Integrated Metabolomics and Network Pharmacology to Reveal the Mechanisms of Forsythia suspensa Extract Against Respiratory Syncytial Virus
Abstract
:1. Introduction
2. Results
2.1. Component Analysis of FS
2.2. FS Significantly Inhibits RSV Infection-Induced Lung Tissue Pathology
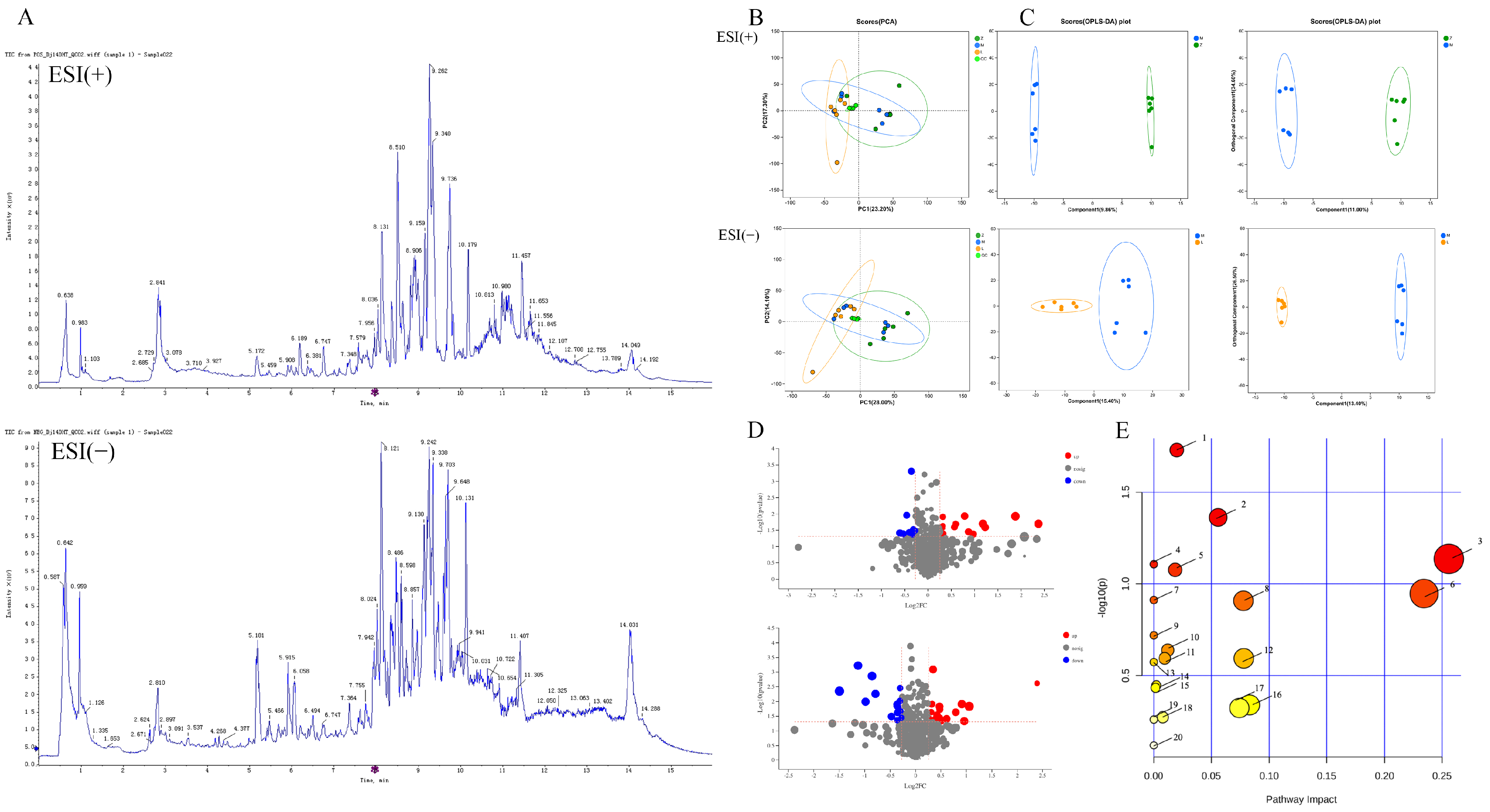
2.3. Metabolomic Analysis
2.3.1. PCA
2.3.2. Identification of Biomarkers
2.3.3. Metabolic Pathway and Network Analysis
2.4. Network Pharmacology Analysis
2.4.1. Interaction Between Compounds and Targets
2.4.2. Enrichment Analysis
2.5. Integrated Pathway Analysis of Targets and Metabolites
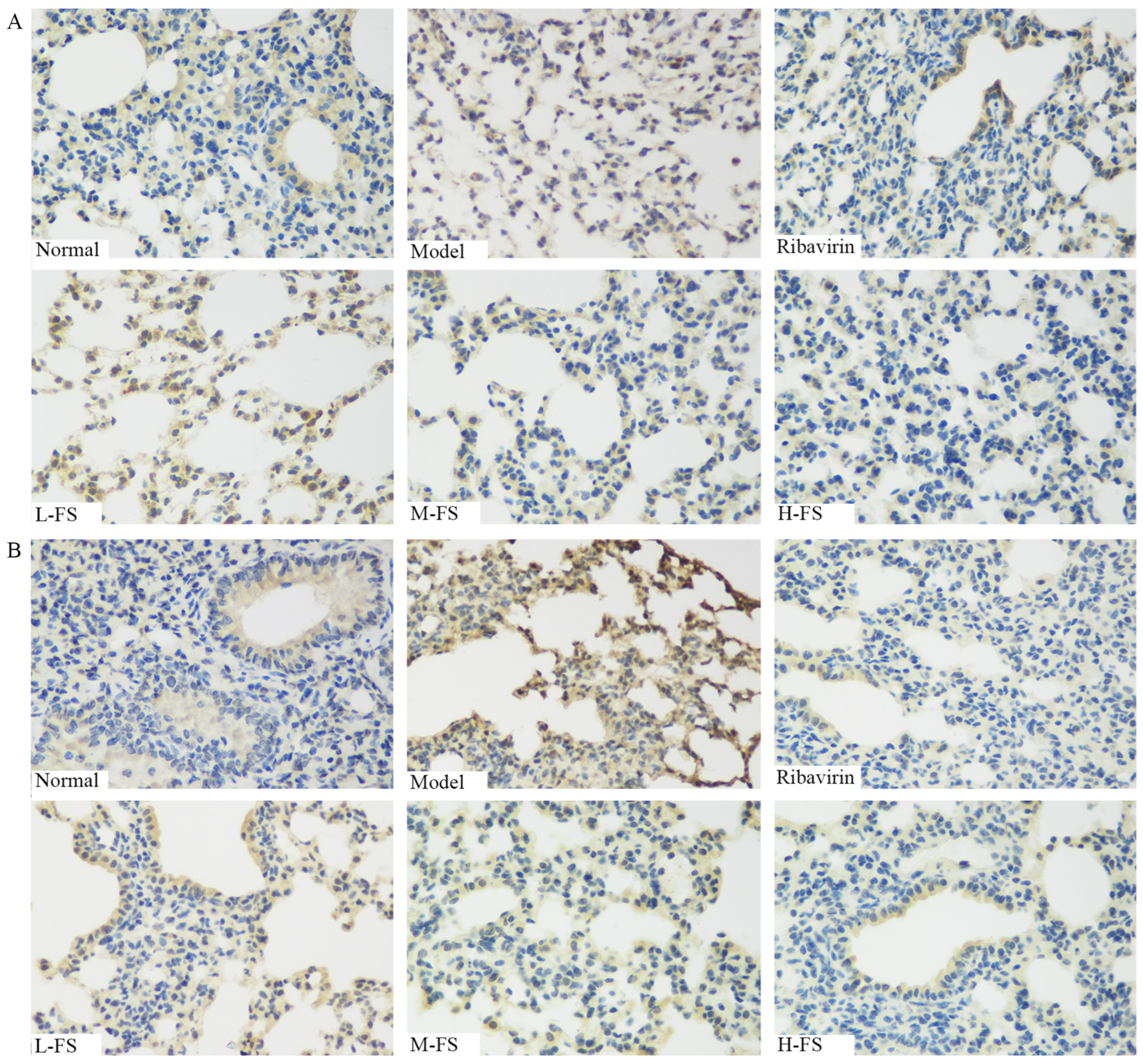
2.6. Immunohistochemistry
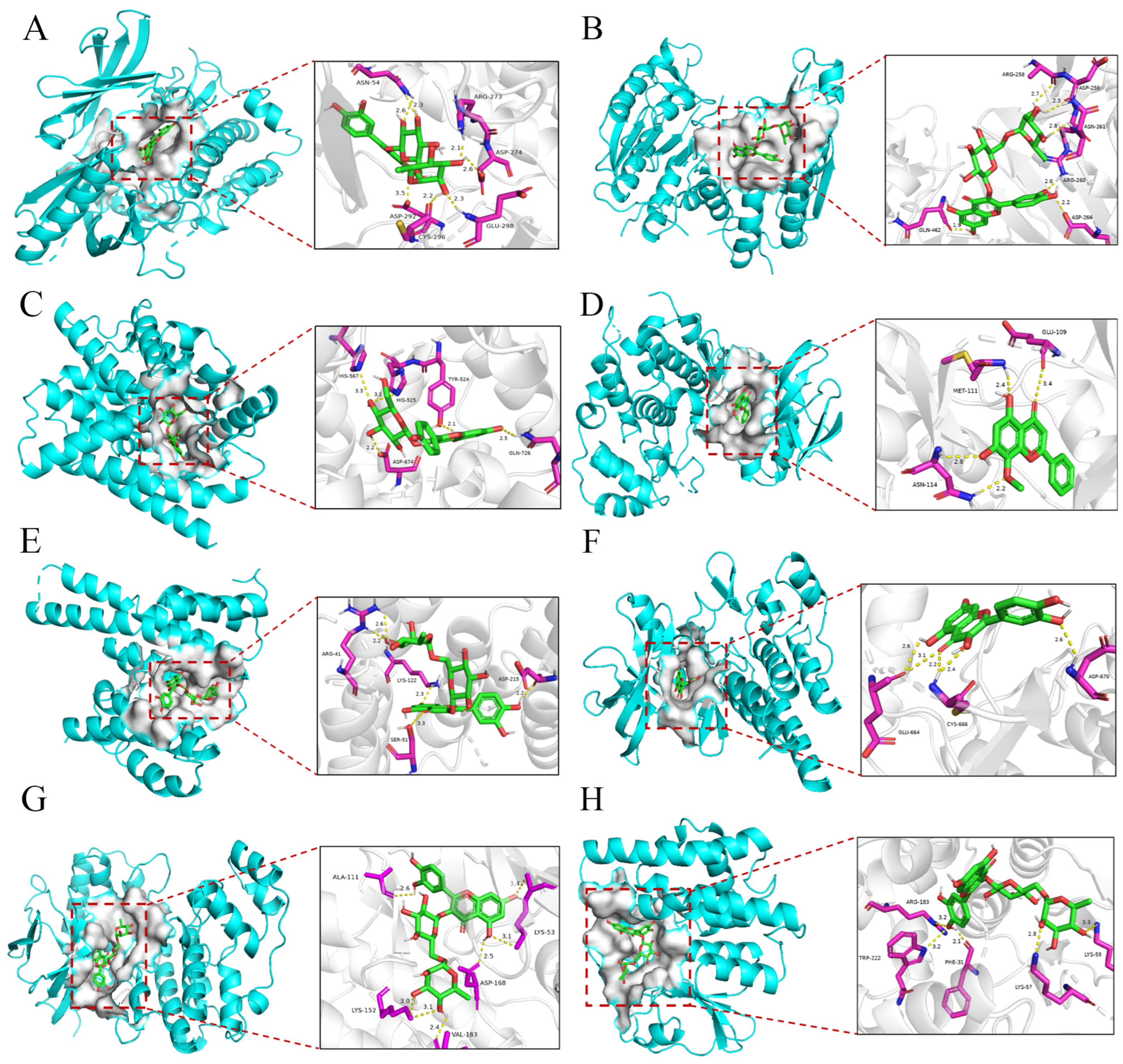
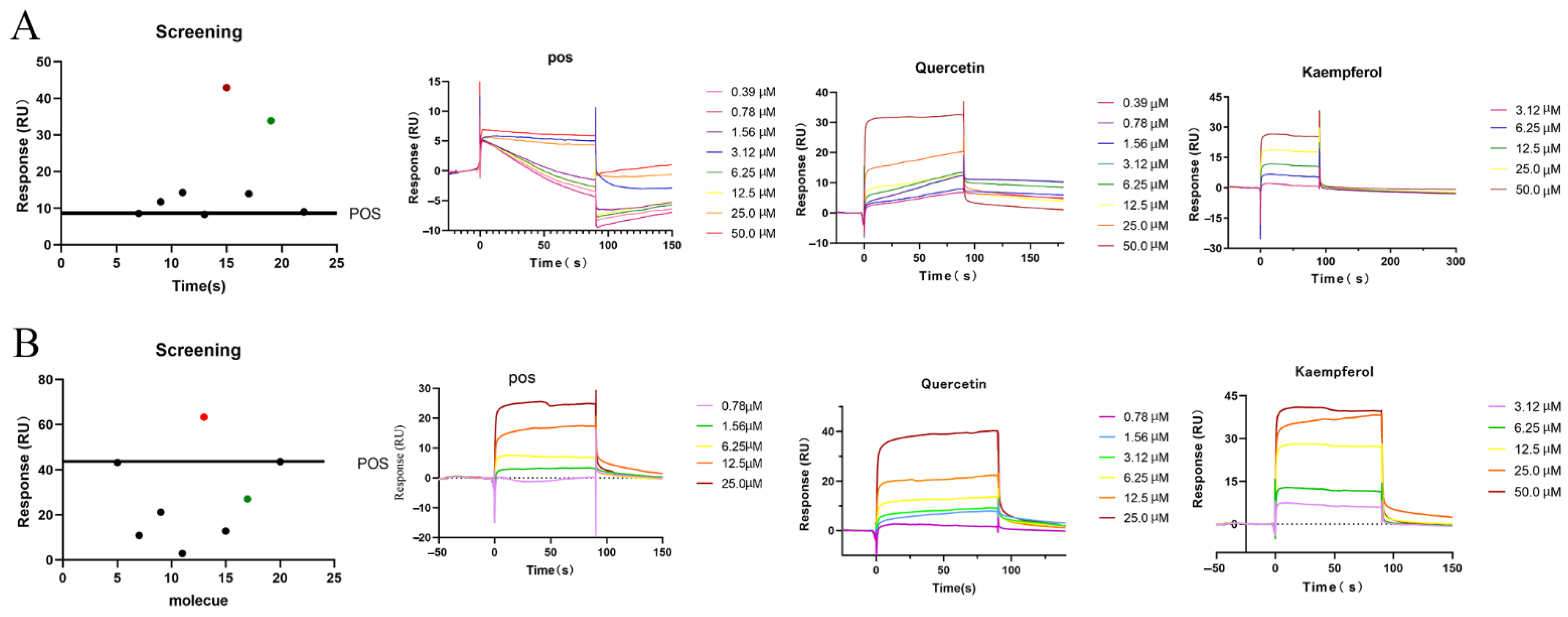
2.7. Interaction Validation
3. Discussion
4. Materials and Methods
4.1. Experimental Materials
4.1.1. Main Instruments
4.1.2. Reagents
4.1.3. Preparation and Component Analysis of FS Samples
4.1.4. RSV Virulent Strain
4.2. Experimental Animals
4.3. Experimental Animal Design
4.3.1. RSV Experimental Model
4.3.2. Dosing Regimen
4.4. Specimen Collection and Processing
4.5. Histopathology and Immunohistochemical Analysis
4.6. Metabolomics Study
4.6.1. Sample Preparation
4.6.2. Instrument Conditions
4.6.3. Multivariate Statistical Analysis
4.7. Network Pharmacology and Metabolomics Integration Analysis
4.8. Molecular Docking
4.9. Biacore Experiment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CysLT | Cysteine leukotriene |
| DAB | 3,3′-Diaminobenzidine |
| DEGs | Differentially Expressed Genes |
| ESI | Electrospray Ionization |
| FC | Fold change |
| FS | Forsythia suspensa extract |
| GO | Gene Ontology |
| HE | Hematoxylin-eosin staining |
| IHC | Immunohistochemistry |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LTC4 | Leukotriene C4 |
| OPLS-DA | Orthogonal Partial Least Squares Discriminant Analysis |
| PC | phosphatidylcholine |
| PCA | Principal Components Analysis |
| PPI | Protein-Protein Interaction |
| QC | Quality control |
| RdRp | RNA-dependent RNA Polymerase |
| RSV | Respiratory syncytial virus |
| SPR | Surface Plasmon Resonance |
| TCM | Traditional Chinese medicine |
| TCMSP | Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform |
| TIC | Total ion chromatograms |
| TLRs | Toll-like receptors |
| UHPLC | Ultra High-Performance Liquid Chromatograph |
References
- Schmidt, H.; Das, A.; Nam, H.; Yang, A.; Ison, M.G. Epidemiology and Outcomes of Hospitalized Adults with Respiratory Syncytial Virus: A 6-Year Retrospective Study. Influenza Other Respir. Viruses 2019, 13, 331–338. [Google Scholar] [CrossRef] [PubMed]
- You, D.; Saravia, J.; Siefker, D.; Shrestha, B.; Cormier, S.A. Crawling with Virus: Translational Insights from a Neonatal Mouse Model on the Pathogenesis of Respiratory Syncytial Virus in Infants. J. Virol. 2016, 90, 2–4. [Google Scholar] [CrossRef]
- Billard, M.-N.; Bont, L.J. Quantifying the RSV Immunity Debt Following COVID-19: A Public Health Matter. Lancet Infect. Dis. 2023, 23, 3–5. [Google Scholar] [CrossRef]
- Ouyang, Y.; Liao, H.; Hu, Y.; Luo, K.; Hu, S.; Zhu, H. Innate Immune Evasion by Human Respiratory Syncytial Virus. Front. Microbiol. 2022, 13, 865592. [Google Scholar] [CrossRef]
- Nair, H.; Nokes, D.J.; Gessner, B.D.; Dherani, M.; Madhi, S.A.; Singleton, R.J.; O’Brien, K.L.; Roca, A.; Wright, P.F.; Bruce, N.; et al. Global Burden of Acute Lower Respiratory Infections Due to Respiratory Syncytial Virus in Young Children: A Systematic Review and Meta-Analysis. Lancet 2010, 375, 1545–1555. [Google Scholar] [CrossRef] [PubMed]
- Borchers, A.T.; Chang, C.; Gershwin, M.E.; Gershwin, L.J. Respiratory Syncytial Virus—A Comprehensive Review. Clin. Rev. Allergy Immunol. 2013, 45, 331–379. [Google Scholar] [CrossRef] [PubMed]
- Paynter, S. Humidity and Respiratory Virus Transmission in Tropical and Temperate Settings. Epidemiol. Infect. 2015, 143, 1110–1118. [Google Scholar] [CrossRef]
- Abu-Raya, B.; Viñeta Paramo, M.; Reicherz, F.; Lavoie, P.M. Why Has the Epidemiology of RSV Changed during the COVID-19 Pandemic? EClinicalMedicine 2023, 61, 102089. [Google Scholar] [CrossRef]
- den Hartog, G.; van Kasteren, P.B.; Schepp, R.M.; Teirlinck, A.C.; van der Klis, F.R.M.; van Binnendijk, R.S. Decline of RSV-Specific Antibodies during the COVID-19 Pandemic. Lancet Infect. Dis. 2023, 23, 23–25. [Google Scholar] [CrossRef]
- Wang, Z.; Xia, Q.; Liu, X.; Liu, W.; Huang, W.; Mei, X.; Luo, J.; Shan, M.; Lin, R.; Zou, D.; et al. Phytochemistry, Pharmacology, Quality Control and Future Research of Forsythia suspensa (Thunb.) Vahl: A Review. J. Ethnopharmacol. 2018, 210, 318–339. [Google Scholar] [CrossRef]
- Dong, Z.; Lu, X.; Tong, X.; Dong, Y.; Tang, L.; Liu, M. Forsythiae Fructus: A Review on Its Phytochemistry, Quality Control, Pharmacology and Pharmacokinetics. Molecules 2017, 22, 1466. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yu, H.; Wang, J.; Lei, W.; Gao, J.; Qiu, X.; Wang, J. The Complete Chloroplast Genome Sequences of the Medicinal Plant Forsythia suspensa (Oleaceae). Int. J. Mol. Sci. 2017, 18, 2288. [Google Scholar] [CrossRef]
- Law, A.H.-Y.; Yang, C.L.-H.; Lau, A.S.-Y.; Chan, G.C.-F. Antiviral Effect of Forsythoside A from Forsythia suspensa (Thunb.) Vahl Fruit against Influenza A Virus through Reduction of Viral M1 Protein. J. Ethnopharmacol. 2017, 209, 236–247. [Google Scholar] [CrossRef]
- Zhao, L.; Xiang, K.-L.; Liu, R.-X.; Xie, Z.-P.; Zhang, S.-M.; Dai, S.-J. Anti-Inflammatory and Anti-Viral Labdane Diterpenoids from the Fruits of Forsythia suspensa. Bioorg. Chem. 2020, 96, 103651. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sun, L.-T.; Zhao, L.; Yue, X.-D.; Dai, S.-J. New C9 -Monoterpenoid Alkaloids Featuring a Rare Skeleton with Anti-Inflammatory and Antiviral Activities from Forsythia suspensa. Chem. Biodivers. 2022, 19, e202100668. [Google Scholar] [CrossRef]
- Li, S.; Zhang, B. Traditional Chinese Medicine Network Pharmacology: Theory, Methodology and Application. Chin. J. Nat. Med. 2013, 11, 110–120. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.-Y.; Zheng, J.-H.; Li, S. TCM Network Pharmacology: A New Trend towards Combining Computational, Experimental and Clinical Approaches. Chin. J. Nat. Med. 2021, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Chen, Y.; Wang, C.; Xiao, W.; Chen, S.; Zhang, S.; Yang, L.; Li, Y. Systems Pharmacology Dissection of Multi-Scale Mechanisms of Action of Huo-Xiang-Zheng-Qi Formula for the Treatment of Gastrointestinal Diseases. Front. Pharmacol. 2018, 9, 1448. [Google Scholar] [CrossRef]
- Zhang, W.; Huai, Y.; Miao, Z.; Qian, A.; Wang, Y. Systems Pharmacology for Investigation of the Mechanisms of Action of Traditional Chinese Medicine in Drug Discovery. Front. Pharmacol. 2019, 10, 743. [Google Scholar] [CrossRef]
- Lin, L.; Yan, H.; Chen, J.; Xie, H.; Peng, L.; Xie, T.; Zhao, X.; Wang, S.; Shan, J. Application of Metabolomics in Viral Pneumonia Treatment with Traditional Chinese Medicine. Chin. Med. 2019, 14, 8. [Google Scholar] [CrossRef]
- Wang, X.; Sun, H.; Zhang, A.; Sun, W.; Wang, P.; Wang, Z. Potential Role of Metabolomics Apporoaches in the Area of Traditional Chinese Medicine: As Pillars of the Bridge between Chinese and Western Medicine. J. Pharm. Biomed. Anal. 2011, 55, 859–868. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, Y.; Jiang, H.; Yang, J.; Wang, Q.; Du, Y.; Xu, H. Integrated Strategy for Accurately Screening Biomarkers Based on Metabolomics Coupled with Network Pharmacology. Talanta 2020, 211, 120710. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Tarcea, V.G.; Karnovsky, A.; Mirel, B.R.; Weymouth, T.E.; Beecher, C.W.; Cavalcoli, J.D.; Athey, B.D.; Omenn, G.S.; Burant, C.F.; et al. Metscape: A Cytoscape Plug-in for Visualizing and Interpreting Metabolomic Data in the Context of Human Metabolic Networks. Bioinformatics 2010, 26, 971–973. [Google Scholar] [CrossRef]
- Nygaard, U.; Hartling, U.B.; Nielsen, J.; Vestergaard, L.S.; Dungu, K.H.S.; Nielsen, J.S.A.; Sellmer, A.; Matthesen, A.T.; Kristensen, K.; Holm, M. Hospital Admissions and Need for Mechanical Ventilation in Children with Respiratory Syncytial Virus before and during the COVID-19 Pandemic: A Danish Nationwide Cohort Study. Lancet Child. Adolesc. Health 2023, 7, 171–179. [Google Scholar] [CrossRef]
- Gajewski, A.; Kośmider, A.; Nowacka, A.; Puk, O.; Wiciński, M. Potential of Herbal Products in Prevention and Treatment of COVID-19. Literature Review. Biomed. Pharmacother. 2021, 143, 112150. [Google Scholar] [CrossRef]
- Shen, P.; Li, J.; Tu, S.; Wu, Y.; Peng, Y.; Chen, G.; Chen, C. Positive Effects of Lianhuaqingwen Granules in COVID-19 Patients: A Retrospective Study of 248 Cases. J. Ethnopharmacol. 2021, 278, 114220. [Google Scholar] [CrossRef]
- Lin, H.; Wang, X.; Liu, M.; Huang, M.; Shen, Z.; Feng, J.; Yang, H.; Li, Z.; Gao, J.; Ye, X. Exploring the Treatment of COVID-19 with Yinqiao Powder Based on Network Pharmacology. Phytother. Res. 2021, 35, 2651–2664. [Google Scholar] [CrossRef] [PubMed]
- Eicher, T.; Kinnebrew, G.; Patt, A.; Spencer, K.; Ying, K.; Ma, Q.; Machiraju, R.; Mathé, A.E.A. Metabolomics and Multi-Omics Integration: A Survey of Computational Methods and Resources. Metabolites 2020, 10, 202. [Google Scholar] [CrossRef]
- Fivash, M.; Towler, E.M.; Fisher, R.J. BIAcore for Macromolecular Interaction. Curr. Opin. Biotechnol. 1998, 9, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Bhattu, M.; Tripathi, A.; Verma, M.; Acevedo, R.; Kumar, P.; Rajput, V.D.; Singh, J. Potential Medicinal Plants to Combat Viral Infections: A Way Forward to Environmental Biotechnology. Environ. Res. 2023, 227, 115725. [Google Scholar] [CrossRef]
- Ottenio de Lourenço, I.; Toscano Pedroso Quintino, E.; Henrique Pereira, M.; Sprengel Lima, C.; Campos Araújo, G.; Octávio Regasini, L.; Alves de Melo, F.; Pereira de Souza, F.; Andres Fossey, M.; Putinhon Caruso, Í. Biophysical Studies of the Interaction of hRSV Non-Structural 1 Protein with Natural Flavonoids and Their Acetylated Derivatives by Spectroscopic Techniques and Computational Simulations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 283, 121751. [Google Scholar] [CrossRef] [PubMed]
- Lopes, B.R.P.; da Costa, M.F.; Genova Ribeiro, A.; da Silva, T.F.; Lima, C.S.; Caruso, I.P.; de Araujo, G.C.; Kubo, L.H.; Iacovelli, F.; Falconi, M.; et al. Quercetin Pentaacetate Inhibits in Vitro Human Respiratory Syncytial Virus Adhesion. Virus Res. 2020, 276, 197805. [Google Scholar] [CrossRef] [PubMed]
- Gomes, D.E.; Caruso, Í.P.; de Araujo, G.C.; de Lourenço, I.O.; de Melo, F.A.; Cornélio, M.L.; Fossey, M.A.; de Souza, F.P. Experimental Evidence and Molecular Modeling of the Interaction between hRSV-NS1 and Quercetin. Int. J. Biol. Macromol. 2016, 85, 40–47. [Google Scholar] [CrossRef]
- Duan, T.; Du, Y.; Xing, C.; Wang, H.Y.; Wang, R.-F. Toll-Like Receptor Signaling and Its Role in Cell-Mediated Immunity. Front. Immunol. 2022, 13, 812774. [Google Scholar] [CrossRef]
- Liu, S.; Gao, L.; Wang, X.; Xing, Y. Respiratory Syncytial Virus Infection Inhibits TLR4 Signaling via Up-Regulation of miR-26b. Cell Biol. Int. 2015, 39, 1376–1383. [Google Scholar] [CrossRef]
- Voelker, D.R.; Numata, M. Phospholipid Regulation of Innate Immunity and Respiratory Viral Infection. J. Biol. Chem. 2019, 294, 4282–4289. [Google Scholar] [CrossRef]
- Canovas, B.; Nebreda, A.R. Diversity and Versatility of P38 Kinase Signalling in Health and Disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 346–366. [Google Scholar] [CrossRef]
- Madkour, M.M.; Anbar, H.S.; El-Gamal, M.I. Current Status and Future Prospects of P38α/MAPK14 Kinase and Its Inhibitors. Eur. J. Med. Chem. 2021, 213, 113216. [Google Scholar] [CrossRef]
- Tian, P.; Wang, Y.; Liu, H.; Yang, Y.; Wu, X.; Wei, H.; Chen, T. Preparation and Evaluation of the Fully Humanized Monoclonal Antibody GD-mAb Against Respiratory Syncytial Virus. Front. Cell Infect. Microbiol. 2019, 9, 275. [Google Scholar] [CrossRef]
- Sanchez, E.L.; Lagunoff, M. Viral Activation of Cellular Metabolism. Virology 2015, 479–480, 609–618. [Google Scholar] [CrossRef]
- Tchesnokov, E.P.; Feng, J.Y.; Porter, D.P.; Götte, M. Mechanism of Inhibition of Ebola Virus RNA-Dependent RNA Polymerase by Remdesivir. Viruses 2019, 11, 326. [Google Scholar] [CrossRef]
- Shan, J.; Qian, W.; Shen, C.; Lin, L.; Xie, T.; Peng, L.; Xu, J.; Yang, R.; Ji, J.; Zhao, X. High-Resolution Lipidomics Reveals Dysregulation of Lipid Metabolism in Respiratory Syncytial Virus Pneumonia Mice. RSC Adv. 2018, 8, 29368–29377. [Google Scholar] [CrossRef] [PubMed]
- Faden, H.; Kaul, T.N.; Ogra, P.L. Activation of Oxidative and Arachidonic Acid Metabolism in Neutrophils by Respiratory Syncytial Virus Antibody Complexes: Possible Role in Disease. J. Infect. Dis. 1983, 148, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Fan, P.; Chen, M.; Xu, Y.; Zhao, D. miRNAs and Leukotrienes in Respiratory Syncytial Virus Infection. Front. Pediatr. 2021, 9, 602195. [Google Scholar] [CrossRef]
- Griese, M. Pulmonary Surfactant in Health and Human Lung Diseases: State of the Art. Eur. Respir. J. 1999, 13, 1455–1476. [Google Scholar] [CrossRef]
- Huang, Q.; Lei, H.; Ding, L.; Wang, Y. Stimulated Phospholipid Synthesis Is Key for Hepatitis B Virus Replications. Sci. Rep. 2019, 9, 12989. [Google Scholar] [CrossRef]
- Akimov, S.A.; Polynkin, M.A.; Jiménez-Munguía, I.; Pavlov, K.V.; Batishchev, O.V. Phosphatidylcholine Membrane Fusion Is pH-Dependent. Int. J. Mol. Sci. 2018, 19, 1358. [Google Scholar] [CrossRef]
- Sun, L.; Chen, A.; Yang, Z.; Chen, J.J.; Guan, W.; Wu, J.; Qin, S.; Zhong, N. Respiratory Syncytial Virus Induces Leukotriene C4 Synthase Expression in Bronchial Epithelial Cells. Respirology 2013, 18 (Suppl. 3), 40–46. [Google Scholar] [CrossRef]
- China Pharmacopoeia Committee. Pharmacopoeia of the People’s Republic of China: Volume I, 2020; China Medical Science Press: Beijing, China, 2020; p. 172. [Google Scholar]
- Eberlein, V.; Ahrends, M.; Bayer, L.; Finkensieper, J.; Besecke, J.K.; Mansuroglu, Y.; Standfest, B.; Lange, F.; Schopf, S.; Thoma, M.; et al. Mucosal Application of a Low-Energy Electron Inactivated Respiratory Syncytial Virus Vaccine Shows Protective Efficacy in an Animal Model. Viruses 2023, 15, 1846. [Google Scholar] [CrossRef]
- Ma, G.; Xu, Z.; Li, C.; Zhou, F.; Hu, B.; Guo, J.; Ke, C.; Chen, L.; Zhang, G.; Lau, H.; et al. Induction of Neutralizing Antibody Responses by AAV5-Based Vaccine for Respiratory Syncytial Virus in Mice. Front. Immunol. 2024, 15, 1451433. [Google Scholar] [CrossRef]
- Attia, S.M.; Al-Khalifa, M.K.; Al-Hamamah, M.A.; Alotaibi, M.R.; Attia, M.S.M.; Ahmad, S.F.; Ansari, M.A.; Nadeem, A.; Bakheet, S.A. Vorinostat Is Genotoxic and Epigenotoxic in the Mouse Bone Marrow Cells at the Human Equivalent Doses. Toxicology 2020, 441, 152507. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Ding, J.; Wang, P.; Zhang, G.; Wang, D.; Ma, Q.; Li, N.; Sun, T. Anti-Respiratory Syncytial Virus Mechanism of Houttuynia Cordata Thunb Exploration Based on Network Pharmacology. Comb. Chem. High Throughput Screen. 2021, 24, 1137–1150. [Google Scholar] [CrossRef] [PubMed]

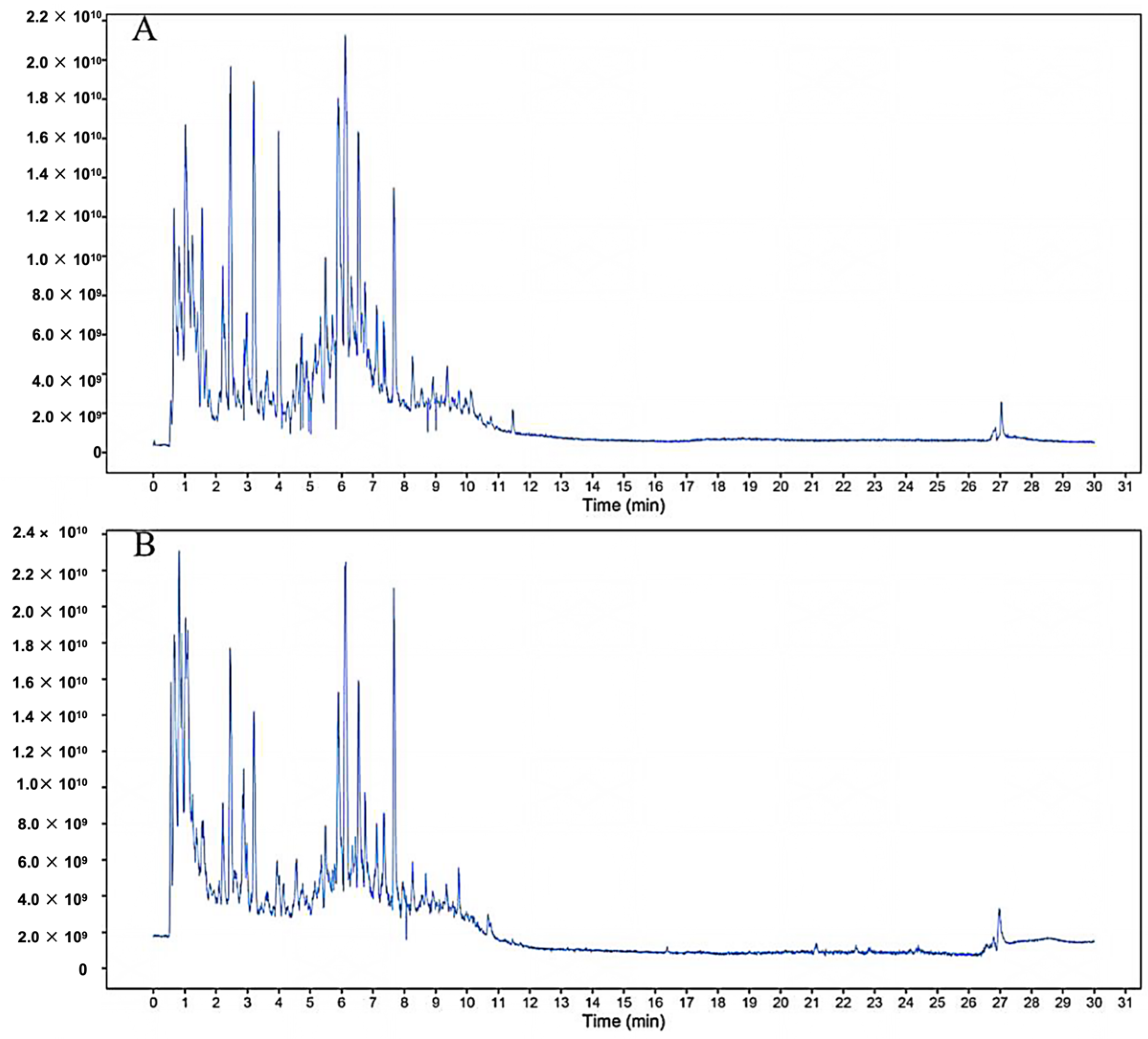



| NO. | Compound | Structure | Molecular Formula | Ion Mode | Mzmed | RT/min | Peak Area |
|---|---|---|---|---|---|---|---|
| 1 | CITRATE | 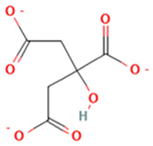 | C6H8O7 | [M − H]− | 191.0 | 0.82 | 19,998,699,402 |
| 2 | Chlorogenic acid | 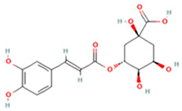 | C16H18O9 | [M − H]− | 353.1 | 3.56 | 8,319,446,995 |
| 3 | 2-(3,4-dihydroxy phenyl)-5,7-dihydroxy-3-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6- [[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxymethyl]oxan-2-yl]oxychromen-4-one | 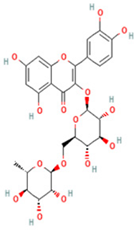 | C27H30O16 | [M + H]+ | 611.2 | 5.91 | 5,364,986,151 |
| 4 | Caffeic acid | 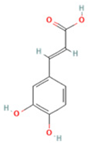 | C9H8O4 | [M−H]− | 179.0 | 4.00 | 3,761,440,238 |
| 5 | Kaempferol | 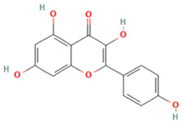 | C15H10O6 | [M + H]+ | 287.1 | 6.38 | 1,078,894,149 |
| 6 | Quercetin-3-O-galactoside | 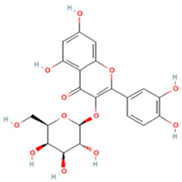 | C21H20O12 | [M − H]− | 463.1 | 6.08 | 488,743,839.5 |
| 7 | Forsythin | 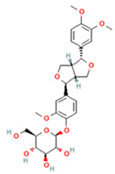 | C27H34O11 | [M + HCOO]− | 579.2 | 7.73 | 324,696,124.3 |
| 8 | Phillygenin | 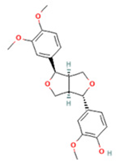 | C21H24O6 | [M + H]+ | 373.2 | 7.77 | 311,462,753.6 |
| 9 | Astragalin | 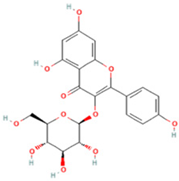 | C21H20O11 | [M + H]+ | 449.1 | 6.19 | 153,726,348 |
| 10 | Cinnamic acid |  | C9H8O2 | [M − H]− | 147.0 | 6.91 | 94,867,226.21 |
| 11 | 4-Methylcatechol | 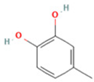 | C7H8O2 | [M − H]− | 123.0 | 2.69 | 92,651,547.51 |
| 12 | Gardenoside |  | C17H24O11 | [M − H]− | 403.1 | 1.07 | 91,742,137.48 |
| 13 | Salidroside | 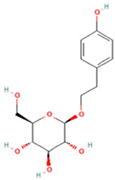 | C14H20O7 | [M − H]− | 299.1 | 2.98 | 90,911,531.36 |
| 14 | Pinoresinol 4-O-glucoside | 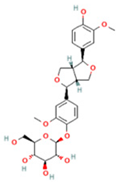 | C26H32O11 | [M − H]− | 519.2 | 6.56 | 80,924,386.53 |
| 15 | 2-Hydroxycinnamic acid, predominantly trans | 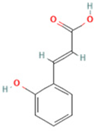 | C9H8O3 | [M + H]+ | 165.1 | 3.82 | 37,618,566.4 |
| 16 | Quercetin | 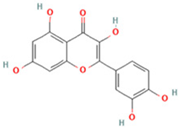 | C15H10O7 | [M − H]− | 301.0 | 7.88 | 31,019,261.86 |
| 17 | Forsythoside E |  | C20H30O12 | [M + H]+ | 463.2 | 3.19 | 26,764,237.96 |
| 18 | (+)-Pinoresinol | 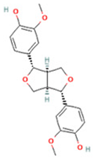 | C20H22O6 | [M − H]− | 357.1 | 6.56 | 18,230,104.05 |
| 19 | arctiin | 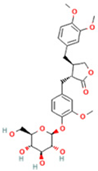 | C27H34O11 | [M − H]− | 533.2 | 9.92 | 14,192,501.17 |
| 20 | Gallic acid | 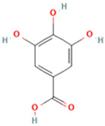 | C7H6O5 | [M − H]− | 169.0 | 4.16 | 10,107,393.13 |
| 21 | Vanillic acid | 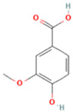 | C8H8O4 | [M + H]+ | 169.0 | 3.92 | 8,778,175.1 |
| 22 | 7-Hydroxycoumarin | 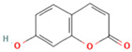 | C9H6O3 | [M − H]− | 161.0 | 2.97 | 7,050,431.305 |
| 23 | Syringic acid |  | C9H10O5 | [M + H]+ | 199.1 | 2.38 | 3,230,285.407 |
| 24 | Ferulic acid | 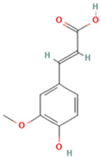 | C10H10O4 | [M + H]+ | 195.1 | 4.40 | 2,614,647.744 |
| 25 | Ursolic acid | 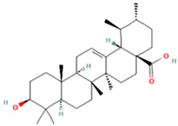 | C30H48O3 | [M + H − H2O]− | 439.4 | 15.67 | 1,532,176.398 |
| NO. | Metabolite | Structure | RT | M/Z | Formula | M vs. Z | L vs. M |
|---|---|---|---|---|---|---|---|
| 1 | L-Proline | 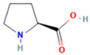 | 0.5312 | 116.0704 | C5H9NO2 | ↑ | ↓ |
| 2 | Spermine | 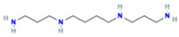 | 0.5507 | 203.2223 | C10H26N4 | ↓ | ↑ |
| 3 | Inosine | 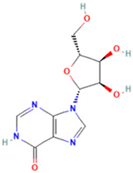 | 0.6451 | 269.0876 | C10H12N4O5 | ↑ | ↓ |
| 4 | Uridine diphosphate glucose | 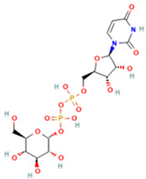 | 0.9191 | 584.0883 | C15H24N2O17P2 | ↑ | ↓ |
| 5 | Uracil |  | 0.9776 | 113.0344 | C4H4N2O2 | ↓ | ↑ |
| 6 | Hypoxanthine | 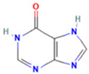 | 1.1476 | 137.0454 | C5H4N4O | ↑ | ↓ |
| 7 | Xanthosine | 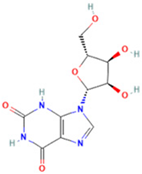 | 1.5070 | 285.0824 | C10H12N4O6 | ↓ | ↑ |
| 8 | Glutathionate(1-) | 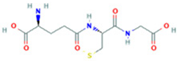 | 3.7882 | 330.0737 | C10H17N3O6S | ↓ | ↑ |
| 9 | Prostaglandin I2 | 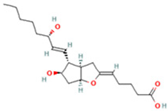 | 5.4683 | 353.232 | C20H32O5 | ↓ | ↓ |
| 10 | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al | 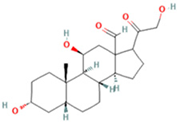 | 5.5731 | 382.2612 | C21H32O5 | ↓ | ↑ |
| 11 | Glycocholic Acid |  | 5.8629 | 466.3164 | C26H43NO6 | ↓ | ↑ |
| 12 | Leukotriene C4 | 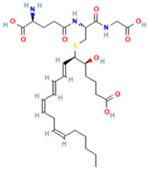 | 6.3286 | 658.3367 | C30H47N3O9S | ↑ | ↓ |
| 13 | PC(18:4(6Z,9Z,12Z,15Z)/ 18:4(6Z,9Z,12Z,15Z)) | 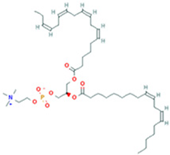 | 7.6699 | 387.7545 | C44H72NO8P | ↓ | ↑ |
| 14 | LysoPA(0:0/18:0) | 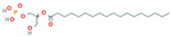 | 9.9456 | 421.2713 | C21H43O7P | ↓ | ↑ |
| NO. | Mol ID | Compound | MW | OB (%) | DL | HL |
|---|---|---|---|---|---|---|
| 26 | MOL000006 | luteolin | 286.25 | 36.16 | 0.25 | 15.94 |
| 27 | MOL000173 | wogonin | 284.28 | 30.68 | 0.23 | 17.75 |
| 28 | MOL000211 | Mairin | 456.78 | 55.38 | 0.78 | 8.87 |
| 29 | MOL000358 | beta-sitosterol | 414.79 | 36.91 | 0.75 | 5.36 |
| 30 | MOL000791 | bicuculline | 367.38 | 69.67 | 0.88 | 15.83 |
| 31 | MOL003281 | 20(S)-dammar-24-ene-3β,20-diol-3-acetate | 486.86 | 40.23 | 0.82 | 9.14 |
| 32 | MOL003283 | (2R,3R,4S)-4-(4-hydroxy-3-methoxy-phenyl)-7-methoxy-2,3-dimethylol-tetralin-6-ol | 360.44 | 66.51 | 0.39 | 1.26 |
| 33 | MOL003290 | (3R,4R)-3,4-bis[(3,4-dimethoxy phenyl)methyl]oxolan-2-one | 386.48 | 52.30 | 0.48 | 3.07 |
| 34 | MOL003295 | (+)-pinoresinol monomethyl ether | 372.45 | 53.08 | 0.57 | 3.02 |
| 35 | MOL003306 | ACon1_001697 | 372.45 | 85.12 | 0.57 | 2.12 |
| 36 | MOL003308 | (+)-pinoresinol monomethyl ether-4-D-beta-glucoside_qt | 372.45 | 61.20 | 0.57 | 2.90 |
| 37 | MOL003315 | 3beta-Acetyl-20,25-epoxydammarane-24alpha-ol | 502.86 | 33.07 | 0.79 | 7.82 |
| 38 | MOL003322 | Forsythinol | 372.45 | 81.25 | 0.57 | 2.72 |
| 39 | MOL003344 | β-amyrin acetate | 468.84 | 42.06 | 0.74 | 1.98 |
| 40 | MOL003347 | hyperforin | 536.87 | 44.03 | 0.60 | 2.15 |
| 41 | MOL003348 | adhyperforin | 550.90 | 44.03 | 0.61 | 0.84 |
| 42 | MOL003365 | Lactucasterol | 426.75 | 40.99 | 0.85 | 5.53 |
| 43 | MOL003370 | Onjixanthone I | 302.30 | 79.16 | 0.30 | 14.86 |
| Metabolic Pathway | Differentially Expressed Gene | Potential Difference Marker |
|---|---|---|
| Arachidonic acid metabolism | PTGS2, ALOX5, ALOX12, CYP1A2, CYP3A4, CYP2C9 | PCs |
| Glycerol phospholipid metabolism | AKR1B1 | Glycerophosphocholine, PCs, LysoPA(0:0/18:0), LysoPC(20:0/0:0) |
| Leukotriene metabolism | ALOX5, CYP1A2, CYP3A4, CYP2C9 | Leukotriene C4 |
| Target | Molecule | Types of Bonds | Residues | Binding Energy (kcal/mol) |
|---|---|---|---|---|
| AKT1 | Rutin | Hydrogen Bond | ASN-54, ARG-273, ASP-274, ASP-292, CYS-296, GLU-298 | −11.6 |
| Hydrophobic Interaction | LEU-264, GLN-79, VAL-270, ASN-53, TRP-80 | |||
| π-Stacking (parallel) | TRP-80 | |||
| CASP8 | Rutin | Hydrogen Bond | ARG-258, ASP-259, ASN-261, ARG-260, ASP-266, GLN-462 | −7.5 |
| Hydrophobic Interaction | TRP-420, VAL-406, ARG-258 | |||
| IL6 | Rutin | Hydrogen Bond | ASP-674, GLN-726, TYR-524, HIS-525, HIS-567 | −10.5 |
| Hydrophobic Interaction | ILE-692, PHE-696, LEU-635 | |||
| π-Stacking (parallel) | PHE-729 | |||
| MAPK8 | Wogonin | Hydrogen Bond | GLU-109, MET-111, ASN-114 | −9.3 |
| Hydrophobic Interaction | LYS-55, VAL-40, ILE-32 | |||
| RELA | Rutin | Hydrogen Bond | LYS-122, SER-51, ASP-215, ARG-41 | −8.1 |
| Hydrophobic Interaction | ILE-46, ILE-219, ASP-215 | |||
| TNF | Quercetin | Hydrogen Bond | GLU-664, CYS-666, ASP-670 | −9.3 |
| Hydrophobic Interaction | ALA-614, VAL-596, LEU-588, ALA-800, LEU-785, PHE-797 | |||
| p38α·MAPK14 | Rutin | Hydrogen Bond | ALA-111, LYS-53, LYS-152, VAL-183, ASP-168 | −10.1 |
| Hydrophobic Interaction | LEU-108, VAL-30, LYS-53, TYR-182 | |||
| TLR4 | Rutin | Hydrogen Bond | ARG-183, TRP-222, PHE-31, LYS-57, LYS-59 | −7.8 |
| Hydrophobic Interaction | PHE-225, ILE-131, VAL-127 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, H.; Ding, J.; Du, Y.; Zhou, X.; Wang, L.; Ding, X.; Cai, W.; Wang, C.; Zhang, M.; Wang, Y.; et al. Integrated Metabolomics and Network Pharmacology to Reveal the Mechanisms of Forsythia suspensa Extract Against Respiratory Syncytial Virus. Int. J. Mol. Sci. 2025, 26, 5244. https://doi.org/10.3390/ijms26115244
Du H, Ding J, Du Y, Zhou X, Wang L, Ding X, Cai W, Wang C, Zhang M, Wang Y, et al. Integrated Metabolomics and Network Pharmacology to Reveal the Mechanisms of Forsythia suspensa Extract Against Respiratory Syncytial Virus. International Journal of Molecular Sciences. 2025; 26(11):5244. https://doi.org/10.3390/ijms26115244
Chicago/Turabian StyleDu, Haitao, Jie Ding, Yaxuan Du, Xinyi Zhou, Lin Wang, Xiaoyan Ding, Wen Cai, Cheng Wang, Mengru Zhang, Yi Wang, and et al. 2025. "Integrated Metabolomics and Network Pharmacology to Reveal the Mechanisms of Forsythia suspensa Extract Against Respiratory Syncytial Virus" International Journal of Molecular Sciences 26, no. 11: 5244. https://doi.org/10.3390/ijms26115244
APA StyleDu, H., Ding, J., Du, Y., Zhou, X., Wang, L., Ding, X., Cai, W., Wang, C., Zhang, M., Wang, Y., & Wang, P. (2025). Integrated Metabolomics and Network Pharmacology to Reveal the Mechanisms of Forsythia suspensa Extract Against Respiratory Syncytial Virus. International Journal of Molecular Sciences, 26(11), 5244. https://doi.org/10.3390/ijms26115244





