Juniper Berry Oil as a Functional Additive in Chitosan–Water Kefiran–Paramylon Porous Sponges: Structural, Physicochemical, and Protein Interaction Insights
Abstract
1. Introduction
2. Results and Discussion
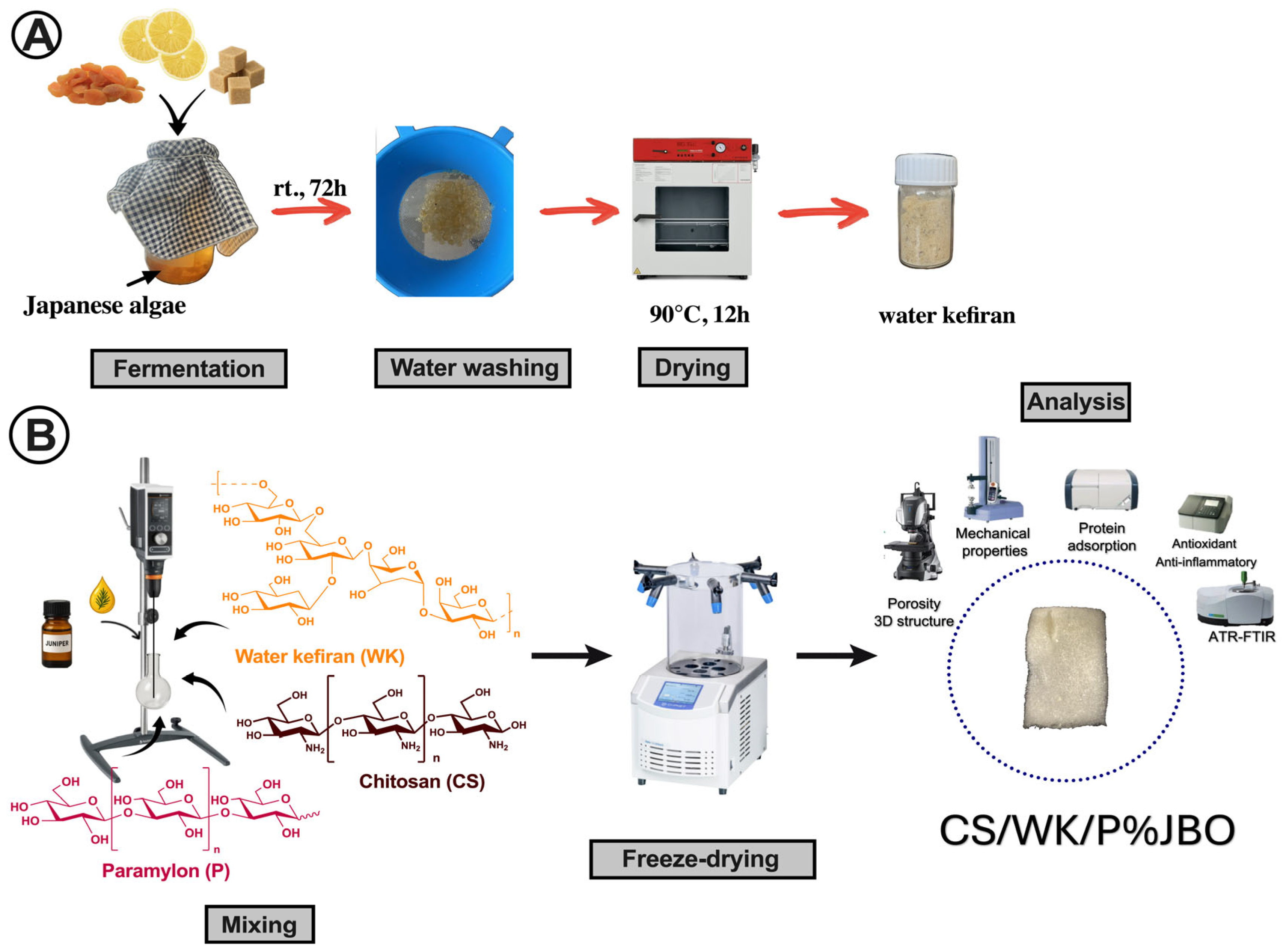
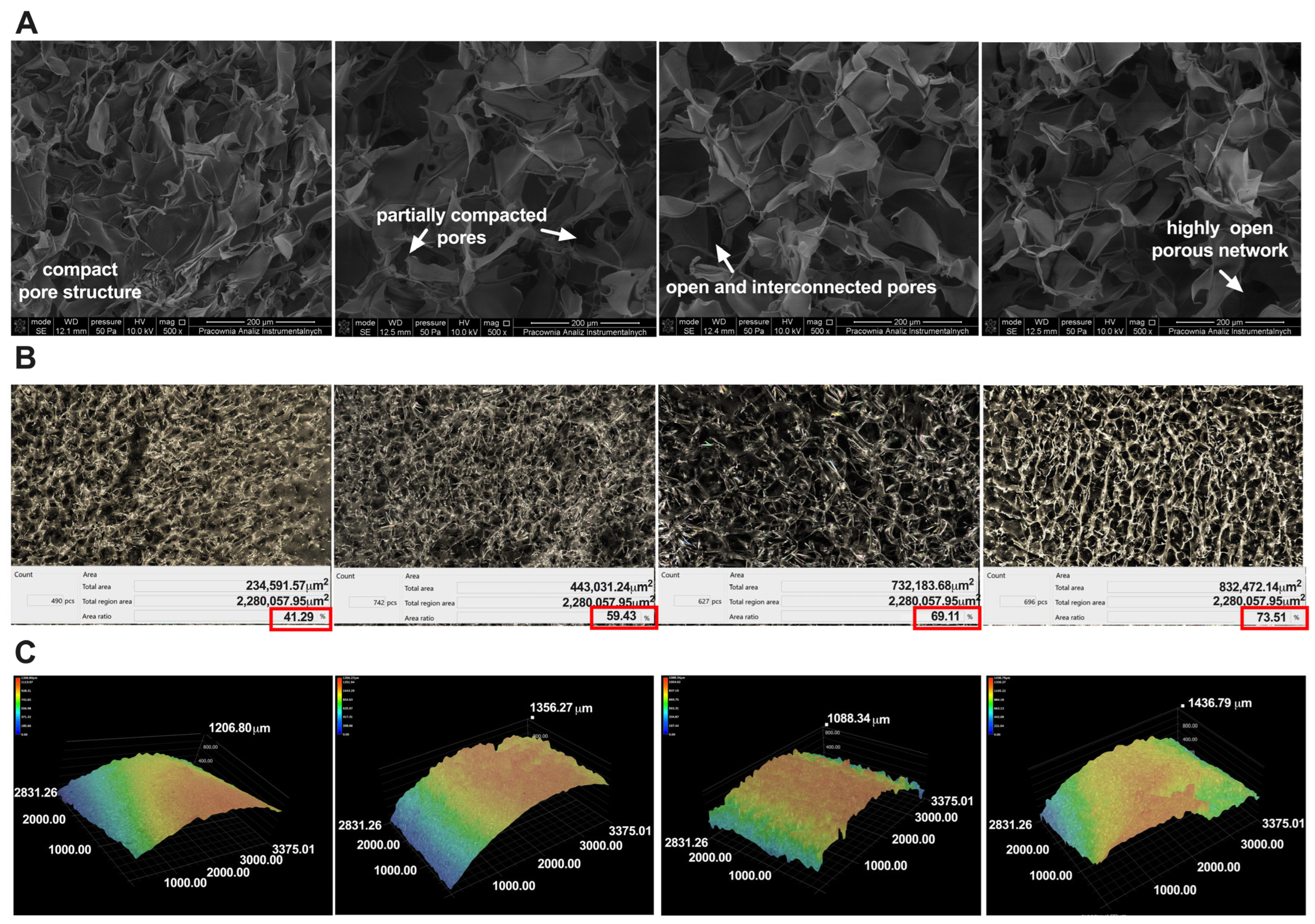
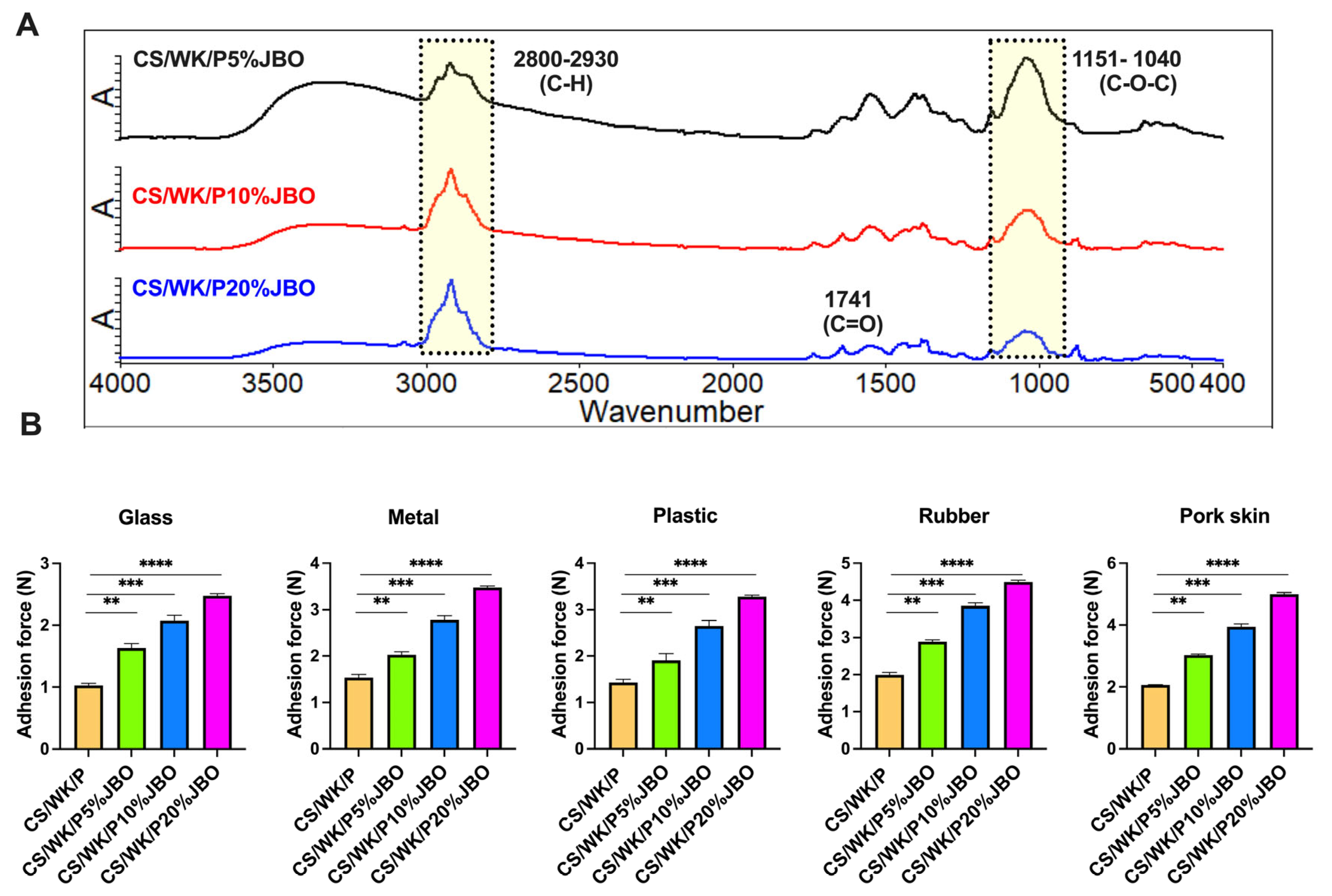
2.1. Preparation and Characterization of the Chitosan–Water Kefiran–Paramylon Sponges Enriched with Juniper Berry Oil (CS/WK/P%JBO)
2.2. Water Vapor Transmission Rate (WVTR)
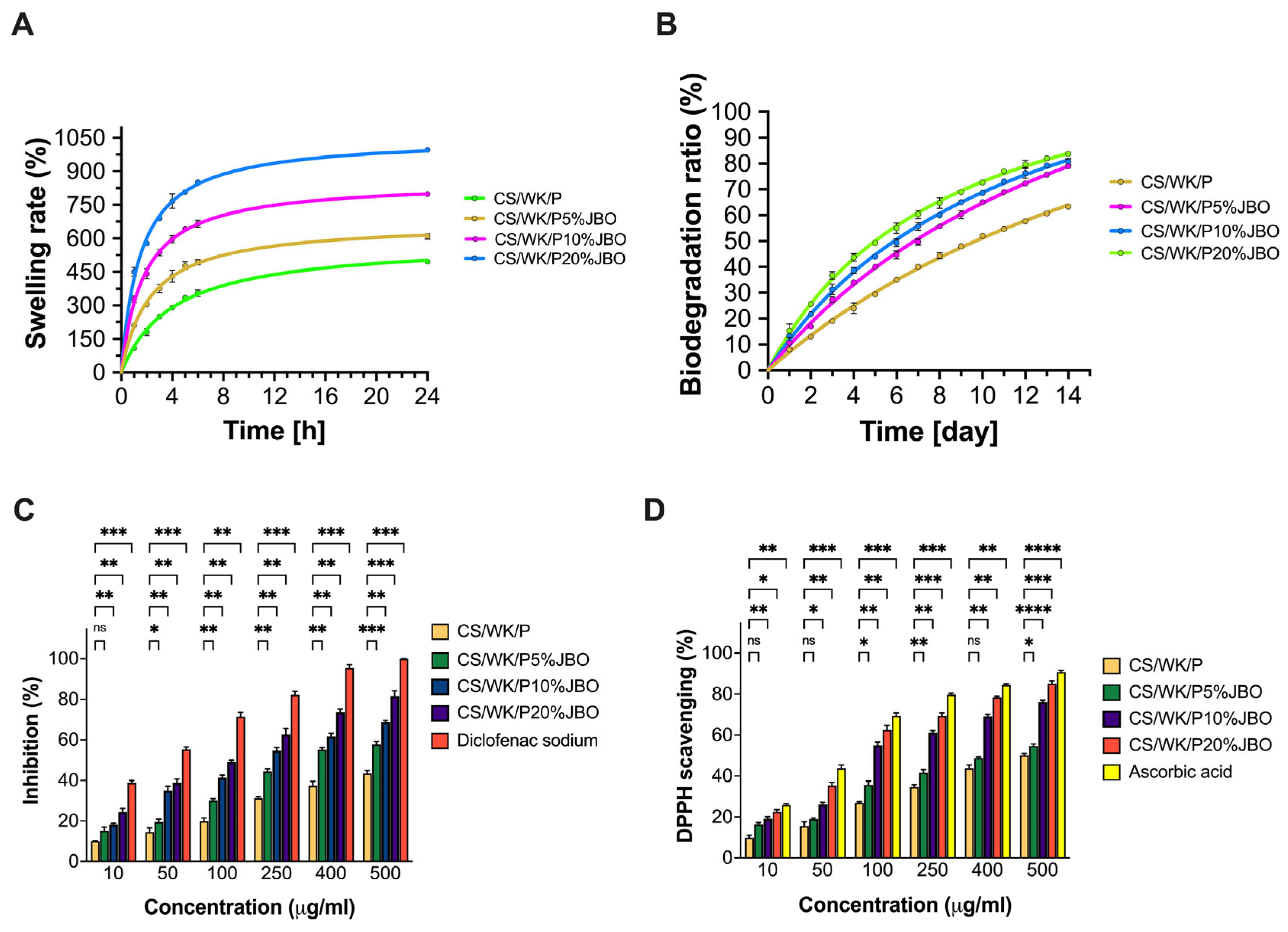
2.3. Swelling Properties
2.4. Biodegradation Ratio
2.5. Anti-Inflammatory Properties
2.6. Antioxidant Properties
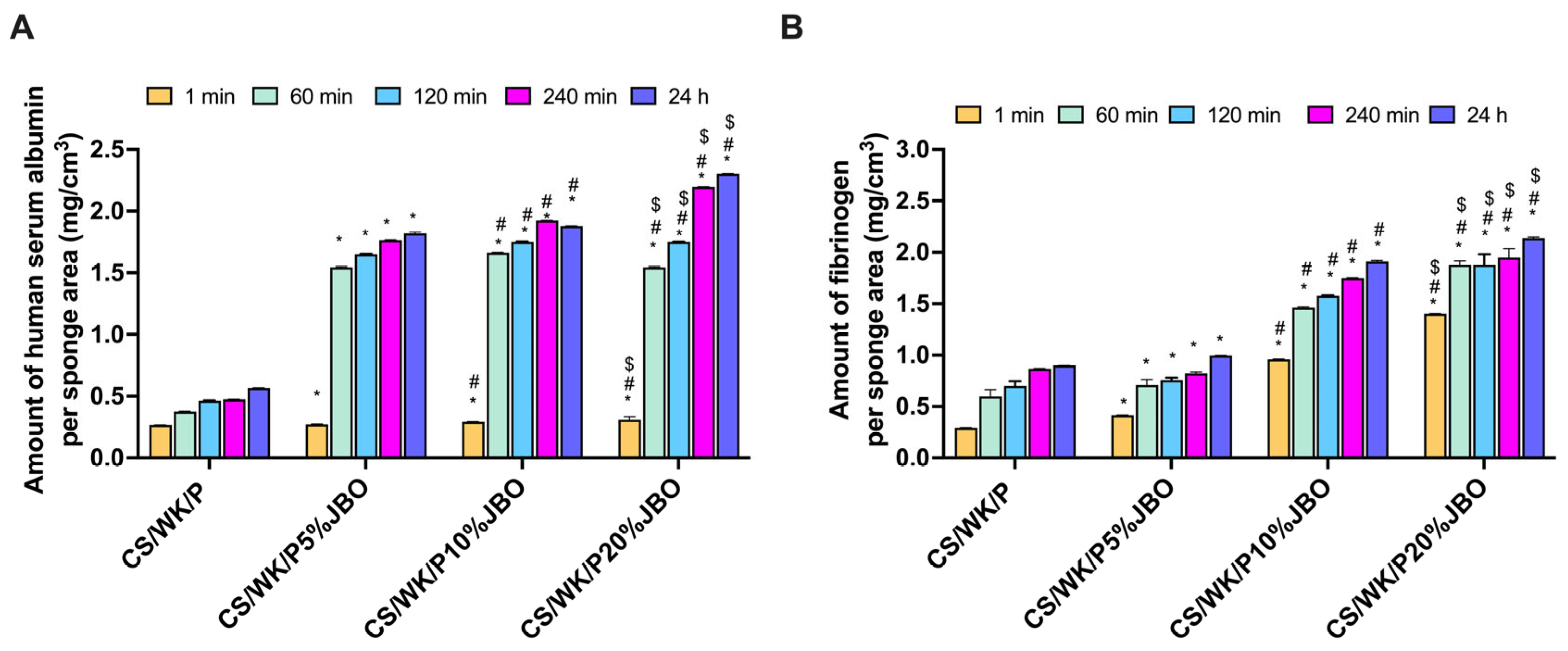
2.7. Protein Adsorption
3. Materials and Methods
3.1. Materials
3.2. Kefir Grain Growth
3.3. Preparation of the Chitosan/Water Kefiran/Paramylon Sponges Enriched with the Juniper Berry Oil (CS/WK/P%JBO)
3.4. Characterization of Chitosan/Water Kefiran/Paramylon Sponges Enriched with Juniper Berry Oil (CS/WK/P%JBO)
3.4.1. Attenuated Total Reflectance Spectroscopy (ATR-FTIR)
3.4.2. Scanning Electron Microscopy (SEM)
3.4.3. 3D Profile and Surface Roughness
3.4.4. Porosity
3.4.5. Mechanical and Adhesive Properties
3.4.6. Swelling Analysis
3.4.7. Biodegradation Analysis
3.4.8. The Water Vapor Transmission Rate (WVTR)
3.4.9. Protein Adsorption
3.4.10. Antioxidant Activity
3.4.11. Anti-Inflammatory Activity
3.4.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khalilov, R.; Bakishzade, A.; Nasibova, A. Future prospects of biomaterials in nanomedicine. Adv. Biol. Earth Sci. 2024, 9, 5–10. [Google Scholar] [CrossRef]
- Farag, M.M. Recent trends on biomaterials for tissue regeneration applications: Review. J. Mater. Sci. 2023, 58, 527–558. [Google Scholar] [CrossRef]
- Mamidi, N.; García, R.G.; Martínez, J.D.H.; Briones, C.M.; Martínez Ramos, A.M.; Tamez, M.F.L.; Del Valle, B.G.; Segura, F.J.M. Recent Advances in Designing Fibrous Biomaterials for the Domain of Biomedical, Clinical, and Environmental Applications. ACS Biomater. Sci. Eng. 2022, 8, 3690–3716. [Google Scholar] [CrossRef]
- Nii, T.; Katayama, Y. Biomaterial-Assisted Regenerative Medicine. Int. J. Mol. Sci. 2021, 22, 8657. [Google Scholar] [CrossRef] [PubMed]
- Hakim, L.K.; Yazdanian, M.; Alam, M.; Abbasi, K.; Tebyaniyan, H.; Tahmasebi, E.; Khayatan, D.; Seifalian, A.; Ranjbar, R.; Yazdanian, A. Biocompatible and Biomaterials Application in Drug Delivery System in Oral Cavity. Evid.-Based Complement. Altern. Med. 2021, 2021, 9011226. [Google Scholar] [CrossRef] [PubMed]
- Hitam, C.N.C.; Jalil, A.A. Recent advances on nanocellulose biomaterials for environmental health photoremediation: An overview. Environ. Res. 2022, 204, 111964. [Google Scholar] [CrossRef]
- Gough, C.R.; Callaway, K.; Spencer, E.; Leisy, K.; Jiang, G.; Yang, S.; Hu, X. Biopolymer-Based Filtration Materials. ACS Omega 2021, 6, 11804–11812. [Google Scholar] [CrossRef]
- Lustenberger, S.; Castro-Muñoz, R. Advanced biomaterials and alternatives tailored as membranes for water treatment and the latest innovative European water remediation projects: A review. Case Stud. Chem. Environ. Eng. 2022, 5, 100205. [Google Scholar] [CrossRef]
- Rezaei Hosseinabadi, S.; Parsapour, A.; Nouri Khorasani, S.; Razavi, S.M.; Hashemibeni, B.; Heidari, F.; Khalili, S. Wound dressing application of castor oil- and CAPA-based polyurethane membranes. Polym. Bull. 2020, 77, 2945–2964. [Google Scholar] [CrossRef]
- Namviriyachote, N.; Muangman, P.; Chinaroonchai, K.; Chuntrasakul, C.; Ritthidej, G.C. Polyurethane-biomacromolecule combined foam dressing containing asiaticoside: Fabrication, characterization and clinical efficacy for traumatic dermal wound treatment. Int. J. Biol. Macromol. 2020, 143, 510–520. [Google Scholar] [CrossRef]
- Jeckson, T.A.; Neo, Y.P.; Sisinthy, S.P.; Gorain, B. Delivery of Therapeutics from Layer-by-Layer Electrospun Nanofiber Matrix for Wound Healing: An Update. J. Pharm. Sci. 2021, 110, 635–653. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Ranjan Dahiya, U.; Yadav, S.; Sharma, P.; Ghosh, D.; Rao, G.K.; Rawat, V.; Kumar, G.; Kumar, A.; Srivastava, C.M. Zinc Oxide Nanoparticles Functionalized on Hydrogel Grafted Silk Fibroin Fabrics as Efficient Composite Dressing. Biomolecules 2020, 10, 710. [Google Scholar] [CrossRef]
- Chelminiak-Dudkiewicz, D.; Machacek, M.; Dlugaszewska, J.; Wujak, M.; Smolarkiewicz-Wyczachowski, A.; Bocian, S.; Mylkie, K.; Goslinski, T.; Marszall, M.P.; Ziegler-Borowska, M. Fabrication and characterization of new levan@CBD biocomposite sponges as potential materials in natural, non-toxic wound dressing applications. Int. J. Biol. Macromol. 2023, 253, 126933. [Google Scholar] [CrossRef] [PubMed]
- Chelminiak-Dudkiewicz, D.; Wujak, M.; Mlynarczyk, D.T.; Dlugaszewska, J.; Mylkie, K.; Smolarkiewicz-Wyczachowski, A.; Ziegler-Borowska, M. Enhancing the porosity of chitosan sponges with CBD by adding antimicrobial violacein. Heliyon 2024, 10, e35389. [Google Scholar] [CrossRef]
- Choi, J.; Jeon, H. Manufacture and Characterization of Alginate-CMC-Dextran Hybrid Double Layer Superabsorbent Scaffolds. Appl. Sci. 2021, 11, 11573. [Google Scholar] [CrossRef]
- de Oliveira, R.S.; Fantaus, S.S.; Guillot, A.J.; Melero, A.; Beck, R.C.R. 3D-Printed Products for Topical Skin Applications: From Personalized Dressings to Drug Delivery. Pharmaceutics 2021, 13, 1946. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, X.; Su, T.; Lu, F.; Chang, Q.; Gao, J. Recent Advances in Macroporous Hydrogels for Cell Behavior and Tissue Engineering. Gels 2022, 8, 606. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, S.; He, Y.; Ou, S.; Yang, Y.; Qu, Y.; Li, J.; Lian, W.; Li, G.; Tian, J.; et al. A cell adhesion-promoting multi-network 3D printing bio-ink based on natural polysaccharide hydrogel. Front. Bioeng. Biotechnol. 2022, 10, 1070566. [Google Scholar] [CrossRef]
- Pandey, R.; Mathur, G. Current Trends in Chitosan Functionalization Methods and Their Applications. Starch-Stärke 2025, 77, 2300248. [Google Scholar] [CrossRef]
- Sebastian, J.; Rouissi, T.; Brar, S.K. Chapter 14—Fungal chitosan: Prospects and challenges. In Handbook of Chitin and Chitosan; Gopi, S., Thomas, S., Pius, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 419–452. [Google Scholar]
- Zhou, Y.; Zhang, Y.; Nie, Y.; Sun, D.; Wu, D.; Ban, L.; Zhang, H.; Yang, S.; Chen, J.; Du, H.; et al. Recent advances and perspectives in functional chitosan-based composites for environmental remediation, energy, and biomedical applications. Prog. Mater. Sci. 2025, 152, 101460. [Google Scholar] [CrossRef]
- Moradi, Z.; Kalanpour, N. Kefiran, a branched polysaccharide: Preparation, properties and applications: A review. Carbohydr. Polym. 2019, 223, 115100. [Google Scholar] [CrossRef] [PubMed]
- Blandón, L.M.; Noseda, M.D.; Islan, G.A.; Castro, G.R.; de Melo Pereira, G.V.; Thomaz-Soccol, V.; Soccol, C.R. Optimization of culture conditions for kefiran production in whey: The structural and biocidal properties of the resulting polysaccharide. Bioact. Carbohydr. Diet. Fibre 2018, 16, 14–21. [Google Scholar] [CrossRef]
- Hasheminya, S.-M.; Dehghannya, J. Novel ultrasound-assisted extraction of kefiran biomaterial, a prebiotic exopolysaccharide, and investigation of its physicochemical, antioxidant and antimicrobial properties. Mater. Chem. Phys. 2020, 243, 122645. [Google Scholar] [CrossRef]
- Tan, K.-X.; Chamundeswari, V.N.; Loo, S.C.J. Prospects of kefiran as a food-derived biopolymer for agri-food and biomedical applications. RSC Adv. 2020, 10, 25339–25351. [Google Scholar] [CrossRef]
- Moradi, M.; Guimarães, J.T.; Sahin, S. Current applications of exopolysaccharides from lactic acid bacteria in the development of food active edible packaging. Curr. Opin. Food Sci. 2021, 40, 33–39. [Google Scholar] [CrossRef]
- Feuzing, F.; Mbakidi, J.P.; Marchal, L.; Bouquillon, S.; Leroy, E. A review of paramylon processing routes from microalga biomass to non-derivatized and chemically modified products. Carbohydr. Polym. 2022, 288, 119181. [Google Scholar] [CrossRef]
- Šantek, B.I.; Felski, M.; Friehs, K.; Lotz, M.; Flaschel, E. Production of paramylon, a β-1,3-glucan, by heterotrophic cultivation of Euglena gracilis on a synthetic medium. Eng. Life Sci. 2009, 9, 23–28. [Google Scholar] [CrossRef]
- Mou, J.-H.; Liu, S.-F.; Yang, L.-L.; Qin, Z.-H.; Yang, Y.-F.; Wang, Z.-Y.; Li, H.-Y.; Lin, C.S.K.; Wang, X. Sustainable paramylon production from food waste by Euglena gracilis using a waste-based cell immobilisation technique. Chem. Eng. J. 2024, 481, 148594. [Google Scholar] [CrossRef]
- Ilangovan, M.; Kabe, T.; Iwata, T. Processing and Evaluation of Bio-Based Paramylon Ester/Poly(butylene succinate) Blends for Industrial Applications. J. Polym. Environ. 2024, 32, 4869–4879. [Google Scholar] [CrossRef]
- Bajac, J.; Nikolovski, B.; Lončarević, I.; Petrović, J.; Bajac, B.; Đurović, S.; Petrović, L. Microencapsulation of juniper berry essential oil (Juniperus communis L.) by spray drying: Microcapsule characterization and release kinetics of the oil. Food Hydrocoll. 2022, 125, 107430. [Google Scholar] [CrossRef]
- Semerdjieva, I.; Zheljazkov, V.D.; Radoukova, T.; Dincheva, I.; Piperkova, N.; Maneva, V.; Astatkie, T.; Kačániová, M. Biological Activity of Essential Oils of Four Juniper Species and Their Potential as Biopesticides. Molecules 2021, 26, 6358. [Google Scholar] [CrossRef] [PubMed]
- Di Santo, M.C.; Alaimo, A.; Domínguez Rubio, A.P.; De Matteo, R.; Pérez, O.E. Biocompatibility analysis of high molecular weight chitosan obtained from Pleoticus muelleri shrimps. Evaluation in prokaryotic and eukaryotic cells. Biochem. Biophys. Rep. 2020, 24, 100842. [Google Scholar] [CrossRef]
- Farasati Far, B.; Naimi-Jamal, M.R.; Safaei, M.; Zarei, K.; Moradi, M.; Yazdani Nezhad, H. A Review on Biomedical Application of Polysaccharide-Based Hydrogels with a Focus on Drug Delivery Systems. Polymers 2022, 14, 5432. [Google Scholar] [CrossRef]
- Jin, M.; Shi, J.; Zhu, W.; Yao, H.; Wang, D.-A. Polysaccharide-Based Biomaterials in Tissue Engineering: A Review. Tissue Eng. Part B Rev. 2020, 27, 604–626. [Google Scholar] [CrossRef] [PubMed]
- Bajac, J.; Zengin, G.; Mitrović, I.; Antić, I.; Radojković, M.; Nikolovski, B.; Terzić, M. Juniper berry essential oils as natural resources of biological and pharmacological high-valuable molecules. Ind. Crops Prod. 2023, 204, 117248. [Google Scholar] [CrossRef]
- Raina, R.; Verma, P.K.; Peshin, R.; Kour, H. Potential of Juniperus communis L as a nutraceutical in human and veterinary medicine. Heliyon 2019, 5, e02376. [Google Scholar] [CrossRef]
- Albrecht, U.W.; Madisch, A. Therapeutic potentials associated with biological properties of Juniper berry oil (Juniperus communis L.) and its therapeutic use in several diseases—A Review. Bioact. Compd. Health Dis. 2022, 5, 174–185. [Google Scholar] [CrossRef]
- Rawat, P.; Dasila, K.; Singh, M.; Kuniyal, J.C. Influence of environmental factors on phytochemical compositions and antioxidant activity of Juniperus communis L. Discov. Environ. 2025, 3, 11. [Google Scholar] [CrossRef]
- Saranti, T.; Melo, P.T.S.; Cerqueira, M.A.; Aouada, F.A.; de Moura, M.R. Performance of Gelatin Films Reinforced with Cloisite Na(+) and Black Pepper Essential Oil Loaded Nanoemulsion. Polymers 2021, 13, 4298. [Google Scholar] [CrossRef]
- Majhy, B.; Priyadarshini, P.; Sen, A.K. Effect of surface energy and roughness on cell adhesion and growth—Facile surface modification for enhanced cell culture. RSC Adv. 2021, 11, 15467–15476. [Google Scholar] [CrossRef]
- Mouhoub, A.; Raouan, S.E.; Guendouz, A.; El Alaoui-Talibi, Z.; Koraichi, S.I.; El Abed, S.; Delattre, C.; El Modafar, C. Antiadhesion effect of the chitosan-based film incorporated with essential oils against foodborne bacteria. Ind. Crops Prod. 2022, 189, 115742. [Google Scholar] [CrossRef]
- Bonilla, J.; Poloni, T.; Lourenço, R.V.; Sobral, P.J.A. Antioxidant potential of eugenol and ginger essential oils with gelatin/chitosan films. Food Biosci. 2018, 23, 107–114. [Google Scholar] [CrossRef]
- Bhatia, S.; Al-Harrasi, A.; Shah, Y.A.; Altoubi, H.W.K.; Kotta, S.; Sharma, P.; Anwer, M.K.; Kaithavalappil, D.S.; Koca, E.; Aydemir, L.Y. Fabrication, Characterization, and Antioxidant Potential of Sodium Alginate/Acacia Gum Hydrogel-Based Films Loaded with Cinnamon Essential Oil. Gels 2023, 9, 337. [Google Scholar] [CrossRef]
- Marangoni Júnior, L.; Vieira, R.P.; Anjos, C.A.R. Kefiran-based films: Fundamental concepts, formulation strategies and properties. Carbohydr. Polym. 2020, 246, 116609. [Google Scholar] [CrossRef]
- Mamand, D.M.; Muhammad, D.S.; Aziz, S.B.; Hama, P.O.; Al-Asbahi, B.A.; Ahmed, A.A.A.; Hassan, J. Enhanced optical properties of chitosan polymer doped with orange peel dye investigated via UV–Vis and FTIR analysis. Sci. Rep. 2025, 15, 3232. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhao, X.; Zhao, X. The Characterization and Functional Properties of Euglena gracilis Paramylon Treated with Different Methods. Int. J. Anal. Chem. 2022, 2022, 7811014. [Google Scholar] [CrossRef]
- Napiórkowska, A.; Szpicer, A.; Wojtasik-Kalinowska, I.; Perez, M.D.T.; González, H.D.; Kurek, M.A. Microencapsulation of Juniper and Black Pepper Essential Oil Using the Coacervation Method and Its Properties after Freeze-Drying. Foods 2023, 12, 4345. [Google Scholar] [CrossRef] [PubMed]
- Agatonovic-Kustrin, S.; Ristivojevic, P.; Gegechkori, V.; Litvinova, T.M.; Morton, D.W. Essential Oil Quality and Purity Evaluation via FT-IR Spectroscopy and Pattern Recognition Techniques. Appl. Sci. 2020, 10, 7294. [Google Scholar] [CrossRef]
- Ordoudi, S.A.; Papapostolou, M.; Kokkini, S.; Tsimidou, M.Z. Diagnostic Potential of FT-IR Fingerprinting in Botanical Origin Evaluation of Laurus nobilis L. Essential Oil is Supported by GC-FID-MS Data. Molecules 2020, 25, 583. [Google Scholar] [CrossRef]
- Predoi, D.; Groza, A.; Iconaru, S.L.; Predoi, G.; Barbuceanu, F.; Guegan, R.; Motelica-Heino, M.S.; Cimpeanu, C. Properties of Basil and Lavender Essential Oils Adsorbed on the Surface of Hydroxyapatite. Materials 2018, 11, 652. [Google Scholar] [CrossRef]
- Czarnecka-Komorowska, D.; Tomasik, M.; Thakur, V.K.; Kostecka, E.; Rydzkowski, T.; Jursa-Kulesza, J.; Bryll, K.; Mysłowski, J.; Gawdzińska, K. Biocomposite composting based on the sugar-protein condensation theory. Ind. Crops Prod. 2022, 183, 114974. [Google Scholar] [CrossRef]
- Sanei-Dehkordi, A.; Fereydouni, N.; Agholi, M.; Ziaei, S.A.; Azadpour, Z.; Zarenezhad, E.; Osanloo, M. Larvicidal Efficacies of Nanoliposomes Containing Alpha-pinene, Citral, Camphor, and Thymol Against Aedes aegypti and Anopheles stephensi Mosquito Vectors. Acta Parasitol. 2025, 70, 56. [Google Scholar] [CrossRef]
- Biswas, A.; do Socorro Rocha Bastos, M.; Furtado, R.F.; Kuzniar, G.; Boddu, V.; Cheng, H.N. Evaluation of the Properties of Cellulose Ester Films that Incorporate Essential Oils. Int. J. Polym. Sci. 2020, 4620868. [Google Scholar] [CrossRef]
- Boro, U.; Priyadarsini, A.; Moholkar, V.S. Synthesis and characterization of poly(lactic acid)/clove essential oil/alkali-treated halloysite nanotubes composite films for food packaging applications. Int. J. Biol. Macromol. 2022, 216, 927–939. [Google Scholar] [CrossRef]
- Yeşilyurt, A.; Mayakrishnan, G.; Parın, U.; Kim, I.S.; Parın, F.N.; Ullah, A. Design and Characterization of Polyvinyl Alcohol/Kappa-Carrageenan Pickering Emulsion Biocomposite Films for Potential Wound Care Applications. J. Biomed. Mater. Res. Part A 2025, 113, e37850. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, R.; Mayakrishnan, V.; Kamaraj, E.; Alrashed, M.M.; Kim, S.-C. Investigation of the Physicochemical and Antibacterial Activities of Plant Extract of Juniperus communis L. With Poly(Butylene Adipate-Co-Terephthalate) Films for Food Packaging. Polym. Adv. Technol. 2024, 35, e70048. [Google Scholar] [CrossRef]
- Bhatia, S.; Jawad, M.; Chinnam, S.; Al-Harrasi, A.; Shah, Y.A.; Khan, T.S.; Al-Azri, M.S.; Koca, E.; Aydemir, L.Y.; Dıblan, S.; et al. Development and Characterization of Potato Starch–Pectin-Based Active Films Enriched With Juniper Berry Essential Oil for Food Packaging Applications. Food Sci. Nutr. 2025, 13, e4688. [Google Scholar] [CrossRef]
- Lee, G.; Seo, H.; Kim, D.; Shin, S.; Kwon, K. All polymeric conductive strain sensors with excellent skin adhesion, recovery, and long-term stability prepared from an anion–zwitterion based hydrogel. RSC Adv. 2023, 13, 1672–1683. [Google Scholar] [CrossRef]
- Sainath, G.; Rohith, P.; Choudhary, B.K. Fatigue Deformation of Polycrystalline Cu Using Molecular Dynamics Simulations. Trans. Indian Inst. Met. 2016, 69, 489–493. [Google Scholar] [CrossRef][Green Version]
- Akhter, R.; Masoodi, F.A.; Wani, T.A. Chitosan, gelatin and pectin based bionanocomposite films with rosemary essential oil as an active ingredient for future foods. Int. J. Biol. Macromol. 2024, 272, 132813. [Google Scholar] [CrossRef]
- Gabrić, D.; Kurek, M.; Ščetar, M.; Brnčić, M.; Galić, K. Characterization of Synthetic Polymer Coated with Biopolymer Layer with Natural Orange Peel Extract Aimed for Food Packaging. Polymers 2023, 15, 2569. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Ullah, S.; Ullah, S.; Shakeel, M.; Afsar, T.; Husain, F.M.; Amor, H.; Razak, S. Innovative biopolymers composite based thin film for wound healing applications. Sci. Rep. 2024, 14, 27415. [Google Scholar] [CrossRef]
- Gheorghita, D.; Grosu, E.; Robu, A.; Ditu, L.M.; Deleanu, I.M.; Gradisteanu Pircalabioru, G.; Raiciu, A.-D.; Bita, A.-I.; Antoniac, A.; Antoniac, V.I. Essential Oils as Antimicrobial Active Substances in Wound Dressings. Materials 2022, 15, 6923. [Google Scholar] [CrossRef]
- Pourhojat, F.; Shariati, S.; Sohrabi, M.; Mahdavi, H.; Asadpour, L. Preparation of antibacterial electrospun Poly lactic-co–glycolic acid nanofibers containing Hypericum perforatum with bedsore healing property and evaluation of its drug release performance. Int. J. Nano Dimens. 2018, 9, 286–297. [Google Scholar]
- Mouro, C.; Gomes, A.P.; Ahonen, M.; Fangueiro, R.; Gouveia, I.C. Chelidonium majus L. Incorporated Emulsion Electrospun PCL/PVA_PEC Nanofibrous Meshes for Antibacterial Wound Dressing Applications. Nanomaterials 2021, 11, 1785. [Google Scholar] [CrossRef]
- Saberian, M.; Seyedjafari, E.; Zargar, S.J.; Mahdavi, F.S.; Sanaei-rad, P. Fabrication and characterization of alginate/chitosan hydrogel combined with honey and aloe vera for wound dressing applications. J. Appl. Polym. Sci. 2021, 138, 51398. [Google Scholar] [CrossRef]
- Karoyo, A.H.; Wilson, L.D. A Review on the Design and Hydration Properties of Natural Polymer-Based Hydrogels. Materials 2021, 14, 1095. [Google Scholar] [CrossRef]
- Pitterou, I.; Kalogeropoulou, F.; Tzani, A.; Tsiantas, K.; Gatou, M.A.; Pavlatou, E.; Batrinou, A.; Fountzoula, C.; Kriebardis, A.; Zoumpoulakis, P.; et al. Development of Alginate Hydrogels Incorporating Essential Oils Loaded in Chitosan Nanoparticles for Biomedical Applications. Molecules 2024, 29, 5318. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Filho, J.G.; de Deus, I.P.B.; Valadares, A.C.F.; Fernandes, C.C.; Estevam, E.B.B.; Egea, M.B. Chitosan Film with Citrus limonia Essential Oil: Physical and Morphological Properties and Antibacterial Activity. Colloids Interfaces 2020, 4, 18. [Google Scholar] [CrossRef]
- Kurowiak, J.; Klekiel, T.; Będziński, R. Biodegradable Polymers in Biomedical Applications: A Review—Developments, Perspectives and Future Challenges. Int. J. Mol. Sci. 2023, 24, 16952. [Google Scholar] [CrossRef]
- Hussein, M.A.M.; Gunduz, O.; Sahin, A.; Grinholc, M.; El-Sherbiny, I.M.; Megahed, M. Dual Spinneret Electrospun Polyurethane/PVA-Gelatin Nanofibrous Scaffolds Containing Cinnamon Essential Oil and Nanoceria for Chronic Diabetic Wound Healing: Preparation, Physicochemical Characterization and In-Vitro Evaluation. Molecules 2022, 27, 2146. [Google Scholar] [CrossRef] [PubMed]
- Argel, S.; Castaño, M.; Jimenez, D.E.; Rodríguez, S.; Vallejo, M.J.; Castro, C.I.; Osorio, M.A. Assessment of Bacterial Nanocellulose Loaded with Acetylsalicylic Acid or Povidone-Iodine as Bioactive Dressings for Skin and Soft Tissue Infections. Pharmaceutics 2022, 14, 1661. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Parker, T.L. Anti-inflammatory activity of Juniper (Juniperus communis) berry essential oil in human dermal fibroblasts. Cogent Med. 2017, 4, 1306200. [Google Scholar] [CrossRef]
- Xiong, S.; Li, R.; Ye, S.; Ni, P.; Shan, J.; Yuan, T.; Liang, J.; Fan, Y.; Zhang, X. Vanillin enhances the antibacterial and antioxidant properties of polyvinyl alcohol-chitosan hydrogel dressings. Int. J. Biol. Macromol. 2022, 220, 109–116. [Google Scholar] [CrossRef]
- Hu, H.; Li, D.; Bai, R.; Zhang, W.; Luo, H.; Yu, E. Chemodiversity and Bioactivity of the Essential Oils of Juniperus and Implication for Taxonomy. Int. J. Mol. Sci. 2023, 24, 15203. [Google Scholar] [CrossRef] [PubMed]
- Shitole, A.A.; Raut, P.W.; Sharma, N.; Giram, P.; Khandwekar, A.P.; Garnaik, B. Electrospun polycaprolactone/hydroxyapatite/ZnO nanofibers as potential biomaterials for bone tissue regeneration. J. Mater. Sci. Mater. Med. 2019, 30, 51. [Google Scholar] [CrossRef]
- Shitole, A.A.; Raut, P.; Giram, P.; Rade, P.; Khandwekar, A.; Garnaik, B.; Sharma, N. Poly (vinylpyrrolidone)-iodine engineered poly (ε-caprolactone) nanofibers as potential wound dressing materials. Mater. Sci. Eng. C 2020, 110, 110731. [Google Scholar] [CrossRef]
- Wu, X.; Chen, K.; Zhang, X.; Wei, R. Shear-induced orientation distribution in fibrinogen adsorption. Phys. Fluids 2025, 37, 021920. [Google Scholar] [CrossRef]
- Coma, M.E.; Peltzer, M.A.; Delgado, J.F.; Salvay, A.G. Water kefir grains as an innovative source of materials: Study of plasticiser content on film properties. Eur. Polym. J. 2019, 120, 109234. [Google Scholar] [CrossRef]
- Buyana, B.; Aderibigbe, B.; Ray, S.S.; Ndinteh, D.; Fonkui, Y. Development, characterization, and in vitro evaluation of water soluble poloxamer/pluronic-mastic gum-gum acacia-based wound dressing. J. Appl. Polym. Sci. 2020, 137, 48728. [Google Scholar] [CrossRef]
- He, Y.; Zhao, W.; Dong, Z.; Ji, Y.; Li, M.; Hao, Y.; Zhang, D.; Yuan, C.; Deng, J.; Zhao, P.; et al. A biodegradable antibacterial alginate/carboxymethyl chitosan/Kangfuxin sponges for promoting blood coagulation and full-thickness wound healing. Int. J. Biol. Macromol. 2021, 167, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, X.; Zeng, L.; Pan, H.; Liu, Z. Allicin-loaded chitosan/polyvinyl alcohol scaffolds as a potential wound dressing material to treat diabetic wounds: An in vitro and in vivo study. J. Drug Deliv. Sci. Technol. 2021, 65, 102734. [Google Scholar] [CrossRef]
- Nowak, A.; Zagórska-Dziok, M.; Perużyńska, M.; Cybulska, K.; Kucharska, E.; Ossowicz-Rupniewska, P.; Piotrowska, K.; Duchnik, W.; Kucharski, Ł.; Sulikowski, T.; et al. Assessment of the Anti-Inflammatory, Antibacterial and Anti-Aging Properties and Possible Use on the Skin of Hydrogels Containing Epilobium angustifolium L. Extracts. Front. Pharmacol. 2022, 13, 896706. [Google Scholar] [CrossRef]
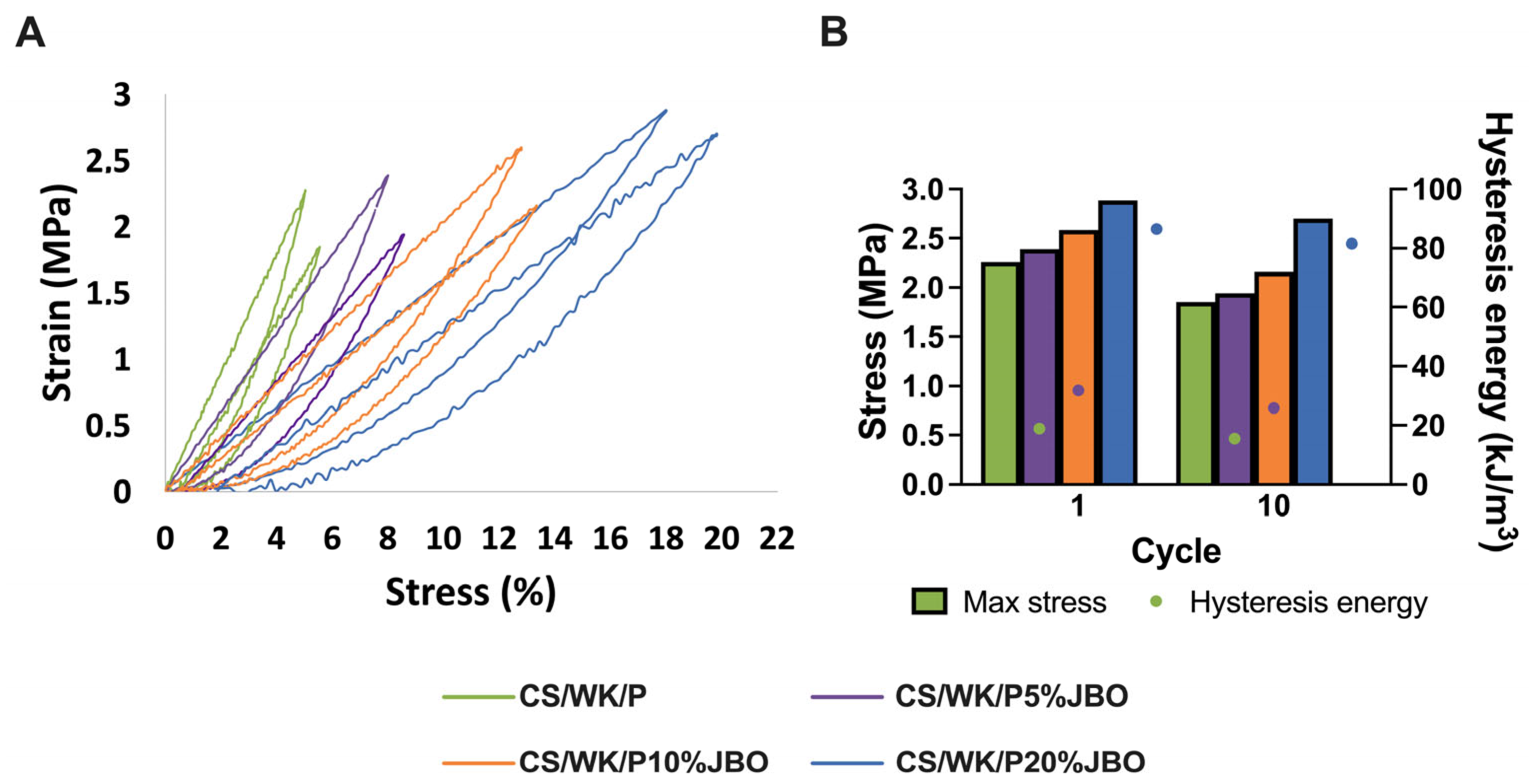
| Sample | Mechanical Properties | ||
|---|---|---|---|
| Tensile Strength [MPa] | Strain [%] | Young’s Modulus [MPa] | |
| CS/WK/P | 2.25 ± 0.05 | 5.03 ± 0.15 | 131.2 ± 1.36 |
| CS/WK/P5%JBO | 2.39 ± 0.04 a | 8.00 ± 0.20 a | 120.9 ± 1.37 a |
| CS/WK/P10%JBO | 2.60 ± 0.03 a,b | 12.80 ± 0.30 a,b | 97.3 ± 0.91 a,b |
| CS/WK/P20%JBO | 2.88 ± 0.05 a,b,c | 18.00 ± 0.30 a,b | 76.2 ± 1.15 a,b,c |
| Sample | WVTR (g·m−2·day−1) | References |
|---|---|---|
| CS/WK/P | 1950 ± 60 | This study |
| CS/WK/P5%JBO | 2020 ± 55 a | This study |
| CS/WK/P10%JBO | 2125 ± 65 a,b | This study |
| CS/WK/P20%JBO | 2250 ± 70 a,b,c | This study |
| Chitosan, carboxymethyl cellulose, tannic acid | 1912.25 ± 13.10 | [63] |
| PVA/PVP, thyme essential oil | 606.8 ± 24.2 | [64] |
| PLGA, Hypericum Perforatum | 1200–3057 | [65] |
| PCL/PVA, C. majus extract | 2019.82 ± 151.01 | [66] |
| Alginian, chitosan, aloe vera | 932.6 ± 9.4065 | [67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chelminiak-Dudkiewicz, D. Juniper Berry Oil as a Functional Additive in Chitosan–Water Kefiran–Paramylon Porous Sponges: Structural, Physicochemical, and Protein Interaction Insights. Int. J. Mol. Sci. 2025, 26, 5314. https://doi.org/10.3390/ijms26115314
Chelminiak-Dudkiewicz D. Juniper Berry Oil as a Functional Additive in Chitosan–Water Kefiran–Paramylon Porous Sponges: Structural, Physicochemical, and Protein Interaction Insights. International Journal of Molecular Sciences. 2025; 26(11):5314. https://doi.org/10.3390/ijms26115314
Chicago/Turabian StyleChelminiak-Dudkiewicz, Dorota. 2025. "Juniper Berry Oil as a Functional Additive in Chitosan–Water Kefiran–Paramylon Porous Sponges: Structural, Physicochemical, and Protein Interaction Insights" International Journal of Molecular Sciences 26, no. 11: 5314. https://doi.org/10.3390/ijms26115314
APA StyleChelminiak-Dudkiewicz, D. (2025). Juniper Berry Oil as a Functional Additive in Chitosan–Water Kefiran–Paramylon Porous Sponges: Structural, Physicochemical, and Protein Interaction Insights. International Journal of Molecular Sciences, 26(11), 5314. https://doi.org/10.3390/ijms26115314






