From Molecular Precision to Clinical Practice: A Comprehensive Review of Bispecific and Trispecific Antibodies in Hematologic Malignancies
Abstract
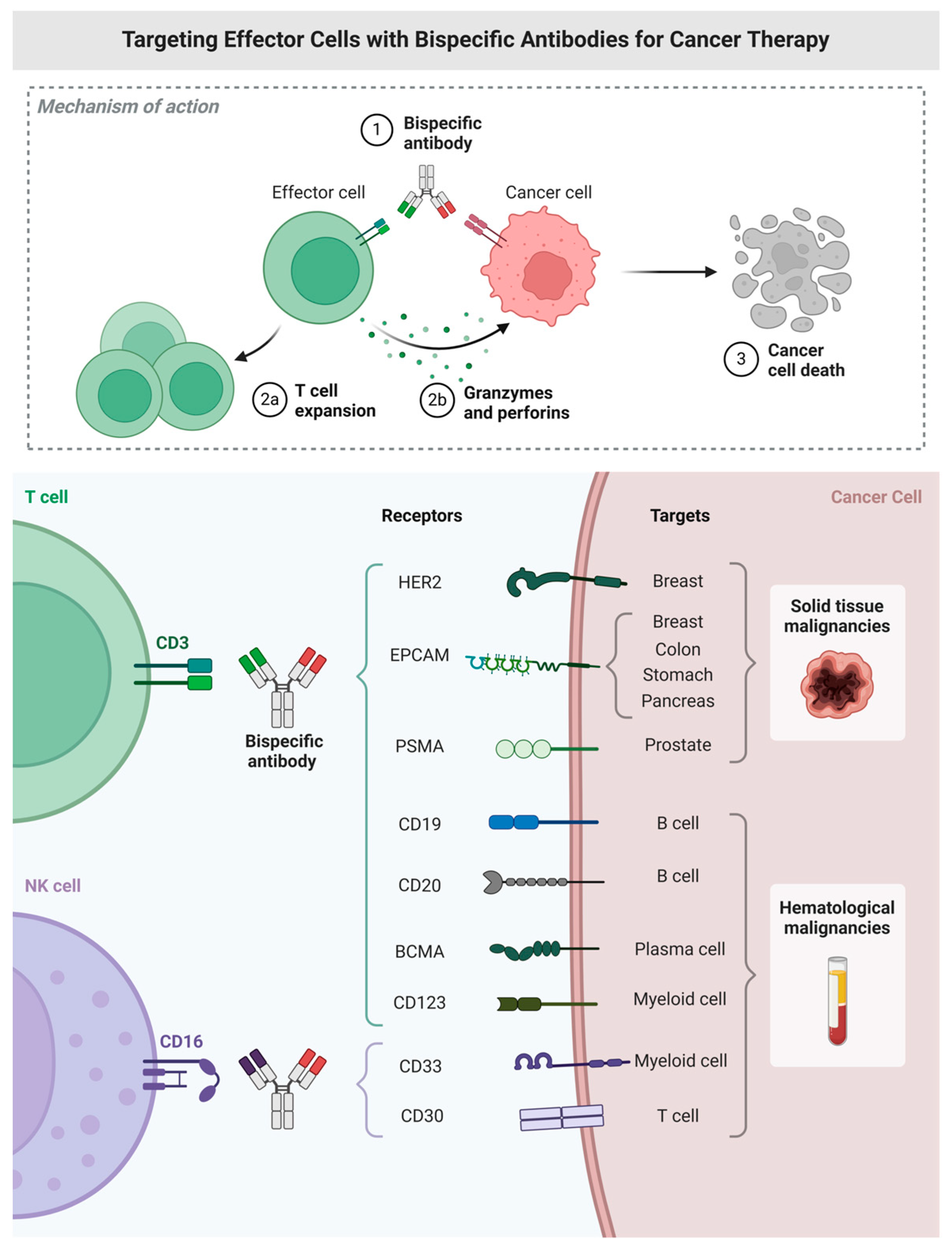
1. Introduction
2. Molecular Design of Bispecific and Trispecific Constructs
3. Therapeutic Indications of Bispecific and Trispecific Antibodies in Hematologic Malignancies
3.1. Acute Lymphoblastic Leukemia (ALL)
3.2. Acute Myeloid Leukemia (AML)
3.3. B-Cell Non-Hodgkin Lymphomas: Diffuse Large B-Cell and Follicular Subtypes
3.4. Multiple Myeloma (MM)
4. Immune-Related Toxicities of Bispecific and Trispecific Antibodies
4.1. Cytokine Release Syndrome (CRS)
4.2. Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS)
4.3. Hematologic Toxicities
4.4. Infections
5. Resistance Mechanisms and Therapeutic Strategies in T-Cell Redirecting Bispecific and Trispecific Antibody Therapy
5.1. Primary and Secondary Resistance
5.2. Biomarkers: Pretreatment and On-Treatment
5.3. Minimal Residual Disease (MRD)
5.4. Retreatment and Approaches to Overcome Resistance
6. Future Directions and Strategic Positioning of Bispecific and Trispecific Antibodies in Hematologic Malignancies
6.1. Disease-Specific Integration and Sequencing
6.2. Beyond Trispecifics: New Multispecific Designs
6.3. Expanding into Solid Tumors
6.4. Artificial Intelligence in Multispecific Antibody Development
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Moreau, P.; Touzeau, C. T-cell–redirecting bispecific antibodies in multiple myeloma: A revolution? Blood J. Am. Soc. Hematol. 2022, 139, 3681–3687. [Google Scholar] [CrossRef] [PubMed]
- Kordic, A.; Phillips, T.J.; Weiss, J. The Current State of Bispecific Antibodies and T-Cell Directed Therapy in NHL. Cancers 2025, 17, 1192. [Google Scholar] [CrossRef] [PubMed]
- Castaneda-Puglianini, O.; Chavez, J.C. Bispecific antibodies for non-Hodgkin’s lymphomas and multiple myeloma. Drugs Context 2021, 10, 2021-2-4. [Google Scholar] [CrossRef] [PubMed]
- Lantz, J.; Pham, N.; Jones, C.; Reed, D.; El Chaer, F.; Keng, M. Blinatumomab in Practice. Curr. Hematol. Malig. Rep. 2024, 19, 1–8. [Google Scholar] [CrossRef]
- Chakraborty, R.; Cheruvalath, H.; Patwari, A.; Szabo, A.; Schinke, C.; Dhakal, B.; Lentzsch, S.; D’Souza, A.; Mohyuddin, G.R.; Julian, K.; et al. Sustained remission following finite duration bispecific antibody therapy in patients with relapsed/refractory myeloma. Blood Cancer J. 2024, 14, 137. [Google Scholar] [CrossRef]
- Li, J.; Slaga, D.; Johnston, J.; Junttila, T.T. IMiDs Augment CD3-Bispecific Antibody–Induced CD8+ T-Cell Cytotoxicity and Expansion by Enhancing IL2 Production. Mol. Cancer Ther. 2023, 22, 659–666. [Google Scholar] [CrossRef]
- Baines, A.C.; Kanapuru, B.; Zhao, J.; Price, L.S.; Zheng, N.; Konicki, R.; Manning, M.L.; Gehrke, B.J.; Theoret, M.R.; Gormley, N.J. FDA Approval Summary: Teclistamab–A Bispecific CD3 T-Cell Engager for Patients with Relapsed or Refractory Multiple Myeloma. Clin. Cancer Res. 2024, 30, 5515–5520. [Google Scholar] [CrossRef]
- Lancman, G.; Richter, J.; Chari, A. Bispecifics, trispecifics, and other novel immune treatments in myeloma. Hematol. Am. Soc. Hematol. Educ. Program 2020, 2020, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Chen, G.; Cao, Y.J. Optimal Structural Designs of Trispecific Antibodies to Enhance Therapeutic Efficacy in Solid Tumors and Hematological Malignancies. Cancer Immunosurveill. Methods Protoc. 2025, 2930, 277. [Google Scholar]
- Schoenfeld, K.; Harwardt, J.; Kolmar, H. Better safe than sorry: Dual targeting antibodies for cancer immunotherapy. Biol. Chem. 2024, 405, 443–459. [Google Scholar] [CrossRef]
- Velasquez, M.P.; Bonifant, C.L.; Gottschalk, S. Redirecting T cells to hematological malignancies with bispecific antibodies. Blood J. Am. Soc. Hematol. 2018, 131, 30–38. [Google Scholar] [CrossRef]
- Restelli, C.; Ruella, M.; Paruzzo, L.; Tarella, C.; Pelicci, P.G.; Colombo, E. Recent advances in immune-based therapies for acute myeloid leukemia. Blood Cancer Discov. 2024, 5, 234–248. [Google Scholar] [CrossRef] [PubMed]
- van de Donk, N.W.; O’Neill, C.; de Ruijter, M.E.; Verkleij, C.P.; Zweegman, S. T-cell redirecting bispecific and trispecific antibodies in multiple myeloma beyond BCMA. Curr. Opin. Oncol. 2023, 35, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, J.K.; Gao, L.; Shouse, G.; Song, J.Y.; Pak, S.; Lee, B.; Chen, B.T.; Kallam, A.; Baird, J.H.; Marcucci, G.; et al. Glofitamab stimulates immune cell infiltration of CNS tumors and induces clinical responses in secondary CNS lymphoma. Blood 2024, 144, 457–461. [Google Scholar]
- Sehn, L.H.; Bartlett, N.L.; Matasar, M.J.; Schuster, S.J.; Assouline, S.E.; Giri, P.; Kuruvilla, J.; Shadman, M.; Cheah, C.Y.; Dietrich, S.; et al. Long-term 3-year follow-up of mosunetuzumab in relapsed or refractory follicular lymphoma after≥ 2 prior therapies. Blood 2025, 145, 708–719. [Google Scholar]
- Thieblemont, C.; Karimi, Y.H.; Ghesquieres, H.; Cheah, C.Y.; Clausen, M.R.; Cunningham, D.; Jurczak, W.; Do, Y.R.; Gasiorowski, R.; Lewis, D.J.; et al. Epcoritamab in relapsed/refractory large B-cell lymphoma: 2-year follow-up from the pivotal EPCORE NHL-1 trial. Leukemia 2024, 38, 2653–2662. [Google Scholar] [CrossRef]
- Qin, X.; Ning, W.; Liu, H.; Liu, X.; Luo, W.; Xia, N. Stepping forward: T-cell redirecting bispecific antibodies in cancer therapy. Acta Pharm. Sin. B 2024, 14, 2361–2377. [Google Scholar] [CrossRef]
- Fleury, I.; MacDonald, D.; Shafey, M.; Christofides, A.; Sehn, L.H. Optimal Use of Bispecific Antibodies for the Treatment of Diffuse Large B-Cell Lymphoma in Canada. Curr. Oncol. 2025, 32, 142. [Google Scholar] [CrossRef] [PubMed]
- Jain, T.; Litzow, M.R. Management of toxicities associated with novel immunotherapy agents in acute lymphoblastic leukemia. Ther. Adv. Hematol. 2020, 11, 2040620719899897. [Google Scholar] [CrossRef]
- Cho, S.F.; Lin, L.; Xing, L.; Wen, K.; Yu, T.; Wahl, J.; Matthes, K.; Munshi, N.; Anderson, K.C.; Arvedson, T.; et al. AMG 701, a half-life extended anti-BCMA BiTE®, potently induces T cell-redirected lysis of human multiple myeloma cells and can be combined with IMiDs to overcome the immunosuppressive bone marrow microenvironment. Clin. Lymphoma Myeloma Leuk. 2019, 19, e54. [Google Scholar] [CrossRef]
- Harrison, S.J.; Minnema, M.C.; Lee, H.C.; Spencer, A.; Kapoor, P.; Madduri, D.; Larsen, J.; Ailawadhi, S.; Kaufman, J.L.; Raab, M.S.; et al. A phase 1 first in human (FIH) study of AMG 701, an anti-B-cell maturation antigen (BCMA) half-life extended (HLE) BiTE®(bispecific T-cell engager) molecule, in relapsed/refractory (RR) multiple myeloma (MM). Blood 2020, 136, 28–29. [Google Scholar] [CrossRef]
- Shirley, M. Glofitamab: First approval. Drugs 2023, 83, 935–941. [Google Scholar] [CrossRef]
- Minson, A.; Dickinson, M. Glofitamab CD20-TCB bispecific antibody. Leuk. Lymphoma 2021, 62, 3098–3108. [Google Scholar] [CrossRef]
- Nooka, A.K.; Rodriguez, C.; Mateos, M.V.; Manier, S.; Chastain, K.; Banerjee, A.; Kobos, R.; Qi, K.; Verona, R.; Doyle, M.; et al. Incidence, timing, and management of infections in patients receiving teclistamab for the treatment of relapsed/refractory multiple myeloma in the MajesTEC-1 study. Cancer 2024, 130, 886–900. [Google Scholar] [CrossRef]
- Moreau, P.; van de Donk, N.W.; Delforge, M.; Einsele, H.; De Stefano, V.; Perrot, A.; Besemer, B.; Pawlyn, C.; Karlin, L.; Manier, S.; et al. Comparative efficacy of teclistamab versus current treatments in real-world clinical practice in the prospective LocoMMotion study in patients with triple-class-exposed relapsed and/or refractory multiple myeloma. Adv. Ther. 2023, 40, 2412–2425. [Google Scholar] [CrossRef] [PubMed]
- Dunai, C.; Ames, E.; Ochoa, M.C.; Fernandez-Sendin, M.; Melero, I.; Simonetta, F.; Baker, J.; Alvarez, M. Killers on the loose: Immunotherapeutic strategies to improve NK cell-based therapy for cancer treatment. Int. Rev. Cell Mol. Biol. 2022, 370, 65–122. [Google Scholar] [PubMed]
- Page, A.; Chuvin, N.; Valladeau-Guilemond, J.; Depil, S. Development of NK cell-based cancer immunotherapies through receptor engineering. Cell. Mol. Immunol. 2024, 21, 315–331. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, L.; Gu, X.; Zhao, J.; Bi, J.; Pan, L. Leveraging T cell co-stimulation for enhanced therapeutic efficacy of trispecific antibodies targeting prostate cancer. J. Immunother. Cancer 2025, 13, e010140. [Google Scholar] [CrossRef]
- Sandeep Shinde, S.H.; Ahmed, S.; Sharma, S.S.; Pande, A.H. Engineered polyspecific antibodies: A new frontier in the field of immunotherapeutics. Immunology 2024, 171, 464–496. [Google Scholar] [CrossRef] [PubMed]
- Thisted, T.; Smith, F.D.; Jiang, Z.G.; Onumajuru, A.; Biesova, Z.; Kleschenko, Y.; Malhotra, K.; Saxena, V.; Mukherjee, A.; van der Horst, E.H. Dual Targeting for Enhanced Tumor Immunity: Conditionally Active CD28xVISTA Bispecific Antibodies Promote Myeloid-Driven T-Cell Activation. bioRxiv 2025. bioRxiv:07.647657. [Google Scholar]
- Lotze, M.T.; Olejniczak, S.H.; Skokos, D. CD28 co-stimulation: Novel insights and applications in cancer immunotherapy. Nat. Rev. Immunol. 2024, 24, 878–895. [Google Scholar] [CrossRef]
- In, H.; Park, M.; Lee, H.; Han, K.H. Immune Cell Engagers: Advancing Precision Immunotherapy for Cancer Treatment. Antibodies 2025, 14, 16. [Google Scholar] [CrossRef] [PubMed]
- Klingemann, H. The NK-92 cell line—30 years later: Its impact on natural killer cell research and treatment of cancer. Cytotherapy 2023, 25, 451–457. [Google Scholar] [CrossRef]
- Maskalenko, N.A.; Zahroun, S.; Tsygankova, O.; Anikeeva, N.; Sykulev, Y.; Campbell, K.S. The FcγRIIIA (CD16) L48-H/R Polymorphism Enhances NK Cell–Mediated Antibody-Dependent Cellular Cytotoxicity by Promoting Serial Killing. Cancer Immunol. Res. 2025, 13, 417–429. [Google Scholar] [CrossRef]
- Tapia-Galisteo, A.; Compte, M.; Álvarez-Vallina, L.; Sanz, L. When three is not a crowd: Trispecific antibodies for enhanced cancer immunotherapy. Theranostics 2023, 13, 1028–1041. [Google Scholar] [CrossRef]
- Zhao, L.; Li, S.; Wei, X.; Qi, X.; Liu, D.; Liu, L.; Wen, F.; Zhang, J.S.; Wang, F.; Liu, Z.L.; et al. A novel CD19/CD22/CD3 trispecific antibody enhances therapeutic efficacy and overcomes immune escape against B-ALL. Blood J. Am. Soc. Hematol. 2022, 140, 1790–1802. [Google Scholar] [CrossRef] [PubMed]
- Kuchnio, A.; Yang, D.; Vloemans, N.; Lowenstein, C.; Cornelissen, I.; Amorim, R.; Han, C.; Sukumaran, S.; Janssen, L.; Suls, T.; et al. Characterization of JNJ-80948543, a novel CD79bxCD20xCD3 trispecific T-cell redirecting antibody for the treatment of b-cell non-Hodgkin lymphoma. Blood 2022, 140, 3105–3106. [Google Scholar] [CrossRef]
- Fontan, L.; Zwolak, A.; Guimerans-Lorenzo, I.; Bekkers, M.; Hein, N.; Vloemans, N.; Trella, E.; Smets, T.; Cornelissen, I.; Assefa, A.; et al. JNJ-87801493 (CD20xCD28), a Potential First-in-Class CD20 Targeted CD28 Costimulatory Bispecific Antibody, Enhances the Activity of B-Cell Targeting T-Cell Engagers in Preclinical Models. Blood 2024, 144, 1408. [Google Scholar] [CrossRef]
- Cao, Z.; Osellame, L.D.; Allan, L.; Scott, A.M. Clinical development of tri-specific antibodies for immune-oncology. Expert Opin. Investig. Drugs 2025. Online ahead of print. [Google Scholar] [CrossRef]
- Horenstein, A.L.; Faini, A.C.; Morandi, F.; Ortolan, E.; Storti, P.; Giuliani, N.; Richardson, P.G.; Malavasi, F. Monoclonal anti-CD38 therapy in human myeloma: Retrospects and prospects. Front. Immunol. 2025, 16, 1519300. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.; Stein, A.; Gökbuget, N.; Fielding, A.K.; Schuh, A.C.; Ribera, J.M.; Wei, A.; Dombret, H.; Foà, R.; Bassan, R.; et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N. Engl. J. Med. 2017, 376, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.A.; Ji, L.; Xu, X.; Devidas, M.; Hogan, L.E.; Borowitz, M.J.; Raetz, E.A.; Zugmaier, G.; Sharon, E.; Bernhardt, M.B.; et al. Effect of postreinduction therapy consolidation with blinatumomab vs. chemotherapy on disease-free survival in children, adolescents, and young adults with first relapse of B-cell acute lymphoblastic leukemia: A randomized clinical trial. JAMA 2021, 325, 833–842. [Google Scholar] [CrossRef]
- Yao, Y.; Hu, Y.; Wang, F. Trispecific antibodies for cancer immunotherapy. Immunology 2023, 169, 389–399. [Google Scholar] [CrossRef]
- Huang, S.; Van Duijnhoven, S.M.; Sijts, A.J.; Van Elsas, A. Bispecific antibodies targeting dual tumor-associated antigens in cancer therapy. J. Cancer Res. Clin. Oncol. 2020, 146, 3111–3122. [Google Scholar] [CrossRef]
- Zabaleta, A.; Blanco, L.; Kim, P.; Bisht, K.; Wang, H.; Van de Velde, H.J.; Lasa, M.; Tamariz-Amador, L.E.; Otero, P.R.; San Miguel, J.; et al. A CD38/CD28xCD3 trispecific T-cell engager (TCE) as a potentially active agent in multiple myeloma patients relapsed and/or refractory (RRMM) to anti-CD38 monoclonal antibodies (mAbs). Blood 2023, 142, 1921. [Google Scholar] [CrossRef]
- Müller, D. Optimized CD19/CD22/CD3 antibody. Blood J. Am. Soc. Hematol. 2022, 140, 1750–1751. [Google Scholar] [CrossRef]
- Uy, G.L.; Aldoss, I.; Foster, M.C.; Sayre, P.H.; Wieduwilt, M.J.; Advani, A.S.; Godwin, J.E.; Arellano, M.L.; Sweet, K.L.; Emadi, A.; et al. Flotetuzumab as salvage immunotherapy for refractory acute myeloid leukemia. Blood J. Am. Soc. Hematol. 2021, 137, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Espinoza-Gutarra, M.R.; Green, S.D.; Zeidner, J.F.; Konig, H. CD123-targeted therapy in acute myeloid leukemia. Expert Rev. Hematol. 2021, 14, 561–576. [Google Scholar] [CrossRef]
- Lee, E.; Lee, S.; Park, S.; Son, Y.G.; Yoo, J.; Koh, Y.; Shin, D.Y.; Lim, Y.; Won, J. Asymmetric anti-CLL-1× CD3 bispecific antibody, ABL602 2+ 1, with attenuated CD3 affinity endows potent antitumor activity but limited cytokine release. J. Immunother. Cancer 2023, 11, e007494. [Google Scholar] [CrossRef]
- Guarnera, L.; Bravo-Perez, C.; Visconte, V. Immunotherapy in acute myeloid leukemia: A literature review of emerging strategies. Bioengineering 2023, 10, 1228. [Google Scholar] [CrossRef] [PubMed]
- Ravandi, F.; Bashey, A.; Stock, W.; Foran, J.M.; Mawad, R.; Egan, D.; Blum, W.; Yang, A.; Pastore, A.; Johnson, C.; et al. Complete responses in relapsed/refractory acute myeloid leukemia (AML) patients on a weekly dosing schedule of vibecotamab (XmAb14045), a CD123 x CD3 T cell-engaging bispecific antibody; initial results of a phase 1 study. Blood 2020, 136, 4–5. [Google Scholar] [CrossRef]
- Sallman, D.A.; Al Malki, M.; Asch, A.S.; Lee, D.J.; Kambhampati, S.; Donnellan, W.B.; Bradley, T.J.; Vyas, P.; Jeyakumar, D.; Marcucci, G.; et al. Tolerability and efficacy of the first-in-class anti-CD47 antibody magrolimab combined with azacitidine in MDS and AML patients: Phase Ib results. J. Clin. Oncol. 2020, 38. [Google Scholar] [CrossRef]
- Leong, S.R.; Sukumaran, S.; Hristopoulos, M.; Totpal, K.; Stainton, S.; Lu, E.; Wong, A.; Tam, L.; Newman, R.; Vuillemenot, B.R.; et al. An anti-CD3/anti–CLL-1 bispecific antibody for the treatment of acute myeloid leukemia. Blood J. Am. Soc. Hematol. 2017, 129, 609–618. [Google Scholar] [CrossRef]
- Arvindam, U.S.; van Hauten, P.M.; Schirm, D.; Schaap, N.; Hobo, W.; Blazar, B.R.; Vallera, D.A.; Dolstra, H.; Felices, M.; Miller, J.S. A trispecific killer engager molecule against CLEC12A effectively induces NK-cell mediated killing of AML cells. Leukemia 2021, 35, 1586–1596. [Google Scholar] [CrossRef]
- Rolin, C.; Zimmer, J.; Seguin-Devaux, C. Bridging the gap with multispecific immune cell engagers in cancer and infectious diseases. Cell. Mol. Immunol. 2024, 21, 643–661. [Google Scholar]
- Roskopf, C.C.; Braciak, T.A.; Fenn, N.C.; Kobold, S.; Fey, G.H.; Hopfner, K.P.; Oduncu, F.S. Dual-targeting triplebody 33-3-19 mediates selective lysis of biphenotypic CD19+ CD33+ leukemia cells. Oncotarget 2016, 7, 22579. [Google Scholar] [CrossRef]
- Bannerji, R.; Allan, J.N.; Arnason, J.E.; Brown, J.R.; Advani, R.; Ansell, S.M.; O’Brien, S.M.; Duell, J.; Martin, P.; Joyce, R.M.; et al. Odronextamab (REGN1979), a human CD20 x CD3 bispecific antibody, induces durable, complete responses in patients with highly refractory B-cell non-Hodgkin lymphoma, including patients refractory to CAR T therapy. Blood 2020, 136, 42–43. [Google Scholar] [CrossRef]
- Hutchings, M. The evolving therapy of DLBCL: Bispecific antibodies. Hematol. Oncol. 2023, 41, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Morschhauser, F.; Fowler, N.H.; Feugier, P.; Bouabdallah, R.; Tilly, H.; Palomba, M.L.; Fruchart, C.; Libby, E.N.; Casasnovas, R.O.; Flinn, I.W.; et al. Rituximab plus lenalidomide in advanced untreated follicular lymphoma. N. Engl. J. Med. 2018, 379, 934–947. [Google Scholar] [CrossRef] [PubMed]
- Budde, L.E.; Sehn, L.H.; Matasar, M.; Schuster, S.J.; Assouline, S.; Giri, P.; Kuruvilla, J.; Canales, M.; Dietrich, S.; Fay, K.; et al. Safety and efficacy of mosunetuzumab, a bispecific antibody, in patients with relapsed or refractory follicular lymphoma: A single-arm, multicentre, phase 2 study. Lancet Oncol. 2022, 23, 1055–1065. [Google Scholar] [PubMed]
- Dickinson, M.J.; Carlo-Stella, C.; Morschhauser, F.; Bachy, E.; Corradini, P.; Iacoboni, G.; Khan, C.; Wróbel, T.; Offner, F.; Trněný, M.; et al. Glofitamab for relapsed or refractory diffuse large B-cell lymphoma. N. Engl. J. Med. 2022, 387, 2220–2231. [Google Scholar]
- Matasar, M.; Bartlett, N.L.; Shadman, M.; Budde, L.E.; Flinn, I.; Gregory, G.P.; Kim, W.S.; Hess, G.; El-Sharkawi, D.; Diefenbach, C.S.; et al. Mosunetuzumab safety profile in patients with relapsed/refractory B-cell non-hodgkin lymphoma: Clinical management experience from a pivotal phase I/II trial. Clin. Lymphoma Myeloma Leuk. 2024, 24, 240–253. [Google Scholar]
- Brody, J.; Falchi, L.; Vitolo, U.; Nijland, M.; Offner, F.; Snauwaert, S.; Patah, P.; Marek, J.; Morehouse, C.; Steele, A.J.; et al. Fixed-Duration Epcoritamab in Combination with Bendamustine+ Rituximab for First-Line Treatment of Follicular Lymphoma: Initial Results from Epcore NHL-2 Arm 3. Blood 2024, 144, 1627. [Google Scholar] [CrossRef]
- Budde, L.E.; Sehn, L.H.; Matasar, M.J.; Schuster, S.J.; Assouline, S.; Giri, P.; Kuruvilla, J.; Canales, M.; Dietrich, S.; Fay, K.; et al. Mosunetuzumab monotherapy is an effective and well-tolerated treatment option for patients with relapsed/refractory (R/R) follicular lymphoma (FL) who have received ≥ 2 prior lines of therapy: Pivotal results from a phase I/II study. Blood 2021, 138, 127. [Google Scholar]
- Linton, K.M.; Wahlin, B.; Leppa, S.; Morschhauser, F.; Elliott, B.; Liu, T.; Stirner, M.C.; Abbas, A.; Falchi, L. Subcutaneous Epcoritamab in Combination with Rituximab and Lenalidomide in Relapsed or Refractory Follicular Lymphoma: Preliminary Phase 1/2 Results. Br. J. Haematol. 2022, 197, 94. [Google Scholar]
- Lu, H.; Oka, A.; Coulson, M.; Polli, J.R.; Aardalen, K.; Ramones, M.; Walker, D.B.; Carrion, A.; Alexander, D.; Klopfenstein, M.; et al. PIT565, a first-in-class anti-CD19, anti-CD3, anti-CD2 trispecific antibody for the treatment of B cell malignancies. Blood 2022, 140, 3148. [Google Scholar] [CrossRef]
- Mazza, I.A.; Barba, P.; Yuda, J.; Palomba, M.L.; Alderuccio, J.P.; De Vriendt, C.; Corradini, P.; Lim, F.L.; Zinzani, P.L.; Jain, N.; et al. A phase 1 study of PIT565, a first-in-class, anti-CD3, anti-CD19, anti-CD2 trispecific antibody in patients with relapsed and/or refractory B-Cell malignancies. Blood 2023, 142, 3099. [Google Scholar] [CrossRef]
- Abou Dalle, I.; Dulery, R.; Moukalled, N.; Ricard, L.; Stocker, N.; El-Cheikh, J.; Mohty, M.; Bazarbachi, A. Bi-and Tri-specific antibodies in non-Hodgkin lymphoma: Current data and perspectives. Blood Cancer J. 2024, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Vasu, S.; Bezerra, E.; Denlinger, N.; Szuminski, N.; Schneider, D.; Dash, P.; Wirthlin, L.; Epperla, N.; Sawalha, Y.; Woyach, J.A.; et al. Initial Results of a First-in-Human, Phase I Study Point-of-Care Manufacturing of Trispecific CAR-T Cells Targeting CD19/20/22 in B-Cell Malignancies. Blood 2024, 144, 2078. [Google Scholar] [CrossRef]
- Qureshi, Z.; Jamil, A.; Altaf, F.; Siddique, R.; Ahmed, F. Efficacy and safety of teclistamab in relapsed or refractory multiple myeloma: A systematic review and meta-analysis. Ann. Hematol. 2024, 103, 4901–4912. [Google Scholar] [CrossRef]
- Tomasson, M.H.; Iida, S.; Niesvizky, R.; Mohty, M.; Bahlis, N.J.; Martinez‐Lopez, J.; Koehne, G.; Rodriguez-Otero, P.; Prince, H.M.; Viqueira, A.; et al. Long-term survival and safety of elranatamab in patients with relapsed or refractory multiple myeloma: Update from the MagnetisMM-3 study. HemaSphere 2024, 8, e136. [Google Scholar]
- Lee, H.C.; Bumma, N.; Richter, J.R.; Dhodapkar, M.V.; Hoffman, J.E.; Suvannasankha, A.; Zonder, J.A.; Shah, M.R.; Lentzsch, S.; Maly, J.J.; et al. LINKER-MM1 study: Linvoseltamab (REGN5458) in patients with relapsed/refractory multiple myeloma. J. Clin. Oncol. 2023, 41, 8006. [Google Scholar] [CrossRef]
- Chari, A.; Minnema, M.C.; Berdeja, J.G.; Oriol, A.; van de Donk, N.W.; Rodríguez-Otero, P.; Askari, E.; Mateos, M.V.; Costa, L.J.; Caers, J.; et al. Talquetamab, a T-cell–redirecting GPRC5D bispecific antibody for multiple myeloma. N. Engl. J. Med. 2022, 387, 2232–2244. [Google Scholar] [CrossRef] [PubMed]
- Trudel, S.; Cohen, A.D.; Krishnan, A.Y.; Fonseca, R.; Spencer, A.; Berdeja, J.G.; Lesokhin, A.; Forsberg, P.A.; Laubach, J.P.; Costa, L.J.; et al. Cevostamab monotherapy continues to show clinically meaningful activity and manageable safety in patients with heavily pre-treated relapsed/refractory multiple myeloma (RRMM): Updated results from an ongoing phase I study. Blood 2021, 138, 157. [Google Scholar] [CrossRef]
- Yan, S.; Ming, X.; Zheng, R.; Zhu, X.; Xiao, Y. Application of GPRC5D Targeting Therapy in Relapsed Refractory Multiple Myeloma. Cancer Med. 2025, 14, e70764. [Google Scholar] [CrossRef]
- van de Donk, N.W.; Vega, G.; Perrot, A.; Anguille, S.; Oriol, A.; Minnema, M.; Kaiser, M.F.; Lee, H.C.; Garfall, A.; Matous, J.V.; et al. First-in-Human Study of JNJ-79635322 (JNJ-5322), a Novel, Next-Generation Trispecific Antibody (TsAb), in Patients (pts) with Relapsed/Refractory Multiple Myeloma (RRMM): Initial Phase 1 Results. Available online: https://meetings.asco.org/abstracts-presentations/243590 (accessed on 5 April 2025).
- Grab, A.L.; Kim, P.S.; John, L.; Bisht, K.; Wang, H.; Baumann, A.; Van de Velde, H.; Sarkar, I.; Shome, D.; Reichert, P.; et al. Pre-Clinical Assessment of SAR442257, a CD38/CD3xCD28 Trispecific T Cell Engager in Treatment of Relapsed/Refractory Multiple Myeloma. Cells 2024, 13, 879. [Google Scholar] [CrossRef]
- Wu, L.; Seung, E.; Xu, L.; Rao, E.; Lord, D.M.; Wei, R.R.; Cortez-Retamozo, V.; Ospina, B.; Posternak, V.; Ulinski, G.; et al. Trispecific antibodies enhance the therapeutic efficacy of tumor-directed T cells through T cell receptor co-stimulation. Nat. Cancer 2020, 1, 86–98. [Google Scholar] [CrossRef]
- van de Donk, N.W.; Zweegman, S. T-cell-engaging bispecific antibodies in cancer. Lancet 2023, 402, 142–158. [Google Scholar] [CrossRef]
- Gökbuget, N.; Dombret, H.; Bonifacio, M.; Reichle, A.; Graux, C.; Faul, C.; Diedrich, H.; Topp, M.S.; Brüggemann, M.; Horst, H.A.; et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood J. Am. Soc. Hematol. 2018, 131, 1522–1531. [Google Scholar] [CrossRef] [PubMed]
- Sangwan, K.; Sharma, V.; Goyal, P.K. Pharmacological profile of novel anti-cancer drugs approved by USFDA in 2022: A review. Curr. Mol. Med. 2024, 24, 734–750. [Google Scholar] [CrossRef] [PubMed]
- Schjesvold, F.; Jelinek, T.; Polgarova, K.; Pour, L.; Yoon, S.S.; Kim, W.S.; Fosså, A.; San-Miguel, J.F.; Canales, M.; Rodríguez-Otero, P.; et al. First-in-Human Phase 1 Study of SAR442257 in Patients with Relapsed/Refractory Multiple Myeloma and Non-Hodgkin Lymphoma. Blood 2024, 144, 1992. [Google Scholar] [CrossRef]
- Martin, T.G.; Mateos, M.V.; Nooka, A.; Banerjee, A.; Kobos, R.; Pei, L.; Qi, M.; Verona, R.; Doyle, M.; Smit, J.; et al. Detailed overview of incidence and management of cytokine release syndrome observed with teclistamab in the MajesTEC-1 study of patients with relapsed/refractory multiple myeloma. Cancer 2023, 129, 2035–2046. [Google Scholar] [CrossRef]
- Abramson, J.S.; Ku, M.; Hertzberg, M.; Huang, H.Q.; Fox, C.P.; Zhang, H.; Yoon, D.H.; Kim, W.S.; Abdulhaq, H.; Townsend, W.; et al. Glofitamab plus gemcitabine and oxaliplatin (GemOx) versus rituximab-GemOx for relapsed or refractory diffuse large B-cell lymphoma (STARGLO): A global phase 3, randomised, open-label trial. Lancet 2024, 404, 1940–1954. [Google Scholar] [CrossRef]
- Thieblemont, C.; Phillips, T.; Ghesquieres, H.; Cheah, C.Y.; Clausen, M.R.; Cunningham, D.; Do, Y.R.; Feldman, T.; Gasiorowski, R.; Jurczak, W.; et al. Epcoritamab, a novel, subcutaneous CD3xCD20 bispecific T-cell–engaging antibody, in relapsed or refractory large B-cell lymphoma: Dose expansion in a phase I/II trial. J. Clin. Oncol. 2023, 41, 2238–2247. [Google Scholar] [CrossRef]
- Nolan-Stevaux, O.; Smith, R. Logic-gated and contextual control of immunotherapy for solid tumors: Contrasting multi-specific T cell engagers and CAR-T cell therapies. Front. Immunol. 2024, 15, 1490911. [Google Scholar] [CrossRef] [PubMed]
- Locke, F.L.; Mahmoudjafari, Z.; Kebriaei, P.; Gardner, R.A.; Frigault, M.J.; Frey, N.; Komanduri, K.V.; Perales, M.A.; Nikiforow, S. Awakening from REMS: ASTCT 80/20 Ongoing Recommendations for Safe Use of Chimeric Antigen Receptor T Cells. Transplant. Cell. Ther. 2025, in press. [Google Scholar] [CrossRef]
- Boutin, L.; Barjon, C.; Lafrance, L.; Senechal, E.; Bourges, D.; Vigne, E.; Scotet, E. Targeting human γδ T cells as a potent and safe alternative to pan-T cells bispecific cell engagers. bioRxiv 2023. bioRxiv:10.548307. [Google Scholar]
- Radtke, K.K.; Bender, B.C.; Li, Z.; Turner, D.C.; Roy, S.; Belousov, A.; Li, C.C. Clinical Pharmacology of Cytokine Release Syndrome with T-Cell–Engaging Bispecific Antibodies: Current Insights and Drug Development Strategies. Clin. Cancer Res. 2025, 31, 245–257. [Google Scholar] [CrossRef]
- Leidy, S.; Snyder, J.; Davis, J.A.; Wesson, W.; Hess, B.; Jacobs, R.; Edmonds, M.; Ahmed, N.; Hoffmann, M. Practical Implications of Multi-Institution Cytokine Release Syndrome (CRS) and Immune Effector Cell-Associated Neurotoxicity (ICANS) Rates in Lymphoma Targeted Bispecific Antibodies (BsAb). Blood 2024, 144, 2350. [Google Scholar] [CrossRef]
- Beltran, H.; Johnson, M.L.; Jain, P.; Schenk, E.L.; Sanborn, R.E.; Thompson, J.R.; Dowlati, A.; Mamdani, H.; Aggarwal, R.R.; Anand, B.S.; et al. Updated results from a phase 1/2 study of HPN328, a tri-specific, half-life (T1/2) extended DLL3-targeting T-cell engager in patients (pts) with small cell lung cancer (SCLC) and other neuroendocrine cancers (NEC). J. Clin. Oncol. 2024, 42. [Google Scholar] [CrossRef]
- van de Donk, N.W.; Moreau, P.; Garfall, A.L.; Bhutani, M.; Oriol, A.; Nooka, A.K.; Martin, T.G.; Rosiñol, L.; Mateos, M.V.; Bahlis, N.J.; et al. Long-term follow-up from MajesTEC-1 of teclistamab, a B-cell maturation antigen (BCMA) x CD3 bispecific antibody, in patients with relapsed/refractory multiple myeloma (RRMM). J. Clin. Oncol. 2023, 41. [Google Scholar] [CrossRef]
- Mohan, M.; Monge, J.; Shah, N.; Luan, D.; Forsberg, M.; Bhatlapenumarthi, V.; Balev, M.; Patwari, A.; Cheruvalath, H.; Bhutani, D.; et al. Teclistamab in relapsed refractory multiple myeloma: Multi-institutional real-world study. Blood Cancer J. 2024, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, M.J.; Carlo-Stella, C.; Morschhauser, F.; Bachy, E.; Cartron, G.; Corradini, P.; Bartlett, N.L.; Iacoboni, G.; Khan, C.; Hertzberg, M.S.; et al. Fixed-duration Glofitamab Monotherapy Continues to Demonstrate Durable Responses in Patients with Relapsed or Refractory Large B-Cell Lymphoma: 3-year Follow-Up From a Pivotal Phase II Study. Blood 2024, 144, 865. [Google Scholar] [CrossRef]
- Paul, S.; Jabbour, E.; Nichols, E.D.; Short, N.J.; Kantarjian, H. Blinatumomab for the treatment of acute lymphoblastic leukemia in a real-world setting: Clinical vignettes. Leuk. Lymphoma 2025, 66, 389–399. [Google Scholar]
- Gao, W.; Yu, J.; Sun, Y.; Song, Z.; Liu, X.; Han, X.; Li, L.; Qiu, L.; Zhou, S.; Qian, Z.; et al. Adverse events in the nervous system associated with blinatumomab: A real-world study. BMC Med. 2025, 23, 72. [Google Scholar] [CrossRef]
- Liu, L.; Krishnan, A. Talquetamab in multiple myeloma. Haematologica 2023, 109, 718. [Google Scholar] [CrossRef]
- Tapia-Galisteo, A.; Álvarez-Vallina, L.; Sanz, L. Bi-and trispecific immune cell engagers for immunotherapy of hematological malignancies. J. Hematol. Oncol. 2023, 16, 83. [Google Scholar] [CrossRef]
- Lee, D.W.; Santomasso, B.D.; Locke, F.L.; Ghobadi, A.; Turtle, C.J.; Brudno, J.N.; Maus, M.V.; Park, J.H.; Mead, E.; Pavletic, S.; et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol. Blood Marrow Transplant. 2019, 25, 625–638. [Google Scholar] [CrossRef]
- Carrara, S.C. Generation of Multispecific Antibodies with Immune Cell Modulating Functions. Ph.D. Thesis, Technische Universität Darmstadt, Darmstadt, Germany, 2023. [Google Scholar]
- Li, H.; Zhao, L.; Sun, Z.; Yao, Y.; Li, L.; Wang, J.; Hua, T.; Ji, S.; Wang, S.; Cheng, H.; et al. Prolonged hematological toxicity in patients receiving BCMA/CD19 CAR-T-cell therapy for relapsed or refractory multiple myeloma. Front. Immunol. 2022, 13, 1019548. [Google Scholar] [CrossRef]
- Moreau, P.; Garfall, A.L.; van de Donk, N.W.; Nahi, H.; San-Miguel, J.F.; Oriol, A.; Nooka, A.K.; Martin, T.; Rosinol, L.; Chari, A.; et al. Teclistamab in relapsed or refractory multiple myeloma. N. Engl. J. Med. 2022, 387, 495–505. [Google Scholar] [CrossRef]
- Dima, D.; Davis, J.A.; Ahmed, N.; Sannareddy, A.; Shaikh, H.; Mahmoudjafari, Z.; Khouri, J.; Kaur, G.; Strouse, C.; Valent, J.; et al. Real-world safety and efficacy of teclistamab for patients with heavily pretreated relapsed-refractory multiple myeloma. Blood 2023, 142, 91. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, X.; Zhang, R.; Jing, Z.; Zhao, C.; Pan, F.; Zheng, B.; Dai, R.; Yang, Y.; Zeng, L. Beyond antibodies and CAR-T: CC312, a first-in-class, anti-CD19, anti-CD3, anti-CD28 trispecific antibody in treatment with relapsed and/or refractory B-cell malignancies. Cancer Res. 2025, 85, 2140. [Google Scholar] [CrossRef]
- Menon, V.; Holkova, B.; Pacaud, L.; Gn, S.; Garton, A.; Pihlgren, M.; Matsuura, T.; van der Graaf, P.H.; Perro, M.; Konto, C. Clinical validation of a quantitative systems pharmacology (QSP) model of ISB 2001 used for deriving first in human (FIH) dose and efficient phase 1 dose escalation design in relapsed refractory multiple myeloma (RRMM) patients. Cancer Res. 2025, 85, 3694. [Google Scholar] [CrossRef]
- Tan, Y.; Li, X.; Yu, F.; Xu, J.; Qian, Z.; Cao, Y.; Yang, X.; Du, Q.; Peng, F.; Han, S.; et al. Abstract LB128: A novel tri-specific T cell engager targeting BCMA and GPRC5D for treatment of multiple myeloma. Cancer Res. 2024, 84, LB128. [Google Scholar] [CrossRef]
- Roth, H.; Rogers, D.; Sanchez, I.; Tyrell, B.; Snyder, A.; Doolan, K.; Doranz, B.; Chambers, R.; Rucker, J. GPRC5D multispecific antibodies with potent anti-tumor activity against multiple myeloma. Cancer Res. 2025, 85, 3406. [Google Scholar] [CrossRef]
- Bangolo, A.; Amoozgar, B.; Mansour, C.; Zhang, L.; Gill, S.; Ip, A.; Cho, C. Comprehensive Review of Early and Late Toxicities in CAR T-Cell Therapy and Bispecific Antibody Treatments for Hematologic Malignancies. Cancers 2025, 17, 282. [Google Scholar] [CrossRef]
- Salvaris, R.; Ong, J.; Gregory, G.P. Bispecific antibodies: A review of development, clinical efficacy and toxicity in B-cell lymphomas. J. Pers. Med. 2021, 11, 355. [Google Scholar] [CrossRef]
- Tan, C.R.; Asoori, S.; Huang, C.Y.; Brunaldi, L.; Popat, R.; Kastritis, E.; Martinez-Lopez, J.; Bansal, R.; Silva Corraes, A.D.; Chhabra, S.; et al. Real-world evaluation of teclistamab for the treatment of relapsed/refractory multiple myeloma (RRMM): An International Myeloma Working Group Study. Blood Cancer J. 2025, 15, 53. [Google Scholar]
- Hutchings, M.; Morschhauser, F.; Iacoboni, G.; Carlo-Stella, C.; Offner, F.C.; Sureda, A.; Salles, G.; Martínez-Lopez, J.; Crump, M.; Thomas, D.N.; et al. Glofitamab, a novel, bivalent CD20-targeting T-cell–engaging bispecific antibody, induces durable complete remissions in relapsed or refractory B-cell lymphoma: A phase I trial. J. Clin. Oncol. 2021, 39, 1959–1970. [Google Scholar] [CrossRef]
- Elemian, S.; Habbas, A.; Jumean, S.; Al Omour, B.; Hamad, M.; Tan, J.Y.; Chan, K.H.; Guron, G.; Shaaban, H. Efficacy and Safety of Mosunetuzumab in Relapsed/Refractory Non-Hodgkin Lymphoma: A Systematic Review. Blood 2024, 144, 6512. [Google Scholar] [CrossRef]
- An, G.; Xing, L.; Chen, W.; Zhang, Y.; Gao, W.; Qiu, L.G.; Wu, G.; Ning, J.; Wei, M.; Li, F. MBS314, a G Protein-Coupled Receptor Family C Group 5 Member D (GPRC5D) x B-Cell Maturation Antigen (BCMA) x CD3 Trispecific Antibody, in Relapsed and/or Refractory Multiple Myeloma (RRMM): Preliminary Results from a Phase I, First-in-Human, Open-Label, Dose Escalation Study. Blood 2024, 144, 3356. [Google Scholar]
- Mazahreh, F.; Mazahreh, L.; Schinke, C.; Thanendrarajan, S.; Zangari, M.; Shaughnessy, J.D., Jr.; Zhan, F.; Van Rhee, F.; Al Hadidi, S. Risk of infections associated with the use of bispecific antibodies in multiple myeloma: A pooled analysis. Blood Adv. 2023, 7, 3069–3074. [Google Scholar] [CrossRef] [PubMed]
- Martino, M.; Gamberi, B.; Antonioli, E.; Aquino, S.; Della Pepa, R.; Malerba, L.; Mangiacavalli, S.; Pezzatti, S.; Bringhen, S.; Zamagni, E. Anti-BCMA CAR-T cell-based therapies and bispecific antibodies in the immunotherapy era: Are we ready for this? Expert Rev. Hematol. 2024, 17, 375–390. [Google Scholar] [CrossRef]
- Yee, A.J. Improving outcomes with anti-BCMA bispecific antibodies with attention to infection. Blood Cancer J. 2024, 14, 110. [Google Scholar] [CrossRef] [PubMed]
- Rafei, H.; Rezvani, K. Mitigating infection risks: The promise and challenge of bispecific antibodies in haematological malignancies. Br. J. Haematol. 2024, 205, 764–766. [Google Scholar] [CrossRef]
- Fu, B.; Liu, R.; Gao, G.; Lin, Z.; He, A. Mechanisms and salvage treatments in patients with multiple myeloma relapsed post-BCMA CAR-T cell therapy. Front. Immunol. 2024, 15, 1433774. [Google Scholar] [CrossRef]
- Lee, H.; Ahn, S.; Maity, R.; Leblay, N.; Ziccheddu, B.; Truger, M.; Chojnacka, M.; Cirrincione, A.; Durante, M.; Tilmont, R.; et al. Mechanisms of antigen escape from BCMA-or GPRC5D-targeted immunotherapies in multiple myeloma. Nat. Med. 2023, 29, 2295–2306. [Google Scholar] [CrossRef]
- Woo, S.R.; Turnis, M.E.; Goldberg, M.V.; Bankoti, J.; Selby, M.; Nirschl, C.J.; Bettini, M.L.; Gravano, D.M.; Vogel, P.; Liu, C.L.; et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012, 72, 917–927. [Google Scholar] [CrossRef]
- Ghermezi, M.; Li, M.; Vardanyan, S.; Harutyunyan, N.M.; Gottlieb, J.; Berenson, A.; Spektor, T.M.; Andreu-Vieyra, C.; Petraki, S.; Sanchez, E.; et al. Serum B-cell maturation antigen: A novel biomarker to predict outcomes for multiple myeloma patients. Haematologica 2016, 102, 785. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, M.L.; Galimberti, S.; Buda, G. Beyond BCMA, why GPRC5D could be the right way: Treatment strategies with immunotherapy at relapse after anti-BCMA agents. Cancer Immunol. Immunother. 2023, 72, 3931–3937. [Google Scholar] [CrossRef]
- Fernández de Larrea, C.; Staehr, M.; Lopez, A.V.; Ng, K.Y.; Chen, Y.; Godfrey, W.D.; Purdon, T.J.; Ponomarev, V.; Wendel, H.G.; Brentjens, R.J.; et al. Defining an optimal dual-targeted CAR T-cell therapy approach simultaneously targeting BCMA and GPRC5D to prevent BCMA escape–driven relapse in multiple myeloma. Blood Cancer Discov. 2020, 1, 146–154. [Google Scholar] [CrossRef]
- Lee, H.; Neri, P.; Ahn, S.; Maity, R.; Leblay, N.; Ziccheddu, B.; Chojnacka, M.; Tilmont, R.; Barakat, E.; Landgren, O.; et al. Role of TNFRSF17 and GPRC5D structural and point mutations in resistance to targeted immunotherapies in multiple myeloma (MM). Blood 2022, 140, 252–253. [Google Scholar]
- Hofmann, M.; Thimme, R.; Schamel, W.W. PD-1 and LAG-3: Synergistic fostering of T cell exhaustion. Signal Transduct. Target. Ther. 2024, 9, 291. [Google Scholar] [PubMed]
- Lichtenegger, F.S.; Rothe, M.; Schnorfeil, F.M.; Deiser, K.; Krupka, C.; Augsberger, C.; Schlüter, M.; Neitz, J.; Subklewe, M. Targeting LAG-3 and PD-1 to enhance T cell activation by antigen-presenting cells. Front. Immunol. 2018, 9, 385. [Google Scholar] [CrossRef] [PubMed]
- Munshi, N.C.; Avet-Loiseau, H.; Rawstron, A.C.; Owen, R.G.; Child, J.A.; Thakurta, A.; Sherrington, P.; Samur, M.K.; Georgieva, A.; Anderson, K.C.; et al. Association of minimal residual disease with superior survival outcomes in patients with multiple myeloma: A meta-analysis. JAMA Oncol. 2017, 3, 28–35. [Google Scholar]
- Garfall, A.L.; Nooka, A.K.; van de Donk, N.W.; Moreau, P.; Bhutani, M.; Oriol, A.; Martin, T.G.; Rosiñol, L.; Mateos, M.V.; Bahlis, N.; et al. MM-336 Long-Term Follow-Up from the Phase 1/2 MajesTEC-1 Trial of Teclistamab in Patients With Relapsed/Refractory Multiple Myeloma (RRMM). Clin. Lymphoma Myeloma Leuk. 2024, 24, S548. [Google Scholar]
- Lesokhin, A.M.; Tomasson, M.H.; Arnulf, B.; Bahlis, N.J.; Miles Prince, H.; Niesvizky, R.; Rodrίguez-Otero, P.; Martinez-Lopez, J.; Koehne, G.; Touzeau, C.; et al. Elranatamab in relapsed or refractory multiple myeloma: Phase 2 MagnetisMM-3 trial results. Nat. Med. 2023, 29, 2259–2267. [Google Scholar] [CrossRef]
- Avet-Loiseau, H.; Ludwig, H.; Landgren, O.; Paiva, B.; Morris, C.; Yang, H.; Zhou, K.; Ro, S.; Mateos, M.V. Minimal residual disease status as a surrogate endpoint for progression-free survival in newly diagnosed multiple myeloma studies: A meta-analysis. Clin. Lymphoma Myeloma Leuk. 2020, 20, e30–e37. [Google Scholar] [CrossRef] [PubMed]
- Perrot, A.; Lauwers-Cances, V.; Corre, J.; Robillard, N.; Hulin, C.; Chretien, M.L.; Dejoie, T.; Maheo, S.; Stoppa, A.M.; Pegourie, B.; et al. Minimal residual disease negativity using deep sequencing is a major prognostic factor in multiple myeloma. Blood J. Am. Soc. Hematol. 2018, 132, 2456–2464. [Google Scholar] [CrossRef]
- Xia, J.; Li, Z.; Xu, K. Immunotherapies targeting GPRC5D in relapsed or refractory multiple myeloma: Latest updates from 2022 ASH Annual Meeting. J. Hematol. Oncol. 2023, 16, 60. [Google Scholar]
- Falchi, L.; Carlo-Stella, C.; Morschhauser, F.; Hutchings, M.; Bachy, E.; Cartron, G.; Khan, C.; Tani, M.; Martinez-Lopez, J.; Bartlett, N.L.; et al. Glofitamab monotherapy in pts with relapsed/refractory (R/R) large B-cell lymphoma (LBCL): Extended follow-up and landmark analyses from a pivotal phase II study. J. Clin. Oncol. 2023, 41. [Google Scholar] [CrossRef]
- Balendran, S.; Tam, C.; Ku, M. T-Cell Engaging Antibodies in Diffuse Large B Cell Lymphoma—An Update. J. Clin. Med. 2023, 12, 6737. [Google Scholar]
- Zinselmeyer, B.H.; Heydari, S.; Sacristán, C.; Nayak, D.; Cammer, M.; Herz, J.; Cheng, X.; Davis, S.J.; Dustin, M.L.; McGavern, D.B. PD-1 promotes immune exhaustion by inducing antiviral T cell motility paralysis. J. Exp. Med. 2013, 210, 757–774. [Google Scholar] [CrossRef] [PubMed]
- Paiva, B.; Gaffney, B.; Burnett, K.; Castiglioni, P.; Angelo, M.; Pierce, D.W.; Boss, I.W. Synergistic antitumor activity of alnuctamab (ALNUC.; BMS-986349; CC-93269), a BCMA 2+ 1 T cell engager (TCE), and celmod agents in multiple myeloma (MM) preclinical models. Blood 2022, 140, 7054–7055. [Google Scholar] [CrossRef]
- Haber, L.; Olson, K.; Kelly, M.P.; Crawford, A.; DiLillo, D.J.; Tavaré, R.; Ullman, E.; Mao, S.; Canova, L.; Sineshchekova, O.; et al. Generation of T-cell-redirecting bispecific antibodies with differentiated profiles of cytokine release and biodistribution by CD3 affinity tuning. Sci. Rep. 2021, 11, 14397. [Google Scholar] [CrossRef] [PubMed]
- Labanca, C.; Martino, E.A.; Vigna, E.; Bruzzese, A.; Mendicino, F.; De Luca, P.; Lucia, E.; Olivito, V.; Fragliasso, V.; Neri, A.; et al. Mosunetuzumab for the treatment of follicular lymphoma. Expert Opin. Biol. Ther. 2024, 24, 1039–1048. [Google Scholar] [CrossRef]
- Sun, L.; Romancik, J.T. The Development and Application of Bispecific Antibodies in B-Cell Non-Hodgkin Lymphoma. J. Pers. Med. 2025, 15, 51. [Google Scholar] [CrossRef]
- Bruzzese, A.; Martino, E.A.; Labanca, C.; Caridà, G.; Mendicino, F.; Lucia, E.; Olivito, V.; Puccio, N.; Neri, A.; Morabito, F.; et al. Therapeutic Strategies for Relapsed or Refractory B-Cell Acute Lymphoblastic Leukemia in Adult Patients: Optimizing the Use of Monoclonal Antibodies. Eur. J. Haematol. 2025, 114, 938–952. [Google Scholar]
- Peter, J.; Toppeta, F.; Trubert, A.; Danhof, S.; Hudecek, M.; Däullary, T. Multi-Targeting CAR-T Cell Strategies to Overcome Immune Evasion in Lymphoid and Myeloid Malignancies. Oncol. Res. Treat. 2025, 48, 265–279. [Google Scholar]
- Cliff, E.R.; Mian, H.; Mohyuddin, G.R. Teclistamab in relapsed or refractory multiple myeloma. N. Engl. J. Med. 2022, 387, 1721–1722. [Google Scholar]
- Shaver, J.; Horton, D.; Halford, Z. Targeting GPRC5D With Talquetamab: A New Frontier in Bispecific Antibody Therapy for Relapsed/Refractory Multiple Myeloma. Ann. Pharmacother. 2025, 59, 350–363. [Google Scholar] [CrossRef]
- Xu, Y.; Cai, Z.; Xia, Z.; Yang, C.; Chen, J.; Zhu, Z.; Jing, X.; Tian, J.; Zhang, N.; Cui, A.; et al. A Phase I First-in-Human, Open-Label Trial to Investigate the Safety, Tolerability, Pharmacokinetics and Preliminary Antitumor Activity of SIM0500, a Humanized GPRC5D-BCMA-CD3 Trispecific Antibody, in Participants with Relapsed or Refractory Multiple Myeloma. Blood 2024, 144, 3381–3382. [Google Scholar]
- Carrara, S.C.; Harwardt, J.; Grzeschik, J.; Hock, B.; Kolmar, H. TriTECM: A tetrafunctional T-cell engaging antibody with built-in risk mitigation of cytokine release syndrome. Front. Immunol. 2022, 13, 1051875. [Google Scholar] [CrossRef]
- Zhang, T.; Lin, Y.; Gao, Q. Bispecific antibodies targeting immunomodulatory checkpoints for cancer therapy. Cancer Biol. Med. 2023, 20, 181–195. [Google Scholar] [CrossRef]
- Desnoyers, L.R.; Vasiljeva, O.; Richardson, J.H.; Yang, A.; Menendez, E.E.; Liang, T.W.; Wong, C.; Bessette, P.H.; Kamath, K.; Moore, S.J.; et al. Tumor-specific activation of an EGFR-targeting probody enhances therapeutic index. Sci. Transl. Med. 2013, 5, 207ra144. [Google Scholar] [CrossRef] [PubMed]
- Grymula, K.; Tarnowski, M.; Wysoczynski, M.; Drukala, J.; Barr, F.G.; Ratajczak, J.; Kucia, M.; Ratajczak, M.Z. Overlapping and distinct role of CXCR7-SDF-1/ITAC and CXCR4-SDF-1 axes in regulating metastatic behavior of human rhabdomyosarcomas. Int. J. Cancer 2010, 127, 2554–2568. [Google Scholar] [CrossRef] [PubMed]
- FDAU. FDA Grants Accelerated Approval to Tarlatamab-Dlle for Extensive Stage Small Cell Lung Cancer. 2024. Available online: https://www.lungcancerstoday.com/post/fda-grants-accelerated-approval-to-tarlatamab-dlle-for-extensive-stage-sclc (accessed on 5 April 2025).
- Hummel, H.D.; Kufer, P.; Grüllich, C.; Seggewiss-Bernhardt, R.; Deschler-Baier, B.; Chatterjee, M.; Goebeler, M.E.; Miller, K.; de Santis, M.; Loidl, W.; et al. Pasotuxizumab, a BiTE® immune therapy for castration-resistant prostate cancer: Phase I, dose-escalation study findings. Immunotherapy 2021, 13, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Gedeon, P.C.; Schaller, T.H.; Chitneni, S.K.; Choi, B.D.; Kuan, C.T.; Suryadevara, C.M.; Snyder, D.J.; Schmittling, R.J.; Szafranski, S.E.; Cui, X.; et al. A rationally designed fully human EGFRvIII: CD3-targeted bispecific antibody redirects human T cells to treat patient-derived intracerebral malignant glioma. Clin. Cancer Res. 2018, 24, 3611–3631. [Google Scholar] [CrossRef]
- Leitao, C.D.; Borras, A.M.; Xu, T.; Oroujeni, M.; Liu, Y.; Westerberg, C.; Clinton, J.; Tolmachev, V.; Orlova, A.; Ståhl, S.; et al. Conditionally activated affibody-based prodrug targeting EGFR demonstrates improved tumour selectivity. J. Control. Release 2023, 357, 185–195. [Google Scholar] [CrossRef]
- Li, D.; Cheng, P.; Wang, J.; Qiu, X.; Zhang, X.; Xu, L.; Liu, Y.; Qin, S. IRF6 is directly regulated by ZEB1 and ELF3, and predicts a favorable prognosis in gastric cancer. Front. Oncol. 2019, 9, 220. [Google Scholar] [CrossRef]
- Marinov, T.M.; Wasdin, P.T.; Jordaan, G.; Janke, A.K.; Abu-Shmais, A.A.; Georgiev, I.S. An expandable synthetic library of human paired antibody sequences. PLOS Comput. Biol. 2025, 21, e1012932. [Google Scholar] [CrossRef]
- Prelaj, A.; Galli, E.G.; Miskovic, V.; Pesenti, M.; Viscardi, G.; Pedica, B.; Mazzeo, L.; Bottiglieri, A.; Provenzano, L.; Spagnoletti, A.; et al. Real-world data to build explainable trustworthy artificial intelligence models for prediction of immunotherapy efficacy in NSCLC patients. Front. Oncol. 2023, 12, 1078822. [Google Scholar] [CrossRef]
- Tran, K.A.; Addala, V.; Koufariotis, L.T.; Zhang, J.; Wood, S.; Leonard, C.; Hoeijmakers, L.L.; Blank, C.U.; Crispin-Ortuzar, M.; Williams, E.D.; et al. Explainable machine learning identifies features and thresholds predictive of immunotherapy response. bioRxiv 2025. bioRxiv:23.643560. [Google Scholar]
- BioCopy. BioCopy Chooses Genedata for AI-Powered Multispecific Antibody Development. 2024. Available online: https://www.genedata.com/company/news/details/press-release/biocopy-advances-ai-driven-antibody-discovery (accessed on 27 April 2025).

| Disease | Agent | Target(s) | Type | Phase/Approval | ORR/CR (%) | Notable Comments |
|---|---|---|---|---|---|---|
| ALL | Blinatumomab | CD19 × CD3 | BsAb (BiTE) | FDA/EMA Approved (R/R, MRD) | ORR: ~44%; CR: ~19% | Approved for Ph-negative ALL; MRD clearance indication. |
| ALL | CD19/CD22/CD3 | CD19, CD22, CD3 | TsAb | Preclinical | N/A | Dual-antigen targeting to overcome CD19 escape. |
| AML | Flotetuzumab | CD123 × CD3 | BsAb (DART) | Phase I/II | ORR: 24% | Investigated in R/R AML; TP53-mutated cohort notable. |
| AML | CLEC12A/CD16/IL-15 (TriKE) | CLEC12A, CD16, IL-15 | TriKE | Preclinical | N/A | NK-cell engagement plus cytokine-driven proliferation. |
| DLBCL | Glofitamab | CD20 × CD3 | BsAb | EMA Approved (Post-CAR-T R/R LBCL) | ORR: 51.6%; CR: 39.4% | Step-up dosing improves safety and durability. |
| DLBCL | Mosunetuzumab | CD20 × CD3 | BsAb | EMA Approved (FL); Phase II (DLBCL) | ORR: 43.2%; CR: 24.8% | Approved for FL; active investigation for DLBCL. |
| DLBCL | CAR20.19.22 | CD20, CD19, CD22 | Tri-CAR | Phase I ongoing | ORR: 75%; CR: 42% | Multitarget CAR-T; antigen escape prevention. |
| FL | Mosunetuzumab | CD20 × CD3 | BsAb | EMA Approved (FL) | ORR: 80%; CR: 60% | First bispecific approved for FL (chemo-free option). |
| MM | Teclistamab | BCMA × CD3 | BsAb | FDA/EMA Approved | ORR: 63%; CR: ≥39.4% | First BsAb for RRMM; subcutaneous administration. |
| MM | Talquetamab | GPRC5D × CD3 | BsAb | FDA Approved (post–BCMA) | ORR: ~73% | Targets GPRC5D; dermatologic toxicity common. |
| MM | SIM0500 | BCMA/GPRC5D/CD3 | TsAb | Phase I ongoing | N/A | Dual-antigen engagement to prevent BCMA escape. |
| MM | SAR442257 | CD38/CD28 × CD3 | TsAb | Phase I ongoing | N/A | CD28-mediated T-cell co-stimulation; early-phase data. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amoozgar, B.; Bangolo, A.; Habibi, M.; Cho, C.; Goy, A. From Molecular Precision to Clinical Practice: A Comprehensive Review of Bispecific and Trispecific Antibodies in Hematologic Malignancies. Int. J. Mol. Sci. 2025, 26, 5319. https://doi.org/10.3390/ijms26115319
Amoozgar B, Bangolo A, Habibi M, Cho C, Goy A. From Molecular Precision to Clinical Practice: A Comprehensive Review of Bispecific and Trispecific Antibodies in Hematologic Malignancies. International Journal of Molecular Sciences. 2025; 26(11):5319. https://doi.org/10.3390/ijms26115319
Chicago/Turabian StyleAmoozgar, Behzad, Ayrton Bangolo, Maryam Habibi, Christina Cho, and Andre Goy. 2025. "From Molecular Precision to Clinical Practice: A Comprehensive Review of Bispecific and Trispecific Antibodies in Hematologic Malignancies" International Journal of Molecular Sciences 26, no. 11: 5319. https://doi.org/10.3390/ijms26115319
APA StyleAmoozgar, B., Bangolo, A., Habibi, M., Cho, C., & Goy, A. (2025). From Molecular Precision to Clinical Practice: A Comprehensive Review of Bispecific and Trispecific Antibodies in Hematologic Malignancies. International Journal of Molecular Sciences, 26(11), 5319. https://doi.org/10.3390/ijms26115319





