Adsorption of Sr2+ from Synthetic Waste Effluents Using Taiwan Zhi-Shin Bentonite
Abstract
1. Introduction
2. Results
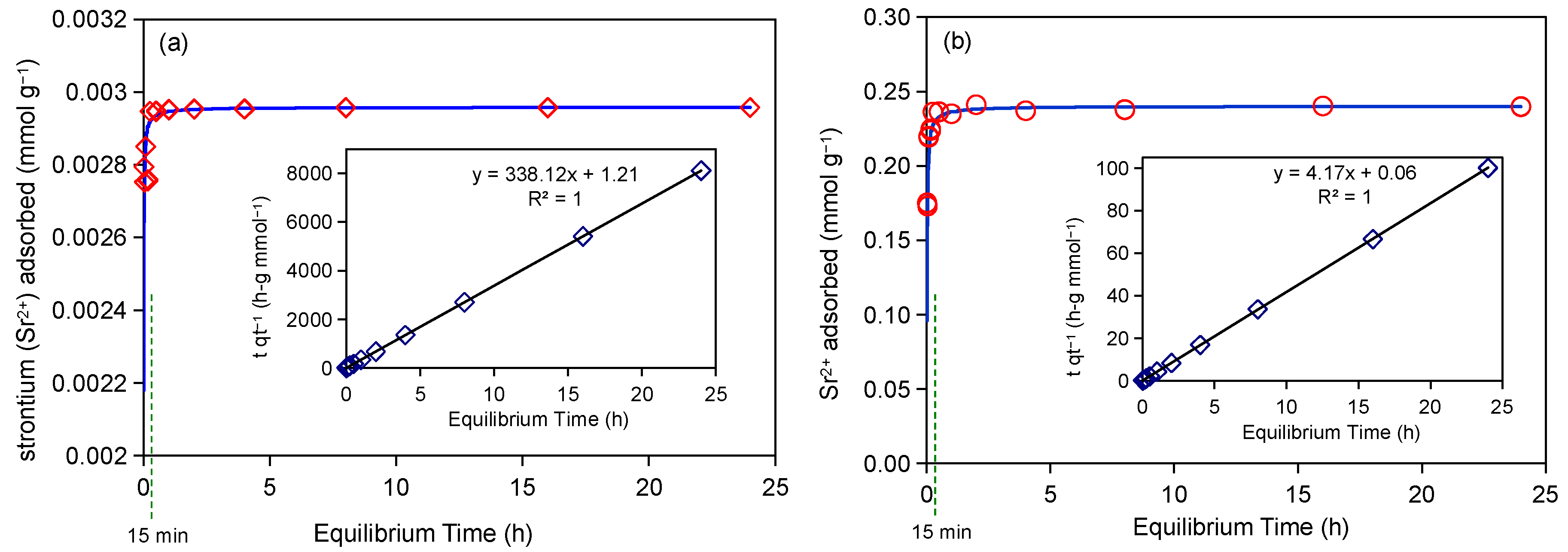
2.1. Adsorption Kinetics Experiment of Strontium (Sr2+)
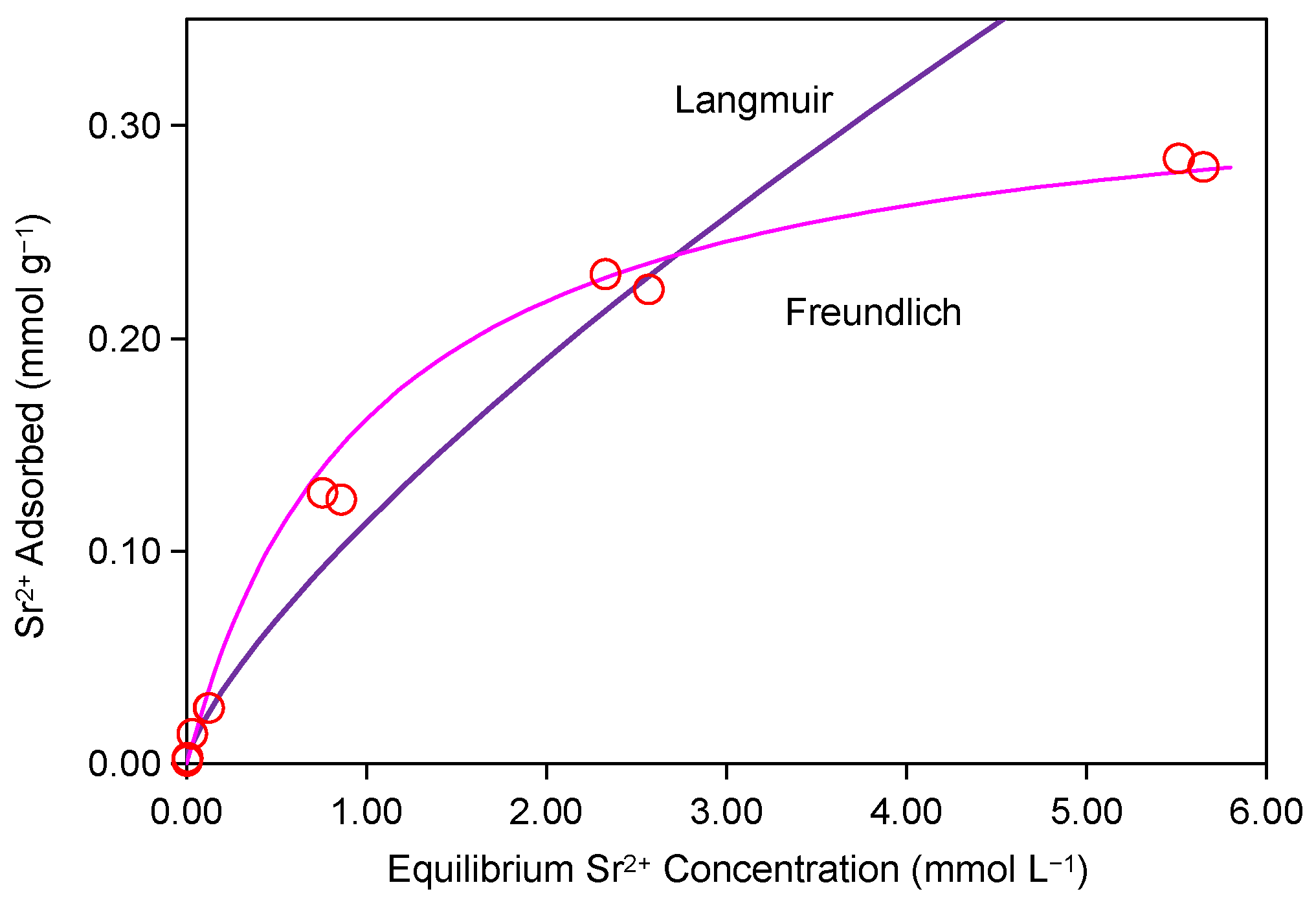
2.2. Adsorption Isotherm Experiment of Sr2+
2.3. Experiments on Influencing Factors of Adsorption of Sr2+
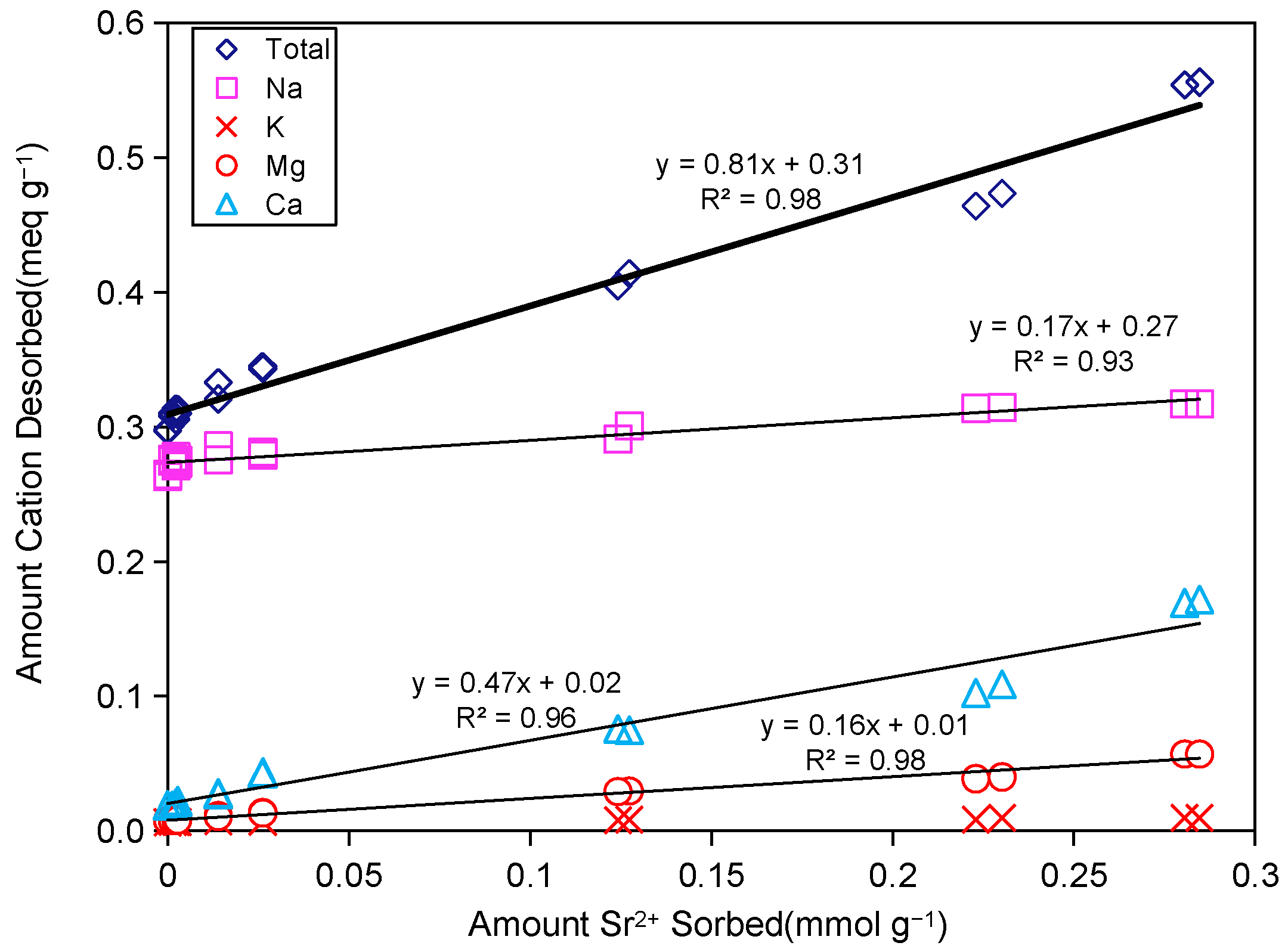
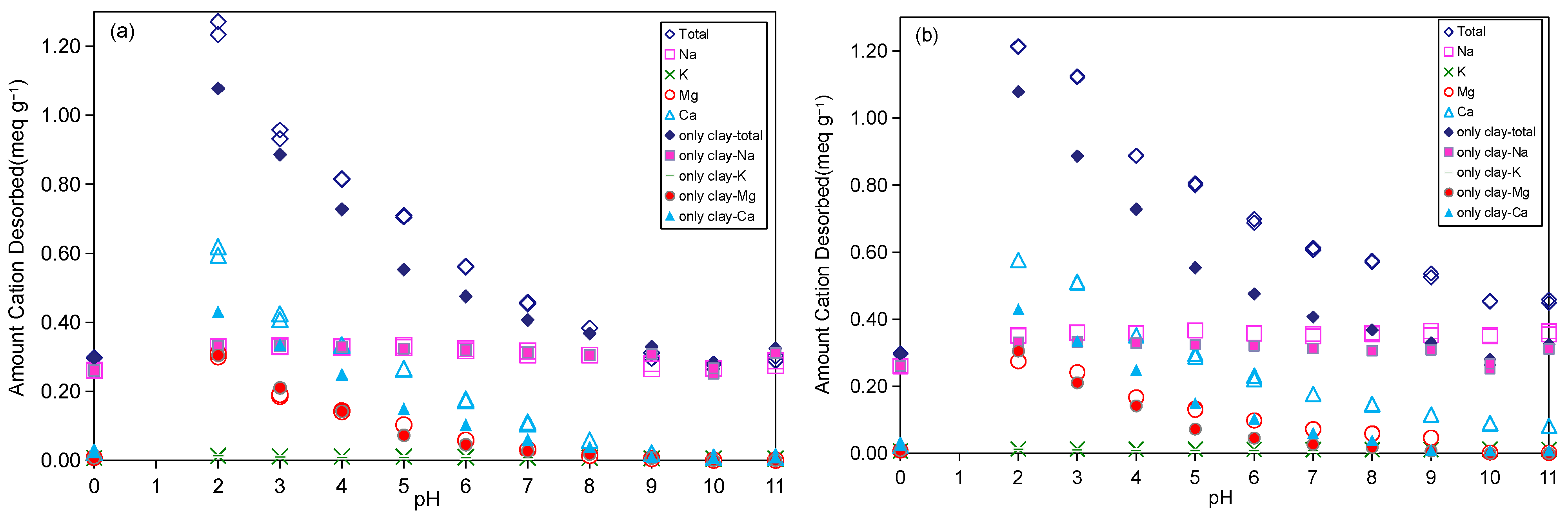
2.3.1. Exchangeable Cation Desorption
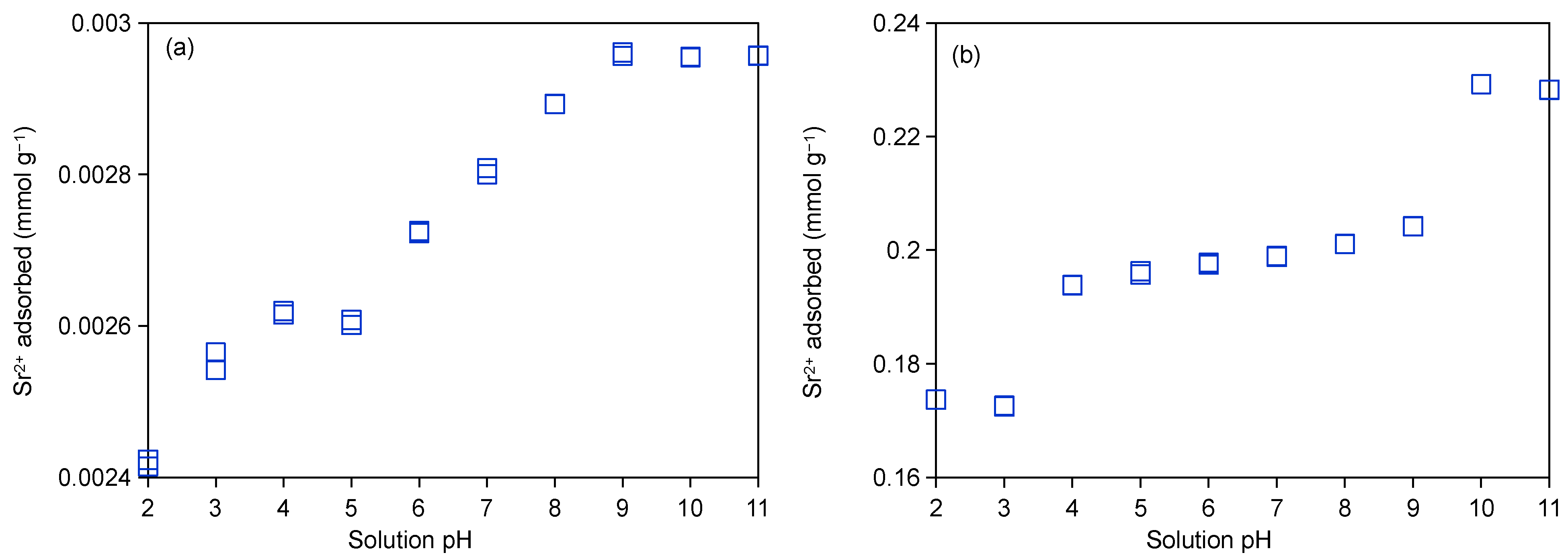
2.3.2. Influence of pH Value
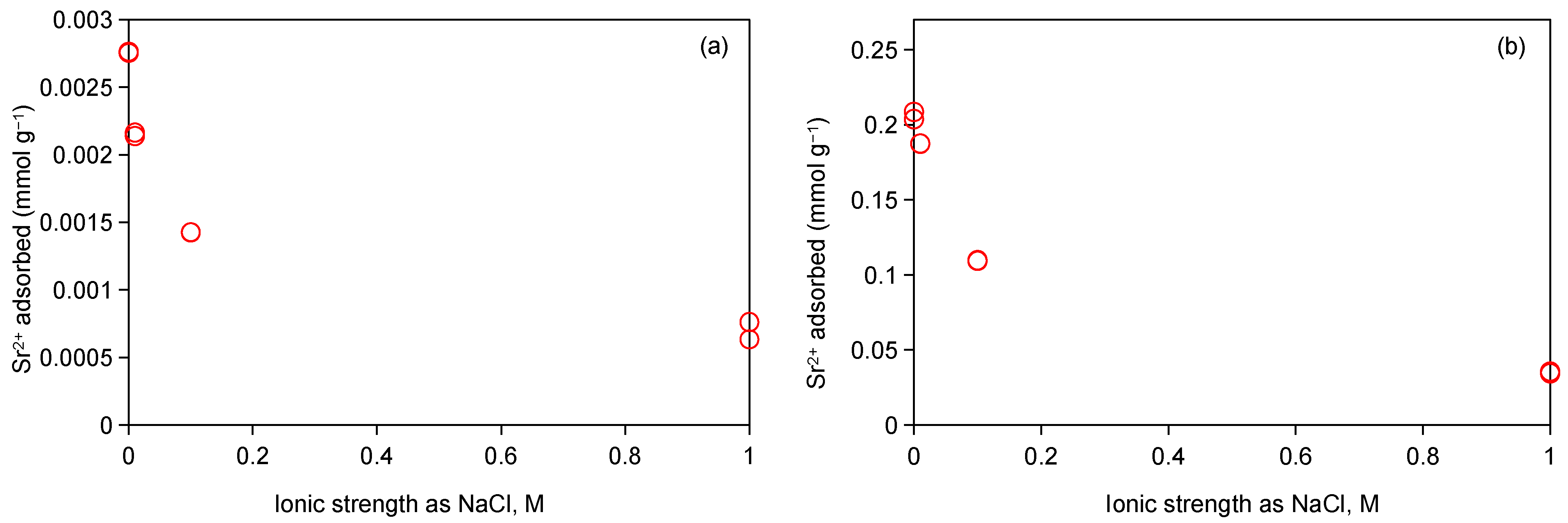
2.3.3. Effect of Ionic Strength on Adsorption
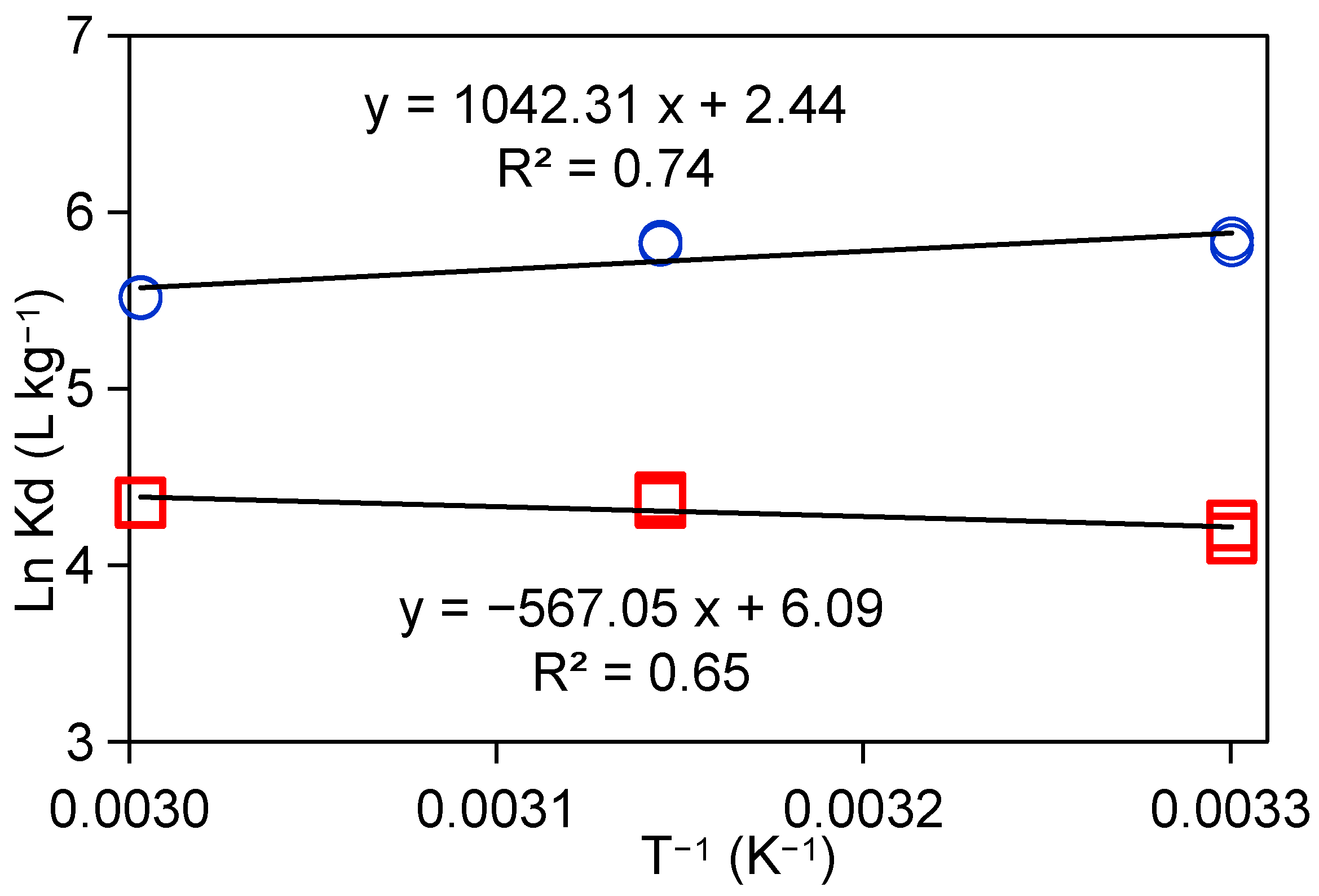
2.3.4. Effect of Temperature on Sr2+ Adsorption
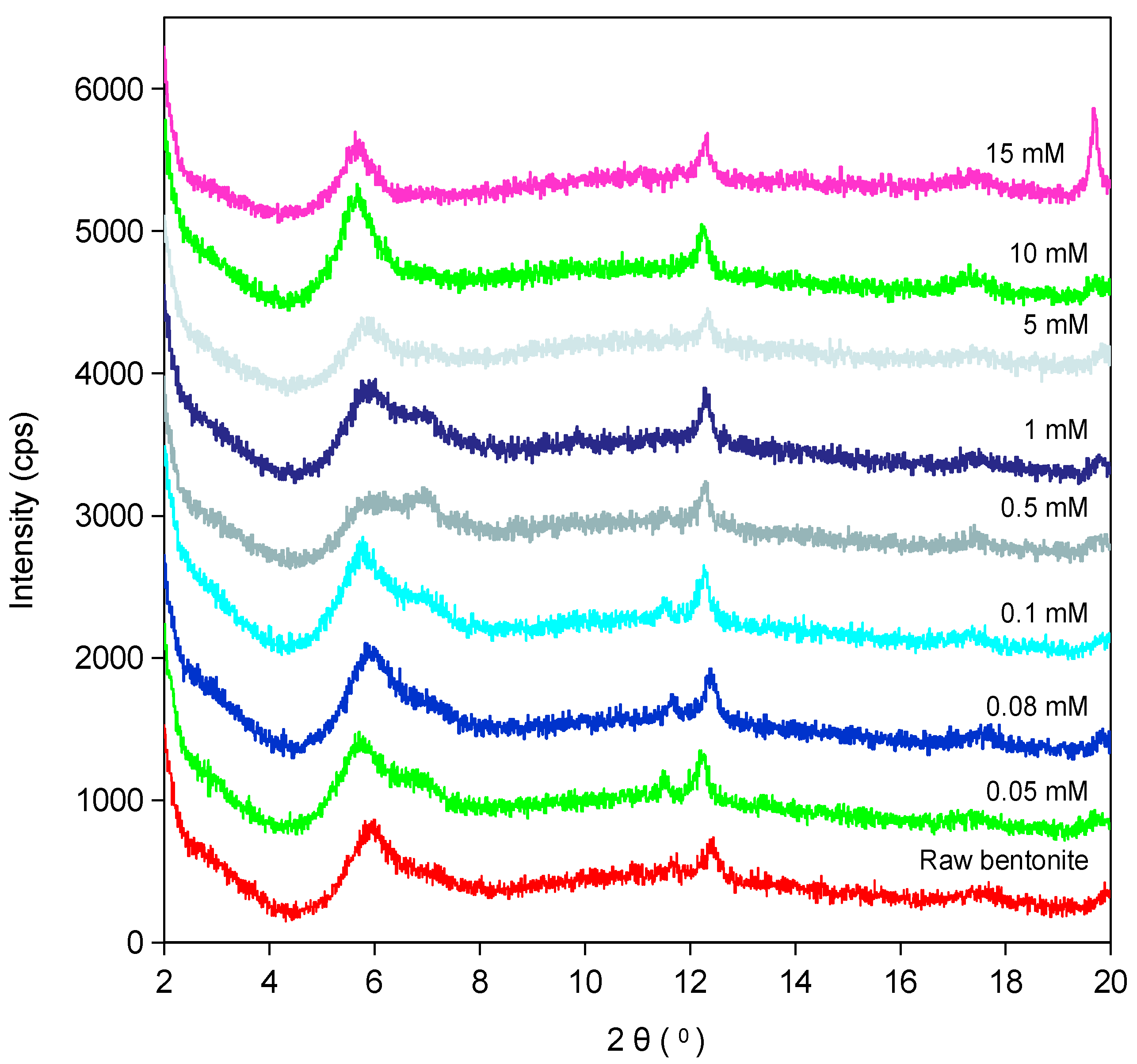
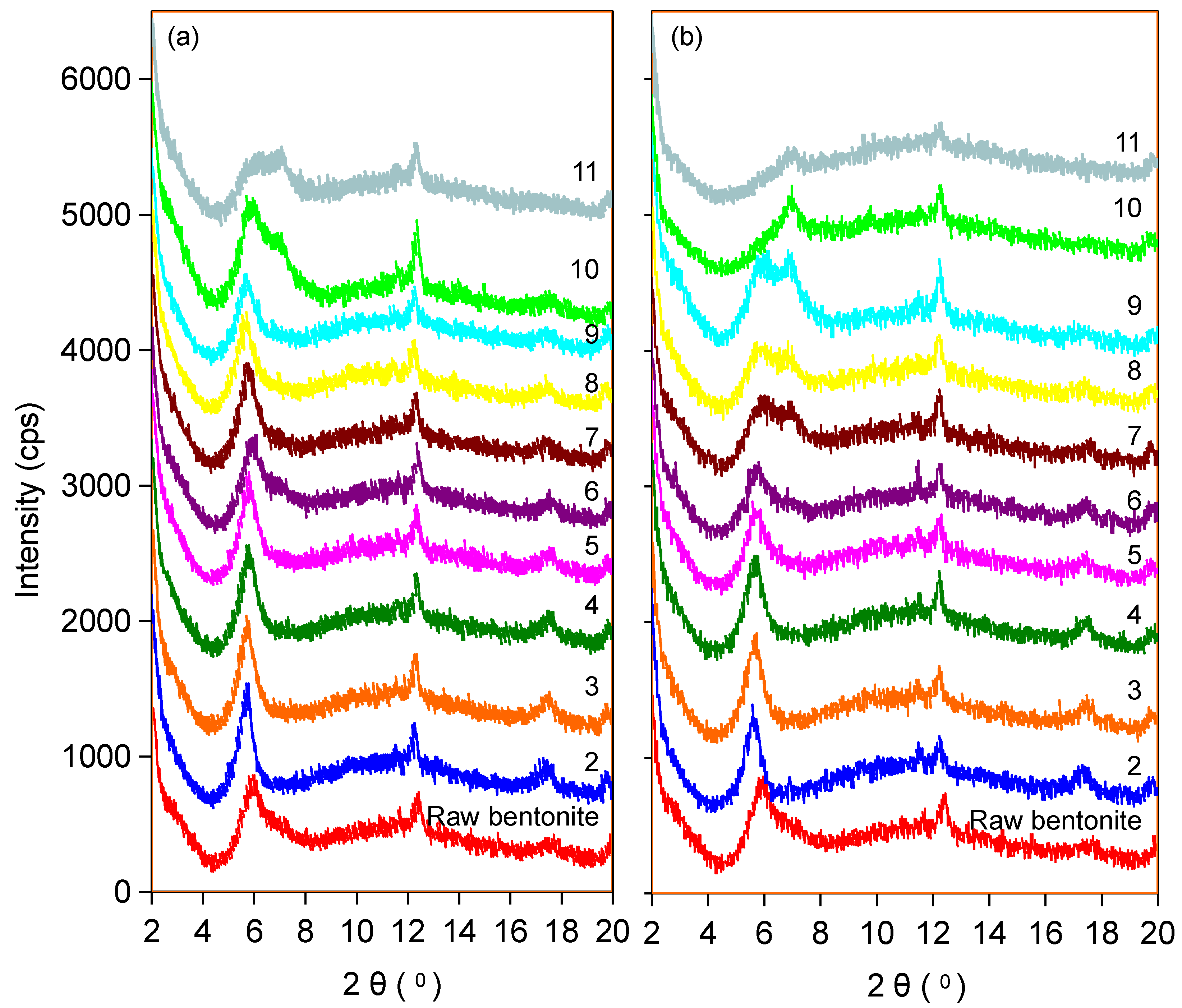
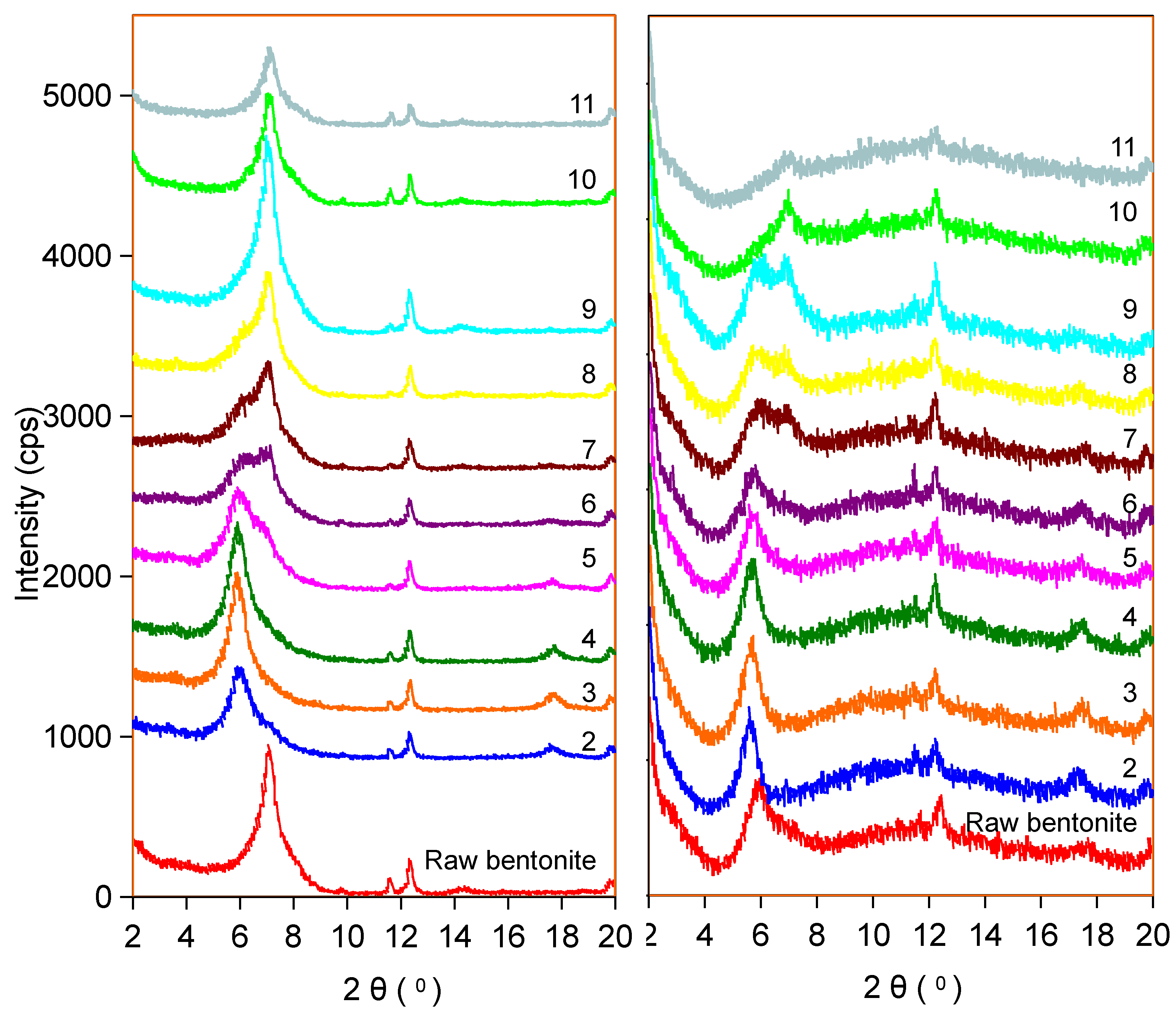
2.4. X-Ray Diffraction
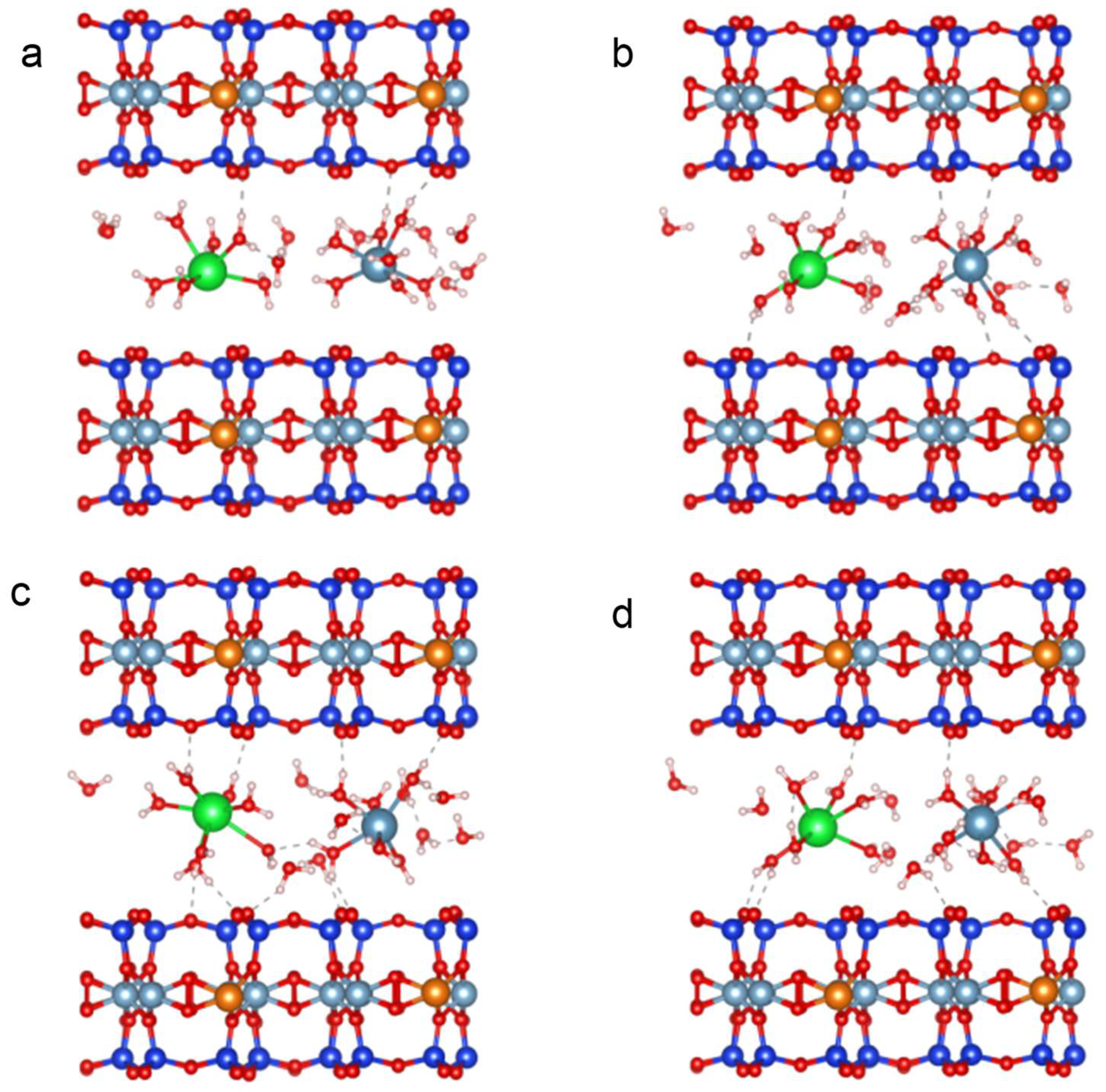
2.5. Molecular Simulation
3. Discussion
4. Materials and Methods
4.1. Materials
4.1.1. Preparation of Simulated Nuclear Waste Liquid
4.1.2. Taiwan Zhi-Shin Bentonite
4.2. Experimental Methods
4.2.1. Batch Experiment
4.2.2. Analytical Methods
4.2.3. Molecular Simulation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mandakhbayar, N.; El-Fiqi, A.; Lee, J.-H.; Kim, H.-W. Evaluation of Strontium-Doped Nanobioactive Glass Cement for Dentin–Pulp Complex Regeneration Therapy. ACS Biomater. Sci. Eng. 2019, 5, 6117–6126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gu, P.; Zhang, Z.; Liu, J.; Dong, L.; Zhang, G. Effective, rapid and selective adsorption of radioactive Sr2+ from aqueous solution by a novel metal sulfide adsorbent. Chem. Eng. J. 2018, 351, 668–677. [Google Scholar] [CrossRef]
- Ghandhi, S.A.; Weber, W.; Melo, D.; Doyle-Eisele, M.; Chowdhury, M.; Guilmette, R.; Amundson, S.A. Effect of 90Sr internal emitter on gene expression in mouse blood. BMC Genom. 2015, 16, 586. [Google Scholar] [CrossRef]
- Ghoti, H.; Amer, J.; Winder, A.; Rachmilewitz, E.; Fibach, E. Oxidative stress in red blood cells, platelets and polymorphonuclear leukocytes from patients with myelodysplastic syndrome. Eur. J. Haematol. 2007, 79, 463–467. [Google Scholar] [CrossRef]
- Aarkrog, A.; Baxter, M.S.; Bettencourt, A.O.; Bojanowski, R.; Bologa, A.; Charmasson, S.; Cunha, I.; Delfanti, R.; Duran, E.; Holm, E.; et al. A comparison of doses from 137Cs and 210Po in marine food: A major international study. J. Environ. Radioactiv. 1997, 34, 69–90. [Google Scholar] [CrossRef]
- Povinec, P.P.; Hirose, K.; Aoyama, M. Radiostrontium in the western North Pacific: Characteristics, behavior, and the Fukushima impact. Environ. Sci. Technol. 2012, 46, 10356–10363. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Yao, F.; Xiong, S.; Wu, Z.; Niu, G.; Lu, T. Strontium in public drinking water and associated public health risks in Chinese cities. Environ. Sci. Pollut. Res. 2021, 28, 23048–23059. [Google Scholar] [CrossRef]
- Adabbo, M.; Caputo, D.; de Gennaro, B.; Pansini, M.; Colella, C. Ion exchange selectivity of phillipsite for Cs and Sr as a function of framework composition. Micropor. Mesopor. Mater. 1999, 28, 315–324. [Google Scholar] [CrossRef]
- Rout, T.K.; Sengupta, D.K.; Kaur, G.; Kumar, S. Enhanced removal of dissolved metal ions in radioactive effluents by flocculation. Int. J. Miner. Process. 2016, 80, 215–222. [Google Scholar] [CrossRef]
- Jia, F.; Li, J.; Wang, J.; Sun, Y. Removal of strontium ions from simulated radioactive wastewater by vacuum membrane distillation. Ann. Nucl. Energy 2017, 103, 363–368. [Google Scholar] [CrossRef]
- Su, Z.; Deng, Z.; Wang, Y.; Ji, C.; Li, F.; Yang, G.; Huang, L. Effects of the Sr/Ca ratio on the bioremediation of strontium based on microbially-induced carbonate precipitation. J. Environ. Chem. Eng. 2023, 11, 108990. [Google Scholar] [CrossRef]
- Chen, K.; Chen, C.; Ren, X.; Alsaedi, A.; Hayat, T. Interaction mechanism between different facet TiO2 and U (VI): Experimental and density-functional theory investigation. Chem. Eng. J. 2019, 359, 944–954. [Google Scholar] [CrossRef]
- Marinović, S.S.; Ajduković, M.J.; Jović-Jovičić, N.J.; Mudrinić, T.M.; Nedić-Vasiljević, B.N.; Banković, P.T.; Milutinović-Nikolić, A.D. Adsorption of strontium on different sodium enriched bentonites. J. Serb. Chem. Soc. 2017, 82, 449–463. [Google Scholar] [CrossRef]
- Ma, H.; Shen, M.; Tong, Y.; Wang, X. Radioactive wastewater treatment technologies: A review. Molecules 2023, 28, 1935. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Parmar, P.; Saraf, M. Radiation, radionuclides and bacteria: An in-perspective review. J. Environ. Radioact. 2017, 180, 27–35. [Google Scholar] [CrossRef]
- Vanhoudt, N.; Vandenhove, H.; Leys, N.; Janssen, P. Potential of higher plants, algae, and cyanobacteria for remediation of radioactively contaminated waters. Chemosphere 2018, 207, 239–254. [Google Scholar] [CrossRef]
- Cheng, Y.; Chuah, G.K. The synthesis and applications of α-zirconium phosphate. Chin Chem. Lett. 2020, 31, 307–310. [Google Scholar] [CrossRef]
- He, W.; Ai, K.; Ren, X.; Wang, S.; Lu, L. Inorganic layered ion-exchangers for decontamination of toxic metal ions in aquatic systems. J. Mater. Chem. A 2017, 5, 19593–19606. [Google Scholar] [CrossRef]
- Zakrzewska-Trznadel, G. Advances in membrane technologies for the treatment of liquid radioactive waste. Desalination 2013, 321, 119–130. [Google Scholar] [CrossRef]
- Liu, C.; Su, G.; Chen, T.; Liang, M.; Zhao, L.; Zhang, D. Preparation of sulfonylcalix [4] arene-loaded XAD-7 resin for strontium (II) adsorption. IOP Conf. Ser. Earth Environ. Sci. 2019, 267, 032084. [Google Scholar] [CrossRef]
- Hafizi, M.; Abolghasemi, H.; Moradi, M.; Milani, S.A. Strontium adsorption from sulfuric acid solution by Dowex 50W-X resins. Chin. J. Chem. Eng. 2011, 19, 267–272. [Google Scholar] [CrossRef]
- Lv, T.T.; Ma, W.; Zhang, D.; Zhang, T.; Tang, J.H.; Zeng, X.; Feng, M.L.; Huang, X.Y. Rapid and highly selective Sr2+ uptake by 3D microporous rare earth oxalates with the facile synthesis, high water stability and radiation resistance. Chem. Eng. J. 2022, 435, 134906. [Google Scholar] [CrossRef]
- Ogata, F.; Kobayashi, Y.; Uematsu, Y.; Nakamura, T.; Kawasaki, N. Zeolite produced from fly ash by thermal treatment in alkaline solution and its capability to adsorb Cs (I) and Sr (II) in aqueous solution. Yakugaku Zasshi-J. Pharm. Soc. Jpn. 2020, 140, 729–737. [Google Scholar] [CrossRef]
- Abu-Nada, A.; Abdala, A.; McKay, G. Isotherm and Kinetic Modeling of Strontium Adsorption on Graphene Oxide. Nanomaterials 2021, 11, 2780. [Google Scholar] [CrossRef] [PubMed]
- Naeimi, S.; Faghihian, H. Modification and magnetization of MOF (HKUST-1) for the removal of Sr2+ from aqueous solutions. Equilibrium, kinetic and thermodynamic modeling studies. Sep. Sci. Technol. 2017, 52, 2899–2908. [Google Scholar] [CrossRef]
- Zheng, B.; Yin, J.; Zhu, L.; Zhou, B.; Shen, H.; Harbottle, D.; Hunter, T.N.; Sheng, Y.; Zhu, Q.; Zhang, H. Thiol-rich and ion-imprinted alginate hydrogel as a highly adsorptive and recyclable filtration membrane for rapid and selective Sr (II) removal. Chem. Eng. J. 2023, 465, 142752. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, L.; Dai, X.; Zhu, L.; Xiao, C.; Xu, L.; Zhang, Z.; Alekseev, E.V.; Wang, Y.; Zhang, C.; et al. Distinctive two-step intercalation of Sr2+ into a coordination polymer with record high 90Sr uptake capabilities. Chem 2019, 5, 977–994. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, J.; Zhao, X.; Li, F.; Jiang, F. Adsorption characteristics of strontium on synthesized antimony silicate. Chem. Eng. J. 2015, 277, 378–387. [Google Scholar] [CrossRef]
- Chegrouche, S.; Mellah, A.; Barkat, M. Removal of strontium from aqueous solutions by adsorption onto activated carbon: Kinetic and thermodynamic studies. Desalinatio 2009, 235, 306–318. [Google Scholar] [CrossRef]
- Moloukhia, H.; Hegazy, W.S.; Abdel-Galil, E.A.; Mahrous, S.S. Removal of Eu3+, Ce3+, Sr2+, and Cs+ ions from radioactive waste solutions by modified activated carbon prepared from coconut shells. Chem. Ecol. 2016, 32, 324–345. [Google Scholar] [CrossRef]
- Van, O.H.; Fripiar, J.J. Data handbook for clay materials and other non-metallic minerals. Soil Sci. 1981, 131, 62. [Google Scholar]
- CMS Industries. Bentonite Manufacturers, Suppliers and Exporters in Taiwan Materials. Available online: https://www.cmsindustries.in/ (accessed on 10 January 2024).
- Murali, M.S.; Mathur, J.N. Sorption characteristics of Am (III), Sr (II) and Cs (I) on bentonite and granite. J. Radioanal. Nucl. Chem. 2002, 254, 129–136. [Google Scholar] [CrossRef]
- Khan, S.A.; Khan, M.A. Sorption of strontium on bentonite. Waste Manag. 1995, 15, 641–650. [Google Scholar] [CrossRef]
- Mohajeri, P.; Smith, C.; Selamat, M.R.; Abdul Aziz, H. Enhancing the adsorption of lead (II) by bentonite enriched with pH-adjusted meranti sawdust. Water 2018, 10, 1875. [Google Scholar] [CrossRef]
- He, H.; Xu, E.; Qiu, Z.; Wu, T.; Wang, S.; Lu, Y.; Chen, G. Phenol adsorption mechanism of organically modified bentonite and its microstructural changes. Sustainability 2022, 14, 1318. [Google Scholar] [CrossRef]
- Zhang, Z.; Gu, P.; Zhang, M.; Yan, S.; Dong, L.; Zhang, G. Synthesis of a robust layered metal sulfide for rapid and effective removal of Sr2+ from aqueous solutions. Chem. Eng. J. 2019, 372, 1205–1215. [Google Scholar] [CrossRef]
- Izosimova, Y.; Gurova, I.; Tolpeshta, I.; Karpukhin, M.; Zakusin, S.; Zakusina, O.; Samburshiy, A.; Krupskaya, V. Adsorption of Cs (I) and Sr (II) on bentonites with different compositions at different pH. Minerals 2022, 12, 862. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, Y.; Hu, J.; Zheng, Q.; Zhao, Y.; Lv, G.; Liao, L. Clay minerals and clay-based materials for heavy metals pollution control. Sci. Total Environ. 2024, 954, 176193. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Wu, P.; Dai, Y.; Long, H.; Zhu, N.; Li, P.; Wu, J.; Dang, Z. Characterization of organo-montmorillonites and comparison for Sr (II) removal: Equilibrium and kinetic studies. Chem. Eng. J. 2012, 191, 288–296. [Google Scholar] [CrossRef]
- Pabalan, R.T.; Bertetti, F.P. Cation-exchange properties of natural zeolites. Rev. Mineral. Geochem. 2001, 45, 453–518. [Google Scholar] [CrossRef]
- Freundlich, H. Über die adsorption in lösungen. Z. Phys. Chem. 1907, 57, 385–470. [Google Scholar] [CrossRef]
- Rahman, R.O.A.; Ibrahim, H.A.; Hanafy, M.; Monem, N.M.A. Assessment of synthetic zeolite Na A–X as sorbing barrier for strontium in a radioactive disposal facility. Chem. Eng. J. 2010, 157, 100–112. [Google Scholar] [CrossRef]
- Molera, M.; Eriksen, T. Diffusion of 22Na+, 85Sr2+, 134Cs+ and 57Co2+ in bentonite clay compacted to different densities: Experiments and modeling. Radiochim. Acta 2002, 90, 753–760. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Wang, J. Cation exchange, interlayer spacing, and thermal analysis of Na/Ca-montmorillonite modified with alkaline and alkaline earth metal ions. J. Therm. Anal. Calorim. 2012, 110, 1199–1206. [Google Scholar] [CrossRef]
- Tong, Y.; Zhang, H.; Li, X.; Jia, Q. Experimental study on sodium modification and purification of GMZ bentonite. Constr. Build. Mater. 2023, 367, 130060. [Google Scholar] [CrossRef]
- Alvarez-Ayuso, E.; Garcia-Sanchez, A. Removal of heavy metals from waste waters by natural and Na-exchanged bentonites. Clays Clay Miner. 2003, 51, 475–480. [Google Scholar] [CrossRef]
- Lee, C.P.; Tsai, S.C.; Wu, M.C.; Tsai, T.L. A study on removal of Cs and Sr from aqueous solution by bentonite–alginate microcapsules. J. Radioanal. Nucl. Chem. 2018, 318, 2381–2387. [Google Scholar] [CrossRef]
- Young, D.A.; Smith, D.E. Simulations of clay mineral swelling and hydration: Dependence upon interlayer ion size and charge. J. Phys. Chem. B 2000, 104, 9163–9170. [Google Scholar] [CrossRef]
- Nilsson, K.K.; Jensen, B.S.; Carlsen, L. The Migration Chemistry of Strontium; European applied research reports nuclear science and technology section; Harwood Academic Publishers: Reading, UK, 1985; pp. 149–200. Available online: https://books.google.rs/books/about/The_Migration_Chemistry_of_Strontium.html?id=gV970AEACAAJ&redir_esc=y (accessed on 21 May 2025).
- Carroll, S.A.; Roberts, S.K.; Criscenti, L.J.; O’Day, P.A. Surface complexation model for strontium sorption to amorphous silica and goethite. Geochem. Trans. 2008, 9, 2. [Google Scholar] [CrossRef]
- Zhang, S. A new nano-sized calcium hydroxide photocatalytic material for the photodegradation of organic dyes. Rsc Adv. 2014, 4, 15835–15840. [Google Scholar] [CrossRef]
- Janusz, W.; Skwarek, E. Study of sorption processes of strontium on the synthetic hydroxyapatite. Adsorption 2016, 22, 697–706. [Google Scholar] [CrossRef]
- Chen, C.C.; Hayes, K.F. X-ray absorption spectroscopy investigation of aqueous Co (II) and Sr (II) sorption at clay–water interfaces. Geochim. Cosmochim. Acta 1999, 63, 3205–3215. [Google Scholar] [CrossRef]
- Edet, U.A.; Ifelebuegu, A.O. Kinetics, isotherms, and thermodynamic modeling of the adsorption of phosphates from model wastewater using recycled brick waste. Processes 2020, 8, 665. [Google Scholar] [CrossRef]
- Ghaemi, A.; Torab-Mostaedi, M.; Ghannadi-Maragheh, M. Characterizations of strontium (II) and barium (II) adsorption from aqueous solutions using dolomite powder. J. Hazard. Mater. 2011, 190, 916–921. [Google Scholar] [CrossRef]
- Yusan, S.; Erenturk, S. Adsorption characterization of strontium on PAN/zeolite composite adsorbent. World J. Nucl. Sci. Technol. 2011, 1, 6–12. [Google Scholar] [CrossRef]
- Kilincarslan Kaygun, A.; Eral, M.; Akyil Erenturk, S. Removal of cesium and strontium using natural attapulgite: Evaluation of adsorption isotherm and thermodynamic data. J. Radioanal. Nucl. Chem. 2017, 311, 1459–1464. [Google Scholar] [CrossRef]
- Khan, S.; Riaz-Ur-Reman; Khan, M. Adsorption of Cs (I), Sr (II) and Co (II) on Al2O3. J. Radioanal. Nucl. Chem. 1995, 190, 81–96. [Google Scholar] [CrossRef]
- Celebi, O.; Kilikli, A.; Erten, H.N. Sorption of radioactive cesium and barium ions onto solid humic acid. J. Hazard. Mater. 2009, 168, 695–703. [Google Scholar] [CrossRef]
- Möller, T.; Clearfield, A.; Harjula, R. Preparation of hydrous mixed metal oxides of Sb, Nb, Si, Ti and W with a pyrochlore structure and exchange of radioactive cesium and strontium ions into the materials. Micropor. Mesopor. Mat. 2002, 54, 187–199. [Google Scholar] [CrossRef]
- İnan, S.; Altaş, Y. Preparation of zirconium–manganese oxide/polyacrylonitrile (Zr–Mn oxide/PAN) composite spheres and the investigation of Sr (II) sorption by experimental design. Chem. Eng. J. 2011, 168, 1263–1271. [Google Scholar] [CrossRef]
- Bérend, I.; Cases, J.M.; François, M.; Uriot, J.P.; Michot, L.; Masion, A.; Thomas, F. Mechanism of adsorption and desorption of water vapor by homoionic montmorillonites: 2. The Li+, Na+, K+, Rb+ and Cs+-exchanged forms. Clays Clay Miner. 1995, 43, 324–336. [Google Scholar] [CrossRef]
- Cases, J.M.; Bérend, I.; François, M.; Uriot, J.P.; Michot, L.J.; Thomas, F. Mechanism of adsorption and desorption of water vapor by homoionic montmorillonite: 3. The Mg2+, Ca2+, Sr2+ and Ba2+ exchanged forms. Clays Clay Miner. 1997, 45, 8–22. [Google Scholar] [CrossRef]
- Sánchez, R.T. Mechanochemical effects on physicochemical parameters of homoionic smectite. Colloids Surf. Physicochem. Eng. Asp. 1997, 127, 135–140. [Google Scholar] [CrossRef]
- Sohrabnezhad, S.; Pourahmad, A.; Razavi, M. Silver bromide in montmorillonite as visible light-driven photocatalyst and the role of montmorillonite. Appl. Phys. A Mater. Sci. Process. 2016, 122, 822. [Google Scholar] [CrossRef]
- Berghout, A.; Tunega, D.; Zaoui, A. Density functional theory (DFT) study of the hydration steps of Na+/Mg2+/Ca2+/Sr2+/Ba2+-exchanged montmorillonites. Clays Clay Miner. 2010, 58, 174–187. [Google Scholar] [CrossRef]
- Singh, B.; Gilkes, R.J. Properties of soil kaolinites from south-western Australia. J. Soil Sci. 1992, 43, 645–667. [Google Scholar] [CrossRef]
- Ning, Z.; Ishiguro, M.; Koopal, L.K.; Sato, T.; Kashiwagi, J.I. Strontium adsorption and penetration in kaolinite at low Sr2+ concentration. Soil Sci. Plant Nutr. 2017, 63, 14–17. [Google Scholar] [CrossRef]
- Keçeli, G. Adsorption kinetics and equilibria of strontium onto kaolinite. Sep. Sci. Technol. 2015, 50, 72–80. [Google Scholar] [CrossRef]
- Holmboe, M.; Wold, S.; Jonsson, M. Porosity investigation of compacted bentonite using XRD profile modeling. J. Contam. Hydrol. 2012, 128, 19–32. [Google Scholar] [CrossRef]
- Ferrage, E.; Lanson, B.; Sakharov, B.A.; Drits, V.A. Investigation of smectite hydration properties by modeling experimental X-ray diffraction patterns: Part I. Montmorillonite hydration properties. Am. Mineral. 2005, 90, 1358–1374. [Google Scholar] [CrossRef]
- Laird, D.A. Influence of layer charge on swelling of smectites. Appl. Clay Sci. 2006, 34, 74–87. [Google Scholar] [CrossRef]
- Saiyouri, N.; Tessier, D.; Hicher, P.Y. Experimental study of swelling in unsaturated compacted clays. Clay Miner. 2004, 39, 469–479. [Google Scholar] [CrossRef]
- Chen, W.C.; Huang, W.H. Effect of groundwater chemistry on the swelling behavior of a Ca-bentonite for deep geological repository. Phys. Chem. Earth. Parts A/B/C 2013, 65, 42–49. [Google Scholar] [CrossRef]
- Sun, L.; Cao, Y.; Li, L.; Zeng, Q. Adsorption Characteristics and Mechanism of Calcium Ions on Different Molybdenite Surfaces via Experiments and DFT Simulations. Separations 2021, 8, 107. [Google Scholar] [CrossRef]
- Yaphary, Y.L.; He, M.; Lu, G.; Zou, F.; Liu, P.; Tsang, D.C.; Leng, Z. Experiment and multiscale molecular simulations on the Cu absorption by biochar-modified asphalt: An insight into removal capability and mechanism of heavy metals from stormwater runoff. Chem. Eng. J. 2023, 462, 142205. [Google Scholar] [CrossRef]
- Xing, X.; Lv, G.; Zhu, W.; He, C.; Liao, L.; Mei, L.; Li, Z.; Li, G. The binding energy between the interlayer cations and montmorillonite layers and its influence on Pb2+ adsorption. Appl. Clay Sci. 2015, 112, 117–122. [Google Scholar] [CrossRef]
- Li, Y.W.; Schulthess, C.P. Ion-exchange modeling of divalent cation adsorption on SWy-3 montmorillonite. Clays Clay Miner. 2021, 69, 167–187. [Google Scholar] [CrossRef]
- Aristilde, L.; Lanson, B.; Charlet, L. Interstratification patterns from the pH-dependent intercalation of a tetracycline antibiotic within montmorillonite layers. Langmuir 2013, 29, 4492–4501. [Google Scholar] [CrossRef]
- Wang, C.J.; Li, Z.; Jiang, W.T.; Jean, J.S.; Liu, C.C. Cation exchange interaction between antibiotic ciprofloxacin and montmorillonite. J. Hazard. Mater. 2010, 183, 309–314. [Google Scholar] [CrossRef]
- Lide, D.R. (Ed.) CRC Handbook of Chemistry and Physics, 85th ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Goo, J.Y.; Kim, B.J.; Kwon, J.S.; Jo, H.Y. Bentonite alteration and retention of cesium and iodide ions by Ca-bentonite in alkaline and saline solutions. Appl. Clay Sci. 2023, 245, 107141. [Google Scholar] [CrossRef]
- Sun, A.; Xu, Q.C.; Xu, S.J.; Shang, G.X.H.; Sun, J. Ultrasonic-assisted dispersive liquid phase microextration with low-volume toxic solvent for the separation of strontium and its application for strontium isotope determination. Asian J. Chem. 2014, 26, 169–172. [Google Scholar] [CrossRef]
- Katz, A.K.; Glusker, J.P.; Beebe, S.A.; Bock, C.W. Calcium ion coordination: A comparison with that of beryllium, magnesium, and zinc. J. Am. Chem. Soc. 1996, 118, 5752–5763. [Google Scholar] [CrossRef]
- Borchert, M.; Wilke, M.; Schmidt, C.; Kvashnina, K.; Jahn, S. Strontium complexation in aqueous solutions and silicate glasses: Insights from high energy-resolution fluorescence detection X-ray spectroscopy and ab-initio modeling. Geochim. Cosmochim. Acta 2014, 142, 535–552. [Google Scholar] [CrossRef]
- Kühne, T.D.; Iannuzzi, M.D.; Ben, M.; Rybkin, V.V.; Seewald, P.; Stein, F.; Hutter, J. CP2K: An electronic structure and molecular dynamics software package-Quickstep: Efficient and accurate electronic structure calculations. J. Chem. Phys. 2020, 152, 194103. [Google Scholar] [CrossRef] [PubMed]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Goedecker, S.; Teter, M.; Hutter, J. Separable dual-space Gaussian pseudopotentials. Phys. Rev. B 1996, 54, 1703. [Google Scholar] [CrossRef]
- VandeVondele, J.; Hutter, J. An efficient orbital transformation method for electronic structure calculations. J. Chem. Phys. 2003, 118, 4365–4369. [Google Scholar] [CrossRef]
- Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 1984, 81, 511–519. [Google Scholar] [CrossRef]
- Hoover, W.G. Canonical dynamics: Equilibrium phase-space distributions. Phys. Rev. A 1985, 31, 1695. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Ali, M.M.S.; Abdel-Galil, E.A.; Hamed, M.M. Removal of strontium radionuclides from liquid scintillation waste and environmental water samples. Appl. Radiat. Isot. 2020, 166, 109357. [Google Scholar] [CrossRef]
- Aytas, S.; Yurtlu, M.; Donat, R. Adsorption characteristic of U (VI) ion onto thermally activated bentonite. J. Hazard. Mater. 2009, 172, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.A.; Abrams, S.; Bradburne, T.; Lester, D.; Storch, P.; Remy, J.; Dindal, A. PFAS Experts Symposium: Statements on regulatory policy, chemistry and analytics, toxicology, transport/fate, and remediation for per-and polyfluoroalkyl substances (PFAS) contamination issues. Remediat. J. 2009, 29, 31–48. [Google Scholar] [CrossRef]
- Rogers, H.; Bowers, J.; Gates-Anderson, D. An isotope dilution–precipitation process for removing radioactive cesium from wastewater. J. Hazard. Mater. 2012, 243, 124–129. [Google Scholar] [CrossRef]
- Oleksiienko, O.; Wolkersdorfer, C.; Sillanpää, M. Titanosilicates in Cation Adsorption and Cation Exchange—A Review. Chem. Eng. J. 2017, 317, 570–585. [Google Scholar] [CrossRef]
| Adsorbents | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| qe exp (mmol g−1) | qmax (mmol g−1) | R2 | KF | n | R2 | |
| Zhi-Shin bentonite | 0.28 | 0.41 | 0.4945 | 0.112 | 1.196 | 0.96 |
| Ci | pH | LnKd (L Kg−1) | ΔG° (kJ mol−1) | ΔH° (kJ mol−1) | ΔS° (KJ mol−1 K−1) | ||||
|---|---|---|---|---|---|---|---|---|---|
| 303 | 318 K | 333 K | 303 K | 318 K | 333 K | ||||
| 0.1 mM-Sr | 9 | 2.43086 | 2.42254 | 2.11603 | −0.62492 | −6.1295 | −6.0099 | −8.67 | −0.01 |
| 10 mM-Sr | 9 | 0.78774 | 0.96798 | 0.9534 | −2.06 | −2.73 | −2.73 | 4.71 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Li, Y.; Yang, Y.; Chang, P.-H. Adsorption of Sr2+ from Synthetic Waste Effluents Using Taiwan Zhi-Shin Bentonite. Int. J. Mol. Sci. 2025, 26, 5298. https://doi.org/10.3390/ijms26115298
Lin Y, Li Y, Yang Y, Chang P-H. Adsorption of Sr2+ from Synthetic Waste Effluents Using Taiwan Zhi-Shin Bentonite. International Journal of Molecular Sciences. 2025; 26(11):5298. https://doi.org/10.3390/ijms26115298
Chicago/Turabian StyleLin, Yihui, Yuhan Li, Yating Yang, and Po-Hsiang Chang. 2025. "Adsorption of Sr2+ from Synthetic Waste Effluents Using Taiwan Zhi-Shin Bentonite" International Journal of Molecular Sciences 26, no. 11: 5298. https://doi.org/10.3390/ijms26115298
APA StyleLin, Y., Li, Y., Yang, Y., & Chang, P.-H. (2025). Adsorption of Sr2+ from Synthetic Waste Effluents Using Taiwan Zhi-Shin Bentonite. International Journal of Molecular Sciences, 26(11), 5298. https://doi.org/10.3390/ijms26115298








