The Effect of Coronary Artery Bypass Surgery on Interleukin-18 Concentration and Biomarkers Related to Vascular Endothelial Glycocalyx Degradation
Abstract
1. Introduction
2. Results
2.1. Demographic and Clinical Data of Patients
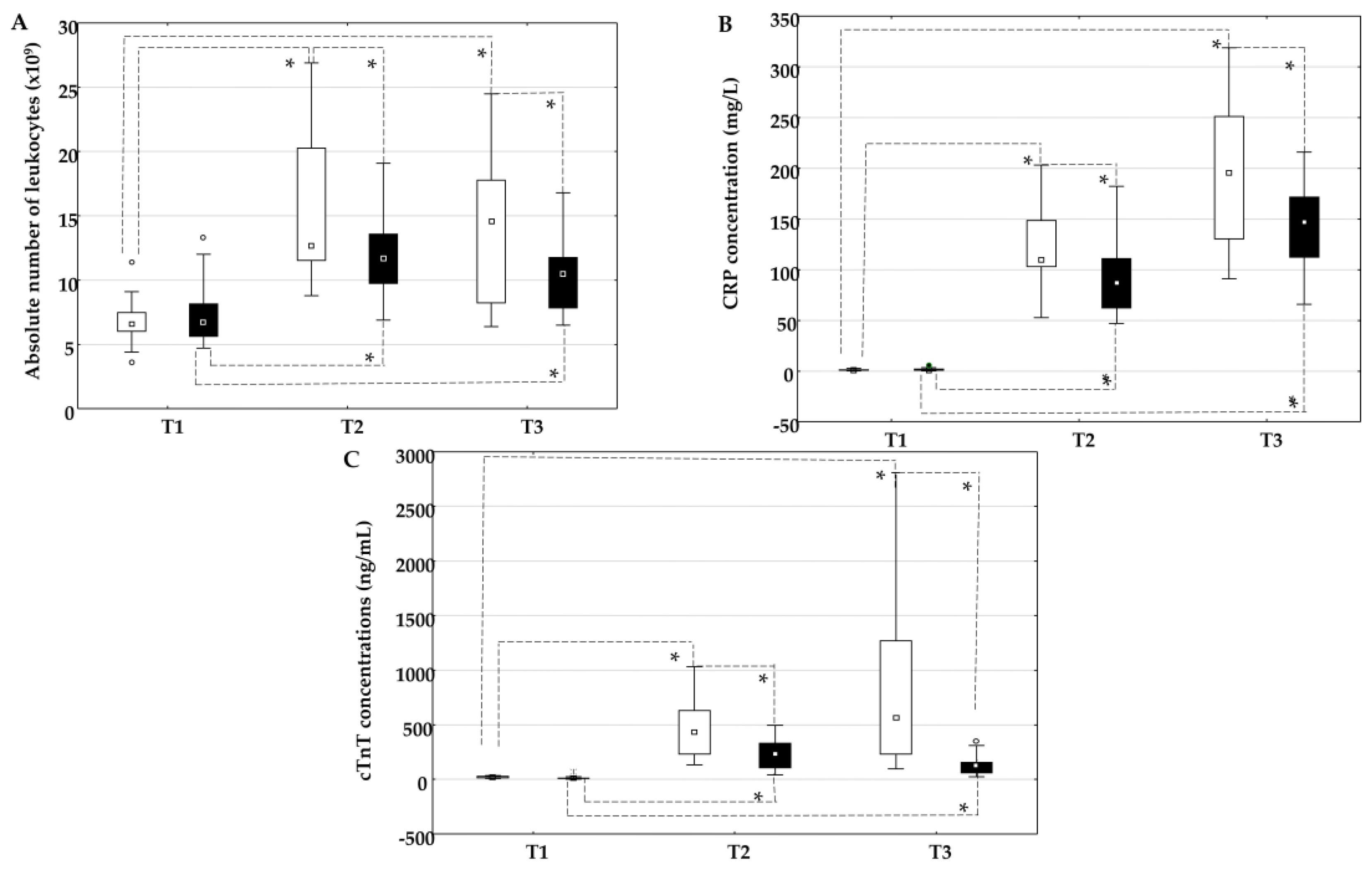
2.2. The Influence of On-Pump and Off-Pump Coronary Artery Bypass Surgery on the Absolute Number of Leukocytes, the Concentration of C-Reactive Protein, and Cardiac Troponin T
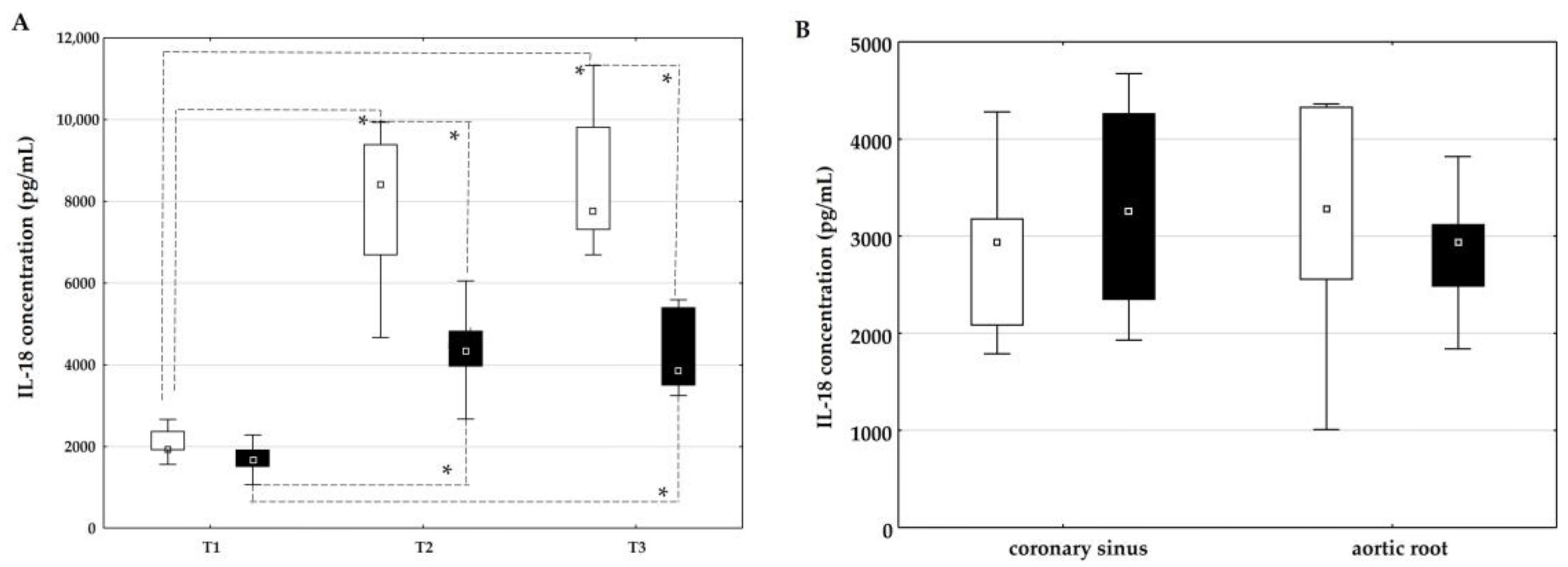
2.3. The Influence of On-Pump and Off-Pump Coronary Artery Bypass Surgery on the Concentration of IL-18
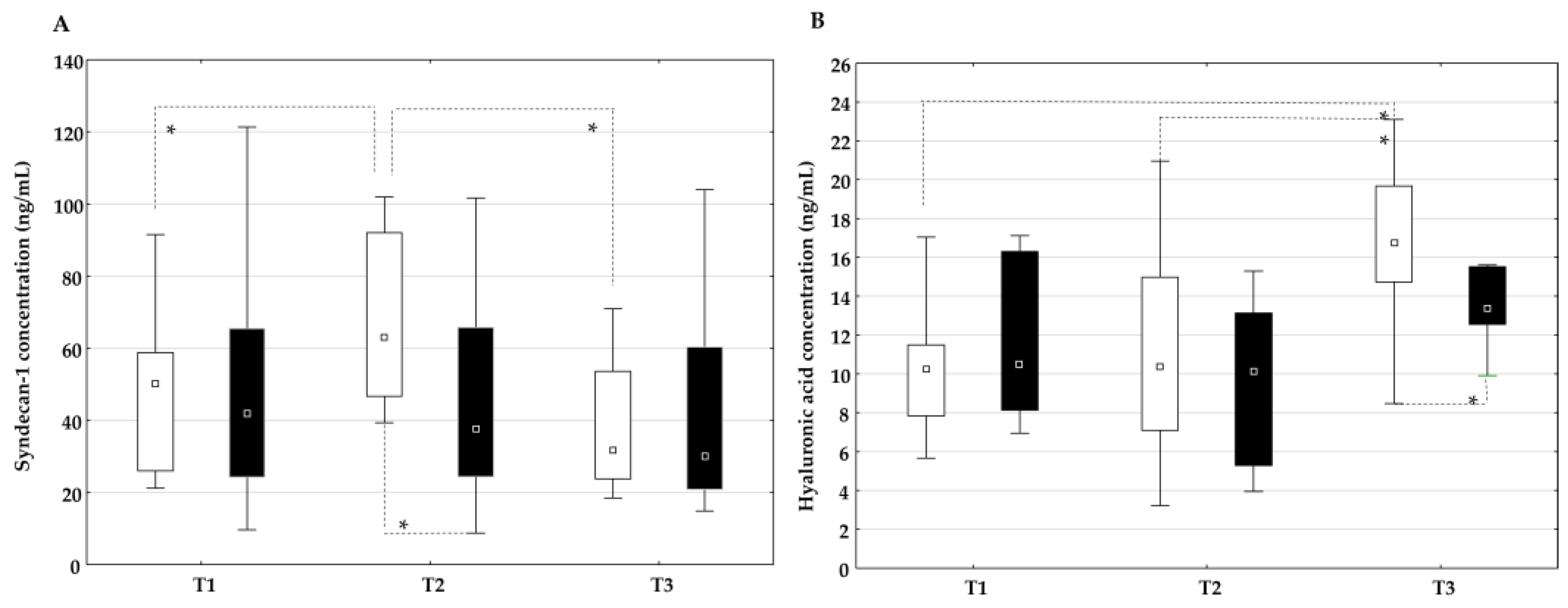
2.4. The Influence of On-Pump and Off-Pump Coronary Artery Bypass Surgery on Endothelial Glycocalyx Degradation Products
2.5. Correlation Between the Plasma Concentration of IL-18 and Markers of the Inflammatory Response, Myocardial Injury, and Endothelial Glycocalyx Degradation Products
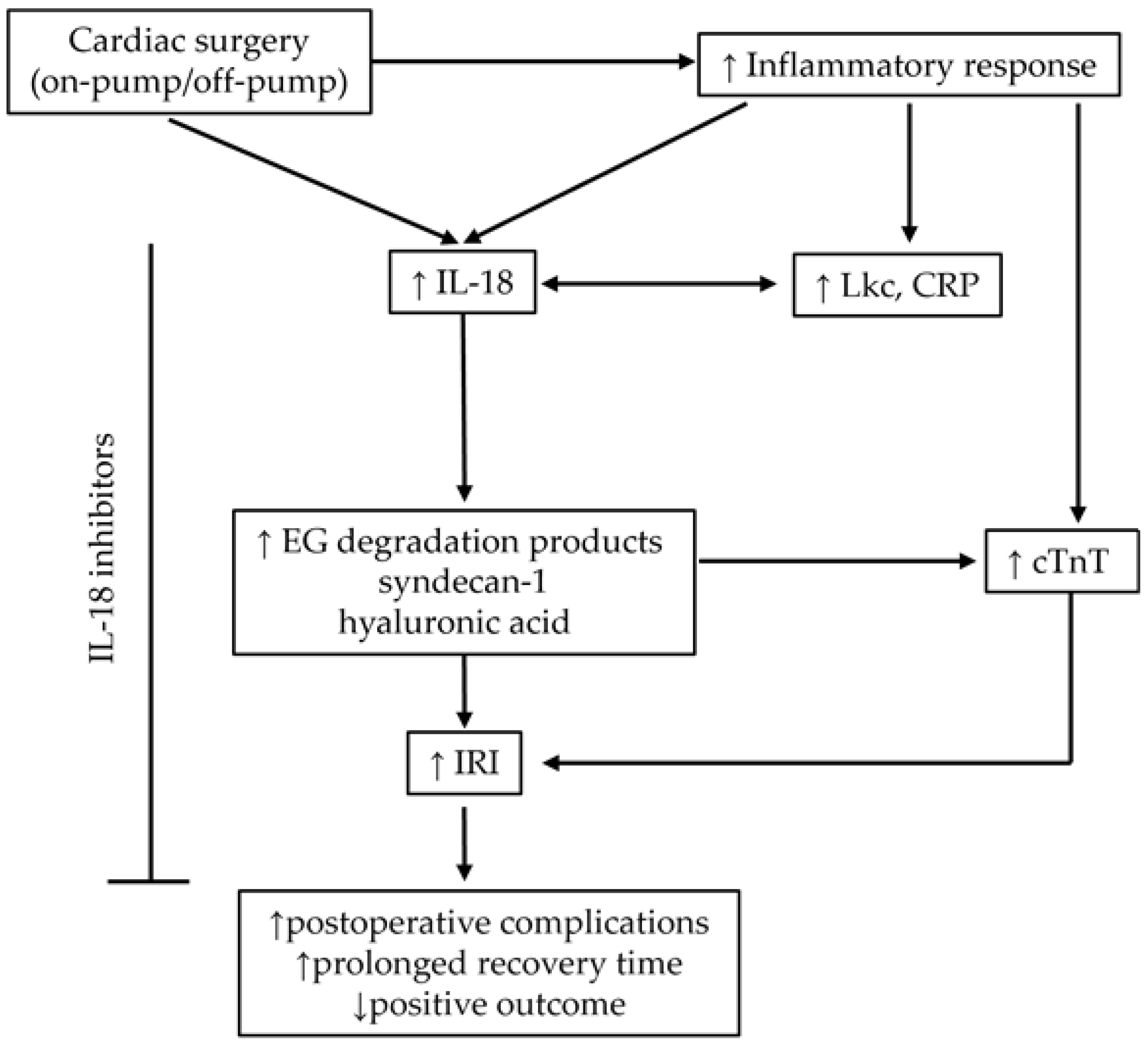
3. Discussion
3.1. Cardiac Surgery and IL-18
3.2. Diagnostic, Prognostic, and Potential Therapeutic Implications of Studied Biomarkers
3.3. Cardiac Surgery and Endothelial Glycocalyx
3.4. Study Limitations
3.5. Future Perspectives of IL-18 in Cardiac Surgery
4. Materials and Methods
4.1. Patients
4.2. Anesthesia and Surgical Procedure
4.3. Blood Collection
4.4. Determination of the Absolute Number of Leukocytes, the Concentration of C-Reactive Protein, and the Concentration of Troponin
4.5. Determination of the Plasma Concentration of Interleukin-18, Syndecan-1, and Hyaluronic Acid
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CABG | Coronary artery bypass grafting |
| CRP | C-reactive protein |
| ED | Endothelial dysfunction |
| EG | Endothelial glycocalyx |
| ICU | Intensive care unit |
| IL | Interleukin |
| IRI | Ischemia–reperfusion injury |
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Theroux, P. Pathophysiology of coronary artery disease. Circulation 2005, 111, 3481–3488. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef]
- Wenger, N.K.; Boden, W.E.; Carabello, B.A.; Carney, R.M.; Cerqueira, M.D.; Criqul, M.H.; Epstein, A.E.; Froelicher, E.S.; Gibbons, G.H.; Hlatky, M.A.; et al. Cardiovascular Disability: Updating the Social Security Listings; National Academies Press (US): Washington, DC, USA, 2010. Available online: https://www.ncbi.nlm.nih.gov/books/NBK209984/ (accessed on 23 March 2025).
- Stevens, J.R.; Zamani, A.; Osborne, J.I.A.; Zamani, R.; Akrami, M. Critical evaluation of stents in coronary angioplasty: A systematic review. Biomed. Eng. Online. 2021, 20, 46. [Google Scholar] [CrossRef] [PubMed]
- Shaefi, S.; Mittel, A.; Loberman, D.; Ramakrishna, H. Off-Pump Versus On-Pump Coronary Artery Bypass Grafting: A Systematic Review and Analysis of Clinical Outcomes. J. Cardiothorac. Vasc. Anesth. 2019, 33, 232–244. [Google Scholar] [CrossRef]
- King, N. On vs. off pump coronary artery bypass grafting: The next chapter. Ann. Transl. Med. 2017, 5, 116. [Google Scholar] [CrossRef]
- Quin, J.A.; Wagner, T.H.; Hattler, B.; Carr, B.M.; Collins, J.; Almassi, G.H.; Grover, F.L.; Shroyer, A.L. Ten-Year Outcomes of Off-Pump vs. On-Pump Coronary Artery By-pass Grafting in the Department of Veterans Affairs: A Randomised Clinical Trial. JAMA. Surg. 2022, 157, 303–310. [Google Scholar] [CrossRef]
- Shroyer, A.L.; Grover, F.L.; Hattler, B.; Collins, J.F.; McDonald, G.O.; Kozora, E.; Lucke, J.C.; Baltz, J.H.; Novitzky, D. Veterans Affairs Randomized On/Off Bypass (ROOBY) Study Group. On-pump versus off-pump coronary-artery bypass surgery. N. Engl. J. Med. 2009, 361, 1827–1837. [Google Scholar] [CrossRef]
- Chikwe, J.; Lee, T.; Itagaki, S.; Adams, D.H.; Egorova, N.N. Long-Term Outcomes After Off-Pump Versus On-Pump Coronary Artery Bypass Grafting by Experienced Surgeons. J. Am. Coll. Cardiol. 2018, 72, 1478–1486. [Google Scholar] [CrossRef]
- Benedetto, U.; Puskas, J.; Kappetein, A.P.; Brown, W.M., 3rd; Horkay, F.; Boonstra, P.W.; Bogáts, G.; Noiseux, N.; Dressler, O.; Angelini, G.D.; et al. Off-Pump Versus On-Pump Bypass Surgery for Left Main Coronary Artery Disease. J. Am. Coll. Cardiol. 2019, 74, 729–740. [Google Scholar] [CrossRef]
- Khan, M.S.; Islam, M.Y.; Ahmed, M.U.; Bawany, F.I.; Khan, A.; Arshad, M.H. On pump coronary artery bypass graft surgery versus off pump coronary artery bypass graft surgery: A review. Glob. J. Health Sci. 2014, 6, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e4–e17. [Google Scholar] [CrossRef]
- Yang, Q.; He, G.W.; Underwood, M.J.; Yu, C.M. Cellular and molecular mechanisms of endothelial ischemia/reperfusion injury: Perspectives and implications for postischemic myocardial protection. Am. J. Transl. Res. 2016, 8, 765–777. [Google Scholar] [PubMed]
- Tong, C.; Zhou, B. Cardioprotective strategies in myocardial ischemia-reperfusion injury: Implications for improving clinical translation. J. Mol. Cell. Cardiol. Plus. 2024, 11, 100278. [Google Scholar] [CrossRef] [PubMed]
- Köse, S.K.; Karahilal, B.; Engin, B.; Aydoğdu, G.; Yağar, S.; Orhan, K. Relationships between Interleukin 18 -607 C/A and -137 G/C, Osteopontin -9250 C/T Genetic Polymorphisms and Systemic Inflammatory Response Syndrome in Coronary Artery Bypass Graft Surgery. Medicina 2024, 60, 724. [Google Scholar] [CrossRef]
- Weymann, A.; Popov, A.F.; Sabashnikov, A.; Ali-Hasan-Al-Saegh, S.; Ryazanov, M.; Tse, G.; Mirhosseini, S.J.; Liu, T.; Lotfaliani, M.; Sedaghat, M.; et al. Baseline and postoperative levels of C-reactive protein and interleukins as inflammatory predictors of atrial fibrillation following cardiac surgery: A systematic review and meta-analysis. Kardiol. Pol. 2018, 76, 440–451. [Google Scholar] [CrossRef]
- Everett, A.D.; Alam, S.S.; Owens, S.L.; Parker, D.M.; Goodrich, C.; Likosky, D.S.; Thiessen-Philbrook, H.; Wyler von Ballmoos, M.; Lobdell, K.; MacKenzie, T.A.; et al. The Association between Cytokines and 365-Day Readmission or Mortality in Adult Cardiac Surgery. J. ExtraCorpor. Technol. 2019, 51, 201–209. [Google Scholar] [CrossRef]
- Nathan, N.; Preux, P.M.; Feiss, P.; Denizot, Y. Plasma interleukin-4, interleukin-10, and interleukin-13 concentrations and complications after coronary artery bypass graft surgery. J. Cardiothorac. Vasc. Anesth. 2000, 14, 156–160. [Google Scholar] [CrossRef]
- Thompson, S.R.; Novick, D.; Stock, C.J.; Sanders, J.; Brull, D.; Cooper, J.; Woo, P.; Miller, G.; Rubinstein, M.; Humphries, S.E. Free Interleukin (IL)-18 levels, and the impact of IL18 and IL18BP genetic variation, in CHD patients and healthy men. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2743–2749. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Prabhu, S.D.; Reddy, V.S.; Boylston, W.H.; Valente, A.J.; Chandrasekar, B. Neutralization of interleukin-18 ameliorates ischemia/reperfusion-induced myocardial injury. J. Biol. Chem. 2009, 284, 7853–7865. [Google Scholar] [CrossRef]
- Kawasaki, D.; Tsujino, T.; Morimoto, S.; Masai, M.; Masutani, M.; Ohyanagi, M.; Kashiwamura, S.; Okamura, H.; Masuyama, T. Plasma interleukin-18 concentration: A novel marker of myocardial ischemia rather than necrosis in humans. Coron. Artery Dis. 2005, 16, 437–441. [Google Scholar] [CrossRef]
- Hartford, M.; Wiklund, O.; Hultén, L.M.; Persson, A.; Karlsson, T.; Herlitz, J.; Hulthe, J.; Caidahl, K. Interleukin-18 as a predictor of future events in patients with acute coronary syndromes. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2039–2046. [Google Scholar] [CrossRef]
- Koch, W.; Wolferstetter, H.; Schatke, A.; Schömig, A.; Kastrati, A. Interleukin 18 gene variation and risk of acute myocardial infarction. Cytokine 2011, 56, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Fix, C.; Bingham, K.; Carver, W. Effects of interleukin-18 on cardiac fibroblast function and gene expression. Cytokine 2011, 53, 19–28. [Google Scholar] [CrossRef]
- O’Brien, L.C.; Mezzaroma, E.; Van Tassell, B.W.; Marchetti, C.; Carbone, S.; Abbate, A.; Toldo, S. Interleukin-18 as a therapeutic target in acute myocardial infarction and heart failure. Mol. Med. 2014, 20, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Ding, J.; Hong, R.; Bai, S.; Wang, Z.; Mo, C.; Hu, Q.; Li, Z.; Guan, Y. Increased interleukin-18 level contributes to the development and severity of ischemic stroke. Aging 2019, 11, 7457–7472. [Google Scholar] [CrossRef]
- Zakkar, M.; Ascione, R.; James, A.F.; Angelini, G.D.; Suleiman, M.S. Inflammation, oxidative stress and postoperative atrial fibrillation in cardiac surgery. Pharmacol. Ther. 2015, 154, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekar, B.; Vemula, K.; Surabhi, R.M.; Li-Weber, M.; Owen Schaub, L.B.; Jensen, L.E.; Mummidi, S. Activation of intrinsic and extrinsic proapoptotic signaling pathways in interleukin-18-mediated human cardiac endothelial cell death. J. Biol. Chem. 2004, 279, 20221–20233. [Google Scholar] [CrossRef]
- Dri, E.; Lampas, E.; Lazaros, G.; Lazarou, E.; Theofilis, P.; Tsioufis, C.; Tousoulis, D. Inflammatory Mediators of Endothelial Dysfunction. Life 2023, 20, 1420. [Google Scholar] [CrossRef]
- Brettner, F.; Chappell, D.; Schwartz, L.; Lukasz, A.; Kümpers, P.; Becker, B.F.; Reichart, B.; Rehm, M.; Bruegger, D. Vascular Endothelial Dysfunction during Cardiac Surgery: On-Pump versus Off-Pump Coronary Surgery. Eur. Surg. Res. 2017, 58, 354–368. [Google Scholar] [CrossRef]
- Hadi, H.A.; Carr, C.S.; Al Suwaidi, J. Endothelial dysfunction: Cardiovascular risk factors, therapy, and outcome. Vasc. Health Risk Manag. 2005, 1, 183–198. [Google Scholar] [PubMed]
- Pisano, C.; Asta, L.; Sbrigata, A.; Balistreri, C.R. A Narrative Review: Syndecans in Aortic Aneurysm Pathogenesis and Course-Biomarkers and Targets? Int. J. Mol. Sci. 2025, 26, 1211. [Google Scholar] [CrossRef]
- Milusev, A.; Rieben, R.; Sorvillo, N. The Endothelial Glycocalyx: A Possible Therapeutic Target in Cardiovascular Disorders. Front. Cardiovasc. Med. 2022, 9, 897087. [Google Scholar] [CrossRef] [PubMed]
- Karampoor, S.; Zahednasab, H.; Farahmand, M.; Mirzaei, R.; Zamani, F.; Tabibzadeh, A.; Bouzari, B.; Ajdarkosh, H.; Nikkhah, M.; Hashemi, M.R.; et al. A possible pathogenic role of Syndecan-1 in the pathogenesis of coronavirus disease 2019 (COVID-19). Int. Immunopharmacol. 2021, 97, 107684. [Google Scholar] [CrossRef]
- Spiess, B.D. Heparin: Effects upon the Glycocalyx and Endothelial Cells. J. ExtraCorpor. Technol. 2017, 49, 192–197. [Google Scholar] [CrossRef]
- Villalba, N.; Baby, S.; Yuan, S.Y. The Endothelial Glycocalyx as a Double-Edged Sword in Microvascular Homeostasis and Pathogenesis. Front. Cell. Dev. Biol. 2021, 9, 711003. [Google Scholar] [CrossRef]
- Dogné, S.; Flamion, B. Endothelial Glycocalyx Impairment in Disease: Focus on Hyaluronan Shedding. Am. J. Pathol. 2020, 190, 768–780. [Google Scholar] [CrossRef]
- Erickson, M.; Stern, R. Chain gangs: New aspects of hyaluronan metabolism. Biochem. Res. Int. 2012, 2012, 893947. [Google Scholar] [CrossRef]
- Goodall, K.J.; Poon, I.K.; Phipps, S.; Hulett, M.D. Soluble heparan sulfate fragments generated by heparanase trigger the release of pro-inflammatory cytokines through TLR-4. PLoS ONE 2014, 9, e109596. [Google Scholar] [CrossRef]
- Passov, A.; Schramko, A.; Salminen, U.S.; Aittomäki, J.; Andersson, S.; Pesonen, E. Endothelial glycocalyx during early reperfusion in patients undergoing cardiac surgery. PLoS ONE 2021, 16, e0251747. [Google Scholar] [CrossRef]
- Abraham, A.S.; Elliott, C.W.; Abraham, M.S.; Ahuja, S. Intra-operative anesthetic induced myocardial protection during cardiothoracic surgery: A literature review. J. Thorac. Dis. 2023, 15, 7042–7049. [Google Scholar] [CrossRef] [PubMed]
- Squiccimarro, E.; Stasi, A.; Lorusso, R.; Paparella, D. Narrative review of the systemic inflammatory reaction to cardiac surgery and cardiopulmonary bypass. Artif. Organs 2022, 46, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Fatehi Hassanabad, A.; Schoettler, F.I.; Kent, W.D.T.; Adams, C.A.; Holloway, D.D.; Ali, I.S.; Novick, R.J.; Ahsan, M.R.; McClure, R.S.; Shanmugam, G.; et al. Cardiac surgery elicits pericardial inflammatory responses that are distinct compared with postcardiopulmonary bypass systemic inflammation. JTCVS Open 2023, 16, 389–400. [Google Scholar] [CrossRef]
- Pfeiler, S.; Winkels, H.; Kelm, M.; Gerdes, N. IL-1 family cytokines in cardiovascular disease. Cytokine 2019, 122, 154215. [Google Scholar] [CrossRef]
- Parolari, A.; Poggio, P.; Myasoedova, V.; Songia, P.; Bonalumi, G.; Pilozzi, A.; Pacini, D.; Alamanni, F.; Tremoli, E. Biomarkers in Coronary Artery Bypass Surgery: Ready for Prime Time and Outcome Prediction? Front. Cardiovasc. Med. 2016, 2, 39. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Zhang, H.; Zhu, S.; Wang, S.; Zhu, K.; Zhao, Y.; Xu, L.; Zhang, P.; Xie, J.; Sun, A.; et al. Interleukin-18 accelerates cardiac inflammation and dysfunction during ischemia/reperfusion injury by transcriptional activation of CXCL16. Cell. Signal. 2021, 87, 110141. [Google Scholar] [CrossRef]
- Kaplanski, G. Interleukin-18: Biological properties and role in disease pathogenesis. Immunol. Rev. 2018, 281, 138–153. [Google Scholar] [CrossRef]
- Ji, Q.; Zeng, Q.; Huang, Y.; Shi, Y.; Lin, Y.; Lu, Z.; Meng, K.; Wu, B.; Yu, K.; Chai, M.; et al. Elevated plasma IL-37, IL-18, and IL-18BP concentrations in patients with acute coronary syndrome. Mediators Inflamm. 2014, 2014, 165742. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Executive Group on Behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition on Myocardial Infarction. Fourth universal definition of myocardial infarction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef]
- Åkerblom, A.; James, S.K.; Lakic, T.G.; Becker, R.C.; Cannon, C.P.; Steg, P.G.; Himmelmann, A.; Katus, H.A.; Storey, R.F.; Wallentin, L.; et al. PLATO Investigators. Interleukin-18 in patients with acute coronary syndromes. Clin. Cardiol. 2019, 42, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Tan, J.; Wang, Y.; Meldrum, K.K.; Dinarello, C.A.; Meldrum, D.R. IL-18 Binding Protein-Expressing Mesenchymal Stem Cells Improve Myocardial Protection after Ischemia or Infarction. Proc. Natl. Acad. Sci. USA 2009, 106, 17499–17504. [Google Scholar] [CrossRef] [PubMed]
- Lurati Buse, G.A.; Koller, M.T.; Grapow, M.; Bolliger, D.; Seeberger, M.; Filipovic, M. The prognostic value of troponin release after adult cardiac surgery—A meta-analysis. Eur. J. Cardiothorac. Surg. 2010, 37, 399–406. [Google Scholar] [CrossRef]
- Paparella, D.; Guida, P.; Caparrotti, S.; Fanelli, V.; Martinelli, G.; Mazzei, V.; Zaccaria, S.; Bisceglia, L.; Scrascia, G. Myocardial damage influences short- and mid-term survival after valve surgery: A prospective multicenter study. J. Thorac. Cardiovasc. Surg. 2014, 148, 2373–2379. [Google Scholar] [CrossRef]
- Domanski, M.J.; Mahaffey, K.; Hasselblad, V.; Brener, S.J.; Smith, P.K.; Hillis, G.; Engoren, M.; Alexander, J.H.; Levy, J.H.; Chaitman, B.R.; et al. Association of myocardial enzyme elevation and survival following coronary artery bypass graft surgery. JAMA 2011, 305, 585–591. [Google Scholar] [CrossRef]
- Devereaux, P.J.; Lamy, A.; Chan, M.T.V.; Allard, R.V.; Lomivorotov, V.V.; Landoni, G.; Zheng, H.; Paparella, D.; McGillion, M.H.; Belley-Côté, E.P.; et al. High-Sensitivity Troponin I after Cardiac Surgery and 30-Day Mortality. N. Engl. J. Med. 2022, 386, 827–836. [Google Scholar] [CrossRef]
- Jin, J.; Fang, F.; Gao, W.; Chen, H.; Wen, J.; Wen, X.; Wen, X.; Chen, J. The Structure and Function of the Glycocalyx and Its Connection With Blood-Brain Barrier. Front. Cell. Neurosci. 2021, 15, 739699. [Google Scholar] [CrossRef]
- Patterson, E.K.; Cepinskas, G.; Fraser, D.D. Endothelial Glycocalyx Degradation in Critical Illness and Injury. Front. Med. 2022, 9, 898592. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.O.; Vasconcelos, V.W.; Lima, J.D.S.; Vieira Neto, J.R.; da Costa, G.E.; Esteves, J.C.; de Sousa, S.C.; Moura, J.A.; Santos, F.R.S.; Leitão Filho, J.M.; et al. Biochemical Changes in Cardiopulmonary Bypass in Cardiac Surgery: New In-sights. J. Pers. Med. 2023, 13, 1506. [Google Scholar] [CrossRef] [PubMed]
- Wahba, A.; Milojevic, M.; Boer, C.; De Somer, F.M.J.J.; Gudbjartsson, T.; van den Goor, J.; Jones, T.J.; Lomivorotov, V.; Merkle, F.; Ranucci, M.; et al. 2019 EACTS/EACTA/EBCP guidelines on cardiopulmonary bypass in adult cardiac surgery. Eur. J. Cardiothorac. Surg. 2020, 57, 210–251. [Google Scholar] [CrossRef]
- Qu, J.; Cheng, Y.; Wu, W.; Yuan, L.; Liu, X. Glycocalyx Impairment in Vascular Disease: Focus on Inflammation. Front. Cell Dev. Biol. 2021, 9, 730621. [Google Scholar] [CrossRef]
- Byrne, R.A.; Fremes, S.; Capodanno, D.; Czerny, M.; Doenst, T.; Emberson, J.R.; Falk, V.; Gaudino, M.; McMurray, J.J.V.; Mehran, R.; et al. 2022 Joint ESC/EACTS review of the 2018 guideline recommendations on the revascularization of left main coronary artery disease in patients at low surgical risk and anatomy suitable for PCI or CABG. Eur. J. Cardiothorac. Surg. 2023, 64, ezad286. [Google Scholar] [CrossRef] [PubMed]

| On-Pump Group (n = 30) | Off-Pump Group (n = 30) | p Value | |
|---|---|---|---|
| Age (years) | 68.4 (59–74.5) | 67 (63–69) | 0.47 |
| Sex (men/women) | 19/11 | 18/12 | 0.39 |
| Length of mechanical ventilation (hours) | 10 (9–13.1) | 10 (9–11) | 0.14 |
| Length of intensive care unit stay (hours) | 24 (24–48) | 24 (24–36) | 0.71 |
| Outcome (discharged/death) | 30/0 | 30/0 | 1 |
| Correlation | Concentration of IL-18 (pg/mL) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| On-Pump Group | Off-Pump Group | |||||||||||
| T1 | T2 | T3 | T1 | T2 | T3 | |||||||
| r | p | r | p | r | p | r | p | r | p | r | p | |
| Absolute number of leukocytes (×109) | 0.70 | 0.18 | 0.74 | 0.013 | 0.83 | 0.04 | 0.18 | 0.61 | 0.71 | 0.019 | 0.41 | 0.02 |
| C-reactive protein concentration (mg/L) | 0.74 | 0.15 | 0.73 | 0.016 | 0.84 | 0.03 | 0.16 | 0.67 | 0.49 | 0.15 | 0.33 | 0.38 |
| Cardiac troponin T concentration (ng/mL) | 0.40 | 0.59 | 0.89 | 0.41 | 0.71 | 0.15 | 0.07 | 0.84 | 0.19 | 0.06 | 0.64 | 0.16 |
| Syndecan-1 concentration (ng/mL) | 0.57 | 0.31 | 0.82 | 0.022 | 0.73 | 0.01 | 0.37 | 0.31 | 0.42 | 0.22 | 0.05 | 0.89 |
| Hyaluronic acid concentration (ng/mL) | 0.19 | 0.81 | 0.25 | 0.45 | 0.32 | 0.52 | 0.21 | 0.61 | 0.12 | 0.72 | 0.25 | 0.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knežević, D.; Batičić, L.; Ćurko-Cofek, B.; Batinac, T.; Ljubačev, A.; Valenčić Seršić, L.; Laškarin, G.; Zdravković, M.; Šoštarič, M.; Sotošek, V. The Effect of Coronary Artery Bypass Surgery on Interleukin-18 Concentration and Biomarkers Related to Vascular Endothelial Glycocalyx Degradation. Int. J. Mol. Sci. 2025, 26, 5453. https://doi.org/10.3390/ijms26125453
Knežević D, Batičić L, Ćurko-Cofek B, Batinac T, Ljubačev A, Valenčić Seršić L, Laškarin G, Zdravković M, Šoštarič M, Sotošek V. The Effect of Coronary Artery Bypass Surgery on Interleukin-18 Concentration and Biomarkers Related to Vascular Endothelial Glycocalyx Degradation. International Journal of Molecular Sciences. 2025; 26(12):5453. https://doi.org/10.3390/ijms26125453
Chicago/Turabian StyleKnežević, Danijel, Lara Batičić, Božena Ćurko-Cofek, Tanja Batinac, Aleksandra Ljubačev, Lara Valenčić Seršić, Gordana Laškarin, Marko Zdravković, Maja Šoštarič, and Vlatka Sotošek. 2025. "The Effect of Coronary Artery Bypass Surgery on Interleukin-18 Concentration and Biomarkers Related to Vascular Endothelial Glycocalyx Degradation" International Journal of Molecular Sciences 26, no. 12: 5453. https://doi.org/10.3390/ijms26125453
APA StyleKnežević, D., Batičić, L., Ćurko-Cofek, B., Batinac, T., Ljubačev, A., Valenčić Seršić, L., Laškarin, G., Zdravković, M., Šoštarič, M., & Sotošek, V. (2025). The Effect of Coronary Artery Bypass Surgery on Interleukin-18 Concentration and Biomarkers Related to Vascular Endothelial Glycocalyx Degradation. International Journal of Molecular Sciences, 26(12), 5453. https://doi.org/10.3390/ijms26125453






