Niacin Modulates SIRT1-Driven Signaling to Counteract Radiation-Induced Neurocognitive and Behavioral Impairments
Abstract
1. Introduction
2. Results
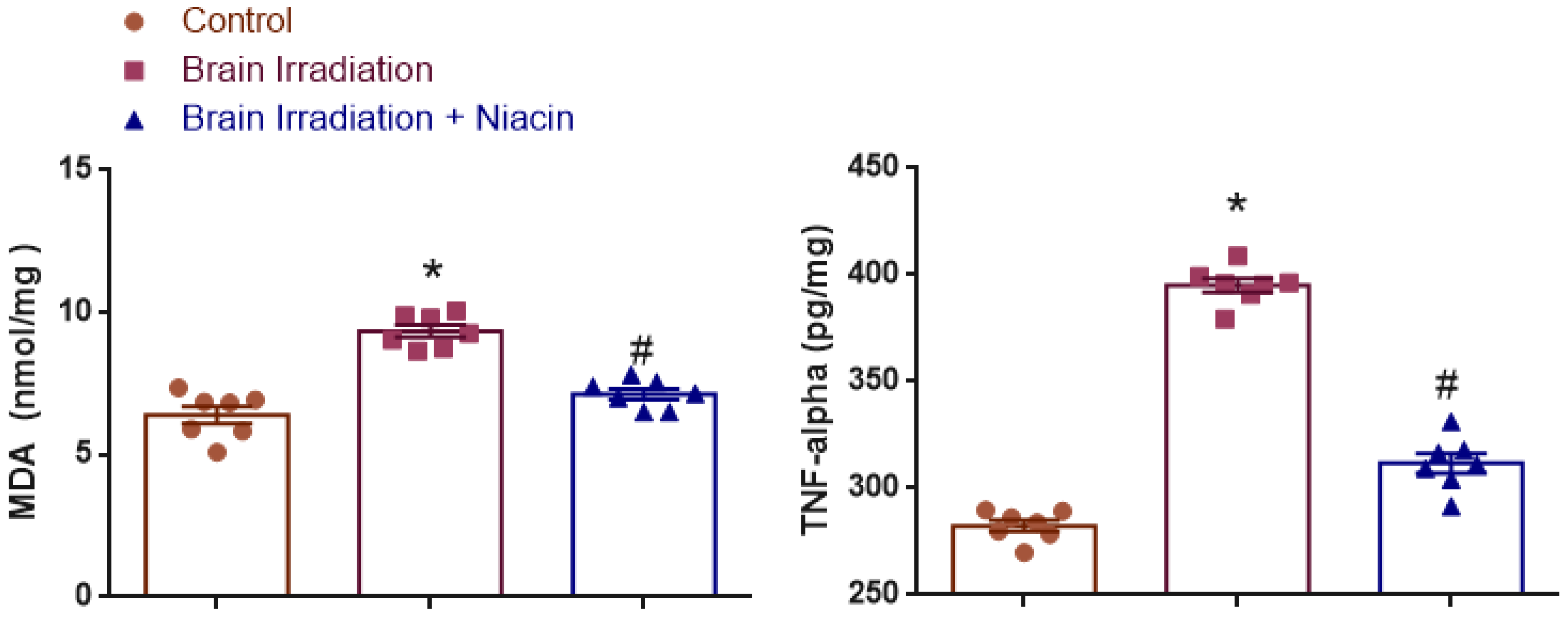
2.1. Oxidative Stress and Inflammation
2.1.1. MDA Analysis
2.1.2. TNF-α Analysis
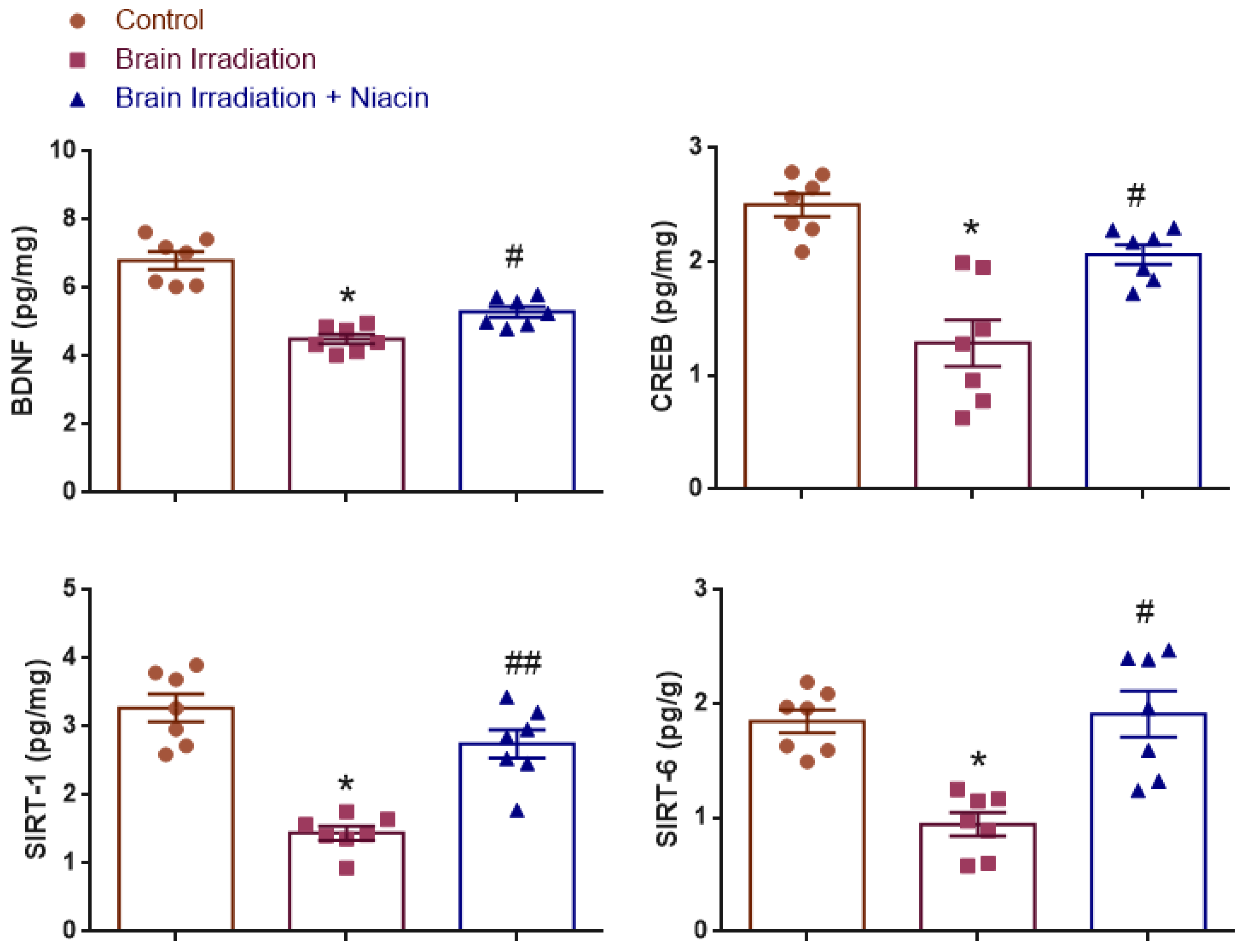
2.2. Neurotrophic and Synaptic Plasticity Markers
2.2.1. BDNF Analysis
2.2.2. CREB Analysis
2.2.3. SIRT1 Analysis
2.2.4. SIRT6 Analysis
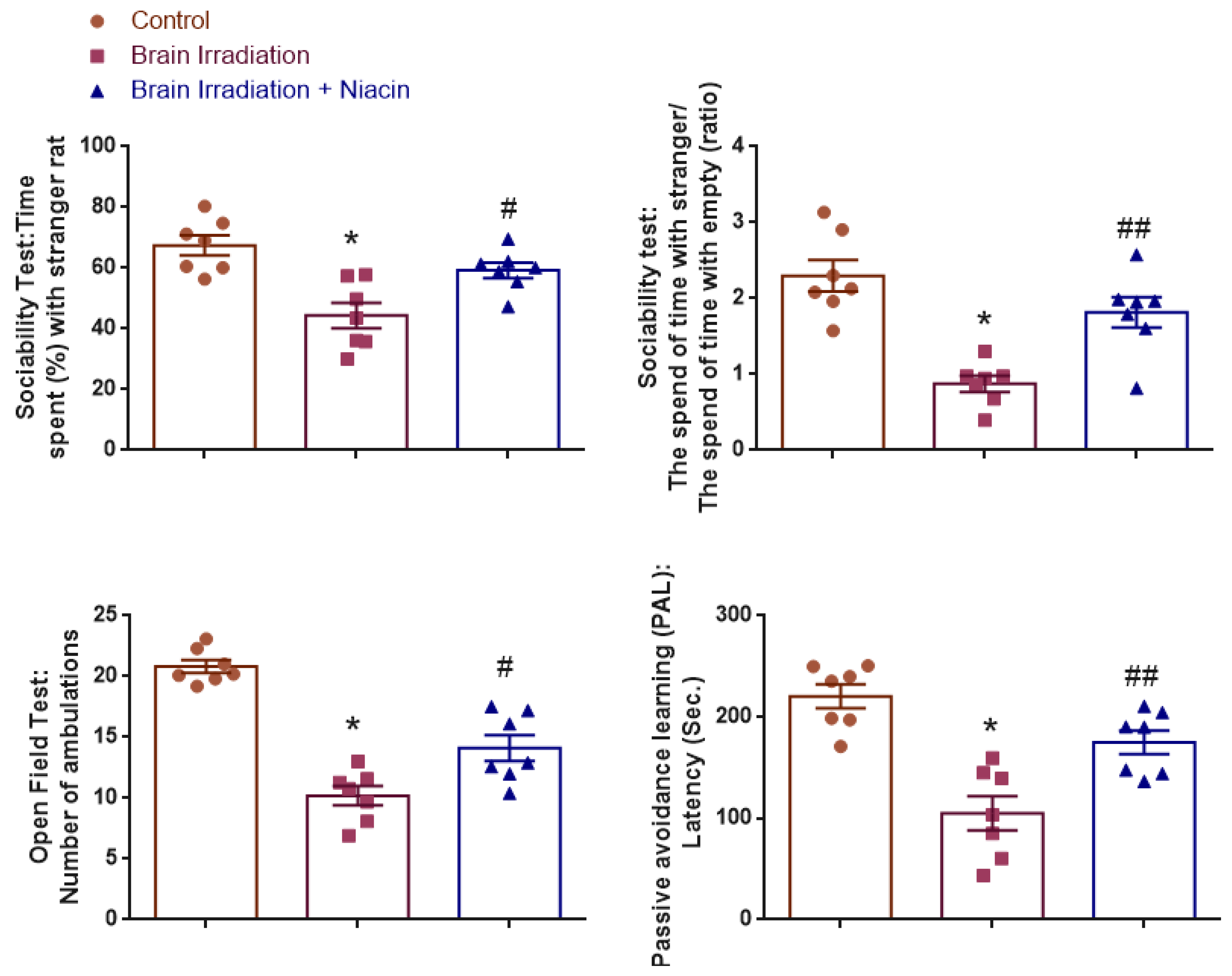
2.3. Behavioral Assessments
2.3.1. Sociability Test
Time Spent (%) with Stranger Rat
The Time Spent with Strangers/the Time Spent Alone (Ratio)
2.3.2. Open Field Test (OFT)
2.3.3. Passive Avoidance Learning (PAL)
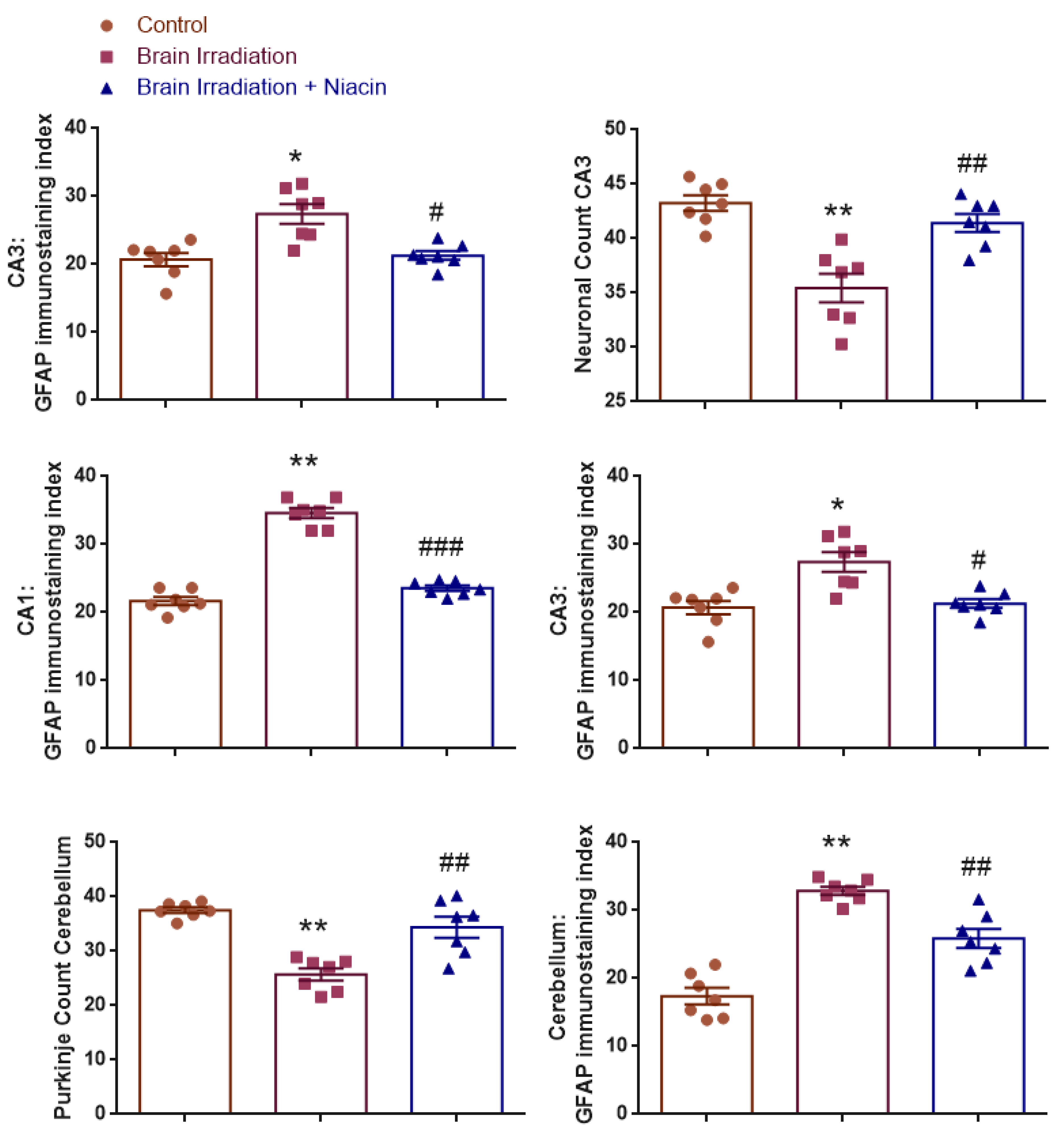
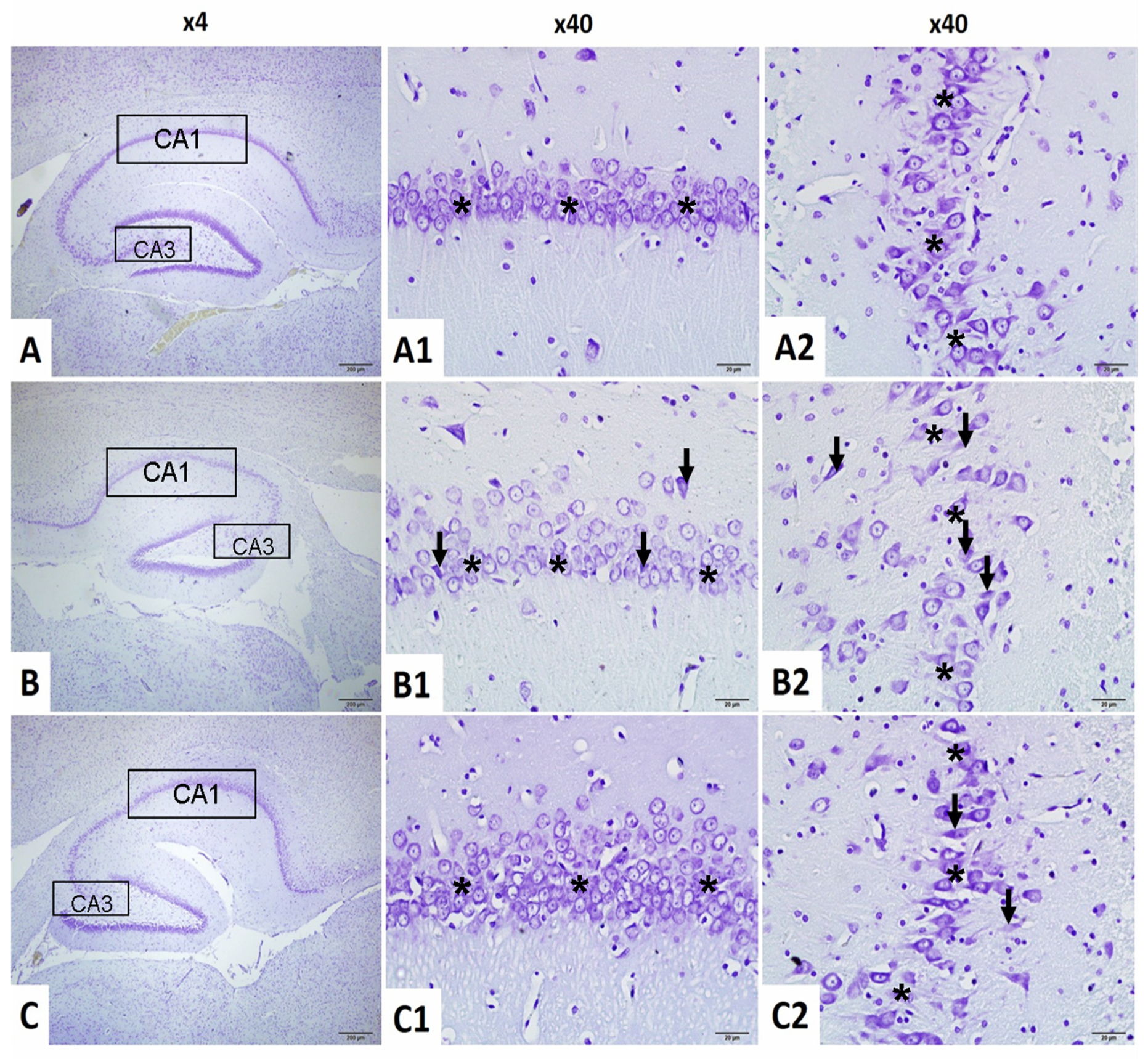
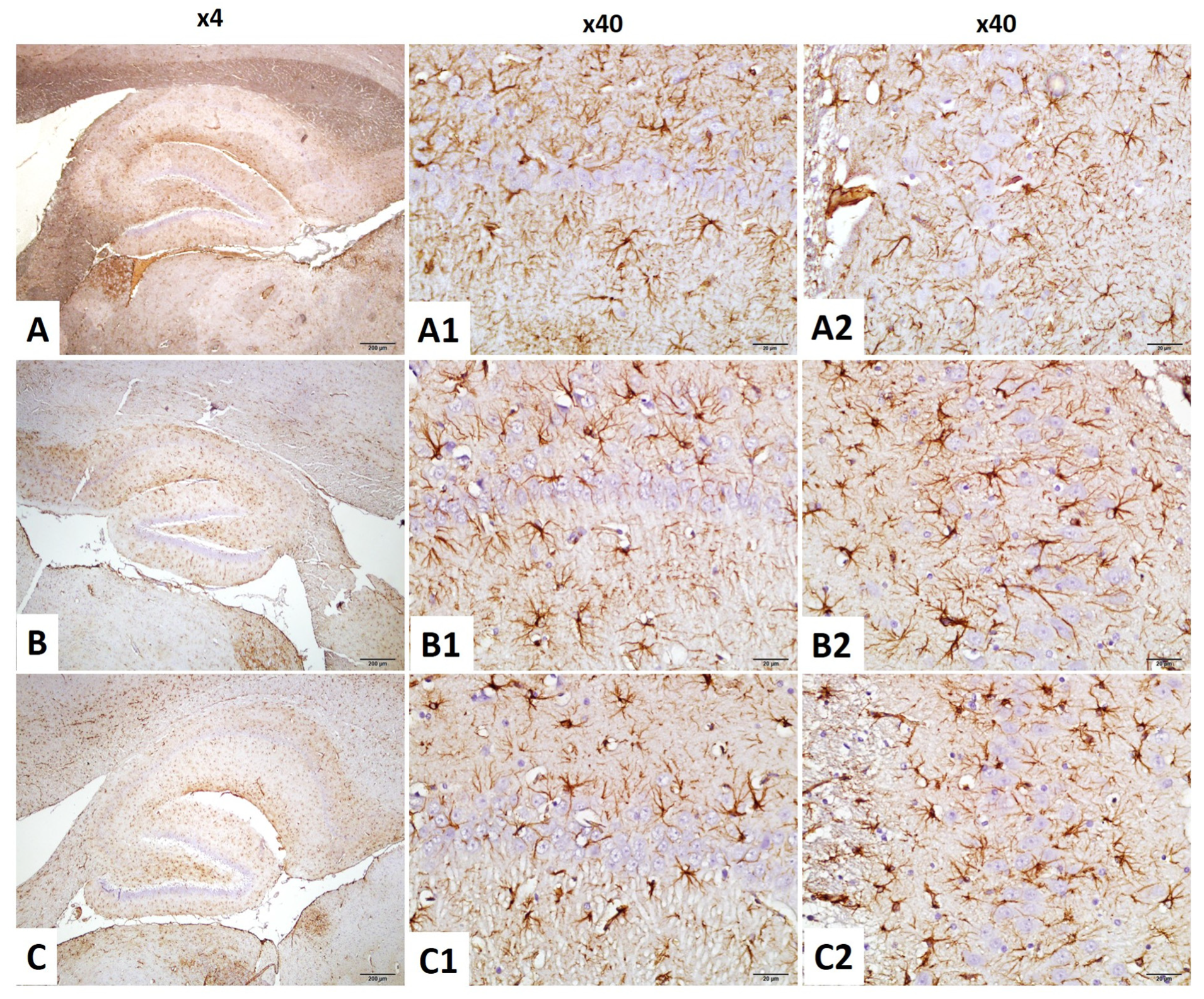
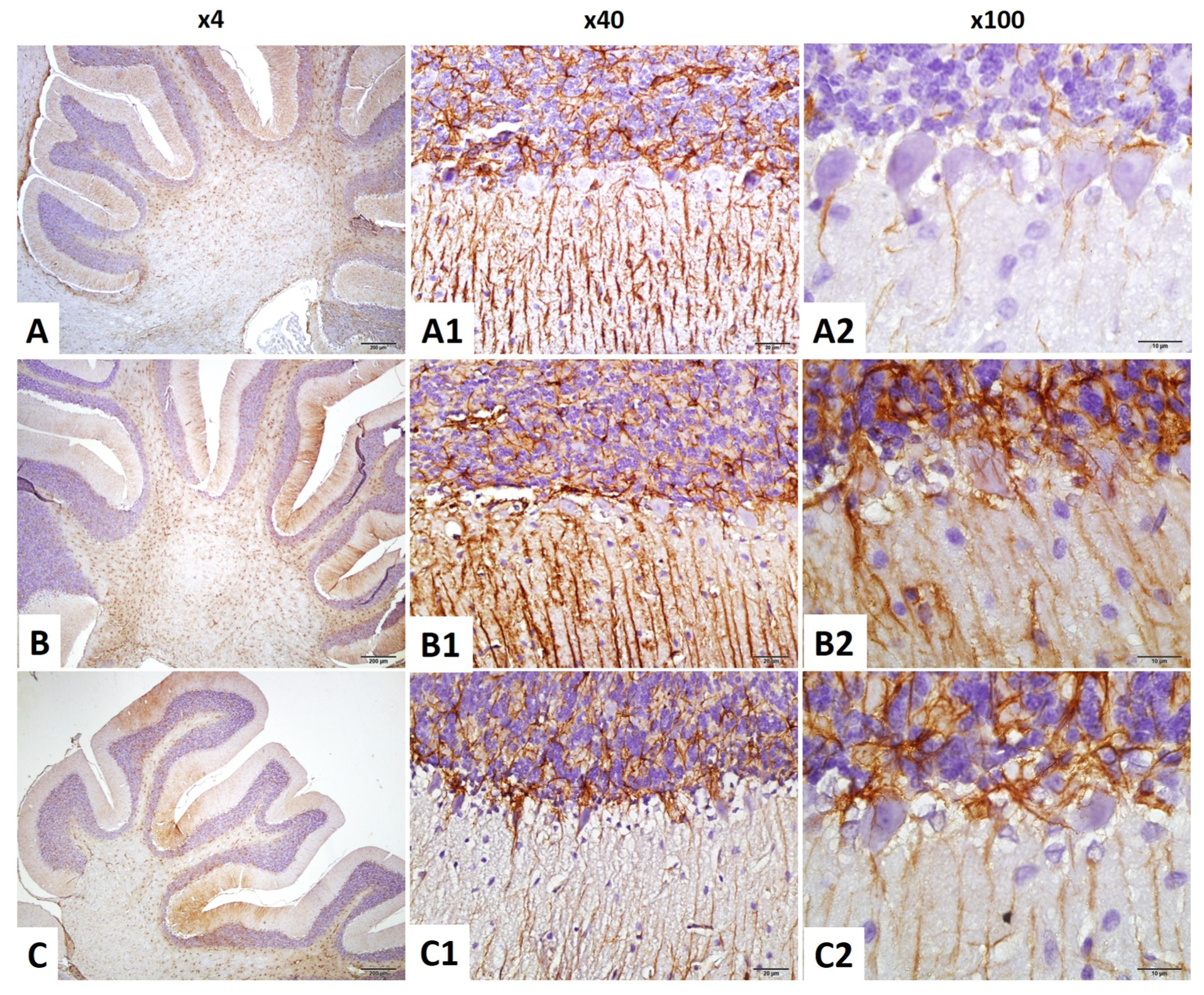
2.4. Histological and Immunohistochemical Findings
2.4.1. Neuronal Count in CA1 Region
2.4.2. Neuronal Count in CA3 Region
2.4.3. CA1–GFAP Immunostaining Index
2.4.4. CA3–GFAP Immunostaining Index
2.4.5. Purkinje Cell Count in the Cerebellum
2.4.6. GFAP Immunostaining Index in the Cerebellum
3. Discussion
4. Materials and Methods
4.1. Animals
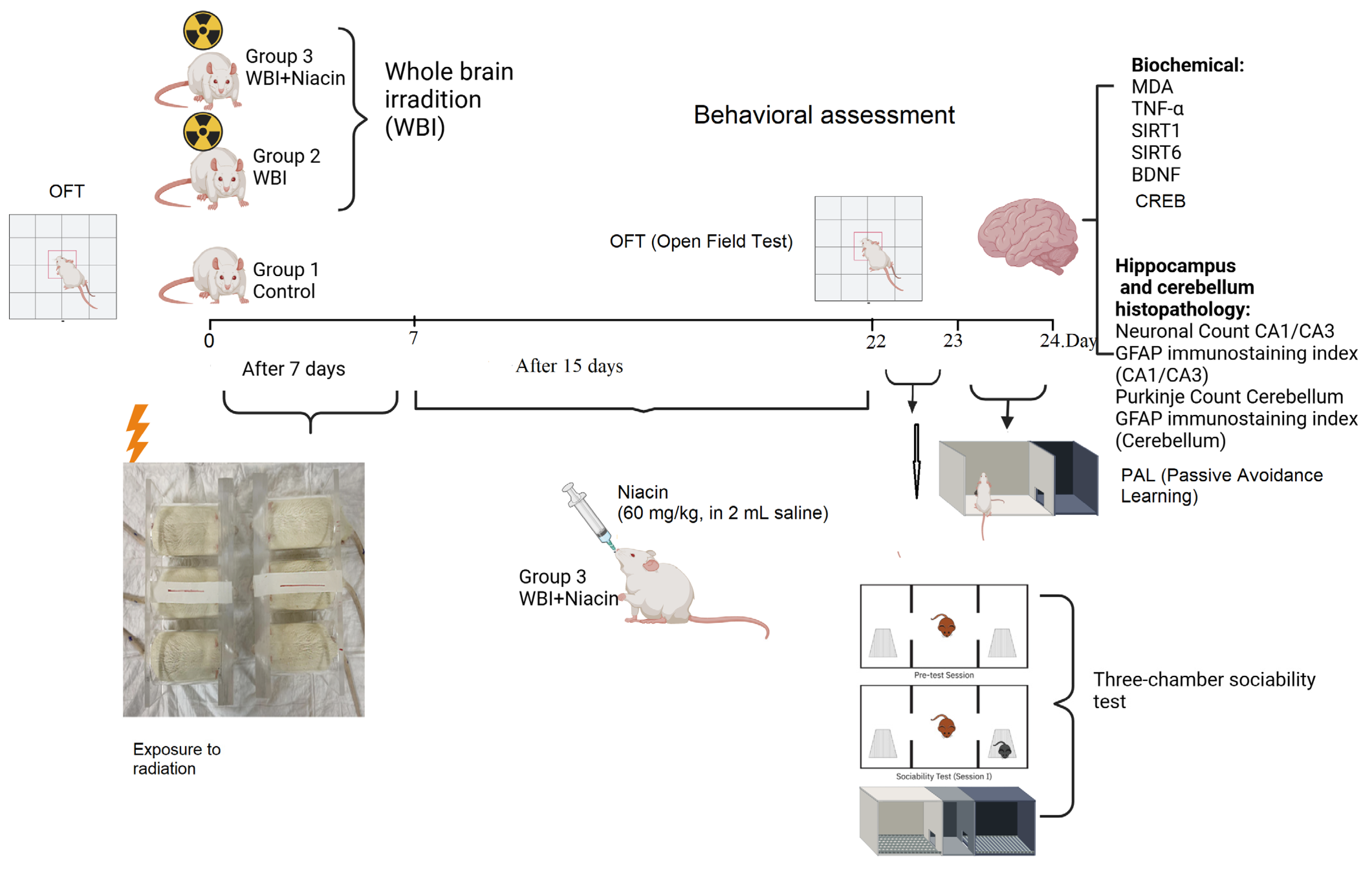
4.2. Experimental Procedures
- Group 1 (Control): Rats that were not exposed to whole-brain irradiation and were maintained under standard laboratory conditions. These animals received 2 mL of saline via oral gavage once daily for 15 consecutive days.
- Group 2 (WBI +Saline): Rats that underwent whole-brain irradiation and subsequently received 2 mL of saline via oral gavage once daily for 15 consecutive days, beginning on Day 7 post-irradiation.
- Group 3 (WBI +Niacin): Rats exposed to whole-brain irradiation and treated with niacin (60 mg/kg/day, dissolved in 2 mL sterile 0.9% NaCl) via oral gavage for 15 consecutive days, starting on Day 7 post-irradiation.
4.3. Irradiation Procedure
4.4. Behavioral Tests
4.4.1. Three-Chamber Sociability Test
4.4.2. Open-Field Test (OFT)
4.5. Passive Avoidance Learning (PAL)
4.6. Hippocampus and Cerebellum Histopathology
Neuronal Counting Procedure in Hippocampus
4.7. Brain Biochemical Analysis
4.8. Measurement of Brain Lipid Peroxidation (MDA)
4.9. Statistical Analysis
5. Conclusions
Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gzell, C.; Back, M.; Wheeler, H.; Bailey, D.; Foote, M. Radiotherapy in Glioblastoma: The Past, the Present and the Future. Clin. Oncol. 2017, 29, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Greene-Schloesser, D.; Robbins, M.E.; Peiffer, A.M.; Shaw, E.G.; Wheeler, K.T.; Chan, M.D. Radiation-induced brain injury: A review. Front. Oncol. 2012, 2, 73. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kim, J.S.; Moon, C.; Son, Y. Profiling of gene expression in the brain associated with anxiety-related behaviors in the chronic phase following cranial irradiation. Sci. Rep. 2022, 12, 13162. [Google Scholar] [CrossRef] [PubMed]
- Crossen, J.R.; Garwood, D.; Glatstein, E.; Neuwelt, E.A. Neurobehavioral sequelae of cranial irradiation in adults: A review of radiation-induced encephalopathy. J. Clin. Oncol. 1994, 12, 627–642. [Google Scholar] [CrossRef]
- Meyers, C.A.; Brown, P.D. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J. Clin. Oncol. 2006, 24, 1305–1309. [Google Scholar] [CrossRef]
- Janda, M.; Steginga, S.; Langbecker, D.; Dunn, J.; Walker, D.; Eakin, E. Quality of life among patients with a brain tumor and their carers. J. Psychosom. Res. 2007, 63, 617–623. [Google Scholar] [CrossRef]
- Krukowski, K.; Grue, K.; Frias, E.S.; Pietrykowski, J.; Jones, T.; Nelson, G.; Rosi, S. Female mice are protected from space radiation-induced maladaptive responses. Brain Behav. Immun. 2018, 74, 106–120. [Google Scholar] [CrossRef]
- Zaleska, M.M.; Floyd, R.A. Regional lipid-peroxidation in rat-brain in vitro- possible role of endogenous iron. Neurochem. Res. 1985, 10, 397–410. [Google Scholar] [CrossRef]
- Gan, L.; Wang, Z.H.; Zhang, H.; Zhou, R.; Sun, C.; Liu, Y.; Si, J.; Liu, Y.Y.; Wang, Z.G. Protective effects of shikonin on brain injury induced by carbon ion beam irradiation in mice. Biomed. Environ. Sci. 2015, 28, 148–151. [Google Scholar]
- Nuszkiewicz, J.; Woźniak, A.; Szewczyk-Golec, K. Ionizing radiation as a source of oxidative stress—The protective role of melatonin and vitamin D. Int. J. Mol. Sci. 2020, 21, 5804. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Samad, N.; Hameed, A.; Manzoor, N.; Shoukat, S.; Irfan, A.; Shazly, G.A.; Khalid, A.; Ejaz, U.; Khaliq, S.; Mateev, E.; et al. Antioxidant and neuro-modulatory effects of niacin prevent D-galactose-induced behavioral deficits and memory impairment. Exp. Gerontol. 2024, 198, 112624. [Google Scholar] [CrossRef] [PubMed]
- Raber, J.; Rola, R.; LeFevour, A.; Morhardt, D.; Curley, J.; Mizumatsu, S.; VandenBerg, S.R.; Fike, J.R. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat. Res. 2004, 162, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Pascoe, M.; Byrne, E.; King, A.; Cooper, D.; Foldvary-Schaefer, N.; Mehra, R.; Armstrong, T.S. Sleep disorders associated with cranial radiation—A systematic review. Neuro-Oncology 2025, 27, 63–76. [Google Scholar] [CrossRef]
- Gürkan, G.; Atasoy, Ö.; Çini, N.; Sever, I.H.; Özkul, B.; Yaprak, G.; Şirin, C.; Uyanıkgil, Y.; Kızmazoğlu, C.; Erdoğan, M.A.; et al. Reparative, Neuroprotective and Anti-neurodegenerative Effects of Granulocyte Colony Stimulating Factor in Radiation-Induced Brain Injury Model. J. Korean Neurosurg. Soc. 2023, 66, 511–524. [Google Scholar] [CrossRef]
- Abdelhamid, A.H.; Mantawy, E.M.; Said, R.S.; El-Demerdash, E. Neuroprotective effects of saxagliptin against radiation-induced cognitive impairment: Insights on Akt/CREB/SIRT1/BDNF signaling pathway. Toxicol. Appl. Pharmacol. 2024, 489, 116994. [Google Scholar] [CrossRef]
- Ji, J.F.; Ji, S.J.; Sun, R.; Li, K.; Zhang, Y.; Zhang, L.Y.; Tian, Y. Forced running exercise attenuates hippocampal neurogenesis impairment and the neurocognitive deficits induced by whole-brain irradiation via the BDNF-mediated pathway. Biochem. Biophys. Res. Commun. 2014, 443, 646–651. [Google Scholar] [CrossRef]
- Tome, W.A.; Gokhan, S.; Gulinello, M.E.; Patrik Brodin, N.; Heard, J.; Mehler, M.F.; Guha, C. Hippocampal-dependent neurocognitive impairment following cranial irradiation observed in pre-clinical models: Current knowledge and possible future directions. Br. J. Radiol. 2016, 89, 20150762. [Google Scholar] [CrossRef]
- Kesner Raymond, P. Behavioral functions of the CA3 subregion of the hippocampus. Learn. Mem. 2007, 14, 771–781. [Google Scholar] [CrossRef]
- Shi, L.; Molina, D.P.; Robbins, M.E.; Wheeler, K.T.; Brunso-Bechtold, J.K. Hippocampal neuron number is unchanged 1 year after fractionated whole-brain irradiation at middle age. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 526–532. [Google Scholar] [CrossRef]
- Cui, L.; Pierce, D.; Light, K.E.; Melchert, R.B.; Fu, Q.; Sree Kumar, K.; Hauer-Jensen, M. Sublethal total body irradiation leads to early cerebellar damage and oxidative stress. Curr. Neurovasc. Res. 2010, 7, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Yang, L.; Liu, Y.Y.; Jiang, L.; Huang, J.F. Association between dietary niacin intake and cognitive function in the elderly: Evidence from NHANES 2011–2014. Food Sci. Nutr. 2023, 11, 4651–4664. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Wen, P.; Fu, Y.; Gao, Y.; Qi, X.; Chen, B.; Tao, Y.; Wu, L.; Xu, A.; Lu, H.; et al. Radiation induces apoptosis primarily through the intrinsic pathway in mammalian cells. Cell. Signal. 2019, 62, 109337. [Google Scholar] [CrossRef] [PubMed]
- Ramanan, S.; Zhao, W.; Riddle, D.R.; Robbins, M.E. Role of PPARs in Radiation-Induced Brain Injury. PPAR Res. 2010, 2010, 234975. [Google Scholar] [CrossRef]
- Alkis, M.E.; Bilgin, H.M.; Akpolat, V.; Dasdag, S.; Yegin, K.; Yavas, M.C.; Akdag, M.Z. Effect of 900-, 1800-, and 2100-MHz radiofrequency radiation on DNA and oxidative stress in brain. Electromagn. Biol. Med. 2019, 38, 32–47. [Google Scholar] [CrossRef]
- Sisakht, M.; Darabian, M.; Mahmoodzadeh, A.; Bazi, A.; Shafiee, S.M.; Mokarram, P.; Khoshdel, Z. The role of radiation-induced oxidative stress as a regulator of radio-adaptive responses. Int. J. Radiat. Biol. 2020, 96, 561–576. [Google Scholar] [CrossRef]
- Beattie, E.C.; Stellwagen, D.; Morishita, W.; Bresnahan, J.C.; Ha, B.K.; Von Zastrow, M.; Beattie, M.S.; Malenka, R.C. Control of synaptic strength by glial TNFα. Science 2002, 295, 2282–2285. [Google Scholar] [CrossRef]
- Chiang, C.S.; McBride, W.H.; Withers, H.R. Radiation-induced astrocytic and microglial responses in mouse brain. Radiother. Oncol. 1993, 29, 60–68. [Google Scholar] [CrossRef]
- Kol, A.; Adamsky, A.; Groysman, M.; Kreisel, T.; London, M.; Goshen, I. Astrocytes contribute to remote memory formation by modulating hippocampal–cortical communication during learning. Nat. Neurosci. 2020, 23, 1229–1239. [Google Scholar] [CrossRef]
- Pang, P.T.; Lu, B. Regulation of late-phase LTP and long-term memory in normal and aging hippocampus: Role of secreted proteins tPA and BDNF. Ageing Res. Rev. 2004, 3, 407–430. [Google Scholar] [CrossRef]
- Ou, L.C.; Gean, P.W. Transcriptional regulation of brain-derived neurotrophic factor in the amygdala during consolidation of fear memory. Mol. Pharmacol. 2007, 72, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.; Yang, M.; Kang, S.; Lee, S.; Kim, J.; Kim, J.; Park, S.; Kim, J.-S.; Jo, S.-K.; Jung, U.; et al. Cranial irradiation regulates CREB-BDNF signaling and variant BDNF transcript levels in the mouse hippocampus. Neurobiol. Learn. Mem. 2015, 121, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Rada, P.; Pardo, V.; Mobasher, M.A.; García-Martínez, I.; Ruiz, L.; González-Rodríguez, Á.; Sanchez-Ramos, C.; Muntané, J.; Alemany, S.; James, L.P.; et al. Sirt1 Controls Acetaminophen Hepatotoxicity by Modulating Inflammation and Oxidative Stress. Antioxid. Redox Signal. 2018, 28, 1187–1208. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Li, Q.; Sheng, B.; Yang, H.; Jiang, W. Sirt1 Inhibits Apoptosis by Promoting Autophagic Flux in Human Nucleus Pulposus Cells in the Key Stage of Degeneration Via Erk Signal Pathway. BioMed Res. Int. 2021, 2021, 8818713. [Google Scholar] [CrossRef]
- Liang, D.; Zhuo, Y.; Guo, Z.; He, L.; Wang, X.; He, Y.; Li, L.; Dai, H. Sirt1/Pgc-1 Pathway Activation Triggers Autophagy/Mitophagy and Attenuates Oxidative Damage in Intestinal Epithelial Cells. Biochimie 2020, 170, 10–20. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Wang, Y.; Chao, Y.; Zhang, J.; Jia, Y.; Tie, J.; Hu, D. Regulation of SIRT1 and its roles in inflammation. Front. Immunol. 2022, 13, 831168. [Google Scholar] [CrossRef]
- Yoshizaki, T.; Schenk, S.; Imamura, T.; Babendure, J.L.; Sonoda, N.; Bae, E.J.; Oh, D.Y.; Lu, M.; Milne, J.C.; Westphal, C.; et al. Sirt1 Inhibits Inflammatory Pathways in Macrophages and Modulates Insulin Sensitivity. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E419–E428. [Google Scholar] [CrossRef]
- Liao, H.; Huang, J.; Liu, J.; Zhu, H.; Chen, Y.; Li, X.; Wen, J.; Yang, Q. Sirt1 regulates microglial activation and inflammation following oxygen-glucose deprivation/reoxygenation injury by targeting the Shh/Gli-1 signaling pathway. Mol. Biol. Rep. 2023, 50, 3317–3327. [Google Scholar] [CrossRef]
- Orecchia, A.; Scarponi, C.; Di Felice, F.; Cesarini, E.; Avitabile, S.; Mai, A.; Mauro, M.L.; Sirri, V.; Zambruno, G.; Albanesi, C.; et al. Sirtinol treatment reduces inflammation in human dermal microvascular endothelial cells. PLoS ONE 2011, 6, e24307. [Google Scholar] [CrossRef]
- Chen, G.D.; Yu, W.D.; Chen, X.P. Sirt1 Activator Represses the Transcription of Tnf−A in Thp−1 Cells of a Sepsis Model Via Deacetylation of H4k16. Mol. Med. Rep. 2016, 14, 5544–5550. [Google Scholar] [CrossRef]
- Kawahara, T.L.; Michishita, E.; Adler, A.S.; Damian, M.; Berber, E.; Lin, M.; McCord, R.A.; Ongaigui, K.C.; Boxer, L.D.; Chang, H.Y.; et al. SIRT6 links histone H3 lysine nine deacetylation to NF-κB-dependent gene expression and organismal life span. Cell 2009, 136, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.; Huang, W.; Zhao, Y.; Fang, X.; Zhang, Y.; Gao, F.; Huang, D.; Wang, B.; Shi, G. Verapamil alleviates myocardial ischemia/reperfusion injury by attenuating oxidative stress via activation of SIRT1. Front. Pharmacol. 2022, 13, 822640. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, K.; Ma, Z.; Liu, D.; Yang, Y.; Sun, M.; Wen, A.; Hao, Y.; Ma, S.; Ren, F.; et al. SIRT1 activation by butein attenuates sepsis-induced brain injury in mice subjected to cecal ligation and puncture via alleviating inflammatory and oxidative stress. Toxicol. Appl. Pharmacol. 2019, 363, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Bai, L.; Ren, Q.; Sun, G.; Si, Y. Protective effects of SIRT6 against lipopolysaccharide (LPS) are mediated by deacetylation of Ku70. Mol. Immunol. 2018, 101, 312–318. [Google Scholar] [CrossRef]
- He, R.; Liu, J.; Wang, B.; Zhang, H.; Xie, S.; Zhang, Y.; Liu, X.; Wang, J.; Wu, D.; Du, L.; et al. X-ray-based ultra-high dose rate FLASH radiotherapy mitigates acute radiation-induced hippocampal injury and inflammation. J. Neurorestoratol. 2025, 13, 100186. [Google Scholar] [CrossRef]
- Ganji, S.H.; Qin, S.; Zhang, L.; Kamanna, V.S.; Kashyap, M.L. Niacin inhibits vascular oxidative stress, redox-sensitive genes, and monocyte adhesion to human aortic endothelial cells. Atherosclerosis 2009, 202, 68–75. [Google Scholar] [CrossRef]
- Ganji, S.; Kamanna, S.; Kamanna, V.S.; Kashyap, M.L. Niacin increases human aortic endothelial Sirt1 activity and nitric oxide: Effect on endothelial function and vascular aging. Am. J. Transl. Res. 2023, 15, 6771. [Google Scholar]
- Jeong, H.; E Cohen, D.; Cui, L.; Supinski, A.; Savas, J.N.; Mazzulli, J.R.; Yates, J.R.; Bordone, L.; Guarente, L.; Krainc, D. Sirt1 mediates neuroprotection from mutant huntingtin by activation of the TORC1 and CREB transcriptional pathway. Nat. Med. 2012, 18, 159–165. [Google Scholar] [CrossRef]
- D’Angelo, S.; Mele, E.; Di Filippo, F.; Viggiano, A.; Meccariello, R. Sirt1 activity in the brain: Simultaneous effects on energy homeostasis and reproduction. Int. J. Environ. Res. Public Health 2021, 18, 1243. [Google Scholar] [CrossRef]
- Yabluchanskiy, A.; Tarantini, S.; Balasubramanian, P.; Kiss, T.; Csipo, T.; Fülöp, G.A.; Lipecz, A.; Ahire, C.; DelFavero, J.; Nyul-Toth, A.; et al. Pharmacological or genetic depletion of senescent astrocytes prevents whole brain irradiation–induced impairment of neurovascular coupling responses protecting cognitive function in mice. Geroscience 2020, 42, 409–428. [Google Scholar] [CrossRef]
- Li, B.; Yabluchanskiy, A.; Tarantini, S.; Allu, S.R.; Sencan-Egilmez, I.; Leng, J.; Alfadhel, M.A.H.; Porter, J.E.; Fu, B.; Ran, C.; et al. Measurements of cerebral microvascular blood flow, oxygenation, and morphology in a mouse model of whole-brain irradiation-induced cognitive impairment by two-photon microscopy and optical coherence tomography: Evidence for microvascular injury in the cerebral white matter. Geroscience 2023, 45, 1491–1510. [Google Scholar] [PubMed]
- Gulej, R.; Nyúl-Tóth, Á.; Ahire, C.; DelFavero, J.; Balasubramanian, P.; Kiss, T.; Tarantini, S.; Benyo, Z.; Pacher, P.; Csik, B.; et al. Elimination of senescent cells by treatment with Navitoclax/ABT263 reverses whole brain irradiation-induced blood-brain barrier disruption in the mouse brain. Geroscience 2023, 45, 2983–3002. [Google Scholar] [CrossRef] [PubMed]
- Kiss, T.; Ungvari, A.; Gulej, R.; Nyúl-Tóth, Á.; Tarantini, S.; Benyo, Z.; Csik, B.; Yabluchanskiy, A.; Mukli, P.; Csiszar, A.; et al. Whole brain irradiation–induced endothelial dysfunction in the mouse brain. Geroscience 2024, 46, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Nyúl-Tóth, Á.; Patai, R.; Csiszar, A.; Ungvari, A.; Gulej, R.; Mukli, P.; Yabluchanskiy, A.; Benyo, Z.; Sotonyi, P.; Prodan, C.I.; et al. Linking peripheral atherosclerosis to blood–brain barrier disruption: Elucidating its role as a manifestation of cerebral small vessel disease in vascular cognitive impairment. Geroscience 2024, 46, 6511–6536. [Google Scholar] [CrossRef]
- Law, M.; Wang, P.C.; Zhou, Z.Y.; Wang, Y. From microcirculation to aging-related diseases: A focus on endothelial SIRT1. Pharmaceuticals 2024, 17, 1495. [Google Scholar] [CrossRef]
- Ahire, C.; Nyul-Toth, A.; DelFavero, J.; Gulej, R.; Faakye, J.A.; Tarantini, S.; Kiss, T.; Kuan-Celarier, A.; Balasubramanian, P.; Ungvari, A.; et al. Accelerated cerebromicrovascular senescence contributes to cognitive decline in a mouse model of paclitaxel (Taxol)-induced chemobrain. Aging Cell 2023, 22, e13832. [Google Scholar] [CrossRef]
- Erbas, O.; Erdogan, M.A.; Khalilnezhad, A.; Gürkan, F.T.; Yiğittürk, G.; Meral, A.; Taskiran, D. Neurobehavioral effects of long-term maternal fructose intake in rat offspring. Int. J. Dev. Neurosci. 2018, 69, 68–79. [Google Scholar] [CrossRef]
- Senkal, E.; Eryigit, U.; Bozkurt, M.F.; Solmaz, V.; Erbas, O. Chronic Testosterone Administration During Pregnancy Causes Autistic-like Traits in Rat Offspring: Possible Relationships with Cerebral Oxytocin, Dopamine, Serotonin and IGF-1 Levels. Res. Square 2021. preprint. [Google Scholar] [CrossRef]
- Liebsch, G.; Linthorst, A.C.; Neumann, I.D.; Reul, J.M.; Holsboer, F.; Landgraf, R. Behavioral, physiological, and neuroendocrine stress responses and differential sensitivity to diazepam in two Wistar rat lines selectively bred for high-and low-anxiety–related behavior. Neuropsychopharmacology 1998, 19, 381–396. [Google Scholar] [CrossRef]
- Aygun, H. Exendin-4 increases absence-like seizures and anxiety–depression-like behaviors in WAG/Rij rats. Epilepsy Behav. 2021, 123, 108246. [Google Scholar] [CrossRef]
- Erbaş, O.; Solmaz, V.; Aksoy, D.; Yavaşoğlu, A.; Sağcan, M.; Taşkıran, D. Cholecalciferol (vitamin D 3) improves cognitive dysfunction and reduces inflammation in a rat fatty liver model of metabolic syndrome. Life Sci. 2014, 103, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, I.; Furini, C.R.; Myskiw, J.C. Fear memory. Physiol Rev. 2016, 96, 695–750. [Google Scholar] [CrossRef] [PubMed]
- Ögren, S.; Stiedl, O. Passive avoidance. In Encyclopedia of Psychopharmacology; Stolerman, I.P., Ed.; Department of Behavioural Pharmacology Institute of Psychiatry King’s College London: London, UK, 2010; Volume 2, pp. 960–967. [Google Scholar]
- Solmaz, V.; Erdoğan, M.A.; Alnak, A.; Meral, A.; Erbaş, O. Erythropoietin shows gender dependent positive effects on social deficits, learning/memory impairments, neuronal loss and neuroinflammation in the lipopolysaccharide induced rat model of autism. Neuropeptides 2020, 83, 102073. [Google Scholar] [CrossRef] [PubMed]
- Markham, J.A.; Juraska, J.M. Social recognition memory: Influence of age, sex, and ovarian hormonal status. Physiol. Behav. 2007, 92, 881–888. [Google Scholar] [CrossRef]
- Zeng, P.Y.; Tsai, Y.H.; Lee, C.L.; Ma, Y.K.; Kuo, T.H. Minimal influence of estrous cycle on studies of female mouse behaviors. Front. Mol. Neurosci. 2023, 16, 1146109. [Google Scholar] [CrossRef]
- Reilly, M.P.; Weeks, C.D.; Topper, V.Y.; Thompson, L.M.; Crews, D.; Gore, A.C. The effects of prenatal PCBs on adult social behavior in rats. Horm. Behav. 2015, 73, 47–55. [Google Scholar] [CrossRef]
- Shirenova, S.D.; Khlebnikova, N.N.; Krupina, N.A. Changes in sociability and preference for social novelty in female rats in prolonged social isolation. Neurosci. Behav. Physiol. 2023, 53, 103–118. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tunç, E.; Aygün, H.; Erdoğan, M.A.; Uyanıkgil, Y.; Erbaş, O. Niacin Modulates SIRT1-Driven Signaling to Counteract Radiation-Induced Neurocognitive and Behavioral Impairments. Int. J. Mol. Sci. 2025, 26, 5285. https://doi.org/10.3390/ijms26115285
Tunç E, Aygün H, Erdoğan MA, Uyanıkgil Y, Erbaş O. Niacin Modulates SIRT1-Driven Signaling to Counteract Radiation-Induced Neurocognitive and Behavioral Impairments. International Journal of Molecular Sciences. 2025; 26(11):5285. https://doi.org/10.3390/ijms26115285
Chicago/Turabian StyleTunç, Erdinç, Hatice Aygün, Mümin Alper Erdoğan, Yiğit Uyanıkgil, and Oytun Erbaş. 2025. "Niacin Modulates SIRT1-Driven Signaling to Counteract Radiation-Induced Neurocognitive and Behavioral Impairments" International Journal of Molecular Sciences 26, no. 11: 5285. https://doi.org/10.3390/ijms26115285
APA StyleTunç, E., Aygün, H., Erdoğan, M. A., Uyanıkgil, Y., & Erbaş, O. (2025). Niacin Modulates SIRT1-Driven Signaling to Counteract Radiation-Induced Neurocognitive and Behavioral Impairments. International Journal of Molecular Sciences, 26(11), 5285. https://doi.org/10.3390/ijms26115285






