PIK3CA Mutations: Are They a Relevant Target in Adult Diffuse Gliomas?
Abstract
1. Introduction
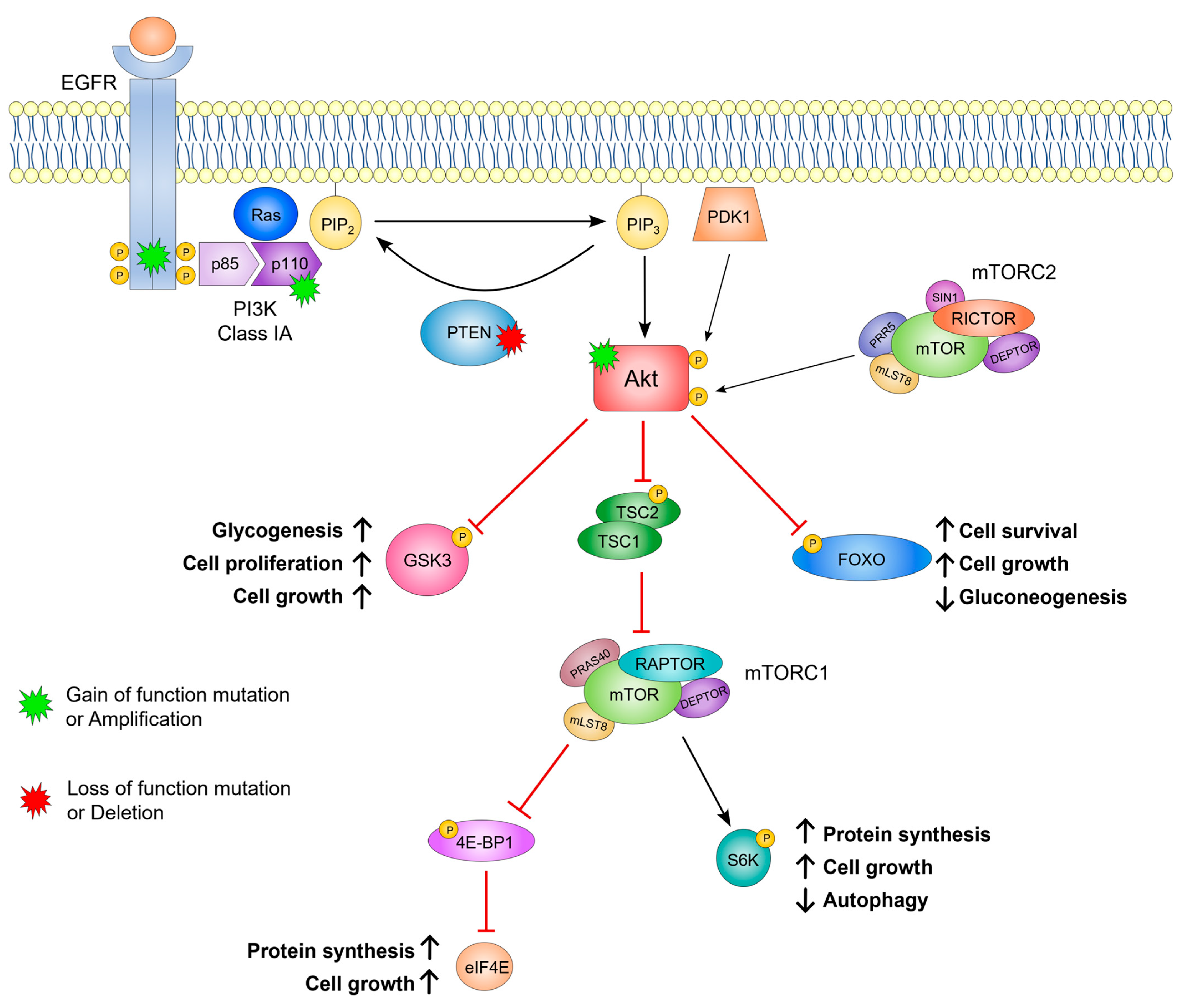
2. The Role of PI3K in Cell Signaling
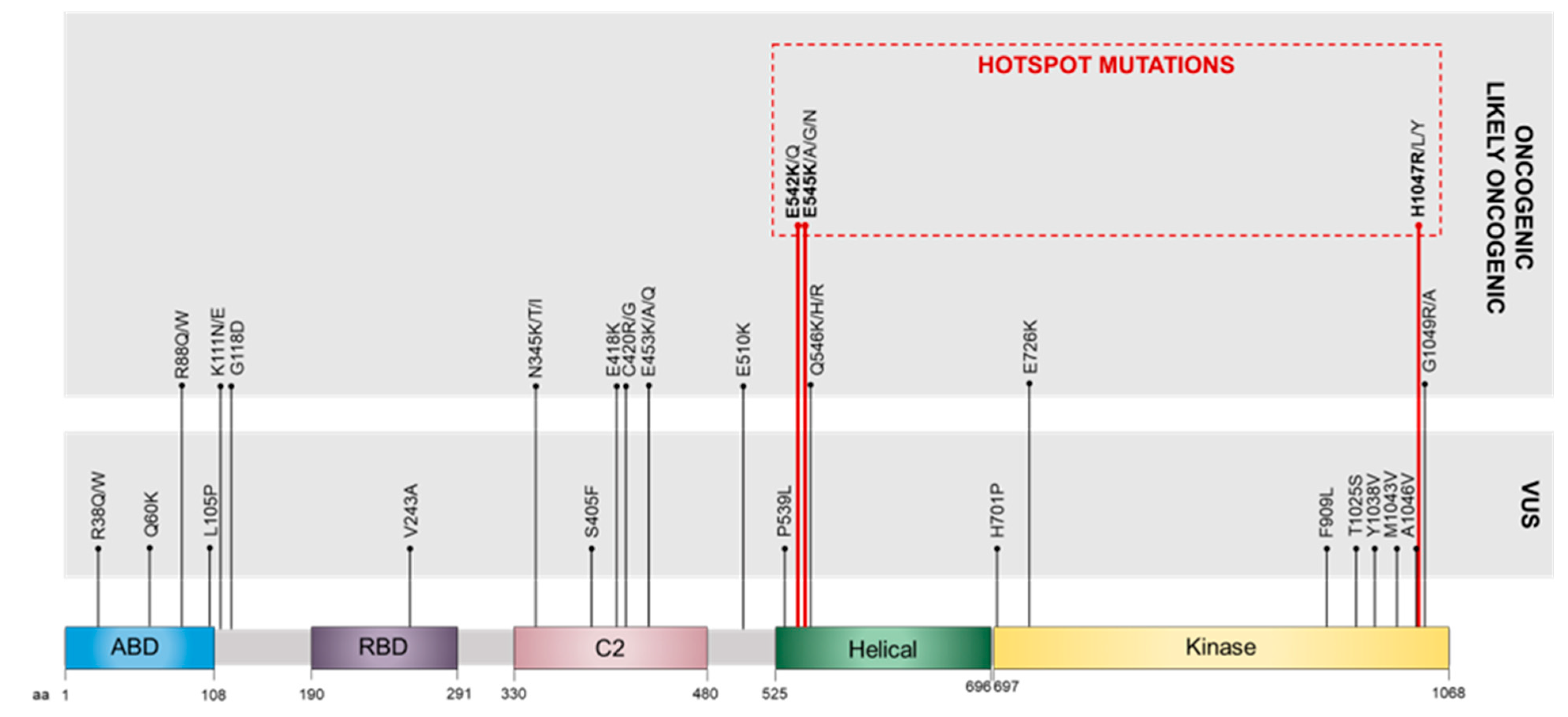
3. PIK3CA Gene Alterations
4. PIK3CA Mutations as Biomarkers in Glioma
4.1. Mutation Frequency
4.2. Clinical and Biological Impact
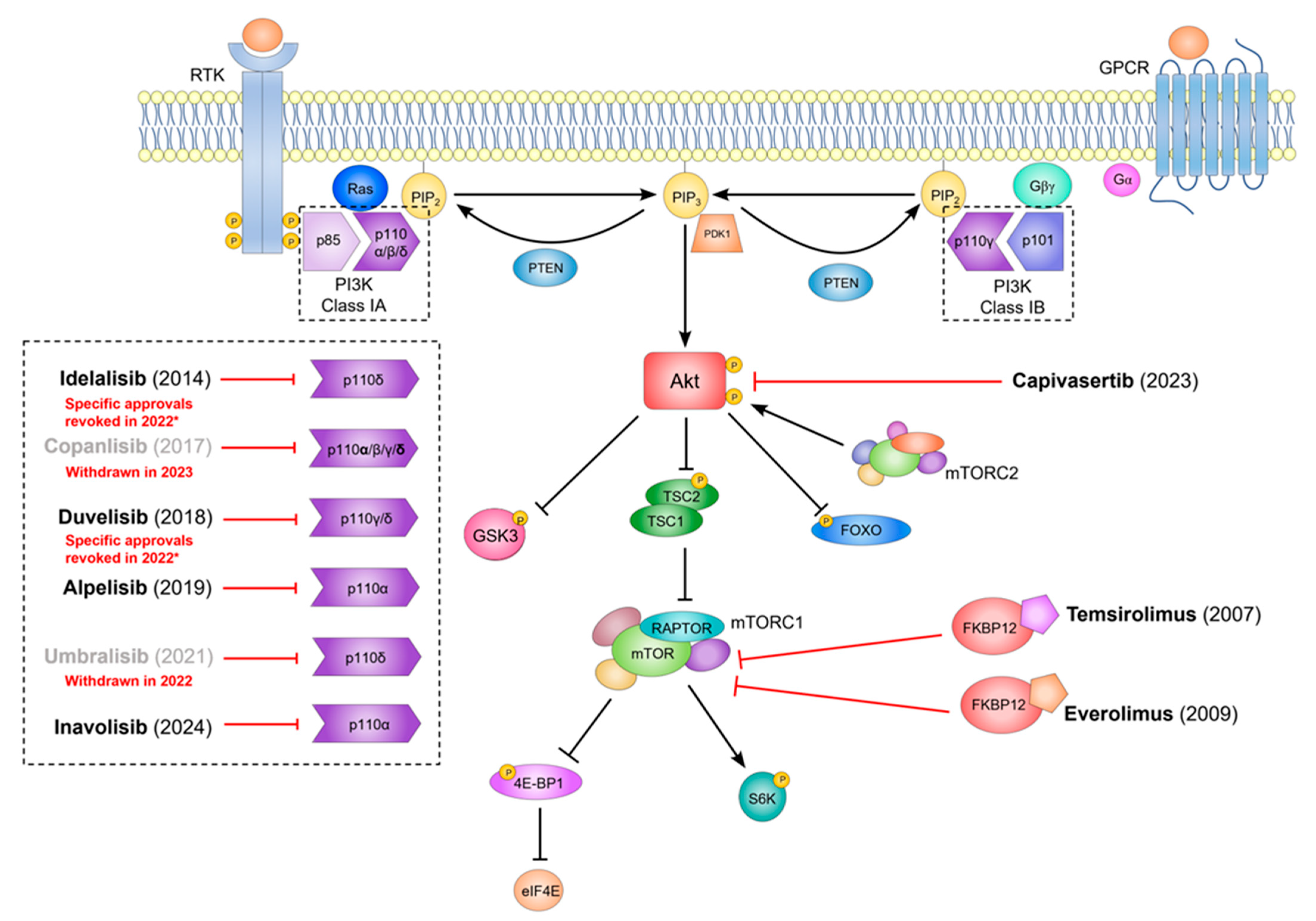
5. Could PI3Kα Inhibitors Offer a New Approach to Target Glioma?
6. Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 4E-BP1 | Eukaryotic Translation Initiation Factor 4E-Binding Protein |
| ABD | Adaptor-Binding Domain |
| Akt | Protein Kinase B |
| ALT | Alanine Aminotransferase |
| AMPK | 5′ Adenosine Monophosphate-Activated Protein Kinase |
| AST | Aspartate Aminotransferase |
| ATRX | Alpha Thalassemia/Mental Retardation Syndrome X-linked |
| BC | Breast Cancer |
| CDKN2A/B | Cyclin-Dependent Kinase Inhibitor 2A/B |
| CLL | Chronic Lymphocytic Leukemia |
| EGFR | Epidermal Growth Factor Receptor |
| eIF4E | Eukaryotic Translation Initiation Factor 4E |
| ERK | Extracellular Signal-Regulated Kinase |
| FDA | U.S. Food and Drug Administration |
| FKBP12 | FK506 Binding Protein-12 |
| FL | Follicular Lymphoma |
| FOXO | Forkhead Box O |
| GBM | Glioblastoma Multiforme |
| GFAP | Glial Fibrillary Acidic Protein |
| GPCR | G-Protein Coupled Receptors |
| GSK3α/β | Glycogen Synthase Kinase 3 α/β |
| HER2/3 | Human Epidermal Growth Factor Receptor 2/3 |
| HR | Hormone Receptor |
| IDH | Isocitrate Dehydrogenase |
| LSSC | Lung Squamous Cell Carcinoma |
| MAPK | Mitogen-Activated Protein Kinase |
| MGMT | O-6-Methylguanine-DNA Methyltransferase |
| mTOR | Mechanistic Target Of Rapamycin |
| mTORC1/2 | Mechanistic Target Of Rapamycin Complex 1/2 |
| NGS | Next-Generation Sequencing |
| OLIG2 | Oligodendrocyte Lineage Transcription Factor 2 |
| PCR-SSCP | Polymerase Chain Reaction—Single Stranded Conformation Polymorphism |
| PDK1 | Phosphatidylinositol-Dependent Kinase 1 |
| PFS | Progression-Free Survival |
| PI | Phosphatidylinositol |
| PI3K | Phosphatidylinositol-4,5-Bisphosphate 3-Kinase |
| PI3P | Phosphatidylinositol-3-Phosphate |
| PIK3CA | Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Alpha |
| PIP2 | Phosphatidylinositol-4,5-Bisphosphate |
| PIP3 | Phosphatidylinositol-3,4,5-Trisphosphate |
| PTEN | Phosphatase and Tensin Homologue |
| RBD | Ras-Binding Domain |
| RTK | Receptor Tyrosine Kinases |
| S6K | Ribosomal Protein S6 Kinase |
| SCC | Squamous Cell Carcinoma |
| SLL | Small Lymphocytic Lymphoma |
| TCGA | The Cancer Genome Atlas |
| TERT | Telomerase Reverse Transcriptase |
| TGFβ | Transforming Growth Factor β |
| TSC1/2 | Tuberous Sclerosis Complex 1/2 |
| VUS | Variants of Uncertain Significance |
| WHO | World Health Organization |
References
- Schaff, L.R.; Mellinghoff, I.K. Glioblastoma and Other Primary Brain Malignancies in Adults: A Review. JAMA 2023, 329, 574–587. [Google Scholar] [CrossRef] [PubMed]
- Price, M.; Ballard, C.; Benedetti, J.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S.; Ostrom, Q.T. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2017–2021. Neuro Oncol. 2024, 26, vi1–vi85. [Google Scholar] [CrossRef] [PubMed]
- Llaguno, S.A.; Chen, J.; Kwon, C.-H.; Jackson, E.L.; Li, Y.; Burns, D.K.; Alvarez-Buylla, A.; Parada, L.F. Malignant Astrocytomas Originate from Neural Stem/Progenitor Cells in a Somatic Tumor Suppressor Mouse Model. Cancer Cell 2009, 15, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Radke, J.; Bortolussi, G.; Pagenstecher, A. Akt and C-Myc Induce Stem-Cell Markers in Mature Primary P53−/− Astrocytes and Render These Cells Gliomagenic in the Brain of Immunocompetent Mice. PLoS ONE 2013, 8, e56691. [Google Scholar] [CrossRef]
- Lindberg, N.; Kastemar, M.; Olofsson, T.; Smits, A.; Uhrbom, L. Oligodendrocyte Progenitor Cells Can Act as Cell of Origin for Experimental Glioma. Oncogene 2009, 28, 2266–2275. [Google Scholar] [CrossRef]
- Velásquez, C.; Mansouri, S.; Mora, C.; Nassiri, F.; Suppiah, S.; Martino, J.; Zadeh, G.; Fernández-Luna, J.L. Molecular and Clinical Insights into the Invasive Capacity of Glioblastoma Cells. J. Oncol. 2019, 2019, 1740763. [Google Scholar] [CrossRef]
- Rouse, C.; Gittleman, H.; Ostrom, Q.T.; Kruchko, C.; Barnholtz-Sloan, J.S. Years of Potential Life Lost for Brain and CNS Tumors Relative to Other Cancers in Adults in the United States, 2010. Neuro Oncol. 2016, 18, 70–77. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Kalokhe, G.; Grimm, S.A.; Chandler, J.P.; Helenowski, I.; Rademaker, A.; Raizer, J.J. Metastatic Glioblastoma: Case Presentations and a Review of the Literature. J. Neuro-Oncol. 2012, 107, 21–27. [Google Scholar] [CrossRef]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma Stem Cells Promote Radioresistance by Preferential Activation of the DNA Damage Response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef]
- Hombach-Klonisch, S.; Mehrpour, M.; Shojaei, S.; Harlos, C.; Pitz, M.; Hamai, A.; Siemianowicz, K.; Likus, W.; Wiechec, E.; Toyota, B.D.; et al. Glioblastoma and Chemoresistance to Alkylating Agents: Involvement of Apoptosis, Autophagy, and Unfolded Protein Response. Pharmacol. Ther. 2018, 184, 13–41. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 WHO Classification of Tumours of the Central Nervous System. Acta Neuropathol. 2007, 114, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella, D.; Webster, B.; Hiroko, K.C.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A Summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Brat, D.J.; Prayson, R.A.; Ryken, T.C.; Olson, J.J. Diagnosis of Malignant Glioma: Role of Neuropathology. J. Neurooncol 2008, 89, 287–311. [Google Scholar] [CrossRef]
- Reifenberger, G.; Wirsching, H.-G.; Knobbe-Thomsen, C.B.; Weller, M. Advances in the Molecular Genetics of Gliomas—Implications for Classification and Therapy. Nat. Rev. Clin. Oncol. 2017, 14, 434–452. [Google Scholar] [CrossRef]
- Brito, C.; Azevedo, A.; Esteves, S.; Marques, A.R.; Martins, C.; Costa, I.; Mafra, M.; Marques, J.M.B.; Roque, L.; Pojo, M. Clinical Insights Gained by Refining the 2016 WHO Classification of Diffuse Gliomas with: EGFR Amplification, TERT Mutations, PTEN Deletion and MGMT Methylation. BMC Cancer 2019, 19, 968. [Google Scholar] [CrossRef]
- Ceccarelli, M.; Barthel, F.P.; Malta, T.M.; Sabedot, T.S.; Salama, S.R.; Murray, B.A.; Morozova, O.; Newton, Y.; Radenbaugh, A.; Pagnotta, S.M.; et al. Molecular Profiling Reveals Biologically Discrete Subsets and Pathways of Progression in Diffuse Glioma. Cell 2016, 164, 550–563. [Google Scholar] [CrossRef]
- Brennan, C.W.; Verhaak, R.G.W.; McKenna, A.; Campos, B.; Noushmehr, H.; Salama, S.R.; Zheng, S.; Chakravarty, D.; Sanborn, J.Z.; Berman, S.H.; et al. The Somatic Genomic Landscape of Glioblastoma. Cell 2013, 155, 462–477. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Beiko, J.; Suki, D.; Hess, K.R.; Fox, B.D.; Cheung, V.; Cabral, M.; Shonka, N.; Gilbert, M.R.; Sawaya, R.; Prabhu, S.S.; et al. IDH1 Mutant Malignant Astrocytomas Are More Amenable to Surgical Resection and Have a Survival Benefit Associated with Maximal Surgical Resection. Neuro Oncol. 2014, 16, 81–91. [Google Scholar] [CrossRef]
- Cairncross, J.G.; Wang, M.; Jenkins, R.B.; Shaw, E.G.; Giannini, C.; Brachman, D.G.; Buckner, J.C.; Fink, K.L.; Souhami, L.; Laperriere, N.J.; et al. Benefit From Procarbazine, Lomustine and Vincristine in Oligodendroglial Tumors Is Associated With Mutation of IDH. J. Clinl Oncol. 2014, 32, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO Guidelines on the Diagnosis and Treatment of Diffuse Gliomas of Adulthood. Nat. Rev. Clin. Oncol. 2021, 18, 170–186. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, R.B.; Blair, H.; Ballman, K.V.; Giannini, C.; Arusell, R.M.; Law, M.; Flynn, H.; Passe, S.; Felten, S.; Brown, P.D.; et al. A t(1;19)(Q10;P10) Mediates the Combined Deletions of 1p and 19q and Predicts a Better Prognosis of Patients with Oligodendroglioma. Cancer Res. 2006, 66, 9852–9861. [Google Scholar] [CrossRef]
- Weller, M.; Weber, R.G.; Willscher, E.; Riehmer, V.; Hentschel, B.; Kreuz, M.; Felsberg, J.; Beyer, U.; Loffler-Wirth, H.; Kaulich, K.; et al. Molecular Classification of Diffuse Cerebral WHO Grade II/III Gliomas Using Genome- and Transcriptome-Wide Profiling Improves Stratification of Prognostically Distinct Patient Groups. Acta Neuropathol. 2015, 129, 679–693. [Google Scholar] [CrossRef] [PubMed]
- Eckel-Passow, J.E.; Lachance, D.H.; Molinaro, A.M.; Walsh, K.M.; Decker, P.A.; Sicotte, H.; Pekmezci, M.; Rice, T.; Kosel, M.L.; Smirnov, I.V.; et al. Glioma Groups Based on 1p/19q, IDH, and TERT Promoter Mutations in Tumors. N. Engl. J. Med. 2015, 372, 2499–2508. [Google Scholar] [CrossRef]
- Lee, Y.; Koh, J.; Kim, S.-I.; Won, J.K.; Park, C.-K.; Choi, S.H.; Park, S.-H. The Frequency and Prognostic Effect of TERT Promoter Mutation in Diffuse Gliomas. Acta Neuropathol. Commun. 2017, 5, 62. [Google Scholar] [CrossRef]
- Lee, J.K.; Wang, J.; Sa, J.K.; Ladewig, E.; Lee, H.-O.; Lee, I.-H.; Kang, H.J.; Rosenbloom, D.S.; Camara, P.G.; Liu, Z.; et al. Spatiotemporal Genomic Architecture Informs Precision Oncology in Glioblastoma. Nat. Genet. 2017, 49, 594–599. [Google Scholar] [CrossRef]
- Tanaka, S.; Batchelor, T.T.; Iafrate, A.J.; Dias-Santagata, D.; Borger, D.R.; Ellisen, L.W.; Yang, D.; Louis, D.N.; Cahill, D.P.; Chi, A.S. PIK3CA Activating Mutations Are Associated with More Disseminated Disease at Presentation and Earlier Recurrence in Glioblastoma. Acta Neuropathol. Commun. 2019, 7, 66. [Google Scholar] [CrossRef]
- Brito, C.; Tomás, A.; Azevedo, A.; Esteves, S.; Mafra, M.; Roque, L.; Pojo, M. PIK3CA Mutations in Diffuse Gliomas: An Update on Molecular Stratification, Prognosis, Recurrence, and Aggressiveness. Clin. Med. Insights Oncol. 2022, 16, 1–10. [Google Scholar] [CrossRef]
- Lai, K.; Killingsworth, M.C.; Lee, C.S. Gene of the Month: PIK3CA. J. Clin. Pathol. 2015, 68, 253–257. [Google Scholar] [CrossRef]
- Engelman, J.A.; Luo, J.; Cantley, L.C. The Evolution of Phosphatidylinositol 3-Kinases as Regulators of Growth and Metabolism. Nat. Rev. Genet. 2006, 7, 606–619. [Google Scholar] [CrossRef]
- Gaidarov, I.; Smith, M.E.K.; Domin, J.; Keen, J.H. The Class II Phosphoinositide 3-Kinase C2α Is Activated by Clathrin and Regulates Clathrin-Mediated Membrane Trafficking. Mol. Cell 2001, 7, 443–449. [Google Scholar] [CrossRef]
- Yoshioka, K.; Yoshida, K.; Cui, H.; Wakayama, T.; Takuwa, N.; Okamoto, Y.; Du, W.; Qi, X.; Asanuma, K.; Sugihara, K.; et al. Endothelial PI3K-C2α, a Class II PI3K, Has an Essential Role in Angiogenesis and Vascular Barrier Function. Nat. Med. 2012, 18, 1560–1569. [Google Scholar] [CrossRef] [PubMed]
- Wurmser, A.E.; Emr, S.D. Novel PtdIns(3)P-Binding Protein Etf1 Functions as an Effector of the Vps34 PtdIns 3-Kinase in Autophagy. J. Cell Biol. 2002, 158, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Nobukuni, T.; Joaquin, M.; Roccio, M.; Dann, S.G.; Kim, S.Y.; Gulati, P.; Byfield, M.P.; Backer, J.M.; Natt, F.; Bos, J.L.; et al. Amino Acids Mediate MTOR/Raptor Signaling through Activation of Class 3 Phosphatidylinositol 3OH-Kinase. Proc. Natl. Acad. Sci. USA 2005, 102, 14238–14243. [Google Scholar] [CrossRef]
- Stoyanov, B.; Volinia, S.; Hanck, T.; Rubio, I.; Loubtchenkov, M.; Malek, D.; Stoyanova, S.; Vanhaesebroeck, B.; Dhand, R.; Nürnberg, B.; et al. Cloning and Characterization of a G Protein-Activated Human Phosphoinositide-3 Kinase. Science 1995, 269, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Stephens, L.R.; Eguinoa, A.; Erdjument-Bromage, H.; Lui, M.; Cooke, F.; Coadwell, J.; Smrcka, A.S.; Thelen, M.; Cadwallader, K.; Tempst, P.; et al. The G Beta Gamma Sensitivity of a PI3K Is Dependent upon a Tightly Associated Adaptor, P101. Cell 1997, 89, 105–114. [Google Scholar] [CrossRef]
- Willis, O.; Choucair, K.; Alloghbi, A.; Stanbery, L.; Mowat, R.; Brunicardi, F.C.; Dworkin, L.; Nemunaitis, J. PIK3CA Gene Aberrancy and Role in Targeted Therapy of Solid Malignancies. Cancer Gene Ther. 2020, 27, 634–644. [Google Scholar] [CrossRef]
- Okkenhaug, K.; Bilancio, A.; Priddle, H.; Sancho, S.; Peskett, E.; Pearce, W.; Meek, S.E.; Salpekar, A.; Waterfield, M.D.; Smith, A.J.H.; et al. Impaired B and T Cell Antigen Receptor Signaling in P110delta PI 3-Kinase Mutant Mice. Science 2002, 297, 1031–1034. [Google Scholar] [CrossRef]
- Burke, J.E. Structural Basis for Regulation of Phosphoinositide Kinases and Their Involvement in Human Disease. Mol. Cell 2018, 71, 653–673. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, Y.; McIlroy, J.; Rordorf-Nikolic, T.; Orr, G.A.; Backer, J.M. Regulation of the P85/P110 Phosphatidylinositol 3′-Kinase: Stabilization and Inhibition of the P110α Catalytic Subunit by the P85 Regulatory Subunit. Mol. Cell Biol. 1998, 18, 1379–1387. [Google Scholar] [CrossRef] [PubMed]
- Klippel, A.; Kavanaugh, W.M.; Pot, D.; Williams, L.T. A Specific Product of Phosphatidylinositol 3-Kinase Directly Activates the Protein Kinase Akt through Its Pleckstrin Homology Domain. Mol. Cell Biol. 1997, 17, 338–344. [Google Scholar] [CrossRef]
- Iida, M.; Harari, P.M.; Wheeler, D.L.; Toulany, M. Targeting AKT/PKB to Improve Treatment Outcomes for Solid Tumors. Mutat. Res. 2020, 819–820, 111690. [Google Scholar] [CrossRef]
- Alessi, D.R.; James, S.R.; Downes, C.P.; Holmes, A.B.; Gaffney, P.R.J.; Reese, C.B.; Cohen, P. Characterization of a 3-Phosphoinositide-Dependent Protein Kinase Which Phosphorylates and Activates Protein Kinase Bα. Curr. Biol. 1997, 7, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Sarbassov, D.D.; Guertin, D.A.; Ali, S.M.; Sabatini, D.M. Phosphorylation and Regulation of Akt/PKB by the Rictor-MTOR Complex. Science 2005, 307, 1098–1101. [Google Scholar] [CrossRef]
- Frame, S.; Cohen, P.; Biondi, R.M. A Common Phosphate Binding Site Explains the Unique Substrate Specificity of GSK3 and Its Inactivation by Phosphorylation. Mol. Cell 2001, 7, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Brunet, A.; Bonni, A.; Zigmond, M.J.; Lin, M.Z.; Juo, P.; Hu, L.S.; Anderson, M.J.; Arden, K.C.; Blenis, J.; Greenberg, M.E. Akt Promotes Cell Survival by Phosphorylating and Inhibiting a Forkhead Transcription Factor. Cell 1999, 96, 857–868. [Google Scholar] [CrossRef]
- Potter, C.J.; Pedraza, L.G.; Xu, T. Akt Regulates Growth by Directly Phosphorylating Tsc2. Nat. Cell Biol. 2002, 4, 658–665. [Google Scholar] [CrossRef]
- Inoki, K.; Li, Y.; Zhu, T.; Wu, J.; Guan, K.-L. TSC2 Is Phosphorylated and Inhibited by Akt and Suppresses MTOR Signalling. Nat. Cell Biol. 2002, 4, 648–657. [Google Scholar] [CrossRef]
- Maehama, T.; Dixon, J.E. The Tumor Suppressor, PTEN/MMAC1, Dephosphorylates the Lipid Second Messenger, Phosphatidylinositol 3,4,5-Trisphosphate. J. Biol. Chem. 1998, 273, 13375–13378. [Google Scholar] [CrossRef]
- Castellano, E.; Molina-Arcas, M.; Krygowska, A.A.; East, P.; Warne, P.; Nicol, A.; Downward, J. RAS Signalling through PI3-Kinase Controls Cell Migration via Modulation of Reelin Expression. Nat. Commun. 2016, 7, 11245. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, S.; Moelling, K. Phosphorylation and Regulation of Raf by Akt (Protein Kinase B). Science 1999, 286, 1741–1744. [Google Scholar] [CrossRef] [PubMed]
- Hawley, S.A.; Ross, F.A.; Gowans, G.J.; Tibarewal, P.; Leslie, N.R.; Hardie, D.G. Phosphorylation by Akt within the ST Loop of AMPK-A1 down-Regulates Its Activation in Tumour Cells. Biochem. J. 2014, 459, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Hawke, D.; Zheng, Y.; Xia, Y.; Meisenhelder, J.; Nika, H.; Mills, G.B.; Kobayashi, R.; Hunter, T.; Lu, Z. Phosphorylation of β-Catenin by AKT Promotes β-Catenin Transcriptional Activity. J. Biol. Chem. 2007, 282, 11221–11229. [Google Scholar] [CrossRef]
- Hamidi, A.; Song, J.; Thakur, N.; Itoh, S.; Marcusson, A.; Bergh, A.; Heldin, C.-H.; Landström, M. TGF-β Promotes PI3K-AKT Signaling and Prostate Cancer Cell Migration through the TRAF6-Mediated Ubiquitylation of P85α. Sci. Signal 2017, 10, eaal4186. [Google Scholar] [CrossRef]
- Millis, S.Z.; Jardim, D.L.; Albacker, L.; Ross, J.S.; Miller, V.A.; Ali, S.M.; Kurzrock, R. Phosphatidylinositol 3-Kinase Pathway Genomic Alterations in 60,991 Diverse Solid Tumors Informs Targeted Therapy Opportunities. Cancer 2019, 125, 1185–1199. [Google Scholar] [CrossRef]
- Kandoth, C.; McLellan, M.D.; Vandin, F.; Ye, K.; Niu, B.; Lu, C.; Xie, M.; Zhang, Q.; McMichael, J.F.; Wyczalkowski, M.A.; et al. Mutational Landscape and Significance across 12 Major Cancer Types. Nature 2013, 502, 333–339. [Google Scholar] [CrossRef]
- Thorpe, L.M.; Yuzugullu, H.; Zhao, J.J. PI3K in Cancer: Divergent Roles of Isoforms, Modes of Activation and Therapeutic Targeting. Nat. Rev. Cancer 2015, 15, 7–24. [Google Scholar] [CrossRef]
- Xiao, W.; Zhang, G.; Chen, B.; Chen, X.; Wen, L.; Lai, J.; Li, X.; Li, M.; Liu, H.; Liu, J.; et al. Mutational Landscape of PI3K-AKT-MTOR Pathway in Breast Cancer: Implications for Targeted Therapeutics. J. Cancer 2021, 12, 4408–4417. [Google Scholar] [CrossRef]
- Samuels, Y.; Wang, Z.; Bardelli, A.; Silliman, N.; Ptak, J.; Szabo, S.; Yan, H.; Gazdar, A.; Powell, S.M.; Riggins, G.J.; et al. High Frequency of Mutations of the PIK3CA Gene in Human Cancers. Science 2004, 304, 554. [Google Scholar] [CrossRef]
- Kang, S.; Bader, A.G.; Vogt, P.K. Phosphatidylinositol 3-Kinase Mutations Identified in Human Cancer Are Oncogenic. Proc. Natl. Acad. Sci. USA 2005, 102, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Samuels, Y.; Diaz, L.A., Jr.; Schmidt-Kittler, O.; Cummins, J.M.; DeLong, L.; Cheong, I.; Rago, C.; Huso, D.L.; Lengauer, C.; Kinzler, K.W.; et al. Mutant PIK3CA Promotes Cell Growth and Invasion of Human Cancer Cells. Cancer Cell 2005, 7, 561–573. [Google Scholar] [CrossRef]
- Bader, A.G.; Kang, S.; Vogt, P.K. Cancer-Specific Mutations in PIK3CA Are Oncogenic in Vivo. Proc. Natl. Acad. Sci. USA 2006, 103, 1475–1479. [Google Scholar] [CrossRef] [PubMed]
- Isakoff, S.J.; Engelman, J.A.; Irie, H.Y.; Luo, J.; Brachmann, S.M.; Pearline, R.V.; Cantley, L.C.; Brugge, J.S. Breast Cancer-Associated PIK3CA Mutations Are Oncogenic in Mammary Epithelial Cells. Cancer Res. 2005, 65, 10992–11000. [Google Scholar] [CrossRef]
- Zhao, L.; Vogt, P.K. Hot-Spot Mutations in P110α of Phosphatidylinositol 3-Kinase (PI3K): Differential Interactions with the Regulatory Subunit P85 and with RAS. Cell Cycle 2010, 9, 596–600. [Google Scholar] [CrossRef]
- Gkeka, P.; Evangelidis, T.; Pavlaki, M.; Lazani, V.; Christoforidis, S.; Agianian, B.; Cournia, Z. Investigating the Structure and Dynamics of the PIK3CA Wild-Type and H1047R Oncogenic Mutant. PLoS Comput. Biol. 2014, 10, e1003895. [Google Scholar] [CrossRef]
- Leontiadou, H.; Galdadas, I.; Athanasiou, C.; Cournia, Z. Insights into the Mechanism of the PIK3CA E545K Activating Mutation Using MD Simulations. Sci. Rep. 2018, 8, 15544. [Google Scholar] [CrossRef] [PubMed]
- Miled, N.; Yan, Y.; Hon, W.C.; Perisic, O.; Zvelebil, M.; Inbar, Y.; Schneidman-Duhovny, D.; Wolfson, H.J.; Backer, J.M.; Williams, R.L. Mechanism of Two Classes of Cancer Mutations in the Phosphoinositide 3-Kinase Catalytic Subunit. Science 2007, 317, 239–242. [Google Scholar] [CrossRef]
- Jenkins, M.L.; Ranga-Prasad, H.; Parson, M.A.H.; Harris, N.J.; Rathinaswamy, M.K.; Burke, J.E. Oncogenic Mutations of PIK3CA Lead to Increased Membrane Recruitment Driven by Reorientation of the ABD, P85 and C-Terminus. Nat. Commun. 2023, 14, 181. [Google Scholar] [CrossRef]
- Gymnopoulos, M.; Elsliger, M.-A.; Vogt, P.K. Rare Cancer-Specific Mutations in PIK3CA Show Gain of Function. Proc. Natl. Acad. Sci. USA 2007, 104, 5569–5574. [Google Scholar] [CrossRef]
- Gabelli, S.B.; Huang, C.-H.; Mandelker, D.; Schmidt-Kittler, O.; Vogelstein, B.; Amzel, L.M. Structural Effects of Oncogenic PI3Ka Mutations. Curr. Top. Microbiol. Immunol. 2010, 347, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Goldstein, L.D.; Durinck, S.; Chen, Y.-J.; Nguyen, T.T.; Kljavin, N.M.; Sokol, E.S.; Stawiski, E.W.; Haley, B.; Ziai, J.; et al. S100a4 Upregulation in Pik3caH1047R;Trp53R270H;MMTV-Cre-Driven Mammary Tumors Promotes Metastasis. Breast Cancer Res. 2019, 21, 152. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Network. Comprehensive Molecular Portraits of Human Breast Tumours. Nature 2012, 490, 61–70. [Google Scholar] [CrossRef]
- Mangone, F.R.; Bobrovnitchaia, I.G.; Salaorni, S.; Manuli, E.; Nagai, M.A. PIK3CA Exon 20 Mutations Are Associated with Poor Prognosis in Breast Cancer Patients. Clinics 2012, 67, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Kalinsky, K.; Jacks, L.M.; Heguy, A.; Patil, S.; Drobnjak, M.; Bhanot, U.K.; Hedvat, C.V.; Traina, T.A.; Solit, D.; Gerald, W.; et al. PIK3CA Mutation Associates with Improved Outcome in Breast Cancer. Clin. Cancer Res. 2009, 15, 5049–5059. [Google Scholar] [CrossRef]
- Li, S.Y.; Rong, M.; Grieu, F.; Iacopetta, B. PIK3CA Mutations in Breast Cancer Are Associated with Poor Outcome. Breast Cancer Res. Treat. 2006, 96, 91–95. [Google Scholar] [CrossRef]
- Loi, S.; Haibe-Kains, B.; Majjaj, S.; Lallemand, F.; Durbecq, V.; Larsimont, D.; Gonzalez-Angulo, A.M.; Pusztai, L.; Symmans, W.F.; Bardelli, A.; et al. PIK3CA Mutations Associated with Gene Signature of Low MTORC1 Signaling and Better Outcomes in Estrogen Receptor-Positive Breast Cancer. Proc. Natl. Acad. Sci. USA 2010, 107, 10208–10213. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Zhu, X.; Sun, Y.; Wang, J.; Zhong, X.; Li, J.; Hu, M.; Zheng, H. Prevalence and Prognostic Role of PIK3CA/AKT1 Mutations in Chinese Breast Cancer Patients. Cancer Res. Treat. 2019, 51, 128–140. [Google Scholar] [CrossRef]
- Stemke-Hale, K.; Gonzalez-Angulo, A.M.; Lluch, A.; Neve, R.M.; Kuo, W.-L.; Davies, M.; Carey, M.; Hu, Z.; Guan, Y.; Sahin, A.; et al. An Integrative Genomic and Proteomic Analysis of PIK3CA, PTEN, and AKT Mutations in Breast Cancer. Cancer Res. 2008, 68, 6084–6091. [Google Scholar] [CrossRef]
- Martínez-Saéz, O.; Chic, N.; Pascual, T.; Adamo, B.; Vidal, M.; González-Farré, B.; Sanfeliu, E.; Schettini, F.; Conte, B.; Brasó-Maristany, F.; et al. Frequency and Spectrum of PIK3CA Somatic Mutations in Breast Cancer. Breast Cancer Res. 2020, 22, 45. [Google Scholar] [CrossRef]
- Reinhardt, K.; Stückrath, K.; Hartung, C.; Kaufhold, S.; Uleer, C.; Hanf, V.; Lantzsch, T.; Peschel, S.; John, J.; Pöhler, M.; et al. PIK3CA-Mutations in Breast Cancer. Breast Cancer Res. Treat. 2022, 196, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; von Minckwitz, G.; Schneeweiss, A.; Paepke, S.; Lehmann, A.; Rezai, M.; Zahm, D.M.; Sinn, P.; Khandan, F.; Eidtmann, H.; et al. PIK3CA Mutations Are Associated with Lower Rates of Pathologic Complete Response to Anti-Human Epidermal Growth Factor Receptor 2 (HER2) Therapy in Primary HER2-Overexpressing Breast Cancer. J. Clin. Oncol. 2014, 32, 3212–3220. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Lee, E.; Park, K.; Park, W.-Y.; Jung, H.H.; Ahn, J.S.; Im, Y.-H.; Park, Y.H. Clinical Implications of Genomic Profiles in Metastatic Breast Cancer with a Focus on TP53 and PIK3CA, the Most Frequently Mutated Genes. Oncotarget 2017, 8, 27997–28007. [Google Scholar] [CrossRef]
- Razavi, P.; Chang, M.T.; Xu, G.; Bandlamudi, C.; Ross, D.S.; Vasan, N.; Cai, Y.; Bielski, C.M.; Donoghue, M.T.A.; Jonsson, P.; et al. The Genomic Landscape of Endocrine-Resistant Advanced Breast Cancers. Cancer Cell 2018, 34, 427–438. [Google Scholar] [CrossRef]
- Sobhani, N.; Roviello, G.; Corona, S.P.; Scaltriti, M.; Ianza, A.; Bortul, M.; Zanconati, F.; Generali, D. The Prognostic Value of PI3K Mutational Status in Breast Cancer: A Meta-Analysis. J. Cell Biochem. 2018, 119, 4287–4292. [Google Scholar] [CrossRef]
- Hart, J.R.; Zhang, Y.; Liao, L.; Ueno, L.; Du, L.; Jonkers, M.; Yates, J.R.; Vogt, P.K. The Butterfly Effect in Cancer: A Single Base Mutation Can Remodel the Cell. Proc. Natl. Acad. Sci. USA 2015, 112, 1131–1136. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.S.; Koren, S.; Leroy, C.; Brinkhaus, H.; Müller, U.; Klebba, I.; Müller, M.; Cardiff, R.D.; Bentires-Alj, M. Expression of PIK3CA Mutant E545K in the Mammary Gland Induces Heterogeneous Tumors but Is Less Potent than Mutant H1047R. Oncogenesis 2013, 2, e74. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Loibl, S.; von Minckwitz, G.; Darb-Esfahani, S.; Lederer, B.; Denkert, C. PIK3CA H1047R Mutation Associated with a Lower Pathological Complete Response Rate in Triple-Negative Breast Cancer Patients Treated with Anthracycline-Taxane–Based Neoadjuvant Chemotherapy. Cancer Res. Treat. 2020, 52, 689–696. [Google Scholar] [CrossRef]
- Mosele, F.; Stefanovska, B.; Lusque, A.; Dien, A.T.; Garberis, I.; Droin, N.; Le Tourneau, L.; Sablin, M.; Lacroix, L.; Enrico, D.; et al. Outcome and Molecular Landscape of Patients with PIK3CA-Mutated Metastatic Breast Cancer. Ann. Oncol. 2020, 31, 377–386. [Google Scholar] [CrossRef]
- Kim, J.W.; Lim, A.R.; You, J.Y.; Lee, J.H.; Song, S.E.; Lee, N.K.; Jung, S.P.; Cho, K.R.; Kim, C.Y.; Park, K.H. PIK3CA Mutation Is Associated with Poor Response to HER2-Targeted Therapy in Breast Cancer Patients. Cancer Res. Treat. 2023, 55, 531–541. [Google Scholar] [CrossRef]
- Cizkova, M.; Dujaric, M.-E.; Lehmann-Che, J.; Scott, V.; Tembo, O.; Asselain, B.; Pierga, J.-Y.; Marty, M.; De Cremoux, P.; Spyratos, F.; et al. Outcome Impact of PIK3CA Mutations in HER2-Positive Breast Cancer Patients Treated with Trastuzumab. Br. J. Cancer 2013, 108, 1807–1809. [Google Scholar] [CrossRef] [PubMed]
- Berns, K.; Horlings, H.M.; Hennessy, B.T.; Madiredjo, M.; Hijmans, E.M.; Beelen, K.; Linn, S.C.; Gonzalez-Angulo, A.M.; Stemke-Hale, K.; Hauptmann, M.; et al. A Functional Genetic Approach Identifies the PI3K Pathway as a Major Determinant of Trastuzumab Resistance in Breast Cancer. Cancer Cell 2007, 12, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Chiu, J.W.; Krzyzanowska, M.K.; Serra, S.; Knox, J.J.; Dhani, N.C.; Mackay, H.; Hedley, D.; Moore, M.; Liu, G.; Burkes, R.L.; et al. Molecular Profiling of Patients with Advanced Colorectal Cancer: Princess Margaret Cancer Centre Experience. Clin. Color. Cancer 2018, 17, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Stec, R.; Semeniuk-Wojtaś, A.; Charkiewicz, R.; Bodnar, L.; Korniluk, J.; Smoter, M.; Chyczewski, L.; Nikliński, J.; Szczylik, C. Mutation of the PIK3CA Gene as a Prognostic Factor in Patients with Colorectal Cancer. Oncol. Lett. 2015, 10, 1423–1429. [Google Scholar] [CrossRef][Green Version]
- Ogino, S.; Liao, X.; Imamura, Y.; Yamauchi, M.; McCleary, N.J.; Ng, K.; Niedzwiecki, D.; Saltz, L.B.; Mayer, R.J.; Whittom, R.; et al. Predictive and Prognostic Analysis of PIK3CA Mutation in Stage III Colon Cancer Intergroup Trial. J. Natl. Cancer Inst. 2013, 105, 1789–1798. [Google Scholar] [CrossRef]
- Day, F.L.; Jorissen, R.N.; Lipton, L.; Mouradov, D.; Sakthianandeswaren, A.; Christie, M.; Li, S.; Tsui, C.; Tie, J.; Desai, J.; et al. PIK3CA and PTEN Gene and Exon Mutation-Specific Clinicopathologic and Molecular Associations in Colorectal Cancer. Clin. Cancer Res. 2013, 19, 3285–3296. [Google Scholar] [CrossRef]
- Lee, C.S.; Song, I.H.; Lee, A.; Kang, J.; Lee, Y.S.; Lee, I.K.; Song, Y.S.; Lee, S.H. Enhancing the Landscape of Colorectal Cancer Using Targeted Deep Sequencing. Sci. Rep. 2021, 11, 8154. [Google Scholar] [CrossRef]
- Fu, X.; Lin, H.; Fan, X.; Zhu, Y.; Wang, C.; Chen, Z.; Tan, X.; Huang, J.; Cai, Y.; Huang, Y. The Spectrum, Tendency and Predictive Value of PIK3CA Mutation in Chinese Colorectal Cancer Patients. Front. Oncol. 2021, 11, 595675. [Google Scholar] [CrossRef]
- Wang, Q.; Shi, Y.; Zhou, K.; Wang, L.; Yan, Z.; Liu, Y.; Xu, L.; Zhao, S.; Chu, H.; Shi, T.; et al. PIK3CA Mutations Confer Resistance to First-Line Chemotherapy in Colorectal Cancer. Cell Death Dis. 2018, 9, 739. [Google Scholar] [CrossRef]
- Ogino, S.; Nosho, K.; Kirkner, G.J.; Shima, K.; Irahara, N.; Kure, S.; Chan, A.T.; Engelman, J.A.; Kraft, P.; Cantley, L.C.; et al. PIK3CA Mutation Is Associated with Poor Prognosis among Patients with Curatively Resected Colon Cancer. J. Clin. Oncol. 2009, 27, 1477–1484. [Google Scholar] [CrossRef]
- Sartore-Bianchi, A.; Martini, M.; Molinari, F.; Veronese, S.; Nichelatti, M.; Artale, S.; Di Nicolantonio, F.; Saletti, P.; De Dosso, S.; Mazzucchelli, L.; et al. PIK3CA Mutations in Colorectal Cancer Are Associated with Clinical Resistance to EGFR-Targeted Monoclonal Antibodies. Cancer Res. 2009, 69, 1851–1857. [Google Scholar] [CrossRef]
- Ranjbar, R.; Mohammadpour, S.; Esfahani, A.T.; Namazian, S.; Yaghob-Taleghani, M.; Baghaei, K.; Tabatabaei, S.A.M.; Pasharavesh, L.; Nazemalhosseini-Mojarad, E. Prevalence and Prognostic Role of PIK3CA E545K Mutation in Iranian Colorectal Cancer Patients. Gastroenterol. Hepatol. Bed Bench 2019, 12, S22–S29. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Shi, Y.; Zhang, S.; Yang, S. PIK3CA Mutation and Clinicopathological Features of Colorectal Cancer: A Systematic Review and Meta-Analysis. Acta Oncol. 2020, 59, 66–74. [Google Scholar] [CrossRef]
- Xu, J.-M.; Wang, Y.; Wang, Y.-L.; Wang, Y.; Liu, T.; Ni, M.; Li, M.-S.; Lin, L.; Ge, F.-J.; Gong, C.; et al. PIK3CA Mutations Contribute to Acquired Cetuximab Resistance in Patients with Metastatic Colorectal Cancer. Clin. Cancer Res. 2017, 23, 4602–4616. [Google Scholar] [CrossRef]
- Polom, K.; Marrelli, D.; Roviello, G.; Pascale, V.; Voglino, C.; Vindigni, C.; Generali, D.; Roviello, F. PIK3CA Mutation in Gastric Cancer and the Role of Microsatellite Instability Status in Mutations of Exons 9 and 20 of the PIK3CA Gene. Adv. Clin. Exp. Med. 2018, 27, 963–969. [Google Scholar] [CrossRef]
- Fang, W.-L.; Huang, K.-H.; Lan, Y.-T.; Lin, C.-H.; Chang, S.-C.; Chen, M.-H.; Chao, Y.; Lin, W.-C.; Lo, S.-S.; Li, A.-F.Y.; et al. Mutations in PI3K/AKT Pathway Genes and Amplifications of PIK3CA Are Associated with Patterns of Recurrence in Gastric Cancers. Oncotarget 2016, 7, 6201–6220. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Yamada, Y.; Taniguchi, H.; Fukahori, M.; Sasaki, Y.; Shoji, H.; Honma, Y.; Iwasa, S.; Takashima, A.; Kato, K.; et al. Clinicopathological Features and Prognostic Roles of KRAS, BRAF, PIK3CA and NRAS Mutations in Advanced Gastric Cancer. BMC Res. Notes 2014, 7, 271. [Google Scholar] [CrossRef]
- Barbi, S.; Cataldo, I.; De Manzoni, G.; Bersani, S.; Lamba, S.; Mattuzzi, S.; Bardelli, A.; Scarpa, A. The Analysis of PIK3CA Mutations in Gastric Carcinoma and Metanalysis of Literature Suggest That Exon-Selectivity Is a Signature of Cancer Type. J. Exp. Clin. Cancer Res. 2010, 29, 32. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-W.; Lee, H.S.; Nam, K.H.; Ahn, S.; Kim, J.W.; Ahn, S.-H.; Park, D.J.; Kim, H.-H.; Lee, K.-W. PIK3CA Mutations Are Associated with Increased Tumor Aggressiveness and Akt Activation in Gastric Cancer. Oncotarget 2017, 8, 90948–90958. [Google Scholar] [CrossRef]
- Harada, K.; Baba, Y.; Shigaki, H.; Ishimoto, T.; Miyake, K.; Kosumi, K.; Tokunaga, R.; Izumi, D.; Ohuchi, M.; Nakamura, K.; et al. Prognostic and Clinical Impact of PIK3CA Mutation in Gastric Cancer: Pyrosequencing Technology and Literature Review. BMC Cancer 2016, 16, 400. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. Comprehensive Molecular Characterization of Gastric Adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Velasco, A.; Bussaglia, E.; Pallares, J.; Dolcet, X.; Llobet, D.; Encinas, M.; Llecha, N.; Palacios, J.; Prat, J.; Matias-Guiu, X. PIK3CA Gene Mutations in Endometrial Carcinoma: Correlation with PTEN and K-RAS Alterations. Hum. Pathol. 2006, 37, 1465–1472. [Google Scholar] [CrossRef]
- Watanabe, T.; Nanamiya, H.; Kojima, M.; Nomura, S.; Furukawa, S.; Soeda, S.; Tanaka, D.; Isogai, T.; Imai, J.-i.; Watanabe, S.; et al. Clinical Relevance of Oncogenic Driver Mutations Identified in Endometrial Carcinoma. Transl. Oncol. 2021, 14, 101010. [Google Scholar] [CrossRef] [PubMed]
- Oda, K.; Stokoe, D.; Taketani, Y.; McCormick, F. High Frequency of Coexistent Mutations of PIK3CA and PTEN Genes in Endometrial Carcinoma. Cancer Res. 2005, 65, 10669–10673. [Google Scholar] [CrossRef]
- Mjos, S.; Werner, H.M.J.; Birkeland, E.; Holst, F.; Berg, A.; Halle, M.K.; Tangen, I.L.; Kusonmano, K.; Mauland, K.K.; Oyan, A.M.; et al. PIK3CA Exon9 Mutations Associate with Reduced Survival, and Are Highly Concordant between Matching Primary Tumors and Metastases in Endometrial Cancer. Sci. Rep. 2017, 7, 10240. [Google Scholar] [CrossRef]
- Nakagaki, T.; Tamura, M.; Kobashi, K.; Omori, A.; Koyama, R.; Idogawa, M.; Ogi, K.; Hiratsuka, H.; Tokino, T.; Sasaki, Y. Targeted Next-Generation Sequencing of 50 Cancer-Related Genes in Japanese Patients with Oral Squamous Cell Carcinoma. Tumor Biol. 2018, 40, 1010428318800180. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Genome Atlas Research Network. Comprehensive Genomic Characterization of Head and Neck Squamous Cell Carcinomas. Nature 2015, 517, 576–582. [Google Scholar] [CrossRef]
- Morris, L.G.T.; Taylor, B.S.; Bivona, T.G.; Gong, Y.; Eng, S.; Brennan, C.W.; Kaufman, A.; Kastenhuber, E.R.; Banuchi, V.E.; Singh, B.; et al. Genomic Dissection of the Epidermal Growth Factor Receptor (EGFR)/PI3K Pathway Reveals Frequent Deletion of the EGFR Phosphatase PTPRS in Head and Neck Cancers. Proc. Natl. Acad. Sci. USA 2011, 108, 19024–19029. [Google Scholar] [CrossRef]
- Agrawal, N.; Frederick, M.J.; Pickering, C.R.; Bettegowda, C.; Chang, K.; Li, R.J.; Fakhry, C.; Xie, T.-X.; Zhang, J.; Wang, J.; et al. Exome Sequencing of Head and Neck Squamous Cell Carcinoma Reveals Inactivating Mutations in NOTCH1. Science 2011, 333, 1154–1157. [Google Scholar] [CrossRef]
- Lui, V.W.Y.; Hedberg, M.L.; Li, H.; Vangara, B.S.; Pendleton, K.; Zeng, Y.; Lu, Y.; Zhang, Q.; Du, Y.; Gilbert, B.R.; et al. Frequent Mutation of the PI3K Pathway in Head and Neck Cancer Defines Predictive Biomarkers. Cancer Discov. 2013, 3, 761–769. [Google Scholar] [CrossRef]
- Yokota, T.; Serizawa, M.; Hosokawa, A.; Kusafuka, K.; Mori, K.; Sugiyama, T.; Tsubosa, Y.; Koh, Y. PIK3CA Mutation Is a Favorable Prognostic Factor in Esophageal Cancer: Molecular Profile by next-Generation Sequencing Using Surgically Resected Formalin-Fixed, Paraffin-Embedded Tissue. BMC Cancer 2018, 18, 826. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Jiang, D.; Zhang, J.; Gavine, P.R.; Xu, S.; Liu, Y.; Xu, C.; Huang, J.; Tan, Y.; Wang, H.; et al. Frequency, Characterization, and Prognostic Analysis of PIK3CA Gene Mutations in Chinese Esophageal Squamous Cell Carcinoma. Hum. Pathol. 2014, 45, 352–358. [Google Scholar] [CrossRef]
- Shigaki, H.; Baba, Y.; Watanabe, M.; Murata, A.; Ishimoto, T.; Iwatsuki, M.; Iwagami, S.; Nosho, K.; Baba, H. PIK3CA Mutation Is Associated with a Favorable Prognosis among Patients with Curatively Resected Esophageal Squamous Cell Carcinoma. Clin. Cancer Res. 2013, 19, 2451–2459. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, Y.; Tang, C.; Jones, L.; Ye, H.; Zhang, G.; Cao, W.; Li, J.; Liu, L.; Liu, Z.; et al. TP53, PIK3CA, FBXW7 and KRAS Mutations in Esophageal Cancer Identified by Targeted Sequencing. Cancer Genom. Proteom. 2016, 13, 231–238. [Google Scholar]
- Sawada, G.; Niida, A.; Uchi, R.; Hirata, H.; Shimamura, T.; Suzuki, Y.; Shiraishi, Y.; Chiba, K.; Imoto, S.; Takahashi, Y.; et al. Genomic Landscape of Esophageal Squamous Cell Carcinoma in a Japanese Population. Gastroenterology 2016, 150, 1171–1182. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.-B.; Chen, Z.-L.; Li, J.-G.; Hu, X.-D.; Shi, X.-J.; Sun, Z.-M.; Zhang, F.; Zhao, Z.-R.; Li, Z.-T.; Liu, Z.-Y.; et al. Genetic Landscape of Esophageal Squamous Cell Carcinoma. Nat. Genet. 2014, 46, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Seo, A.N.; Kang, B.W.; Bae, H.I.; Kwon, O.K.; Park, K.B.; Lee, S.S.; Chung, H.Y.; Yu, W.; Jeon, S.W.; Kang, H.; et al. Exon 9 Mutation of PIK3CA Associated with Poor Survival in Patients with Epstein-Barr Virus-Associated Gastric Cancer. Anticancer. Res. 2019, 39, 2145–2154. [Google Scholar] [CrossRef]
- Bredin, H.K.; Krakstad, C.; Hoivik, E.A. PIK3CA Mutations and Their Impact on Survival Outcomes of Patients with Endometrial Cancer: A Systematic Review and Meta-Analysis. PLoS ONE 2023, 18, e0283203. [Google Scholar] [CrossRef]
- Broderick, D.K.; Di, C.; Parrett, T.J.; Samuels, Y.R.; Cummins, J.M.; Mclendon, R.E.; Fults, D.W.; Velculescu, V.E.; Bigner, D.D.; Yan, H. Mutations of PIK3CA in Anaplastic Oligodendrogliomas, High-Grade Astrocytomas, and Medulloblastomas. Cancer Res. 2004, 64, 5048–5050. [Google Scholar] [CrossRef]
- Gallia, G.L.; Rand, V.; Siu, I.-M.; Eberhart, C.G.; James, C.D.; Marie, S.K.N.; Oba-Shinjo, S.M.; Carlotti, C.G.; Caballero, O.L.; Simpson, A.J.G.; et al. PIK3CA Gene Mutations in Pediatric and Adult Glioblastoma Multiforme. Mol. Cancer Res. 2006, 4, 709–714. [Google Scholar] [CrossRef]
- Verhaak, R.G.W.; Hoadley, K.A.; Purdom, E.; Wang, V.; Qi, Y.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Mesirov, J.P.; et al. Integrated Genomic Analysis Identifies Clinically Relevant Subtypes of Glioblastoma Characterized by Abnormalities in PDGFRA, IDH1, EGFR and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef]
- Hartmann, C.; Bartels, G.; Gehlhaar, C.; Holtkamp, N.; von Deimling, A. PIK3CA Mutations in Glioblastoma Multiforme. Acta Neuropathol. 2005, 109, 639–642. [Google Scholar] [CrossRef]
- Knobbe, C.B.; Trampe-Kieslich, A.; Reifenberger, G. Genetic Alteration and Expression of the Phosphoinositol-3-Kinase/Akt Pathway Genes PIK3CA and PIKE in Human Glioblastomas. Neuropathol. Appl. Neurobiol. 2005, 31, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, H.-D.; Niu, W.; Pan, H. New Molecular Prognostic Factors of Adult Diffuse Lower-Grade Gliomas in Post-2016 Molecular Era: A Retrospective Analysis from Single Center. Br. J. Neurosurg. 2021, 4, 1580–1587. [Google Scholar] [CrossRef]
- Mueller, W.; Mizoguchi, M.; Silen, E.; D’Amore, K.; Nutt, C.L.; Louis, D.N. Mutations of the PIK3CA Gene Are Rare in Human Glioblastoma. Acta Neuropathol. 2005, 109, 654–655. [Google Scholar] [CrossRef] [PubMed]
- Saadeh, F.S.; Morsi, R.Z.; El-Kurdi, A.; Nemer, G.; Mahfouz, R.; Charafeddine, M.; Khoury, J.; Najjar, M.W.; Khoueiry, P.; Assi, H.I. Correlation of Genetic Alterations by Whole-Exome Sequencing with Clinical Outcomes of Glioblastoma Patients from the Lebanese Population. PLoS ONE 2020, 15, e0242793. [Google Scholar] [CrossRef]
- Hartmann, C.; Devermann, L.; Gehlhaar, C.; Holtkamp, N.; von Deimling, A. PIK3CA Mutations in Oligodendroglial Tumours. Neuropathol. Appl. Neurobiol. 2006, 32, 209–212. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Genome Atlas Research Network. Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas. N. Engl. J. Med. 2015, 372, 2481–2498. [Google Scholar] [CrossRef]
- Dono, A.; Alfaro-Munoz, K.; Yan, Y.; Lopez-Garcia, C.A.; Soomro, Z.; Williford, G.; Takayasu, T.; Robell, L.; Majd, N.K.; de Groot, J.; et al. Molecular, Histological, and Clinical Characteristics of Oligodendrogliomas: A Multi-Institutional Retrospective Study. Neurosurgery 2022, 90, 515–522. [Google Scholar] [CrossRef]
- Baker, C.L.; Vaughn, C.P.; Samowitz, W.S. A PIK3CA Pyrosequencing-Based Assay That Excludes Pseudogene Interference. J. Mol. Diagn. 2012, 14, 56–60. [Google Scholar] [CrossRef]
- Ang, D.; O’Gara, R.; Schilling, A.; Beadling, C.; Warrick, A.; Troxell, M.L.; Corless, C.L. Novel Method for PIK3CA Mutation Analysis: Locked Nucleic Acid-PCR Sequencing. J. Mol. Diagn. 2013, 15, 312–318. [Google Scholar] [CrossRef] [PubMed]
- McNeill, R.S.; Stroobant, E.E.; Smithberger, E.; Canoutas, D.A.; Butler, M.K.; Shelton, A.K.; Patel, S.D.; Limas, J.C.; Skinner, R.; Bash, R.E.; et al. PIK3CA Missense Mutations Promote Glioblastoma Pathogenesis, but Do Not Enhance Targeted PI3K Inhibition. PLoS ONE 2018, 13, e0200014. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, K.; Nakamura, T.; Juratli, T.A.; Williams, E.A.; Matsushita, Y.; Miyake, S.; Nishi, M.; Miller, J.J.; Tummala, S.S.; Fink, A.L.; et al. PI3K/AKT/MTOR Pathway Alterations Promote Malignant Progression and Xenograft Formation in Oligodendroglial Tumors. Clin. Cancer Res. 2019, 25, 4375–4387. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Lin, C.-C.J.; Hatcher, A.; Lozzi, B.; Kong, K.; Huang-Hobbs, E.; Cheng, Y.-T.; Beechar, V.B.; Zhu, W.; Zhang, Y.; et al. PIK3CA Variants Selectively Initiate Brain Hyperactivity during Gliomagenesis. Nature 2020, 578, 166–171. [Google Scholar] [CrossRef]
- Alqahtani, A.; Ayesh, H.S.K.; Halawani, H. PIK3CA Gene Mutations in Solid Malignancies: Association with Clinicopathological Parameters and Prognosis. Cancers 2020, 12, 93. [Google Scholar] [CrossRef]
- Houghton, P.J. Everolimus. Clin. Cancer Res. 2010, 16, 1368–1372. [Google Scholar] [CrossRef]
- Kwitkowski, V.E.; Prowell, T.M.; Ibrahim, A.; Farrell, A.T.; Justice, R.; Mitchell, S.S.; Sridhara, R.; Pazdur, R. FDA Approval Summary: Temsirolimus as Treatment for Advanced Renal Cell Carcinoma. Oncologist 2010, 15, 428–435. [Google Scholar] [CrossRef]
- Shirley, M. Capivasertib: First Approval. Drugs 2024, 84, 337–346. [Google Scholar] [CrossRef]
- Dhillon, S.; Keam, S.J. Umbralisib: First Approval. Drugs 2021, 81, 857–866. [Google Scholar] [CrossRef]
- Markham, A. Idelalisib: First Global Approval. Drugs 2014, 74, 1701–1707. [Google Scholar] [CrossRef]
- Markham, A. Copanlisib: First Global Approval. Drugs 2017, 77, 2057–2062. [Google Scholar] [CrossRef]
- Blair, H.A. Duvelisib: First Global Approval. Drugs 2018, 78, 1847–1853. [Google Scholar] [CrossRef] [PubMed]
- Markham, A. Alpelisib: First Global Approval. Drugs 2019, 79, 1249–1253. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.A. Inavolisib: First Approval. Drugs 2025, 85, 271–278. [Google Scholar] [CrossRef]
- Banerjee, T.; Kim, M.S.; Haslam, A.; Prasad, V. Clinical Trials Portfolio and Regulatory History of Idelalisib in Indolent Non-Hodgkin Lymphoma: A Systematic Review and Meta-Analysis. JAMA Intern. Med. 2023, 183, 435–441. [Google Scholar] [CrossRef]
- Benjamin, D.J.; Prasad, V. PI3K Inhibitors in Haematological Malignancies. Lancet Oncol. 2022, 23, e362–e363. [Google Scholar] [CrossRef]
- Skånland, S.S.; Okkenhaug, K.; Davids, M.S. PI3K Inhibitors in Hematology: When One Door Closes…. Clin. Cancer Res. 2024, 30, 3667–3675. [Google Scholar] [CrossRef] [PubMed]
- André, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef]
- André, F.; Ciruelos, E.M.; Juric, D.; Loibl, S.; Campone, M.; Mayer, I.A.; Rubovszky, G.; Yamashita, T.; Kaufman, B.; Lu, Y.S.; et al. Alpelisib plus Fulvestrant for PIK3CA-Mutated, Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor-2–Negative Advanced Breast Cancer: Final Overall Survival Results from SOLAR-1. Ann. Oncol. 2021, 32, 208–217. [Google Scholar] [CrossRef]
- Savas, P.; Lo, L.L.; Luen, S.J.; Blackley, E.F.; Callahan, J.; Moodie, K.; van Geelen, C.T.; Ko, Y.A.; Weng, C.F.; Wein, L.; et al. Alpelisib Monotherapy for PI3K-Altered, Pretreated Advanced Breast Cancer: A Phase II Study. Cancer Discov. 2022, 12, 2058–2073. [Google Scholar] [CrossRef]
- Vasan, N.; Razavi, P.; Johnson, J.L.; Shao, H.; Shah, H.; Antoine, A.; Ladewig, E.; Gorelick, A.; Lin, T.-Y.; Toska, E.; et al. Double PIK3CA Mutations in Cis Increase Oncogenicity and Sensitivity to PI3Kα Inhibitors. Science 2019, 366, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Ni, J.; McFaline-Figueroa, J.R.; Wang, Y.; Bronson, R.T.; Ligon, K.L.; Wen, P.Y.; Roberts, T.M.; Zhao, J.J. Divergent Roles of PI3K Isoforms in PTEN-Deficient Glioblastomas. Cell Rep. 2020, 32, 108196. [Google Scholar] [CrossRef] [PubMed]
- Eckerdt, F.D.; Bell, J.B.; Gonzalez, C.; Oh, M.S.; Perez, R.E.; Mazewski, C.; Fischietti, M.; Goldman, S.; Nakano, I.; Platanias, L.C. Combined PI3Kα-MTOR Targeting of Glioma Stem Cells. Sci. Rep. 2020, 10, 21873. [Google Scholar] [CrossRef]
- Passarelli, A.; Carbone, V.; Pignata, S.; Mazzeo, R.; Lorusso, D.; Scambia, G.; Canova, S.; Di Palma, T.; Tasca, G.; Mantiero, M.; et al. Alpelisib for PIK3CA-Mutated Advanced Gynecological Cancers: First Clues of Clinical Activity. Gynecol. Oncol. 2024, 183, 61–67. [Google Scholar] [CrossRef]
- Bogani, G.; Chiappa, V.; Bini, M.; Ronzulli, D.; Indini, A.; Conca, E.; Raspagliesi, F. BYL719 (Alpelisib) for the Treatment of PIK3CA-Mutated, Recurrent/Advanced Cervical Cancer. Tumori 2023, 109, 244–248. [Google Scholar] [CrossRef]
- Wei, Y.; Lin, S.; Zhi, W.; Chu, T.; Liu, B.; Peng, T.; Xu, M.; Ding, W.; Cao, C.; Wu, P. Genomic Analysis of Cervical Carcinoma Identifies Alpelisib as a Therapeutic Option for PIK3CA-Mutant Cervical Carcinoma via the PI3K/AKT Pathway. J. Med. Virol. 2023, 95, e28656. [Google Scholar] [CrossRef]
- Xu, H.; Chen, K.; Shang, R.; Chen, X.; Zhang, Y.; Song, X.; Evert, M.; Zhong, S.; Li, B.; Calvisi, D.F.; et al. Alpelisib Combination Treatment as Novel Targeted Therapy against Hepatocellular Carcinoma. Cell Death Dis. 2021, 12, 920. [Google Scholar] [CrossRef]
- Kim, K.H.; Hwang, S.; Kim, M.K.; Park, K.-U.; Yun, T.; Lee, K.-W.; Kim, J.H.; Keam, B.; Cho, B.C.; Oh, S.Y.; et al. Differential Efficacy of Alpelisib by PIK3CA Mutation Site in Head and Neck Squamous Cell Carcinoma: An Analysis from the KCSG HN 15-16 TRIUMPH Trial. Cancer Res. Treat. 2025. [Google Scholar] [CrossRef] [PubMed]
- Razak, A.R.A.; Wang, H.M.; Chang, J.Y.; Ahn, M.J.; Munster, P.; Blumenschein, G.; Solomon, B.; Lim, D.W.T.; Hong, R.L.; Pfister, D.; et al. A Phase 1b/2 Study of Alpelisib in Combination with Cetuximab in Patients with Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma. Target. Oncol. 2023, 18, 853–868. [Google Scholar] [CrossRef]
- Jin, N.; Keam, B.; Cho, J.; Lee, M.J.; Kim, H.R.; Torosyan, H.; Jura, N.; Ng, P.K.S.; Mills, G.B.; Li, H.; et al. Therapeutic Implications of Activating Noncanonical PIK3CA Mutations in Head and Neck Squamous Cell Carcinoma. J. Clin. Investig. 2021, 131, e150335. [Google Scholar] [CrossRef]
- García-Carracedo, D.; Cai, Y.; Qiu, W.; Saeki, K.; Friedman, R.A.; Lee, A.; Li, Y.; Goldberg, E.M.; Stratikopoulos, E.E.; Parsons, R.; et al. PIK3CA and P53 Mutations Promote 4NQO-Initated Head and Neck Tumor Progression and Metastasis in Mice. Mol. Cancer Res. 2020, 18, 822–834. [Google Scholar] [CrossRef]
- Shi, R.; Li, M.; Raghavan, V.; Tam, S.; Cabanero, M.; Pham, N.-A.; Shepherd, F.A.; Moghal, N.; Tsao, M.-S. Targeting the CDK4/6-Rb Pathway Enhances Response to PI3K Inhibition in PIK3CA-Mutant Lung Squamous Cell Carcinoma. Clin. Cancer Res. 2018, 24, 5990–6000. [Google Scholar] [CrossRef]
- Baselga, J.; Im, S.-A.; Iwata, H.; Cortés, J.; De Laurentiis, M.; Jiang, Z.; Arteaga, C.L.; Jonat, W.; Clemons, M.; Ito, Y.; et al. Buparlisib plus Fulvestrant versus Placebo plus Fulvestrant in Postmenopausal, Hormone Receptor-Positive, HER2-Negative, Advanced Breast Cancer (BELLE-2): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 2017, 18, 904–916. [Google Scholar] [CrossRef] [PubMed]
- Welt, A.; Wiesweg, M.; Theurer, S.; Abenhardt, W.; Groschek, M.; Müller, L.; Schröder, J.; Tewes, M.; Chiabudini, M.; Potthoff, K.; et al. Buparlisib in Combination with Tamoxifen in Pretreated Patients with Hormone Receptor-Positive, HER2-Negative Advanced Breast Cancer Molecularly Stratified for PIK3CA Mutations and Loss of PTEN Expression. Cancer Med. 2020, 9, 4527–4539. [Google Scholar] [CrossRef]
- Garrido-Castro, A.C.; Saura, C.; Barroso-Sousa, R.; Guo, H.; Ciruelos, E.; Bermejo, B.; Gavilá, J.; Serra, V.; Prat, A.; Paré, L.; et al. Phase 2 Study of Buparlisib (BKM120), a Pan-Class I PI3K Inhibitor, in Patients with Metastatic Triple-Negative Breast Cancer. Breast Cancer Res. 2020, 22, 120. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Kato, K.; Hara, H.; Takahashi, S.; Muro, K.; Nishina, T.; Wakabayashi, M.; Nomura, S.; Sato, A.; Ohtsu, A.; et al. Phase II Study of BKM120 in Patients with Advanced Esophageal Squamous Cell Carcinoma (EPOC1303). Esophagus 2022, 19, 702–710. [Google Scholar] [CrossRef]
- Wen, P.Y.; Touat, M.; Alexander, B.M.; Mellinghoff, I.K.; Ramkissoon, S.; McCluskey, C.S.; Pelton, K.; Haidar, S.; Basu, S.S.; Gaffey, S.C.; et al. Buparlisib in Patients With Recurrent Glioblastoma Harboring Phosphatidylinositol 3-Kinase Pathway Activation: An Open-Label, Multicenter, Multi-Arm, Phase II Trial. J. Clin. Oncol. 2019, 37, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, M.; Clement, P.M.; Campone, M.; Gil-Gil, M.J.; DeGroot, J.; Chinot, O.; Idbaih, A.; Gan, H.; Raizer, J.; Wen, P.Y.; et al. Buparlisib plus Carboplatin or Lomustine in Patients with Recurrent Glioblastoma: A Phase Ib/II, Open-Label, Multicentre, Randomised Study. ESMO Open 2020, 5, e000672. [Google Scholar] [CrossRef]
- Zhang, S.; Peng, X.; Li, X.; Liu, H.; Zhao, B.; Elkabets, M.; Liu, Y.; Wang, W.; Wang, R.; Zhong, Y.; et al. BKM120 Sensitizes Glioblastoma to the PARP Inhibitor Rucaparib by Suppressing Homologous Recombination Repair. Cell Death Dis. 2021, 12, 546. [Google Scholar] [CrossRef]
- Kim, H.R.; Kang, H.N.; Yun, M.R.; Ju, K.Y.; Choi, J.W.; Jung, D.M.; Pyo, K.H.; Hong, M.H.; Ahn, M.J.; Sun, J.M.; et al. Mouse–Human Co-Clinical Trials Demonstrate Superior Anti-Tumour Effects of Buparlisib (BKM120) and Cetuximab Combination in Squamous Cell Carcinoma of Head and Neck. Br. J. Cancer 2020, 123, 1720–1729. [Google Scholar] [CrossRef]
- Soulières, D.; Faivre, S.; Mesía, R.; Remenár, É.; Li, S.H.; Karpenko, A.; Dechaphunkul, A.; Ochsenreither, S.; Kiss, L.A.; Lin, J.C.; et al. Buparlisib and Paclitaxel in Patients with Platinum-Pretreated Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck (BERIL-1): A Randomised, Double-Blind, Placebo-Controlled Phase 2 Trial. Lancet Oncol. 2017, 18, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Soulières, D.; Licitra, L.; Mesía, R.; Remenar, E.; Li, S.H.; Karpenko, A.; Chol, M.; Wang, Y.A.; Solovieff, N.; Bourdeau, L.; et al. Molecular Alterations and Buparlisib Efficacy in Patients with Squamous Cell Carcinoma of the Head and Neck: Biomarker Analysis from BERIL-1. Clin. Cancer Res. 2018, 24, 2505–2516. [Google Scholar] [CrossRef]
- Turner, N.C.; Im, S.-A.; Saura, C.; Juric, D.; Loibl, S.; Kalinsky, K.; Schmid, P.; Loi, S.; Sunpaweravong, P.; Musolino, A.; et al. Inavolisib-Based Therapy in PIK3CA-Mutated Advanced Breast Cancer. N. Engl. J. Med. 2024, 391, 1584–1596. [Google Scholar] [CrossRef] [PubMed]
- Bedard, P.L.; Jhaveri, K.L.; Accordino, M.K.; Cervantes, P.A.; Gambardella, V.; Hamilton, E.; Italiano, P.A.; Kalinsky, P.K.; Krop, P.I.E.; Oliveira, M.; et al. Inavolisib plus Letrozole or Fulvestrant in PIK3CA-Mutated, Hormone Receptor-Positive, HER2-Negative Advanced or Metastatic Breast Cancer (GO39374): An Open-Label, Multicentre, Dose-Escalation and Dose-Expansion Phase 1/1b Study. Eur. J. Cancer 2025, 221, 115397. [Google Scholar] [CrossRef]
- Song, K.W.; Edgar, K.A.; Hanan, E.J.; Hafner, M.; Oeh, J.; Merchant, M.; Sampath, D.; Nannini, M.A.; Hong, R.; Phu, L.; et al. RTK-Dependent Inducible Degradation of Mutant PI3Kα Drives GDC-0077 (Inavolisib) Efficacy. Cancer Discov. 2022, 12, 204–219. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.Y.; Frederick De Groot, J.; Battiste, J.; Goldlust, S.A.; Garner, J.S.; Friend, J.; Simpson, J.A.; Damek, D.; Olivero, A.; Cloughesy, T.F. Paxalisib in Patients with Newly Diagnosed Glioblastoma with Unmethylated MGMT Promoter Status: Final Phase 2 Study Results. J. Clin. Oncol. 2022, 40, 2047. [Google Scholar] [CrossRef]
- Guo, T.; Wu, C.; Zhang, J.; Yu, J.; Li, G.; Jiang, H.; Zhang, X.; Yu, R.; Liu, X. Dual Blockade of EGFR and PI3K Signaling Pathways Offers a Therapeutic Strategy for Glioblastoma. Cell Commun. Signal 2023, 21, 363. [Google Scholar] [CrossRef]
- Heffron, T.P.; Ndubaku, C.O.; Salphati, L.; Alicke, B.; Cheong, J.; Drobnick, J.; Edgar, K.; Gould, S.E.; Lee, L.B.; Lesnick, J.D.; et al. Discovery of Clinical Development Candidate GDC-0084, a Brain Penetrant Inhibitor of PI3K and MTOR. ACS Med. Chem. Lett. 2016, 7, 351–356. [Google Scholar] [CrossRef]
- Salphati, L.; Alicke, B.; Heffron, T.P.; Shahidi-Latham, S.; Nishimura, M.; Cao, T.; Carano, R.A.; Cheong, J.; Greve, J.; Koeppen, H.; et al. Brain Distribution and Efficacy of the Brain Penetrant PI3K Inhibitor GDC-0084 in Orthotopic Mouse Models of Human Glioblastoma. Drug Metab. Disp. 2016, 44, 1881–1889. [Google Scholar] [CrossRef]
- Shi, F.; Guo, H.; Zhang, R.; Liu, H.; Wu, L.; Wu, Q.; Liu, J.; Liu, T.; Zhang, Q. The PI3K Inhibitor GDC-0941 Enhances Radiosensitization and Reduces Chemoresistance to Temozolomide in GBM Cell Lines. Neuroscience 2017, 346, 298–308. [Google Scholar] [CrossRef]
- Zumsteg, Z.S.; Morse, N.; Krigsfeld, G.; Gupta, G.; Higginson, D.S.; Lee, N.Y.; Morris, L.; Ganly, I.; Shiao, S.L.; Powell, S.N.; et al. Taselisib (GDC-0032), a Potent β-Sparing Small Molecule Inhibitor of PI3K, Radiosensitizes Head and Neck Squamous Carcinomas Containing Activating PIK3CA Alterations. Clin. Cancer Res. 2016, 22, 2009–2019. [Google Scholar] [CrossRef]
- Wirtz, E.D.; Hoshino, D.; Maldonado, A.T.; Tyson, D.R.; Weaver, A.M. Response of Head and Neck Squamous Cell Carcinoma Cells Carrying PIK3CA Mutations to Selected Targeted Therapies. JAMA Otolaryngol. Head Neck Surg. 2015, 141, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Varkaris, A.; Pazolli, E.; Gunaydin, H.; Wang, Q.; Pierce, L.; Boezio, A.A.; Bulku, A.; Dipietro, L.; Fridrich, C.; Frost, A.; et al. Discovery and Clinical Proof-of-Concept of RLY-2608, a First-in-Class Mutant-Selective Allosteric PI3Kα Inhibitor That Decouples Antitumor Activity from Hyperinsulinemia. Cancer Discov. 2024, 14, 241–257. [Google Scholar] [CrossRef]
- Dent, S.; Cortés, J.; Im, Y.H.; Diéras, V.; Harbeck, N.; Krop, I.E.; Wilson, T.R.; Cui, N.; Schimmoller, F.; Hsu, J.Y.; et al. Phase III Randomized Study of Taselisib or Placebo with Fulvestrant in Estrogen Receptor-Positive, PIK3CA-Mutant, HER2-Negative, Advanced Breast Cancer: The SANDPIPER Trial. Ann. Oncol. 2021, 32, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Dickler, M.N.; Saura, C.; Richards, D.A.; Krop, I.E.; Cervantes, A.; Bedard, P.L.; Patel, M.R.; Pusztai, L.; Oliveira, M.; Cardenas, A.K.; et al. Phase II Study of Taselisib (GDC-0032) in Combination with Fulvestrant in Patients with HER2-Negative, Hormone Receptor–Positive Advanced Breast Cancer. Clin. Cancer Res. 2018, 24, 4380–4387. [Google Scholar] [CrossRef]
- Grinshpun, A.; Ren, S.; Graham, N.; DeMeo, M.K.; Wrabel, E.; Carter, J.; Tayob, N.; Pereslete, A.; Hamilton, E.; Juric, D.; et al. Phase Ib Dose-Escalation Trial of Taselisib (GDC-0032) in Combination with HER2-Directed Therapies in Patients with Advanced HER2+ Breast Cancer. ESMO Open 2024, 9, 103465. [Google Scholar] [CrossRef] [PubMed]
- Langer, C.J.; Redman, M.W.; Wade, J.L.; Aggarwal, C.; Bradley, J.D.; Crawford, J.; Stella, P.J.; Knapp, M.H.; Miao, J.; Minichiello, K.; et al. SWOG S1400B (NCT02785913), a Phase II Study of GDC-0032 (Taselisib) for Previously Treated PI3K-Positive Patients with Stage IV Squamous Cell Lung Cancer (Lung-MAP Sub-Study). J. Thorac. Oncol. 2019, 14, 1839–1846. [Google Scholar] [CrossRef]
- Blackwell, K.; Burris, H.; Gomez, P.; Lynn Henry, N.; Isakoff, S.; Campana, F.; Gao, L.; Jiang, J.; Macé, S.; Tolaney, S.M. Phase I/II Dose-Escalation Study of PI3K Inhibitors Pilaralisib or Voxtalisib in Combination with Letrozole in Patients with Hormone-Receptor-Positive and HER2-Negative Metastatic Breast Cancer Refractory to a Non-Steroidal Aromatase Inhibitor. Breast Cancer Res. Treat. 2015, 154, 287–297. [Google Scholar] [CrossRef]
- Wen, P.Y.; Omuro, A.; Ahluwalia, M.S.; Fathallah-Shaykh, H.M.; Mohile, N.; Lager, J.J.; Laird, A.D.; Tang, J.; Jiang, J.; Egile, C.; et al. Phase I Dose-Escalation Study of the PI3K/MTOR Inhibitor Voxtalisib (SAR245409, XL765) plus Temozolomide with or without Radiotherapy in Patients with High-Grade Glioma. Neuro Oncol. 2015, 17, 1275–1283. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, G.; Liang, H. Dual PI3K/MTOR Inhibitor, XL765, Suppresses Glioblastoma Growth by Inducing ER Stress Dependent Apoptosis. Onco Targets Ther. 2019, 12, 5415–5424. [Google Scholar] [CrossRef]
- Batsios, G.; Viswanath, P.; Subramani, E.; Najac, C.; Gillespie, A.M.; Santos, R.D.; Molloy, A.R.; Pieper, R.O.; Ronen, S.M. PI3K/MTOR Inhibition of IDH1 Mutant Glioma Leads to Reduced 2HG Production That Is Associated with Increased Survival. Sci. Rep. 2019, 9, 10521. [Google Scholar] [CrossRef] [PubMed]
- Arend, R.C.; Davis, A.M.; Chimiczewski, P.; O’Malley, D.M.; Provencher, D.; Vergote, I.; Ghamande, S.; Birrer, M.J. EMR 20006-012: A Phase II Randomized Double-Blind Placebo Controlled Trial Comparing the Combination of Pimasertib (MEK Inhibitor) with SAR245409 (PI3K Inhibitor) to Pimasertib Alone in Patients with Previously Treated Unresectable Borderline or Low Grade Ovarian Cancer. Gynecol. Oncol. 2020, 156, 301–307. [Google Scholar] [CrossRef]
- Inaba, K.; Oda, K.; Ikeda, Y.; Sone, K.; Miyasaka, A.; Kashiyama, T.; Fukuda, T.; Uehara, Y.; Arimoto, T.; Kuramoto, H.; et al. Antitumor Activity of a Combination of Dual PI3K/MTOR Inhibitor SAR245409 and Selective MEK1/2 Inhibitor Pimasertib in Endometrial Carcinomas. Gynecol. Oncol. 2015, 138, 323–331. [Google Scholar] [CrossRef]
- Gravina, G.L.; Mancini, A.; Scarsella, L.; Colapietro, A.; Jitariuc, A.; Vitale, F.; Marampon, F.; Ricevuto, E.; Festuccia, C. Dual PI3K/MTOR Inhibitor, XL765 (SAR245409), Shows Superior Effects to Sole PI3K [XL147 (SAR245408)] or MTOR [Rapamycin] Inhibition in Prostate Cancer Cell Models. Tumor Biol. 2016, 37, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Ma, F.; Liu, B.; Guan, X.; Li, L.; Li, C.; Qian, H.; Xu, B. Everolimus in Hormone Receptor-Positive Metastatic Breast Cancer: PIK3CA Mutation H1047R Was a Potential Efficacy Biomarker in a Retrospective Study. BMC Cancer 2019, 19, 442. [Google Scholar] [CrossRef]
- Varkaris, A.; Fece de la Cruz, F.; Martin, E.E.; Norden, B.L.; Chevalier, N.; Kehlmann, A.M.; Leshchiner, I.; Barnes, H.; Ehnstrom, S.; Stavridi, A.M.; et al. Allosteric PI3Kα Inhibition Overcomes On-Target Resistance to Orthosteric Inhibitors Mediated by Secondary PIK3CA Mutations. Cancer Discov. 2024, 14, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Moynahan, M.E.; Chen, D.; He, W.; Sung, P.; Samoila, A.; You, D.; Bhatt, T.; Patel, P.; Ringeisen, F.; Hortobagyi, G.N.; et al. Correlation between PIK3CA Mutations in Cell-Free DNA and Everolimus Efficacy in HR+, HER2-Advanced Breast Cancer: Results from BOLERO-2. Br. J. Cancer 2017, 116, 726–730. [Google Scholar] [CrossRef]
- De Santis, M.C.; Gulluni, F.; Campa, C.C.; Martini, M.; Hirsch, E. Targeting PI3K Signaling in Cancer: Challenges and Advances. BBA Rev. Cancer 2019, 1871, 361–366. [Google Scholar] [CrossRef]
- Furet, P.; Guagnano, V.; Fairhurst, R.A.; Imbach-Weese, P.; Bruce, I.; Knapp, M.; Fritsch, C.; Blasco, F.; Blanz, J.; Aichholz, R.; et al. Discovery of NVP-BYL719 a Potent and Selective Phosphatidylinositol-3 Kinase Alpha Inhibitor Selected for Clinical Evaluation. Bioorg. Med. Chem. Lett. 2013, 23, 3741–3748. [Google Scholar] [CrossRef]
- Chakrabarty, A.; Sánchez, V.; Kuba, M.G.; Rinehart, C.; Arteaga, C.L. Feedback Upregulation of HER3 (ErbB3) Expression and Activity Attenuates Antitumor Effect of PI3K Inhibitors. Proc. Natl. Acad. Sci. USA 2012, 109, 2718–2723. [Google Scholar] [CrossRef]
- Serra, V.; Scaltriti, M.; Prudkin, L.; Eichhorn, P.J.A.; Ibrahim, Y.H.; Chandarlapaty, S.; Markman, B.; Rodriguez, O.; Guzman, M.; Rodriguez, S.; et al. PI3K Inhibition Results in Enhanced HER Signaling and Acquired ERK Dependency in HER2-Overexpressing Breast Cancer. Oncogene 2011, 30, 2547–2557. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, B.D.; Pauli, C.; Xing, D.; Wang, D.G.; Li, X.; Wu, D.; Amadiume, S.C.; Goncalves, M.D.; Hodakoski, C.; Lundquist, M.R.; et al. Suppression of Insulin Feedback Enhances the Efficacy of PI3K Inhibitors. Nature 2018, 560, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Le, X.; Antony, R.; Razavi, P.; Treacy, D.J.; Luo, F.; Ghandi, M.; Castel, P.; Scaltriti, M.; Baselga, J.; Garraway, L.A. Systematic Functional Characterization of Resistance to PI3K Inhibition in Breast Cancer. Cancer Discov. 2016, 6, 1134–1147. [Google Scholar] [CrossRef]
- Juric, D.; Castel, P.; Griffith, M.; Griffith, O.L.; Won, H.H.; Ellis, H.; Ebbesen, S.H.; Ainscough, B.J.; Ramu, A.; Iyer, G.; et al. Convergent Loss of PTEN Leads to Clinical Resistance to a PI(3)Kα Inhibitor. Nature 2015, 518, 240–244. [Google Scholar] [CrossRef]
- Elkabets, M.; Vora, S.; Juric, D.; Morse, N.; Mino-Kenudson, M.; Muranen, T.; Tao, J.; Campos, A.B.; Rodon, J.; Ibrahim, Y.H.; et al. MTORC1 Inhibition Is Required for Sensitivity to PI3K P110α Inhibitors in PIK3CA-Mutant Breast Cancer. Sci. Transl. Med. 2013, 5, 196ra99. [Google Scholar] [CrossRef]
- Schwartz, S.; Wongvipat, J.; Trigwell, C.B.; Hancox, U.; Carver, B.S.; Rodrik-Outmezguine, V.; Will, M.; Yellen, P.; de Stanchina, E.; Baselga, J.; et al. Feedback Suppression of PI3Kα Signaling in PTEN-Mutated Tumors Is Relieved by Selective Inhibition of PI3Kβ. Cancer Cell 2015, 27, 109–122. [Google Scholar] [CrossRef]
- Costa, C.; Ebi, H.; Martini, M.; Beausoleil, S.A.; Faber, A.C.; Jakubik, C.T.; Huang, A.; Wang, Y.; Nishtala, M.; Hall, B.; et al. Measurement of PIP3 Levels Reveals an Unexpected Role for P110β in Early Adaptive Responses to P110α-Specific Inhibitors in Luminal Breast Cancer. Cancer Cell 2015, 27, 97–108. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, B.; Qiao, Y.; Zhang, Y.; Tian, Y.; Jiang, J.; Ma, D.; Kong, B. Phosphoinositide-3-Kinase Inhibition Enhances Radiosensitization of Cervical Cancer in Vivo. Int. J. Gynecol. Cancer 2011, 21, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Cui, G.-B.; Zhang, J.; Zhang, F.; Zhou, Y.-A.; Jian, T.; Li, X.-F. Inhibition of PI3 Kinases Enhances the Sensitivity of Non-Small Cell Lung Cancer Cells to Ionizing Radiation. Oncol. Rep. 2010, 24, 1683–1689. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. Comprehensive Genomic Characterization Defines Human Glioblastoma Genes and Core Pathways. Nature 2008, 455, 1061–1068. [Google Scholar] [CrossRef]
- Srividya, M.R.; Thota, B.; Shailaja, B.C.; Arivazhagan, A.; Thennarasu, K.; Chandramouli, B.A.; Hegde, A.S.; Santosh, V. Homozygous 10q23/PTEN Deletion and Its Impact on Outcome in Glioblastoma: A Prospective Translational Study on a Uniformly Treated Cohort of Adult Patients. Neuropathology 2011, 31, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Wee, S.; Wiederschain, D.; Maira, S.-M.; Loo, A.; Miller, C.; DeBeaumont, R.; Stegmeier, F.; Yao, Y.-M.; Lengauer, C. PTEN-Deficient Cancers Depend on PIK3CB. Proc. Natl. Acad. Sci. USA 2008, 105, 13057–13062. [Google Scholar] [CrossRef] [PubMed]
| Classification | Glioma Subgroup | Sample Size | Method | Region Evaluated | Mutation Frequency | Prognostic Effect (U/M) | Reference |
|---|---|---|---|---|---|---|---|
| Histological | GBM | 30 (Multifocal) | Illumina HiSeq | All coding exons | 30% | Unfavorable (U) | [27] |
| 130 (Solitary) | 10% | ||||||
| 15 | Sanger sequencing | All coding exons | 27% | --- | [60] | ||
| 60 | Illumina HiSeq | All coding exons | 21.7% | --- | [136] | ||
| 38 | Sanger sequencing | Exons 2, 3, 5, 6, 8, 10, 13, 14, 19 and 21 * | 18% | --- | [130] | ||
| 291 | Illumina HiSeq | All coding exons | 11% | --- | [18] | ||
| 157 | SNaPshot® multiplex system | 6 known hotspots | 8.3% | Unfavorable (M) | [28] | ||
| 70 | PCR-SSCP direct sequencing | All coding exons | 7% | --- | [132] | ||
| 116 | Affymetrix microarray | All coding exons | 6% | --- | [131] | ||
| 105 | Sanger sequencing | Exons 10 and 21 * | 5% | --- | [129] | ||
| 97 | PCR-SSCP direct sequencing | Exons 2, 3, 5, 6, 8, 10, 13, 14, 19 and 21 * | 5% | --- | [133] | ||
| 40 | Ion semiconductor sequencing | All coding exons | 5% | --- | [134] | ||
| 30 | PCR-SSCP | All coding exons | 0% | --- | [135] | ||
| Astrocytoma | 52 | Ion semiconductor sequencing | All coding exons | 9.6% | Unfavorable (U) | [134] | |
| 31 | Sanger sequencing | Exons 10 and 21 * | 3% | --- | [129] | ||
| Oligodendroglioma | 21 | Sanger sequencing | Exons 10 and 21 * | 14% | --- | [129] | |
| 66 | PCR-SSCP direct sequencing | Exons 2, 10 and 21 * | 5% | --- | [137] | ||
| 17 | Ion semiconductor sequencing | All coding exons | 0% | --- | [134] | ||
| Molecular | GBM, IDH-wildtype | 567 (TCGA) | Illumina HiSeq | Exons 10 and 21* | 2% | Not significant (M) | [29] |
| 239 | Sanger sequencing | 3% | |||||
| GBM, IDH-mutant | 25 (TCGA) | Illumina HiSeq | Exons 10 and 21 * | 8% | --- | [29] | |
| 11 | Sanger sequencing | 9% | |||||
| Astrocytoma, IDH-wildtype | 39 | Sanger sequencing | Exons 10 and 21 * | 10% | --- | [29] | |
| Astrocytoma, IDH-mutant | 56 | Sanger sequencing | Exons 10 and 21* | 5% | --- | [29] | |
| 1p/19q codeleted + IDH-mutant | 84 | Illumina HiSeq | All coding exons | 20% | --- | [138] | |
| 107 | Illumina HiSeq | All coding exons | 14% | Unfavorable (M) | [139] | ||
| 49 | Sanger sequencing | Exons 10 and 21 * | 10% | --- | [29] |
| Inhibitor | Cancer Type | Cancer Subtype | Study Type | Treatment | Outcome | PIK3CA Status Dependent? | Reference |
|---|---|---|---|---|---|---|---|
| Alpelisib PI3Kα (orthosteric) | Breast | HR+ HER2- | Clinical-PhIII | +Fulvestrant | ■ Favorable | ♦ Yes | [158,159] |
| HR+ HER2- PI3K altered | Clinical-PhII | Monotherapy | ■ Favorable | N.E. (All-mut) | [160] | ||
| Triple negative | Clinical-PhII | Monotherapy | ■ No benefit | N.E. | |||
| --- | Preclinical-in vitro | Monotherapy | ■ Sensitive | ♦ Yes | [161] | ||
| Brain | GBM | Preclinical-in vivo | ±PI3Kβ inhibitor (AZD6482) | ■ Sensitive | N.E. | [162] | |
| Preclinical-in vitro | ±mTOR inhibitor (OSI-027) | ■ Sensitive | N.E. (WT-only) | [163] | |||
| Gynecological | PIK3CA-mut | Clinical-obs | Monotherapy | ■ Favorable | N.E. (All-mut) | [164] | |
| PIK3CA-mut cervical | Clinical | Monotherapy | ■ Favorable | N.E. (All-mut) | [165] | ||
| Cervical | Preclinical-in vitro | Monotherapy | ■ Sensitive | ♦ Yes | [166] | ||
| Liver | HCC | Preclinical in vivo + in vitro | ±mTOR inhibitor (MLN0128) | ■ Sensitive | ♦ Yes | [167] | |
| Head and neck | PI3K altered SCC | Clinical-PhII | Monotherapy | ■ Favorable | ♦ Yes | [168] | |
| SCC | Clinical-PhIb/II | +Cetuximab | ■ No benefit | N.E. | [169] | ||
| Preclinical-in vivo | Monotherapy | ■ Sensitive | ♦ Yes | [170] | |||
| Preclinical-in vivo | +Cisplatin | ■ Favorable | N.E. (All-mut) | [171] | |||
| Lung | SCC | Preclinical-in vivo | ±CDK4/6 inhibitors | ■ Favorable | ♦ Yes | [172] | |
| Buparlisib Pan-PI3K (mainly PI3Kα) | Breast | HR+ HER2- | Clinical-PhIII | +Fulvestrant | ■ Favorable | ♦ Yes | [173] |
| Clinical-PhII | +Tamoxifen | ■ Favorable | ♦ Yes | [174] | |||
| Triple negative | Clinical-PhII | Monotherapy | ■ Minimal benefit | N.E. | [175] | ||
| Esophagus | SCC | Clinical-PhII | Monotherapy | ■ Favorable | N.E. | [176] | |
| Brain | GBM | Clinical-PhII | Monotherapy | ■ Minimal benefit | ♦ No | [177] | |
| Clinical-PhIb/II | +Carboplatin or lomustine | ■ Minimal benefit | N.E. | [178] | |||
| Preclinical in vivo + in vitro | ±PARP inhibitor (rucaparib) | ■ Favorable | N.E. (WT-only) | [179] | |||
| Preclinical-in vivo | Monotherapy | ■ Minimal benefit | N.E. | [162] | |||
| Preclinical-in vitro | ±MEK inhibitor (selumetinib) | ■ Sensitive | ♦ No | [142] | |||
| Head and neck | SCC | Clinical-PhII | ±Cetuximab | ■ Favorable | N.E. (WT-only) | [180] | |
| Clinical-PhII | +Paclitaxel | ■ Favorable | ♦ No | [181,182] | |||
| Lung | SCC | Preclinical-in vivo | ±CDK4/6 inhibitors | ■ Favorable | ♦ Yes | [172] | |
| Inavolisib PI3Kα (orthosteric) | Breast | HR+ HER2- PIK3CA-mut | Clinical-PhIII | +Palbociclib-Fulvestrant | ■ Favorable | N.E. (All-mut) | [183] |
| Clinical-PhIb/II | +Letrozole or Fulvestrant | ■ Favorable | N.E. (All-mut) | [184] | |||
| --- | Preclinical in vivo + in vitro | ±Palbociclib and/or fulvestrant | ■ Favorable | ♦ Yes | [185] | ||
| Preclinical-in vitro | Monotherapy | ■ Sensitive | ♦ Yes | [161] | |||
| Paxalisib Pan-PI3K/ mTOR | Brain | GBM | Clinical-PhII | Monotherapy | ■ Favorable | N.E. | [186] |
| Preclinical in vivo + in vitro | ±EGFR inhibitor (AZD-9291) | ■ Favorable | N.E. (WT-only) | [187] | |||
| Preclinical-in vivo | Monotherapy | ■ Sensitive | N.E. | [188,189] | |||
| Pictilisib PI3Kα/δ | Colon | --- | Preclinical-in vitro | Monotherapy | ■ Sensitive | ♦ No | [185] |
| Brain | GBM | Preclinical-in vitro | ±Temozolomide | ■ Sensitive | N.E. | [190] | |
| Head and neck | SCC | Preclinical-in vitro | Monotherapy | ■ Sensitive | ♦ No | [191,192] | |
| RLY-2608 PI3Kα (allosteric) | Breast | HR+ HER2- PIK3CA-mut | Clinical-case | +Fulvestrant | ■ Favorable | N.E. (All-mut) | [193] |
| --- | Preclinical in vivo + in vitro | ±Fulvestrant | ■ Favorable | ♦ Yes | |||
| Taselisib PI3Kα/δ/γ | Breast | HR+ HER2- | Clinical-PhIII | +Fulvestrant | ■ Minimal benefit | ♦ No | [194] |
| Clinical-PhII | +Fulvestrant | ■ Favorable | ♦ Yes | [195] | |||
| Clinical-PhIb | +HER2 inhibitors | ■ Favorable | ♦ No | [196] | |||
| --- | Preclinical-in vivo | Monotherapy | ■ Sensitive | N.E. (All-mut) | [185] | ||
| Colon | --- | Preclinical-in vitro | Monotherapy | ■ Sensitive | ♦ Yes | ||
| Head and neck | SCC | Preclinical in vivo + in vitro | Monotherapy | ■ Favorable | ♦ Yes | [191] | |
| Lung | SCC | Clinical-PhII | Monotherapy | ■ No benefit | N.E. (All-mut) | [197] | |
| Voxtalisib Pan-PI3K/ mTOR | Breast | HR+ HER2- | Clinical-PhI/II | +Letrozole | ■ Minimal benefit | ♦ No | [198] |
| Brain | GBM | Clinical-PhI | +Temozolomide | ■ Favorable | N.E. | [199] | |
| Preclinical in vivo + in vitro | ±Temozolomide | ■ Favorable | N.E. (WT-only) | [200] | |||
| Low-grade glioma | Preclinical in vivo + in vitro | Monotherapy | ■ Favorable | N.E. (WT-only) | [201] | ||
| Gynecological | Ovarian | Clinical-PhII | +MEK inhibitor (pimasertib) | ■ Minimal benefit | N.E. | [202] | |
| Endometrial | Preclinical-in vitro | ±MEK inhibitor (pimasertib) | ■ Sensitive | ♦ No | [203] | ||
| Prostate | --- | Preclinical-in vitro | Monotherapy | ■ Sensitive | N.E. | [204] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomás, A.; Pojo, M. PIK3CA Mutations: Are They a Relevant Target in Adult Diffuse Gliomas? Int. J. Mol. Sci. 2025, 26, 5276. https://doi.org/10.3390/ijms26115276
Tomás A, Pojo M. PIK3CA Mutations: Are They a Relevant Target in Adult Diffuse Gliomas? International Journal of Molecular Sciences. 2025; 26(11):5276. https://doi.org/10.3390/ijms26115276
Chicago/Turabian StyleTomás, Ana, and Marta Pojo. 2025. "PIK3CA Mutations: Are They a Relevant Target in Adult Diffuse Gliomas?" International Journal of Molecular Sciences 26, no. 11: 5276. https://doi.org/10.3390/ijms26115276
APA StyleTomás, A., & Pojo, M. (2025). PIK3CA Mutations: Are They a Relevant Target in Adult Diffuse Gliomas? International Journal of Molecular Sciences, 26(11), 5276. https://doi.org/10.3390/ijms26115276






