Platelet-Rich Plasma and Platelet-Rich Fibrin in Endodontics: A Scoping Review
Abstract
1. Introduction
2. Results
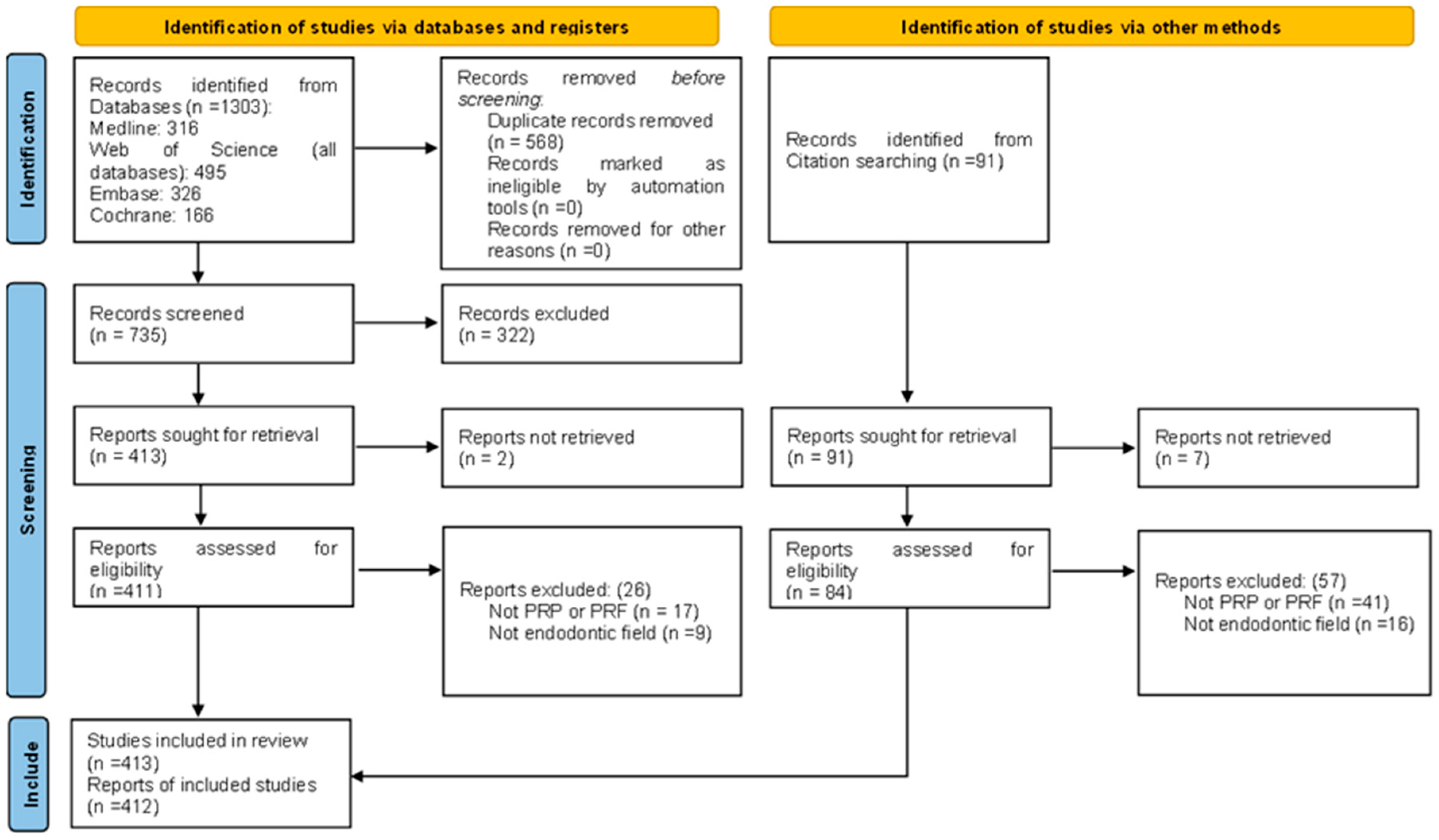
2.1. Study Selection
2.2. Characteristics of the Included Studies
2.3. Endodontic Procedures
2.3.1. Pulpotomy and Pulp Capping
2.3.2. Regenerative Endodontic Procedures in Immature Teeth
2.3.3. Apexification
2.3.4. Apical Surgery
2.3.5. Reimplantation of Avulsed Tooth and Intentional Reimplantation
2.3.6. Autotransplantation
2.3.7. Biological Effects
2.3.8. Endo-Perio Lesions
2.3.9. Root Fracture
2.3.10. Management of Root Perforation
2.3.11. Mechanical Properties
2.3.12. Management of Root Resorption
2.3.13. Regeneration of Mature Teeth
3. Discussion
4. Materials and Methods
4.1. Protocol Registration
4.2. Review Questions
4.3. Information Sources
4.4. Search Strategy
4.5. Eligibility Criteria
4.6. Selection Process
4.7. Data Extraction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| A-PRF | Advanced platelet-rich fibrin |
| CGF | Concentrated growth factors |
| IGF-1 | Insulin-like growth factor 1 |
| IGF-2 | Insulin-like growth factor 2 |
| I-PRF | Injectable platelet-rich fibrin |
| IL-1 | Interleukin-1 |
| IL-4 | Interleukin-4 |
| IL-6 | Interleukin-6 |
| L-PRF | Leucocyte platelet-rich fibrin |
| MeSH | Medical subject headings |
| MTA | Mineral trioxide aggregate |
| OSF | Open science framework |
| P-PRP | Pure platelet-rich plasma |
| PCC | Population, concept, context |
| PDGF | Platelet-derived growth factor |
| PRF | Platelet-rich fibrin |
| PRISMA-ScR | Preferred reporting items for systematic reviews and meta-analysis protocols, Scoping review extension guidelines |
| PRP | Platelet-rich plasma |
| RCT | Randomized controlled trial |
| RET | Regenerative endodontic treatment |
| RPM | Revolution per minute |
| T-PRF | Titanium platelet-rich fibrin |
| TGF-B1 | Transforming growth factor B1 |
| VEGF | Vascular endothelial growth factor |
References
- Morotomi, T.; Washio, A.; Kitamura, C. Current and Future Options for Dental Pulp Therapy. Jpn. Dent. Sci. Rev. 2019, 55, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.E.; Garcia-Godoy, F.; Hargreaves, K.M. Regenerative Endodontics: A Review of Current Status and a Call for Action. J. Endod. 2007, 33, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Gathani, K.; Raghavendra, S. Scaffolds in Regenerative Endodontics: A Review. Dent. Res. J. (Isfahan) 2016, 13, 379–386. [Google Scholar] [CrossRef]
- Zhang, W.; Yelick, P.C. Tooth Repair and Regeneration: Potential of Dental Stem Cells. Trends Mol. Med. 2021, 27, 501–511. [Google Scholar] [CrossRef]
- Hotwani, K.; Sharma, K. Platelet Rich Fibrin—A Novel Acumen into Regenerative Endodontic Therapy. Restor. Dent. Endod. 2014, 39, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.A. Evolution, Current Status and Advances in Application of Platelet Concentrate in Periodontics and Implantology. World J. Clin. Cases 2017, 5, 159–171. [Google Scholar] [CrossRef]
- Whitman, D.H.; Berry, R.L.; Green, D.M. Platelet Gel: An Autologous Alternative to Fibrin Glue With Applications in Oral and Maxillofacial Surgery. J. Oral Maxillofac. Surg. 1997, 55, 1294–1299. [Google Scholar] [CrossRef]
- Umakanth, K.; Balaji Ganesh, S.; Smiline Girija, A. Applications Of Platelet Concentrates In Endodontics—A Review. Int. J. Pharm. Res. 2020, 12, 2102–2107. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; Rasmusson, L.; Albrektsson, T. Classification of Platelet Concentrates: From Pure Platelet-Rich Plasma (P-PRP) to Leucocyte- and Platelet-Rich Fibrin (L-PRF). Trends Biotechnol. 2009, 27, 158–167. [Google Scholar] [CrossRef]
- Arshad, S.; Tehreem, F.; Rehab khan, M.; Ahmed, F.; Marya, A.; Karobari, M.I. Platelet-Rich Fibrin Used in Regenerative Endodontics and Dentistry: Current Uses, Limitations, and Future Recommendations for Application. Int. J. Dent. 2021, 2021, 4514598. [Google Scholar] [CrossRef]
- Elver, A.; Caymaz, M.G. Novel Approaches to the Use of Platelet-Rich Fibrin: A Literature Review. Saudi Dent. J. 2023, 35, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Pietruszka, P.; Chruścicka, I.; Duś-Ilnicka, I.; Paradowska-Stolarz, A. PRP and PRF—Subgroups and Divisions When Used in Dentistry. J. Pers. Med. 2021, 11, 944. [Google Scholar] [CrossRef]
- Fujioka-Kobayashi, M.; Miron, R.J.; Hernandez, M.; Kandalam, U.; Zhang, Y.; Choukroun, J. Optimized Platelet-Rich Fibrin With the Low-Speed Concept: Growth Factor Release, Biocompatibility, and Cellular Response. J. Periodontol. 2017, 88, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Bolhari, B.; Meraji, N.; Ghorbanzadeh, A.; Sarraf, P.; Moayeri, R. Applications of Fibrin-Based Products in Endodontics: A Literature Review. Dent. Hypotheses 2019, 10, 85–90. [Google Scholar] [CrossRef]
- Eid, A.; Mancino, D.; Rekab, M.S.; Haikel, Y.; Kharouf, N. Effectiveness of Three Agents in Pulpotomy Treatment of Permanent Molars with Incomplete Root Development: A Randomized Controlled Trial. Healthcare 2022, 10, 431. [Google Scholar] [CrossRef]
- Riaz, A.; Shah, F.A. Regenerating the Pulp–Dentine Complex Using Autologous Platelet Concentrates: A Critical Appraisal of the Current Histological Evidence. Tissue Eng. Regen. Med. 2021, 18, 37–48. [Google Scholar] [CrossRef]
- Alsolaihim, A.; Alsolaihim, A.; Alsolaihim, N.; Alowais, L. Biomimetic Regenerative Materials in Restorative Dentistry and Endodontics. J. Int. Oral. Health 2023, 15, 250–256. [Google Scholar] [CrossRef]
- Khurshid, Z.; Asiri, F.Y.I.; Najeeb, S.; Ratnayake, J. The Impact of Autologous Platelet Concentrates on the Periapical Tissues and Root Development of Replanted Teeth: A Systematic Review. Materials 2022, 15, 2776. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, B.; Huang, C.; Ye, R. Intentional Replantation of a Second Premolar with Internal Resorption and Root Fracture: A Case Report. J. Contemp. Dent. Pract. 2021, 22, 562–567. [Google Scholar] [CrossRef]
- Kapoor, S. Surgical Management of a Non-Healing Intra-Alveolar Root Fracture Associated with Pulpal Calcification and Root Resorption: A Case Report. J. Clin. Diagn. Res. 2015, 9, ZD03–ZD05. [Google Scholar] [CrossRef]
- Mohammed, S.E.; Gawdat, S.I.; Ibrahim, S.M. Role of Prf With Mta and Theracal After Pulpotomy In Relieving Pain and Maintaining the Vitality of the Remaining Radicular Pulp Tissue in Permanent Posterior Teeth with Closed Root Apices: “Randomized Controlled Trial”. J. Pharm. Negat. Results 2022, 13, 2559–2569. [Google Scholar] [CrossRef]
- Mandviwala, D.K.; Arora, A.V.; Kapoor, S.V.; Shah, P.B. Internal Root Resorption: A Rare Complication of Vital Pulp Therapy Using Platelet-Rich Fibrin. J. Oral. Maxillofac. Pathol. 2022, 26, 132. [Google Scholar] [CrossRef]
- Ctri Pulpotomy in Mature Permanent Tooth Using Various Biomaterials. 2023. Available online: https://trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2023/06/053989 (accessed on 5 January 2024).
- Ctri Comparasion of Success Rate of Pulpotomy with Biodentine Using PRF Membrane and Collagen Scaffold in Permanent Molars. 2023. Available online: https://trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2023/03/050834 (accessed on 5 January 2024).
- Naik, S.V.; Attiguppe, P.; Prakash, A.J. Comparative Evaluation of the Regenerative Potential of Blood Clot and Platelet-Rich Fibrin in Young Permanent Teeth Based on the Revised American Academy of Endodontics Clinical Considerations for Regenerative Procedure: 2016. Int. J. Clin. Pediatr. Dent. 2023, 16, S149–S154. [Google Scholar] [CrossRef]
- Das, S.; Srivastava, R.; Thosar, N.R.; Khubchandani, M.; Ragit, R.; Malviya, N. Regenerative Endodontics-Reviving the Pulp the Natural Way: A Case Report. Cureus 2023, 15, e36587. [Google Scholar] [CrossRef]
- Kumar, J.K.; Surendranath, P.; Eswaramoorthy, R. Regeneration of Immature Incisor Using Platelet Rich Fibrin: Report of a Novel Clinical Application. BMC Oral Health 2023, 23, 69. [Google Scholar] [CrossRef]
- Ríos-Osorio, N.; Caviedes-Bucheli, J.; Jimenez-Peña, O.; Orozco-Agudelo, M.; Mosquera-Guevara, L.; Jiménez-Castellanos, F.; Muñoz-Alvear, H. Comparative Outcomes of Platelet Concentrates and Blood Clot Scaffolds for Regenerative Endodontic Procedures: A Systematic Review of Randomized Controlled Clinical Trials. J. Clin. Exp. Dent. 2023, 15, e239–e249. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zheng, L.; Daraqel, B.; Liu, J.; Hu, Y. The Efficacy of Concentrated Growth Factor and Platelet-Rich Fibrin as Scaffolds in Regenerative Endodontic Treatment Applied to Immature Permanent Teeth: A Retrospective Study. BMC Oral Health 2023, 23, 482. [Google Scholar] [CrossRef]
- Biradar, N.; Ragulakollu, R.; Bogishetty, C.; Tej, G.; Gandham, S.; Vardhan, P. Combination Therapy of Antibiotics and Platelet-Rich Fibrin for Apical Closure: Case Series. Int. J. Clin. Pediatr. Dent. 2023, 16, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Pruthi, P.J.; Goel, S.; Yadav, P.; Nawal, R.R.; Talwar, S. Novel Application of a Calcium Silicate—based Cement and Platelet-Rich Fibrin in Complex Endodontic Cases: A Case Series. Gen. Dent. 2020, 68, 46–49. [Google Scholar]
- Murray, P.E. Platelet-Rich Plasma and Platelet-Rich Fibrin Can Induce Apical Closure More Frequently Than Blood-Clot Revascularization for the Regeneration of Immature Permanent Teeth: A Meta-Analysis of Clinical Efficacy. Front. Bioeng. Biotechnol. 2018, 6, 139. [Google Scholar] [CrossRef]
- Hugar, S.M.; Gokhale, N.; Soneta, S.P.; Joshi, R.S.; Dialani, P.K.; Saxena, N. Evaluation of the Treatment Protocols in the Management of Pulpally Involved Young Permanent Teeth in Children: A Systematic Review and Meta-Analysis. Int. J. Clin. Pediatr. Dent. 2022, 15, S103–S113. [Google Scholar] [CrossRef] [PubMed]
- Santhakumar, M.; Yayathi, S.; Retnakumari, N. A Clinicoradiographic Comparison of the Effects of Platelet-Rich Fibrin Gel and Platelet-Rich Fibrin Membrane as Scaffolds in the Apexification Treatment of Young Permanent Teeth. J. Indian Soc. Pedod. Prev. Dent. 2018, 36, 65–70. [Google Scholar] [CrossRef] [PubMed]
- di Lauro, A.E.; Valletta, A.; Aliberti, A.; Cangiano, M.; Dolce, P.; Sammartino, G.; Gasparro, R. The Effectiveness of Autologous Platelet Concentrates in the Clinical and Radiographic Healing after Endodontic Surgery: A Systematic Review. Materials 2023, 16, 7187. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Jain, A.K.; Rao, R.D.; Sivasailam, S.; Jain, R. Effect of Platelet-Rich Fibrin on Periapical Healing and Resolution of Clinical Symptoms in Patients Following Periapical Surgery: A Systematic Review and Meta-Analysis. J. Conserv. Dent. 2023, 26, 366–376. [Google Scholar] [CrossRef]
- Nct Post-Operative Evaluation of Endodontic Microsurgeries Done Using a Piezoelectric Ultrasonic Technique: An in Vivo Study. 2023. Available online: https://clinicaltrials.gov/show/NCT05863728 (accessed on 5 January 2024).
- Govindaraju, L.; Antony, D.P.; S, P. Surgical Management of Radicular Cyst With the Application of a Natural Platelet Concentrate: A Case Report. Cureus 2023, 15, e33992. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.-L.; Jia, L.-N.; Wang, J.-J.; Zhang, M. Rescuing “Hopeless” Avulsed Teeth Using Autologous Platelet-Rich Fibrin Following Delayed Reimplantation: Two Case Reports. World J. Clin. Cases 2023, 11, 635–644. [Google Scholar] [CrossRef]
- Parthasarathy, R.; Srinivasan, S.; C, V.; Thanikachalam, Y.; Ramachandran, A. An Interdisciplinary Management of Avulsed Maxillary Incisors: A Case Report. Cureus 2022, 14, e23891. [Google Scholar] [CrossRef]
- Behnaz, M.; Izadi, S.S.; Mashhadi Abbas, F.; Dianat, O.; Sadeghabadi, S.; Akbarzadeh, T.; Haeri, A.; Kazem, M.; Younessian, F. The Impact of Platelet-rich Fibrin (PRF) on Delayed Tooth Replantation: A Preliminary Animal Study. Aust. Endod. J. 2021, 47, 457–466. [Google Scholar] [CrossRef]
- Gaviño Orduña, J.F.; García García, M.; Dominguez, P.; Caviedes Bucheli, J.; Martin Biedma, B.; Abella Sans, F.; Manzanares Céspedes, M.C. Successful Pulp Revascularization of an Autotransplantated Mature Premolar with Fragile Fracture Apicoectomy and Plasma Rich in Growth Factors: A 3-year Follow-up. Int. Endod. J. 2020, 53, 421–433. [Google Scholar] [CrossRef]
- Iqbal, A.; Riaz, A.; Waheed, A.; Khan, S.U.; Nawadat, K.; Islam, S. Reorienting Goals in Endodontic Therapy: Pulp Revitalization, on the Brink of a Paradigm Shift. J. Pak. Med. Assoc. 2021, 71, 2589–2595. [Google Scholar] [CrossRef]
- Moraschini, V.; Miron, R.J.; Mourão, C.F.d.A.B.; Louro, R.S.; Sculean, A.; da Fonseca, L.A.M.; Calasans Maia, M.D.; Shibli, J.A. Antimicrobial Effect of Platelet-rich Fibrin: A Systematic Review of in Vitro Evidence-based Studies. Periodontol. 2000 2024, 94, 131–142. [Google Scholar] [CrossRef]
- Panda, P.; Govind, S.; Sahoo, S.K.; Pattanaik, S.; Mallikarjuna, R.M.; Nalawade, T.; Saraf, S.; Khaldi, N.A.; Jahdhami, S.A.; Shivagange, V.; et al. Analysis of Pulp Tissue Viability and Cytotoxicity of Pulp Capping Agents. J. Clin. Med. 2023, 12, 539. [Google Scholar] [CrossRef] [PubMed]
- Khatri, S.; Mathew, S.; Nagaraja, S.; Hegde, S.; Ghosh, S.; Ravichandran, K. Comparative Evaluation of PH and Ca+ Ion Release from MTA on Interaction with Platelet-Rich Fibrin and Blood Clot: An in Vitro Study. F1000Research 2023, 12, 364. [Google Scholar] [CrossRef] [PubMed]
- Sandra, F.; Sutanto, A.; Wulandari, W.; Lambertus, R.; Celinna, M.; Dewi, N.M.; Ichwan, S.J.A. Crucial Triad in Pulp-Dentin Complex Regeneration: Dental Stem Cells, Scaffolds, and Signaling Molecules. Indones. Biomed. J. 2023, 15, 25–46. [Google Scholar] [CrossRef]
- Thakur, V.; Mittal, S.; Tewari, S.; Kamboj, M.; Duhan, J.; Sangwan, P.; Kumar, V.; Gupta, A. Comparative Histological Evaluation of Two PRF Formulations (PRF High and PRF Medium) on Quality of Life and Healing Outcome of Apicomarginal Defects: A Randomized Clinical Trial. J. Cranio-Maxillofac. Surg. 2023, 51, 166–177. [Google Scholar] [CrossRef]
- Makkad, R.S. Platelet-Rich Fibrin and Titanium-Prepared Platelet-Rich Fibrin in Endoperio Lesion Management. Bioinformation 2023, 19, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Oktawati, S.; Siswanto, H.; Mardiana, A.; Supiaty; Neormansyah, I.; Basir, I. Endodontic–Periodontic Lesion Management: A Systematic Review. Med. Clín. Práct. 2020, 3, 100098. [Google Scholar] [CrossRef]
- Onicas, M.I.; Narita, L.E.; Mester, A.; Onisor, F.; Mancini, L. Platelet-Rich Fibrin: A Viable Therapy for Endodontic-Periodontal Lesions? A Preliminary Assessment. Appl. Sci. 2021, 11, 7081. [Google Scholar] [CrossRef]
- Ardila, C.M.; Vivares-Builes, A.M. Clinical Efficacy of Treatment of Endodontic-Periodontal Lesions: A Systematic Scoping Review of Experimental Studies. Int. J. Environ. Res. Public Health 2022, 19, 13649. [Google Scholar] [CrossRef]
- Arango-Gómez, E.; Nino-Barrera, J.L.; Nino, G.; Jordan, F.; Sossa-Rojas, H. Pulp Revascularization with and without Platelet-Rich Plasma in Two Anterior Teeth with Horizontal Radicular Fractures: A Case Report. Restor. Dent. Endod. 2019, 44, e35. [Google Scholar] [CrossRef]
- Mohamed, D.A.-A.; Abdelwahab, S.A.; Mahmoud, R.H.; Taha, R.M. Radiographic and Immuno-Histochemical Evaluation of Root Perforation Repair Using MTA with or without Platelet-Rich Fibrin or Concentrated Growth Factors as an Internal Matrix in Dog’s Teeth: In Vivo Animal Study. Clin. Oral. Investig. 2023, 27, 5103–5119. [Google Scholar] [CrossRef] [PubMed]
- Teja, K.; Ramesh, S. Nonsurgical Management of Strip Perforation Using Platelet-Rich Fibrin and MTA by Matrix Concept—A Case Report with One Year Follow-Up. Contemp. Clin. Dent. 2021, 12, 84–87. [Google Scholar] [CrossRef]
- Córdova-Malca, F.; Coaguila-Llerena, H.; Garré-Arnillas, L.; Rayo-Iparraguirre, J.; Faria, G. Endodontic Micro-Resurgery and Guided Tissue Regeneration of a Periapical Cyst Associated to Recurrent Root Perforation: A Case Report. Restor. Dent. Endod. 2022, 47, e35. [Google Scholar] [CrossRef]
- Nagaraja, S.; Mathew, S.; Rajaram, R.; Pushpalatha, C.; Abraham, A.; Chandanala, S. Evaluation of Histological and PH Changes in Platelet-Rich Fibrin and Platelet-Rich Fibrin Matrix: A In Vitro Study. Contemp. Clin. Dent. 2019, 10, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Chhaya, D.; Vaidya, N.; Patel, V.; Chudasama, K.; Doshi, S.; Kumar, P. Evaluation and Comparison of Mechanical Properties of Platelet-Rich Fibrin Membrane, Fish Collagen Membrane, Bovine Collagen Membrane and Chorionic Membrane—An SEM Study. Indian J. Dent. Res. 2022, 33, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Johns, D.; Shivashankar, V.; Maroli, R.; Joseph, R. Invasive Cervical Root Resorption: Engineering the Lost Tissue by Regeneration. Contemp. Clin. Dent. 2013, 4, 536–539. [Google Scholar] [CrossRef]
- Gupta, G.; Agarwal, A.; Ansari, A.A.; Singh, R.K. Non-Surgical Management of a Large Periapical Lesion with Internal Resorption Using PRF, Hydroxyapatite and MTA. BMJ Case Rep. 2022, 15, e248907. [Google Scholar] [CrossRef]
- Nalawade, T.M.; Telgi, C.R.; Arora, G.; Rachappa, M. Platelet Rich Plasma and Bone Graft for Rehabilitation of Luxation Injuries to Permanent Incisors. J. Adv. Oral. Res. 2011, 2, 41–44. [Google Scholar] [CrossRef]
- Dadpe, A.M. Regenerative Endodontic Procedures in Teeth with Root Resorption: A Systematic Review. Eur. Endod. J. 2023, 8, 170–186. [Google Scholar] [CrossRef]
- Eldessoky, A.E.; Khalefa, M.M.; Abu-Seida, A.M. Regenerative Endodontic Therapy in Mature Teeth with Necrotic Pulp and Apical Periodontitis Using Two Disinfection Protocols. BMC Oral Health 2023, 23, 163. [Google Scholar] [CrossRef]
- Ahmed, Y.E.; Ahmed, G.M.; Ghoneim, A.G. Evaluation of Postoperative Pain and Healing Following Regenerative Endodontics Using Platelet-rich Plasma versus Conventional Endodontic Treatment in Necrotic Mature Mandibular Molars with Chronic Periapical Periodontitis. A Randomized Clinical Trial. Int. Endod. J. 2023, 56, 404–418. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Lin, Y.; Xu, X.; Chen, Z.; Xiang, Y.; Yang, L.; Zhang, W.; Xiao, S.; Chen, X. Clinical Observation of Autologous Platelet Rich Fibrin Assisted Revascularization of Mature Permanent Teeth. Head Face Med. 2023, 19, 9. [Google Scholar] [CrossRef]
- Li, J.; Zheng, L.; Daraqel, B.; Liu, J.; Hu, Y. Treatment Outcome of Regenerative Endodontic Procedures for Necrotic Immature and Mature Permanent Teeth: A Systematic Review and Meta-Analysis Based on Randomised Controlled Trials. Oral Health Prev. Dent. 2023, 21, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.-Y.; Tan, Y.; Peng, Q.; Zuo, J.; Li, N. Novel Applications of Platelet Concentrates in Tissue Regeneration (Review). Exp. Ther. Med. 2021, 21, 226. [Google Scholar] [CrossRef] [PubMed]
- Cecerska-Heryć, E.; Goszka, M.; Serwin, N.; Roszak, M.; Grygorcewicz, B.; Heryć, R.; Dołęgowska, B. Applications of the Regenerative Capacity of Platelets in Modern Medicine. Cytokine Growth Factor. Rev. 2022, 64, 84–94. [Google Scholar] [CrossRef]
- Niemczyk, W.; Kępa, M.; Żurek, J.; Aboud, A.; Skaba, D.; Wiench, R. Comparative Evaluation of Platelet-Rich Fibrin (PRF) and Concentrated Growth Factor (CGF) as Carriers for Antibiotics—In Vitro Study. Int. J. Mol. Sci. 2025, 26, 4303. [Google Scholar] [CrossRef]
- Niemczyk, W.; Żurek, J.; Niemczyk, S.; Kępa, M.; Zięba, N.; Misiołek, M.; Wiench, R. Antibiotic-Loaded Platelet-Rich Fibrin (AL-PRF) as a New Carrier for Antimicrobials: A Systematic Review of In Vitro Studies. Int. J. Mol. Sci. 2025, 26, 2140. [Google Scholar] [CrossRef]
- Hoveizi, E.; Naddaf, H.; Ahmadianfar, S.; Bernardi, S. Using Odontoblasts Derived from Dog Endometrial Stem Cells Encapsulated in Fibrin Gel Associated with BMP-2 in a Rat Pulp-Capping Model. Curr. Issues Mol. Biol. 2023, 45, 2984–2999. [Google Scholar] [CrossRef]
- Singh, R.K.; Shakya, V.K.; Khanna, R.; Singh, B.P.; Jindal, G.; Kirubakaran, R.; Sequeira-Byron, P. Interventions for Managing Immature Permanent Teeth with Necrotic Pulps. Cochrane Database Syst. Rev. 2017, 2017, CD012709. [Google Scholar] [CrossRef]
- Wei, X.; Yang, M.; Yue, L.; Huang, D.; Zhou, X.; Wang, X.; Zhang, Q.; Qiu, L.; Huang, Z.; Wang, H.; et al. Expert Consensus on Regenerative Endodontic Procedures. Int. J. Oral Sci. 2022, 14, 55. [Google Scholar] [CrossRef]
- Law, A.S. Considerations for Regeneration Procedures. J. Endod. 2013, 39, S44–S56. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.; Nathwani, S.; Bunyan, R. Autotransplantation of Teeth: An Evidence-Based Approach. Br. Dent. J. 2018, 224, 861–864. [Google Scholar] [CrossRef]
- Patel, S.; Krastl, G.; Weiger, R.; Lambrechts, P.; Tjäderhane, L.; Gambarini, G.; Teng, P. ESE Position Statement on Root Resorption. Int. Endod. J. 2023, 56, 792–801. [Google Scholar] [CrossRef]
- Clauder, T. Present Status and Future Directions—Managing Perforations. Int. Endod. J. 2022, 55, 872–891. [Google Scholar] [CrossRef] [PubMed]
- Dikensoy, O. Importance of Reading and Publishing Case Reports. Turk. Thorac. J. 2019, 20, 265–266. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.; Godfrey, C.; Mcinerney, P.; Munn, Z.; Trico, A.; Khalil, H. Chapter 11: Scoping Reviews. In JBI Manual for Evidence Synthesis; Aromataris, E., Munn, Z., Eds.; JBI: Adelaide, Australia, 2020. [Google Scholar]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Booth, A. Searching for Qualitative Research for Inclusion in Systematic Reviews: A Structured Methodological Review. Syst. Rev. 2016, 5, 74. [Google Scholar] [CrossRef]

| n | % | |
|---|---|---|
| STUDY TYPE | ||
| Case report | 93 | 22.52% |
| Narrative review | 66 | 13.98% |
| Randomized controlled trial | 62 | 15.01% |
| Randomized controlled trial protocol | 52 | 12.59% |
| Systematic review | 42 | 10.17% |
| Animal | 34 | 8.23% |
| Case-series | 27 | 6.54% |
| In vitro | 24 | 5.81% |
| Cohort | 5 | 1.21% |
| Case-control | 3 | 0.73% |
| Scoping review | 3 | 0.73% |
| Umbrella review | 2 | 0.48% |
| Total (Studies) | 413 | 100% |
| TYPE OF PROCEDURE | ||
| Regeneration of immature teeth | 185 | 44.90% |
| Apical surgery | 80 | 19.42% |
| Pulpotomy/Pulp capping | 56 | 13.59% |
| Regeneration of mature teeth | 39 | 9.47% |
| Biological properties evaluation | 38 | 9.22% |
| Apexification | 20 | 4.85% |
| Endo-perio lesions | 16 | 3.88% |
| Intentional reimplantation | 13 | 3.16% |
| Perforation | 10 | 2.43% |
| Resorption | 5 | 1.21% |
| Root fracture | 3 | 0.73% |
| Auto transplant | 2 | 0.49% |
| Mechanical properties evaluation | 2 | 0.49% |
| Total (Articles) | 412 | 100% |
| TOP 10 JOURNALS | ||
| Journal of Endodontics | 55 | 13.35% |
| International Endodontic Journal | 20 | 4.85% |
| Journal of Conservative Dentistry | 19 | 4.61% |
| Journal of Clinical and Diagnostic Research | 17 | 3.13% |
| Contemporary Clinical Dentistry | 10 | 2.43% |
| International Journal of Pediatric Dentistry | 8 | 1.94% |
| Restorative Dentistry and Endodontics | 8 | 1.94% |
| The Journal of Contemporary Dental Practice | 7 | 1.70% |
| Indian Journal of Dental Research | 7 | 1.70% |
| BMC Oral Health | 6 | 1.46% |
| Total (Articles) | 412 | 100% |
| TOP 10 FIRST AUTHORS | ||
| Johns, D. A. | 4 | 0.97% |
| Jadhav, G.R. | 4 | 0.97% |
| Torabinejad, M. | 4 | 0.97% |
| Meschi, N. | 4 | 0.97% |
| Chen, Y. | 3 | 0.73% |
| Alawwad, M. | 3 | 0.73% |
| Bezgin, T. | 3 | 0.73% |
| Shivashankar, V. Y. | 3 | 0.73% |
| Nagaraja, S. | 3 | 0.73% |
| Hiremath, H. | 3 | 0.73% |
| Total (Articles) | 412 | 100% |
| PUBLICATION YEAR | ||
| 2023 | 41 | 9.95% |
| 2022 | 53 | 12.86% |
| 2021 | 43 | 10.44% |
| 2020 | 56 | 13.59% |
| 2019 | 31 | 7.52% |
| 2018 | 21 | 5.10% |
| 2017 | 34 | 8.25% |
| 2016 | 34 | 8.25% |
| 2015 | 27 | 6.55% |
| 2014 | 21 | 5.01% |
| 2013 | 21 | 5.01% |
| 2012 | 13 | 3.16% |
| 2011 | 9 | 2.18% |
| 2010 | 3 | 0.73% |
| 2009 | 3 | 0.73% |
| 2008 | 1 | 0.24% |
| 2007 | 1 | 0.24% |
| 2006 | 0 | 0% |
| 2005 | 0 | 0% |
| 2004 | 1 | 0.24% |
| Total (Articles) | 412 | 100% |
| PCC | |
|---|---|
| Population | Patients and experimental models of endodontic treatment |
| Concept | PRF and PRP application |
| Context | Endodontic treatment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rebimbas Guerreiro, S.; Marto, C.M.; Paula, A.; Pereira, J.R.d.A.; Carrilho, E.; Marques-Ferreira, M.; Vicente Paulo, S. Platelet-Rich Plasma and Platelet-Rich Fibrin in Endodontics: A Scoping Review. Int. J. Mol. Sci. 2025, 26, 5479. https://doi.org/10.3390/ijms26125479
Rebimbas Guerreiro S, Marto CM, Paula A, Pereira JRdA, Carrilho E, Marques-Ferreira M, Vicente Paulo S. Platelet-Rich Plasma and Platelet-Rich Fibrin in Endodontics: A Scoping Review. International Journal of Molecular Sciences. 2025; 26(12):5479. https://doi.org/10.3390/ijms26125479
Chicago/Turabian StyleRebimbas Guerreiro, Simão, Carlos Miguel Marto, Anabela Paula, Joana Rita de Azevedo Pereira, Eunice Carrilho, Manuel Marques-Ferreira, and Siri Vicente Paulo. 2025. "Platelet-Rich Plasma and Platelet-Rich Fibrin in Endodontics: A Scoping Review" International Journal of Molecular Sciences 26, no. 12: 5479. https://doi.org/10.3390/ijms26125479
APA StyleRebimbas Guerreiro, S., Marto, C. M., Paula, A., Pereira, J. R. d. A., Carrilho, E., Marques-Ferreira, M., & Vicente Paulo, S. (2025). Platelet-Rich Plasma and Platelet-Rich Fibrin in Endodontics: A Scoping Review. International Journal of Molecular Sciences, 26(12), 5479. https://doi.org/10.3390/ijms26125479







