Unveiling the Involvement of Extracellular Vesicles in Breast Cancer’s Organotrophic Metastasis: Molecular Mechanisms and Translational Prospects
Abstract
1. Introduction
1.1. Breast Cancer Classification and Metastasis
1.2. EVs and Tumor Metastasis
2. EVs and Organ-Specific Metastasis of Breast Cancer
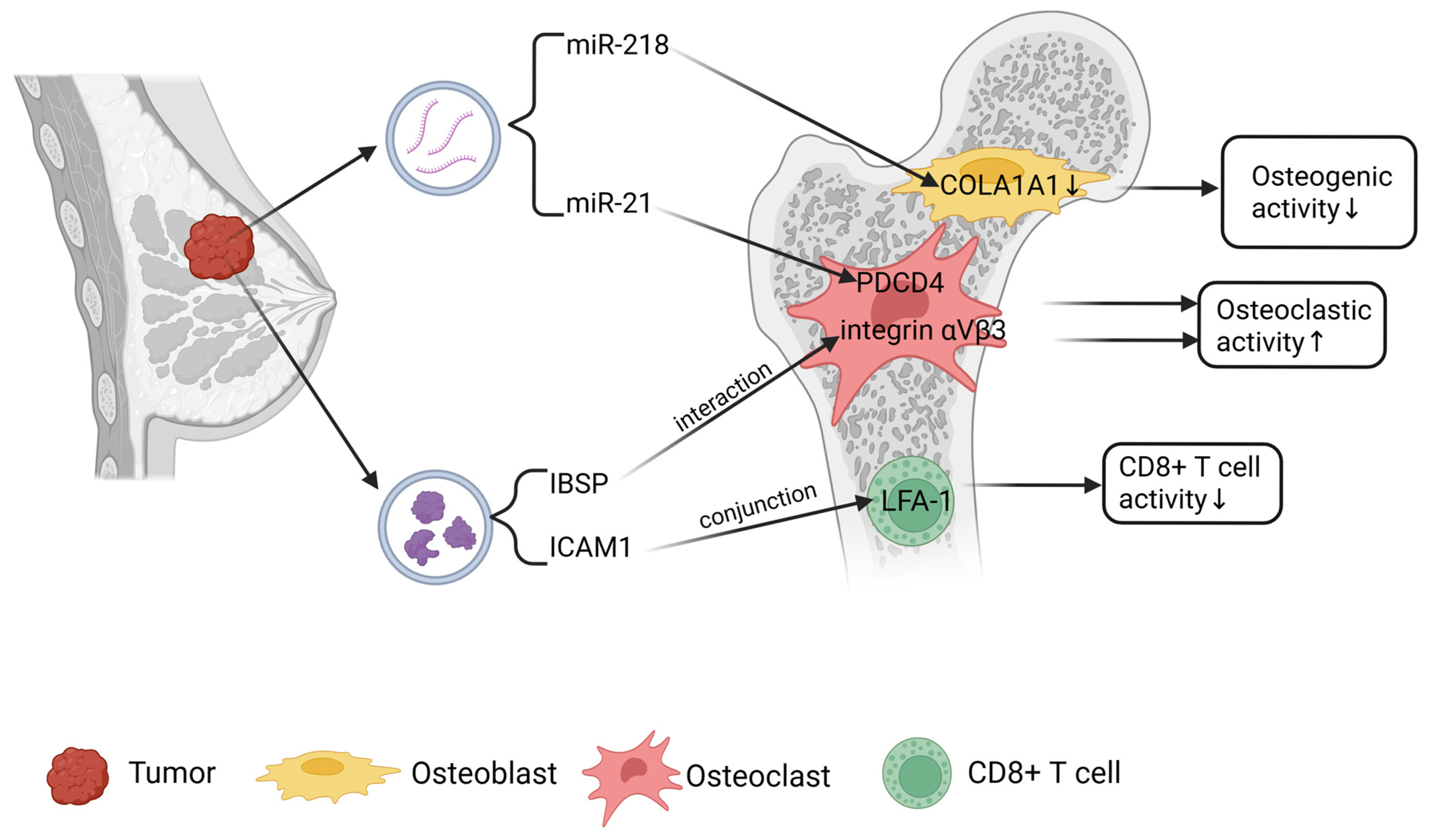
2.1. Bone Metastasis of Breast Cancer
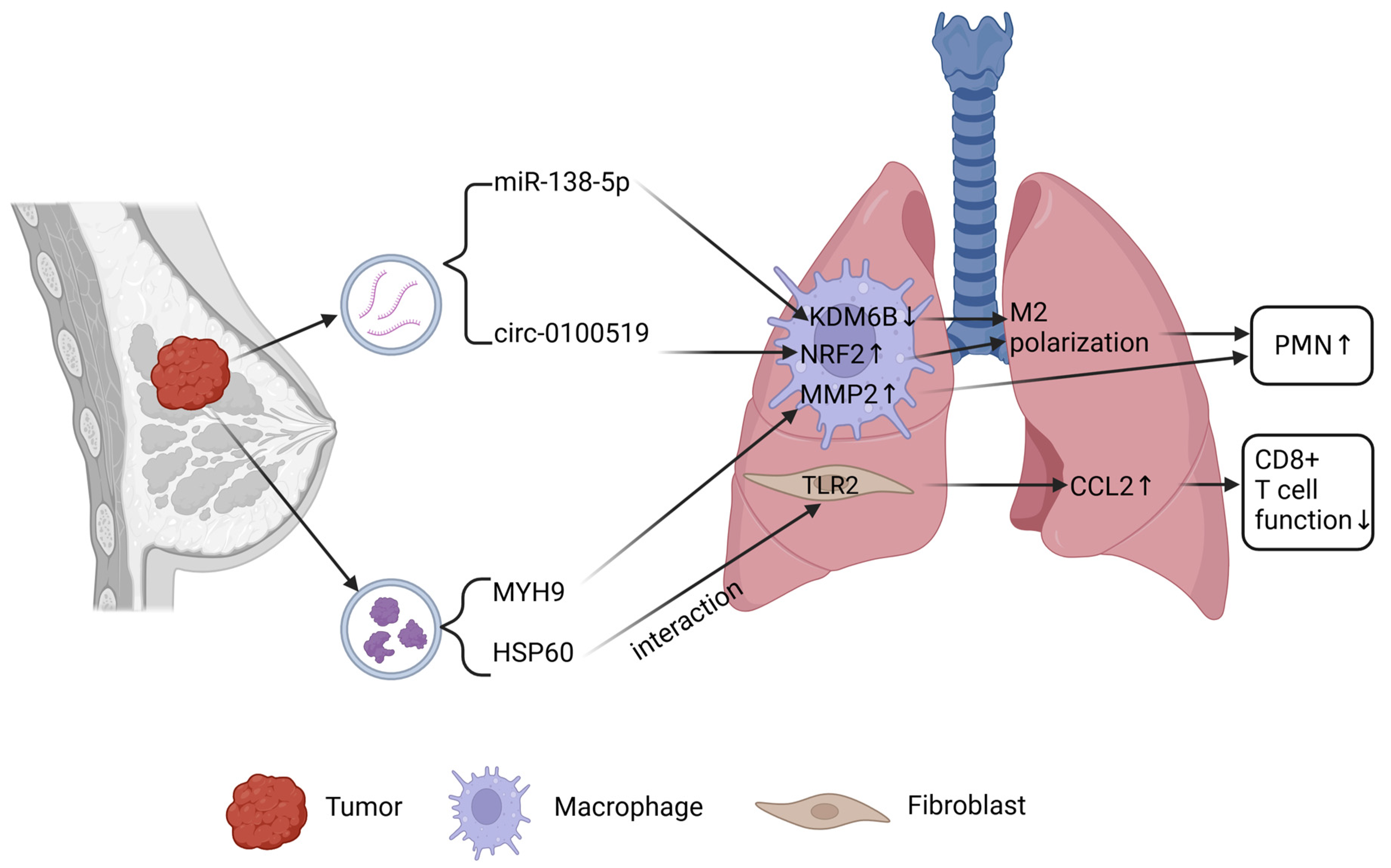
2.2. Lung Metastasis of Breast Cancer
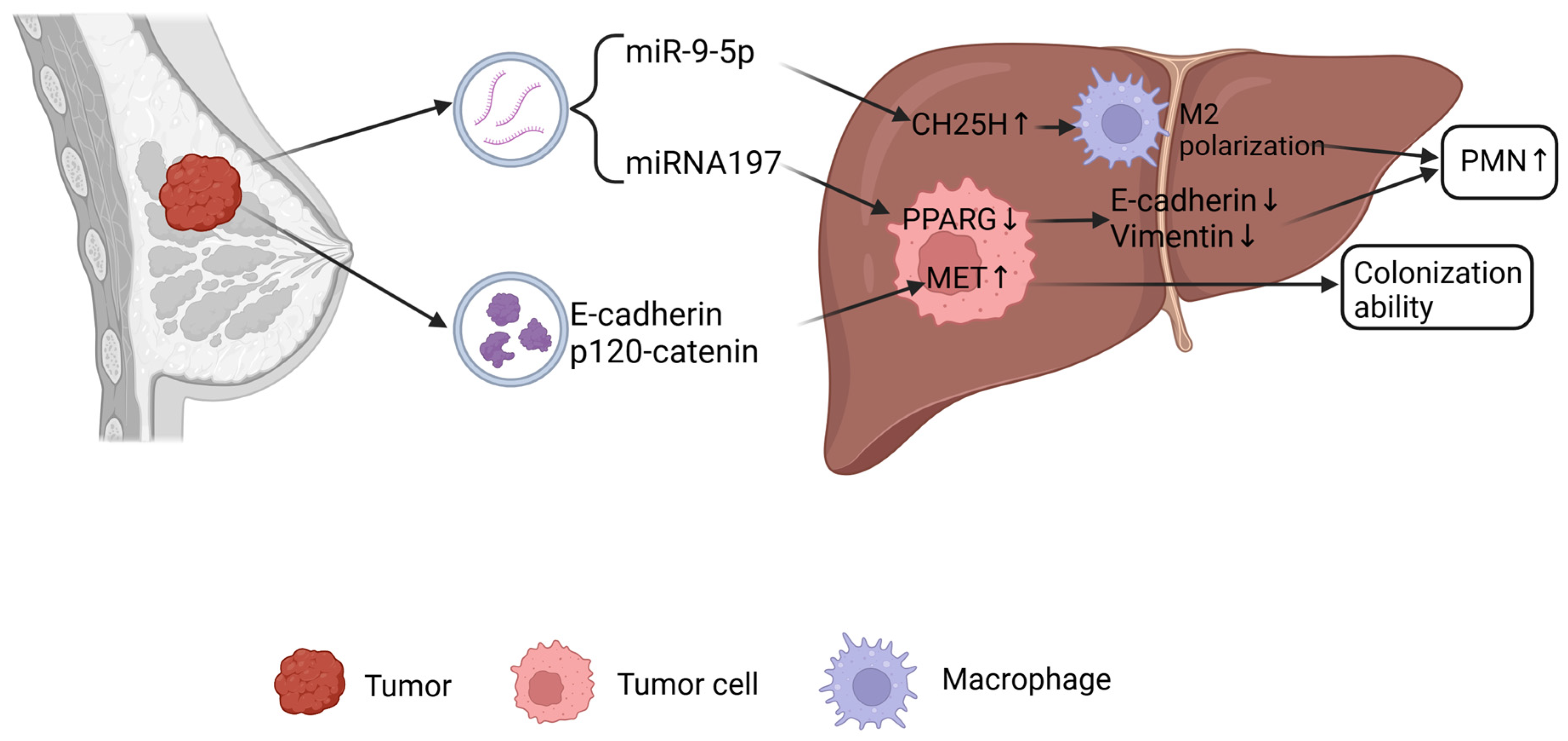
2.3. Liver Metastasis of Breast Cancer
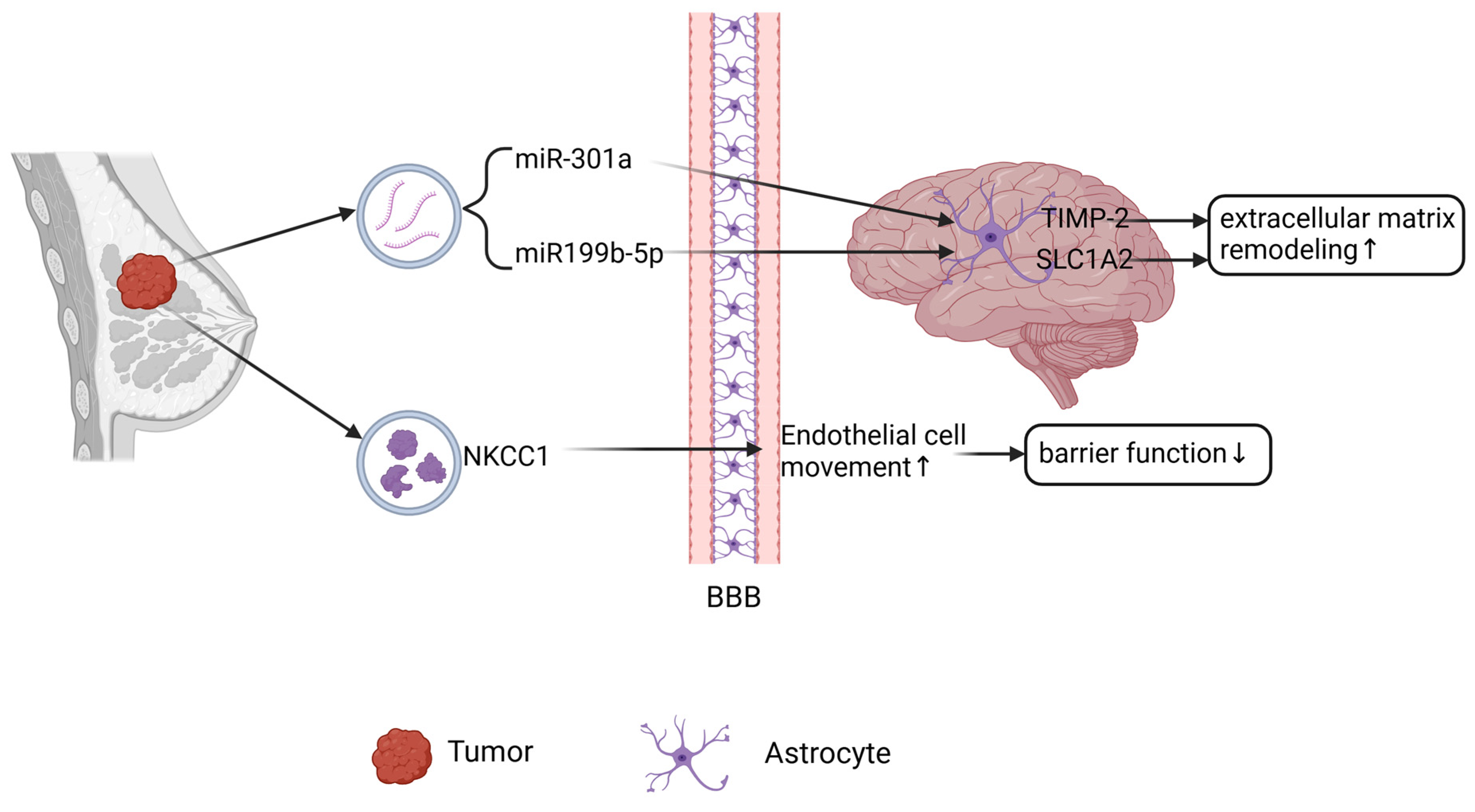
2.4. Brain Metastasis of Breast Cancer
3. Clinical Diagnostics and Applications
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, J.; Harper, A.; McCormack, V.; Sung, H.; Houssami, N.; Morgan, E.; Mutebi, M.; Garvey, G.; Soerjomataram, I.; Fidler-Benaoudia, M.M. Global Patterns and Trends in Breast Cancer Incidence and Mortality across 185 Countries. Nat. Med. 2025, 31, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.S.; Conant, E.F.; Soo, M.S. Molecular Subtypes of Breast Cancer: A Review for Breast Radiologists. J. Breast Imaging 2021, 3, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Shen, L.; Luo, M.; Zhang, K.; Li, J.; Yang, Q.; Zhu, F.; Zhou, D.; Zheng, S.; Chen, Y.; et al. Circulating Tumor Cells: Biology and Clinical Significance. Signal Transduct. Target. Ther. 2021, 6, 404. [Google Scholar] [CrossRef]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast Cancer. Nat. Rev. Dis. Prim. 2019, 5, 66. [Google Scholar] [CrossRef]
- Wu, Q.; Li, J.; Zhu, S.; Wu, J.; Chen, C.; Liu, Q.; Wei, W.; Zhang, Y.; Sun, S. Breast Cancer Subtypes Predict the Preferential Site of Distant Metastases: A SEER Based Study. Oncotarget 2017, 8, 27990–27996. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Liu, Y.R.; Ji, P.; Hu, X.; Shao, Z.M. Impact of Molecular Subtypes on Metastatic Breast Cancer Patients a SEER Population-Based Study. Sci. Rep. 2017, 7, 45411. [Google Scholar] [CrossRef]
- Gerstberger, S.; Jiang, Q.; Ganesh, K. Metastasis. Cell 2023, 186, 1564–1579. [Google Scholar] [CrossRef]
- Loric, S.; Denis, J.A.; Desbene, C.; Sabbah, M.; Conti, M. Extracellular Vesicles in Breast Cancer: From Biology and Function to Clinical Diagnosis and Therapeutic Management. Int. J. Mol. Sci. 2023, 24, 7208. [Google Scholar] [CrossRef]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Dong, M.; Liu, Q.; Xu, Y.; Zhang, Q. Extracellular Vesicles: The Landscape in the Progression, Diagnosis, and Treatment of Triple-Negative Breast Cancer. Front. Cell Dev. Biol. 2022, 10, 842898. [Google Scholar] [CrossRef]
- Becker, A.; Thakur, B.K.; Weiss, J.M.; Kim, H.S.; Peinado, H.; Lyden, D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell 2016, 30, 836–848. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Shi, K.; Yang, S.; Liu, J.; Zhou, Q.; Wang, G.; Song, J.; Li, Z.; Zhang, Z.; Yuan, W. Effect of Exosomal miRNA on Cancer Biology and Clinical Applications. Mol. Cancer 2018, 17, 147. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-K.; Lee, J.; Kim, S.R.; Choi, D.-S.; Yoon, Y.J.; Kim, J.H.; Go, G.; Nhung, D.; Hong, K.; Jang, S.C.; et al. EVpedia: A Community Web Portal for Extracellular Vesicles Research. Bioinformatics 2015, 31, 933–939. [Google Scholar] [CrossRef]
- Kim, D.-K.; Kang, B.; Kim, O.Y.; Choi, D.; Lee, J.; Kim, S.R.; Go, G.; Yoon, Y.J.; Kim, J.H.; Jang, S.C.; et al. EVpedia: An Integrated Database of High-Throughput Data for Systemic Analyses of Extracellular Vesicles. J. Extracell. Vesicles 2013, 2, 20384. [Google Scholar] [CrossRef] [PubMed]
- Lowry, M.C.; Gallagher, W.M.; O’Driscoll, L. The Role of Exosomes in Breast Cancer. Clin. Chem. 2015, 61, 1457–1465. [Google Scholar] [CrossRef]
- Syn, N.; Wang, L.; Sethi, G.; Thiery, J.-P.; Goh, B.-C. Exosome-Mediated Metastasis: From Epithelial–Mesenchymal Transition to Escape from Immunosurveillance. Trends Pharmacol. Sci. 2016, 37, 606–617. [Google Scholar] [CrossRef]
- Gangoda, L.; Liem, M.; Ang, C.; Keerthikumar, S.; Adda, C.G.; Parker, B.S.; Mathivanan, S. Proteomic Profiling of Exosomes Secreted by Breast Cancer Cells with Varying Metastatic Potential. Proteomics 2017, 17, 1600370. [Google Scholar] [CrossRef]
- Macedo, F.; Ladeira, K.; Pinho, F.; Saraiva, N.; Bonito, N.; Pinto, L.; Gonçalves, F. Bone Metastases: An Overview. Oncol. Rev. 2017, 11, 321. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, B.; Fan, Q. Mechanisms of Breast Cancer Bone Metastasis. Cancer Lett. 2010, 292, 1–7. [Google Scholar] [CrossRef]
- Tsuzuki, S.; Park, S.H.; Eber, M.R.; Peters, C.M.; Shiozawa, Y. Skeletal Complications in Cancer Patients with Bone Metastases. Int. J. Urol. 2016, 23, 825–832. [Google Scholar] [CrossRef]
- Brook, N.; Brook, E.; Dharmarajan, A.; Dass, C.R.; Chan, A. Breast Cancer Bone Metastases: Pathogenesis and Therapeutic Targets. Int. J. Biochem. Cell Biol. 2018, 96, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Hill, P.A.; Tumber, A.; Papaioannou, S.; Meikle, M.C. The Cellular Actions of Interleukin-11 on Bone Resorption in Vitro. Endocrinology 1998, 139, 1564–1572. [Google Scholar] [CrossRef]
- Lee, A.W.-M.; States, D.J. Both Src-Dependent and -Independent Mechanisms Mediate Phosphatidylinositol 3-Kinase Regulation of Colony-Stimulating Factor 1-Activated Mitogen-Activated Protein Kinases in Myeloid Progenitors. Mol. Cell. Biol. 2000, 20, 6779–6798. [Google Scholar] [CrossRef]
- Kim, B.; Kim, H.; Jung, S.; Moon, A.; Noh, D.; Lee, Z.H.; Kim, H.J.; Kim, H. A CTGF-RUNX2-RANKL Axis in Breast and Prostate Cancer Cells Promotes Tumor Progression in Bone. J. Bone Miner. Res. 2020, 35, 155–166. [Google Scholar] [CrossRef]
- Liu, X.; Cao, M.; Palomares, M.; Wu, X.; Li, A.; Yan, W.; Fong, M.Y.; Chan, W.-C.; Wang, S.E. Metastatic Breast Cancer Cells Overexpress and Secrete miR-218 to Regulate Type I Collagen Deposition by Osteoblasts. Breast Cancer Res. 2018, 20, 127–139. [Google Scholar] [CrossRef]
- Hashimoto, K.; Ochi, H.; Sunamura, S.; Kosaka, N.; Mabuchi, Y.; Fukuda, T.; Yao, K.; Kanda, H.; Ae, K.; Okawa, A.; et al. Cancer-Secreted Hsa-miR-940 Induces an Osteoblastic Phenotype in the Bone Metastatic Microenvironment via Targeting ARHGAP1 and FAM134A. Proc. Natl. Acad. Sci. USA 2018, 115, 2204–2209. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Qian, N.; Ling, S.; Li, Y.; Sun, W.; Li, J.; Du, R.; Zhong, G.; Liu, C.; Yu, G.; et al. Breast Cancer Exosomes Contribute to Pre-Metastatic Niche Formation and Promote Bone Metastasis of Tumor Cells. Theranostics 2021, 11, 1429–1445. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhu, Y.; Li, L.; Zhou, S.; Yin, G.; Yu, G.; Cui, H. Breast Cancer Cell-derived Exosomal miR-20a-5p Promotes the Proliferation and Differentiation of Osteoclasts by Targeting SRCIN1. Cancer Med. 2019, 8, 5687–5701. [Google Scholar] [CrossRef]
- Wu, K.; Feng, J.; Lyu, F.; Xing, F.; Sharma, S.; Liu, Y.; Wu, S.-Y.; Zhao, D.; Tyagi, A.; Deshpande, R.P.; et al. Exosomal miR-19a and IBSP Cooperate to Induce Osteolytic Bone Metastasis of Estrogen Receptor-Positive Breast Cancer. Nat. Commun. 2021, 12, 5196–5214. [Google Scholar] [CrossRef]
- Puppo, M.; Croset, M.; Ceresa, D.; Valluru, M.K.; Canuas Landero, V.G.; Hernandez Guadarrama, M.; Iuliani, M.; Pantano, F.; Dawn Ottewell, P.; Clézardin, P. Protective Effects of miR-24-2-5p in Early Stages of Breast Cancer Bone Metastasis. Breast Cancer Res. 2024, 26, 186–205. [Google Scholar] [CrossRef]
- Liu, X.; Ma, R.; Wei, F.; Wang, M.; Jiang, Y.; Zheng, P.; Cao, Z. Tumor-Derived Exosomal lncRNA-MIR193BHG Promotes Bone Metastasis of Breast Cancer by Targeting the miR-489-3p/DNMT3A Signaling Axis in Osteoclasts. J. Transl. Med. 2025, 23, 142–160. [Google Scholar] [CrossRef]
- Chen, M.; Fu, Z.; Wu, C. Tumor-Derived Exosomal ICAM1 Promotes Bone Metastasis of Triple-Negative Breast Cancer by Inducing CD8+ T Cell Exhaustion. Int. J. Biochem. Cell Biol. 2024, 175, 106637–106647. [Google Scholar] [CrossRef] [PubMed]
- Tiedemann, K.; Sadvakassova, G.; Mikolajewicz, N.; Juhas, M.; Sabirova, Z.; Tabariès, S.; Gettemans, J.; Siegel, P.M.; Komarova, S.V. Exosomal Release of L-Plastin by Breast Cancer Cells Facilitates Metastatic Bone Osteolysis. Transl. Oncol. 2019, 12, 462–474. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, I.C.; Groom, A.C.; Chambers, A.F. Cancer Spread and Micrometastasis Development: Quantitative Approaches for in Vivo Models. Bioessays 2002, 24, 885–893. [Google Scholar] [CrossRef]
- Stott, S.L.; Hsu, C.-H.; Tsukrov, D.I.; Yu, M.; Miyamoto, D.T.; Waltman, B.A.; Rothenberg, S.M.; Shah, A.M.; Smas, M.E.; Korir, G.K.; et al. Isolation of Circulating Tumor Cells Using a Microvortex-Generating Herringbone-Chip. Proc. Natl. Acad. Sci. USA 2010, 107, 18392–18397. [Google Scholar] [CrossRef]
- Xun, J.; Du, L.; Gao, R.; Shen, L.; Wang, D.; Kang, L.; Chen, C.; Zhang, Z.; Zhang, Y.; Yue, S.; et al. Cancer-Derived Exosomal miR-138-5p Modulates Polarization of Tumor-Associated Macrophages through Inhibition of KDM6B. Theranostics 2021, 11, 6847–6859. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, M.; Zhang, X.; Ji, J.; Zhang, H.; Shen, L.; Zhu, Y.; Liu, X. Exosomal Circ-0100519 Promotes Breast Cancer Progression via Inducing M2 Macrophage Polarisation by USP7/NRF2 Axis. Clin. Transl. Med. 2024, 14, e1763. [Google Scholar] [CrossRef]
- Feng, L.; Weng, J.; Yao, C.; Wang, R.; Wang, N.; Zhang, Y.; Tanaka, Y.; Su, L. Extracellular Vesicles Derived from SIPA1high Breast Cancer Cells Enhance Macrophage Infiltration and Cancer Metastasis through Myosin-9. Biology 2022, 11, 543. [Google Scholar] [CrossRef]
- Sun, X.; Wang, X.; Yan, C.; Zheng, S.; Gao, R.; Huang, F.; Wei, Y.; Wen, Z.; Chen, Y.; Zhou, X.; et al. Tumor Cell–Released LC3-positive EVs Promote Lung Metastasis of Breast Cancer through Enhancing Premetastatic Niche Formation. Cancer Sci. 2022, 113, 3405–3416. [Google Scholar] [CrossRef]
- Qi, M.; Xia, Y.; Wu, Y.; Zhang, Z.; Wang, X.; Lu, L.; Dai, C.; Song, Y.; Xu, K.; Ji, W.; et al. Lin28B-High Breast Cancer Cells Promote Immune Suppression in the Lung Pre-Metastatic Niche via Exosomes and Support Cancer Progression. Nat. Commun. 2022, 13, 897. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, J.; Wang, M.; Yang, J.; Zhu, S.; Li, L.; Hu, X.; Wang, Z.; Pang, L.; Li, P.; et al. Chronic Stress-induced and Tumor Derived SP1+ Exosomes Polarizing IL-1β+ Neutrophils to Increase Lung Metastasis of Breast Cancer. Adv. Sci. 2025, 12, 2310266. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Zong, S.; Wang, Y.; Zhou, Y.; Wang, K.; Shi, F.; Wang, J.; Feng, M.; Luo, W.; Zhang, L.; et al. Breast Cancer-Derived CAV1 Promotes Lung Metastasis by Regulating Integrin A6β4 and the Recruitment and Polarization of Tumor-Associated Neutrophils. Int. J. Biol. Sci. 2024, 20, 5695–5714. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Li, Q.; Liu, G.; Huang, X.; Li, A.; Guo, H.; Qi, L.; Zhang, J.; Song, J.; Su, X.; et al. Endosomal Protein DENND10/FAM45A Integrates Extracellular Vesicle Release with Cancer Cell Migration. BMC Biol. 2024, 22, 154. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Gu, J.; Wang, Y.; Wu, H.; Cheng, W.; Wang, Q.; Zheng, G.; Wang, X. Long Non-coding RNA NEAT1 Transported by Extracellular Vesicles Contributes to Breast Cancer Development by Sponging microRNA-141-3p and Regulating KLF12. Cell Biosci. 2021, 11, 68. [Google Scholar] [CrossRef]
- Duan, S.; Nordmeier, S.; Byrnes, A.E.; Buxton, I.L.O. Extracellular Vesicle-Mediated Purinergic Signaling Contributes to Host Microenvironment Plasticity and Metastasis in Triple Negative Breast Cancer. Int. J. Mol. Sci. 2021, 22, 597. [Google Scholar] [CrossRef]
- Furusato, B.; Mohamed, A.; Uhlén, M.; Rhim, J.S. CXCR4 and Cancer: CXCR4 and Cancer. Pathol. Int. 2010, 60, 497–505. [Google Scholar] [CrossRef]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.J.; Nieto, M.A. Epithelial-Mesenchymal Transitions in Development and Disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef]
- Auguste, P.; Fallavollita, L.; Wang, N.; Burnier, J.; Bikfalvi, A.; Brodt, P. The Host Inflammatory Response Promotes Liver Metastasis by Increasing Tumor Cell Arrest and Extravasation. Am. J. Pathol. 2007, 170, 1781–1792. [Google Scholar] [CrossRef]
- Li, L.; Xiong, Y.; Wang, N.; Zhu, M.; Gu, Y. Breast Cancer Stem Cells-Derived Extracellular Vesicles Affect PPARG Expression by Delivering MicroRNA-197 in Breast Cancer Cells. Clin. Breast Cancer 2022, 22, 478–490. [Google Scholar] [CrossRef]
- Li, M.-X.; Hu, S.; Lei, H.-H.; Yuan, M.; Li, X.; Hou, W.-K.; Huang, X.-J.; Xiao, B.-W.; Yu, T.-X.; Zhang, X.-H.; et al. Tumor-Derived miR-9-5p-Loaded EVs Regulate Cholesterol Homeostasis to Promote Breast Cancer Liver Metastasis in Mice. Nat. Commun. 2024, 15, 10539–10559. [Google Scholar] [CrossRef]
- Heo, W.; Lee, W.; Cheun, J.H.; Lee, E.-S.; Li, S.; Kim, H.S.; Son, H.-Y.; Kim, J.H.; Woo, Y.D.; Chung, D.H.; et al. Triple-Negative Breast Cancer-Derived Extracellular Vesicles Promote a Hepatic Premetastatic Niche via a Cascade of Microenvironment Remodeling. Mol. Cancer Res. 2023, 21, 726–740. [Google Scholar] [CrossRef] [PubMed]
- Voglstaetter, M.; Thomsen, A.R.; Nouvel, J.; Koch, A.; Jank, P.; Navarro, E.G.; Gainey-Schleicher, T.; Khanduri, R.; Groß, A.; Rossner, F.; et al. Tspan8 Is Expressed in Breast Cancer and Regulates E-cadherin/Catenin Signalling and Metastasis Accompanied by Increased Circulating Extracellular Vesicles. J. Pathol. 2019, 248, 421–437. [Google Scholar] [CrossRef] [PubMed]
- Simsek, M.; Aliyev, A.; Baydas, T.; Besiroglu, M.; Demir, T.; Shbair, A.T.; Seker, M.; Turk, H.M. Breast Cancer Patients with Brain Metastases: A Cross-Sectional Study. Breast J. 2022, 2022, 5763810. [Google Scholar] [CrossRef]
- Darlix, A.; Louvel, G.; Fraisse, J.; Jacot, W.; Brain, E.; Debled, M.; Mouret-Reynier, M.A.; Goncalves, A.; Dalenc, F.; Delaloge, S.; et al. Impact of Breast Cancer Molecular Subtypes on the Incidence, Kinetics and Prognosis of Central Nervous System Metastases in a Large Multicentre Real-Life Cohort. Br. J. Cancer 2019, 121, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Hosonaga, M.; Saya, H.; Arima, Y. Molecular and Cellular Mechanisms Underlying Brain Metastasis of Breast Cancer. Cancer Metastasis Rev. 2020, 39, 711–720. [Google Scholar] [CrossRef]
- Busatto, S.; Song, T.; Kim, H.J.; Hallinan, C.; Lombardo, M.N.; Stemmer-Rachamimov, A.O.; Lee, K.; Moses, M.A. Breast Cancer-derived Extracellular Vesicles Modulate the Cytoplasmic and Cytoskeletal Dynamics of Blood-brain Barrier Endothelial Cells. J. Extracell. Vesicles 2025, 14, e70038. [Google Scholar] [CrossRef]
- Rodrigues, G.; Hoshino, A.; Kenific, C.M.; Matei, I.R.; Steiner, L.; Freitas, D.; Kim, H.S.; Oxley, P.R.; Scandariato, I.; Casanova-Salas, I.; et al. Tumour Exosomal CEMIP Protein Promotes Cancer Cell Colonization in Brain Metastasis. Nat. Cell Biol. 2019, 21, 1403–1412. [Google Scholar] [CrossRef]
- Morad, G.; Daisy, C.C.; Otu, H.H.; Libermann, T.A.; Dillon, S.T.; Moses, M.A. Cdc42-Dependent Transfer of Mir301 from Breast Cancer-Derived Extracellular Vesicles Regulates the Matrix Modulating Ability of Astrocytes at the Blood–Brain Barrier. Int. J. Mol. Sci. 2020, 21, 3851. [Google Scholar] [CrossRef]
- Ruan, X.; Yan, W.; Cao, M.; Daza, R.A.M.; Fong, M.Y.; Yang, K.; Wu, J.; Liu, X.; Palomares, M.; Wu, X.; et al. Breast Cancer Cell-Secreted miR-199b-5p Hijacks Neurometabolic Coupling to Promote Brain Metastasis. Nat. Commun. 2024, 15, 4549–4565. [Google Scholar] [CrossRef]
- Sirkisoon, S.R.; Wong, G.L.; Aguayo, N.R.; Doheny, D.L.; Zhu, D.; Regua, A.T.; Arrigo, A.; Manore, S.G.; Wagner, C.; Thomas, A.; et al. Breast Cancer Extracellular Vesicles-Derived miR-1290 Activates Astrocytes in the Brain Metastatic Microenvironment via the FOXA2→CNTF Axis to Promote Progression of Brain Metastases. Cancer Lett. 2022, 540, 215726–215740. [Google Scholar] [CrossRef]
- Xing, F.; Liu, Y.; Wu, S.-Y.; Wu, K.; Sharma, S.; Mo, Y.-Y.; Feng, J.; Sanders, S.; Jin, G.; Singh, R.; et al. Loss of XIST in Breast Cancer Activates MSN-c-Met and Reprograms Microglia via Exosomal miRNA to Promote Brain Metastasis. Cancer Res. 2018, 78, 4316–4330. [Google Scholar] [CrossRef] [PubMed]
- Caby, M.-P.; Lankar, D.; Vincendeau-Scherrer, C.; Raposo, G.; Bonnerot, C. Exosomal-like Vesicles Are Present in Human Blood Plasma. Int. Immunol. 2005, 17, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, T.; Sugimachi, K.; Iinuma, H.; Takahashi, Y.; Kurashige, J.; Sawada, G.; Ueda, M.; Uchi, R.; Ueo, H.; Takano, Y.; et al. Exosomal microRNA in Serum Is a Novel Biomarker of Recurrence in Human Colorectal Cancer. Br. J. Cancer 2015, 113, 275–281. [Google Scholar] [CrossRef]
- Raj, D.A.A.; Fiume, I.; Capasso, G.; Pocsfalvi, G. A Multiplex Quantitative Proteomics Strategy for Protein Biomarker Studies in Urinary Exosomes. Kidney Int. 2012, 81, 1263–1272. [Google Scholar] [CrossRef]
- Lässer, C.; Seyed Alikhani, V.; Ekström, K.; Eldh, M.; Torregrosa Paredes, P.; Bossios, A.; Sjöstrand, M.; Gabrielsson, S.; Lötvall, J.; Valadi, H. Human Saliva, Plasma and Breast Milk Exosomes Contain RNA: Uptake by Macrophages. J. Transl. Med. 2011, 9, 9. [Google Scholar] [CrossRef]
- Dai, S.; Wei, D.; Wu, Z.; Zhou, X.; Wei, X.; Huang, H.; Li, G. Phase I Clinical Trial of Autologous Ascites-Derived Exosomes Combined with GM-CSF for Colorectal Cancer. Mol. Ther. 2008, 16, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Street, J.M.; Barran, P.E.; Mackay, C.L.; Weidt, S.; Balmforth, C.; Walsh, T.S.; Chalmers, R.T.; Webb, D.J.; Dear, J.W. Identification and Proteomic Profiling of Exosomes in Human Cerebrospinal Fluid. J. Transl. Med. 2012, 10, 5. [Google Scholar] [CrossRef]
- Lee, J.-E.; Moon, P.-G.; Cho, Y.-E.; Kim, Y.-B.; Kim, I.-S.; Park, H.; Baek, M.-C. Identification of EDIL3 on Extracellular Vesicles Involved in Breast Cancer Cell Invasion. J. Proteom. 2016, 131, 17–28. [Google Scholar] [CrossRef]
- Risha, Y.; Minic, Z.; Ghobadloo, S.M.; Berezovski, M.V. The Proteomic Analysis of Breast Cell Line Exosomes Reveals Disease Patterns and Potential Biomarkers. Sci. Rep. 2020, 10, 13572. [Google Scholar] [CrossRef]
- Syn, N.L.; Wang, L.; Chow, E.K.-H.; Lim, C.T.; Goh, B.-C. Exosomes in Cancer Nanomedicine and Immunotherapy: Prospects and Challenges. Trends Biotechnol. 2017, 35, 665–676. [Google Scholar] [CrossRef]
- Zhao, L.; Gu, C.; Gan, Y.; Shao, L.; Chen, H.; Zhu, H. Exosome-Mediated siRNA Delivery to Suppress Postoperative Breast Cancer Metastasis. J. Control. Release 2020, 318, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Gai, C.; Li, Z.; Ding, D.; Zheng, J.; Zhang, W.; Lv, S.; Li, W. Targeted Exosome-Encapsulated Erastin Induced Ferroptosis in Triple Negative Breast Cancer Cells. Cancer Sci. 2019, 110, 3173–3182. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Cao, X.; Cai, H.; Feng, P.; Chen, X.; Zhu, Y.; Yang, Y.; An, W.; Yang, Y.; Jie, J. The Exosomes Derived from CAR-T Cell Efficiently Target Mesothelin and Reduce Triple-Negative Breast Cancer Growth. Cell. Immunol. 2021, 360, 104262. [Google Scholar] [CrossRef] [PubMed]
| Metastatic Site | EV-Carried Molecules | Mechanisms | Function |
|---|---|---|---|
| Bone | miR-218 | Inhibits COL1A1 expression, promotes bone resorption | Promote |
| miR-940 | Enhances osteogenic differentiation of MSCs | Promote | |
| miR-21 | Activates osteoclast differentiation pathways | Promote | |
| miR-20a-5p | Suppresses SRCIN1, promotes osteoclast proliferation | Promote | |
| miR-19a | Inhibits PTEN, activates NF-κB/AKT pathways | Promote | |
| ICAM1 | Induces CD8+ T cell exhaustion | Promote | |
| IBSP | Enhances migration of osteoclast precursors | Promote | |
| L-plastin | Activates calcium signaling and NFATC1 | Promote | |
| miR-24-2-5p | Inhibits osteoclast differentiation and tumor migration | Inhibit | |
| Lung | miR-138-5p | Induces M2 macrophage polarization | Promote | Promote |
| circ-0100519 | Stabilizes NRF2, promotes pro-inflammatory factors | Promote | |
| MYH9 | Stimulates MMP2/VEGFA secretion | Promote | Promote | |
| LC3+ EVs (TRAPs) | Activates TLR2-MyD88-NF-κB pathway | Promote | |
| Low let-7 EVs | Activates STAT3, polarizes N2 neutrophils | Promote | |
| SP1+ EVs | Activates TLR4-NF-κB pathway | Promote | Promote | |
| CAV1+ EVs | Promotes N2 neutrophil polarization | Promote | |
| DENND10-deficient EVs | Reduces ECM components, impairs cell adhesion | Inhibit | |
| NEAT1 | Activates EMT and invasion-related genes | Promote | Promote | |
| Liver | miR-197 | Suppresses PPARG, induces EMT | Promote | Promote |
| miR-9-5p | Modulates cholesterol metabolism | Promote | |
| CX3CL1-inducing EVs | Recruits CX3CR1+ macrophages | Promote | |
| Tspan8+ EVs | Induces MET to enhance colonization | Promote | |
| Brain | CEMIP+ EVs | Promotes tumor endothelial adhesion | Promote |
| miR-301a-3p | Suppresses TIMP-2, remodels ECM | Promote | |
| miR-199b-5p | Alters neuro-metabolic microenvironment | Promote | |
| miR-1290/1246 | Induces M2 astrocyte polarization | Promote | |
| XIST-deficient EVs | Suppresses TIMP-2, activates immunosuppression | Promote |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shang, H.; Zhang, Y.; Chao, T. Unveiling the Involvement of Extracellular Vesicles in Breast Cancer’s Organotrophic Metastasis: Molecular Mechanisms and Translational Prospects. Int. J. Mol. Sci. 2025, 26, 5430. https://doi.org/10.3390/ijms26125430
Shang H, Zhang Y, Chao T. Unveiling the Involvement of Extracellular Vesicles in Breast Cancer’s Organotrophic Metastasis: Molecular Mechanisms and Translational Prospects. International Journal of Molecular Sciences. 2025; 26(12):5430. https://doi.org/10.3390/ijms26125430
Chicago/Turabian StyleShang, Haotian, Yumin Zhang, and Tengfei Chao. 2025. "Unveiling the Involvement of Extracellular Vesicles in Breast Cancer’s Organotrophic Metastasis: Molecular Mechanisms and Translational Prospects" International Journal of Molecular Sciences 26, no. 12: 5430. https://doi.org/10.3390/ijms26125430
APA StyleShang, H., Zhang, Y., & Chao, T. (2025). Unveiling the Involvement of Extracellular Vesicles in Breast Cancer’s Organotrophic Metastasis: Molecular Mechanisms and Translational Prospects. International Journal of Molecular Sciences, 26(12), 5430. https://doi.org/10.3390/ijms26125430







