Polymorphisms in Necroptosis Pathway Genes: Novel Prognostic Markers for Multiple Myeloma Treatment Outcomes
Abstract
1. Introduction
2. Results
2.1. Distribution of RIPK1, RIPK3, and MAPKAPK2 Genotypes in MM Patients and Healthy Controls
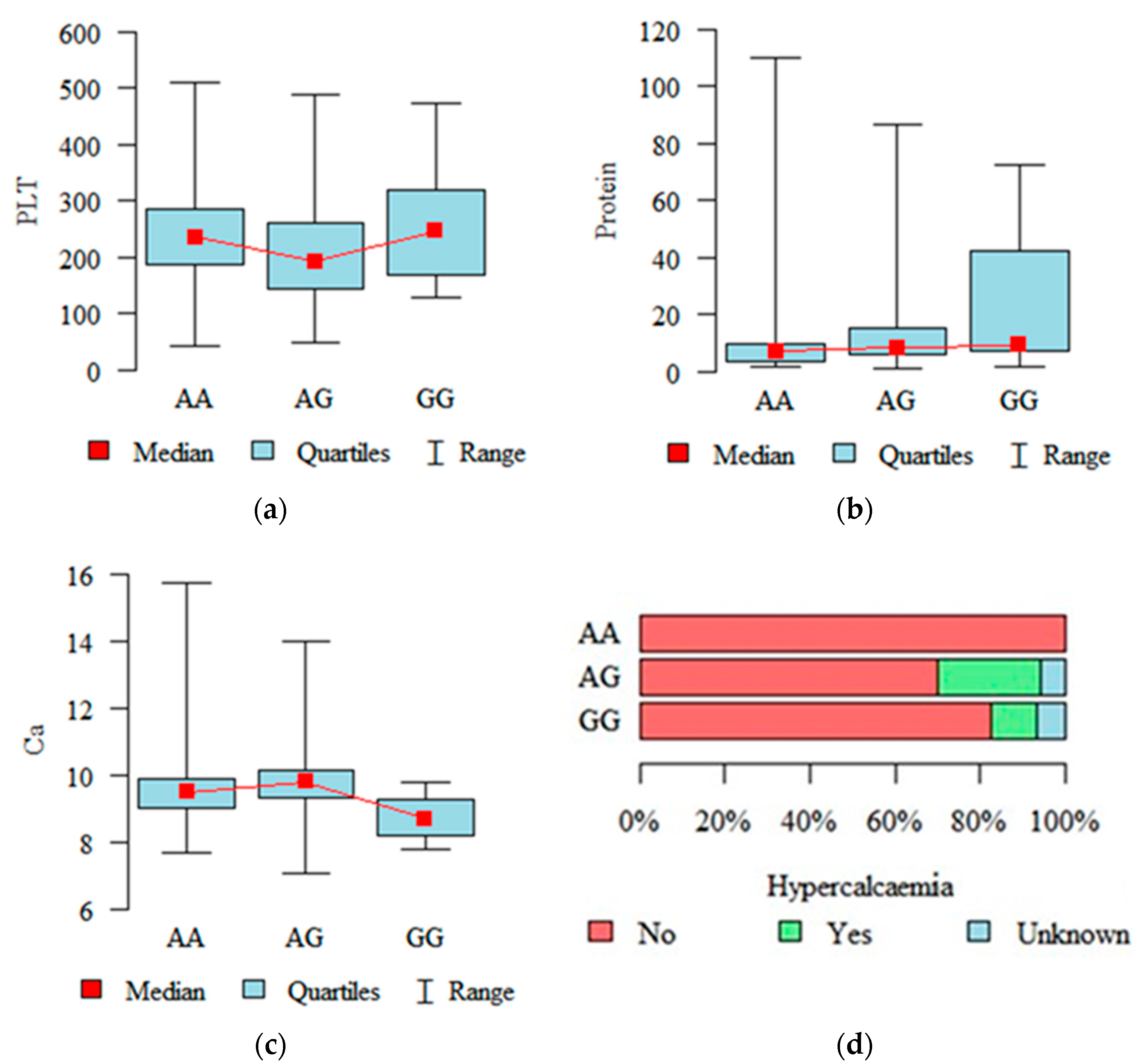
2.2. Associations with Diagnostic and Prognostic Parameters
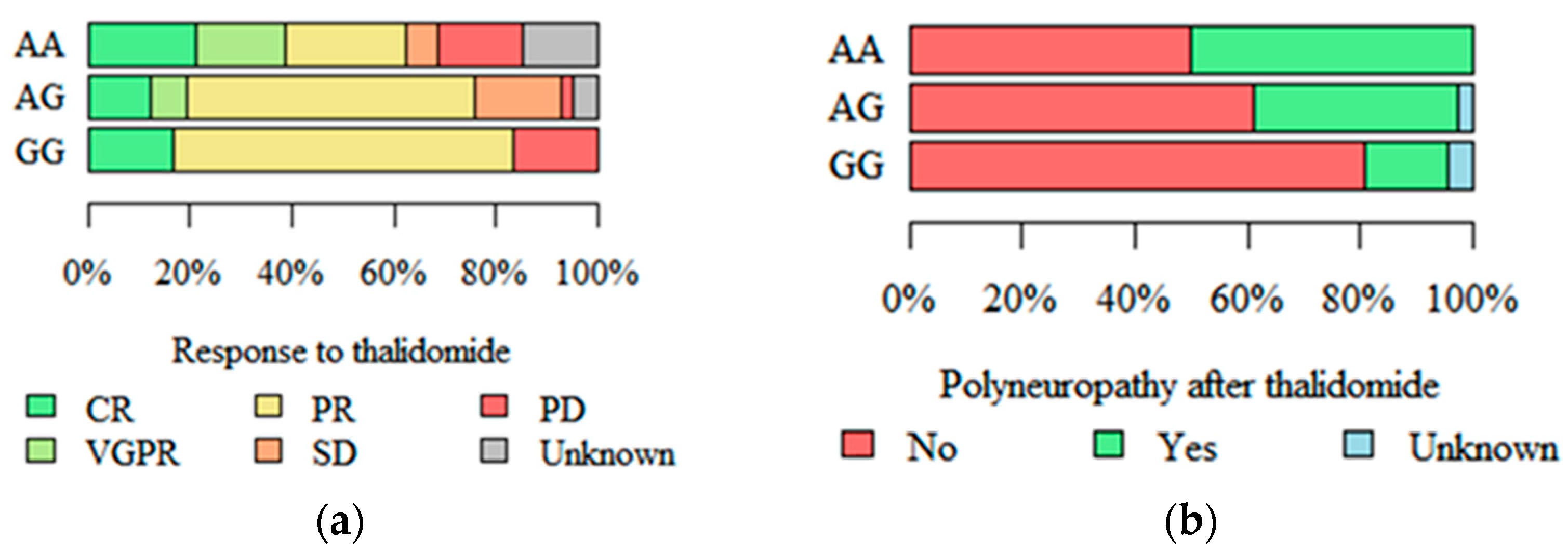
2.3. Response to Treatment and Peripheral Polyneuropathy
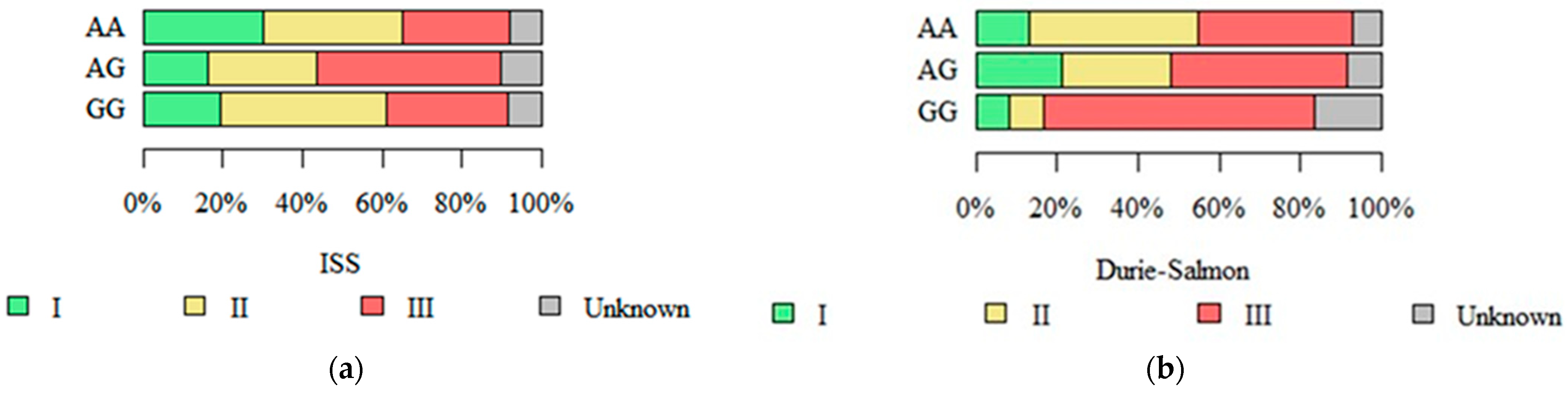
2.4. Stage in Durie–Salmon, ISS, and R-ISS Systems
2.5. Survival Analysis
2.5.1. Overall Survival
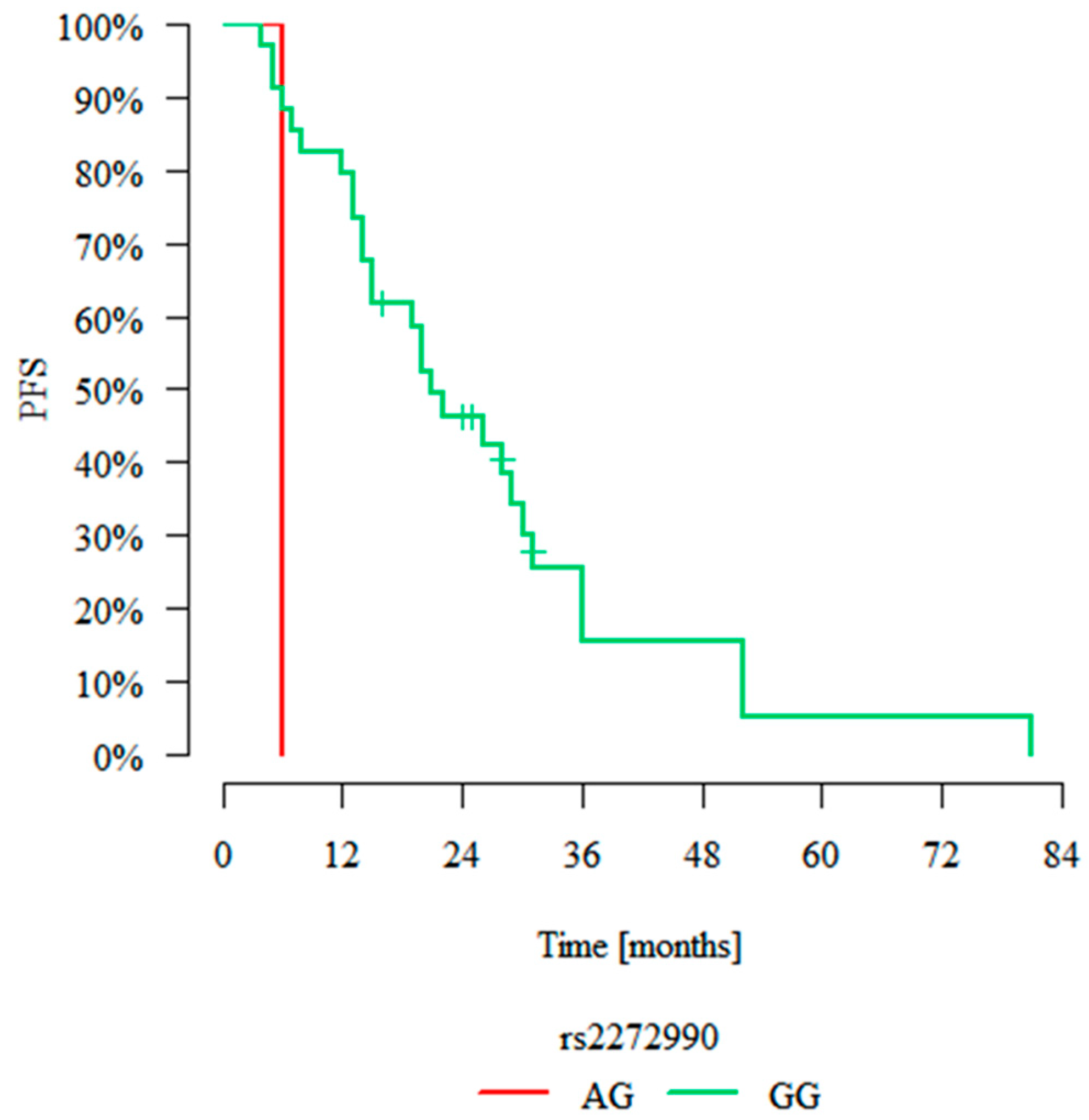
2.5.2. Progression-Free Survival
3. Discussion
4. Materials and Methods
4.1. General Characteristic of Population
4.2. Genotyping
4.3. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MM | Multiple myeloma |
| ISS | International Staging System |
| TNF-α | Tumour necrosis factor alpha |
| RIPK1 | Receptor-interacting serine/threonine kinase 1 |
| RIPK3 | Receptor-interacting serine/threonine kinase 3 |
| MLKL | Mixed lineage kinase domain-like pseudokinase |
| DAMP | Damage-associated nuclear pattern |
| MAPKAPK2 | MAP kinase-activated protein kinase 2 |
| SNP | Single-nucleotide polymorphism |
| CR | Complete remission |
| PR | Partial remission |
| VGPR | Very good partial remission |
| SD | Stable disease |
| PD | Progressive disease |
| OS | Overall survival |
| PFS | Progression-free survival |
| DLBCL | Diffuse large B cell lymphoma |
| DFS | Disease-free survival |
| TMN | Tumour, nodes, metastases |
| SHSP1 | Small heat shock protein 1 |
| auto-HSCT | Autologous haematopoietic stem cell transplantation |
References
- Cid Ruzafa, J.; Merinopoulou, E.; Baggaley, R.F.; Leighton, P.; Werther, W.; Felici, D.; Cox, A. Patient population with multiple myeloma and transitions across different lines of therapy in the USA: An epidemiologic model. Pharmacoepidemiol. Drug Saf. 2016, 25, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Zhao, D.; Hatanpaa, K.J.; Mickey, B.E.; Saha, D.; Boothman, D.A.; Story, M.D.; Wong, E.T.; Burma, S.; Georgescu, M.M.; et al. RIP1 activates PI3K-Akt via a dual mechanism involving NF-kappaB-mediated inhibition of the mTOR-S6K-IRS1 negative feedback loop and down-regulation of PTEN. Cancer Res. 2009, 69, 4107–4111. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, Y.; Kikuchi, J. Molecular pathogenesis of multiple myeloma. Int. J. Clin. Oncol. 2015, 20, 413–422. [Google Scholar] [CrossRef]
- Durie, B.G.; Salmon, S.E. A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer 1975, 36, 842–854. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Fan, Z.; Luo, G.; Yang, C.; Huang, Q.; Fan, K.; Cheng, H.; Jin, K.; Ni, Q.; Yu, X.; et al. The role of necroptosis in cancer biology and therapy. Mol. Cancer 2019, 18, 100. [Google Scholar] [CrossRef]
- Palumbo, A.; Anderson, K. Multiple myeloma. N. Engl. J. Med. 2011, 364, 1046–1060. [Google Scholar] [CrossRef]
- Terpos, E.; Katodritou, E.; Roussou, M.; Pouli, A.; Michalis, E.; Delimpasi, S.; Parcharidou, A.; Kartasis, Z.; Zomas, A.; Symeonidis, A.; et al. High serum lactate dehydrogenase adds prognostic value to the international staging system even in the era of novel agents. Eur. J. Haematol. 2010, 85, 114–119. [Google Scholar] [CrossRef]
- Adams, J. Proteasome inhibitors as new anticancer drugs. Curr. Opin. Oncol. 2002, 14, 628–634. [Google Scholar] [CrossRef]
- Arastu-Kapur, S.; Anderl, J.L.; Kraus, M.; Parlati, F.; Shenk, K.D.; Lee, S.J.; Muchamuel, T.; Bennett, M.K.; Driessen, C.; Ball, A.J.; et al. Nonproteasomal targets of the proteasome inhibitors bortezomib and carfilzomib: A link to clinical adverse events. Clin. Cancer Res. 2011, 17, 2734–2743. [Google Scholar] [CrossRef]
- Ludwig, H.; Durie, B.G.; Bolejack, V.; Turesson, I.; Kyle, R.A.; Blade, J.; Fonseca, R.; Dimopoulos, M.; Shimizu, K.; San Miguel, J.; et al. Myeloma in patients younger than age 50 years presents with more favorable features and shows better survival: An analysis of 10,549 patients from the International Myeloma Working Group. Blood 2008, 111, 4039–4047. [Google Scholar] [CrossRef]
- Holstein, S.A.; McCarthy, P.L. Immunomodulatory drugs in multiple myeloma: Mechanisms of action and clinical experience. Drugs 2017, 77, 505–520. [Google Scholar] [CrossRef] [PubMed]
- Vandenabeele, P.; Galluzzi, L.; Vanden Berghe, T.; Kroemer, G. Molecular mechanisms of necroptosis: An ordered cellular explosion. Nat. Rev. Mol. Cell Biol. 2010, 11, 700–714. [Google Scholar] [CrossRef]
- Ea, C.K.; Deng, L.; Xia, Z.P.; Pineda, G.; Chen, Z.J. Activation of IKK by TNFalpha requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol. Cell 2006, 22, 245–257. [Google Scholar] [CrossRef]
- Micheau, O.; Tschopp, J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 2003, 114, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Wegner, K.W.; Saleh, D.; Degterov, A. Complex Pathologic Roles of RIPK1 and RIPK3: Moving Beyond Necroptosis. Trends Pharmacol. Sci. 2017, 38, 202–225. [Google Scholar] [CrossRef]
- Annibaldi, A.; Wicky, J.S.; Vanden Berghe, T.; Swatek, K.N.; Ruan, J.; Liccardi, G.; Bianchi, K.; Elliott, P.R.; Choi, S.M.; Van Coillie, S.; et al. Ubiquitin-Mediated Regulation of RIPK1 Kinase Activity Independent of IKK and MK2. Mol. Cell 2018, 15, 566–580. [Google Scholar] [CrossRef] [PubMed]
- Wani, T.H.; Chakrabarty, A.; Shibata, N.; Yamazaki, H.; Guengerich, F.P.; Chowdhury, G. The Dihydroxy Metabolite of the Teratogen Thalidomide Causes Oxidative DNA Damage. Chem. Res. Toxicol. 2017, 30, 1622–1628. [Google Scholar] [CrossRef] [PubMed]
- Tummers, B.; Green, D.R. Caspase-8; regulating life and death. Immunol. Rev. 2017, 277, 76–89. [Google Scholar] [CrossRef]
- Weeks, S.D.; Muranova, L.K.; Heirbaut, M.; Beelen, S.; Strelkov, S.V.; Gusev, N.B. Characterization of human small heat shock protein HSPB1 α-crystallin domain localised mutants associated with hereditary motor neuron diseases. Sci. Rep. 2018, 8, 688. [Google Scholar] [CrossRef]
- Maliński, M.; Cichocki, M. Inhibicja aktywności proteasomu jako nowa strategia w terapii i chemioprewencji nowotworów. Postep. Hig. Med. Dosw. 2013, 67, 90–106. [Google Scholar] [CrossRef]
- Vercammen, D.; Brouckaert, G.; Denecker, G.; Van de Craen, M.; Declercq, W.; Fiers, W.; Vandenabeele, P. Dual signaling of the Fas receptor: Initiation of both apoptotic and necrotic cell death pathways. J. Exp. Med. 1998, 188, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Hitomi, J.; Christofferson, D.E.; Ng, A.; Yao, J.; Degterev, A.; Xavier, R.J.; Yuan, J. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell 2008, 135, 1311–1323. [Google Scholar] [CrossRef] [PubMed]
- Moquin, D.M.; McQuade, T.; Chan, F.K. CYLD deubiquitinates RIP1 in the TNFα-induced necrosome to facilitate kinase activation and programmed necrosis. PLoS ONE 2013, 8, e76841. [Google Scholar] [CrossRef]
- O’Donnell, M.A.; Perez-Jimenez, E.; Oberst, A.; Ng, A.; Massoumi, R.; Xavier, R.; Green, D.R.; Ting, A.T. Caspase 8 inhibits programmed necrosis by processing CYLD. Nat. Cell Biol. 2011, 13, 1437–1442. [Google Scholar] [CrossRef]
- Li, D.; Xu, T.; Cao, Y.; Wang, H.; Li, L.; Chen, S.; Wang, X.; Shen, Z. A cytosolic heat shock protein 90 and cochaperone CDC37 complex is required for RIP3 activation during necroptosis. Proc. Natl. Acad. Sci. USA 2015, 112, 5017–5022. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; McQuade, T.; Siemer, A.B.; Napetschnig, J.; Moriwaki, K.; Hsiao, Y.S.; Damko, E.; Moquin, D.; Walz, T.; McDermott, A.; et al. The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell 2012, 150, 339–350. [Google Scholar] [CrossRef]
- Rodriguez, D.A.; Weinlich, R.; Brown, S.; Guy, C.; Fitzgerald, P.; Dillon, C.P.; Oberst, A.; Quarato, G.; Low, J.; Cripps, J.G.; et al. Characterization of RIPK3-mediated phosphorylation of the activation loop of MLKL during necroptosis. Cell Death Differ. 2016, 23, 76–88. [Google Scholar] [CrossRef]
- Chen, W.; Wu, J.; Li, L.; Zhang, Z.; Ren, J.; Liang, Y.; Chen, F.; Yang, C.; Zhou, Z.; Su, S.S.; et al. Ppm1b negatively regulates necroptosis through dephosphorylating Rip3. Nat. Cell Biol. 2015, 17, 434–444. [Google Scholar] [CrossRef]
- Raninga, P.V.; Di Trapani, G.; Vuckovic, S.; Tonissen, K.F. Targeted knockdown of DJ-1 induces multiple myeloma cell death via KLF6 upregulation. Apoptosis 2016, 12, 1422–1437. [Google Scholar] [CrossRef]
- Murphy, J.M.; Czabotar, P.E.; Hildebrand, J.M.; Lucet, I.S.; Zhang, J.G.; Alvarez-Diaz, S.; Lewis, R.; Lalaoui, N.; Metcalf, D.; Webb, A.I.; et al. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity 2013, 39, 443–453. [Google Scholar] [CrossRef]
- Quarato, G.; Guy, C.S.; Grace, C.R.; Llambi, F.; Nourse, A.; Rodriguez, D.A.; Wakefield, R.; Frase, S.; Moldoveanu, T.; Green, D.R. Sequential engagement of distinct MLKL phosphatidylinositol-binding sites executes necroptosis. Mol. Cell 2016, 61, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Sun, J.; Yoon, J.S.; Zhang, Y.; Zheng, L.; Murphy, E.; Mattson, M.P.; Lenardo, M.J. Mitochondrial protein PGAM5 regulates mitophagic protection against cell necroptosis. PLoS ONE 2016, 11, e0147792. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, A.; Vandenabeele, P.; Krysko, D.V. Necroptosis: The Release of Damage-Associated Molecular Patterns and Its Physiological Relevance. Immunity 2013, 38, 209–223. [Google Scholar] [CrossRef]
- Iwama, K.; Chihara, D.; Tsuda, K.; Ugai, T.; Sugihara, H.; Nishida, Y.; Yamakura, M.; Takeuchi, M.; Matsue, K. Normalization of free light chain kappa/lambda ratio is a robust prognostic indicator of favorable outcome in patients with multiple myeloma. Eur. J. Haematol. 2012, 90, 134–141. [Google Scholar] [CrossRef]
- Degterev, A.; Maki, J.L.; Yuan, J. Activity and specificity of necrostatin-1, small-molecule inhibitor of RIP1 kinase. Cell Death Differ. 2013, 20, 366. [Google Scholar] [CrossRef]
- Butrym, A.; Rybka, J.; Łacina, P.; Gębura, K.; Frontkiewicz, D.; Bogunia-Kubik, K.; Mazur, G. Polymorphisms within beta-catenin encoding gene affect multiple myeloma development and treatment. Leuk. Res. 2015, 39, 1462–1466. [Google Scholar] [CrossRef]
- Łacina, P.; Butrym, A.; Mazur, G.; Bogunia-Kubik, K. BSG and MCT1 Genetic Variants Influence Survival in Multiple Myeloma Patients. Genes 2018, 9, 226. [Google Scholar] [CrossRef] [PubMed]
- Dratwa, M.; Łacina, P.; Butrym, A.; Porzuczek, D.; Mazur, G.; Bogunia-Kubik, K. Telomere length and hTERT genetic variants as potential prognostic markers in multiple myeloma. Sci. Rep. 2023, 13, 15792. [Google Scholar] [CrossRef]
- Galluzzi, L.; Kepp, O.; Chan, F.K.; Kroemer, G. Necroptosis: Mechanisms and Relevance to Disease. Annu. Rev. Pathol. 2017, 12, 103–130. [Google Scholar] [CrossRef]
- Rajkumar, S.V.; Kumar, S. Multiple Myeloma: Diagnosis and Treatment. Mayo Clin. Proc. 2016, 91, 101–119. [Google Scholar] [CrossRef]
- Ali, M.; Mocarski, E.S. Proteasome inhibition blocks necroptosis by attenuating death complex aggregation. Cell Death Dis. 2018, 9, 346. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Taylor, J.A. SNPinfo: Integrating GWAS and candidate gene information into functional SNP selection for genetic association studies. Nucleic Acids Res. 2009, 37, W600–W605. [Google Scholar] [CrossRef]
- Hosgood, H.D., 3rd; Baris, D.; Zhang, Y.; Berndt, S.I.; Menashe, I.; Morton, L.M.; Lee, K.M.; Yeager, M.; Zahm, S.H.; Chanock, S.; et al. Genetic variation in cell cycle and apoptosis related genes and multiple myeloma risk. Leuk. Res. 2009, 33, 1609–1614. [Google Scholar] [CrossRef]
- Hunt, R.; Sauna, Z.E.; Ambudkar, S.V.; Gottesman, M.M.; Kimchi-Sarfaty, C. Silent (synonymous) SNPs: Should we care about them? Methods Mol. Biol. 2009, 578, 23–39. [Google Scholar] [PubMed]
- Chae, Y.S.; Kim, J.G.; Sohn, S.K.; Moon, J.H.; Kim, S.N.; Lee, S.J.; Park, T.I.; Lee, M.H. Lymphotoxin alfa and receptor-interacting protein kinase 1 gene polymorphisms may correlate with prognosis in patients with diffuse large B cell lymphoma treated with R-CHOP. Cancer Chemother. Pharmacol. 2010, 65, 571–577. [Google Scholar] [CrossRef]
- Feng, X.; Song, Q.; Yu, A.; Tang, H.; Peng, Z.; Wang, X. Receptor-interacting protein kinase 3 is a predictor of survival and plays a tumor suppressive role in colorectal cancer. Neoplasma 2015, 62, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Li, G.; Cai, M.; Qian, Y.; Wang, L.; Xiao, L.; Thaiss, F.; Shi, B. Expression and genetic polymorphism of necroptosis related protein RIPK1 is correlated with severe hepatic ischemia-reperfusion injury and prognosis after hepatectomy in hepatocellular carcinoma patients. Cancer Biomark. 2017, 20, 23–29. [Google Scholar] [CrossRef]
- Piechnik, A.; Giannopoulos, K. Mechanizmy działania leków immunomodulujących w szpiczaku plazmocytowym. Hematologia 2011, 2, 105–115. [Google Scholar]
- Mitsiades, N.; Mitsiades, C.S.; Poulaki, V.; Chauhan, D.; Richardson, P.G.; Hideshima, T.; Munshi, N.C.; Treon, S.P.; Anderson, K.C. Apoptotic signaling induced by immunomodulatory thalidomide analogs in human multiple myeloma cells: Therapeutic implications. Blood 2002, 99, 4525–4530. [Google Scholar] [CrossRef]
- McCormick, K.D.; Ghosh, A.; Trivedi, S.; Wang, L.; Coyne, C.B.; Ferris, R.L.; Sarkar, S.N. Innate immune signaling through differential RIPK1 expression promote tumor progression in head and neck squamous cell carcinoma. Carcinogenesis 2016, 37, 522–529. [Google Scholar] [CrossRef]
- Seifert, L.; Werba, G.; Tiwari, S.; Giao Ly, N.N.; Alothman, S.; Alqunaibit, D.; Avanzi, A.; Barilla, R.; Daley, D.; Greco, S.H.; et al. The necrosome promotes pancreatic oncogenesis via CXCL1 and Mincle-induced immune suppression. Nature 2016, 532, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Mellors, P.W.; Binder, M.; Buadi, F.K.; Lacy, M.Q.; Gertz, M.A.; Dispenzieri, A.; Hayman, S.R.; Kapoor, P.; Gonsalves, W.I.; Hwa, Y.L.; et al. Development of thrombocytopenia during first-line treatment and survival outcomes in newly diagnosed multiple myeloma. Leuk. Lymphoma 2019, 60, 2960–2967. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; San Miguel, J.; Sonneveld, P.; Mateos, M.V.; Zamagni, E.; Avet-Loiseau, H.; Hajek, R.; Dimopoulos, M.A.; Ludwig, H.; Einsele, H.; et al. Multiple myeloma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Cerhan, J.R.; Ansell, S.M.; Fredericksen, Z.S.; Kay, N.E.; Liebow, M.; Call, T.G.; Dogan, A.; Cunningham, J.M.; Wang, A.H.; Liu-Mares, W.; et al. Genetic variation in 1253 immune and inflammation genes and risk of non-Hodgkin lymphoma. Blood 2007, 110, 4455–4463. [Google Scholar] [CrossRef]
- Keifer, J.A.; Guttridge, D.C.; Ashburner, B.P.; Baldwin, A.S., Jr. Inhibition of NF-kappa B activity by thalidomide through suppression of IkappaB kinase activity. J. Biol. Chem. 2001, 276, 22382–22387. [Google Scholar] [CrossRef]
- Höckendorf, U.; Yabal, M.; Herold, T.; Munkhbaatar, E.; Rott, S.; Jilg, S.; Kauschinger, J.; Magnani, G.; Reisinger, F.; Heuser, M.; et al. RIPK3 restricts myeloid Leukemogenesis by promoting cell death and differentiation of leukemia initiating cells. Cancer Cell 2016, 30, 75–91. [Google Scholar] [CrossRef]
- Nugues, A.-L.; El Bouazzati, H.; Hétuin, D.; Berthon, C.; Loyens, A.; Bertrand, E.; Jouy, N.; Idziorek, T.; Quesnel, B. RIP3 is downregulated in human myeloid leukemia cells and modulates apoptosis and caspase-mediated p65/RelA cleavage. Cell Death Dis. 2014, 5, e1384. [Google Scholar] [CrossRef]
- Li, N.; Lin, N.; Yao, X.; Wang, H.; Zhou, L.; Guo, Y. Association of MK2 and ZFP36 gene polymorphisms with high density lipoprotein cholesterol in Uygur population from Hetian area of Xinjiang. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 2013, 30, 345–351. [Google Scholar]
- Lin, N.; Li, N.F.; Yao, X.G.; Wang, H.M.; Liang, D.P.; Guo, Y.Y.; Zhou, L. Association of MK2 gene polymorphisms with low-density lipoprotein cholesterol and tumor necrosis factor-alpha in Uygur population from Hetian area of Xinjiang. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2013, 35, 52–57. [Google Scholar]
- Wang, X.; Wang, Y.; Ding, Z.J.; Yue, B.; Zhang, P.Z.; Chen, X.D.; Chen, X.; Chen, J.; Chen, F.Q.; Chen, Y.; et al. The role of RIP3 mediated necroptosis in ouabain-induced spiral ganglion neurons injuries. Neurosci. Lett. 2014, 578, 111–116. [Google Scholar] [CrossRef]
- Iannielli, A.; Bido, S.; Folladori, L.; Segnali, A.; Cancellieri, C.; Maresca, A.; Massimino, L.; Rubio, A.; Morabito, G.; Caporali, L. Pharmacological Inhibition of Necroptosis Protects from Dopaminergic Neuronal Cell Death in Parkinson’s Disease Models. Cell Rep. 2018, 22, 2066–2079. [Google Scholar] [CrossRef] [PubMed]
- Pu, S.; Li, S.; Xu, Y.; Wu, J.; Lv, Y.; Du, D. Role of receptor-interacting protein 1/receptor-interacting protein 3 in inflammation and necrosis following chronic constriction injury of the sciatic nerve. Neuroreport 2018, 29, 1373–1378. [Google Scholar] [CrossRef] [PubMed]
- Arrázola, M.S.; Saquel, C.; Catalán, R.J.; Barrientos, S.A.; Hernandez, D.E.; Martínez, N.W.; Catenaccio, A.; Court, F.A. Axonal Degeneration Is Mediated by Necroptosis Activation. J. Neurosci. 2019, 39, 3832–3844. [Google Scholar] [CrossRef] [PubMed]
- Tamilarasan, K.P.; Kolluru, G.K.; Rajaram, M.; Indhumathy, M.; Saranya, R.; Chatterjee, S. Thalidomide attenuates nitric oxide mediated angiogenesis by blocking migration of endothelial cells. BMC Cell Biol. 2007, 7, 17. [Google Scholar] [CrossRef]
| MM Patients | Control Group | |
|---|---|---|
| RIPK1 rs2272990 | ||
| GG | 188 | 94 |
| AG | 17 | 6 |
| AA | 0 | 0 |
| RIPK1 rs9391981 | ||
| GG | 189 | 92 |
| CG | 16 | 8 |
| CC | 0 | 0 |
| RIPK3 rs724165 | ||
| AG | 106 | 52 |
| AA | 63 | 34 |
| GG | 36 | 13 |
| RIPK3 rs3212243 | ||
| AA | 122 | 54 |
| AG | 71 | 44 |
| GG | 12 | 2 |
| MAPKAPK2 rs4073250 | ||
| GG | 130 | 58 |
| AG | 67 | 31 |
| AA | 4 | 8 |
| MAPKAPK2 rs45514798 | ||
| GG | 139 | 73 |
| AG | 62 | 24 |
| AA | 4 | 3 |
| Feature | HR | 95% CI | p-Value | ||
|---|---|---|---|---|---|
| Sex | female | 1 | ref. | ||
| male | 0.423 | 0.105 | 1.703 | 0.226 | |
| Age | [years] | 1.246 | 1.115 | 1.392 | <0.001 |
| Durie–Salmon | I | 1 | ref. | ||
| II | 2.077 | 0.228 | 18.927 | 0.517 | |
| III | 23.261 | 2.007 | 269.590 | 0.012 | |
| ISS | I | 1 | ref. | ||
| II | 1.656 | 0.201 | 13.608 | 0.639 | |
| III | 0.102 | 0.004 | 2.518 | 0.163 | |
| Type of light chain | Lambda | 1 | ref. | ||
| Kappa | 12.533 | 2.016 | 77.919 | 0.007 | |
| Type of heavy chain | A | 1 | ref. | ||
| G | 12.533 | 0.058 | 1.202 | 0.085 | |
| Haemoglobin | 0.920 | 0.597 | 1.417 | 0.706 | |
| Hypercalcaemia | No | 1 | ref. | ||
| Yes | 0.185 | 0.015 | 2.240 | 0.185 | |
| Renal insufficiency | No | 1 | ref. | ||
| Yes | 1.154 | 0.225 | 5.933 | 0.864 | |
| Osteolysis | No | 1 | ref. | ||
| Yes | 4.186 | 0.851 | 20.591 | 0.078 | |
| RIPK1 rs2272990 | GG | 1 | ref. | ||
| AG | No deaths—impossible to calculate | ||||
| RIPK1 rs9391981 | GG | 1 | ref. | ||
| CG | 71.614 | 2.333 | 2198.070 | 0.014 | |
| RIPK3 rs724165 | AA | 1 | ref. | ||
| AG | 0.567 | 0.089 | 3.600 | 0.548 | |
| GG | <0.001 | <0.001 | 0.144 | 0.009 | |
| RIPK3 rs3212243 | AA | 1 | ref. | ||
| AG | 5.199 | 0.679 | 39.795 | 0.112 | |
| GG | 1389.587 | 3.493 | 552,848.394 | 0.018 | |
| MAPKAPK2 rs4073250 | GG | 1 | ref. | ||
| AG | 1.029 | 0.214 | 4.941 | 0.971 | |
| AA | 30.671 | 1.356 | 693.526 | 0.031 | |
| MAPKAPK2 rs45514798 | GG | 1 | ref. | ||
| AG | 2.233 | 0.488 | 10.230 | 0.301 | |
| AA | No deaths—impossible to calculate | ||||
| Feature | HR | 95% CI | p-Value | ||
|---|---|---|---|---|---|
| Sex | female | 1 | ref. | ||
| male | 1.009 | 0.531 | 1.918 | 0.978 | |
| Age | [years] | 1.019 | 0.985 | 1.055 | 0.275 |
| Durie–Salmon | I | 1 | ref. | ||
| II | 0.648 | 0.244 | 1.720 | 0.384 | |
| III | 0.753 | 0.266 | 2.125 | 0.591 | |
| ISS | I | 1 | ref. | ||
| II | 1.666 | 0.636 | 4.366 | 0.299 | |
| III | 1.139 | 0.353 | 3.677 | 0.828 | |
| Type of light chain | Lambda | 1 | ref. | ||
| Kappa | 1.639 | 0.797 | 3.370 | 0.179 | |
| Type of heavy chain | A | 1 | ref. | ||
| G | 1.050 | 0.515 | 2.143 | 0.893 | |
| Haemoglobin | 0.897 | 0.738 | 1.092 | 0.279 | |
| Hypercalcaemia | No | 1 | ref. | ||
| Yes | 1.373 | 0.476 | 3.956 | 0.557 | |
| Renal insufficiency | No | 1 | ref. | ||
| Yes | 1.226 | 0.655 | 2.293 | 0.524 | |
| Osteolysis | No | 1 | ref. | ||
| Yes | 0.885 | 0.467 | 1.679 | 0.709 | |
| Response to the first line of treatment | CR | 1 | ref. | ||
| VGPR | 0.872 | 0.281 | 2.706 | 0.813 | |
| PR | 1.112 | 0.502 | 2.463 | 0.793 | |
| SD | 1.046 | 0.406 | 2.700 | 0.925 | |
| PD | 0.357 | 0.085 | 1.503 | 0.160 | |
| RIPK1 rs2272990 | GG | 1 | ref. | ||
| AG | 0.676 | 0.059 | 7.732 | 0.753 | |
| RIPK1 rs9391981 | GG | 1 | ref. | ||
| CG | 1.207 | 0.1 | 14.611 | 0.882 | |
| RIPK3 rs724165 | AA | 1 | ref. | ||
| AG | 1.513 | 0.697 | 3.285 | 0.295 | |
| GG | 0.645 | 0.218 | 1.909 | 0.428 | |
| RIPK3 rs3212243 | AA | 1 | ref. | ||
| AG | 0.786 | 0.382 | 1.619 | 0.514 | |
| GG | 2.265 | 0.453 | 11.316 | 0.319 | |
| MAPKAPK2 rs4073250 | GG | 1 | ref. | ||
| AG | 1.125 | 0.548 | 2.308 | 0.749 | |
| AA | 6.919 | 1.277 | 37.473 | 0.025 | |
| MAPKAPK2 rs45514798 | GG | 1 | ref. | ||
| AG | 1.884 | 0.945 | 3.757 | 0.072 | |
| AA | 3.060 | 0.264 | 35.46 | 0.371 | |
| N = 205 | |
|---|---|
| Sex | |
| female | 102 (49.8%) |
| male | 103 (50.2%) |
| Age | |
| median | 65 years |
| range | 37–88 years |
| Immunoglobulin secretion | |
| IgG | 118 (57.6%) |
| IgA | 45 (22.0%) |
| IgM | 1 (0.5%) |
| IgD | 1 (0.5%) |
| IgM and IgG | 1 (0.5%) |
| Light chain | 15 (7.3%) |
| Plasma cell leukemia | 1 (0.5%) |
| Non-secretory | 23 (11.2%) |
| ISS stage | |
| I | 44 (21.5%) |
| II | 67 (32.7%) |
| III | 80 (39.0%) |
| unknown | 14 (6.8%) |
| Durie–Salmon stage | |
| I | 33 (16.1%) |
| II | 72 (35.1%) |
| III | 88 (42.9%) |
| unknown | 12 (5.9%) |
| Therapy | |
| thalidomide | 91 (44.4%) |
| bortezomib | 91 (44.4%) |
| lenalidomide | 62 (30.2%) |
| auto-HSCT | 58 (28.3%) |
| Other parameters | |
| renal insufficiency | 55 (26.8%) |
| hypercalcaemia | 32 (15.6%) |
| osteolytic lesions | 99 (48.3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokołowski, M.; Łacina, P.; Bogunia-Kubik, K.; Mazur, G.; Butrym, A. Polymorphisms in Necroptosis Pathway Genes: Novel Prognostic Markers for Multiple Myeloma Treatment Outcomes. Int. J. Mol. Sci. 2025, 26, 5237. https://doi.org/10.3390/ijms26115237
Sokołowski M, Łacina P, Bogunia-Kubik K, Mazur G, Butrym A. Polymorphisms in Necroptosis Pathway Genes: Novel Prognostic Markers for Multiple Myeloma Treatment Outcomes. International Journal of Molecular Sciences. 2025; 26(11):5237. https://doi.org/10.3390/ijms26115237
Chicago/Turabian StyleSokołowski, Marcin, Piotr Łacina, Katarzyna Bogunia-Kubik, Grzegorz Mazur, and Aleksandra Butrym. 2025. "Polymorphisms in Necroptosis Pathway Genes: Novel Prognostic Markers for Multiple Myeloma Treatment Outcomes" International Journal of Molecular Sciences 26, no. 11: 5237. https://doi.org/10.3390/ijms26115237
APA StyleSokołowski, M., Łacina, P., Bogunia-Kubik, K., Mazur, G., & Butrym, A. (2025). Polymorphisms in Necroptosis Pathway Genes: Novel Prognostic Markers for Multiple Myeloma Treatment Outcomes. International Journal of Molecular Sciences, 26(11), 5237. https://doi.org/10.3390/ijms26115237






