Divergent Functions of Rap1A and Rap1B in Endothelial Biology and Disease
Abstract
1. Introduction
2. New Insights into Rap1-Mediated Adhesion
2.1. Spatial Regulation of Rap1 Effector Targeting Towards Integrins or Cadherins
2.2. Physiological Relevance of Rap1-Integrin Signaling in Postnatal Lung Development
3. Isoform-Specific Functions of Rap1A and Rap1B
3.1. Rap1B as a VEGFR2 Co-Activator in Angiogenesis and NO Signaling
3.1.1. Angiogenesis and VEGFR2 Signaling
3.1.2. NO Release
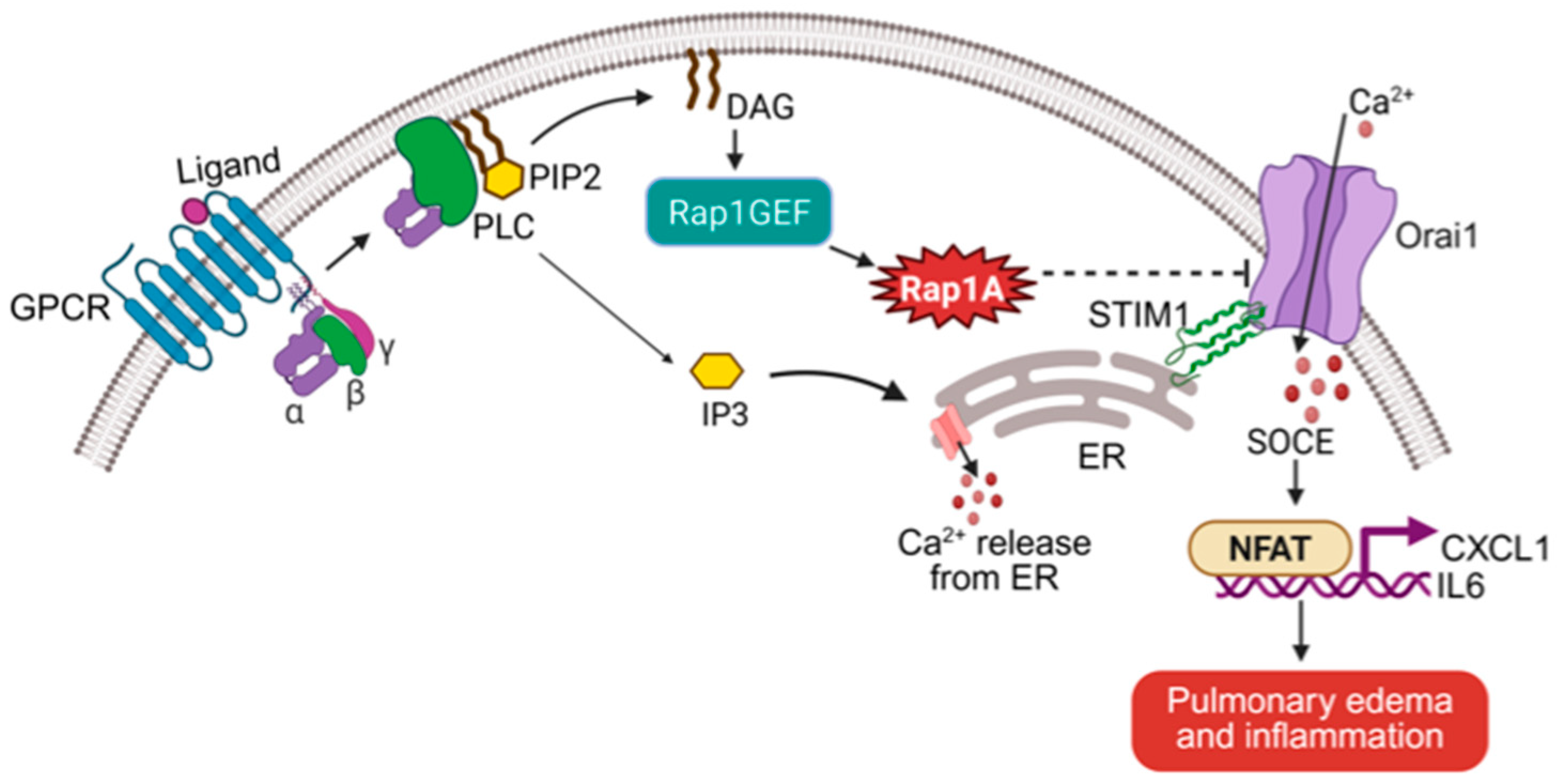
3.2. Rap1A as a Calcium Homeostasis Regulator via SOCE Suppression
3.3. Distinct Anti-Inflammatory Mechanisms of Rap1 Isoforms
3.3.1. Rap1B, NF-κB, and Vascular Immunosuppression
3.3.2. Rap1A, SOCE, and NFAT Transcription
4. Molecular Basis for Isoform Divergence
HVR–Directed Membrane Microdomain Targeting
5. Dynamic Regulation of Rap1 Activity
5.1. Positive Regulation: GEF Diversity and Functional Contexts
5.2. Negative Regulation: GAPs, Sequestration, and Inhibitory Scaffolds
5.3. Ubiquitination as a Mechanism for Effector Switching
5.4. microRNA Control of Rap1
6. Therapeutic Implications
6.1. Targeting Rap1 in Physiological Disorders: Lung, Retina, and Brain
6.2. Pathological Rap1 Signaling in Tumor Vasculature and Beyond
7. Conclusions and Future Directions. Toward Isoform-Specific Therapeutics in Vascular Disease
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AF-6 | Afadin-6 |
| AM | Astragalus membranaceus |
| Ca2+ | Calcium |
| CRAC | Calcium release-activated calcium |
| cAMP | Cyclic adenosine monophosphate |
| ER | Endoplasmic reticulum |
| eNOS | Endothelial nitric oxide synthase |
| ESCC | Esophageal squamous cell carcinoma |
| ECM | Extracellular matrix |
| ERK | Extracellular signal-regulated kinase |
| FLRT2 | Fibronectin leucine-rich transmembrane protein 2 |
| GPCR | G protein-coupled receptor |
| GAPs | GTPase-activating proteins |
| GEFs | Guanine nucleotide exchange factors |
| HUVECs | Human umbilical vein endothelial cells |
| HVRs | Hypervariable regions |
| IP3 | Inositol 1,4,5-trisphosphate |
| ICAM-1 | Intercellular adhesion molecule 1 |
| IL-6 | Interleukin-6 |
| LPHN2 | Latrophilin-2 |
| LNP | Lipid nanoparticle |
| MAPK | Mitogen-activated protein kinase |
| NFAT | Nuclear factor of activated T cells |
| NO | Nitric oxide |
| NLRP3 | NLR family pyrin domain containing 3 |
| PA | Phosphatidic acid |
| PIP3 | Phosphatidylinositol (3,4,5)-trisphosphate |
| PIP2 | Phosphatidylinositol 4,5-bisphosphate |
| PI4P | Phosphatidylinositol 4-phosphate |
| PS | Phosphatidylserine |
| PI3K | Phosphoinositide 3-kinase |
| PLC | Phospholipase C |
| PDGFR | Platelet-derived growth factor receptor |
| PECAM-1 | Platelet and endothelial cell adhesion molecule 1 |
| PBD | Polybasic domain |
| RASIP1 | Ras-Interacting Protein 1 |
| SHANK | SH3 and multiple ankyrin repeat domains protein |
| SOCE | Store-operated calcium entry |
| STIM1 | Stromal interaction molecule 1 |
| TNF | Tumor necrosis factor |
| VCAM-1 | Vascular cell adhesion molecule 1 |
| VCI | Vascular cognitive impairment |
| VEGF | Vascular endothelial growth factor |
| VEGFR2 | Vascular endothelial growth factor receptor 2 |
| YAP | Yes-associated protein |
References
- Wennerberg, K.; Rossman, K.L.; Der, C.J. The Ras superfamily at a glance. J. Cell Sci. 2005, 118 Pt 5, 843–846. [Google Scholar] [CrossRef]
- Wittinghofer, A.; Herrmann, C. Ras-effector interactions, the problem of specificity. FEBS Lett. 1995, 369, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Wittchen, E.S.; Aghajanian, A.; Burridge, K. Isoform-specific differences between Rap1A and Rap1B GTPases in the formation of endothelial cell junctions. Small GTPases 2011, 2, 65–76. [Google Scholar] [CrossRef]
- Lakshmikanthan, S.; Sobczak, M.; Li Calzi, S.; Shaw, L.; Grant, M.B.; Chrzanowska-Wodnicka, M. Rap1B promotes VEGF-induced endothelial permeability and is required for dynamic regulation of the endothelial barrier. J. Cell Sci. 2018, 131, jcs207605. [Google Scholar] [CrossRef]
- Bos, J.L.; de Bruyn, K.; Enserink, J.; Kuiperij, B.; Rangarajan, S.; Rehmann, H.; Riedl, J.; de Rooij, J.; van Mansfeld, F.; Zwartkruis, F. The role of Rap1 in integrin-mediated cell adhesion. Biochem. Soc. Trans. 2003, 31 Pt 1, 83–86. [Google Scholar] [CrossRef]
- Chrzanowska-Wodnicka, M. Rap1 in endothelial biology. Curr. Opin. Hematol. 2017, 24, 248–255. [Google Scholar] [CrossRef]
- Pannekoek, W.J.; Post, A.; Bos, J.L. Rap1 signaling in endothelial barrier control. Cell Adh. Migr. 2014, 8, 100–107. [Google Scholar] [CrossRef]
- Kosuru, R.; Romito, O.; Sharma, G.P.; Ferraresso, F.; Ghadrdoost Nakhchi, B.; Yang, K.; Mammoto, T.; Mammoto, A.; Kastrup, C.J.; Zhang, D.X.; et al. Rap1A Modulates Store-Operated Calcium Entry in the Lung Endothelium: A Novel Mechanism Controlling NFAT-Mediated Vascular Inflammation and Permeability. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 2271–2287. [Google Scholar] [CrossRef]
- Bos, J.L. Epac proteins: Multi-purpose cAMP targets. Trends Biochem. Sci. 2006, 31, 680–686. [Google Scholar] [CrossRef]
- de Rooij, J.; Zwartkruis, F.J.; Verheijen, M.H.; Cool, R.H.; Nijman, S.M.; Wittinghofer, A.; Bos, J.L. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 1998, 396, 474–477. [Google Scholar] [CrossRef]
- Kosuru, R.; Chrzanowska, M. Integration of Rap1 and Calcium Signaling. Int. J. Mol. Sci. 2020, 21, 1616. [Google Scholar] [CrossRef]
- Randhawa, P.K.; Rylova, S.; Heinz, J.Y.; Kiser, S.; Fried, J.H.; Dunworth, W.P.; Anderson, A.L.; Barber, A.T.; Chappell, J.C.; Roberts, D.M.; et al. The Ras activator RasGRP3 mediates diabetes-induced embryonic defects and affects endothelial cell migration. Circ. Res. 2011, 108, 1199–1208. [Google Scholar] [CrossRef]
- Roberts, D.M.; Anderson, A.L.; Hidaka, M.; Swetenburg, R.L.; Patterson, C.; Stanford, W.L.; Bautch, V.L. A vascular gene trap screen defines RasGRP3 as an angiogenesis-regulated gene required for the endothelial response to phorbol esters. Mol. Cell Biol. 2004, 24, 10515–10528. [Google Scholar] [CrossRef]
- Tang, S.; Chen, T.; Yu, Z.; Zhu, X.; Yang, M.; Xie, B.; Li, N.; Cao, X.; Wang, J. RasGRP3 limits Toll-like receptor-triggered inflammatory response in macrophages by activating Rap1 small GTPase. Nat. Commun. 2014, 5, 4657. [Google Scholar] [CrossRef]
- Araya, M.K.; Chen, W.; Ke, Y.; Zhou, Y.; Gorfe, A.A.; Hancock, J.F.; Liu, J. Differential Lipid Binding Specificities of RAP1A and RAP1B are Encoded by the Amino Acid Sequence of the Membrane Anchors. J. Am. Chem. Soc. 2024, 146, 19782–19791. [Google Scholar] [CrossRef]
- Glading, A.; Han, J.; Stockton, R.A.; Ginsberg, M.H. KRIT-1/CCM1 is a Rap1 effector that regulates endothelial cell cell junctions. J. Cell Biol. 2007, 179, 247–254. [Google Scholar] [CrossRef]
- Wilson, C.W.; Ye, W. Regulation of vascular endothelial junction stability and remodeling through Rap1-Rasip1 signaling. Cell Adh. Migr. 2014, 8, 76–83. [Google Scholar] [CrossRef]
- Pannekoek, W.J.; Vliem, M.J.; Bos, J.L. Multiple Rap1 effectors control Epac1-mediated tightening of endothelial junctions. Small GTPases 2020, 11, 346–353. [Google Scholar] [CrossRef]
- Tawa, H.; Rikitake, Y.; Takahashi, M.; Amano, H.; Miyata, M.; Satomi-Kobayashi, S.; Kinugasa, M.; Nagamatsu, Y.; Majima, T.; Ogita, H.; et al. Role of afadin in vascular endothelial growth factor- and sphingosine 1-phosphate-induced angiogenesis. Circ. Res. 2010, 106, 1731–1742. [Google Scholar] [CrossRef]
- Calderwood, D.A.; Zent, R.; Grant, R.; Rees, D.J.; Hynes, R.O.; Ginsberg, M.H. The Talin head domain binds to integrin beta subunit cytoplasmic tails and regulates integrin activation. J. Biol. Chem. 1999, 274, 28071–28074. [Google Scholar] [CrossRef]
- Lagarrigue, F.; Vikas Anekal, P.; Lee, H.S.; Bachir, A.I.; Ablack, J.N.; Horwitz, A.F.; Ginsberg, M.H. A RIAM/lamellipodin-talin-integrin complex forms the tip of sticky fingers that guide cell migration. Nat. Commun. 2015, 6, 8492. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Lim, C.J.; Puzon-McLaughlin, W.; Shattil, S.J.; Ginsberg, M.H. RIAM activates integrins by linking talin to ras GTPase membrane-targeting sequences. J. Biol. Chem. 2009, 284, 5119–5127. [Google Scholar] [CrossRef]
- Zhu, L.; Yang, J.; Bromberger, T.; Holly, A.; Lu, F.; Liu, H.; Sun, K.; Klapproth, S.; Hirbawi, J.; Byzova, T.V.; et al. Structure of Rap1b bound to talin reveals a pathway for triggering integrin activation. Nat. Commun. 2017, 8, 1744. [Google Scholar] [CrossRef]
- Camp, D.; Haage, A.; Solianova, V.; Castle, W.M.; Xu, Q.A.; Lostchuck, E.; Goult, B.T.; Tanentzapf, G. Direct binding of Talin to Rap1 is required for cell-ECM adhesion in Drosophila. J. Cell Sci. 2018, 131, jcs225144. [Google Scholar] [CrossRef]
- Gingras, A.R.; Lagarrigue, F.; Cuevas, M.N.; Valadez, A.J.; Zorovich, M.; McLaughlin, W.; Lopez-Ramirez, M.A.; Seban, N.; Ley, K.; Kiosses, W.B.; et al. Rap1 binding and a lipid-dependent helix in talin F1 domain promote integrin activation in tandem. J. Cell Biol. 2019, 218, 1799–1809. [Google Scholar] [CrossRef]
- Liao, Z.; Gingras, A.R.; Lagarrigue, F.; Ginsberg, M.H.; Shattil, S.J. Optogenetics-based localization of talin to the plasma membrane promotes activation of β3 integrins. J. Biol. Chem. 2021, 296, 100675. [Google Scholar] [CrossRef] [PubMed]
- Lilja, J.; Zacharchenko, T.; Georgiadou, M.; Jacquemet, G.; De Franceschi, N.; Peuhu, E.; Hamidi, H.; Pouwels, J.; Martens, V.; Nia, F.H.; et al. SHANK proteins limit integrin activation by directly interacting with Rap1 and R-Ras. Nat. Cell Biol. 2017, 19, 292–305. [Google Scholar] [CrossRef]
- Liao, Z.; Shattil, S.J. Talin, a Rap1 effector for integrin activation at the plasma membrane, also promotes Rap1 activity by disrupting sequestration of Rap1 by SHANK3. J. Cell Sci. 2025, 138, jcs-263595. [Google Scholar] [CrossRef] [PubMed]
- Langenhan, T.; Aust, G.; Hamann, J. Sticky signaling--adhesion class G protein-coupled receptors take the stage. Sci. Signal 2013, 6, re3. [Google Scholar] [CrossRef]
- Camillo, C.; Facchinello, N.; Villari, G.; Mana, G.; Gioelli, N.; Sandri, C.; Astone, M.; Tortarolo, D.; Clapero, F.; Gays, D.; et al. LPHN2 inhibits vascular permeability by differential control of endothelial cell adhesion. J. Cell Biol. 2021, 220, e202006033. [Google Scholar] [CrossRef]
- Moztarzadeh, S.; Sepic, S.; Hamad, I.; Waschke, J.; Radeva, M.Y.; García-Ponce, A. Cortactin is in a complex with VE-cadherin and is required for endothelial adherens junction stability through Rap1/Rac1 activation. Sci. Rep. 2024, 14, 1218. [Google Scholar] [CrossRef]
- Lua, B.L.; Low, B.C. BPGAP1 interacts with cortactin and facilitates its translocation to cell periphery for enhanced cell migration. Mol. Biol. Cell 2004, 15, 2873–2883. [Google Scholar] [CrossRef]
- Chrzanowska-Wodnicka, M.; White, G.C., 2nd; Quilliam, L.A.; Whitehead, K.J. Small GTPase Rap1 Is Essential for Mouse Development and Formation of Functional Vasculature. PLoS ONE 2015, 10, e0145689. [Google Scholar] [CrossRef]
- Lakshmikanthan, S.; Zheng, X.; Nishijima, Y.; Sobczak, M.; Szabo, A.; Vasquez-Vivar, J.; Zhang, D.X.; Chrzanowska-Wodnicka, M. Rap1 promotes endothelial mechanosensing complex formation, NO release and normal endothelial function. EMBO Rep. 2015, 16, 628–637. [Google Scholar] [CrossRef]
- Watanabe-Takano, H.; Kato, K.; Oguri-Nakamura, E.; Ishii, T.; Kobayashi, K.; Murata, T.; Tsujikawa, K.; Miyata, T.; Kubota, Y.; Hanada, Y.; et al. Endothelial cells regulate alveolar morphogenesis by constructing basement membranes acting as a scaffold for myofibroblasts. Nat. Commun. 2024, 15, 1622. [Google Scholar] [CrossRef]
- Branchfield, K.; Li, R.; Lungova, V.; Verheyden, J.M.; McCulley, D.; Sun, X. A three-dimensional study of alveologenesis in mouse lung. Dev. Biol. 2016, 409, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Jayadev, R.; Chi, Q.; Keeley, D.P.; Hastie, E.L.; Kelley, L.C.; Sherwood, D.R. α-Integrins dictate distinct modes of type IV collagen recruitment to basement membranes. J. Cell Biol. 2019, 218, 3098–3116. [Google Scholar] [CrossRef] [PubMed]
- Chrzanowska-Wodnicka, M.; Kraus, A.E.; Gale, D.; White, G.C., 2nd; Vansluys, J. Defective angiogenesis, endothelial migration, proliferation, and MAPK signaling in Rap1b-deficient mice. Blood 2008, 111, 2647–2656. [Google Scholar] [CrossRef]
- Lakshmikanthan, S.; Sobczak, M.; Chun, C.; Henschel, A.; Dargatz, J.; Ramchandran, R.; Chrzanowska-Wodnicka, M. Rap1 promotes VEGFR2 activation and angiogenesis by a mechanism involving integrin αvβ3. Blood 2011, 118, 2015–2026. [Google Scholar] [CrossRef]
- Sharma, G.P.; Kosuru, R.; Lakshmikanthan, S.; Zheng, S.; Chen, Y.; Burns, R.; Xin, G.; Cui, W.; Chrzanowska, M. Endothelial Rap1B mediates T-cell exclusion to promote tumor growth: A novel mechanism underlying vascular immunosuppression. Angiogenesis 2023, 26, 265–278. [Google Scholar] [CrossRef]
- Garcia, V.; Sessa, W.C. Endothelial NOS: Perspective and recent developments. Br. J. Pharmacol. 2019, 176, 189–196. [Google Scholar] [CrossRef]
- Lakshmikanthan, S.; Zieba, B.J.; Ge, Z.D.; Momotani, K.; Zheng, X.; Lund, H.; Artamonov, M.V.; Maas, J.E.; Szabo, A.; Zhang, D.X.; et al. Rap1b in smooth muscle and endothelium is required for maintenance of vascular tone and normal blood pressure. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1486–1494. [Google Scholar] [CrossRef]
- Kosuru, R.; Singh, B.; Lakshmikanthan, S.; Nishijima, Y.; Vasquez-Vivar, J.; Zhang, D.X.; Chrzanowska, M. Distinct Signaling Functions of Rap1 Isoforms in NO Release From Endothelium. Front. Cell Dev. Biol. 2021, 9, 687598. [Google Scholar]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.S. Store-Operated Calcium Channels: From Function to Structure and Back Again. Cold Spring Harb. Perspect. Biol. 2020, 12, a035055. [Google Scholar] [CrossRef]
- Prakriya, M.; Lewis, R.S. Store-Operated Calcium Channels. Physiol. Rev. 2015, 95, 1383–1436. [Google Scholar] [CrossRef]
- Putney, J.W., Jr. A model for receptor-regulated calcium entry. Cell Calcium 1986, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. The Inositol Trisphosphate/Calcium Signaling Pathway in Health and Disease. Physiol. Rev. 2016, 96, 1261–1296. [Google Scholar] [CrossRef]
- Liou, J.; Kim, M.L.; Heo, W.D.; Jones, J.T.; Myers, J.W.; Ferrell, J.E., Jr.; Meyer, T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol. 2005, 15, 1235–1241. [Google Scholar] [CrossRef]
- Roos, J.; DiGregorio, P.J.; Yeromin, A.V.; Ohlsen, K.; Lioudyno, M.; Zhang, S.; Safrina, O.; Kozak, J.A.; Wagner, S.L.; Cahalan, M.D.; et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell Biol. 2005, 169, 435–445. [Google Scholar] [CrossRef]
- Trebak, M.; Putney, J.W., Jr. ORAI Calcium Channels. Physiology 2017, 32, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Abdullaev, I.F.; Bisaillon, J.M.; Potier, M.; Gonzalez, J.C.; Motiani, R.K.; Trebak, M. Stim1 and Orai1 mediate CRAC currents and store-operated calcium entry important for endothelial cell proliferation. Circ. Res. 2008, 103, 1289–1299. [Google Scholar] [CrossRef]
- Emrich, S.M.; Yoast, R.E.; Trebak, M. Physiological Functions of CRAC Channels. Annu. Rev. Physiol. 2022, 84, 355–379. [Google Scholar] [CrossRef]
- Gwack, Y.; Feske, S.; Srikanth, S.; Hogan, P.G.; Rao, A. Signalling to transcription: Store-operated Ca2+ entry and NFAT activation in lymphocytes. Cell Calcium 2007, 42, 145–156. [Google Scholar] [CrossRef]
- Martín-Aragón Baudel, M.; Nieves-Cintrón, M.; Navedo, M.F. Rap1 Brings the A Game to Control Lung Endothelium. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 2288–2290. [Google Scholar] [CrossRef]
- Singh, B.; Kosuru, R.; Lakshmikanthan, S.; Sorci-Thomas, M.G.; Zhang, D.X.; Sparapani, R.; Vasquez-Vivar, J.; Chrzanowska, M. Endothelial Rap1 (Ras-Association Proximate 1) Restricts Inflammatory Signaling to Protect From the Progression of Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 638–650. [Google Scholar] [CrossRef]
- Bokoch, G.M. Biology of the Rap proteins, members of the ras superfamily of GTP-binding proteins. Biochem. J. 1993, 289 Pt 1, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Maltese, W.A. Posttranslational modification of proteins by isoprenoids in mammalian cells. Faseb J. 1990, 4, 3319–3328. [Google Scholar] [CrossRef] [PubMed]
- Kawata, M.; Farnsworth, C.C.; Yoshida, Y.; Gelb, M.H.; Glomset, J.A.; Takai, Y. Posttranslationally processed structure of the human platelet protein smg p21B: Evidence for geranylgeranylation and carboxyl methylation of the C-terminal cysteine. Proc. Natl. Acad. Sci. USA 1990, 87, 8960–8964. [Google Scholar] [CrossRef]
- Buss, J.E.; Quilliam, L.A.; Kato, K.; Casey, P.J.; Solski, P.A.; Wong, G.; Clark, R.; McCormick, F.; Bokoch, G.M.; Der, C.J. The COOH-Terminal Domain of the Rap1A (Krev-1) Protein Is Isoprenylated and Supports Transformation by an H-Ras:Rap1A Chimeric Protein. Mol. Cell. Biol. 1991, 11, 1523–1530. [Google Scholar]
- Prior, I.A.; Hancock, J.F. Ras trafficking, localization and compartmentalized signalling. Semin. Cell Dev. Biol. 2012, 23, 145–153. [Google Scholar] [CrossRef]
- Busquets-Hernández, C.; Triola, G. Palmitoylation as a Key Regulator of Ras Localization and Function. Front. Mol. Biosci. 2021, 8, 659861. [Google Scholar] [CrossRef]
- Ntantie, E.; Gonyo, P.; Lorimer, E.L.; Hauser, A.D.; Schuld, N.; McAllister, D.; Kalyanaraman, B.; Dwinell, M.B.; Auchampach, J.A.; Williams, C.L. An adenosine-mediated signaling pathway suppresses prenylation of the GTPase Rap1B and promotes cell scattering. Sci. Signal 2013, 6, ra39. [Google Scholar] [CrossRef]
- Hancock, J.F. Ras proteins: Different signals from different locations. Nat. Rev. Mol. Cell Biol. 2003, 4, 373–3845. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Hancock, J.F. Ras nanoclusters: Versatile lipid-based signaling platforms. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2015, 1853, 841–849. [Google Scholar] [CrossRef]
- Zhou, Y.; Hancock, J.F. Deciphering lipid codes: K-Ras as a paradigm. Traffic 2018, 19, 157–165. [Google Scholar] [CrossRef]
- Cox, A.D.; Fesik, S.W.; Kimmelman, A.C.; Luo, J.; Der, C.J. Drugging the undruggable RAS: Mission possible? Nat. Rev. Drug Discov. 2014, 13, 828–851. [Google Scholar] [CrossRef]
- McCormick, F. Progress in targeting RAS with small molecule drugs. Biochem. J. 2019, 476, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Stalnecker, C.A.; Der, C.J. RAS, wanted dead or alive: Advances in targeting RAS mutant cancers. Sci. Signal. 2020, 13, eaay6013. [Google Scholar] [CrossRef]
- Liu, J.; Arora, N.; Zhou, Y. RAS GTPases and Interleaflet Coupling in the Plasma Membrane. Cold Spring Harb. Perspect. Biol. 2023, 15, a041414. [Google Scholar] [CrossRef]
- Ke, Y.; Gannaban, R.; Liu, J.; Zhou, Y. STIM1 and lipid interactions at ER-PM contact sites. Am. J. Physiol.-Cell Physiol. 2025, 328, C107–C114. [Google Scholar] [CrossRef]
- Bos, J.L.; de Rooij, J.; Reedquist, K.A. Rap1 signalling: Adhering to new models. Nat. Rev. Mol. Cell Biol. 2001, 2, 369–377. [Google Scholar] [CrossRef]
- Pannekoek, W.J.; Kooistra, M.R.; Zwartkruis, F.J.; Bos, J.L. Cell-cell junction formation: The role of Rap1 and Rap1 guanine nucleotide exchange factors. Biochim. Biophys. Acta 2009, 1788, 790–796. [Google Scholar] [CrossRef]
- Fukuhara, S.; Sakurai, A.; Sano, H.; Yamagishi, A.; Somekawa, S.; Takakura, N.; Saito, Y.; Kangawa, K.; Mochizuki, N. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol. Cell Biol. 2005, 25, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Cullere, X.; Shaw, S.K.; Andersson, L.; Hirahashi, J.; Luscinskas, F.W.; Mayadas, T.N. Regulation of vascular endothelial barrier function by Epac, a cAMP-activated exchange factor for Rap GTPase. Blood 2005, 105, 1950–1955. [Google Scholar] [CrossRef] [PubMed]
- Pannekoek, W.-J.; van Dijk, J.J.; Chan, O.Y.A.; Huveneers, S.; Linnemann, J.R.; Spanjaard, E.; Brouwer, P.M.; van der Meer, A.J.; Zwartkruis, F.J.; Rehmann, H. Epac1 and PDZ-GEF cooperate in Rap1 mediated endothelial junction control. Cell. Signal. 2011, 23, 2056–2064. [Google Scholar] [CrossRef] [PubMed]
- Birukova, A.A.; Tian, X.; Tian, Y.; Higginbotham, K.; Birukov, K.G. Rap-afadin axis in control of Rho signaling and endothelial barrier recovery. Mol. Biol. Cell 2013, 24, 2678–2688. [Google Scholar] [CrossRef] [PubMed]
- Sequera, C.; Manzano, S.; Guerrero, C.; Porras, A. How Rap and its GEFs control liver physiology and cancer development. C3G alterations in human hepatocarcinoma. Hepat. Oncol. 2018, 5, Hep05. [Google Scholar] [CrossRef]
- Khan, A.; Ni, W.; Baltazar, T.; Lopez-Giraldez, F.; Pober, J.S.; Pierce, R.W. ArhGEF12 activates Rap1A and not RhoA in human dermal microvascular endothelial cells to reduce tumor necrosis factor-induced leak. Faseb J. 2022, 36, e22254. [Google Scholar] [CrossRef]
- Wang, Y.; He, H.; Srivastava, N.; Vikarunnessa, S.; Chen, Y.B.; Jiang, J.; Cowan, C.W.; Zhang, X. Plexins are GTPase-activating proteins for Rap and are activated by induced dimerization. Sci. Signal. 2012, 5, ra6. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pascoe, H.G.; Brautigam, C.A.; He, H.; Zhang, X. Structural basis for activation and non-canonical catalysis of the Rap GTPase activating protein domain of plexin. Elife 2013, 2, e01279. [Google Scholar] [CrossRef] [PubMed]
- Valdembri, D.; Regano, D.; Maione, F.; Giraudo, E.; Serini, G. Class 3 semaphorins in cardiovascular development. Cell Adh. Migr. 2016, 10, 641–651. [Google Scholar] [CrossRef]
- Yang, D.S.; Roh, S.; Jeong, S. The axon guidance function of Rap1 small GTPase is independent of PlexA RasGAP activity in Drosophila. Dev. Biol. 2016, 418, 258–267. [Google Scholar] [CrossRef]
- Mehta, V.; Pang, K.L.; Rozbesky, D.; Nather, K.; Keen, A.; Lachowski, D.; Kong, Y.; Karia, D.; Ameismeier, M.; Huang, J.; et al. The guidance receptor plexin D1 is a mechanosensor in endothelial cells. Nature 2020, 578, 290–295. [Google Scholar] [CrossRef]
- Genova, T.; Grolez, G.P.; Camillo, C.; Bernardini, M.; Bokhobza, A.; Richard, E.; Scianna, M.; Lemonnier, L.; Valdembri, D.; Munaron, L.; et al. TRPM8 inhibits endothelial cell migration via a non-channel function by trapping the small GTPase Rap1. J. Cell Biol. 2017, 216, 2107–2130. [Google Scholar] [CrossRef]
- White, C.D.; Erdemir, H.H.; Sacks, D.B. IQGAP1 and its binding proteins control diverse biological functions. Cell. Signal. 2012, 24, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Noritake, J.; Watanabe, T.; Sato, K.; Wang, S.; Kaibuchi, K. IQGAP1: A key regulator of adhesion and migration. J. Cell Sci. 2005, 118 Pt 10, 2085–2092. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, G.; Sun, G. Actin-binding protein, IQGAP1, regulates LPS-induced RPMVECs hyperpermeability and ICAM-1 upregulation via Rap1/Src signalling pathway. Cell. Signal. 2021, 85, 110067. [Google Scholar] [CrossRef]
- Sewduth, R.N.; Carai, P.; Ivanisevic, T.; Zhang, M.; Jang, H.; Lechat, B.; Van Haver, D.; Impens, F.; Nussinov, R.; Jones, E.; et al. Spatial Mechano-Signaling Regulation of GTPases through Non-Degradative Ubiquitination. Adv. Sci. 2023, 10, 2303367. [Google Scholar] [CrossRef]
- Behm-Ansmant, I.; Rehwinkel, J.; Izaurralde, E. MicroRNAs silence gene expression by repressing protein expression and/or by promoting mRNA decay. In Cold Spring Harbor Symposia on Quantitative Biology; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2006; pp. 523–530. [Google Scholar]
- Das, S.; Bryan, K.; Buckley, P.G.; Piskareva, O.; Bray, I.M.; Foley, N.; Ryan, J.; Lynch, J.; Creevey, L.; Fay, J. Modulation of neuroblastoma disease pathogenesis by an extensive network of epigenetically regulated microRNAs. Oncogene 2013, 32, 2927–2936. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, X.; Lin, L.; Chen, H.; Yu, S.; Li, D. MicroRNA-149 is associated with clinical outcome in human neuroblastoma and modulates cancer cell proliferation through Rap1 independent of MYCN amplification. Biochimie 2017, 139, 1–8. [Google Scholar] [CrossRef] [PubMed]
- She, X.; Yu, Z.; Cui, Y.; Lei, Q.; Wang, Z.; Xu, G.; Xiang, J.; Wu, M.; Li, G. miR-128 and miR-149 enhance the chemosensitivity of temozolomide by Rap1B-mediated cytoskeletal remodeling in glioblastoma. Oncol. Rep. 2014, 32, 957–964. [Google Scholar] [CrossRef]
- Banerjee, R.; Russo, N.; Liu, M.; Van Tubergen, E.; D’Silva, N.J. Rap1 and its regulatory proteins: The tumor suppressor, oncogene, tumor suppressor gene axis in head and neck cancer. Small GTPases 2012, 3, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Mani, R.S.; Russo, N.; Scanlon, C.S.; Tsodikov, A.; Jing, X.; Cao, Q.; Palanisamy, N.; Metwally, T.; Inglehart, R.C.; et al. The tumor suppressor gene rap1GAP is silenced by miR-101-mediated EZH2 overexpression in invasive squamous cell carcinoma. Oncogene 2011, 30, 4339–4349. [Google Scholar] [CrossRef]
- Lu, J.; He, M.L.; Wang, L.; Chen, Y.; Liu, X.; Dong, Q.; Chen, Y.C.; Peng, Y.; Yao, K.T.; Kung, H.F.; et al. MiR-26a inhibits cell growth and tumorigenesis of nasopharyngeal carcinoma through repression of EZH2. Cancer Res. 2011, 71, 225–233. [Google Scholar] [CrossRef]
- Kramer, M.C.; Liang, D.; Tatomer, D.C.; Gold, B.; March, Z.M.; Cherry, S.; Wilusz, J.E. Combinatorial control of Drosophila circular RNA expression by intronic repeats, hnRNPs, and SR proteins. Genes Dev. 2015, 29, 2168–2182. [Google Scholar] [CrossRef]
- Hansen, T.B.; Jensen, T.I.; Clausen, B.H.; Bramsen, J.B.; Finsen, B.; Damgaard, C.K.; Kjems, J. Natural RNA circles function as efficient microRNA sponges. Nature 2013, 495, 384–388. [Google Scholar] [CrossRef]
- Qu, S.; Yang, X.; Li, X.; Wang, J.; Gao, Y.; Shang, R.; Sun, W.; Dou, K.; Li, H. Circular RNA: A new star of noncoding RNAs. Cancer Lett. 2015, 365, 141–148. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, J.; Zou, C.; Xie, X.; Wang, Y.; Wang, B.; Zhao, Z.; Tu, J.; Wang, X.; Li, H.; et al. Microarray Expression Profile and Functional Analysis of Circular RNAs in Osteosarcoma. Cell. Physiol. Biochem. 2017, 43, 969–985. [Google Scholar] [CrossRef]
- Dahiya, N.; Atreya, C.D. RAP1 Downregulation by miR-320c Reduces Platelet Activation in Ex-vivo Storage. Microrna 2019, 8, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Khor, E.S.; Noor, S.M.; Wong, P.F. MiR-107 inhibits the sprouting of intersegmental vessels of zebrafish embryos. Protoplasma 2022, 259, 691–702. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Guan, X.; Du, Y.; Liu, G.; Li, Y.; Wei, Z.; Shi, C.; Yang, J.; Hou, T. Screening of differentially expressed miRNAs during osteogenic/odontogenic differentiation of human dental pulp stem cells exposed to mechanical stress. Am. J. Transl. Res. 2021, 13, 11126–11143. [Google Scholar]
- Birukova, A.A.; Zagranichnaya, T.; Fu, P.; Alekseeva, E.; Chen, W.; Jacobson, J.R.; Birukov, K.G. Prostaglandins PGE(2) and PGI(2) promote endothelial barrier enhancement via PKA- and Epac1/Rap1-dependent Rac activation. Exp. Cell Res. 2007, 313, 2504–2520. [Google Scholar] [CrossRef]
- Birukova, A.A.; Zebda, N.; Fu, P.; Poroyko, V.; Cokic, I.; Birukov, K.G. Association between adherens junctions and tight junctions via Rap1 promotes barrier protective effects of oxidized phospholipids. J. Cell Physiol. 2011, 226, 2052–2062. [Google Scholar] [CrossRef]
- Ke, Y.; Karki, P.; Zhang, C.; Li, Y.; Nguyen, T.; Birukov, K.G.; Birukova, A.A. Mechanosensitive Rap1 activation promotes barrier function of lung vascular endothelium under cyclic stretch. Mol. Biol. Cell 2019, 30, 959–974. [Google Scholar] [CrossRef] [PubMed]
- Ramasubramanian, B.; Kim, J.; Ke, Y.; Li, Y.; Zhang, C.O.; Promnares, K.; Tanaka, K.A.; Birukov, K.G.; Karki, P.; Birukova, A.A. Mechanisms of pulmonary endothelial permeability and inflammation caused by extracellular histone subunits H3 and H4. Faseb J. 2022, 36, e22470. [Google Scholar] [CrossRef]
- Yang, H.; Han, R.-y.; Gong, R.-w.; Zhang, Y.-j.; Yang, S.-s.; Xu, G.-z.; Liu, W. CST3 alleviates retinal vascular leakage by regulating the Rap1 signaling pathway. Exp. Eye Res. 2024, 247, 110042. [Google Scholar] [CrossRef]
- Ramos, C.J.; Lin, C.; Liu, X.; Antonetti, D.A. The EPAC-Rap1 pathway prevents and reverses cytokine-induced retinal vascular permeability. J. Biol. Chem. 2018, 293, 717–730. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Fan, Z.; Xue, W.; Sun, F.; Zhu, H.; Huang, D.; Wang, Z.; Dong, L. Vitexin regulates Epac and NLRP3 and ameliorates chronic cerebral hypoperfusion injury. Can. J. Physiol. Pharmacol. 2021, 99, 1079–1087. [Google Scholar] [CrossRef]
- Lin, L.; Chen, W.; Yao, C.; Wu, L.; Yan, Q.; Cai, X.; Zhu, S.; Lao, Y.; Zhang, G.; Lan, X.; et al. Exploring the target and molecular mechanism of Astragalus membranaceus in the treatment of vascular cognitive impairment based on network pharmacology and molecular docking. Medicine 2023, 102, e33063. [Google Scholar] [CrossRef] [PubMed]
- Ghadrdoost Nakhchi, B.; Kosuru, R.; Chrzanowska, M. Towards Targeting Endothelial Rap1B to Overcome Vascular Immunosuppression in Cancer. Int. J. Mol. Sci. 2024, 25, 9853. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Guo, Z.; Song, M.; Pan, Q.; Zhao, J.; Huang, Y.; Han, Y.; Ouyang, D.; Yang, C.; Chen, H.; et al. Lenvatinib improves anti-PD-1 therapeutic efficacy by promoting vascular normalization via the NRP-1-PDGFRβ complex in hepatocellular carcinoma. Front. Immunol. 2023, 14, 1212577. [Google Scholar] [CrossRef]
- Qian, J.; Olbrecht, S.; Boeckx, B.; Vos, H.; Laoui, D.; Etlioglu, E.; Wauters, E.; Pomella, V.; Verbandt, S.; Busschaert, P.; et al. A pan-cancer blueprint of the heterogeneous tumor microenvironment revealed by single-cell profiling. Cell Res. 2020, 30, 745–762. [Google Scholar] [CrossRef]
- Sha, Y.; Hong, H.; Cai, W.; Sun, T. Single-Cell Transcriptomics of Endothelial Cells in Upper and Lower Human Esophageal Squamous Cell Carcinoma. Curr. Oncol. 2022, 29, 7680–7694. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Yang, K.; Zou, Y.; Huo, R. Identification of Key microRNAs and Genes in Infantile Hemangiomas. Front. Genet. 2022, 13, 766561. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, L.; Ye, J.; Xu, C.; Wu, B.; Wu, J.; Hong, T. Identification of core genes of craniopharyngioma angiogenesis based on single-cell nuclear transcriptome sequencing. Cell Mol. Biol. 2024, 70, 136–141. [Google Scholar] [CrossRef]
- Li, D.; Jiu, J.; Liu, H.; Yan, X.; Li, X.; Yan, L.; Zhang, J.; Fan, Z.; Li, S.; Du, G.; et al. Tissue-engineered mesenchymal stem cell constructs alleviate tendinopathy by suppressing vascularization. Bioact. Mater. 2024, 36, 474–489. [Google Scholar] [CrossRef]
- Xu, L.; Li, C. Network-Based Analysis Reveals Gene Signature in Tip Cells and Stalk Cells. Anticancer. Agents Med. Chem. 2022, 22, 1571–1581. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosuru, R.; Chrzanowska, M. Divergent Functions of Rap1A and Rap1B in Endothelial Biology and Disease. Int. J. Mol. Sci. 2025, 26, 5372. https://doi.org/10.3390/ijms26115372
Kosuru R, Chrzanowska M. Divergent Functions of Rap1A and Rap1B in Endothelial Biology and Disease. International Journal of Molecular Sciences. 2025; 26(11):5372. https://doi.org/10.3390/ijms26115372
Chicago/Turabian StyleKosuru, Ramoji, and Magdalena Chrzanowska. 2025. "Divergent Functions of Rap1A and Rap1B in Endothelial Biology and Disease" International Journal of Molecular Sciences 26, no. 11: 5372. https://doi.org/10.3390/ijms26115372
APA StyleKosuru, R., & Chrzanowska, M. (2025). Divergent Functions of Rap1A and Rap1B in Endothelial Biology and Disease. International Journal of Molecular Sciences, 26(11), 5372. https://doi.org/10.3390/ijms26115372






