Flow Cytometric Quantification of Mitochondrial Properties: A High-Throughput Approach for Single Organelle Analysis
Abstract
1. Introduction
2. Results
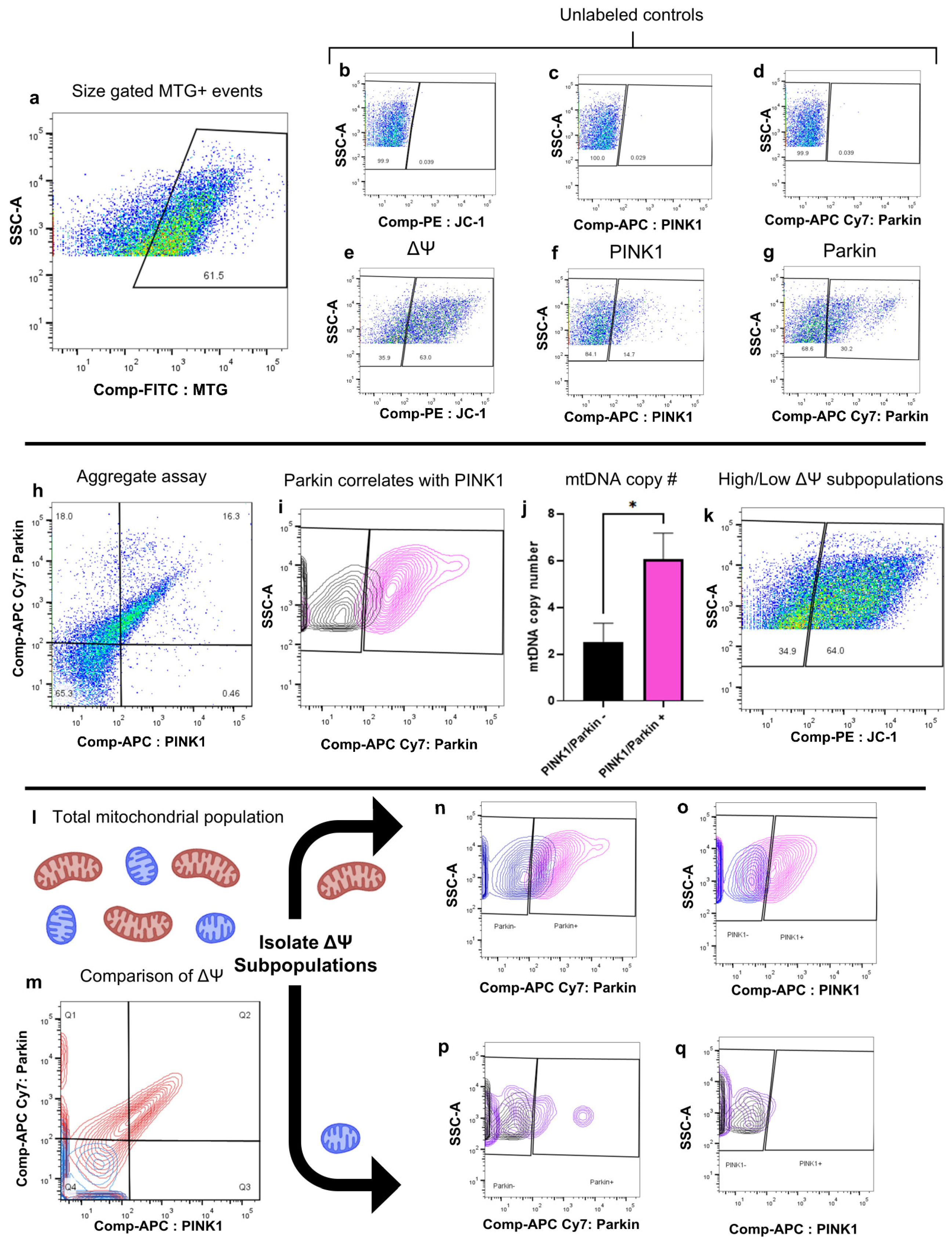
2.1. Development of a Biological Assay for the Quantitative Assessment of Mitophagy in Individual Mitochondria
2.2. Flow Cytometry-Based Assessment of Mitochondria-Containing Extracellular Vesicles in THP1 Macrophages
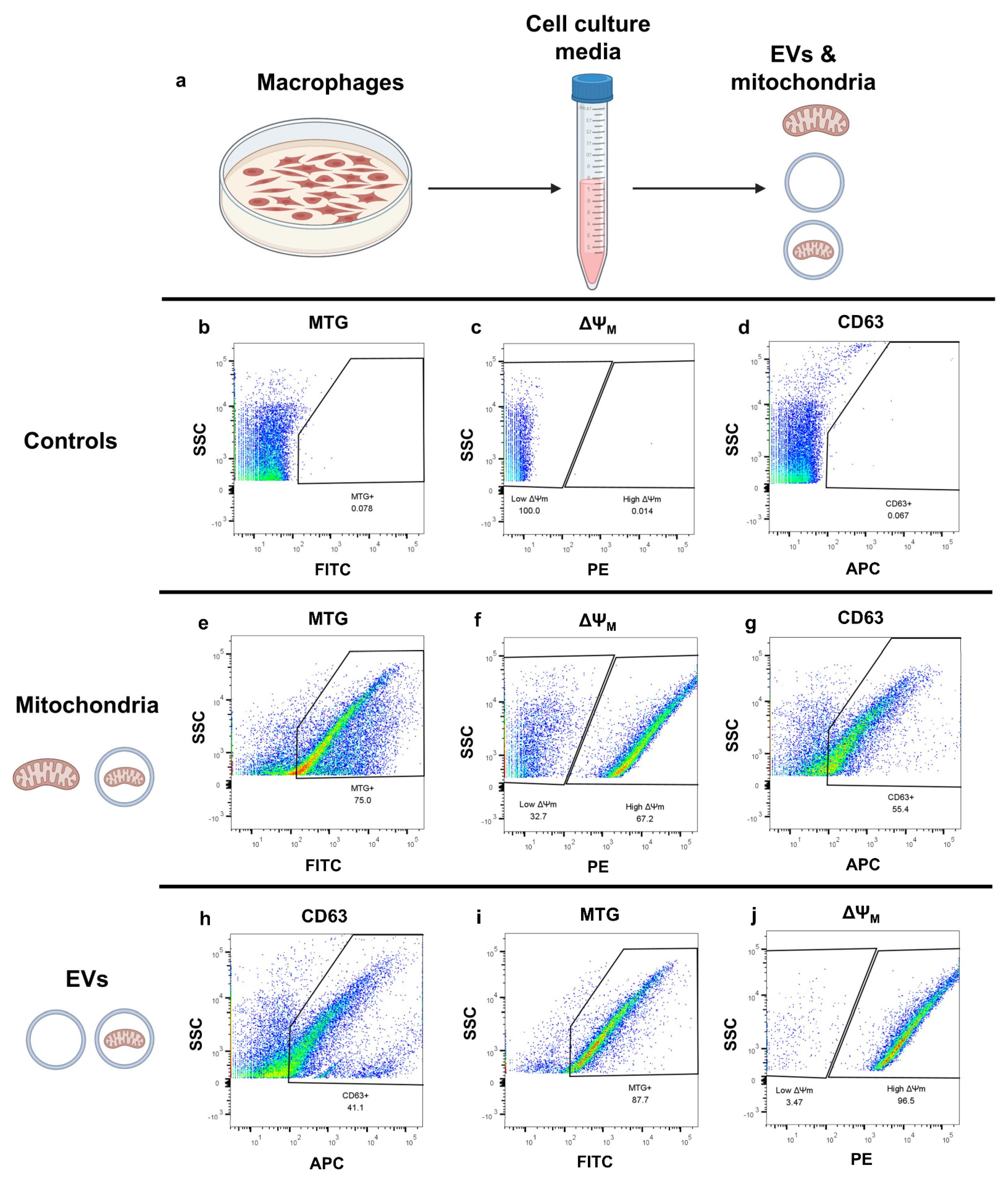
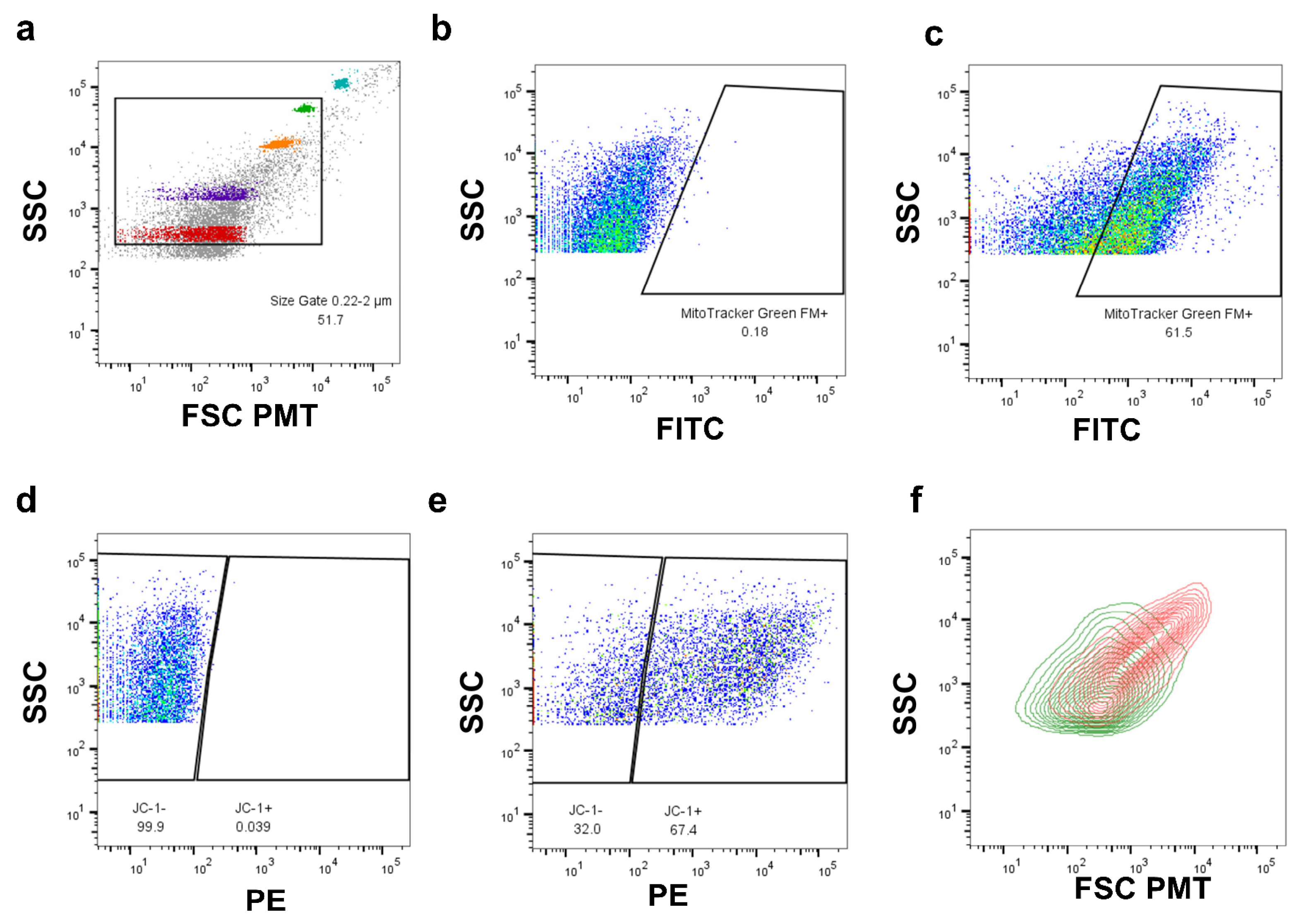
2.3. Flow Cytometry-Based Analysis of Intact, Cell-Free Mitochondria from Blood
2.4. Result Summary
3. Discussion
4. Materials and Methods
4.1. Animals and Cell Lines
4.2. Sample Generation
4.2.1. From Blood (Cell-Free Mitochondria)
4.2.2. From Plated Cells
4.2.3. From Cell Culture Media
4.3. Flow Cytometric Analysis and Sorting
4.4. Downstream Applications
4.4.1. Single-Molecule PCR (smPCR)
4.4.2. ATP Assay
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FAMS | Fluorescence-activated mitochondria sorting |
| ΔΨM | Mitochondrial membrane potential |
| mtDNA | Mitochondrial DNA |
| ROS | Reactive oxygen species |
| EV | Extracellular vesicle |
| PolG | Polymerase gamma |
| MTG | MitoTracker Green FM |
| PINK1 | Phosphatase and tensin homolog (PTEN)-induced putative kinase 1 |
| smPCR | Single-molecule PCR |
| SEM | Standard error of the mean |
References
- MacDonald, J.A.; Bothun, A.M.; Annis, S.N.; Sheehan, H.; Ray, S.; Gao, Y.; Ivanov, A.R.; Khrapko, K.; Tilly, J.L.; Woods, D.C. A nanoscale, multi-parametric flow cytometry-based platform to study mitochondrial heterogeneity and mitochondrial DNA dynamics. Commun. Biol. 2019, 2, 258. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, J.A.; Fowle, W.H.; Woods, D.C. New insights on mitochondrial heterogeneity observed in prepared mitochondrial samples following a method for freeze-fracture and scanning electron microscopy. Micron 2017, 101, 25–31. [Google Scholar] [CrossRef]
- Woods, D.C. Mitochondrial Heterogeneity: Evaluating Mitochondrial Subpopulation Dynamics in Stem Cells. Stem Cells Int. 2017, 2017, 7068567. [Google Scholar] [CrossRef]
- Vafai, S.B.; Mootha, V.K. Mitochondrial disorders as windows into an ancient organelle. Nature 2012, 491, 374–383. [Google Scholar] [CrossRef]
- Benard, G.; Bellance, N.; James, D.; Parrone, P.; Fernandez, H.; Letellier, T.; Rossignol, R. Mitochondrial bioenergetics and structural network organization. J. Cell Sci. 2007, 120, 838–848. [Google Scholar] [CrossRef] [PubMed]
- Wai, T.; Langer, T. Mitochondrial Dynamics and Metabolic Regulation. Trends Endocrinol. Metab. 2016, 27, 105–117. [Google Scholar] [CrossRef]
- Alberico, H.C.; Woods, D.C. Role of Granulosa Cells in the Aging Ovarian Landscape: A Focus on Mitochondrial and Metabolic Function. Front. Physiol. 2022, 12, 800739. [Google Scholar] [CrossRef]
- Palade, G.E. An Electron Microscope Study of the Mitochondrial Structure. J. Histochem. Cytochem. 1953, 1, 188–211. [Google Scholar] [CrossRef] [PubMed]
- Zick, M.; Rabl, R.; Reichert, A.S. Cristae formation—Linking ultrastructure and function of mitochondria. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2009, 1793, 5–19. [Google Scholar] [CrossRef]
- Whitworth, A.J.; Pallanck, L.J. PINK1/Parkin mitophagy and neurodegeneration—What do we really know in vivo? Curr. Opin. Genet. Dev. 2017, 44, 47–53. [Google Scholar] [CrossRef]
- Jin, S.M.; Youle, R.J. The accumulation of misfolded proteins in the mitochondrial matrix is sensed by PINK1 to induce PARK2/Parkin-mediated mitophagy of polarized mitochondria. Autophagy 2013, 9, 1750–1757. [Google Scholar] [CrossRef] [PubMed]
- de Castro, I.P.; Costa, A.C.; Lam, D.; Tufi, R.; Fedele, V.; Moisoi, N.; Dinsdale, D.; Deas, E.; Loh, S.H.Y.; Martins, L.M. Genetic analysis of mitochondrial protein misfolding in Drosophila melanogaster. Cell Death Differ. 2012, 19, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Bartolomé, A.; García-Aguilar, A.; Asahara, S.-I.; Kido, Y.; Guillén, C.; Pajvani, U.B.; Benito, M. MTORC1 Regulates both General Autophagy and Mitophagy Induction after Oxidative Phosphorylation Uncoupling. Mol. Cell. Biol. 2017, 37, e00441-17. [Google Scholar] [CrossRef]
- Kaguni, L.S. DNA Polymerase γ, The Mitochondrial Replicase. Annu. Rev. Biochem. 2004, 73, 293–320. [Google Scholar] [CrossRef]
- Trifunovic, A.; Wredenberg, A.; Falkenberg, M.; Spelbrink, J.N.; Rovio, A.T.; Bruder, C.E.; Bohlooly-Y, M.; Gidlöf, S.; Oldfors, A.; Wibom, R.; et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 2004, 429, 417–423. [Google Scholar] [CrossRef]
- Kujoth, G.C.; Hiona, A.; Pugh, T.D.; Someya, S.; Panzer, K.; Wohlgemuth, S.E.; Hofer, T.; Seo, A.Y.; Sullivan, R.; Jobling, W.A.; et al. Mitochondrial DNA Mutations, Oxidative Stress, and Apoptosis in Mammalian Aging. Science 2005, 309, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Faraci, C.; Annis, S.; Jin, J.; Li, H.; Khrapko, K.; Woods, D.C. Impact of exercise on oocyte quality in the POLG mitochondria! DNA mutator mouse. Reproduction 2018, 156, 185–194. [Google Scholar] [CrossRef]
- Haggadone, M.D.; Peters-Golden, M. Microenvironmental Influences on Extracellular Vesicle-Mediated Communication in the Lung. Trends Mol. Med. 2018, 24, 963–975. [Google Scholar] [CrossRef]
- Ginini, L.; Billan, S.; Fridman, E.; Gil, Z. Insight into Extracellular Vesicle-Cell Communication: From Cell Recognition to Intracellular Fate. Cells 2022, 11, 1375. [Google Scholar] [CrossRef]
- Rabas, N.; Palmer, S.; Mitchell, L.; Ismail, S.; Gohlke, A.; Riley, J.S.; Tait, S.W.; Gammage, P.; Soares, L.L.; Macpherson, I.R.; et al. PINK1 drives production of mtDNA-containing extracellular vesicles to promote invasiveness. J. Cell Biol. 2021, 220, e202006049. [Google Scholar] [CrossRef]
- Phinney, D.G.; Di Giuseppe, M.; Njah, J.; Sala, E.; Shiva, S.; St Croix, C.M.; Stolz, D.B.; Watkins, S.C.; Di, Y.P.; Leikauf, G.D.; et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat. Commun. 2015, 6, 8472. [Google Scholar] [CrossRef] [PubMed]
- Morrison, T.J.; Jackson, M.V.; Cunningham, E.K.; Kissenpfennig, A.; McAuley, D.F.; O’Kane, C.M.; Krasnodembskaya, A.D. Mesenchymal Stromal Cells Modulate Macrophages in Clinically Relevant Lung Injury Models by Extracellular Vesicle Mitochondrial Transfer. Am. J. Respir. Crit. Care Med. 2017, 196, 1275–1286. [Google Scholar] [CrossRef]
- She, Z.; Xie, M.; Hun, M.; Abdirahman, A.S.; Li, C.; Wu, F.; Luo, S.; Wan, W.; Wen, C.; Tian, J. Immunoregulatory Effects of Mitochondria Transferred by Extracellular Vesicles. Front. Immunol. 2021, 11, 628576. [Google Scholar] [CrossRef]
- Gonzalez-Delgado, R.; Muñoz, N.M.; Carlos-Alcalde, W.; Cho, M.S.; Lee, H.; Jin, J.; Serpas, V.; Gorlova, O.; Sheth, R.A.; Afshar-Kharghan, V. Role of circulating mitochondria in venous thrombosis in glioblastoma. J. Thromb. Haemost. 2023, 21, 2202–2212. [Google Scholar] [CrossRef] [PubMed]
- Rosa, H.S.; Ajaz, S.; Gnudi, L.; Malik, A.N. A case for measuring both cellular and cell-free mitochondrial DNA as a disease biomarker in human blood. FASEB J. 2020, 34, 12278–12288. [Google Scholar] [CrossRef]
- Mahmoud, E.H.; Fawzy, A.; Ahmad, O.K.; Ali, A.M. Plasma Circulating Cell-free Nuclear and Mitochondrial DNA as Potential Biomarkers in the Peripheral Blood of Breast Cancer Patients. Asian Pac. J. Cancer Prev. 2016, 16, 8299–8305. [Google Scholar] [CrossRef] [PubMed]
- Lazo, S.; Hooten, N.N.; Green, J.; Eitan, E.; Mode, N.A.; Liu, Q.; Zonderman, A.B.; Ezike, N.; Mattson, M.P.; Ghosh, P.; et al. Mitochondrial DNA in extracellular vesicles declines with age. Aging Cell 2020, 20, e13283. [Google Scholar] [CrossRef]
- Berezhnov, A.V.; Soutar, M.P.; Fedotova, E.I.; Frolova, M.S.; Plun-Favreau, H.; Zinchenko, V.P.; Abramov, A.Y. Intracellular pH Modulates Autophagy and Mitophagy. J. Biol. Chem. 2016, 291, 8701–8708. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, M.; Liu, S.; Guo, J.; Lu, Y.; Cheng, J.; Liu, J. Macrophage-derived extracellular vesicles: Diverse mediators of pathology and therapeutics in multiple diseases. Cell Death Dis. 2020, 11, 924. [Google Scholar] [CrossRef]
- Gangadaran, P.; Rajendran, R.L.; Oh, J.M.; Hong, C.M.; Jeong, S.Y.; Lee, S.-W.; Lee, J.; Ahn, B.-C. Extracellular vesicles derived from macrophage promote angiogenesis In vitro and accelerate new vasculature formation In vivo. Exp. Cell Res. 2020, 394, 112146. [Google Scholar] [CrossRef]
- McCully, J.D.; Levitsky, S.; del Nido, P.J.; Cowan, D.B. Mitochondrial transplantation for therapeutic use. Clin. Transl. Med. 2016, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Capelluto, F.; Alberico, H.; Ledo-Hopgood, P.; Tilly, J.L.; Woods, D.C. Lineage-Mismatched Mitochondrial Replacement in an Inducible Mitochondrial Depletion Model Effectively Restores the Original Proteomic Landscape of Recipient Cells. Adv. Biol. 2023, 7, e2200246. [Google Scholar] [CrossRef]
- Seo, B.J.; Yoon, S.H.; Do, J.T. Mitochondrial Dynamics in Stem Cells and Differentiation. Int. J. Mol. Sci. 2018, 19, 3893. [Google Scholar] [CrossRef]
- Khacho, M.; Slack, R.S. Mitochondrial activity in the regulation of stem cell self-renewal and differentiation. Curr. Opin. Cell Biol. 2017, 49, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Woods, D.C.; Khrapko, K.; Tilly, J.L. Influence of Maternal Aging on Mitochondrial Heterogeneity, Inheritance, and Function in Oocytes and Preimplantation Embryos. Genes 2018, 9, 265. [Google Scholar] [CrossRef]
- Seo, A.Y.; Joseph, A.-M.; Dutta, D.; Hwang, J.C.Y.; Aris, J.P.; Leeuwenburgh, C. New insights into the role of mitochondria in aging: Mitochondrial dynamics and more. J. Cell Sci. 2010, 123, 2533–2542. [Google Scholar] [CrossRef]
- Salas, A.; Yao, Y.-G.; Macaulay, V.; Vega, A.; Carracedo, Á.; Bandelt, H.-J. A Critical Reassessment of the Role of Mitochondria in Tumorigenesis. PLoS Med. 2005, 2, e296. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, L.R.; Liang, B.C. Mutagenesis, tumorigenicity, and apoptosis: Are the mitochondria involved? Mutat. Res./Fundam. Mol. Mech. Mutagen. 1998, 398, 19–26. [Google Scholar] [CrossRef]
- Badrinath, N.; Yoo, S.Y. Mitochondria in cancer: In the aspects of tumorigenesis and targeted therapy. Carcinogenesis 2018, 39, 1419–1430. [Google Scholar] [CrossRef]

| Experimental Aim | Experimental Approach | Result |
|---|---|---|
| Development of a biological assay for the quantitative assessment of mitophagy in individual mitochondria without the use of membrane uncouplers. | Pol G mutant fibroblast mitochondria were assessed for ΔΨM, PINK1, and Parkin expression using FAMS. Individual mitochondria were sorted and assessed for mtDNA copy number. | PINK1 and Parkin expression was detected on a per organelle basis without the use of membrane uncouplers and were shown to colocalize at different rates based on mitochondrial subpopulation. |
| Assessment of spent cell culture media to determine whether detected cell-free mitochondria were free-floating or contained within extracellular vesicles. | Following a 24 h incubation of THP-1 macrophages, spent media were collected, ensured to be free of cells, and assessed for the presence of free mitochondria and extracellular vesicles using FAMS. | Both free-floating and vesicle-enclosed mitochondria were detected. The detection of vesicle-enclosed mitochondria raises the question of whether these organelles are being intentionally excreted by the macrophages in vitro. |
| Test for the presence of intact, cell-free mitochondria in mouse blood plasma. | Plasma was collected and tested for the presence of respiratory-competent mitochondria using FAMS. Sorted mitochondria were pooled and tested for the ability to produce ATP. | Cell-free, respiratory-competent mitochondria were detected in mouse plasma using FAMS. Their ability to respire was confirmed by ATP assay. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piasecki, A.J.; Sheehan, H.C.; Tilly, J.L.; Woods, D.C. Flow Cytometric Quantification of Mitochondrial Properties: A High-Throughput Approach for Single Organelle Analysis. Int. J. Mol. Sci. 2025, 26, 5481. https://doi.org/10.3390/ijms26125481
Piasecki AJ, Sheehan HC, Tilly JL, Woods DC. Flow Cytometric Quantification of Mitochondrial Properties: A High-Throughput Approach for Single Organelle Analysis. International Journal of Molecular Sciences. 2025; 26(12):5481. https://doi.org/10.3390/ijms26125481
Chicago/Turabian StylePiasecki, Andrew J., Hannah C. Sheehan, Jonathan L. Tilly, and Dori C. Woods. 2025. "Flow Cytometric Quantification of Mitochondrial Properties: A High-Throughput Approach for Single Organelle Analysis" International Journal of Molecular Sciences 26, no. 12: 5481. https://doi.org/10.3390/ijms26125481
APA StylePiasecki, A. J., Sheehan, H. C., Tilly, J. L., & Woods, D. C. (2025). Flow Cytometric Quantification of Mitochondrial Properties: A High-Throughput Approach for Single Organelle Analysis. International Journal of Molecular Sciences, 26(12), 5481. https://doi.org/10.3390/ijms26125481







