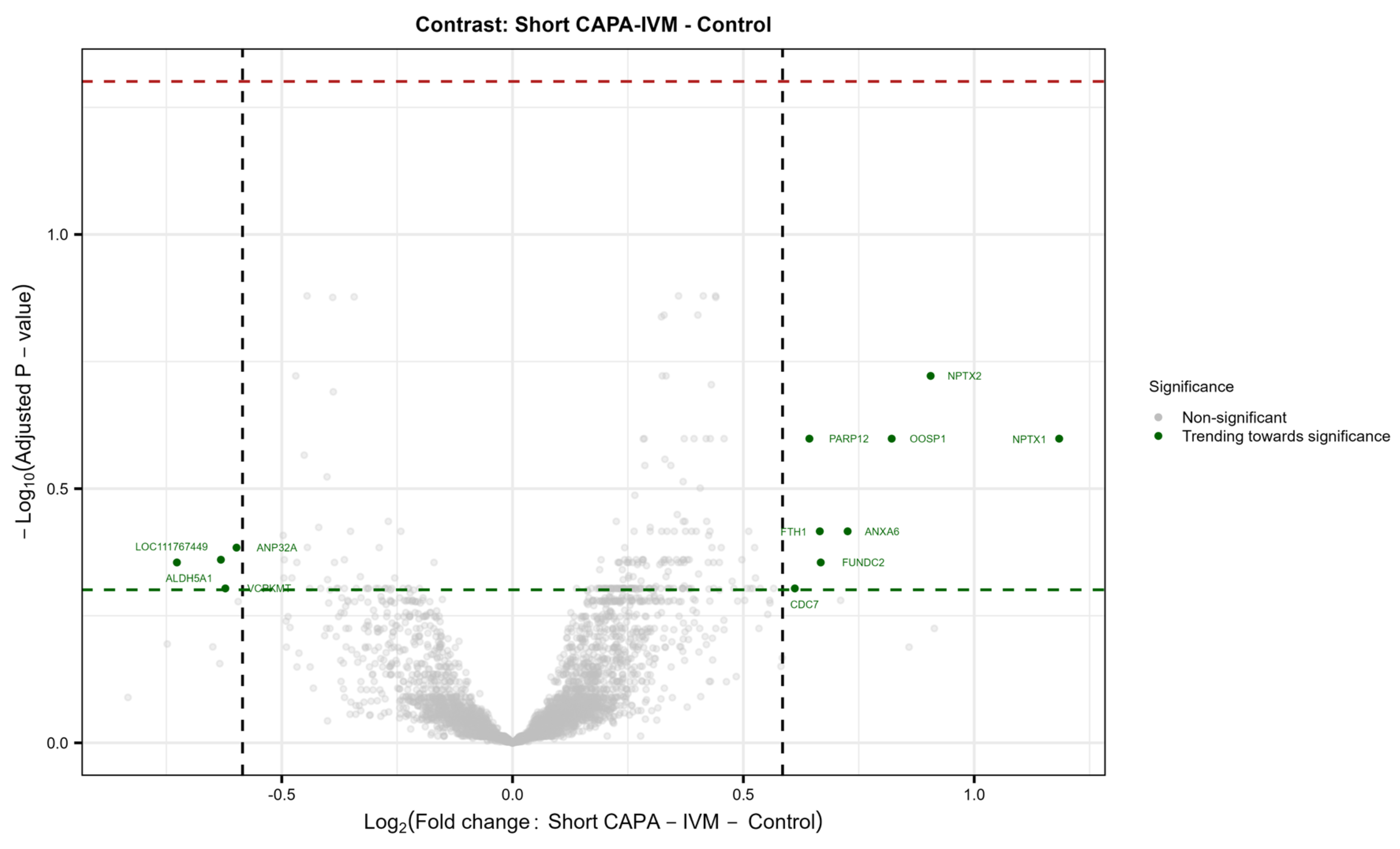
In total, 44 in vitro-matured oocytes (three replicates; control = 23; short CAPA-IVM = 21) were individually analyzed by mass spectrometry-based proteomics. The total number of reliably identified and quantified protein groups (hereafter referred to as proteins) in each sample is summarized in Figure S1B. A total of 4919 proteins were identified from single equine in vitro-matured oocytes across the entire dataset (Table S1). Our results show no significantly differentially abundant (DA) proteins in the short CAPA-IVM group compared to the control group. Furthermore, most of the variance in the dataset was correlated to the sample replicate rather than the treatment condition (Figure S1C,D). Nonetheless, we observed a trend of differential expression for certain proteins in the short CAPA-IVM group (Figure S2), mainly related to oocyte maturation fertilization (WEE2), oocyte activation (ANXA6), meiotic progression (CDC7), spindle–chromosomal stability (PARP12), and cellular stress management (FTH1) (Figure 3).
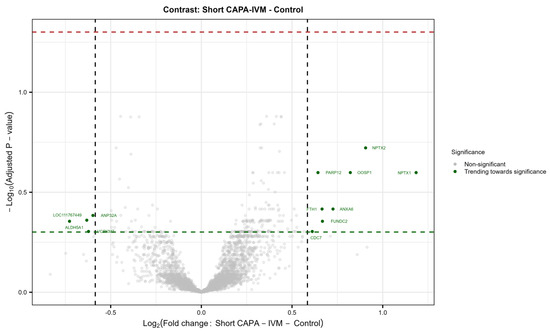
Figure 3.
Volcano plot showing no differentially abundant proteins (DAPs) between matured oocytes from the control and short CAPA-IVM group (p. adj. ≤ 0.05, FC ≥ 1.5). Protein names indicated show IDs trending toward significance in the treatment group (Short CAPA-IVM). (p ≤ 0.05, FC ≥ 1.5). The green dashed line indicates the 0.05 p-value cutoff, whereas the red dashed line indicates the 0.05 adjusted p-value cutoff.
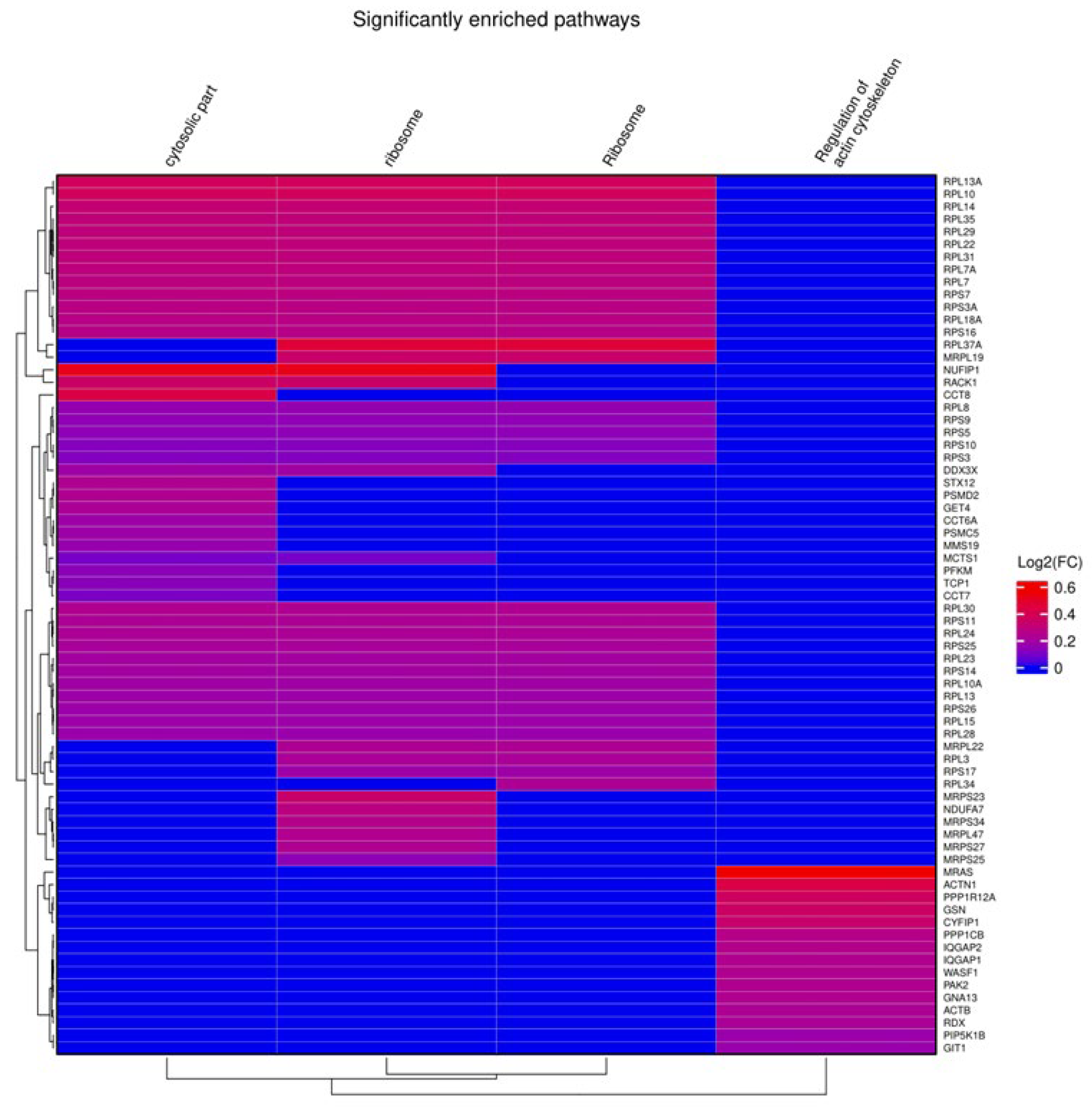
From gene set enrichment analysis (GSEA) against Gene Ontology (GO) terms and pathways from the Kyoto Encyclopedia of Genes and Genomes (KEGG), we identified four enriched gene sets with positive enrichment scores in short CAPA-IVM, related to cytoskeleton regulation (Bta04810, FDR of 0.044), ribosomal function (GO:0005840, Bta03010, FDR of, respectively, 0.021 and 0.014), and cytosolic components (GO:0044445, FDR of 0.035) (Figure 4).
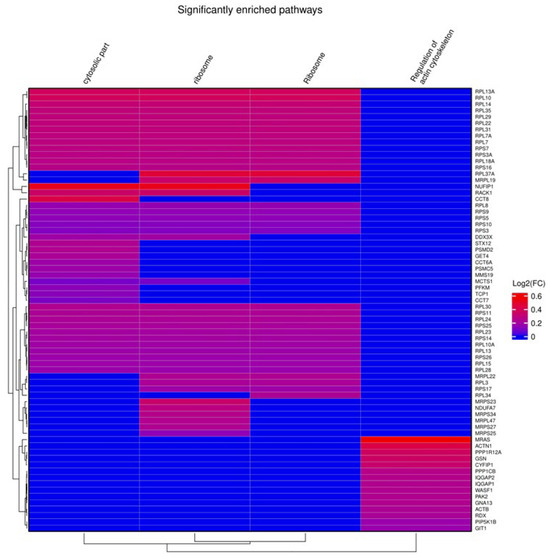
Figure 4.
Gene set enrichment analysis (GSEA) of short CAPA-IVM oocytes compared to control oocytes. The figure shows GSEA results using a heat map, including the list of genes (right side of the heat map) involved in the different pathways (on top of the heat map).
3. Discussion
Optimizing IVM protocols remains a key challenge in human and animal ART, particularly in enhancing oocyte developmental competence [4,15]. Our findings highlight the potential influence of pre-maturation culture duration on extended IVM culture and its impact on oocyte quality [43,44,45,46,47]. We observed that CNP-based CAPA-IVM holds the potential to improve equine oocyte quality and developmental potential. Moreover, proteome trends observed in this and recently published studies [48,49] offer new perspectives on optimizing the IVM protocol, both for horses and humans.
A 24 h pre-maturation in CAPA-IVM yielded a marked improvement in maturation without compromising oocyte survival. This increase in maturation rates has been demonstrated in bovine [50], murine [51], and human oocytes [26], where extended pre-maturation culture resulted in improved nuclear maturity. Nevertheless, despite improved maturation rates, we observed discernible aging-related morphological features such as granular cytoplasm and a large perivitelline space [52,53,54] in these oocytes, which ultimately resulted in poor embryo development. A trend towards compromised developmental potential was observed in the long CAPA-IVM group, as evidenced by lower cleavage rates (41% vs. 62%, p = 0.050) and blastocyst formation rates (3% vs. 13%, p = 0.114).
Our observation on the compromised oocyte morphology in long CAPA-IVM is further supported by a study on equine oocytes where longer pre-maturation duration resulted in compromised gap junctions in COCs [40]. Gap junction (GJ)-mediated communication with cumulus cells is crucial in supporting oocyte metabolism, compensating for its limited ability to metabolize glucose and uptake certain amino acids [55,56,57]. Furthermore, the passage of cyclic nucleotides via GJs safeguards prophase arrest in meiotically competent gametes, allowing for continued cytoplasmic growth and the acquisition of developmental competence [24,50,58,59,60]. Moreover, in porcine oocytes [61], it was demonstrated that prolonged IVM showed poor oocyte quality marked by high ROS levels, apoptosis, and compromised developmental competency compared to good-quality oocytes matured within standard maturation time.
It has been reported that a large perivitelline space in equine oocytes is associated with compromised developmental potential [62]. Previous investigations on human oocytes indicated that extended in vitro maturation culture was found to be associated with altered mitochondrial function, enhanced oxidative stress, and spindle abnormalities [63,64,65,66], which may lead to compromised development [66,67] and higher aneuploidy rates [68]. An equine study showed that prolonging IVM to 48 h significantly decreased cleavage and blastocyst rates [69]. These results demonstrate that while long CAPA-IVM may be beneficial in terms of improved maturation, the accompanying effect of oocyte aging affects the oocyte’s potential to support normal embryogenesis. Hence, it suggests the necessity of optimizing the timing of premature culture to improve cytoplasmic maturity without inducing ageing.
In the next setting, we reduced the duration of the pre-maturation culture to 6 h, as previously investigated on caprine [70], bovine [45], and equine oocytes [40]. As previously reported by Lodde et al. [40], a 6 h cilostamide-based pre-maturation helps maintaining open gap junctions and improves embryo quality in term of cells number per blastocyst. It has been shown in equine oocytes that GJ-based coupling is severely compromised if pre-maturation culture is extended beyond 10 h [40]. Nonetheless, no overall increase in blastocyst rate was reported with the use of cilostamide [40]. Our findings from the short CAPA-IVM condition indicate that a shorter pre-maturation culture duration offers an effective balance between promoting oocyte maturation and supporting subsequent embryo development. Specifically, we observed significantly improved maturation rates accompanied by enhanced embryonic development in the short CAPA-IVM group. These outcomes are consistent with previously published data in both caprine [70] and equine models [40], further supporting the utility of short pre-maturation protocols in optimizing IVM efficiency.
These results are consistent with the existing literature on human [43], murine [44], and bovine [45,46,47] IVM studies where shorter pre-maturation cultures improved embryo quality and developmental potential. This could be attributed to reduced accumulation of stress-related components and relatively better conserved mitochondrial function [64]. However, the exact duration of pre-maturation culture seems species-specific [15].
Calcium-imaging analysis reveals that oocytes exposed to short CAPA-IVM show a tendency for a higher calcium releasing potential (AU) than those matured under control conditions. Previously, only few studies have performed live calcium imaging to determine the calcium-releasing potential of equine IVM oocytes. Overall, the calcium oscillation patterns were inconsistent and were only observed in a small fraction of injected oocytes [71,72]. This may partially explain the suboptimal embryo outcomes frequently observed in equine ARTs, as aberrant calcium-releasing mechanisms can impair fertilization efficiency and lead to early embryonic arrest [73]. Calcium signaling is essential for key developmental processes, including meiotic progression, pronuclear formation, maternal mRNA recruitment, and early embryogenesis [74]. However, a higher number of oocytes were activated even without detectable calcium oscillations [71], comparable with our results showing a higher cleavage rate (79% and 50%) on culturing presumed embryos after calcium imaging, despite a lower number of oocytes showing detectable calcium release (50% and 46%) in the short CAPA-IVM and control groups, respectively. This may represent a species-specific characteristic of equine oocytes, where not all oocytes activate immediately. Notably, injection of the same equine sperm into mouse oocytes resulted in more consistent and robust calcium release, suggesting the delay may be inherent to the oocyte rather than the sperm [72]. To further test the possible insufficient delivery of oocyte activation factor, Bedford et al. [72] permeabilized the sperm membrane by sonication or treatment with Triton-x before ICSI. Contrary to improved calcium oscillations in other species [75,76,77], it does not improve the Ca2+ pattern, suggesting the oocyte’s inability to process the oocyte activation factor delivered by spermatozoa.
Shallow whole-genome sequencing of the developed equine blastocysts showed high euploidy rates across different maturation conditions, with 100% in short CAPA-IVM and long CAPA-IVM and 91% in control groups. These results indicate that the prolonged maturation culture in CAPA-IVM does not affect chromosomal stability, which is similar to previously reported data on human PCOS patients, where CAPA-IVM did not affect euploidy rates [27]. The occurrence of high euploidy rates in equine blastocysts is comparable with a recently reported study by De Coster et al. 2024 [78] where 12 out of 14 analyzed blastocysts showed normal euploid profiles. However, in the same study, it was shown that five of six arrested in vitro-produced (IVP) embryos exhibited chromosomal abnormalities, which could be the reason for their embryonic arrest. Chromosomal integrity is crucial for embryo viability and positive pregnancy outcomes, as aneuploidy is one of the major reasons for implantation failure and pregnancy loss across various species [79,80]. Altogether, it can be assumed that high euploidy rates in developed blastocysts could be a contributing factor for satisfactory pregnancy, i.e., ~70% [38], and live birth rates, i.e., ~60% [81], in equine breeding programs. Therefore, CAPA-IVM’s ability to retain an overall high euploidy rate further encourages its utility in clinical OPU-ICSI programs to improve ART outcomes.
To further develop a deeper understanding of the molecular mechanisms of extended IVM, for the first time, we performed single-oocyte proteomics on in vitro-matured equine oocytes. The data retrieved from this analysis comprehensively describe the proteomic landscape of these oocytes. After filtering, a total of 4919 proteins were reliably identified and quantified in a single equine in vitro-matured oocyte. We noticed that the oocyte proteome does not undergo significant changes at the individual protein level, though four significantly enriched gene sets were observed in short CAPA-IVM oocytes. Moreover, our proteome study revealed a trend of differentially expressed proteins in equine oocytes following short CAPA-IVM, mainly associated with oocyte maturation, activation, and developmental competence. Since we only compared pre-selected matured oocytes from different in vitro maturation groups, proteomic differences leading to failed maturation will not be apparent. Additionally, given that the oocyte is transcriptionally silent during the maturation process, transcripts produced earlier might not be fully translated at the M-II stage. Hence, proteomic differences could become more pronounced in later phases of embryo development, explaining the observed discrepancy in development outcomes. Therefore, proteome analysis of subsequent embryos may offer a more comprehensive understanding of CAPA-IVM’s impact on oocyte potential. Furthermore, it would be interesting to compare the proteomes of in vitro-matured oocytes to those of in vivo-matured ones, as a “golden standard” or positive control, together with proteomic analysis of subsequently developed embryos.
Our GSEA revealed four significant enrichments of pathways associated with ribosomal function, cytosolic components, and cytoskeletal organization in the short CAPA-IVM oocytes, suggesting the presence of a molecular environment conducive to cytoplasmic maturation. The enrichment of cytoskeletal pathways (Bta04810, FDR = 0.044) indicates enhanced spindle organization and actin filament remodeling, which are involved in oocyte polarity, chromosome alignment, and asymmetric division [82]. Notably, PPP1R12A, a regulator of myosin phosphatase and a member of this gene set, is implicated in CNP-cGMP-PKG signaling, a key pathway in maintaining oocyte arrest [83]. The presence of PPP1R12A in this enriched gene set suggests that CAPA-IVM may influence cytoskeletal arrangement via cyclic nucleotide-mediated pathways, essential for better structural integrity and spindle–chromosome complex [83].
Likewise, the upregulation of ribosomal function-related pathways (GO:0005840, Bta03010, FDR = 0.021 and 0.014, respectively) suggests that short CAPA-IVM supports translational activity required for maternal mRNA processing and protein synthesis during maturation [84]. Since oocytes rely on stored maternal transcripts to drive early embryonic development, an effective ribosomal activity might be instrumental for the improved developmental potential observed in our study [84]. The enrichment of cytosolic component-related pathways (GO:0044445, FDR = 0.035) further supports the idea that intracellular organization, protein stability, and metabolic regulation are improved in short CAPA-IVM oocytes, creating a more supportive environment for maturation and fertilization.
Overall, short CAPA-IVM demonstrates a promising approach to synchronize nuclear and cytoplasmic maturation while limiting stress-induced damage. Future research should focus on validating these findings through functional assays to understand their effect on oocyte biology in a wider context. Furthermore, comparative single-oocyte omics analyses across species could provide valuable insights into conserved and species-specific mechanisms, facilitating the refinement and enhancement of in vitro maturation protocols.
4. Materials and Methods
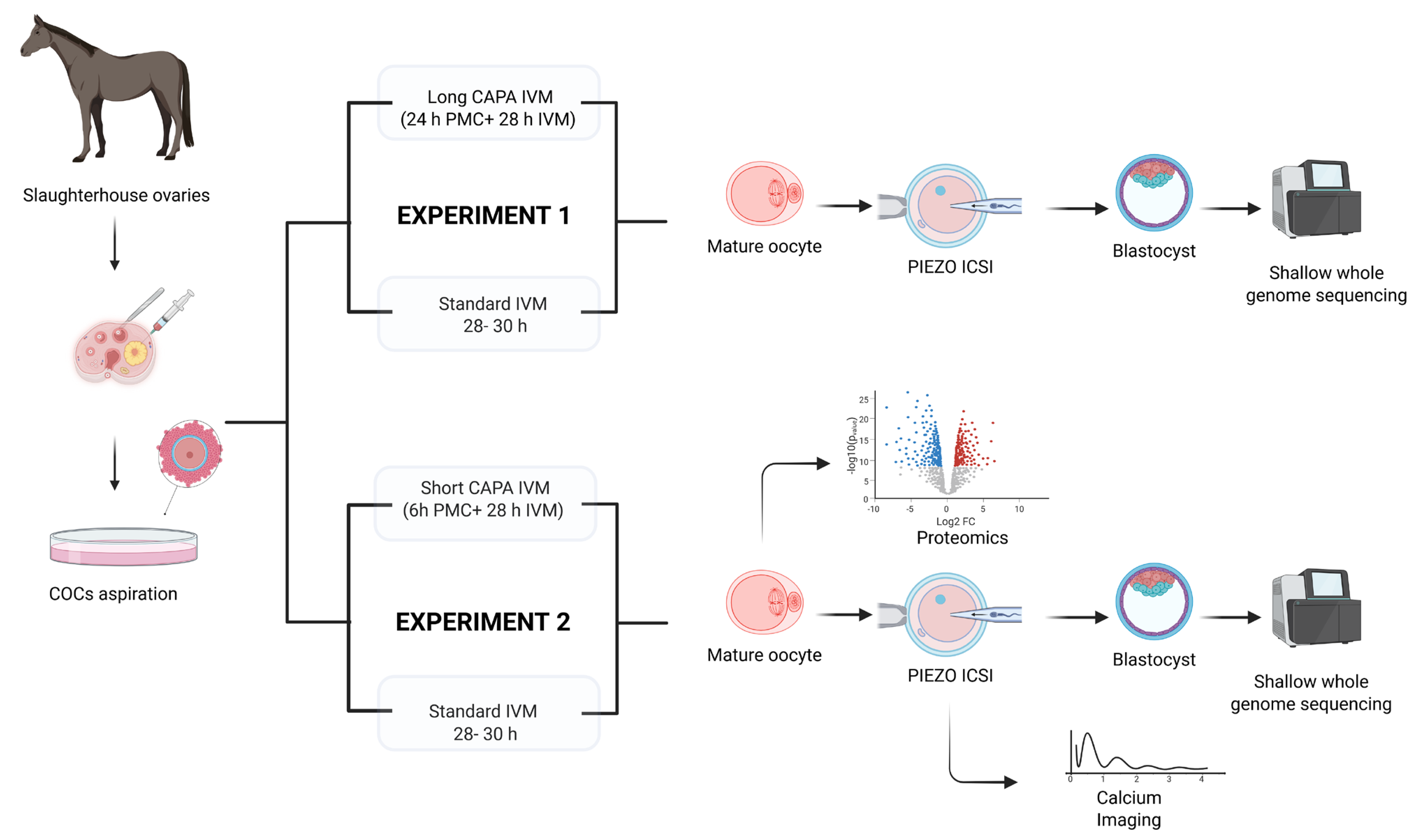
The experimental design followed for this study is shown in Figure 5.
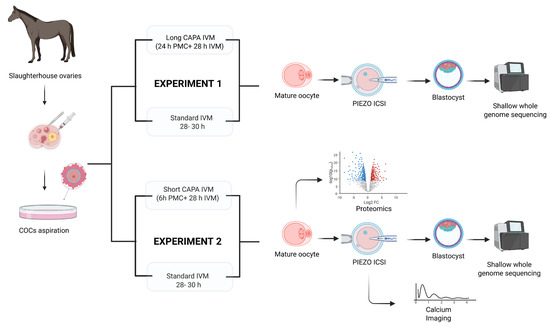
Figure 5.
Experimental design for evaluating CAPA-IVM in equine oocytes in two different settings: Experiment 1 compared long CAPA-IVM with standard IVM but was discontinued due to its negative impact on oocyte morphology and embryo development. Experiment 2 compared short CAPA-IVM with standard IVM. Mature oocytes were further analyzed for embryo development after piezo ICSI (intracytoplasmic sperm injection), calcium releasing potential, and by single-oocyte proteomics. Shallow whole-genome sequencing was performed on developed blastocysts to detect chromosomal aberrations.
4.1. Collection of Equine COCs
Equine ovaries were collected from a local abattoir in Belgium and transported to the laboratory in an insulated box at ambient temperature within 1 h of collection. Ovaries were cleaned by removing surrounding tissue and washed twice with prewarmed normal saline (38.2 °C; NaCl 0.9%). All follicles ranging from 5 to 30 mm were aspirated using a 16-gauge needle attached to a vacuum pump (Craft Suction Pump, Rocket Medical, Washington, UK) (100 mm Hg), scraped with the aspirating needle, and flushed with prewarmed flushing medium (Equiplus, Minitube, Tiefenbach, Germany). The aspirated fluid was collected in sterilized glass bottles and searched under a stereomicroscope (Olympus SZX7®, Olympus Corp. Shinjuku, Tokyo, Japan) for the presence of COCs at room temperature (22 °C). During recovery, COCs were washed and kept in prewarmed TCM-199 with Hank’s salts (38.2; Gibco, Life Technologies, Merelbeke, Belgium) supplemented with 10% v/v FBS (Gibco, Life Technologies, Merelbeke, Belgium) and 0.1% gentamycin v/v (Sigma-Aldrich, Bornem, Belgium) before transferring to the maturation culture. Only COCs with compact and fully surrounding cumulus cells were considered for further IVM.
4.2. In Vitro Maturation and Fertilization
The recovered COCs were either placed in a pre-maturation culture with IVM medium containing 200 nM CNP (Bio-Techne, Dublin, Ireland) for 6 h (short CAPA-IVM) or 24 h (long CAPA-IVM) before IVM, or directly transferred to IVM culture (Medium 199 with Earl’s salts (Gibco, Life Technologies, Merelbeke, Belgium), supplemented with 10% FBS (v/v) (Gibco, Life Technologies, Merelbeke, Belgium), 9.8 μg/mL follicle-stimulating hormone, and 1.96 μg/mL luteinizing hormone (Stimufol, Reprobiol, Ouffet, Belgium)) as a control. In vitro maturation was performed in groups of 10–20 COCs in 500 μL maturation medium under oil (CooperSurgical, Venlo, The Netherlands) at 38.2 °C in 5% CO2-containing air for 28–30 h. Denudation was performed by enzymatic treatment [0.1% bovine hyaluronidase (Sigma-Aldrich, Bornem, Belgium) in TCM-199 with Hank’s salts (Gibco, Life Technologies, Merelbeke, Belgium) supplemented with 10% FBS (Gibco, Life Technologies, Merelbeke, Belgium) and 0.1% gentamycin (Sigma-Aldrich, Bornem, Belgium))] and mechanical stripping using a STRIPPER pipettor and 170 μm and 135 µm capillaries (MXL3-STR-CGR, MXL3-175 and MXL3-135; Cooper Surgical, Trumbull, CT, USA). The nuclear maturity of the oocyte was assessed by the presence of the first polar body.
For oocyte fertilization, ICSI was performed as described previously [78,85]. Briefly, a small piece of straw with frozen semen from a proven fertile stallion was thawed in 1 mL G-MOPS (38.2 °C; Vitrolife, Londerzeel, Belgium) and centrifuged (400× g/3 min; 23 °C). After the first centrifugation step, the supernatant was discarded, and the pellet was resuspended in 1 mL G-MOPS. After the second centrifugation step, the supernatant was discarded, and the pellet was resuspended in 200 μL G-MOPS. At the time of ICSI, a small volume (1 μL) of the resuspended sperm was added to a 5 μL droplet of 7% polyvinylpyrrolidone (CooperSurgical, Venlo, The Netherlands). Metaphase (M-II) oocytes were injected with piezo-drilled ICSI (PrimeTech, Nakamukaihara Tsuchiura Ibaraki, Japan; speed 3–4, intensity 6–8), and presumptive zygotes were cultured for 7–10 days in 20 μL droplets of DMEM/F-12 (Gibco, Life Technologies, Merelbeke, Belgium) with 10% FBS and 0.1% gentamycin under oil at 38.2 °C in a humidified atmosphere of 5% O2, 5% CO2, and 90% N2. The cleavage rate was evaluated on day 3 after ICSI, and blastocyst formation was monitored from day 7 to day 10 post-ICSI.
4.3. Calcium Imaging of In Vitro-Matured Oocytes
Calcium imaging was performed on equine in vitro-matured oocytes, as described previously [86]. Briefly, oocytes were exposed to 7.5 μΜ fura-2-AM (Teflabs, Austin, TX, USA) in an embryo culture medium for 30 min under standard culture conditions. Then, oocytes were injected with a single sperm via piezo-drilled ICSI using an inverted microscope(Olympus IX73; Olympus Shinjuku, Tokyo, Japan). The selected spermatozoa were demembranated with 0.2% lysolecithin (Instruchemie, Delfzijl, The Netherlands) for 1 min to ensure efficient delivery of the oocyte activation factor. ICSI was performed within 30 min, and oocytes were transferred to an inverted epifluorescence microscope (Olympus IX71, Olympus Soft Imaging Solutions GmbH, Antwerpen, Belgium) in a glass-bottom dish (MatTek Corporation, Ashland, MA, USA) under standard culture conditions.
Calcium release was recorded for five consecutive hours, with a 10× objective and a filter switch (Lambda DG-4 filter switch, Sutter Instrument Company, Novato, CA, USA) to provide excitation alternating between 340 and 380 nm. Calcium data were analyzed using Clampfit 10.2 software (Axon Laboratories, Molecular Devices UK Ltd., Winnersh, UK). The total amount of calcium released (in arbitrary units (AU)) was calculated as the product of the mean amplitude (maximum fluorescence intensity per peak) per mean frequency (number of calcium spikes) of all oocytes injected per condition (including the oocytes showing no calcium peaks).
4.4. Genetic Analysis of Developed Embryos
Embryos reaching the blastocyst stage were immediately collected for genetic analysis and later analyzed by shallow whole-genome sequencing as described [87]. Briefly, the zona pellucida of each blastocyst was removed by treating with EmbryoMax® Acidic Tyrode′s Solution (Merck Life Science, Overijse, Belgium) for 1 min. After zona pellucida removal, blastocysts were thoroughly washed and collected in Dulbecco’s phosphate-buffered saline (DPBS) and stored at −20 °C until processing for genetic analysis. Whole-genome amplification (WGA) was performed with the SurePlex DNA amplification kit (Rubicon Genomics Inc., Ann Arbor, MI, USA). DNA was fragmented to ~200 bp using a M220 Focused ultrasonicator Instrument (Covaris, Woburn, MA, USA), and subsequent library preparation was performed with the NEXTflex™ Rapid DNA-Seq Library Prep Kit for Illumina Sequencing (Bioo Scientific, Uden, The Netherlands). Agencourt AMPure XP beads (Beckman Coulter, Suarlée, Belgium) were used for purification. Template preparation was performed on the cBot™ System (Illumina, San Diego, CA, USA), using 2.5 nM of equimolar pooled libraries, and sequencing was performed on a Hiseq3000 sequencer (Illumina, San Diego, CA, USA). CNV detection data was analyzed using the WisecondorX and Vivar software [88,89].
4.5. Single-Oocyte Proteome Sample Preparation
Single-oocyte proteomics was performed as described [90]. In three replicates, individual in vitro-matured oocytes were sampled in 2 µL of DPBS in a twin.tec® PCR Plate 384 LoBind® (Eppendorf 0030129547, Hamburg, Germany) and stored frozen at −80 °C until further processing. Samples were lysed by three thaw–freeze cycles at 80 °C followed by −70 °C for 15 min each, with centrifugation at 1500 g for 10 s after each thawing step. During each incubation step, plates were tightly sealed using plastic plate seals (Diversified Biotech, Dedham, MA, USA). After the first thawing step, 1 µL of 15% LC-MS-grade DMSO (Thermo Scientific, Rockford, IL, USA) was added to each sample. Proteins were then digested overnight at 37 °C in 3 µL of digestion buffer (100 mM triethylammonium bicarbonate (TEAB) (Merck, Darmstadt, Germany), 0.1% n-Dodecyl-B-D-maltoside (Sigma-Aldrich, Steinheim, Germany), 0.02% ProteaseMAX (Promega, Madison, WI, USA), 13.33 ng/µL Trypsin/Lys-C (Promega, Madison, WI, USA)). Subsequently, samples were acidified with 1 µL of 5% trifluoracetic acid (TFA) (Biosolve, Dieuze, France). Samples were transferred to a twin.tec® PCR Plate 384 LoBind® using protein low-binding p10 tips (Socorex, Langenhagen, Germany); in doing so, sample volumes were estimated. Sample volumes were brought to 15 µL using 0.1% TFA. The plate was tightly sealed using an aluminum plate seal (Excel Scientific, Victorville, CA, USA) and stored at −70 °C until data acquisition at the VIB proteomics core facility.
4.6. Proteomics Data Analysis
Raw data files were searched using DIA-NN v1.9.2. The search engine was supplied with the equine reference proteome (Equus caballus: UP000002281 with 69,434 entries) for library generation, allowing for one missed cleavage using Trypsin/P as the protease and up to two variable modifications, with N-terminal methionine excision, methionine oxidation, and N-terminal acetylation set as variable modifications. Peptide length and charge ranges were set to 7–30 and 1–4, respectively. Precursor and fragment ion ranges were set to 400–1000 and 100–1700, respectively. Precursors were filtered at 1% FDR, with quantUMS (high precision), cross-run retention time-based normalization, and match between runs enabled.
Downstream data analysis was performed with R version 4.4.0 within the RStudio environment version 2024.09.0+375. Statistical analysis was performed using the MSqRob2 and QFeatures packages [42,43]. Plotting was performed using the ggplot2 package [44]. Gene set enrichment analysis (GSEA) was performed using the WebGestaltR package [45]. Briefly, only precursors identified in at least five control and five treated samples, proteins for which at least two peptides were identified, and which were identified in each technical batch, were considered for statistical modelling. Precursor quantities were log2-transformed, median-normalized, and then aggregated into protein abundances using the median polish procedure implemented in MSqRob2. Differential expression analysis was performed using MSqRob2. p-values were adjusted using the Benjamini–Hochberg method with an FDR of 0.05. GSEA was performed using the -log2(fold change) × sign(fold change) as a ranking metric, with an FDR of 0.05. The queried database consists of KEGG pathways [91] and the biological process, cellular component and molecular function gene ontologies of a size between 50 and 200 genes. Mass spectrometry raw data is available via the PRIDE data repository (Accession number PXD060584; reviewer username: reviewer_pxd060584@ebi.ac.uk, reviewer password: IPzt5yEKzlBb).
4.7. Statistical Analysis
The chi-square (χ2) test was applied to compare the categorical variables expressed in percentages. The average day of blastocyst formation and calcium-releasing potential (AU) were compared among groups using the Mann–Whitney U test. p-values < 0.05 were considered as statistically significant. Statistical analysis was performed using GraphPad Prism version 10.0.2 (GraphPad Software, Boston, MA, USA).
5. Conclusions
Our research encompasses crucial implications for optimizing IVM systems in both human and equine ARTs. The reciprocal knowledge from both human and equine models in ART research has substantial potential to accelerate progress in both fields. CAPA-IVM, particularly with optimal pre-maturation culture duration, offers a promising approach for enhancing oocyte maturation and embryo development in equine ART. The variable effect of short and long CAPA-IVM on oocyte quality and developmental potential highlights the importance of pre-maturation culture duration to obtain optimal outcomes. The proteomic profiles of IVM MII oocytes from both groups shed light on subtle differences which may impact further development. The high euploidy rates across different culture conditions further highlight the ability of CAPA-IVM as an alternative to traditional IVM, improving oocyte developmental potential with retained genetic integrity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms26125495/s1.
Author Contributions
M.F.-I.-A. designed and performed the experiments, collected samples, analyzed data, and wrote the manuscript. D.A.-V., Q.A.A. and M.H. contributed to the embryology experiments. B.H. and K.S. designed and supervised the experiments and reviewed and edited the manuscript. B.M. contributed to the genetic analysis and interpretation of data. K.G., M.B. and E.A. contributed to proteomics experiments, analysis, and interpretation of results. All authors, including A.V.S., S.M.C.d.S.L., D.S. and C.D.R. contributed to the data curation, formal analysis of data and revision of the manuscript. B.H. and K.S. were responsible for final editing and approval. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by HEC, Pakistan (PD/HEC/HRD/OSS-III/BLG-B2/2021/19320); BOF (Bijzonder Onderzoeksfonds) GOA (Geconcerteerde onderzoeksacties), 2018000504 (GOA030-18 BOF); BOF starting grant to BH (BOF.STG.2021.0042.01); and FWO (Research Foundation-Flanders) 1177425N funding.
Institutional Review Board Statement
All experiments included in this study were performed on slaughterhouse-derived ovaries; hence, no prior ethical approval was required.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study were included in the manuscript and its Supplementary Information Files. Raw data are available from the corresponding author upon reasonable request. The mass spectrometry proteomics data and analysis scripts have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD060584 (https://www.ebi.ac.uk/pride/archive accessed on 8 June 2025).
Acknowledgments
The authors thank everyone at G-FaST and RBU for their constant support in executing this project. We also extend our sincere thanks to Arantxa Cardona Barberán, Krishna Chaitanya Pavani, and Andrei Rybouchkin for generously sharing their expertise during the experimental phase.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Gong, X.; Li, H.; Zhao, Y. The Improvement and Clinical Application of Human Oocyte In Vitro Maturation (IVM). Reprod. Sci. 2022, 29, 2127–2135. [Google Scholar] [CrossRef] [PubMed]
- Maclellan, L.; Carnevale, E.; Da Silva, M.C.; Scoggin, C.; Bruemmer, J.; Squires, E. Pregnancies from vitrified equine oocytes collected from super-stimulated and non-stimulated mares. Theriogenology 2002, 58, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Hinrichs, K. Immature oocyte collection and maturation. In Equine Reproduction, 2nd ed.; McKinnon, A.O., Squires, E.L., Vaala, W.E., Varner, D.D., Eds.; Wiley-Blackwell: Ames, IA, USA, 2011; Volume 2, pp. 2931–2935. [Google Scholar]
- He, M.; Zhang, T.; Yang, Y.; Wang, C. Mechanisms of Oocyte Maturation and Related Epigenetic Regulation. Front. Cell Dev. Biol. 2021, 9, 654028. [Google Scholar] [CrossRef] [PubMed]
- Scott, T.J.; Carnevale, E.; Maclellan, L.; Scoggin, C.; Squires, E. Embryo development rates after transfer of oocytes matured in vivo, in vitro, or within oviducts of mares. Theriogenology 2001, 55, 705–715. [Google Scholar] [CrossRef]
- Foss, R.; Ortis, H.; Hinrichs, K. Effect of potential oocyte transport protocols on blastocyst rates after intracytoplasmic sperm injection in the horse. Equine Vet. J. 2013, 45, 39–43. [Google Scholar] [CrossRef]
- Hyttel, P.; Fair, T.; Callesen, H.; Greve, T. Oocyte growth, capacitation and final maturation in cattle. Theriogenology 1997, 47, 23–32. [Google Scholar] [CrossRef]
- Hendriksen, P.; Vos, P.; Steenweg, W.; Bevers, M.; Dieleman, S. Bovine follicular development and its effect on the in vitro competence of oocytes. Theriogenology 2000, 53, 11–20. [Google Scholar] [CrossRef]
- Sun, G.-Y.; Gong, S.; Kong, Q.-Q.; Li, Z.-B.; Wang, J.; Xu, M.-T.; Luo, M.-J.; Tan, J.-H. Role of AMP-activated protein kinase during postovulatory aging of mouse oocytes. Biol. Reprod. 2020, 103, 534–547. [Google Scholar] [CrossRef]
- Norris, R.P.; Ratzan, W.J.; Freudzon, M.; Mehlmann, L.M.; Krall, J.; Movsesian, M.A.; Wang, H.; Ke, H.; Nikolaev, V.O.; Jaffe, L.A. Cyclic GMP from the surrounding somatic cells regulates cyclic AMP and meiosis in the mouse oocyte. Development 2009, 136, 1869–1878. [Google Scholar] [CrossRef]
- Vaccari, S.; Weeks, J.L.; Hsieh, M.; Menniti, F.S.; Conti, M. Cyclic GMP signaling is involved in the luteinizing hormone-dependent meiotic maturation of mouse oocytes. Biol. Reprod. 2009, 81, 595–604. [Google Scholar] [CrossRef]
- Hyttel, P.; Greve, T.; Callesen, H. Ultrastructural aspects of oocyte maturation and fertilization in cattle. J. Reprod. Fertil. Suppl. 1989, 38, 35–47. [Google Scholar] [PubMed]
- Zhu, S.; Wang, Q. Metabolic control of oocyte development. Biol. Reprod. 2022, 107, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Leal, G.R.; Monteiro, C.A.S.; Souza-Fabjan, J.M.G.; de Paula Vasconcelos, C.O.; Nogueira, L.A.G.; Ferreira, A.M.R.; Serapião, R.V. Role of cAMP modulator supplementations during oocyte in vitro maturation in domestic animals. Anim. Reprod. Sci. 2018, 199, 1–14. [Google Scholar] [CrossRef]
- Gilchrist, R.B.; Ho, T.M.; De Vos, M.; Sanchez, F.; Romero, S.; Ledger, W.L.; Anckaert, E.; Vuong, L.N.; Smitz, J. A fresh start for IVM: Capacitating the oocyte for development using pre-IVM. Hum. Reprod. Update 2023, 30, 3–25. [Google Scholar] [CrossRef]
- Zuccotti, M.; Giorgi Rossi, P.; Martinez, A.; Garagna, S.; Forabosco, A.; Redi, C.A. Meiotic and developmental competence of mouse antral oocytes. Biol. Reprod. 1998, 58, 700–704. [Google Scholar] [CrossRef]
- Funahashi, H.; Cantley, T.C.; Day, B.N. Synchronization of meiosis in porcine oocytes by exposure to dibutyryl cyclic adenosine monophosphate improves developmental competence following in vitro fertilization. Biol. Reprod. 1997, 57, 49–53. [Google Scholar] [CrossRef]
- Luciano, A.M.; Franciosi, F.; Modina, S.C.; Lodde, V. Gap junction-mediated communications regulate chromatin remodeling during bovine oocyte growth and differentiation through cAMP-dependent mechanism (s). Biol. Reprod. 2011, 85, 1252–1259. [Google Scholar] [CrossRef]
- Nogueira, D.; Cortvrindt, R.; De Matos, D.; Vanhoutte, L.; Smitz, J. Effect of phosphodiesterase type 3 inhibitor on developmental competence of immature mouse oocytes in vitro. Biol. Reprod. 2003, 69, 2045–2052. [Google Scholar] [CrossRef][Green Version]
- Somfai, T.; Kikuchi, K.; Onishi, A.; Iwamoto, M.; Fuchimoto, D.-I.; Papp, Á.B.; Sato, E.; Nagai, T. Meiotic arrest maintained by cAMP during the initiation of maturation enhances meiotic potential and developmental competence and reduces polyspermy of IVM/IVF porcine oocytes. Zygote 2003, 11, 199–206. [Google Scholar] [CrossRef]
- Albuz, F.; Sasseville, M.; Lane, M.; Armstrong, D.; Thompson, J.; Gilchrist, R. Simulated physiological oocyte maturation (SPOM): A novel in vitro maturation system that substantially improves embryo yield and pregnancy outcomes. Hum. Reprod. 2010, 25, 2999–3011. [Google Scholar] [CrossRef]
- Guixue, Z.; Luciano, A.; Coenen, K.; Gandolfi, F.; Sirard, M. The influence of cAMP before or during bovine oocyte maturation on embryonic developmental competence. Theriogenology 2001, 55, 1733–1743. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Su, Y.-Q.; Sugiura, K.; Xia, G.; Eppig, J.J. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science 2010, 330, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Franciosi, F.; Coticchio, G.; Lodde, V.; Tessaro, I.; Modina, S.C.; Fadini, R.; Dal Canto, M.; Renzini, M.M.; Albertini, D.F.; Luciano, A.M. Natriuretic peptide precursor C delays meiotic resumption and sustains gap junction-mediated communication in bovine cumulus-enclosed oocytes. Biol. Reprod. 2014, 91, 61, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Kirillova, A.; Bunyaeva, E.; Van Ranst, H.; Khabas, G.; Farmakovskaya, M.; Kamaletdinov, N.; Nazarenko, T.; Abubakirov, A.; Sukhikh, G.; Smitz, J.E.J. Improved maturation competence of ovarian tissue oocytes using a biphasic in vitro maturation system for patients with gynecological malignancy: A study on sibling oocytes. J. Assist. Reprod. Genet. 2021, 38, 1331–1340. [Google Scholar] [CrossRef]
- Sanchez, F.; Le, A.H.; Ho, V.N.A.; Romero, S.; Van Ranst, H.; De Vos, M.; Gilchrist, R.B.; Ho, T.M.; Vuong, L.N.; Smitz, J. Biphasic in vitro maturation (CAPA-IVM) specifically improves the developmental capacity of oocytes from small antral follicles. J. Assist. Reprod. Genet. 2019, 36, 2135–2144. [Google Scholar] [CrossRef]
- Sánchez, F.; Lolicato, F.; Romero, S.; De Vos, M.; Van Ranst, H.; Verheyen, G.; Anckaert, E.; Smitz, J.E.J. An improved IVM method for cumulus-oocyte complexes from small follicles in polycystic ovary syndrome patients enhances oocyte competence and embryo yield. Hum. Reprod. 2017, 32, 2056–2068. [Google Scholar] [CrossRef]
- Akin, N.; Le, A.H.; Ha, U.D.T.; Romero, S.; Sanchez, F.; Pham, T.D.; Nguyen, M.H.N.; Anckaert, E.; Ho, T.M.; Smitz, J.; et al. Positive effects of amphiregulin on human oocyte maturation and its molecular drivers in patients with polycystic ovary syndrome. Hum. Reprod. 2022, 37, 30–43. [Google Scholar] [CrossRef]
- Vuong, L.N.; Ho, V.N.; Ho, T.M.; Dang, V.Q.; Phung, T.H.; Giang, N.H.; Le, A.H.; Pham, T.D.; Wang, R.; Smitz, J. In-vitro maturation of oocytes versus conventional IVF in women with infertility and a high antral follicle count: A randomized non-inferiority controlled trial. Hum. Reprod. 2020, 35, 2537–2547. [Google Scholar] [CrossRef]
- Vuong, L.N.; Le, A.H.; Ho, V.N.; Pham, T.D.; Sanchez, F.; Romero, S.; De Vos, M.; Ho, T.M.; Gilchrist, R.B.; Smitz, J. Live births after oocyte in vitro maturation with a prematuration step in women with polycystic ovary syndrome. J. Assist. Reprod. Genet. 2020, 37, 347–357. [Google Scholar] [CrossRef]
- Vuong, L.N.; Nguyen, L.K.; Le, A.H.; Pham, H.H.; Ho, V.N.; Le, H.L.; Pham, T.D.; Dang, V.Q.; Phung, T.H.; Smitz, J. Fresh embryo transfer versus freeze-only after in vitro maturation with a pre-maturation step in women with high antral follicle count: A randomized controlled pilot study. J. Assist. Reprod. Genet. 2021, 38, 1293–1302. [Google Scholar] [CrossRef]
- McLaughlin, M.; Albertini, D.; Wallace, W.; Anderson, R.; Telfer, E. Metaphase II oocytes from human unilaminar follicles grown in a multi-step culture system. MHR Basic Sci. Reprod. Med. 2018, 24, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Lawson, M.S.; Bean, Y.; Ting, A.Y.; Pejovic, T.; De Geest, K.; Moffitt, M.; Mitalipov, S.M.; Xu, J. Matrix-free 3D culture supports human follicular development from the unilaminar to the antral stage in vitro yielding morphologically normal metaphase II oocytes. Hum. Reprod. 2021, 36, 1326–1338. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Jia, L.; Zeng, H.; Sun, P.; Su, W.; Li, T.; Liang, X.; Fang, C. Neurotrophin-4 promotes in vitro development and maturation of human secondary follicles yielding metaphase II oocytes and successful blastocyst formation. Hum. Reprod. Open 2024, 2024, hoae005. [Google Scholar] [CrossRef]
- Cuervo-Arango, J.; Claes, A.N.; Stout, T.A. Mare and stallion effects on blastocyst production in a commercial equine ovum pick-up–intracytoplasmic sperm injection program. Reprod. Fertil. Dev. 2019, 31, 1894–1903. [Google Scholar] [CrossRef]
- Choi, Y.H.; Love, L.B.; Varner, D.D.; Hinrichs, K. Blastocyst development in equine oocytes with low meiotic competence after suppression of meiosis with roscovitine prior to in vitro maturation. Zygote 2006, 14, 1–8. [Google Scholar] [CrossRef]
- Choi, Y.H.; Love, L.B.; Varner, D.D.; Hinrichs, K. Holding immature equine oocytes in the absence of meiotic inhibitors: Effect on germinal vesicle chromatin and blastocyst development after intracytoplasmic sperm injection. Theriogenology 2006, 66, 955–963. [Google Scholar] [CrossRef]
- Broothaers, K.; Pascottini, O.B.; Hedia, M.; Angel-Velez, D.; De Coster, T.; Peere, S.; Polfliet, E.; Van den Branden, E.; Govaere, J.; Van Soom, A.; et al. Oocyte holding and in vitro maturation duration between 28 and 34 hours do not affect equine OPU-ICSI outcomes. Theriogenology 2025, 233, 64–69. [Google Scholar] [CrossRef]
- Metcalf, E.S.; Masterson, K.R.; Battaglia, D.; Thompson, J.G.; Foss, R.; Beck, R.; Cook, N.L.; O’Leary, T. Conditions to optimise the developmental competence of immature equine oocytes. Reprod. Fertil. Dev. 2020, 32, 1012–1021. [Google Scholar] [CrossRef]
- Lodde, V.; Colleoni, S.; Tessaro, I.; Corbani, D.; Lazzari, G.; Luciano, A.M.; Galli, C.; Franciosi, F. A prematuration approach to equine IVM: Considering cumulus morphology, seasonality, follicle of origin, gap junction coupling and large-scale chromatin configuration in the germinal vesicle. Reprod. Fertil. Dev. 2019, 31, 1793–1804. [Google Scholar] [CrossRef]
- Benammar, A.; Derisoud, E.; Vialard, F.; Palmer, E.; Ayoubi, J.M.; Poulain, M.; Chavatte-Palmer, P. The Mare: A Pertinent Model for Human Assisted Reproductive Technologies? Animals 2021, 11, 2304. [Google Scholar] [CrossRef]
- Carnevale, E.M.; Catandi, G.D.; Fresa, K. Equine Aging and the Oocyte: A Potential Model for Reproductive Aging in Women. J. Equine Vet. Sci. 2020, 89, 103022. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, D.; Ron-El, R.; Friedler, S.; Schachter, M.; Raziel, A.; Cortvrindt, R.; Smitz, J. Meiotic Arrest In Vitro by Phosphodiesterase 3-Inhibitor Enhances Maturation Capacity of Human Oocytes and Allows Subsequent Embryonic Development1. Biol. Reprod. 2006, 74, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Santiquet, N.W.; Greene, A.F.; Becker, J.; Barfield, J.P.; Schoolcraft, W.B.; Krisher, R.L. A pre-in vitro maturation medium containing cumulus oocyte complex ligand-receptor signaling molecules maintains meiotic arrest, supports the cumulus oocyte complex and improves oocyte developmental competence. Mol. Hum. Reprod. 2017, 23, 594–606. [Google Scholar] [CrossRef] [PubMed]
- Morishita, N.; Shibata, K.; Matsunaga, R.; Ochi, M.; Horiuchi, T. Novel CAPA-IVM using dibutyryl-cAMP (DBCAMP) and C-type natriuretic peptide (CNP): Bovine model study for human IVM of oocytes. Fertil. Steril. 2020, 114, e353. [Google Scholar] [CrossRef]
- Farghaly, T.; Khalifa, E.; Mostafa, S.; Hussein, M.; Bedaiwy, M.; Ahmady, A. The effect of temporary meiotic attenuation on the in vitro maturation outcome of bovine oocytes. Vitr. Cell. Dev. Biology. Anim. 2015, 51, 662–671. [Google Scholar] [CrossRef]
- Abdel-Ghani, M.A.; Sakaguchi, K.; Kanno, C.; Yanagawa, Y.; Katagiri, S.; Nagano, M. Effects of pre-maturational culture duration on developmental competence of bovine small-sized oocytes. J. Reprod. Dev. 2018, 64, 365–369. [Google Scholar] [CrossRef]
- Galatidou, S.; Petelski, A.A.; Pujol, A.; Lattes, K.; Latorraca, L.B.; Fair, T.; Popovic, M.; Vassena, R.; Slavov, N.; Barragán, M. Single-cell proteomics reveals decreased abundance of proteostasis and meiosis proteins in advanced maternal age oocytes. Mol. Hum. Reprod. 2024, 30, gaae023. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, H.; Huang, C.; Zeng, Q.; Tian, C.; He, F.; Yang, Y. Simple One-step Vial-based Pretreatment for Deep Single-cell Proteomics and Its Application to Oocyte Aging. bioRxiv 2024. [Google Scholar] [CrossRef]
- Soto-Heras, S.; Paramio, M.-T.; Thompson, J.G. Effect of pre-maturation with C-type natriuretic peptide and 3-isobutyl-1-methylxanthine on cumulus-oocyte communication and oocyte developmental competence in cattle. Anim. Reprod. Sci. 2019, 202, 49–57. [Google Scholar] [CrossRef]
- Gong, X.; Shen, L.; Zhang, H.; Ai, J.; Gilchrist, R.B.; Zhao, Y. CAPA-IVM improves the cytoplasmic quality of in vitro-matured oocytes from unstimulated mice. Theriogenology 2023, 212, 117–128. [Google Scholar] [CrossRef]
- Guelman, V.; Patrizio, P. The oocyte. In A Color Atlas for Human Assisted Reproduction; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2003; pp. 25–47. [Google Scholar]
- Takahashi, T.; Igarashi, H.; Amita, M.; Hara, S.; Kurachi, H. Cellular and molecular mechanisms of various types of oocyte aging. Reprod. Med. Biol. 2011, 10, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Ma, S.; Liu, X.; Miao, D.; Chang, Z.; Luo, M.; Tan, J. Fate of the first polar bodies in mouse oocytes. Mol. Reprod. Dev. Inc. Gamete Res. 2004, 69, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Gilula, N.B.; Epstein, M.L.; Beers, W.H. Cell-to-cell communication and ovulation. A study of the cumulus-oocyte complex. J. Cell Biol. 1978, 78, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Colonna, R.; Mangia, F. Mechanisms of amino acid uptake in cumulus-enclosed mouse oocytes. Biol. Reprod. 1983, 28, 797–803. [Google Scholar] [CrossRef]
- Luciano, A.M.; Franciosi, F.; Dieci, C.; Lodde, V. Changes in large-scale chromatin structure and function during oogenesis: A journey in company with follicular cells. Anim. Reprod. Sci. 2014, 149, 3–10. [Google Scholar] [CrossRef]
- Gharibi, S.; Hajian, M.; Ostadhosseini, S.; Hosseini, S.; Forouzanfar, M.; Nasr-Esfahani, M. Effect of phosphodiesterase type 3 inhibitor on nuclear maturation and in vitro development of ovine oocytes. Theriogenology 2013, 80, 302–312. [Google Scholar] [CrossRef]
- Li, H.; Sutton-McDowall, M.; Wang, X.; Sugimura, S.; Thompson, J.; Gilchrist, R. Extending prematuration with cAMP modulators enhances the cumulus contribution to oocyte antioxidant defence and oocyte quality via gap junctions. Hum. Reprod. 2016, 31, 810–821. [Google Scholar] [CrossRef]
- Romero, S.; Sánchez, F.; Lolicato, F.; Van Ranst, H.; Smitz, J. Immature oocytes from unprimed juvenile mice become a valuable source for embryo production when using C-type natriuretic peptide as essential component of culture medium. Biol. Reprod. 2016, 95, 64. [Google Scholar] [CrossRef]
- Lin, T.; Lee, J.E.; Kang, J.W.; Oqani, R.K.; Cho, E.S.; Kim, S.B.; Il Jin, D. Melatonin supplementation during prolonged in vitro maturation improves the quality and development of poor-quality porcine oocytes via anti-oxidative and anti-apoptotic effects. Mol. Reprod. Dev. 2018, 85, 665–681. [Google Scholar] [CrossRef]
- Altermatt, J.L.; Suh, T.K.; Stokes, J.E.; Carnevale, E.M. Effects of age and equine follicle-stimulating hormone (eFSH) on collection and viability of equine oocytes assessed by morphology and developmental competency after intracytoplasmic sperm injection (ICSI). Reprod. Fertil. Dev. 2009, 21, 615–623. [Google Scholar] [CrossRef]
- Li, X.; Mu, Y.; Elshewy, N.; Ding, D.; Zou, H.; Chen, B.; Wei, Z.; Cao, Y.; Zhou, P.; Zhang, Z. Comparison of IVF and IVM outcomes in the same patient treated with a modified IVM protocol along with an oocytes-maturing system containing melatonin: A pilot study. Life Sci. 2021, 264, 118706. [Google Scholar] [CrossRef] [PubMed]
- Xi, G.; An, L.; Jia, Z.; Tan, K.; Zhang, J.; Wang, Z.; Zhang, C.; Miao, K.; Wu, Z.; Tian, J. Natriuretic peptide receptor 2 (NPR2) localized in bovine oocyte underlies a unique mechanism for C-type natriuretic peptide (CNP)-induced meiotic arrest. Theriogenology 2018, 106, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Di Nisio, V.; Antonouli, S.; Damdimopoulou, P.; Salumets, A.; Cecconi, S. on behalf of SIERR. In vivo and in vitro postovulatory aging: When time works against oocyte quality? J. Assist. Reprod. Genet. 2022, 39, 905–918. [Google Scholar] [CrossRef] [PubMed]
- Son, W.-Y.; Lee, S.-Y.; Lim, J.-H. Fertilization, cleavage and blastocyst development according to the maturation timing of oocytes in in vitro maturation cycles. Hum. Reprod. 2005, 20, 3204–3207. [Google Scholar] [CrossRef]
- Yu, Y.; Yan, J.; Liu, Z.C.; Yan, L.Y.; Li, M.; Zhou, Q.; Qiao, J. Optimal timing of oocyte maturation and its relationship with the spindle assembly and developmental competence of in vitro matured human oocytes. Fertil. Steril. 2011, 96, 73–78.e71. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Ata, B.; Son, W.-Y.; Buckett, W.M.; Tan, S.-L.; Ao, A. Chromosome abnormality rates in human embryos obtained from in-vitro maturation and IVF treatment cycles. Reprod. Biomed. Online 2010, 21, 552–559. [Google Scholar] [CrossRef]
- Carrera, C.A.; González, C.; Díaz, R.M.; Gutierrez, A.M.; Hinrichs, K. Prolonged maturation reduces cleavage and blastocyst rates after ICSI. J. Equine Vet. Sci. 2023, 125, 104637. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, Q.; Cai, J.; Zhao, X.; Ma, B. Effect of C-Type Natriuretic Peptide on Maturation and Developmental Competence of Goat Oocytes Matured In Vitro. PLoS ONE 2015, 10, e0132318. [Google Scholar] [CrossRef]
- Bedford, S.J.; Kurokawa, M.; Hinrichs, K.; Fissore, R.A. Intracellular calcium oscillations and activation in horse oocytes injected with stallion sperm extracts or spermatozoa. Reproduction 2003, 126, 489–499. [Google Scholar] [CrossRef]
- Bedford, S.J.; Kurokawa, M.; Hinrichs, K.; Fissore, R.A. Patterns of Intracellular Calcium Oscillations in Horse Oocytes Fertilized by Intracytoplasmic Sperm Injection: Possible Explanations for the Low Success of This Assisted Reproduction Technique in the Horse1. Biol. Reprod. 2004, 70, 936–944. [Google Scholar] [CrossRef]
- Yeste, M.; Jones, C.; Amdani, S.N.; Coward, K. Oocyte activation and fertilisation: Crucial contributors from the sperm and oocyte. Signal.-Mediat. Control Cell Div. Oogenesis Oocyte-Embryo Dev. 2017, 59, 213–239. [Google Scholar]
- Zafar, M.I.; Lu, S.; Li, H. Sperm-oocyte interplay: An overview of spermatozoon’s role in oocyte activation and current perspectives in diagnosis and fertility treatment. Cell Biosci. 2021, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, M.; Fissore, R.A. ICSI-generated mouse zygotes exhibit altered calcium oscillations, inositol 1,4,5-trisphosphate receptor-1 down-regulation, and embryo development. Mol. Hum. Reprod. 2003, 9, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Dozortsev, D.; Rybouchkin, A.; De Sutter, P.; Dhont, M. Sperm plasma membrane damage prior to intracytoplasmic sperm injection: A necessary condition for sperm nucleus decondensation. Hum. Reprod. 1995, 10, 2960–2964. [Google Scholar] [CrossRef]
- Yanagida, K.; Katayose, H.; Hirata, S.; Yazawa, H.; Hayashi, S.; Sato, A. Influence of sperm immobilization on onset of Ca2+ oscillations after ICSI. Hum. Reprod. 2001, 16, 148–152. [Google Scholar] [CrossRef]
- De Coster, T.; Zhao, Y.; Tšuiko, O.; Demyda-Peyrás, S.; Van Soom, A.; Vermeesch, J.; Smits, K. Genome-wide equine preimplantation genetic testing enabled by simultaneous haplotyping and copy number detection. Sci. Rep. 2024, 14, 2003. [Google Scholar] [CrossRef]
- Gu, C.; Li, K.; Li, R.; Li, L.; Li, X.; Dai, X.; He, Y. Chromosomal aneuploidy associated with clinical characteristics of pregnancy loss. Front. Genet. 2021, 12, 667697. [Google Scholar] [CrossRef]
- Shilton, C.A.; Kahler, A.; Davis, B.W.; Crabtree, J.R.; Crowhurst, J.; McGladdery, A.J.; Wathes, D.C.; Raudsepp, T.; de Mestre, A.M. Whole genome analysis reveals aneuploidies in early pregnancy loss in the horse. Sci. Rep. 2020, 10, 13314. [Google Scholar] [CrossRef]
- Claes, A.; Stout, T.A.E. Success rate in a clinical equine in vitro embryo production program. Theriogenology 2022, 187, 215–218. [Google Scholar] [CrossRef]
- Dunkley, S.; Scheffler, K.; Mogessie, B. Cytoskeletal form and function in mammalian oocytes and zygotes. Curr. Opin. Cell Biol. 2022, 75, 102073. [Google Scholar] [CrossRef]
- Egbert, J.R.; Silbern, I.; Uliasz, T.F.; Lowther, K.M.; Yee, S.-P.; Urlaub, H.; Jaffe, L.A. Phosphatases modified by LH signaling in ovarian follicles: Testing their role in regulating the NPR2 guanylyl cyclase†. Biol. Reprod. 2023, 110, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Lin, Q.; Zhang, Y.; Liu, J.; Zheng, Z. Research Progress of Ribosomal Proteins in Reproductive Development. Int. J. Mol. Sci. 2024, 25, 13151. [Google Scholar] [CrossRef] [PubMed]
- Angel-Velez, D.; De Coster, T.; Azari-Dolatabad, N.; Fernandez-Montoro, A.; Benedetti, C.; Bogado Pascottini, O.; Woelders, H.; Van Soom, A.; Smits, K. New alternative mixtures of cryoprotectants for equine immature oocyte vitrification. Animals 2021, 11, 3077. [Google Scholar] [CrossRef]
- Leemans, B.; Gadella, B.M.; Stout, T.A.E.; Heras, S.; Smits, K.; Ferrer-Buitrago, M.; Claes, E.; Heindryckx, B.; De Vos, W.H.; Nelis, H.; et al. Procaine Induces Cytokinesis in Horse Oocytes via a pH-Dependent Mechanism1. Biol. Reprod. 2015, 93, 1–17. [Google Scholar] [CrossRef]
- Popovic, M.; Dheedene, A.; Christodoulou, C.; Taelman, J.; Dhaenens, L.; Van Nieuwerburgh, F.; Deforce, D.; Van den Abbeel, E.; De Sutter, P.; Menten, B. Chromosomal mosaicism in human blastocysts: The ultimate challenge of preimplantation genetic testing? Hum. Reprod. 2018, 33, 1342–1354. [Google Scholar] [CrossRef]
- Sante, T.; Vergult, S.; Volders, P.-J.; Kloosterman, W.P.; Trooskens, G.; De Preter, K.; Dheedene, A.; Speleman, F.; De Meyer, T.; Menten, B. ViVar: A comprehensive platform for the analysis and visualization of structural genomic variation. PLoS ONE 2014, 9, e113800. [Google Scholar] [CrossRef]
- Raman, L.; Dheedene, A.; De Smet, M.; Van Dorpe, J.; Menten, B. WisecondorX: Improved copy number detection for routine shallow whole-genome sequencing. Nucleic Acids Res. 2019, 47, 1605–1614. [Google Scholar] [CrossRef]
- Fernández Montoro, A.; Araftpoor, E.; De Coster, T.; Angel-Velez, D.; Bühler, M.; Hedia, M.; Gevaert, K.; Van Soom, A.; Pavani, K.C.; Smits, K. Decoding bull fertility in vitro: A proteomics exploration from sperm to blastocyst. Reproduction 2025, 169, e240296. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Matsuura, Y.; Ishiguro-Watanabe, M. KEGG: Biological systems database as a model of the real world. Nucleic Acids Res. 2024, 53, D672–D677. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).