A Case Report to Reflect on the Origins of MMRd Mesonephric-like Ovarian Adenocarcinoma: Can It Be Defined as a Mϋllerian Neoplasm?
Abstract
1. Introduction
2. Materials and Methods
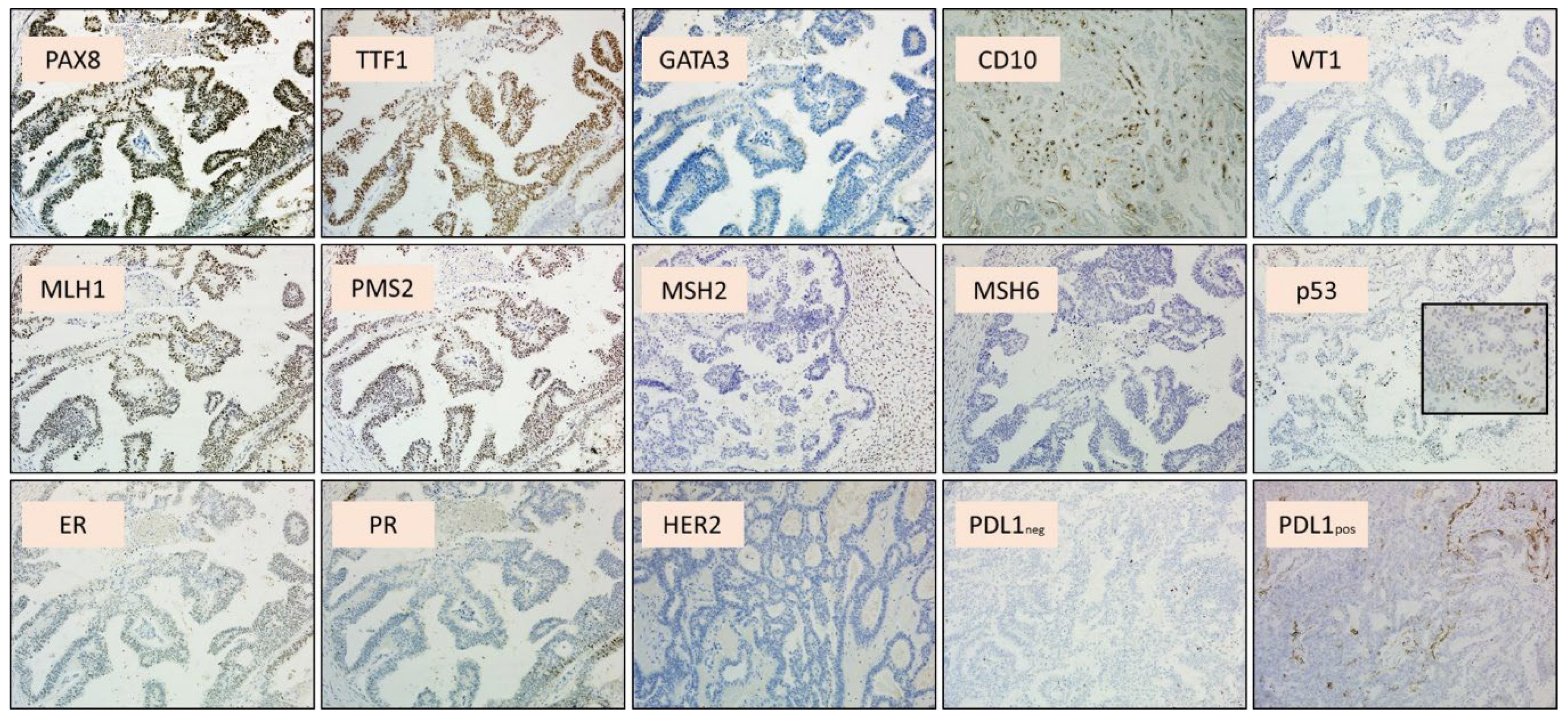
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Howitt, B.E.; Nucci, M.R. Mesonephric proliferations of the female genital tract. Pathology 2018, 50, 141–150. [Google Scholar] [CrossRef] [PubMed]
- McFarland, M.; Quick, C.M.; McCluggage, W.G. Hormone receptor-negative, thyroid transcription factor 1-positive uterine and ovarian adenocarcinomas: Report of a series of mesonephric-like adenocarcinomas. Histopathology 2016, 68, 1013–1020. [Google Scholar] [CrossRef]
- Koh, H.H.; Park, E.; Kim, H.S. Mesonephric-like Adenocarcinoma of the Ovary: Clinicopathological and Molecular Characteristics. Diagnostics 2022, 12, 326. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McCluggage, W.G. Progress in the pathological arena of gynecological cancers. Int. J. Gynecol. Obstet. 2021, 155, 107–114. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- WHO Classification of Tumours Editorial Board. Female genital tumours. In WHO Classification of Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- Kenny, S.L.; McBride, H.A.; Jamison, J.; McCluggage, W.G. Mesonephric adenocarcinomas of the uterine cervix and corpus: HPV-negative neoplasms that are commonly PAX8, CA125, and HMGA2 positive and that may be immunoreactive with TTF1 and hepatocyte nuclear factor 1-β. Am. J. Surg. Pathol. 2012, 36, 799–807. [Google Scholar] [CrossRef] [PubMed]
- McCluggage, W.; FRCPath, G. Mesonephric-like Adenocarcinoma of the Female Genital Tract: From Morphologic Observations to a Well-characterized Carcinoma with Aggressive Clinical Behavior. Adv. Anat. Pathol. 2022, 29, 208–216. [Google Scholar] [CrossRef]
- Mirkovic, J.; Olkhov-Mitsel, E.; Amemiya, Y.; Al-Hussaini, M.; Nofech-Mozes, S.; Djordjevic, B.; Kupets, R.; Seth, A.; McCluggage, W.G. Mesonephric-like adenocarcinoma of the female genital tract: Novel observations and detailed molecular characterisation of mixed tumours and mesonephric-like carcinosarcomas. Histopathology 2023, 82, 978–990. [Google Scholar] [CrossRef]
- Angelico, G.; Salvatorelli, L.; Tinnirello, G.; Santoro, A.; Zannoni, G.F.; Puzzo, L.; Magro, G. The first evidence of mismatch repair deficiency in mesonephric-like adenocarcinoma of the endometrium: Clinicopathological and molecular features of a case emphasising a possible endometrioid carcinogenesis. Histopathology 2024, 84, 1068–1070. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.H.; Howitt, B.E.; Kolin, D.L. From morphology to methylome: Epigenetic studies of Müllerian mesonephric-like adenocarcinoma reveal similarities to cervical mesonephric adenocarcinoma†. J. Pathol. 2024, 263, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Kommoss, F.K.; Lee, C.H.; Tessier-Cloutier, B.; Gilks, C.B.; Stewart, C.J.; von Deimling, A.; Köbel, M. Mesonephric-like adenocarcinoma harbours characteristic copy number variations and a distinct DNA methylation signature closely related to mesonephric adenocarcinoma of the cervix. J. Pathol. 2024, 262, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Moslemi, M.; Moradi, Y.; Dehghanbanadaki, H.; Afkhami, H.; Khaledi, M.; Sedighimehr, N.; Fathi, J.; Sohrabi, E. The association between ATM variants and risk of breast cancer: A systematic review and meta-analysis. BMC Cancer 2021, 21, 27. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marani, C.; Akaev, I.; Yeoh, C.C.; Walsh, E.; Rahimi, S. Cervical malignant mixed mesonephric tumour: A case report with local recurrence after six-years and next-generation sequencing analysis with particular reference to the ataxia telangiectasia mutated gene. Exp. Ther. Med. 2021, 21, 394. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seay, K.; Akanbi, T.; Bustamante, B.; Chaudhary, S.; Goldberg, G.L. Mesonephric-like adenocarcinoma of the ovary with co-existent endometriosis: A case report and review of the literature. Gynecol. Oncol. Rep. 2020, 34, 100657. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, H.G.; Kim, H.; Yeo, M.K.; Won, K.Y.; Kim, Y.S.; Han, G.H.; Kim, H.S.; Na, K. Mesonephric-like Adenocarcinoma of the Uterine Corpus: Comprehensive Analyses of Clinicopathological, Molecular, and Prognostic Characteristics With Retrospective Review of 237 Endometrial Carcinoma Cases. Cancer Genom. Proteom. 2022, 19, 526–539. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kwak, E.L.; Moberg, K.H.; Wahrer, D.C.; Quinn, J.E.; Gilmore, P.M.; Graham, C.A.; Hariharan, I.K.; Harkin, D.P.; Haber, D.A.; Bell, D.W. Infrequent mutations of Archipelago (hAGO, hCDC4, Fbw7) in primary ovarian cancer. Gynecol Oncol. 2005, 98, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Spruck, C.H.; Strohmaier, H.; Sangfelt, O.; Müller, H.M.; Hubalek, M.; Müller-Holzner, E.; Marth, C.; Widschwendter, M.; Reed, S.I. hCDC4 gene mutations in endometrial cancer. Cancer Res. 2002, 62, 4535–4539. [Google Scholar] [PubMed]
- Urick, M.E.; Yu, E.J.; Bell, D.W. High-risk endometrial cancer proteomic profiling reveals that FBXW7 mutation alters L1CAM and TGM2 protein levels. Cancer 2021, 127, 2905–2915. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Santoro, A.; Angelico, G.; Travaglino, A.; Inzani, F.; Arciuolo, D.; Valente, M.; D’Alessandris, N.; Scaglione, G.; Fiorentino, V.; Raffone, A.; et al. New Pathological and Clinical Insights in Endometrial Cancer in View of the Updated ESGO/ESTRO/ESP Guidelines. Cancers 2021, 13, 2623. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Köbel, M.; Yang, R.Z.; Kang, E.Y.; Al-Shamma, Z.; Cook, L.S.; Kinloch, M.; Carey, M.S.; Hopkins, L.; Nelson, G.S.; McManus, K.J.; et al. Survey of NF1 inactivation by surrogate immunohistochemistry in ovarian carcinomas. Gynecol. Oncol. 2023, 178, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.I.; Shah, N.; Tse, J.Y.; Killian, J.K.; Hemmerich, A.; Edgerly, C.; Haberberger, J.; Severson, E.A.; Huang, R.S.P.; Ramkissoon, S.H.; et al. Molecular profiling of mesonephric and mesonephric-like carcinomas of cervical, endometrial and ovarian origin. Gynecol. Oncol. Rep. 2020, 34, 100652. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Norris, E.J.; Jones, W.D.; Surleac, M.D.; Petrescu, A.J.; Destephanis, D.; Zhang, Q.; Hamadeh, I.; Kneisl, J.S.; Livasy, C.A.; Ganapathi, R.N.; et al. Clonal lineage of high grade serous ovarian cancer in a patient with neurofibromatosis type 1. Gynecol. Oncol. Rep. 2018, 23, 41–44. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Euscher, E.D.; Bassett, R.; Duose, D.Y.; Lan, C.; Wistuba, I.; Ramondetta, L.; Ramalingam, P.; Malpica, A. Mesonephric-like Carcinoma of the Endometrium: A Subset of Endometrial Carcinoma with an Aggressive Behavior. Am. J. Surg. Pathol. 2020, 44, 429–443. [Google Scholar] [CrossRef] [PubMed]
- Mjos, S.; Werner, H.M.J.; Birkeland, E.; Holst, F.; Berg, A.; Halle, M.K.; Tangen, I.L.; Kusonmano, K.; Mauland, K.K.; Oyan, A.M.; et al. PIK3CA exon9 mutations associate with reduced survival, and are highly concordant between matching primary tumors and metastases in endometrial cancer. Sci. Rep. 2017, 7, 10240. [Google Scholar] [CrossRef]
- Remmerie, M.; Janssens, V. PP2A: A Promising Biomarker and Therapeutic Target in Endometrial Cancer. Front. Oncol. 2019, 9, 462. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Flaherty, C. PPP2R1A Mutations Associated with Prolonged OS in High-Risk Endometrial Cancer. News Article, 31 January 2025. [Google Scholar]
- Wang, H.; Shang, Y.; Wang, E.; Xu, X.; Zhang, Q.; Qian, C.; Yang, Z.; Wu, S.; Zhang, T. MST1 mediates neuronal loss and cognitive deficits: A novel therapeutic target for Alzheimer’s disease. Prog. Neurobiol. 2022, 214, 102280. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Wang, Y.; Sun, L.; Zhou, S.; Xu, J.; Xing, D. MST1: A future novel target for cardiac diseases. Int. J. Biol. Macromol. 2023, 239, 124296. [Google Scholar] [CrossRef] [PubMed]
- Addante, F.; d’Amati, A.; Santoro, A.; Angelico, G.; Inzani, F.; Arciuolo, D.; Travaglino, A.; Raffone, A.; D’Alessandris, N.; Scaglione, G.; et al. Mismatch Repair Deficiency as a Predictive and Prognostic Biomarker in Endometrial Cancer: A Review on Immunohistochemistry Staining Patterns and Clinical Implications. Int. J. Mol. Sci. 2024, 25, 1056. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Balakrishnan, K.; Chen, Y.; Dong, J. Amplification of Hippo Signaling Pathway Genes Is Governed and Implicated in the Serous Subtype-Specific Ovarian Carcino-Genesis. Cancers 2024, 16, 1781. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cai, L.; Yang, C.; Gu, Y.; Dong, L.; Feng, W. Case Report: The first report of PPP2R1A mutations in mesonephric-like adenocarcinoma of endometrial carcinoma. Front. Oncol. 2023, 13, 1212648. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Santoro, A.; Angelico, G.; Narducci, N.; Travaglino, A.; Piermattei, A.; Inzani, F.; Arciuolo, D.; Addante, F.; Urtueta, B.P.; Tinnirello, G.; et al. Mesonephric-like adenocarcinoma of the endometrium: Detailed molecular characterization of the first case showing a morphological continuum from mesonephric-like metaplasia and atypical mesonephric-like hyperplasia to adenocarcinoma. Virchows Arch. 2025. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Alessandris, N.; Santoro, A.; Valente, M.; Scaglione, G.; Angelico, G.; Urtueta, B.P.; Narducci, N.; Duranti, S.; Addante, F.; Minucci, A.; et al. A Case Report to Reflect on the Origins of MMRd Mesonephric-like Ovarian Adenocarcinoma: Can It Be Defined as a Mϋllerian Neoplasm? Int. J. Mol. Sci. 2025, 26, 5245. https://doi.org/10.3390/ijms26115245
D’Alessandris N, Santoro A, Valente M, Scaglione G, Angelico G, Urtueta BP, Narducci N, Duranti S, Addante F, Minucci A, et al. A Case Report to Reflect on the Origins of MMRd Mesonephric-like Ovarian Adenocarcinoma: Can It Be Defined as a Mϋllerian Neoplasm? International Journal of Molecular Sciences. 2025; 26(11):5245. https://doi.org/10.3390/ijms26115245
Chicago/Turabian StyleD’Alessandris, Nicoletta, Angela Santoro, Michele Valente, Giulia Scaglione, Giuseppe Angelico, Belen Padial Urtueta, Nadine Narducci, Simona Duranti, Francesca Addante, Angelo Minucci, and et al. 2025. "A Case Report to Reflect on the Origins of MMRd Mesonephric-like Ovarian Adenocarcinoma: Can It Be Defined as a Mϋllerian Neoplasm?" International Journal of Molecular Sciences 26, no. 11: 5245. https://doi.org/10.3390/ijms26115245
APA StyleD’Alessandris, N., Santoro, A., Valente, M., Scaglione, G., Angelico, G., Urtueta, B. P., Narducci, N., Duranti, S., Addante, F., Minucci, A., & Zannoni, G. F. (2025). A Case Report to Reflect on the Origins of MMRd Mesonephric-like Ovarian Adenocarcinoma: Can It Be Defined as a Mϋllerian Neoplasm? International Journal of Molecular Sciences, 26(11), 5245. https://doi.org/10.3390/ijms26115245






