Anticancer Potential of Halogen Derivatives of Methyl 6-Acetyl-5-Hydroxy-2-Methyl-1-Benzofuran-3-Carboxylate
Abstract
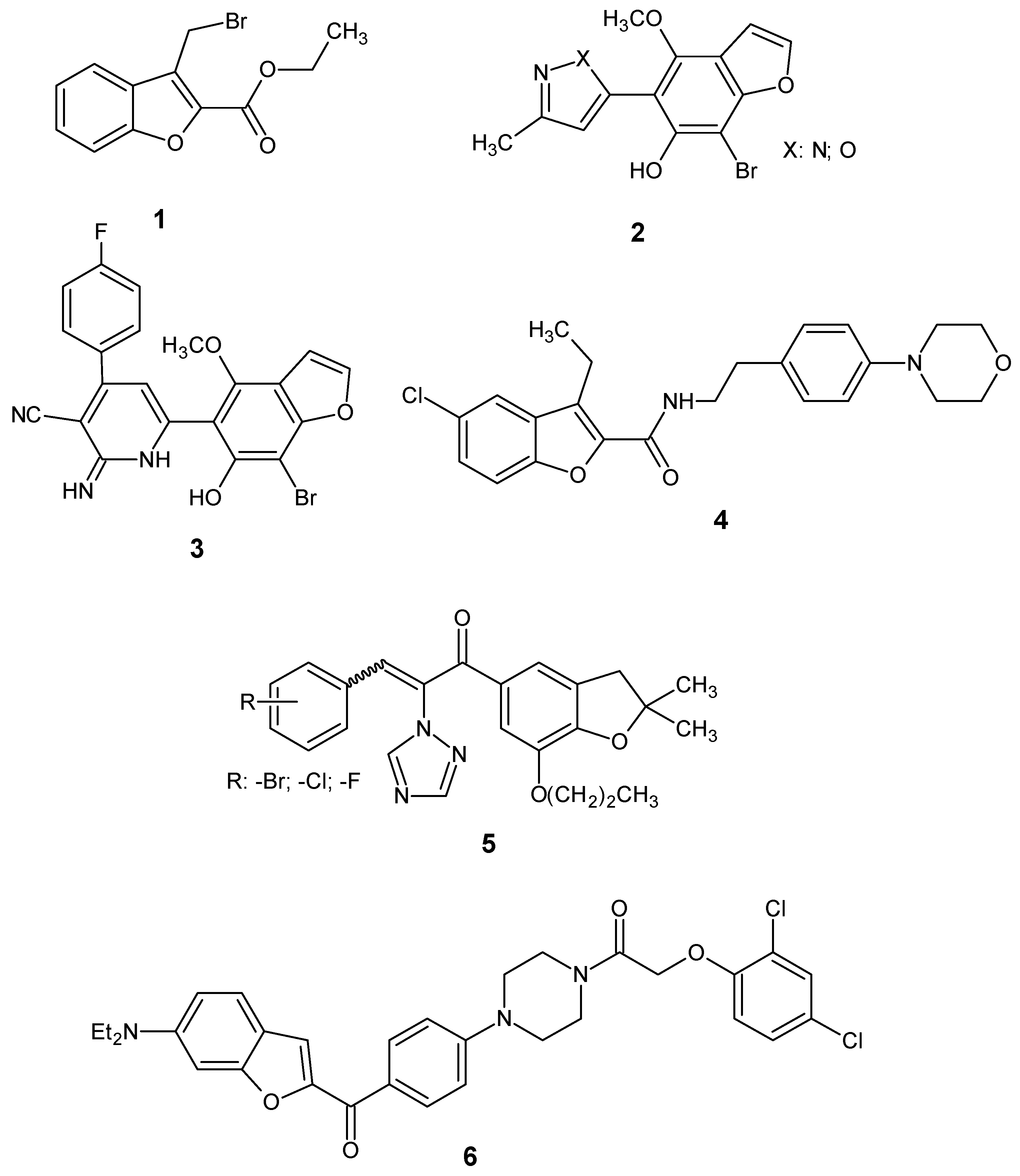
1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biological Evaluation
2.2.1. In Vitro Cytotoxic Activity
2.2.2. Antiproliferative Activity
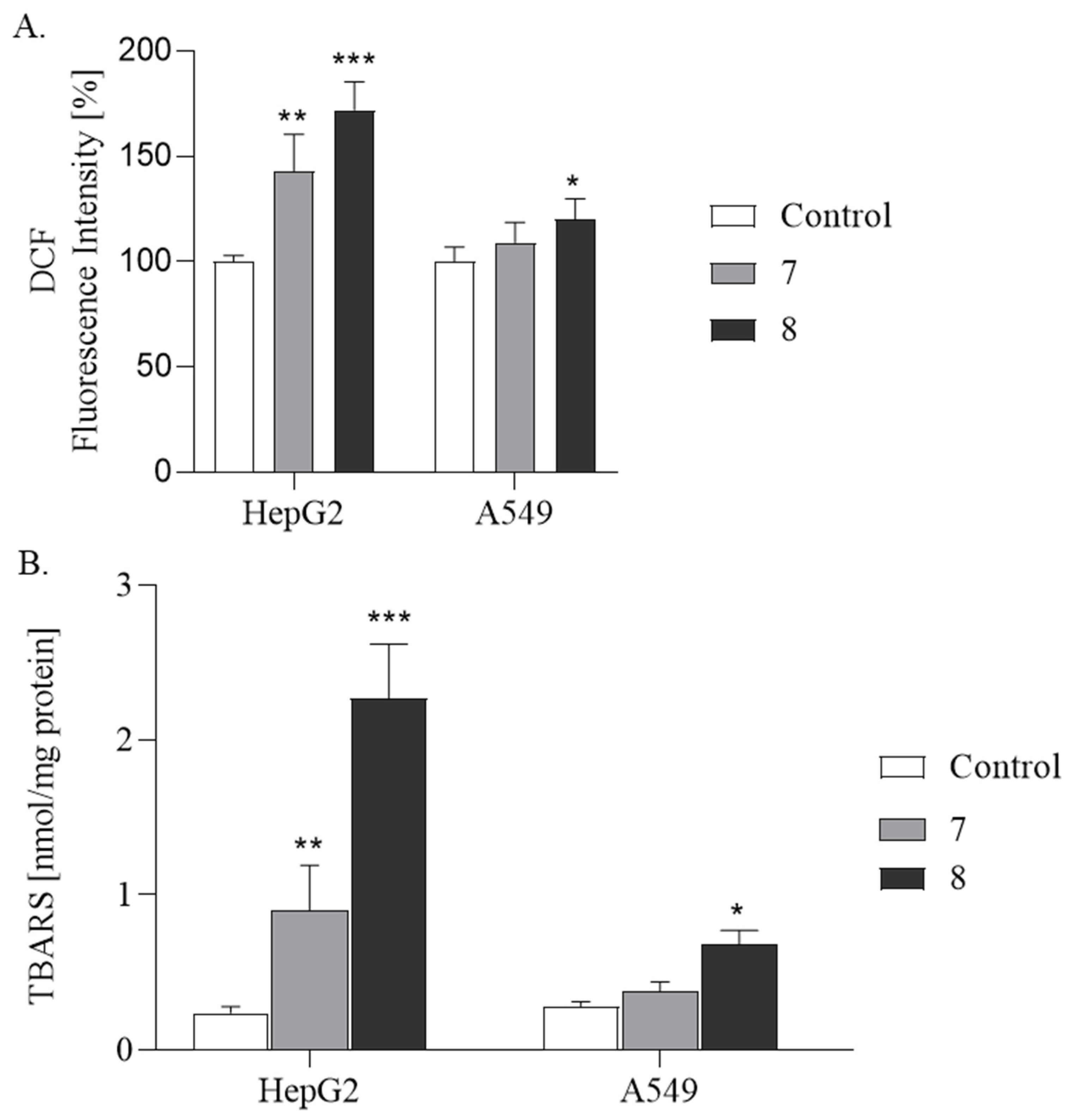
2.2.3. ROS Production and Lipid Peroxidation Studies
2.2.4. Interleukin-6 Assay
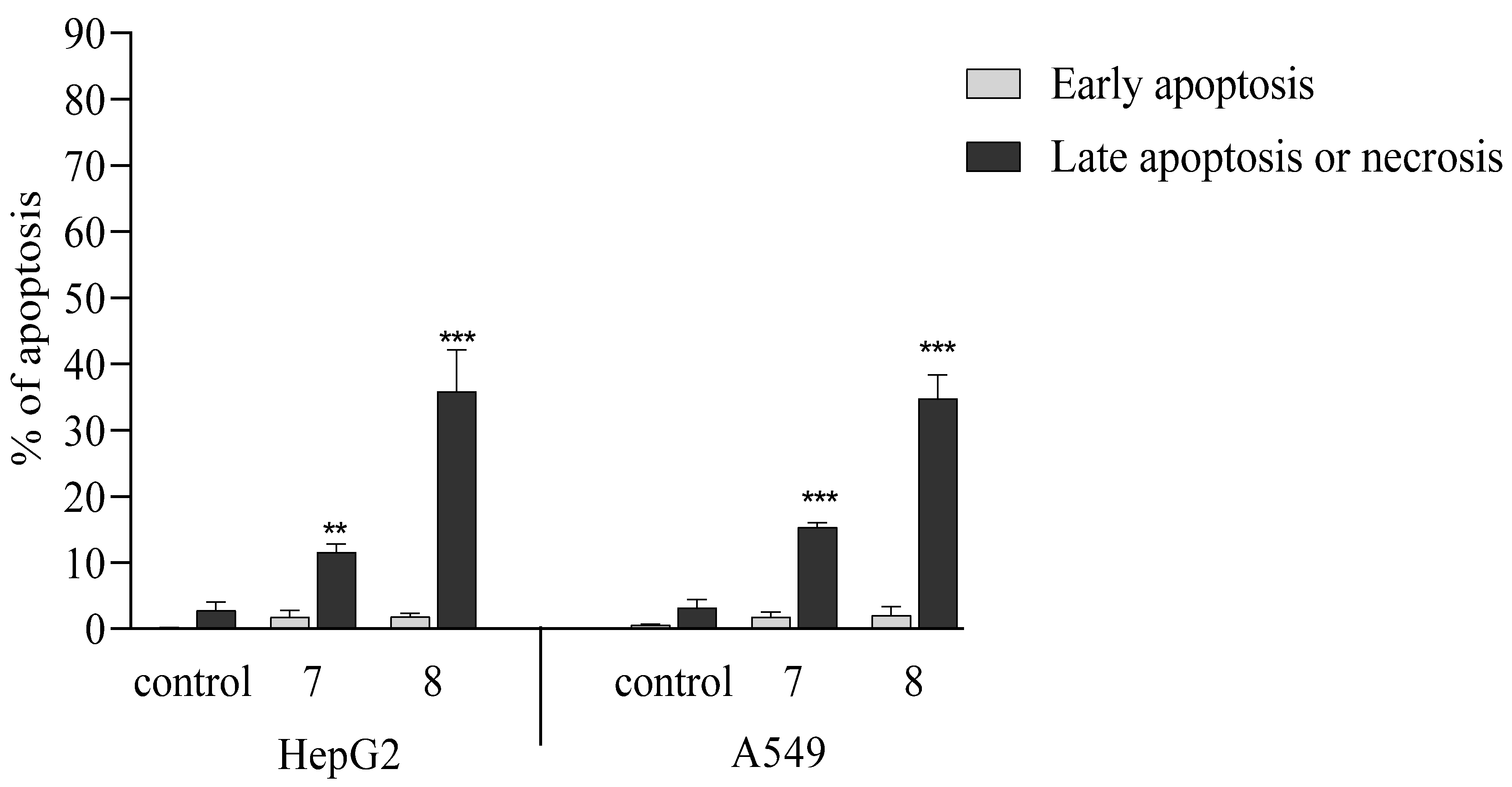
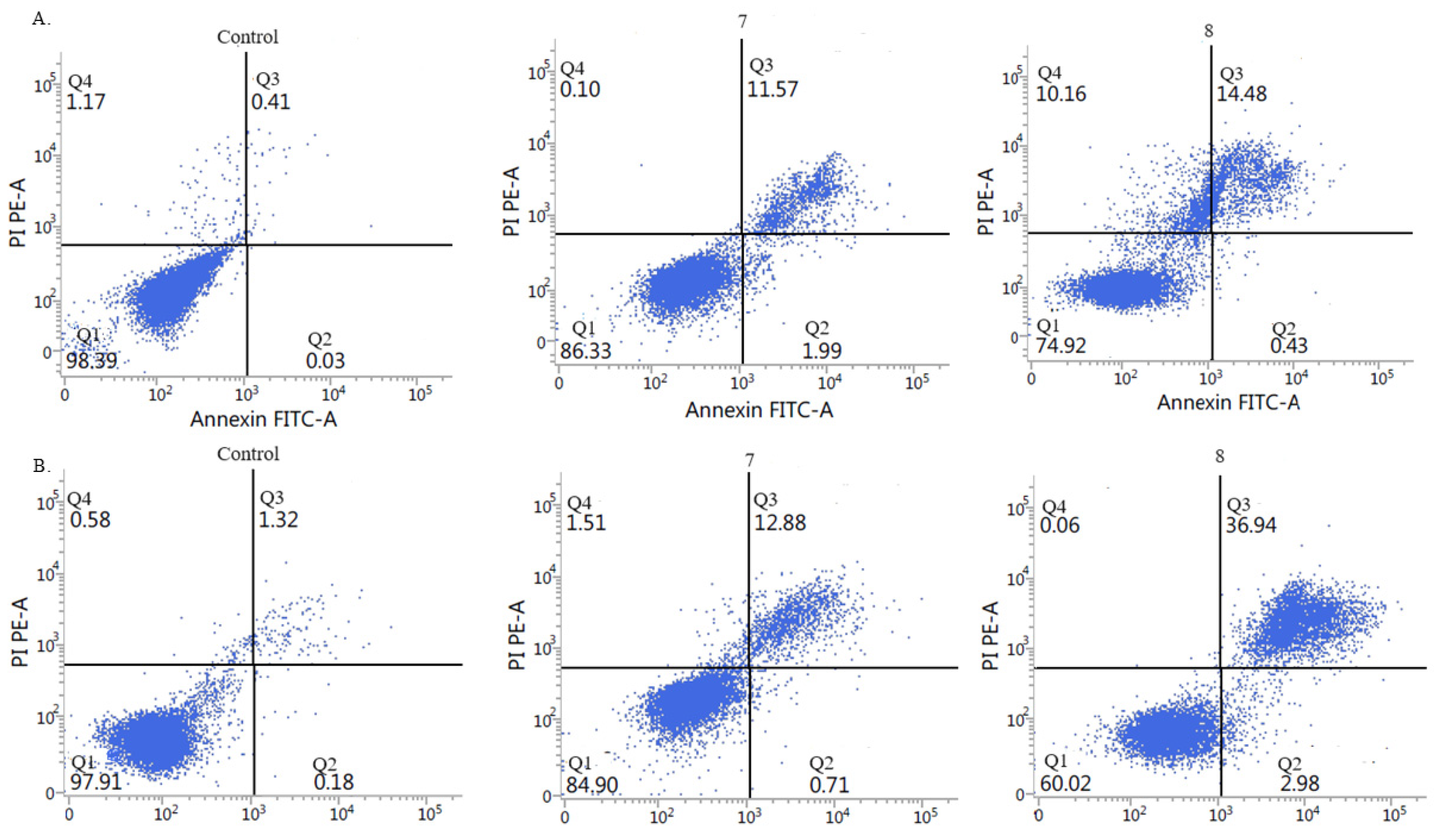
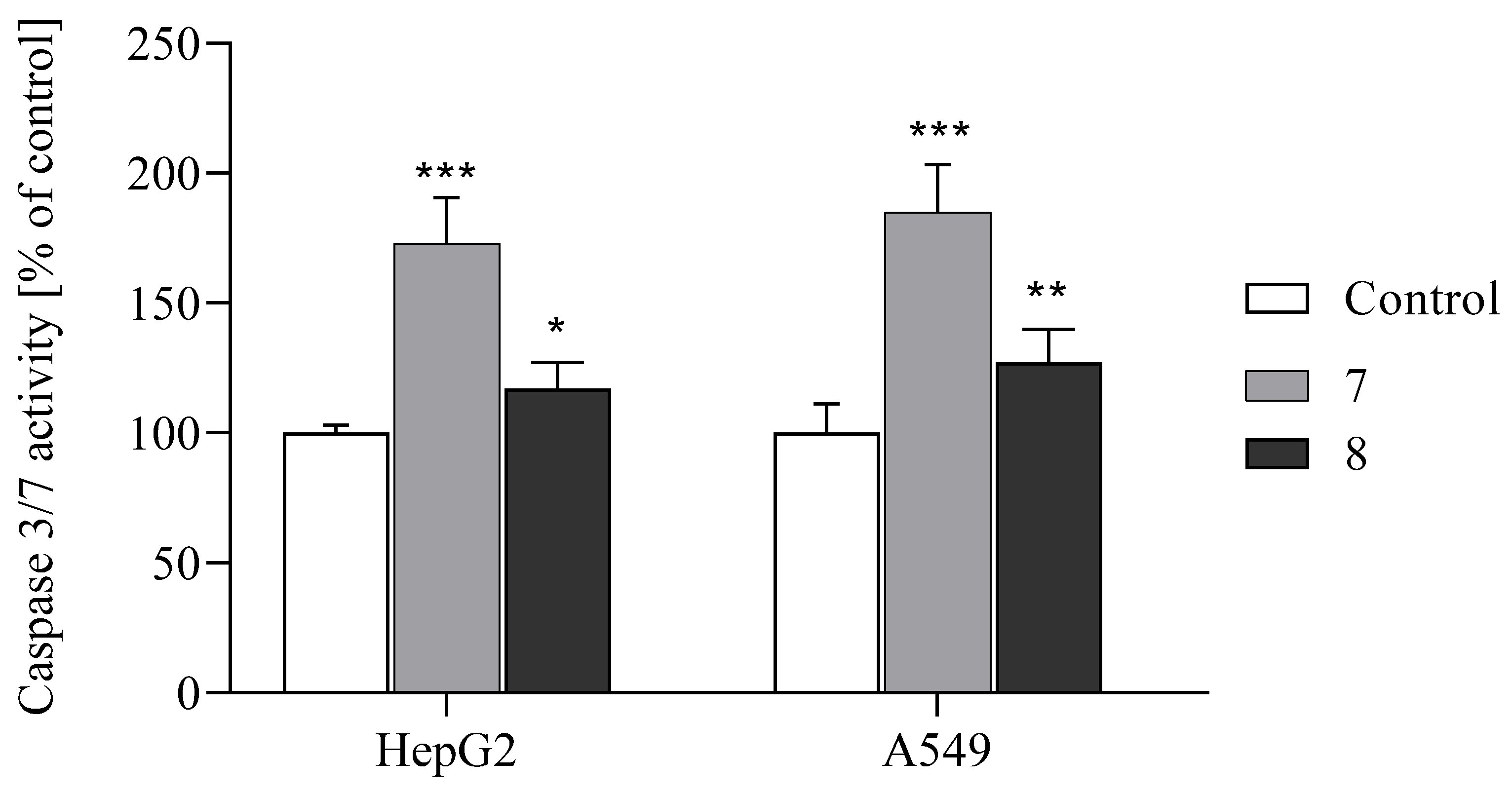
2.2.5. Activation of Apoptosis
2.2.6. Test Benzo [B] Furans Cause G2/M Cell Cycle Arrest
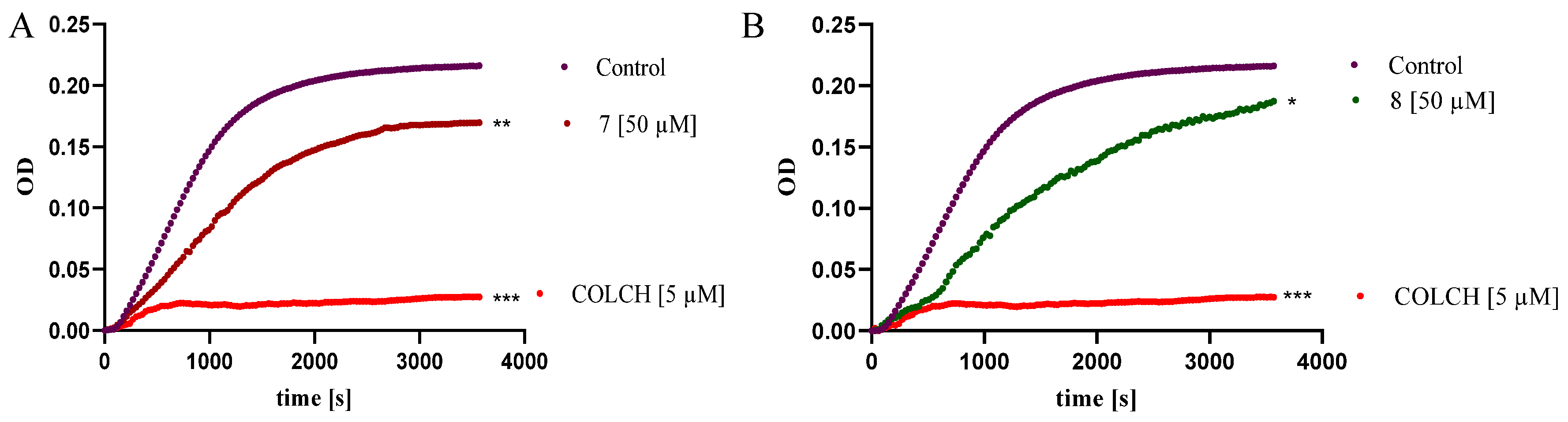
2.2.7. Benzo [B] Furans Inhibit Tubulin Polymerization In Vitro
3. Materials and Methods
3.1. Chemistry
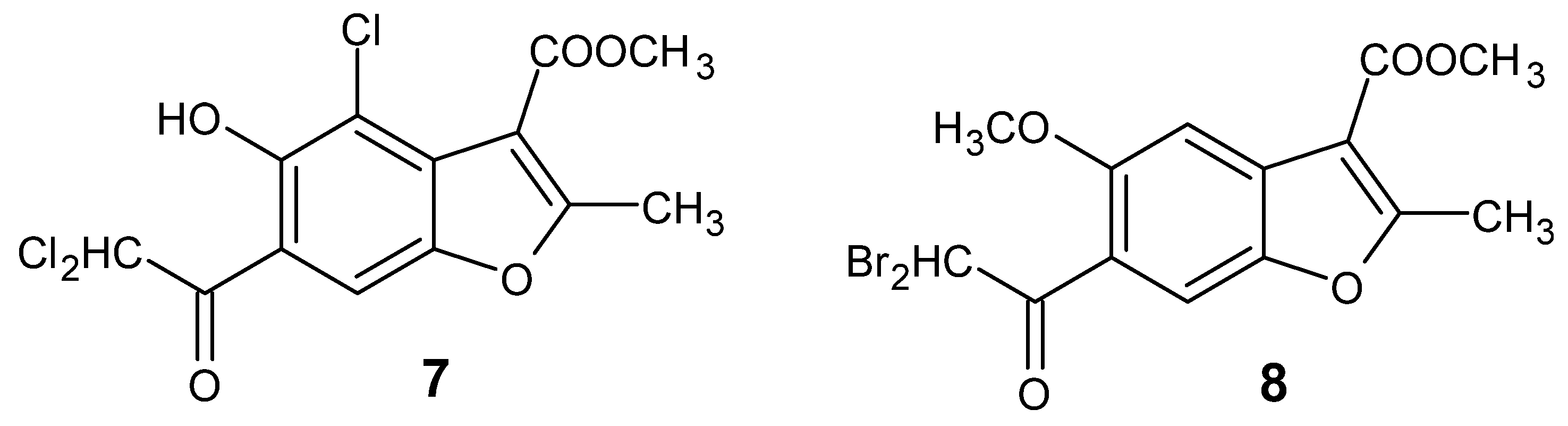
3.1.1. Synthesis of Methyl 4-Chloro-6-(Dichloroacetyl)-5-Hydroxy-2-Methyl-1-Benzofuran-3-Carboxylate (7, Figure 2)
3.1.2. Synthesis of Methyl 6-(Dibromoacetyl)-5-Methoxy-2-Methyl-1-Benzofuran-3-Carboxylate (8, Figure 2)
3.2. Biological Studies
3.2.1. Human Cell Line, MTT Assay
3.2.2. Measurement of Live Cell Number and Viability (%) by Trypan Blue Exclusion Assay
3.2.3. Measurement of Cell Apoptosis by Flow Cytometry Annexin V-FITC Binding Assay
3.2.4. Cell Cycle Analysis
3.2.5. Caspases-3 and -7 Activity Assay
3.2.6. DCFH-DA Assay
3.2.7. TBA Assay
3.2.8. Interleukin-6
3.2.9. Tubulin Polymerization
3.2.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khanam, H.; Shamsuzzaman. Bioactive Benzofuran derivatives: A review. Eur. J. Med. Chem. 2015, 97, 483–504. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.H.; Hu, Y.H.; Yang, J.; Liu, T.; Sun, J.; Wang, X.J. Natural source, bioactivity and synthesis of benzofuran derivatives. RSC Adv. 2019, 9, 27510–27540. [Google Scholar] [CrossRef] [PubMed]
- Nevagi, R.J.; Dighe, S.N.; Dighe, S.N. Biological and medicinal significance of benzofuran. Eur. J. Med. Chem. 2015, 97, 561–581. [Google Scholar] [CrossRef] [PubMed]
- Farhat, J.; Alzyoud, L.; Alwahsh, M.; Al-Omari, B. Structure-Activity Relationship of Benzofuran Derivatives with Potential Anticancer Activity. Cancers 2022, 14, 2196. [Google Scholar] [CrossRef]
- Asadi, P.; Khodarahmi, G.; Jahanian-Najafabadi, A.; Saghaie, L.; Hassanzadeh, F. Biologically Active Heterocyclic Hybrids Based on Quinazolinone, Benzofuran and Imidazolium Moieties: Synthesis, Characterization, Cytotoxic and Antibacterial Evaluation. Chem. Biodivers. 2017, 14, e1600411. [Google Scholar] [CrossRef]
- Mao, Z.W.; Zheng, X.; Lin, Y.P.; Hu, C.Y.; Wang, X.L.; Wan, C.P.; Rao, G.X. Design, synthesis and anticancer activity of novel hybrid compounds between benzofuran and N-aryl piperazine. Bioorg. Med. Chem. Lett. 2016, 26, 3421–3424. [Google Scholar] [CrossRef]
- Yang, X.D.; Wan, W.C.; Deng, X.Y.; Li, Y.; Yang, L.J.; Li, L.; Zhang, H.B. Design, synthesis and cytotoxic activities of novel hybrid compounds between 2-phenylbenzofuran and imidazole. Bioorg. Med. Chem. Lett. 2012, 22, 2726–2729. [Google Scholar] [CrossRef]
- Ma, Y.; Zheng, X.; Gao, H.; Wan, C.; Rao, G.; Mao, Z. Design, Synthesis, and Biological Evaluation of Novel Benzofuran Derivatives Bearing N-Aryl Piperazine Moiety. Molecules 2016, 21, 1684. [Google Scholar] [CrossRef]
- Salome, C.; Ribeiro, N.; Chavagnan, T.; Thuaud, F.; Serova, M.; de Gramont, A.; Faivre, S.; Raymond, E.; Desaubry, L. Benzofuran derivatives as anticancer inhibitors of mTOR signaling. Eur. J. Med. Chem. 2014, 81, 181–191. [Google Scholar] [CrossRef]
- Abdelfatah, S.; Berg, A.; Huang, Q.; Yang, L.J.; Hamdoun, S.; Klinger, A.; Greten, H.J.; Fleischer, E.; Berg, T.; Wong, V.K.W.; et al. MCC1019, a selective inhibitor of the Polo-box domain of Polo-like kinase 1 as novel, potent anticancer candidate. Acta Pharm. Sin. B 2019, 9, 1021–1034. [Google Scholar] [CrossRef]
- Amin, K.M.; Syam, Y.M.; Anwar, M.M.; Ali, H.I.; Abdel-Ghani, T.M.; Serry, A.M. Synthesis and molecular docking study of new benzofuran and furo[3,2-g]chromone-based cytotoxic agents against breast cancer and p38alpha MAP kinase inhibitors. Bioorg. Chem. 2018, 76, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Youssif, B.G.M.; Mohamed, A.M.; Osman, E.E.A.; Abou-Ghadir, O.F.; Elnaggar, D.H.; Abdelrahman, M.H.; Treamblu, L.; Gomaa, H.A.M. 5-Chlorobenzofuran-2-carboxamides: From allosteric CB1 modulators to potential apoptotic antitumor agents. Eur. J. Med. Chem. 2019, 177, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, Z.H.; Hu, A.X.; Yan, X.W.; Ding, N.; Ye, J. Design, Synthesis, and Antitumor Activity of (E,Z)-1-(dihydrobenzofuran-5-yl)-3-phenyl-2-(1,2,4-triazol-1-yl)-2-propen-1-ones. Chem. Biol. Drug Des. 2015, 86, 1339–1350. [Google Scholar] [CrossRef]
- Kossakowski, J.; Krawiecka, M.; Kuran, B.; Stefanska, J.; Wolska, I. Synthesis and preliminary evaluation of the antimicrobial activity of selected 3-benzofurancarboxylic acid derivatives. Molecules 2010, 15, 4737–4749. [Google Scholar] [CrossRef] [PubMed]
- Krawiecka, M.; Kuran, B.; Kossakowski, J.; Cieslak, M.; Kazmierczak- Baranska, J.; Krolewska, K.; Nawrot, B. Synthesis and Cytotoxic Properties of Halogen and Aryl-/Heteroarylpiperazinyl Derivatives of Benzofurans. Anti-Cancer Agents Med. Chem. 2015, 15, 115–121. [Google Scholar] [CrossRef]
- Krolewska-Golinska, K.; Cieslak, M.J.; Sobczak, M.; Dolot, R.; Radzikowska-Cieciura, E.; Napiorkowska, M.; Wybranska, I.; Nawrot, B. Novel Benzo[B]Furans with Anti-Microtubule Activity Upregulate Expression of Apoptotic Genes and Arrest Leukemia Cells in G2/M Phase. Anti-Cancer Agents Med. Chem. 2019, 19, 375–388. [Google Scholar] [CrossRef]
- Napiorkowska, M.; Cieslak, M.; Kazmierczak-Baranska, J.; Krolewska-Golinska, K.; Nawrot, B. Synthesis of New Derivatives of Benzofuran as Potential Anticancer Agents. Molecules 2019, 24, 1529. [Google Scholar] [CrossRef] [PubMed]
- Napiorkowska, M.; Otto-Slusarczyk, D.; Kurpios-Piec, D.; Stukan, I.; Gryzik, M.; Wojda, U. BM7, a derivative of benzofuran, effectively fights cancer by promoting cancer cell apoptosis and impacting IL-6 levels. Eur. J. Pharmacol. 2024, 978, 176751. [Google Scholar] [CrossRef]
- Napiorkowska, M.; Kumaravel, P.; Amboo Mahentheran, M.; Kiernozek-Kalinska, E.; Grosicka-Maciag, E. New Derivatives of 1-(3-Methyl-1-Benzofuran-2-yl)Ethan-1-one: Synthesis and Preliminary Studies of Biological Activity. Int. J. Mol. Sci. 2024, 25, 1999. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, H.S.; Seo, Y.R. Understanding of ROS-Inducing Strategy in Anticancer Therapy. Oxidative Med. Cell. Longev. 2019, 2019, 5381692. [Google Scholar] [CrossRef] [PubMed]
- Chand, K.; Rajeshwari; Hiremathad, A.; Singh, M.; Santos, M.A.; Keri, R.S. A review on antioxidant potential of bioactive heterocycle benzofuran: Natural and synthetic derivatives. Pharmacol. Rep. 2017, 69, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Rindhe, S.S.; Rode, M.A.; Karale, B.K. New benzofuran derivatives as an antioxidant agent. Indian. J. Pharm. Sci. 2010, 72, 231–235. [Google Scholar] [CrossRef]
- Kumar, K.; Mishra, J.P.N.; Singh, R.P. Usnic acid induces apoptosis in human gastric cancer cells through ROS generation and DNA damage and causes up-regulation of DNA-PKcs and γ-H2A.X phosphorylation. Chem.-Biol. Interact. 2020, 315, 108898. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Park, C.; Lee, Y.; Kim, S.; Bose, S.; Choi, M.; Kumar, A.S.; Jung, J.K.; Lee, H. Neuroprotective and antioxidant effects of novel benzofuran-2-carboxamide derivatives. Biomol. Ther. 2015, 23, 275–282. [Google Scholar] [CrossRef]
- Gao, C.; Sun, X.; Wu, Z.; Yuan, H.; Han, H.; Huang, H.; Shu, Y.; Xu, M.; Gao, R.; Li, S.; et al. A Novel Benzofuran Derivative Moracin N Induces Autophagy and Apoptosis Through ROS Generation in Lung Cancer. Front. Pharmacol. 2020, 11, 391. [Google Scholar] [CrossRef]
- Ahmad, J.; Ahamed, M.; Akhtar, M.J.; Alrokayan, S.A.; Siddiqui, M.A.; Musarrat, J.; Al-Khedhairy, A.A. Apoptosis induction by silica nanoparticles mediated through reactive oxygen species in human liver cell line HepG2. Toxicol. Appl. Pharmacol. 2012, 259, 160–168. [Google Scholar] [CrossRef]
- Zhang, Q.; Bao, J.; Yang, J. Genistein-triggered anticancer activity against liver cancer cell line HepG2 involves ROS generation, mitochondrial apoptosis, G2/M cell cycle arrest and inhibition of cell migration. Arch. Med. Sci. 2019, 15, 1001–1009. [Google Scholar] [CrossRef]
- Zou, S.; Wu, Y.; Wen, M.; Liu, J.; Chen, M.; Yuan, J.; Zhou, B. Potential Molecular Mechanism of Illicium simonsii Maxim Petroleum Ether Fraction in the Treatment of Hepatocellular Carcinoma. Pharmaceuticals 2024, 17, 806. [Google Scholar] [CrossRef]
- Gall, J.I.; Goncalves Alves, A.; Carraro Junior, L.R.; da Silva Teixeira Rech, T.; Dos Santos Neto, J.S.; Alves, D.; Pereira Soares, M.S.; Spohr, L.; Spanevello, R.M.; Bruning, C.A.; et al. Insights into serotonergic and antioxidant mechanisms involved in antidepressant-like action of 2-phenyl-3-(phenylselanyl)benzofuran in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 102, 109956. [Google Scholar] [CrossRef]
- Scibetta, S.; Miceli, M.; Iuliano, M.; Stefanuto, L.; Carbone, E.; Piscopo, P.; Petrozza, V.; Romeo, G.; Mangino, G.; Calogero, A.; et al. In Vitro Evaluation of the Antioxidant Capacity of 3,3-Disubstituted-3H-benzofuran-2-one Derivatives in a Cellular Model of Neurodegeneration. Life 2024, 14, 422. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.A.; Dawood, K.M. Anticancer therapeutic potential of benzofuran scaffolds. RSC Adv. 2023, 13, 11096–11120. [Google Scholar] [CrossRef]
- Kumari, N.; Dwarakanath, B.S.; Das, A.; Bhatt, A.N. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumor Biol. 2016, 37, 11553–11572. [Google Scholar] [CrossRef]
- Shi, W.; Men, L.; Pi, X.; Jiang, T.; Peng, D.; Huo, S.; Luo, P.; Wang, M.; Guo, J.; Jiang, Y.; et al. Shikonin suppresses colon cancer cell growth and exerts synergistic effects by regulating ADAM17 and the IL-6/STAT3 signaling pathway. Int. J. Oncol. 2021, 59, 99. [Google Scholar] [CrossRef] [PubMed]
- Lamkanfi, M.; Kanneganti, T.D. Caspase-7: A protease involved in apoptosis and inflammation. Int. J. Biochem. Cell Biol. 2010, 42, 21–24. [Google Scholar] [CrossRef]
- Coskun, D.; Erkisa, M.; Ulukaya, E.; Coskun, M.F.; Ari, F. Novel 1-(7-ethoxy-1-benzofuran-2-yl) substituted chalcone derivatives: Synthesis, characterization and anticancer activity. Eur. J. Med. Chem. 2017, 136, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Sithara, T.; Arun, K.B.; Syama, H.P.; Reshmitha, T.R.; Nisha, P. Morin Inhibits Proliferation of SW480 Colorectal Cancer Cells by Inducing Apoptosis Mediated by Reactive Oxygen Species Formation and Uncoupling of Warburg Effect. Front. Pharmacol. 2017, 8, 640. [Google Scholar] [CrossRef]
- Giordano, C.; Rovito, D.; Barone, I.; Mancuso, R.; Bonofiglio, D.; Giordano, F.; Catalano, S.; Gabriele, B.; Ando, S. Benzofuran-2-acetic ester derivatives induce apoptosis in breast cancer cells by upregulating p21(Cip/WAF1) gene expression in p53-independent manner. DNA Repair 2017, 51, 20–30. [Google Scholar] [CrossRef]
- Vaali-Mohammed, M.A.; Abdulla, M.H.; Matou-Nasri, S.; Eldehna, W.M.; Meeramaideen, M.; Elkaeed, E.B.; El-Watidy, M.; Alhassan, N.S.; Alkhaya, K.; Al Obeed, O. The Anticancer Effects of the Pro-Apoptotic Benzofuran-Isatin Conjugate (5a) Are Associated with p53 Upregulation and Enhancement of Conventional Chemotherapeutic Drug Efficiency in Colorectal Cancer Cell Lines. Front. Pharmacol. 2022, 13, 923398. [Google Scholar] [CrossRef]
- Hranjec, M.; Sović, I.; Ratkaj, I.; Pavlović, G.; Ilić, N.; Valjalo, L.; Pavelić, K.; Kraljević Pavelić, S.; Karminski-Zamola, G. Antiproliferative potency of novel benzofuran-2-carboxamides on tumour cell lines: Cell death mechanisms and determination of crystal structure. Eur. J. Med. Chem. 2013, 59, 111–119. [Google Scholar] [CrossRef]
- El-Khouly, O.A.; Henen, M.A.; El-Sayed, M.A.; Shabaan, M.I.; El-Messery, S.M. Synthesis, anticancer and antimicrobial evaluation of new benzofuran based derivatives: PI3K inhibition, quorum sensing and molecular modeling study. Bioorg. Med. Chem. 2021, 31, 115976. [Google Scholar] [CrossRef] [PubMed]
- El-Khouly, O.A.; Henen, M.A.; El-Sayed, M.A.A.; El-Messery, S.M. Design, synthesis and computational study of new benzofuran hybrids as dual PI3K/VEGFR2 inhibitors targeting cancer. Sci. Rep. 2022, 12, 17104. [Google Scholar] [CrossRef]
- Li, Q.; Jian, X.E.; Chen, Z.R.; Chen, L.; Huo, X.S.; Li, Z.H.; You, W.W.; Rao, J.J.; Zhao, P.L. Synthesis and biological evaluation of benzofuran-based 3,4,5-trimethoxybenzamide derivatives as novel tubulin polymerization inhibitors. Bioorg. Chem. 2020, 102, 104076. [Google Scholar] [CrossRef]
- Wang, F.; Feng, K.-R.; Zhao, J.-Y.; Zhang, J.-W.; Shi, X.-W.; Zhou, J.; Gao, D.; Lin, G.-Q.; Tian, P. Identification of novel STAT3 inhibitors bearing 2-acetyl-7-phenylamino benzofuran scaffold for antitumour study. Bioorg. Med. Chem. 2020, 28, 115822. [Google Scholar] [CrossRef]
- Mokenapelli, S.; Thalari, G.; Vadiyaala, N.; Yerrabelli, J.R.; Irlapati, V.K.; Gorityala, N.; Sagurthi, S.R.; Chitneni, P.R. Synthesis, cytotoxicity, and molecular docking of substituted 3-(2-methylbenzofuran-3-yl)-5-(phenoxymethyl)-1,2,4-oxadiazoles. Arch. Pharm. 2020, 353, e2000006. [Google Scholar] [CrossRef]
- Romagnoli, R.; Baraldi, P.G.; Carrion, M.D.; Cara, C.L.; Cruz-Lopez, O.; Tolomeo, M.; Grimaudo, S.; Di Cristina, A.; Pipitone, M.R.; Balzarini, J.; et al. Design, synthesis and structure-activity relationship of 2-(3′,4′,5′-trimethoxybenzoyl)-benzo[b]furan derivatives as a novel class of inhibitors of tubulin polymerization. Bioorg. Med. Chem. 2009, 17, 6862–6871. [Google Scholar] [CrossRef] [PubMed]
- Pieters, L.; Van Dyck, S.; Gao, M.; Bai, R.; Hamel, E.; Vlietinck, A.; Lemiere, G. Synthesis and biological evaluation of dihydrobenzofuran lignans and related compounds as potential antitumor agents that inhibit tubulin polymerization. J. Med. Chem. 1999, 42, 5475–5481. [Google Scholar] [CrossRef] [PubMed]
- Perez, E.A. Microtubule inhibitors: Differentiating tubulin-inhibiting agents based on mechanisms of action, clinical activity, and resistance. Mol. Cancer Ther. 2009, 8, 2086–2095. [Google Scholar] [CrossRef]
- Dogan, E.; Kara, H.G.; Kosova, B.; Cetintas, V.B. Targeting Apoptosis to Overcome Chemotherapy Resistance. In Metastasis [Internet]; Sergi, C.M., Ed.; Exon Publications: Brisbane, Australia, 2022; Chapter 12. Available online: https://www.ncbi.nlm.nih.gov/books/NBK580869/ (accessed on 4 June 2025). [CrossRef]
- Zhang, J.; Wang, L.; Zhang, Y. Downregulation of NIMA-related kinase-7 inhibits cell proliferation by inducing cell cycle arrest in human retinoblastoma cells. Exp. Ther. Med. 2018, 15, 1360–1366. [Google Scholar] [CrossRef]
- Dominguez-Brauer, C.; Thu, K.L.; Mason, J.M.; Blaser, H.; Bray, M.R.; Mak, T.W. Targeting Mitosis in Cancer: Emerging Strategies. Mol. Cell 2015, 60, 524–536. [Google Scholar] [CrossRef]
- Shapiro, G.I.; Harper, J.W. Anticancer drug targets: Cell cycle and checkpoint control. J. Clin. Investig. 1999, 104, 1645–1653. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.A. Mechanism of action of antitumor drugs that interact with microtubules and tubulin. Curr. Med. Chem. Anticancer Agents 2002, 2, 1–17. [Google Scholar] [CrossRef] [PubMed]
- LeBel, C.P.; Ischiropoulos, H.; Bondy, S.C. Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem. Res. Toxicol. 1992, 5, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [CrossRef]


| Compound | Cancer Cells | Normal Cells | ||||||
|---|---|---|---|---|---|---|---|---|
| IC50, µM b | ||||||||
| SW480 c | SW620 d | HCT116 e | HepG2 f | PC3 g | A549 h | MDA i | HUVEC j | |
| 7 | 33.2 ± 1.2 | 26.5 ± 1.8 | 36.9 ± 4.1 | 11 ± 3.2 | 43 ± 2.1 | 6.3 ± 2.5 | 29.5 ± 1.5 | >1000 [15] |
| 8 | 27.6 ± 1.1 | 10.8 ± 0.9 | 58.3 ± 7.9 | 3.8 ± 0.5 | 33.2 ± 1.1 | 3.5 ± 0.6 | 23.2 ± 2.7 | >1000 [15] |
| Dx k | 0.75 ± 0.1 | 0.26 ± 0.1 | 0.6 ± 0.02 | 0.4 ± 0.1 | 0.59 ± 0.1 | 0.63 ± 0.2 | 0.83 ± 0.03 | 1.0 ± 0.03 |
| Cp k | 10.4 ± 0.9 | 6.70 ± 1.1 | 0.6 ± 0.02 | 4.5 ± 1.2 | 13.2 ± 2.1 | 7.10 ± 1.3 | 3.95 ± 1.1 | 12.0 ± 1.8 |
| Cancer Cell Line | Compound | Cell Number × 106 | Cell Number (%) | Viability (%) |
|---|---|---|---|---|
| HepG2 | - | 1.2 ± 0.45 | 100 | 94 ± 1.52 |
| 7 | 0.6 ± 0.02 ** | 56.8 | 92 ± 4.93 | |
| 8 | 0.47 ± 0.01 *** | 39.4 | 55 ± 1.10 | |
| A549 | - | 1.8 ± 0.90 | 100 | 97 ± 2.52 |
| 7 | 0.6 ± 0.10 ** | 33.9 | 91 ± 1.65 | |
| 8 | 0.1 ± 0.02 *** | 6.21 | 85 ± 7.10 |
| Step | Description |
|---|---|
| Cell Lines Used | SW480 (primary colon cancer), SW620 (lymph node colon cancer), HCT116 (colon carcinoma), PC3 (metastatic prostate cancer), HepG2 (liver cancer), A549 (lung cancer), MDA-MB-231 (breast cancer). |
| Cell Source | All cell lines were obtained from ATCC (Manassas, VA, USA). |
| Culture Media |
|
| Media Supplements | 10% FBS (Sigma-Aldrich, St. Louis, MO, USA), 20 mM HEPES (Biowest, Nuaillé, France), 100 U/mL penicillin, 100 μg/mL streptomycin (Gibco, Grand Island, NY, USA). |
| Culture Conditions | 37 °C, 5% CO2, humidified incubator; cells used at 80–90% confluence. |
| Seeding for Assay | 1 × 104 cells/well in 96-well plates; adhered for 24 h. |
| Treatment | Cells treated with compounds 7 and 8 at various concentrations (1–100 μM) for 72 h. |
| Cytotoxicity Assay | MTT assay (0.5 mg/mL), incubation for 4 h. |
| Detection | Formazan dissolved in DMSO/isopropanol (1:1, v/v); absorbance measured at 570 nm using a MultiscanGo spectrophotometer (ThermoFisher, Waltham, MA, USA). |
| Data Analysis | % cytotoxicity = [A]/[B] × 100, where [A] = treated absorbance, [B] = control absorbance [54]. IC50 calculated using GraphPad Prism 8.0.1. |
| Step | Description |
|---|---|
| Cell Seeding | 1 × 105 cells per well were seeded in 12-well plates. |
| Incubation | Cells were cultured for 24 h at 37 °C with 5% CO2 to allow for adherence. |
| Treatment | Cells were treated with compounds 7 or 8 at their respective IC50 concentrations. Untreated cells served as controls. |
| Incubation Post Treatment | Cells were incubated for 72 h. |
| Washing | Medium was removed; cells were washed twice with PBS. |
| Cell Harvesting | Cells were trypsinized. |
| Viability and Count Assessment | Cell number and viability were assessed using the trypan blue exclusion assay and an automated cell counter (Countess, Invitrogen). |
| Reproducibility | All experiments were performed in triplicate. |
| Step | Description |
|---|---|
| Cell Seeding | 1 × 104 cells/well in black 96-well plates. |
| Initial Incubation | 24 h at 37 °C with 5% CO2 for cell attachment. |
| Treatment | Cells treated with test compounds at their IC50 concentrations; incubated for an additional 24 h under the same conditions. |
| Probe Incubation | Cells washed with PBS, then incubated with 5 μM DCFH-DA for 30 min at 37 °C in the dark. |
| Controls |
|
| Fluorescence Measurement | Measured using a Microplate Spectrofluorometer (BioTek Synergy, BioTek Instruments, Winooski, VT, USA):
|
| Output | ROS level was quantified in terms of fluorescence intensity (FI). |
| Step | Description |
|---|---|
| Cell Lines Used | HepG2 and A549. |
| Cell Seeding | 5 × 104 cells/well in 12-well plates. |
| Initial Incubation | 24 h at 37 °C, 5% CO2 to allow for cell attachment. |
| Treatment | Cells treated with test compounds at IC50 concentrations for 48 h. Untreated cells served as control. |
| TBARS Measurement | Post treatment, TBARS levels assessed by reading absorbance at 532 nm using a Thermo Scientific MultiscanGo spectrophotometer. |
| Quantification | TBARS expressed as nanomoles of MDA equivalents per milligram of protein, using the molar extinction coefficient of 1.56 × 105 M−1 × cm−1. |
| Protein Determination | Protein content measured by the Bradford assay (separate cultures), with absorbance read at 595 nm (MultiscanGo, ThermoFisher Scientific, Carlsbad, CA, USA). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Napiórkowska, M.; Grosicka-Maciąg, E.; Podsadni, P.; Otto-Ślusarczyk, D. Anticancer Potential of Halogen Derivatives of Methyl 6-Acetyl-5-Hydroxy-2-Methyl-1-Benzofuran-3-Carboxylate. Int. J. Mol. Sci. 2025, 26, 5493. https://doi.org/10.3390/ijms26125493
Napiórkowska M, Grosicka-Maciąg E, Podsadni P, Otto-Ślusarczyk D. Anticancer Potential of Halogen Derivatives of Methyl 6-Acetyl-5-Hydroxy-2-Methyl-1-Benzofuran-3-Carboxylate. International Journal of Molecular Sciences. 2025; 26(12):5493. https://doi.org/10.3390/ijms26125493
Chicago/Turabian StyleNapiórkowska, Mariola, Emilia Grosicka-Maciąg, Piotr Podsadni, and Dagmara Otto-Ślusarczyk. 2025. "Anticancer Potential of Halogen Derivatives of Methyl 6-Acetyl-5-Hydroxy-2-Methyl-1-Benzofuran-3-Carboxylate" International Journal of Molecular Sciences 26, no. 12: 5493. https://doi.org/10.3390/ijms26125493
APA StyleNapiórkowska, M., Grosicka-Maciąg, E., Podsadni, P., & Otto-Ślusarczyk, D. (2025). Anticancer Potential of Halogen Derivatives of Methyl 6-Acetyl-5-Hydroxy-2-Methyl-1-Benzofuran-3-Carboxylate. International Journal of Molecular Sciences, 26(12), 5493. https://doi.org/10.3390/ijms26125493






