Sustainable Nanotechnology Strategies for Modulating the Human Gut Microbiota
Abstract
1. Introduction
2. Methodology of the Review
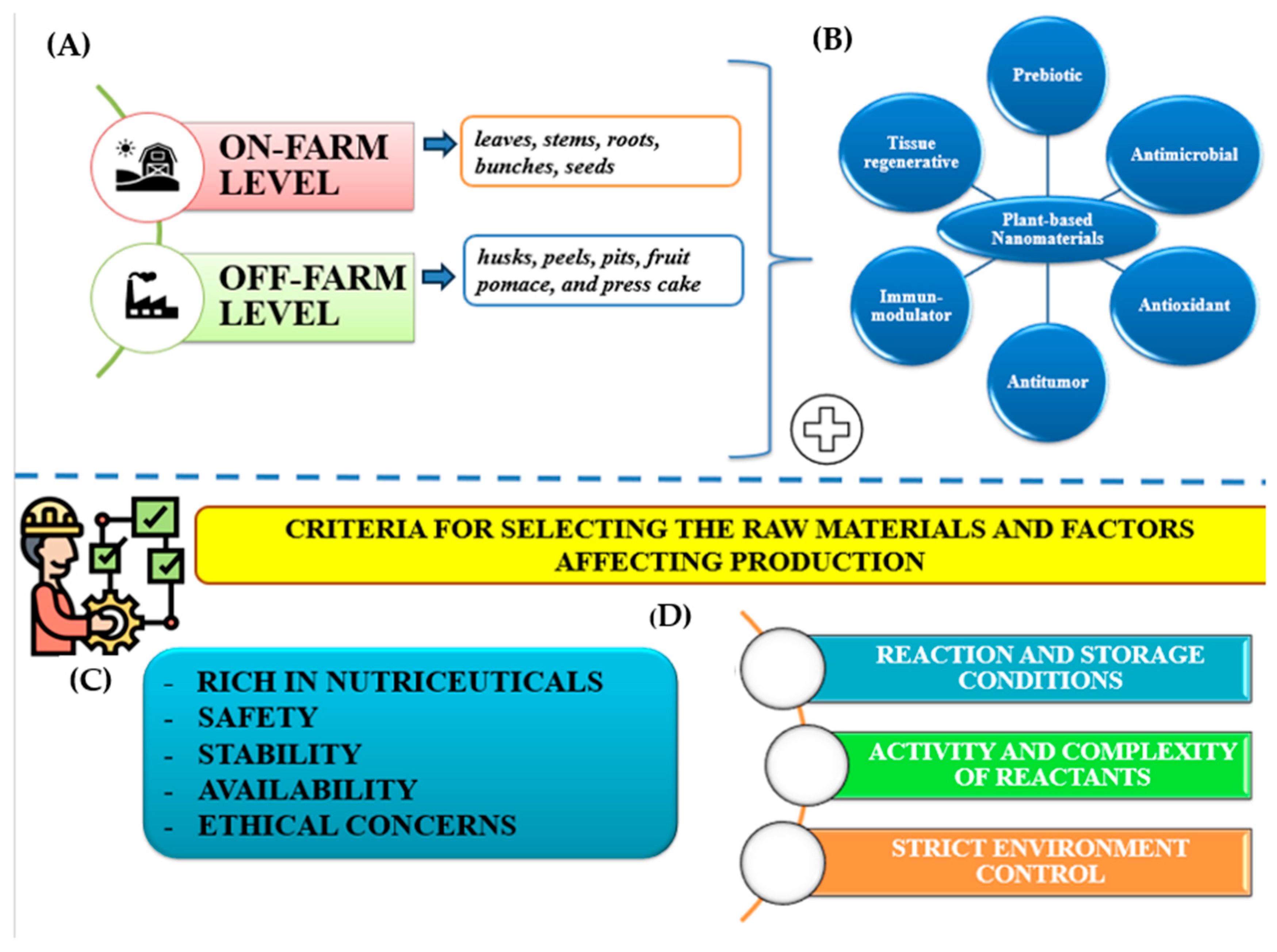
3. The Agro-Journey of Nanotechnology
3.1. Sustainable Agro-Applications of Nanomaterials
3.2. Medicinal and Pharmacological Value of Sustainable Nanomaterials
| Nanoparticles (NPs)/Nanomaterials | Agro-Waste Kind | Suggested Applications | Ref. |
|---|---|---|---|
| Potassium-doped graphene oxide | Oak (Quercus ilex) fruit seeds | Antimicrobial activity | [65] |
| Graphene oxide-nano zero-valent iron | Sugarcane bagasse | Photo-catalytic removal of antibiotics in water | [62] |
| Al2O3 nanocatalyst | Quercus incana L. seeds | Feedstock for producing nanocatalysts and biodiesel | [66] |
| Gold nanoparticles (AuNPs) | Dried plum peel | Antibacterial activity against positive and Gram-negative bacteria | [67] |
| Wurtzite ZnO-nanoparticles | Sugarcane press mud | Photo-catalytic activity in the agricultural and environmental fields | [68] |
| Niobium oxide-NPs (Nb2O5-NPs) | Pecan nutshell, Carya illinoinensis | Antioxidant activity | [69] |
| Nanochitosan | Shrimp shell waste biomass | Antibacterial activity | [64] |
| Titanium dioxide-NPs (TiO2-NPs) | Banana pseudostem | Removal of Indigo Carmine dye | [70] |
| Nanomaterials of cellulose and lignin | Sorghum biomass | Accelerating the industrial and economic prospects of bio-based biorefineries | [71] |
| ZnO-NPs | Corn stalk pith | Bio-filters for the purification of water | [72] |
| Silver nanoparticles | Durian peel (Durio zibethinus Murr.) | Antibacterial activity against both negative- and positive-gram bacteria | [73] |
| ZnO-activated carbon nanoparticles | Plantain peel | Effective adsorbents for treatment of wastewater pollution | [74] |
| Calcium borate nanoparticles | Sugarcane bagasse | Enhancing seed germination and development | [75] |
4. Antimicrobial Activity of Plant-Byproduct-Based Nanomaterials
4.1. Nanomaterials as Antimicrobial Agents Against Antibiotic Resistance
4.2. The Role of Plant Byproducts in Nanoparticle Synthesis
4.3. Green Synthesis of Nanoparticles Using Plant Byproducts
- -
- Rigopoulos et al. [108] employed olive mill waste (Olea europaea) as a reducing agent in silver nanoparticle synthesis through a factorial experimental design, reporting strong antibacterial efficacy against E. coli and S. aureus;
- -
- Other notable examples include cellulose/ZnO nanoparticles derived from peanut shells (Arachis hypogaea), which showed enhanced antimicrobial effects, especially against yeast [109];
- -
- Silver nanoparticles synthesized from tomato peel (Solanum lycopersicum) demonstrated considerable antibacterial activity [110].
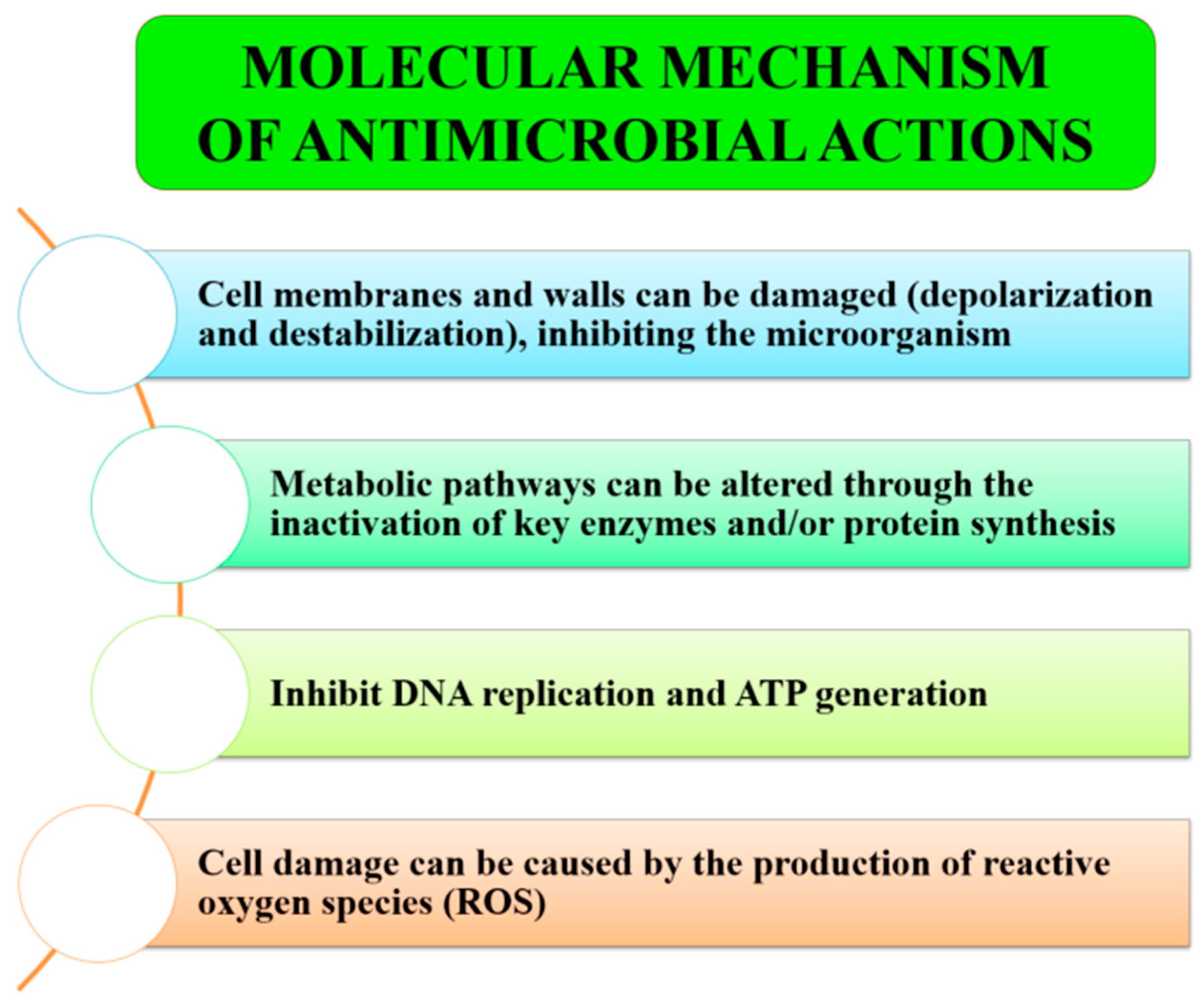
4.4. Mechanisms of Antimicrobial Action
4.5. Factors Affecting Antimicrobial Efficacy
4.6. Comparative Summary of Antimicrobial Activity
4.7. Challenges and Opportunities
5. Exploring the Prebiotic Potential of Plant-Based Nanomaterials
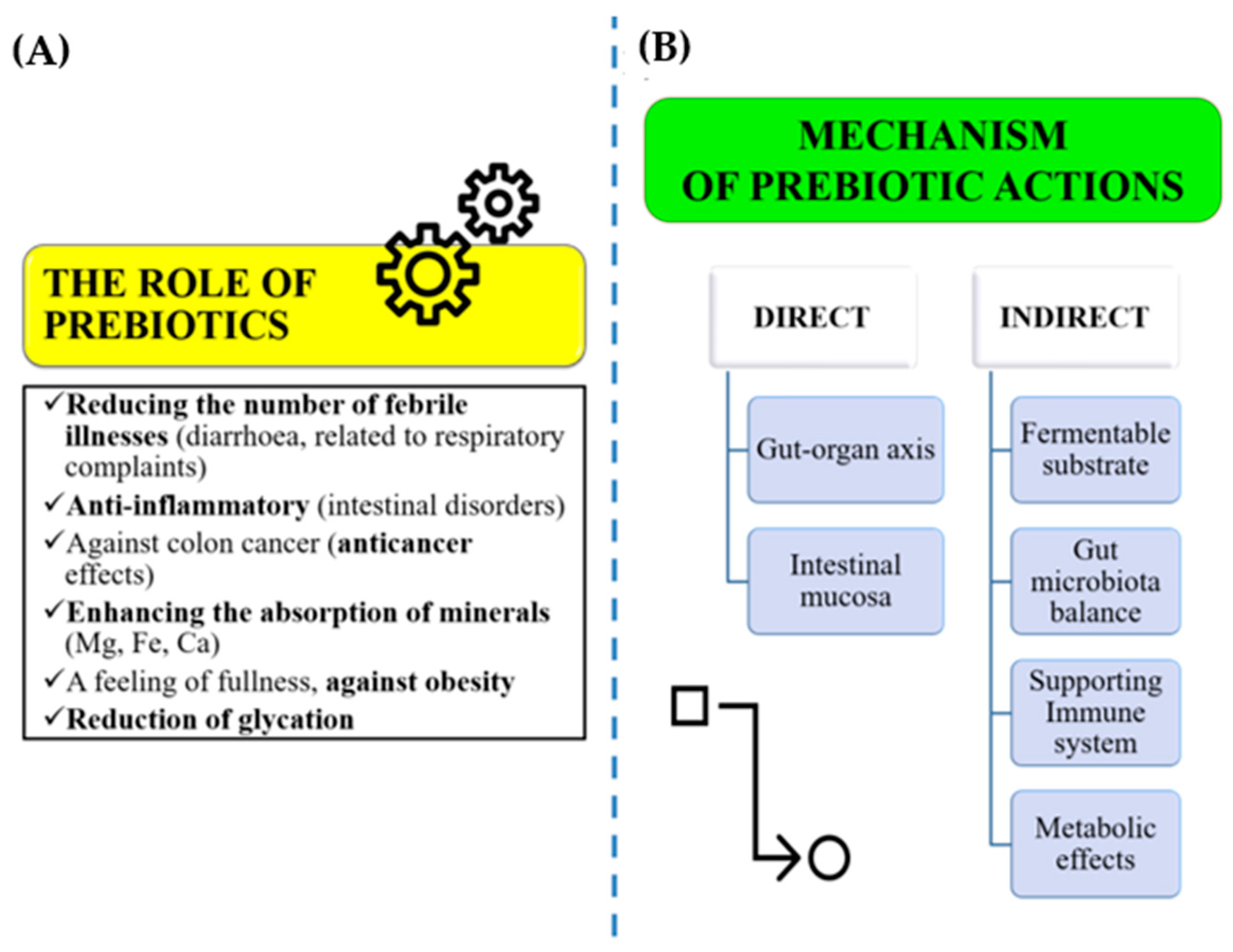
5.1. The Role of Prebiotics
5.2. Plant-Based Byproduct Nanomaterials as a Strong Prebiotic
6. Exploring Preclinical and Early Clinical Evidence for Plant-Derived Nanoparticles
7. Composition of Human Gut Microbiota and Infectious Diseases
7.1. Gut Microbiota and Its Role in Health
7.2. Composition and Diversity of Gut Microbiota
7.3. Gut Microbiota and Infectious Diseases
8. Nanostrategies to Develop Disease Resistance
9. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dalbanjan, N.P.; Eelager, M.P.; Korgaonkar, K.; Gonal, B.N.; Kadapure, A.J.; Arakera, S.B.; Praveen Kumar, S.K. Descriptive Review on Conversion of Waste Residues into Valuable Bionanocomposites for a Circular Bioeconomy. Nano-Struct. Nano-Objects 2024, 39, 101265. [Google Scholar] [CrossRef]
- Bassey, K.E. From Waste to Wonder: Developing Engineered Nanomaterials for Multifaceted Applications. GSC Adv. Res. Rev. 2024, 20, 109–123. [Google Scholar] [CrossRef]
- Amutha Gokul, T.; Ramesh Kumar, K.; Venkatachalam, K.; Suresh Babu, R.; Veeramanikandan, V.; Sagadevan, S.; Balaji, P. Plant-Based Nanostructure for Wound Healing—An Emerging Paradigm for Effective Therapy. Inorg. Chem. Commun. 2024, 162, 112162. [Google Scholar] [CrossRef]
- Kaur, H.; Wadhwa, K. Exploration of New Plant-Based Nanoparticles with Potential Antifungal Activity and Their Mode of Action. In Advances in Antifungal Drug Development; Manzoor, N., Ed.; Springer Nature: Singapore, 2024; pp. 345–371. ISBN 978-981-97-5164-8. [Google Scholar]
- Khanrah, J.; Rawani, A. Evaluation of in Vitro Anthelmintic Activity of Crude Extract and Synthesized Green Silver Nanoparticles of the Leaves of Mammea Americana L. J. Parasit. Dis. 2024, 48, 537–550. [Google Scholar] [CrossRef]
- Liu, C.; Yu, Y.; Fang, L.; Wang, J.; Sun, C.; Li, H.; Zhuang, J.; Sun, C. Plant-Derived Nanoparticles and Plant Virus Nanoparticles: Bioactivity, Health Management, and Delivery Potential. Crit. Rev. Food Sci. Nutr. 2024, 64, 8875–8891. [Google Scholar] [CrossRef]
- Thomas, S.; Gonsalves, R.A.; Jose, J.; Zyoud, S.H.; Prasad, A.R.; Garvasis, J. Plant-Based Synthesis, Characterization Approaches, Applications and Toxicity of Silver Nanoparticles: A Comprehensive Review. J. Biotechnol. 2024, 394, 135–149. [Google Scholar] [CrossRef]
- Amiri, S.; Nezamdoost-Sani, N.; Mostashari, P.; McClements, D.J.; Marszałek, K.; Mousavi Khaneghah, A. Effect of the Molecular Structure and Mechanical Properties of Plant-Based Hydrogels in Food Systems to Deliver Probiotics: An Updated Review. Crit. Rev. Food Sci. Nutr. 2024, 64, 2130–2156. [Google Scholar] [CrossRef]
- Jia, H.; Jia, Y.; Ren, F.; Liu, H. Enhancing Bioactive Compounds in Plant-Based Foods: Influencing Factors and Technological Advances. Food Chem. 2024, 460, 140744. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Shi, Y.; Song, H.; Yu, C. Antibiotic-Free Antibacterial Strategies Enabled by Nanomaterials: Progress and Perspectives. Adv. Mater. 2020, 32, 1904106. [Google Scholar] [CrossRef]
- Pilmis, B.; Le Monnier, A.; Zahar, J.-R. Gut Microbiota, Antibiotic Therapy and Antimicrobial Resistance: A Narrative Review. Microorganisms 2020, 8, 269. [Google Scholar] [CrossRef]
- Xin, Q.; Shah, H.; Nawaz, A.; Xie, W.; Akram, M.Z.; Batool, A.; Tian, L.; Jan, S.U.; Boddula, R.; Guo, B.; et al. Antibacterial Carbon-Based Nanomaterials. Adv. Mater. 2019, 31, 1804838. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kadam, A.; Shinde, S.; Saratale, R.G.; Patra, J.; Ghodake, G. Recent Developments in Nanotechnology Transforming the Agricultural Sector: A Transition Replete with Opportunities. J. Sci. Food Agric. 2018, 98, 849–864. [Google Scholar] [CrossRef] [PubMed]
- Rațu, R.N.; Veleșcu, I.D.; Stoica, F.; Usturoi, A.; Arsenoaia, V.N.; Crivei, I.C.; Postolache, A.N.; Lipșa, F.D.; Filipov, F.; Florea, A.M. Application of Agri-Food By-Products in the Food Industry. Agriculture 2023, 13, 1559. [Google Scholar] [CrossRef]
- Baraketi, S.; Khwaldia, K. Nanoparticles from Agri-Food by-Products: Green Technology Synthesis and Application in Food Packaging. Curr. Opin. Green Sustain. Chem. 2024, 49, 100953. [Google Scholar] [CrossRef]
- Ahmed, K.J.A.J.; Bornare, D.; Jaiswal, S. Review on Extraction of Melanoidins from Coffee Waste and Value Addition in Food. J. Curr. Res. Food Sci. 2024, 5, 157–161. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Badawy, M.T.; Ahmed, F.K.; Kalia, A.; Abd-Elsalam, K.A. Fruit Peel Waste-to-Wealth: Bionanomaterials Production and Their Applications in Agroecosystems. In Agri-Waste and Microbes for Production of Sustainable Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2022; pp. 231–257. ISBN 978-0-12-823575-1. [Google Scholar]
- Arun, G.; Eyini, M.; Gunasekaran, P. Green Synthesis of Silver Nanoparticles Using the Mushroom Fungus Schizophyllum Commune and Its Biomedical Applications. Biotechnol. Bioprocess Eng. 2014, 19, 1083–1090. [Google Scholar] [CrossRef]
- Sanyasi, S.; Majhi, R.K.; Kumar, S.; Mishra, M.; Ghosh, A.; Suar, M.; Satyam, P.V.; Mohapatra, H.; Goswami, C.; Goswami, L. Polysaccharide-Capped Silver Nanoparticles Inhibit Biofilm Formation and Eliminate Multi-Drug-Resistant Bacteria by Disrupting Bacterial Cytoskeleton with Reduced Cytotoxicity towards Mammalian Cells. Sci. Rep. 2016, 6, 24929. [Google Scholar] [CrossRef]
- Preethi, B.; Karmegam, N.; Manikandan, S.; Vickram, S.; Subbaiya, R.; Rajeshkumar, S.; Gomadurai, C.; Govarthanan, M. Nanotechnology-Powered Innovations for Agricultural and Food Waste Valorization: A Critical Appraisal in the Context of Circular Economy Implementation in Developing Nations. Process Saf. Environ. Prot. 2024, 184, 477–491. [Google Scholar] [CrossRef]
- Mengqi, Z.; Shi, A.; Ajmal, M.; Ye, L.; Awais, M. Comprehensive Review on Agricultural Waste Utilization and High-Temperature Fermentation and Composting. Biomass Conv. Bioref. 2023, 13, 5445–5468. [Google Scholar] [CrossRef]
- Han, K.; Xu, J.; Xie, F.; Crowther, J.; Moon, J.J. Engineering Strategies to Modulate the Gut Microbiome and Immune System. J. Immunol. 2024, 212, 208–215. [Google Scholar] [CrossRef]
- Kumar, P.; Saini, S.; Chand, M.; Om Sharma, H. Advance Biotechnological, Pharmaceutical, and Medicinal Applications of Chitinases. In Sustainable Production Innovations; Patel, A.K., Sharma, A.K., Eds.; Wiley: Hoboken, NJ, USA, 2024; pp. 223–231. ISBN 978-1-119-79190-4. [Google Scholar]
- Kumar, R.; Kaur, A.; Sharma, S.; Naveen; Bharti, H.; Kumar, R. Advancements and Challenges in Agriculture Waste Management: A Comprehensive: Review. Educ. Adm. Theory Pract. 2024, 30, 7253–7273. [Google Scholar] [CrossRef]
- Afzaal, M.; Saeed, F.; Shah, Y.A.; Hussain, M.; Rabail, R.; Socol, C.T.; Hassoun, A.; Pateiro, M.; Lorenzo, J.M.; Rusu, A.V. Human Gut Microbiota in Health and Disease: Unveiling the Relationship. Front. Microbiol. 2022, 13, 999001. [Google Scholar] [CrossRef]
- Al Hakeem, W.G.; Acevedo Villanueva, K.Y.; Selvaraj, R.K. The Development of Gut Microbiota and Its Changes Following C. Jejuni Infection in Broilers. Vaccines 2023, 11, 595. [Google Scholar] [CrossRef] [PubMed]
- Bedu-Ferrari, C.; Biscarrat, P.; Langella, P.; Cherbuy, C. Prebiotics and the Human Gut Microbiota: From Breakdown Mechanisms to the Impact on Metabolic Health. Nutrients 2022, 14, 2096. [Google Scholar] [CrossRef]
- Myhrstad, M.C.W.; Tunsjø, H.; Charnock, C.; Telle-Hansen, V.H. Dietary Fiber, Gut Microbiota, and Metabolic Regulation—Current Status in Human Randomized Trials. Nutrients 2020, 12, 859. [Google Scholar] [CrossRef]
- Chikezie Ogbu, C.; Nnaemeka Okey, S. Agro-Industrial Waste Management: The Circular and Bioeconomic Perspective. In Agricultural Waste—New Insights; Ahmad, F., Sultan, M., Eds.; IntechOpen: London, UK, 2023; ISBN 978-1-80356-965-9. [Google Scholar]
- Almaraz-Sánchez, I.; Amaro-Reyes, A.; Acosta-Gallegos, J.A.; Mendoza-Sánchez, M. Processing Agroindustry By-Products for Obtaining Value-Added Products and Reducing Environmental Impact. J. Chem. 2022, 2022, 1–13. [Google Scholar] [CrossRef]
- Barahira, D.S.; Okudoh, V.I.; Eloka-Eboka, A.C. Suitability of Crop Residues as Feedstock for Biofuel Production in South Africa: A Sustainable Win-Win Scenario. J. Oleo Sci. 2021, 70, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Flores-Contreras, E.A.; González-González, R.B.; Pablo Pizaña-Aranda, J.J.; Parra-Arroyo, L.; Rodríguez-Aguayo, A.A.; Iñiguez-Moreno, M.; González-Meza, G.M.; Araújo, R.G.; Ramírez-Gamboa, D.; Parra-Saldívar, R.; et al. Agricultural Waste as a Sustainable Source for Nanoparticle Synthesis and Their Antimicrobial Properties for Food Preservation. Front. Nanotechnol. 2024, 6, 1346069. [Google Scholar] [CrossRef]
- Coronado-Apodaca, K.G.; Rodríguez-De Luna, S.E.; Araújo, R.G.; Oyervides-Muñoz, M.A.; González-Meza, G.M.; Parra-Arroyo, L.; Sosa-Hernandez, J.E.; Iqbal, H.M.N.; Parra-Saldivar, R. Occurrence, Transport, and Detection Techniques of Emerging Pollutants in Groundwater. MethodsX 2023, 10, 102160. [Google Scholar] [CrossRef]
- Anvari, S.; Aguado, R.; Jurado, F.; Fendri, M.; Zaier, H.; Larbi, A.; Vera, D. Analysis of Agricultural Waste/Byproduct Biomass Potential for Bioenergy: The Case of Tunisia. Energy Sustain. Dev. 2024, 78, 101367. [Google Scholar] [CrossRef]
- Gamiz-Conde, A.K.; Burelo, M.; Franco-Urquiza, E.A.; Martínez-Franco, E.; Luna-Barcenas, G.; Bravo-Alfaro, D.A.; Treviño-Quintanilla, C.D. Development and Properties of Bio-Based Polymer Composites Using PLA and Untreated Agro-Industrial Residues. Polym. Test 2024, 139, 108576. [Google Scholar] [CrossRef]
- Ali, Z.; Abdullah, M.; Yasin, M.T.; Amanat, K.; Ahmad, K.; Ahmed, I.; Qaisrani, M.M.; Khan, J. Organic Waste-to-Bioplastics: Conversion with Eco-Friendly Technologies and Approaches for Sustainable Environment. Environ. Res. 2024, 244, 117949. [Google Scholar] [CrossRef] [PubMed]
- Enokida, C.H.; Tapparo, D.C.; Antes, F.G.; Radis Steinmetz, R.L.; Magrini, F.E.; Sophiatti, I.V.M.; Paesi, S.; Kunz, A. Anaerobic Codigestion of Livestock Manure and Agro-Industrial Waste in a CSTR Reactor: Operational Aspects, Digestate Characteristics, and Microbial Community Dynamics. Renew. Energy 2025, 238, 121865. [Google Scholar] [CrossRef]
- Haque, F.; Fan, C.; Lee, Y.-Y. From Waste to Value: Addressing the Relevance of Waste Recovery to Agricultural Sector in Line with Circular Economy. J. Clean. Prod. 2023, 415, 137873. [Google Scholar] [CrossRef]
- Sodhi, G.K.; Kaur, G.; George, N.; Walia, H.K.; Sillu, D.; Rath, S.K.; Saxena, S.; Rios-Solis, L.; Dwibedi, V. Waste to Wealth: Microbial-Based Valorization of Grape Pomace for Nutraceutical, Cosmetic, and Therapeutic Applications to Promote Circular Economy. Process Saf. Environ. Prot. 2024, 188, 1464–1478. [Google Scholar] [CrossRef]
- Adel, R.; Fahim, I.S.; Bakhoum, E.S.; Ahmed, A.M.; AbdelSalam, S.S. Sustainable Nanocellulose Coating for EPS Geofoam Extracted from Agricultural Waste. Waste Manag. 2025, 191, 135–146. [Google Scholar] [CrossRef]
- El-Ramady, H.; El-Henawy, A.; Amer, M.; Omara, A.E.-D.; Elsakhawy, T.; Elbasiouny, H.; Elbehiry, F.; Abou Elyazid, D.; El-Mahrouk, M. Agricultural Waste and Its Nano-Management: Mini Review. Egypt. J. Soil Sci. 2020, 60, 349–364. [Google Scholar] [CrossRef]
- Tang, Y.; Zhao, W.; Gao, L.; Zhu, G.; Jiang, Y.; Rui, Y.; Zhang, P. Harnessing Synergy: Integrating Agricultural Waste and Nanomaterials for Enhanced Sustainability. Environ. Pollut. 2024, 341, 123023. [Google Scholar] [CrossRef] [PubMed]
- Chen, X. Economic Potential of Biomass Supply from Crop Residues in China. Appl. Energy 2016, 166, 141–149. [Google Scholar] [CrossRef]
- Cho, E.J.; Trinh, L.T.P.; Song, Y.; Lee, Y.G.; Bae, H.-J. Bioconversion of Biomass Waste into High Value Chemicals. Bioresour. Technol. 2020, 298, 122386. [Google Scholar] [CrossRef]
- Kassim, F.O.; Thomas, C.L.P.; Afolabi, O.O.D. Integrated Conversion Technologies for Sustainable Agri-Food Waste Valorization: A Critical Review. Biomass Bioenergy 2022, 156, 106314. [Google Scholar] [CrossRef]
- Khoshnevisan, B.; Duan, N.; Tsapekos, P.; Awasthi, M.K.; Liu, Z.; Mohammadi, A.; Angelidaki, I.; Tsang, D.C.; Zhang, Z.; Pan, J.; et al. A Critical Review on Livestock Manure Biorefinery Technologies: Sustainability, Challenges, and Future Perspectives. Renew. Sustain. Energy Rev. 2021, 135, 110033. [Google Scholar] [CrossRef]
- Mannaa, M.; Mansour, A.; Park, I.; Lee, D.-W.; Seo, Y.-S. Insect-Based Agri-Food Waste Valorization: Agricultural Applications and Roles of Insect Gut Microbiota. Environ. Sci. Ecotechnol. 2024, 17, 100287. [Google Scholar] [CrossRef] [PubMed]
- Yukesh Kannah, R.; Merrylin, J.; Poornima Devi, T.; Kavitha, S.; Sivashanmugam, P.; Kumar, G.; Rajesh Banu, J. Food Waste Valorization: Biofuels and Value Added Product Recovery. Bioresour. Technol. Rep. 2020, 11, 100524. [Google Scholar] [CrossRef]
- Seberini, A. Economic, Social and Environmental World Impacts of Food Waste on Society and Zero Waste as a Global Approach to Their Elimination. SHS Web Conf. 2020, 74, 03010. [Google Scholar] [CrossRef]
- Sarangi, P.K.; Singh, A.K.; Srivastava, R.K.; Gupta, V.K. Recent Progress and Future Perspectives for Zero Agriculture Waste Technologies: Pineapple Waste as a Case Study. Sustainability 2023, 15, 3575. [Google Scholar] [CrossRef]
- Yang, M.; Chen, L.; Wang, J.; Msigwa, G.; Osman, A.I.; Fawzy, S.; Rooney, D.W.; Yap, P.-S. Circular Economy Strategies for Combating Climate Change and Other Environmental Issues. Environ. Chem. Lett. 2023, 21, 55–80. [Google Scholar] [CrossRef]
- Geetha, K.; Akshitha, S.; Kiran, R.; Tadikonda, R. An Over View of Nano Technology in Waste Management. Nanoscience 2023, 8, a281–a289. [Google Scholar]
- Liu, Z.; De Souza, T.S.P.; Holland, B.; Dunshea, F.; Barrow, C.; Suleria, H.A.R. Valorization of Food Waste to Produce Value-Added Products Based on Its Bioactive Compounds. Processes 2023, 11, 840. [Google Scholar] [CrossRef]
- Khosravi, A.; Zarepour, A.; Iravani, S.; Varma, R.S.; Zarrabi, A. Sustainable Synthesis: Natural Processes Shaping the Nanocircular Economy. Environ. Sci. Nano 2024, 11, 688–707. [Google Scholar] [CrossRef]
- Mohammadzadeh, V.; Barani, M.; Amiri, M.S.; Taghavizadeh Yazdi, M.E.; Hassanisaadi, M.; Rahdar, A.; Varma, R.S. Applications of Plant-Based Nanoparticles in Nanomedicine: A Review. Sustain. Chem. Pharm. 2022, 25, 100606. [Google Scholar] [CrossRef]
- Nguyen, N.T.T.; Nguyen, L.M.; Nguyen, T.T.T.; Nguyen, T.T.; Nguyen, D.T.C.; Tran, T.V. Formation, Antimicrobial Activity, and Biomedical Performance of Plant-Based Nanoparticles: A Review. Environ. Chem. Lett. 2022, 20, 2531–2571. [Google Scholar] [CrossRef] [PubMed]
- Wazeer, H.; Shridhar Gaonkar, S.; Doria, E.; Pagano, A.; Balestrazzi, A.; Macovei, A. Plant-Based Biostimulants for Seeds in the Context of Circular Economy and Sustainability. Plants 2024, 13, 1004. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Agarwal, U.P.; Ciesielski, P.N.; Himmel, M.E.; Gao, R.; Deng, Y.; Morits, M.; Österberg, M. Towards Sustainable Production and Utilization of Plant-Biomass-Based Nanomaterials: A Review and Analysis of Recent Developments. Biotechnol. Biofuels 2021, 14, 114. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, M.; Sharma, R.; Saini, A.; Khanna, V.; Singh, G. Nanomaterials Mediated Valorization of Agriculture Waste Residue for Biohydrogen Production. Int. J. Hydrogen Energy 2024, 52, 1241–1253. [Google Scholar] [CrossRef]
- Sonu; Rani, G.M.; Pathania, D.; Abhimanyu; Umapathi, R.; Rustagi, S.; Huh, Y.S.; Gupta, V.K.; Kaushik, A.; Chaudhary, V. Agro-Waste to Sustainable Energy: A Green Strategy of Converting Agricultural Waste to Nano-Enabled Energy Applications. Sci. Total Environ. 2023, 875, 162667. [Google Scholar] [CrossRef]
- Phiri, R.; Mavinkere Rangappa, S.; Siengchin, S. Agro-Waste for Renewable and Sustainable Green Production: A Review. J. Clean. Prod. 2024, 434, 139989. [Google Scholar] [CrossRef]
- Ainomugisha, S.; Matovu, M.; Manga, M. Application of Green Agro-Based Nanoparticles in Cement-Based Construction Materials: A Systematic Review. J. Build. Eng. 2024, 87, 108955. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Oladzadabbasabadi, N.; Dheyab, M.A.; Lalabadi, M.A.; Sheibani, S.; Ghasemlou, M.; Esmaeili, Y.; Barrow, C.J.; Naebe, M.; Timms, W. Emerging Trends in Smart and Sustainable Nano-Biosensing: The Role of Green Nanomaterials. Ind. Crops Prod. 2025, 223, 120108. [Google Scholar] [CrossRef]
- Jha, A.K.; Chakraborty, S.; Biswas, J.K. Green Synthesis of Low-Cost Graphene Oxide-Nano Zerovalent Iron Composite from Solid Waste for Photocatalytic Removal of Antibiotics. iScience 2024, 27, 111486. [Google Scholar] [CrossRef]
- Chatterjee, S.; Fosso-Kankeu, E. A Review on Biodegradable Polymers Production from Agricultural Wastes: A Green, Sustainable and Eco-Friendly Approach. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2024; p. B9780323954860000429. ISBN 978-0-12-803581-8. [Google Scholar]
- Rahman, M.d.M.; Maniruzzaman, M.; Saha, R.K. A Green Route of Antibacterial Films Production from Shrimp (Penaeus monodon) Shell Waste Biomass Derived Chitosan: Physicochemical, Thermomechanical, Morphological and Antimicrobial Activity Analysis. S. Afr. J. Chem. Eng. 2025, 51, 153–169. [Google Scholar] [CrossRef]
- Garwal, K.; Tewari, C.; Arya, T.; Rawat, J.; Pande, V.; Basak, S.; Bose, M.; Jung, Y.C.; Sahoo, N.G. Green Synthesis of Agro-Waste–Derived Potassium-Doped Graphene Oxide for Antimicrobial Activity. Plant Nano Biol. 2024, 10, 100119. [Google Scholar] [CrossRef]
- Munir, M.; Ahmad, M.; Abdullah Alsahli, A.; Zhang, L.; Islamov, S.; Sultana, S.; Ussemane Mussagy, C.; Mustafa, A.; Munir, M.; Chaudhry, B.; et al. Harnessing Non-Edible Quercus Incana Seeds for Sustainable and Clean Biodiesel Production Using Seed-Derived Green Al2O3 Nanocatalyst. Sustain. Energy Technol. Assess. 2024, 72, 104025. [Google Scholar] [CrossRef]
- Vorobyova, V.; Skiba, M.; Vinnichuk, K.; Vasyliev, G. Synthesis of Gold Nanoparticles Using Plum Waste Extract with Green Solvents. Sustain. Chem. Environ. 2024, 6, 100086. [Google Scholar] [CrossRef]
- Verma, L.M.; Kumar, A.; Bashir, A.U.; Gangwar, U.; Ingole, P.P.; Sharma, S. Phase Controlled Green Synthesis of Wurtzite (P. 63 Mc.) ZnO Nanoparticles: Interplay of Green Ligands with Precursor Anions, Anisotropy and Photocatalysis. Nanoscale Adv. 2024, 6, 155–169. [Google Scholar] [CrossRef]
- Brum, L.F.W.; Da Silva, M.D.C.R.; Dos Santos, C.; Pavoski, G.; Espinosa, D.C.R.; Da Silva, W.L. Green Synthesis of Niobium (V) Oxide Nanoparticles Using Pecan Nutshell (Carya illinoinensis) and Evaluation of Its Antioxidant Activity. Catal. Today 2025, 445, 115106. [Google Scholar] [CrossRef]
- Sasirekha, D.; Baskaralingam, P.; Arafath, K.A.Y.; Sivanesan, S. Agro-Waste Mediate to Synthesize the Solar Light Active Titanium Dioxide Nanoparticles with Enhanced Efficacy of Pollutant Removal. Optik 2024, 304, 171716. [Google Scholar] [CrossRef]
- Srinivasan, S.; Venkatachalam, S. One Pot Green Process for Facile Fractionation of Sorghum Biomass to Lignin, Cellulose and Hemicellulose Nanoparticles Using Deep Eutectic Solvent. Int. J. Biol. Macromol. 2024, 277, 134295. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Y.; Li, M.; Li, B.; Mao, X.; Wang, Q.; Tang, X.; Zhang, H.; Peng, L.; Gao, X. Sustainable Water Sterilization by Nano-ZnO Using Anisotropic Polysaccharide Columns Derived from Agro-Waste Stalk. Chem. Eng. J. 2024, 496, 153757. [Google Scholar] [CrossRef]
- Nguyen, P.A.T.; Nguyen, T.T.T.; Vo, D.K.N. Pectin from Durian Peel (Durio zibethinus Murr.)—A Novel Reducing and Stabilizing Agent: Physicochemical Properties, Green Synthesis of Silver Nanoparticles and Antimicrobial Properties. Carbohydr. Polym. Technol. Appl. 2024, 8, 100623. [Google Scholar] [CrossRef]
- Dada, A.O.; Inyinbor, A.A.; Tokula, B.E.; Bayode, A.A.; Obayomi, K.S.; Ajanaku, C.O.; Adekola, F.A.; Ajanaku, K.O.; Pal, U. Zinc Oxide Decorated Plantain Peel Activated Carbon for Adsorption of Cationic Malachite Green Dye: Mechanistic, Kinetics and Thermodynamics Modeling. Environ. Res. 2024, 252, 119046. [Google Scholar] [CrossRef] [PubMed]
- Smitha, J.K.; Bhaskar, S.P.; Jose, A.; Geetha, T. Synthesis of Nano Calcium Borate Using Sugarcane Bagasse Extract as a Capping Agent and Its Potential to Promote Germination in Cowpea Seeds. Results Chem. 2024, 10, 101722. [Google Scholar] [CrossRef]
- Abid, N.; Khan, A.M.; Shujait, S.; Chaudhary, K.; Ikram, M.; Imran, M.; Haider, J.; Khan, M.; Khan, Q.; Maqbool, M. Synthesis of Nanomaterials Using Various Top-down and Bottom-up Approaches, Influencing Factors, Advantages, and Disadvantages: A Review. Adv. Colloid. Interface Sci. 2022, 300, 102597. [Google Scholar] [CrossRef] [PubMed]
- Usman, K.A.S.; Maina, J.W.; Seyedin, S.; Conato, M.T.; Payawan, L.M.; Dumée, L.F.; Razal, J.M. Downsizing Metal–Organic Frameworks by Bottom-up and Top-down Methods. NPG Asia Mater. 2020, 12, 58. [Google Scholar] [CrossRef]
- Srikar, S.K.; Giri, D.D.; Pal, D.B.; Mishra, P.K.; Upadhyay, S.N. Green Synthesis of Silver Nanoparticles: A Review. GSC 2016, 6, 34–56. [Google Scholar] [CrossRef]
- McClements, D.J.; Öztürk, B. Utilization of Nanotechnology to Improve the Application and Bioavailability of Phytochemicals Derived from Waste Streams. J. Agric. Food Chem. 2022, 70, 6884–6900. [Google Scholar] [CrossRef]
- Prado-Acebo, I.; Cubero-Cardoso, J.; Lu-Chau, T.A.; Eibes, G. Integral Multi-Valorization of Agro-Industrial Wastes: A Review. Waste Manag. 2024, 183, 42–52. [Google Scholar] [CrossRef]
- Arun Kumar, N.B.; Sirajudeen, J.; Nagaswarupa, H.P.; Anil Kumar, M.R.; Ravi Kumar, C.R.; Gurushantha, K.; Shashi Shekhar, T.R.; Anantharaju, K.S.; Vishnu Mahesh, K.R.; Sharma, S.C.; et al. Photocatalytic and Photoluminescence Studies of ZnO Nanomaterials by Banana Peel Powder. Mater. Today Proc. 2017, 4, 11827–11836. [Google Scholar] [CrossRef]
- Zayed, M.; Ghazal, H.; Othman, H.A.; Hassabo, A.G. Synthesis of Different Nanometals Using Citrus Sinensis Peel (Orange Peel) Waste Extraction for Valuable Functionalization of Cotton Fabric. Chem. Pap. 2022, 76, 639–660. [Google Scholar] [CrossRef]
- Kim, T.Y.; De, R.; Choi, I.; Kim, H.; Hahn, S.K. Multifunctional Nanomaterials for Smart Wearable Diabetic Healthcare Devices. Biomaterials 2024, 310, 122630. [Google Scholar] [CrossRef]
- Zakaria, N.Z.J.; Rozali, S.; Mubarak, N.M.; Ibrahim, S. A Review of the Recent Trend in the Synthesis of Carbon Nanomaterials Derived from Oil Palm By-Product Materials. Biomass Conv. Bioref. 2024, 14, 13–44. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, V.; Roy, A.; Kaur, K.; Rai, A.K.; Rustagi, S. Recent Advances in Nanomaterial-Based Drug Delivery Systems. Nano-Struct. Nano-Objects 2024, 37, 101103. [Google Scholar] [CrossRef]
- Jing, H.H.; Shati, A.A.; Alfaifi, M.Y.; Elbehairi, S.E.I.; Sasidharan, S. The Future of Plant Based Green Carbon Dots as Cancer Nanomedicine: From Current Progress to Future Perspectives and Beyond. J. Adv. Res. 2024, 67, 133–159. [Google Scholar] [CrossRef]
- Fu, W.; Sun, S.; Cheng, Y.; Ma, J.; Hu, Y.; Yang, Z.; Yao, H.; Zhang, Z. Opportunities and Challenges of Nanomaterials in Wound Healing: Advances, Mechanisms, and Perspectives. Chem. Eng. J. 2024, 495, 153640. [Google Scholar] [CrossRef]
- Osazee, F.O.; Mokobia, K.E.; Ifijen, I.H. The Urgent Need for Tungsten-Based Nanoparticles as Antibacterial Agents. Biomed. Mater. Devices 2024, 2, 614–629. [Google Scholar] [CrossRef]
- Lata, S.; Bhardwaj, S.; Garg, R. Nanomaterials for Sensing and Biosensing: Applications in Agri-Food Diagnostics. Int. J. Environ. Anal. Chem. 2024, 104, 4868–4885. [Google Scholar] [CrossRef]
- Suseem, S.R. Plant-Based Carbon Dots Are a Sustainable Alternative to Conventional Nanomaterials for Biomedical and Sensing Applications. Nano Express 2024, 5, 012002. [Google Scholar] [CrossRef]
- Bhat, R. Sustainability Challenges in the Valorization of Agri-Food Wastes and by-Products. In Valorization of Agri-Food Wastes and By-Products; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–27. ISBN 978-0-12-824044-1. [Google Scholar]
- Anand, U.; Carpena, M.; Kowalska-Góralska, M.; Garcia-Perez, P.; Sunita, K.; Bontempi, E.; Dey, A.; Prieto, M.A.; Proćków, J.; Simal-Gandara, J. Safer Plant-Based Nanoparticles for Combating Antibiotic Resistance in Bacteria: A Comprehensive Review on Its Potential Applications, Recent Advances, and Future Perspective. Sci. Total Environ. 2022, 821, 153472. [Google Scholar] [CrossRef] [PubMed]
- Datta, D.; Prajapati, B.; Jethva, H.; Agrawal, K.; Singh, S.; Prajapati, B.G. Value-Added Nanocellulose Valorized from Fruit Peel Waste for Potential Dermal Wound Healing and Tissue Regenerative Applications. Regen. Eng. Transl. Med. 2024, 11, 88–111. [Google Scholar] [CrossRef]
- Yusuf, A.; Al Jitan, S.; Garlisi, C.; Palmisano, G. A Review of Recent and Emerging Antimicrobial Nanomaterials in Wastewater Treatment Applications. Chemosphere 2021, 278, 130440. [Google Scholar] [CrossRef]
- Lei, W.; Qi, M.; Tan, P.; Yang, S.; Fan, L.; Li, H.; Gao, Z. Impact of Polyphenol-Loaded Edible Starch Nanomaterials on Antioxidant Capacity and Gut Microbiota. Int. J. Biol. Macromol. 2024, 265, 130979. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Rana, D.S.; Kumar, S.; Singh, R.K.; Thakur, S.; Singh, D. Coconut Husk Waste-Derived Nitrogen-Doped Mesoporous Carbon Nanomaterial as an Efficient and Sustainable Supercapacitor. Energy Technol. 2024, 12, 2400294. [Google Scholar] [CrossRef]
- Taymaz, E.R.; Uslu, M.E. Innovations in Biocompatible Materials: Exploring the Potential of Cellulose Nanocrystals from Grape Pomace. Chem. Pap. 2024, 78, 5445–5455. [Google Scholar] [CrossRef]
- Shaheen, T.I.; Hussien, G.M.; Mekawey, A.A.; Ghalia, H.H.; El Mokadem, M.T. Facile Extraction of Nanosized β-Glucans from Edible Mushrooms and Their Antitumor Activities. J. Food Compos. Anal. 2022, 111, 104607. [Google Scholar] [CrossRef]
- Gao, Y.; Guo, X.; Liu, Y.; Fang, Z.; Zhang, M.; Zhang, R.; You, L.; Li, T.; Liu, R.H. A Full Utilization of Rice Husk to Evaluate Phytochemical Bioactivities and Prepare Cellulose Nanocrystals. Sci. Rep. 2018, 8, 10482. [Google Scholar] [CrossRef]
- Santos, A.C.; Rodrigues, D.; Sequeira, J.A.D.; Pereira, I.; Simões, A.; Costa, D.; Peixoto, D.; Costa, G.; Veiga, F. Nanotechnological Breakthroughs in the Development of Topical Phytocompounds-Based Formulations. Int. J. Pharm. 2019, 572, 118787. [Google Scholar] [CrossRef]
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014; ISBN 978-92-4-156474-8. [Google Scholar]
- Barros, C.H.N.; Hiebner, D.W.; Fulaz, S.; Vitale, S.; Quinn, L.; Casey, E. Synthesis and Self-Assembly of Curcumin-Modified Amphiphilic Polymeric Micelles with Antibacterial Activity. J. Nanobiotechnol. 2021, 19, 104. [Google Scholar] [CrossRef]
- Fang, G.; Li, W.; Shen, X.; Perez-Aguilar, J.M.; Chong, Y.; Gao, X.; Chai, Z.; Chen, C.; Ge, C.; Zhou, R. Differential Pd-Nanocrystal Facets Demonstrate Distinct Antibacterial Activity against Gram-Positive and Gram-Negative Bacteria. Nat. Commun. 2018, 9, 129. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, L. Nanomaterials Arising amid Antibiotic Resistance. Nat. Rev. Microbiol. 2021, 19, 5–6. [Google Scholar] [CrossRef]
- Angamuthu, S.; Thangaswamy, S.; Raju, A.; Husain, F.M.; Ahmed, B.; Al-Shabib, N.A.; Hakeem, M.J.; Shahzad, S.A.; Abudujayn, S.A.; Alomar, S.Y. Biogenic Preparation and Characterization of Silver Nanoparticles from Seed Kernel of Mangifera Indica and Their Antibacterial Potential against Shigella Spp. Molecules 2023, 28, 2468. [Google Scholar] [CrossRef]
- Hani, U.; Kidwan, F.N.; Albarqi, L.A.; Al-qahtani, S.A.; AlHadi, R.M.; AlZaid, H.A.; Haider, N.; Ansari, M.A. Biogenic Silver Nanoparticle Synthesis Using Orange Peel Extract and Its Multifaceted Biomedical Application. Bioprocess. Biosyst. Eng. 2024, 47, 1363–1375. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Nadar, T.; Lakkakula, J.; Wagh, N.S. Biogenic Synthesis of Nanomaterials: Bioactive Compounds as Reducing, and Capping Agents. In Biogenic Nanomaterials for Environmental Sustainability: Principles, Practices, and Opportunities; Shah, M.P., Bharadvaja, N., Kumar, L., Eds.; Environmental Science and Engineering; Springer International Publishing: Cham, Switzerland, 2024; pp. 147–188. ISBN 978-3-031-45955-9. [Google Scholar]
- Ovais, M.; Khalil, A.T.; Islam, N.U.; Ahmad, I.; Ayaz, M.; Saravanan, M.; Shinwari, Z.K.; Mukherjee, S. Role of Plant Phytochemicals and Microbial Enzymes in Biosynthesis of Metallic Nanoparticles. Appl. Microbiol. Biotechnol. 2018, 102, 6799–6814. [Google Scholar] [CrossRef] [PubMed]
- Rigopoulos, N.; Gkaliouri, C.M.; Ioannou, Z.; Giaouris, E.; Sakavitsi, V.; Gournis, D. Green Synthesis of Silver Nanoparticles Using Olive Mill Wastewater and Olive Stones Extract and Testing Their Antimicrobial Activities against Escherichia Coli and Staphylococcus Epidermidis. Nano Express 2024, 5, 015026. [Google Scholar] [CrossRef]
- Terea, H.; Selloum, D.; Rebiai, A.; Bouafia, A.; Ben Mya, O. Preparation and Characterization of Cellulose/ZnO Nanoparticles Extracted from Peanut Shells: Effects on Antibacterial and Antifungal Activities. Biomass Conv. Bioref. 2024, 14, 19489–19500. [Google Scholar] [CrossRef]
- Meenu, B.; Raman, M.; Sreelakshmi, P.U.; Mathew, P.T. Effect of Mushroom Chitosan Coating on the Quality and Storability of Tomato (Solanum lycopersicum L.). J. Postharvest Technol. 2023, 11, 133–144. [Google Scholar]
- Erdem Büyükkiraz, M.; Kesmen, Z. Antimicrobial Peptides (AMPs): A Promising Class of Antimicrobial Compounds. J. Appl. Microbiol. 2022, 132, 1573–1596. [Google Scholar] [CrossRef]
- Thapliyal, D.; Tewari, K.; Verma, S.; Bhargava, C.K.; Sen, P.; Mehra, A.; Rana, S.; Verros, G.D.; Arya, R.K. Introduction: The Evolution of Functional Coatings from Protection to Innovation. In Functional Coatings for Biomedical, Energy, and Environmental Applications; Arya, R.K., Verros, G.D., Davim, J.P., Eds.; Wiley: Hoboken, NJ, USA, 2024; pp. 1–30. ISBN 978-1-394-26314-1. [Google Scholar]
- Flores-Félix, J.D. Abstracts of the 4th International Electronic Conference on Agronomy. In Proceedings of the IECAG 2024, Basel, Switzerland, 2–5 December 2024; MDPI: Basel, Switzerland, 2025; p. 5. [Google Scholar]
- Poprac, P.; Jomova, K.; Simunkova, M.; Kollar, V.; Rhodes, C.J.; Valko, M. Targeting Free Radicals in Oxidative Stress-Related Human Diseases. Trends Pharmacol. Sci. 2017, 38, 592–607. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.A.; Pérez De La Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Martinet, W.; De Meyer, G.R.Y.; Herman, A.G.; Kockx, M.M. Reactive Oxygen Species Induce RNA Damage in Human Atherosclerosis. Eur. J. Clin. Investig. 2004, 34, 323–327. [Google Scholar] [CrossRef]
- Thirumoorthy, G.; Balasubramanian, B.; George, J.A.; Nizam, A.; Nagella, P.; Srinatha, N.; Pappuswamy, M.; Alanazi, A.M.; Meyyazhagan, A.; Rengasamy, K.R.R.; et al. Phytofabricated Bimetallic Synthesis of Silver-Copper Nanoparticles Using Aerva Lanata Extract to Evaluate Their Potential Cytotoxic and Antimicrobial Activities. Sci. Rep. 2024, 14, 1270. [Google Scholar] [CrossRef]
- Mickymaray, S. Efficacy and Mechanism of Traditional Medicinal Plants and Bioactive Compounds against Clinically Important Pathogens. Antibiotics 2019, 8, 257. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Park, C.; Jang, H.-J.; Kim, B.; Bae, H.-W.; Chung, I.-Y.; Kim, E.S.; Cho, Y.-H. Antibacterial Strategies Inspired by the Oxidative Stress and Response Networks. J. Microbiol. 2019, 57, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Ezerskyte, E.; Butkiene, G.; Katelnikovas, A.; Klimkevicius, V. Development of Biocompatible, UV and NIR Excitable Nanoparticles with Multiwavelength Emission and Enhanced Colloidal Stability. ACS Mater. Au 2025, 5, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Apalowo, O.E.; Adegoye, G.A.; Obuotor, T.M. Microbial-Based Bioactive Compounds to Alleviate Inflammation in Obesity. Curr. Issues Mol. Biol. 2024, 46, 1810–1831. [Google Scholar] [CrossRef]
- Tang, S.; Zheng, J. Antibacterial Activity of Silver Nanoparticles: Structural Effects. Adv. Healthc. Mater. 2018, 7, 1701503. [Google Scholar] [CrossRef]
- Bazzi, W.; Abou Fayad, A.G.; Nasser, A.; Haraoui, L.-P.; Dewachi, O.; Abou-Sitta, G.; Nguyen, V.-K.; Abara, A.; Karah, N.; Landecker, H.; et al. Heavy Metal Toxicity in Armed Conflicts Potentiates AMR in A. Baumannii by Selecting for Antibiotic and Heavy Metal Co-Resistance Mechanisms. Front. Microbiol. 2020, 11, 68. [Google Scholar] [CrossRef]
- Rudramurthy, G.R.; Swamy, M.K. Potential Applications of Engineered Nanoparticles in Medicine and Biology: An Update. J. Biol. Inorg. Chem. 2018, 23, 1185–1204. [Google Scholar] [CrossRef]
- Shumbula, N.P.; Ndala, Z.B.; Nkabinde, S.S.; Nchoe, O.; Mpelane, S.; Shumbula, P.M.; Muthwa, S.F.; Mdluli, P.S.; Mente, P.; Njengele-Tetyana, Z.; et al. Investigation of Antimicrobial Activity and Cytotoxicity of Silver Nanoparticle Synthesized Using Dopamine as a Reducing and Capping Agent. ChemistrySelect 2024, 9, e202303328. [Google Scholar] [CrossRef]
- Alnehia, A.; Al-Sharabi, A.; Al-Hammadi, A.H.; Al-Odayni, A.-B.; Alramadhan, S.A.; Alodeni, R.M. Phyto-Mediated Synthesis of Silver-Doped Zinc Oxide Nanoparticles from Plectranthus Barbatus Leaf Extract: Optical, Morphological, and Antibacterial Properties. Biomass Conv. Bioref. 2024, 14, 17041–17053. [Google Scholar] [CrossRef]
- Govindasamy, G.A.; Sreekantan, S.; Saharudin, K.A.; Poliah, R.; Ong, M.T.; Thavamany, P.J.; Sahgal, G.; Tan, A.A. Composition-Dependent Physicochemical and Bactericidal Properties of Dual Cu-TiO2 Nanoparticles Incorporated in Polypropylene. BioNanoScience 2024, 14, 770–782. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Biswas, R.; Mondal, S.; Ansari, M.A.; Sarkar, T.; Condiuc, I.P.; Trifas, G.; Atanase, L.I. Chitosan and Its Derivatives as Nanocarriers for Drug Delivery. Molecules 2025, 30, 1297. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Liu, H.; Li, M.; Jiang, X.; Yu, H.; Sun, D. An Efficient Metal–Organic Framework-Based Drug Delivery Platform for Synergistic Antibacterial Activity and Osteogenesis. J. Colloid. Interface Sci. 2023, 640, 521–539. [Google Scholar] [CrossRef]
- Skłodowski, K.; Chmielewska-Deptuła, S.J.; Piktel, E.; Wolak, P.; Wollny, T.; Bucki, R. Metallic Nanosystems in the Development of Antimicrobial Strategies with High Antimicrobial Activity and High Biocompatibility. Int. J. Mol. Sci. 2023, 24, 2104. [Google Scholar] [CrossRef]
- Pereira, Y.; Lagniel, G.; Godat, E.; Baudouin-Cornu, P.; Junot, C.; Labarre, J. Chromate Causes Sulfur Starvation in Yeast. Toxicol. Sci. 2008, 106, 400–412. [Google Scholar] [CrossRef]
- Sharma, R.; Sharma, N.; Prashar, A.; Hansa, A.; Asgari Lajayer, B.; Price, G.W. Unraveling the Plethora of Toxicological Implications of Nanoparticles on Living Organisms and Recent Insights into Different Remediation Strategies: A Comprehensive Review. Sci. Total Environ. 2024, 906, 167697. [Google Scholar] [CrossRef] [PubMed]
- Wyszogrodzka, G.; Marszałek, B.; Gil, B.; Dorożyński, P. Metal-Organic Frameworks: Mechanisms of Antibacterial Action and Potential Applications. Drug Discov. Today 2016, 21, 1009–1018. [Google Scholar] [CrossRef]
- Tiki, Y.L.; Tolesa, L.D.; Tiwikrama, A.H.; Chala, T.F. Ginger (Zingiber officinale)-Mediated Green Synthesis of Silver-Doped Tin Oxide Nanoparticles and Evaluation of Its Antimicrobial Activity. ACS Omega 2024, 9, 11443–11452. [Google Scholar] [CrossRef]
- El-Abeid, S.E.; Mosa, M.A.; El-Tabakh, M.A.M.; Saleh, A.M.; El-Khateeb, M.A.; Haridy, M.S.A. Antifungal Activity of Copper Oxide Nanoparticles Derived from Zizyphus Spina Leaf Extract against Fusarium Root Rot Disease in Tomato Plants. J. Nanobiotechnol 2024, 22, 28. [Google Scholar] [CrossRef]
- Mazumder, D.; Mittal, R.; Nath, S.K. Green Synthesis of Silver Nanoparticles from Waste Vigna Mungo Plant and Evaluation of Its Antioxidant and Antibacterial Activity. Biomass Conv. Bioref. 2024, 15, 5839–5850. [Google Scholar] [CrossRef]
- Ahirwar, B.; Ahirwar, D.; Jain, R.; Agrawal, B.; Sahu, P.; Sakure, K.; Badwaik, H. Biofabricated Green Synthesized Hibiscus Silver Nanoparticles Potentiate Antibacterial Activity and Cytotoxicity in Human Lung Cancer Cells. Appl. Biochem. Biotechnol. 2024, 196, 7128–7144. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, S.; Siddiqui, V.U.; Bala, S.; Mishra, N.; Mishra, A.; Lawrence, R.; Bansal, P.; Khan, A.R.; Khan, T. Biogenic Synthesis of Copper Oxide Nanoparticles from Aloe Vera: Antibacterial Activity, Molecular Docking, and Photocatalytic Dye Degradation. ACS Omega 2024, 9, 30190–30204. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Rauf, A.; Khalil, A.A.; Luqman, M.; Islam, M.R.; Hemeg, H.A.; Ahmad, Z.; Al-Awthan, Y.S.; Bahattab, O.; Quradha, M.M. Peganum Harmala L. Extract-Based Gold (Au) and Silver (Ag) Nanoparticles (NPs): Green Synthesis, Characterization, and Assessment of Antibacterial and Antifungal Properties. Food Sci. Nutr. 2024, 12, 4459–4472. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, D.; Verduin, J.J.; Shah, M.; Sharma, S.B.; Poinern, G.E.J. A Review of Current Research into the Biogenic Synthesis of Metal and Metal Oxide Nanoparticles via Marine Algae and Seagrasses. J. Nanosci. 2017, 2017, 8013850. [Google Scholar] [CrossRef]
- Rana, A.; Yadav, K.; Jagadevan, S. A Comprehensive Review on Green Synthesis of Nature-Inspired Metal Nanoparticles: Mechanism, Application and Toxicity. J. Clean. Prod. 2020, 272, 122880. [Google Scholar] [CrossRef]
- Li, L.; Ma, C.; Yang, Y.; Wang, B.; Liu, X.; Wang, Y.; Bian, X.; Zhang, G.; Zhang, N. Exploring the Potential of Plant-Derived Metal Ion Binding Peptides: Preparation, Structure-Activity Relationship, and Biological Activities. Trends Food Sci. Technol. 2024, 152, 104650. [Google Scholar] [CrossRef]
- Najmi, A.; Javed, S.A.; Al Bratty, M.; Alhazmi, H.A. Modern Approaches in the Discovery and Development of Plant-Based Natural Products and Their Analogues as Potential Therapeutic Agents. Molecules 2022, 27, 349. [Google Scholar] [CrossRef]
- Zheng, G.; Zhang, B.; Yu, H.; Song, Z.; Xu, X.; Zheng, Z.; Zhao, K.; Zhao, J.; Zhao, Y. Therapeutic Applications and Potential Biological Barriers of Nano-Delivery Systems in Common Gastrointestinal Disorders: A Comprehensive Review. Adv. Compos. Hybrid. Mater. 2025, 8, 227. [Google Scholar] [CrossRef]
- Han, Y.; Guo, X.; Ji, Z.; Guo, Y.; Ma, W.; Du, H.; Guo, Y.; Xiao, H. Colon Health Benefits of Plant-Derived Exosome-like Nanoparticles via Modulating Gut Microbiota and Immunity. Crit. Rev. Food Sci. Nutr. 2025, 150, 1–21. [Google Scholar] [CrossRef]
- Rahman, M.A.; Jalouli, M.; Yadab, M.K.; Al-Zharani, M. Progress in Drug Delivery Systems Based on Nanoparticles for Improved Glioblastoma Therapy: Addressing Challenges and Investigating Opportunities. Cancers 2025, 17, 701. [Google Scholar] [CrossRef]
- Patadiya, A.; Mehta, D.; Karuppiah, N. Bridging Nature and Nanotechnology: A Review on the Potential of Herbal Nanoparticles in Medicine. E3S Web Conf. 2025, 619, 05005. [Google Scholar] [CrossRef]
- Slavin, J. Fiber and Prebiotics: Mechanisms and Health Benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef] [PubMed]
- Victoria Obayomi, O.; Folakemi Olaniran, A.; Olugbemiga Owa, S. Unveiling the Role of Functional Foods with Emphasis on Prebiotics and Probiotics in Human Health: A Review. J. Funct. Foods 2024, 119, 106337. [Google Scholar] [CrossRef]
- Brosseau, C.; Selle, A.; Palmer, D.J.; Prescott, S.L.; Barbarot, S.; Bodinier, M. Prebiotics: Mechanisms and Preventive Effects in Allergy. Nutrients 2019, 11, 1841. [Google Scholar] [CrossRef]
- Tör\Hos, G.; El-Ramady, H.; Prokisch, J.; Velasco, F.; Llanaj, X.; Nguyen, D.H.; Peles, F. Modulation of the Gut Microbiota with Prebiotics and Antimicrobial Agents from Pleurotus Ostreatus Mushroom. Foods 2023, 12, 2010. [Google Scholar] [CrossRef] [PubMed]
- Holkem, A.T.; Silva, M.P.D.; Favaro-Trindade, C.S. Probiotics and Plant Extracts: A Promising Synergy and Delivery Systems. Crit. Rev. Food Sci. Nutr. 2023, 63, 9561–9579. [Google Scholar] [CrossRef]
- Kustyawati, M.E.; Fadhallah, E.G.; Hidayati, S.; Pramesti, A.; Hidayat, L. The Potential of Oil Palm Mesocarp Fiber Waste as a Prebiotic Material—Chemical and Microbial Evaluation Using Probiotic Saccharomyces Cerevisiae, Lactobacillus Casei and Escherichia Coli. Ecol. Eng. Environ. Technol. 2024, 25, 339–346. [Google Scholar] [CrossRef]
- Wang, M.; Chen, X.; Zhou, L.; Li, Y.; Yang, J.; Ji, N.; Xiong, L.; Sun, Q. Prebiotic Effects of Resistant Starch Nanoparticles on Growth and Proliferation of the Probiotic Lactiplantibacillus Plantarum Subsp. Plantarum. LWT 2022, 154, 112572. [Google Scholar] [CrossRef]
- Lopes, V.R.; Strømme, M.; Ferraz, N. In Vitro Biological Impact of Nanocellulose Fibers on Human Gut Bacteria and Gastrointestinal Cells. Nanomaterials 2020, 10, 1159. [Google Scholar] [CrossRef]
- Liu, Y.; Weng, P.; Liu, Y.; Wu, Z.; Wang, L.; Liu, L. Citrus Pectin Research Advances: Derived as a Biomaterial in the Construction and Applications of Micro/Nano-Delivery Systems. Food Hydrocoll. 2022, 133, 107910. [Google Scholar] [CrossRef]
- Pereira, B.; Marcondes, W.F.; Carvalho, W.; Arantes, V. High Yield Biorefinery Products from Sugarcane Bagasse: Prebiotic Xylooligosaccharides, Cellulosic Ethanol, Cellulose Nanofibrils and Lignin Nanoparticles. Bioresour. Technol. 2021, 342, 125970. [Google Scholar] [CrossRef] [PubMed]
- Lopes, S.A.; Roque-Borda, C.A.; Duarte, J.L.; Di Filippo, L.D.; Borges Cardoso, V.M.; Pavan, F.R.; Chorilli, M.; Meneguin, A.B. Delivery Strategies of Probiotics from Nano- and Microparticles: Trends in the Treatment of Inflammatory Bowel Disease—An Overview. Pharmaceutics 2023, 15, 2600. [Google Scholar] [CrossRef] [PubMed]
- Karnwal, A.; Jassim, A.Y.; Mohammed, A.A.; Sharma, V.; Al-Tawaha, A.R.M.S.; Sivanesan, I. Nanotechnology for Healthcare: Plant-Derived Nanoparticles in Disease Treatment and Regenerative Medicine. Pharmaceuticals 2024, 17, 1711. [Google Scholar] [CrossRef]
- Ahmed, O.; Sibuyi, N.R.S.; Fadaka, A.O.; Madiehe, M.A.; Maboza, E.; Meyer, M.; Geerts, G. Plant Extract-Synthesized Silver Nanoparticles for Application in Dental Therapy. Pharmaceutics 2022, 14, 380. [Google Scholar] [CrossRef]
- Pacyga, K.; Pacyga, P.; Szuba, E.; Viscardi, S.; Topola, E.; Duda-Madej, A. Nanotechnology Meets Phytotherapy: A Cutting-Edge Approach to Treat Bacterial Infections. Int. J. Mol. Sci. 2025, 26, 1254. [Google Scholar] [CrossRef] [PubMed]
- Barathi, S.; Ramalingam, S.; Krishnasamy, G.; Lee, J. Exploring the Biomedical Frontiers of Plant-Derived Nanoparticles: Synthesis and Biological Reactions. Pharmaceutics 2024, 16, 923. [Google Scholar] [CrossRef]
- Abdellatif, A.A.H.; Mostafa, M.A.H.; Konno, H.; Younis, M.A. Exploring the Green Synthesis of Silver Nanoparticles Using Natural Extracts and Their Potential for Cancer Treatment. 3 Biotech 2024, 14, 274. [Google Scholar] [CrossRef]
- Agbarya, A.; Ruimi, N.; Epelbaum, R.; Ben-Arye, E.; Mahajna, J. Natural Products as Potential Cancer Therapy Enhancers: A Preclinical Update. SAGE Open Med. 2014, 2, 2050312114546924. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.S.E.; Tay, H.L.; Tan, S.H.; Lee, T.H.; Ng, T.M.; Lye, D.C. Gut Microbiota Modulation: Implications for Infection Control and Antimicrobial Stewardship. Adv. Ther. 2020, 37, 4054–4067. [Google Scholar] [CrossRef]
- Kesharwani, R.K.; Kumar, P.; Keservani, R.K. (Eds.) The Nature of Nutraceuticals: History, Properties, Sources, and Nanotechnology, 1st ed.; Apple Academic Press: Palm Bay, FL, USA, 2025; ISBN 978-1-003-51896-9. [Google Scholar]
- Gargiullo, L.; Del Chierico, F.; D’Argenio, P.; Putignani, L. Gut Microbiota Modulation for Multidrug-Resistant Organism Decolonization: Present and Future Perspectives. Front. Microbiol. 2019, 10, 1704. [Google Scholar] [CrossRef]
- Pérez-Cobas, A.E.; Artacho, A.; Knecht, H.; Ferrús, M.L.; Friedrichs, A.; Ott, S.J.; Moya, A.; Latorre, A.; Gosalbes, M.J. Differential Effects of Antibiotic Therapy on the Structure and Function of Human Gut Microbiota. PLoS ONE 2013, 8, e80201. [Google Scholar] [CrossRef] [PubMed]
- Kamel, M.; Aleya, S.; Alsubih, M.; Aleya, L. Microbiome Dynamics: A Paradigm Shift in Combatting Infectious Diseases. J. Pers. Med. 2024, 14, 217. [Google Scholar] [CrossRef]
- Ahlawat, S.; Asha; Sharma, K.K. Gut–Organ Axis: A Microbial Outreach and Networking. Lett. Appl. Microbiol. 2021, 72, 636–668. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the Human Gut Microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Piccioni, A.; Cicchinelli, S.; Valletta, F.; De Luca, G.; Longhitano, Y.; Candelli, M.; Ojetti, V.; Sardeo, F.; Navarra, S.; Covino, M.; et al. Gut Microbiota and Autoimmune Diseases: A Charming Real World Together with Probiotics. Curr. Med. Chem. 2022, 29, 3147–3159. [Google Scholar] [CrossRef] [PubMed]
- Hugon, P.; Dufour, J.-C.; Colson, P.; Fournier, P.-E.; Sallah, K.; Raoult, D. A Comprehensive Repertoire of Prokaryotic Species Identified in Human Beings. Lancet Infect. Dis. 2015, 15, 1211–1219. [Google Scholar] [CrossRef]
- Ghosh, S.; Pramanik, S. Structural Diversity, Functional Aspects and Future Therapeutic Applications of Human Gut Microbiome. Arch. Microbiol. 2021, 203, 5281–5308. [Google Scholar] [CrossRef]
- Gorvitovskaia, A.; Holmes, S.P.; Huse, S.M. Interpreting Prevotella and Bacteroides as Biomarkers of Diet and Lifestyle. Microbiome 2016, 4, 15. [Google Scholar] [CrossRef]
- Hidalgo-Cantabrana, C.; Delgado, S.; Ruiz, L.; Ruas-Madiedo, P.; Sánchez, B.; Margolles, A. Bifidobacteria and Their Health-Promoting Effects. In Bugs as Drugs; Britton, R.A., Cani, P.D., Eds.; ASM Press: Washington, DC, USA, 2018; pp. 73–98. ISBN 978-1-68367-080-3. [Google Scholar]
- Hoegenauer, C.; Hammer, H.F.; Mahnert, A.; Moissl-Eichinger, C. Methanogenic Archaea in the Human Gastrointestinal Tract. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 805–813. [Google Scholar] [CrossRef]
- Kreulen, I.A.M.; De Jonge, W.J.; Van Den Wijngaard, R.M.; Van Thiel, I.A.M. Candida Spp. in Human Intestinal Health and Disease: More than a Gut Feeling. Mycopathologia 2023, 188, 845–862. [Google Scholar] [CrossRef]
- Manrique, P.; Dills, M.; Young, M. The Human Gut Phage Community and Its Implications for Health and Disease. Viruses 2017, 9, 141. [Google Scholar] [CrossRef] [PubMed]
- Hamad, I.; Raoult, D.; Bittar, F. Repertory of Eukaryotes (Eukaryome) in the Human Gastrointestinal Tract: Taxonomy and Detection Methods. Parasite Immunol. 2016, 38, 12–36. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-Millán, M.J.; Pérez-Matute, P.; Oteo, J.A. Gut Microbiota: A Key Player in Health and Disease. A Review Focused on Obesity. J. Physiol. Biochem. 2015, 71, 509–525. [Google Scholar] [CrossRef] [PubMed]
- Belizário, J.E.; Faintuch, J. Microbiome and Gut Dysbiosis. In Metabolic Interaction in Infection; Silvestre, R., Torrado, E., Eds.; Experientia Supplementum; Springer International Publishing: Cham, Switzerland, 2018; Volume 109, pp. 459–476. ISBN 978-3-319-74931-0. [Google Scholar]
- Cai, Y.; Chen, L.; Zhang, S.; Zeng, L.; Zeng, G. The Role of Gut Microbiota in Infectious Diseases. WIREs Mech. Dis. 2022, 14, e1551. [Google Scholar] [CrossRef]
- Sun, M.; He, C.; Cong, Y.; Liu, Z. Regulatory Immune Cells in Regulation of Intestinal Inflammatory Response to Microbiota. Mucosal Immunol. 2015, 8, 969–978. [Google Scholar] [CrossRef]
- Calo-Mata, P.; Ageitos, J.M.; Böhme, K.; Barros-Velázquez, J. Intestinal Microbiota: First Barrier Against Gut-Affecting Pathogens. In New Weapons to Control Bacterial Growth; Villa, T.G., Vinas, M., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 281–314. ISBN 978-3-319-28366-1. [Google Scholar]
- Iacob, S.; Iacob, D.G. Infectious Threats, the Intestinal Barrier, and Its Trojan Horse: Dysbiosis. Front. Microbiol. 2019, 10, 1676. [Google Scholar] [CrossRef]
- Ciabattini, A.; Olivieri, R.; Lazzeri, E.; Medaglini, D. Role of the Microbiota in the Modulation of Vaccine Immune Responses. Front. Microbiol. 2019, 10, 1305. [Google Scholar] [CrossRef] [PubMed]
- Fishbein, S.R.S.; Mahmud, B.; Dantas, G. Antibiotic Perturbations to the Gut Microbiome. Nat. Rev. Microbiol. 2023, 21, 772–788. [Google Scholar] [CrossRef]
- Hong, L.; Lee, S.-M.; Kim, W.-S.; Choi, Y.-J.; Oh, S.-H.; Li, Y.-L.; Choi, S.-H.; Chung, D.H.; Jung, E.; Kang, S.-K.; et al. Synbiotics Containing Nanoprebiotics: A Novel Therapeutic Strategy to Restore Gut Dysbiosis. Front. Microbiol. 2021, 12, 715241. [Google Scholar] [CrossRef]
- Kumari, T.; Bag, K.K.; Das, A.B.; Deka, S.C. Synergistic Role of Prebiotics and Probiotics in Gut Microbiome Health: Mechanisms and Clinical Applications. Food Bioeng. 2024, 3, 407–424. [Google Scholar] [CrossRef]
- Mohamadzadeh, M.; Fazeli, A.; Shojaosadati, S.A. Polysaccharides and Proteins-Based Bionanocomposites for Microencapsulation of Probiotics to Improve Stability and Viability in the Gastrointestinal Tract: A Review. Int. J. Biol. Macromol. 2024, 259, 129287. [Google Scholar] [CrossRef]
- De Lima, J.S.; Leão, A.D.; De Jesus Oliveira, A.C.; Chaves, L.L.; Ramos, R.K.L.G.; Rodrigues, C.F.C.; Soares-Sobrinho, J.L.; Soares, M.F.D.L.R. Potential of Plant-Based Polysaccharides as Therapeutic Agents in Ulcerogenic Diseases of the Gastrointestinal Tract: A Review. Int. J. Biol. Macromol. 2024, 281, 136399. [Google Scholar] [CrossRef]
- Peng, P.; Feng, T.; Yang, X.; Nie, C.; Yu, L.; Ding, R.; Zhou, Q.; Jiang, X.; Li, P. Gastrointestinal Microenvironment Responsive Nanoencapsulation of Probiotics and Drugs for Synergistic Therapy of Intestinal Diseases. ACS Nano 2023, 17, 14718–14730. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Y. A Review on the Potential Reuse of Functional Polysaccharides Extracted from the By-Products of Mushroom Processing. Food Bioprocess. Technol. 2020, 13, 217–228. [Google Scholar] [CrossRef]
- Kumbhar, P.; Kolekar, K.; Patil, R.; Rhatwal, R.; Singh, S.K.; Dua, K.; Patravale, V.; Disouza, J. Advanced Drug Delivery Approaches Containing Synbiotics. In Synbiotics in Human Health: Biology to Drug Delivery; Dua, K., Ed.; Springer Nature: Singapore, 2024; pp. 459–472. ISBN 978-981-99-5574-9. [Google Scholar]
- Torres-Giner, S.; Prieto, C.; Lagaron, J.M. Nanomaterials to Enhance Food Quality, Safety, and Health Impact. Nanomaterials 2020, 10, 941. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Nazhand, A.; Lucarini, M.; Atanasov, A.G.; Souto, E.B.; Novellino, E.; Capasso, R.; Santini, A. An Updated Overview on Nanonutraceuticals: Focus on Nanoprebiotics and Nanoprobiotics. Int. J. Mol. Sci. 2020, 21, 2285. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Chang, L.; Gao, P.; Li, J.; Lu, X.; Hua, M.; Li, X.; Liu, X.; Lan, Y. Synbiotics Containing Sea Buckthorn Polysaccharides Ameliorate DSS-Induced Colitis in Mice via Regulating Th17/Treg Homeostasis through Intestinal Microbiota and Their Production of BA Metabolites and SCFAs. Int. J. Biol. Macromol. 2024, 276, 133794. [Google Scholar] [CrossRef]
- Zhu, R.; Yuan, W.; Xia, A.; Sun, X.; Yan, W.; Wu, T.; Wang, G.; Li, Y.; Yin, Q.; Li, Y. Inulin-Based Nanoparticle Modulates Gut Microbiota and Immune Microenvironment for Improving Colorectal Cancer Therapy. Adv. Funct. Mater. 2024, 34, 2407685. [Google Scholar] [CrossRef]
- Badawy, M.T.; Mostafa, M.; Khalil, M.S.; Abd-Elsalam, K.A. Agri-Food and Environmental Applications of Bionanomaterials Produced from Agri-Waste and Microbes. In Agri-Waste and Microbes for Production of Sustainable Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2022; pp. 441–463. ISBN 978-0-12-823575-1. [Google Scholar]
- Mandal, M.; Sarkar, A. Green Syntheses of Nanoparticles from Plant Growth–Promoting Microorganisms and Their Application in the Agri-Food Industries. In Nanotechnology and Nanomaterials in the Agri-Food Industries; Elsevier: Amsterdam, The Netherlands, 2024; pp. 185–204. ISBN 978-0-323-99682-2. [Google Scholar]
- Hu, Q.; Li, J.; Wang, T.; Xu, X.; Duan, Y.; Jin, Y. Polyphenolic Nanoparticle-Modified Probiotics for Microenvironment Remodeling and Targeted Therapy of Inflammatory Bowel Disease. ACS Nano 2024, 18, 12917–12932. [Google Scholar] [CrossRef]
- He, H.; Qin, Q.; Xu, F.; Chen, Y.; Rao, S.; Wang, C.; Jiang, X.; Lu, X.; Xie, C. Oral Polyphenol-Armored Nanomedicine for Targeted Modulation of Gut Microbiota–Brain Interactions in Colitis. Sci. Adv. 2023, 9, eadf3887. [Google Scholar] [CrossRef]
- Rasyida, A.; Rizkha Pradipta, T.; Tri Wicaksono, S.; Mitha Pratiwi, V.; Widya Rakhmawati, Y. Preliminary Study of Alginates Extracted from Brown Algae (Sargassum Sp.) Available in Madura Island as Composite Based Hydrogel Materials. Mater. Sci. Forum 2019, 964, 240–245. [Google Scholar] [CrossRef]
- Bealer, E.J.; Onissema-Karimu, S.; Rivera-Galletti, A.; Francis, M.; Wilkowski, J.; Salas-de La Cruz, D.; Hu, X. Protein–Polysaccharide Composite Materials: Fabrication and Applications. Polymers 2020, 12, 464. [Google Scholar] [CrossRef] [PubMed]
- Törős, G.; Béni, Á.; Balláné, A.K.; Semsey, D.; Ferroudj, A.; Prokisch, J. Production of Myco-Nanomaterial Products from Pleurotus Ostreatus (Agaricomycetes) Mushroom via Pyrolysis. Pharmaceutics 2025, 17, 591. [Google Scholar] [CrossRef] [PubMed]

| Size Range | Type of NPs | Plant Species | Precursors Used | Shape & Size | Inhibition Against | Method | Ref. |
|---|---|---|---|---|---|---|---|
| <15 nm | Silver-Copper NPs | Aerva lanata | AgNO3 & CuSO4 | Semi-spherical cluster, avg. 9.5 nm | S. aureus, P. aeruginosa | Agar well diffusion | [117] |
| Silver-Tin Oxide NPs | Zingiber officinale | SnCl4 & AgNO3 | Cubic crystal, avg. 9.5 nm | S. aureus, E. coli, F. oxysporum, F. graminearum | Disc diffusion | [136] | |
| 15–30 nm | Copper Oxide NPs | Zizyphus spina-christi | CuSO4 | Spherical, 13.4–30.9 nm | F. solani | Agar dilution | [137] |
| Silver NPs | Vigna mungo | AgNO3 | Cubic, avg. 24.49 nm | E. coli, S. aureus | Agar well diffusion | [138] | |
| Silver NPs | Hibiscus sabdariffa | AgNO3 | Spherical, avg. 21.22 ± 5.17 nm | E. coli, S. aureus | Disc diffusion | [139] | |
| Copper Oxide NPs | Aloe barbadensis | Cu(NO3)2·3H2O | Shape unspecified, <20 nm | L. monocytogenes, K. pneumoniae, S. typhi, P. aeruginosa | Agar well diffusion | [140] | |
| >30 nm | Silver NPs | Peganum harmala | AgNO3 | Oval, 42–72 nm | S. typhi, P. aeruginosa, E. coli, B. subtilis, S. aureus, C. albicans, A. niger, P. notatum | Micro dilution | [141] |
| Gold NPs | Peganum harmala | HauCl4 | Spherical, 12.6–35.7 nm | S. typhi, P. aeruginosa, E. coli, B. subtilis, S. aureus, C. albicans, A. niger, P. notatum | Micro dilution | [141] |
| Extract Source | Nanoparticle Type | Application | Study Phase | Key Findings | Ref. |
|---|---|---|---|---|---|
| Neem Leaf | Silver (AgNPs) | Wound healing in diabetic ulcers | Pilot Clinical | Reduced wound size and microbial load | [162] |
| Pomegranate Peel | Silver (AgNPs) | Oral antimicrobial rinse | Early Clinical | Biofilm reduction in healthy volunteers | [163] |
| Green Tea | Gold/AgNPs | Oral biocompatibility, antimicrobial | Phase I | No adverse effects; biofilm inhibition | [164] |
| Neem+ Green Tea | Various NPs | General antimicrobial, wound care | Preclinical | Biocompatibility, microbial inhibition | [165] |
| Neem+ Pomegranate) | Silver NPs | Cancer models | Preclinical | Anticancer activity, ROS-mediated apoptosis | [166] |
| Green Tea (Catechins) | Polymer NPs | Oncology–breast/prostate cancer | Early Clinical | Enhanced efficacy, fewer side effects | [167] |
| Application Field | Description | Ref. |
|---|---|---|
| Nanotechnology in Agriculture | - Enhancing disease resistance and promoting human health by transforming waste into valuable therapeutic tools. | [30] |
| Nanoencapsulation Technologies | - Enhances stability, bioavailability, and delivery of prebiotics, probiotics, and synbiotics. Byproducts, such as cellulose and metal oxides, provide antimicrobial properties that benefit food preservation. | [30] |
| Gut Microbiota and Disease Resistance | - Target pathogenic microbes while preserving beneficial gut bacteria, such as Bifidobacteria and Lactobacilli. This helps maintain a balanced microbiome and supports gut health. | [193,194] |
| Prebiotic Nanoencapsulation | - Nanoencapsulation of prebiotics, such as inulin and fructooligosaccharides, enhances their stability and bioavailability, improving their effectiveness in the digestive system. | [195,196] |
| Probiotic Nanoencapsulation | - Probiotics can be protected using nanotechnology, such as alginate-based coatings and bionanocomposites, which increase their effectiveness by protecting them during gastric transit and enhancing their lifespan. | [197,198] |
| Synbiotic Nanoencapsulation | - Synbiotics (a combination of prebiotics and probiotics) are encapsulated using nanotechnology to improve their stability and therapeutic effects. They can potentially restore microbial balance and prevent infections. | [193,199] |
| Functional Foods and Bioactive Components | - Functional foods enriched with nanoparticles, such as polyphenols and carotenoids, improve gut health by enhancing bioavailability and promoting balance in the gut microbiome. | [200,201] |
| Nanoenhanced Functional Foods | - These foods boost the production of short-chain fatty acids (SCFAs) and other beneficial metabolites. They help lower inflammation, improve gut barrier function, and contribute to disease resistance. | [202,203] |
| Nanoagricultural Developments | - Innovations in nanoagriculture alter the gut environment by promoting beneficial bacteria growth, suppressing pathogens, and enhancing immune responses, leading to better disease resistance. | [204,205] |
| Bioactive Chemicals Synergy | - Nanoparticles combined with bioactive chemicals like polyphenols and prebiotics synergize to reduce oxidative stress, inflammation, and dysbiosis, helping to prevent and treat gastrointestinal illnesses. | [206,207] |
| Innovations in Probiotic Delivery | - Techniques such as alginate-based coatings, bionanocomposites, and pullulan nanoparticles derived from agricultural waste improve probiotics’ stability and antimicrobial properties. | [208,209] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Törős, G.; Gulyás, G.; El-Ramady, H.; Alibrahem, W.; Muthu, A.; Gangakhedkar, P.; Atieh, R.; Prokisch, J. Sustainable Nanotechnology Strategies for Modulating the Human Gut Microbiota. Int. J. Mol. Sci. 2025, 26, 5433. https://doi.org/10.3390/ijms26125433
Törős G, Gulyás G, El-Ramady H, Alibrahem W, Muthu A, Gangakhedkar P, Atieh R, Prokisch J. Sustainable Nanotechnology Strategies for Modulating the Human Gut Microbiota. International Journal of Molecular Sciences. 2025; 26(12):5433. https://doi.org/10.3390/ijms26125433
Chicago/Turabian StyleTörős, Gréta, Gabriella Gulyás, Hassan El-Ramady, Walaa Alibrahem, Arjun Muthu, Prasad Gangakhedkar, Reina Atieh, and József Prokisch. 2025. "Sustainable Nanotechnology Strategies for Modulating the Human Gut Microbiota" International Journal of Molecular Sciences 26, no. 12: 5433. https://doi.org/10.3390/ijms26125433
APA StyleTörős, G., Gulyás, G., El-Ramady, H., Alibrahem, W., Muthu, A., Gangakhedkar, P., Atieh, R., & Prokisch, J. (2025). Sustainable Nanotechnology Strategies for Modulating the Human Gut Microbiota. International Journal of Molecular Sciences, 26(12), 5433. https://doi.org/10.3390/ijms26125433










