Outcome of Sleep Rehabilitation in Autistic Children with Sleep Disorders Is Linked to Melatonin Receptor Genes SNPs
Abstract
1. Introduction
2. Results
2.1. Distribution of MT1 and MT2 SNPs Alleles and Genotypes in Children with ASD, Their Siblings and HCs
2.2. SDSC Scores at T0 in Children with ASD and Their Siblings
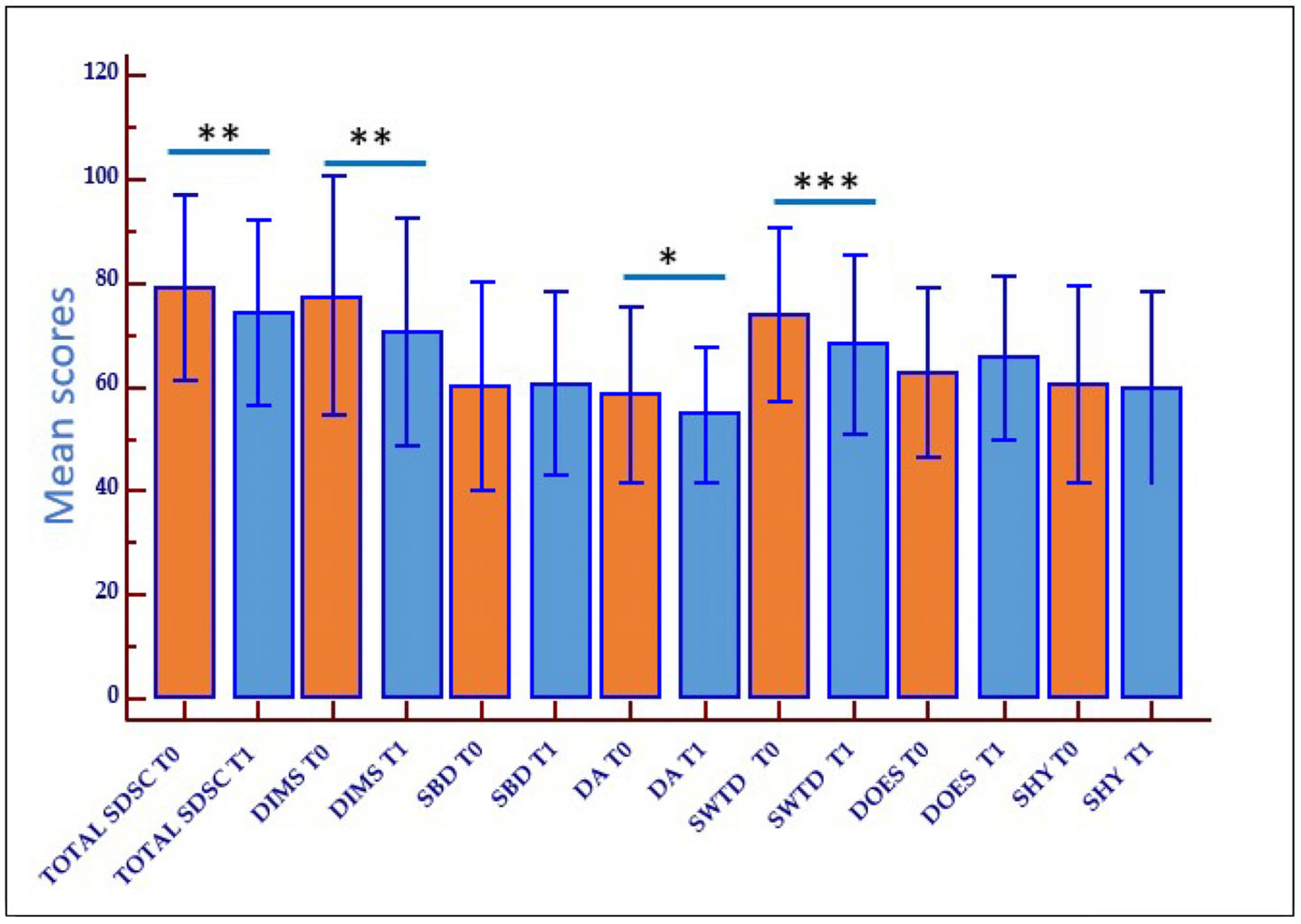
2.3. Outcome of Rehabilitation on Sleep Disturbances (Behavioral Therapy and Sleep Hygiene)
2.4. Effect of MT1 and MT2 Receptors Polymorphisms on Rehabilitation Outcome
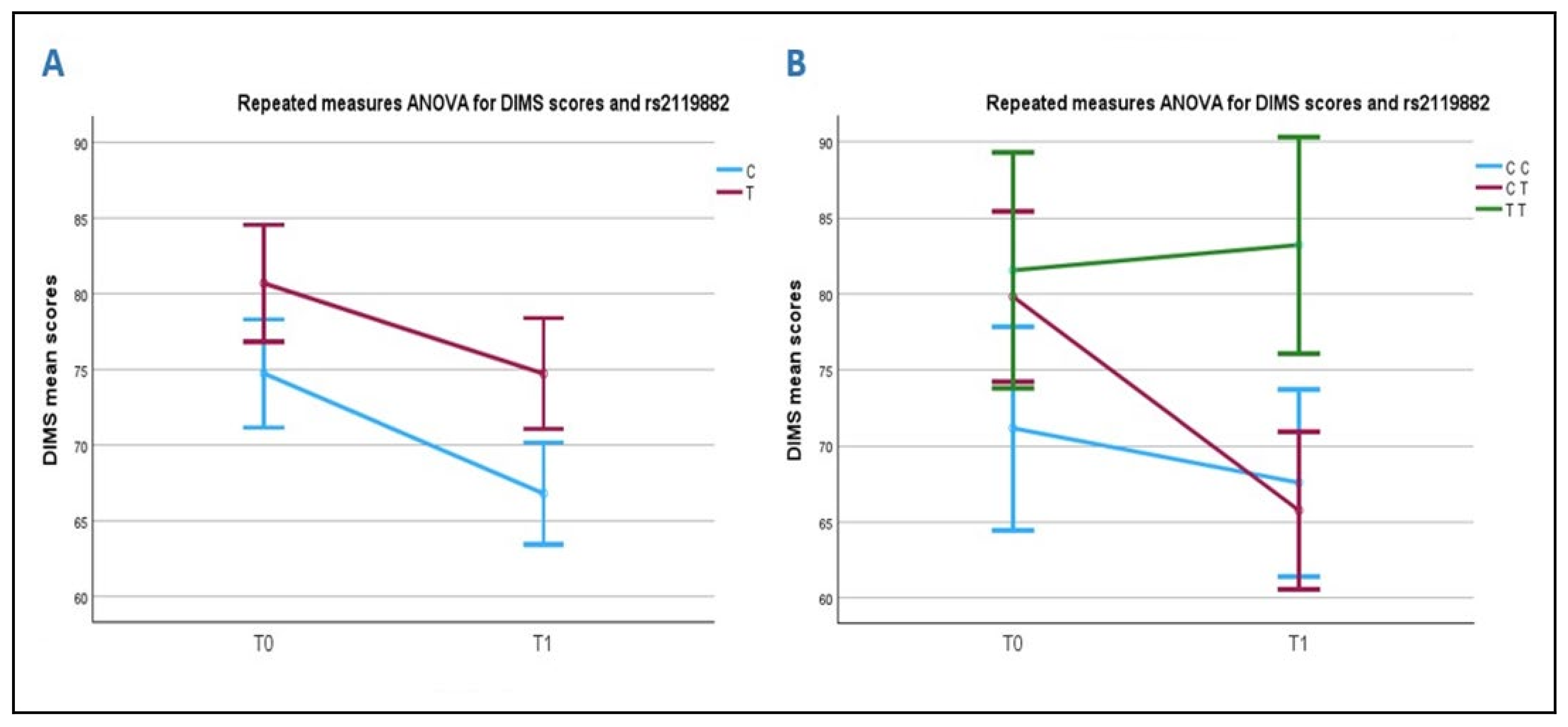
2.4.1. Association Between DIMS Scale and the rs2119882 (T/C) Polymorphism of the MT1 Receptor Gene
2.4.2. Association Between DA Subscales and the MT1 Receptor Gene rs2119882 (T/C) Polymorphism
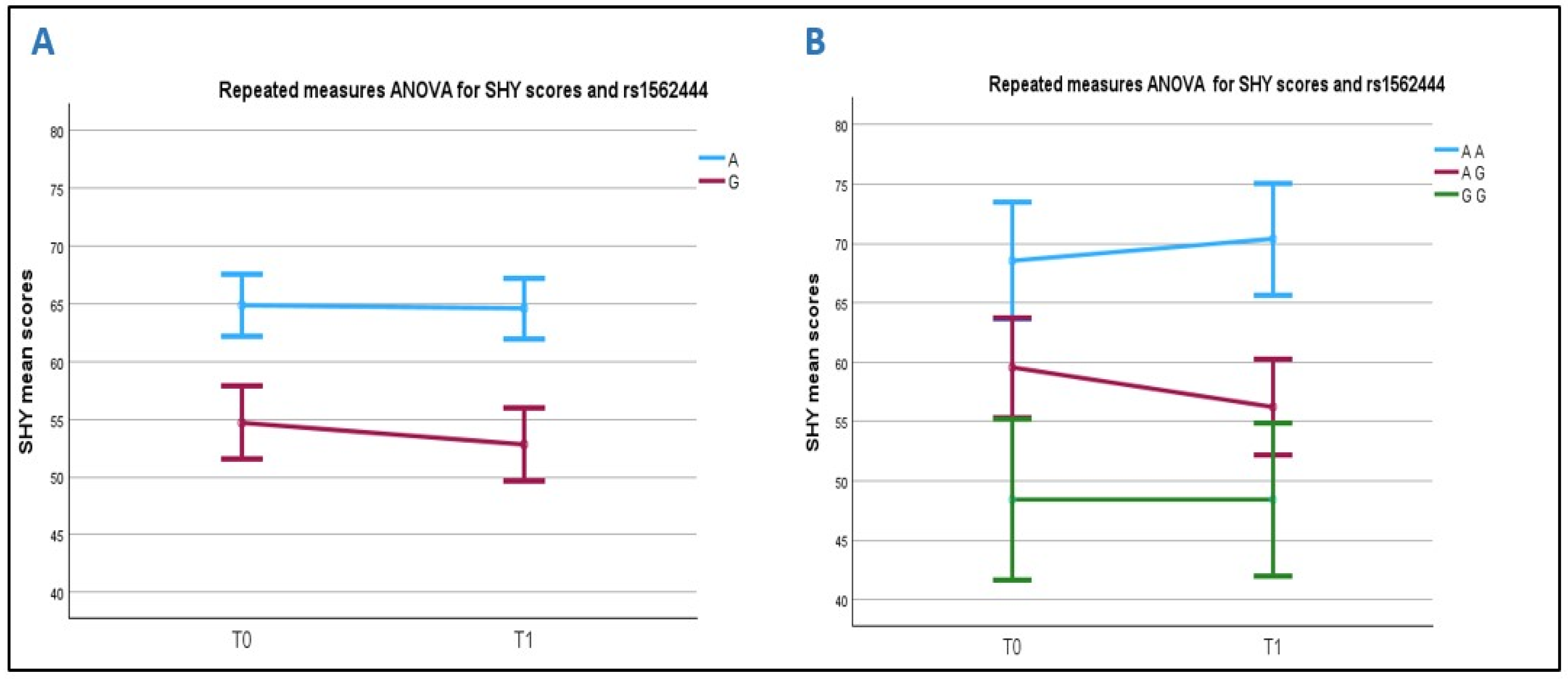
2.4.3. Association Between SHY Score and the MT2 Receptor Gene rs1562444 (A/G) Polymorphism
2.5. MT2 rs10830963 (G) Allele, ASD Severity, and Total SDSC and Subscales Scores
3. Discussion
Limitations of the Study
4. Materials and Methods
4.1. ASD Patients, Siblings and Healthy Controls
4.2. Melatonin Receptors SNPs Genotyping
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association (APA). Diagnostic and Statistical Manual of Mental Disorders (DSM-V-TR), 5th ed.; American Psychiatry Association Publishing: Washington, DC, USA, 2022. [Google Scholar] [CrossRef]
- Schwichtenberg, A.J.; Janis, A.; Lindsay, A.; Desai, H.; Sahu, A.; Kellerman, A.; Chong, P.L.H.; Abel, E.A.; Yatcilla, J.K. Sleep in Children with Autism Spectrum Disorder: A Narrative Review and Systematic Update. Curr. Sleep Med. Rep. 2022, 8, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, J.H.; Yi, J.H.; Kim, J.Y.; Solmi, M.; Cortese, S.; Smith, L.; Koyanagi, A.; Shin, J.I.; Cheon, K.-A.; et al. Correlations between sleep problems, core symptoms, and behavioral problems in children and adolescents with autism spectrum disorder: A systematic review and meta-analysis. Eur. Child Adolesc. Psychiatry 2024, 33, 1539–1549. [Google Scholar] [CrossRef]
- Cohen, S.; Conduit, R.; Lockley, S.W.; Rajaratnam, S.M.; Cornish, K.M. The relationship between sleep and behavior in autism spectrum disorder (ASD): A review. J. Neurodev. Disord. 2014, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Bruni, O.; Breda, M.; Mammarella, V.; Mogavero, M.P.; Ferri, R. Sleep and circadian disturbances in children with neurodevelopmental disorders. Nat. Rev. Neurol. 2025, 21, 103–120. [Google Scholar] [CrossRef]
- Carta, A.; Fucà, E.; Guerrera, S.; Napoli, E.; Valeri, G.; Vicari, S. Characterization of Clinical Manifestations in the Co-occurring Phenotype of Attention Deficit/Hyperactivity Disorder and Autism Spectrum Disorder. Front. Psychol. 2020, 11, 861. [Google Scholar] [CrossRef] [PubMed]
- McLay, L.L.; France, K.G.; Blampied, N.M.; Hunter, J.E.; van Deurs, J.R.; Woodford, E.; Gibbs, R.; Lang, R. Collateral Child and Parent Effects of Function Based Behavioral Interventions for Sleep Problems in Children and Adolescents with Autism. J. Autism Dev. Disord. 2022, 52, 2258–2273. [Google Scholar] [CrossRef]
- Nir, I.; Meir, D.; Zilber, N.; Knobler, H.; Hadjez, J.; Lerner, Y. Brief report: Circadian melatonin, thyroid-stimulating hormone, prolactin, and cortisol levels in serum of young adults with autism. J. Autism Dev. Disord. 1995, 25, 641–654. [Google Scholar] [CrossRef]
- Hu, V.W.; Sarachana, T.; Kim, K.S.; Nguyen, A.; Kulkarni, S.; Steinberg, M.E.; Luu, T.; Lai, Y.; Lee, N.H. Gene expression profiling differentiates autism case-controls and phenotypic variants of autism spectrum disorders: Evidence for circadian rhythm dysfunction in severe autism. Autism Res. 2009, 2, 78–97. [Google Scholar] [CrossRef]
- Tordjman, S.; Anderson, G.M.; Bellissant, E.; Botbol, M.; Charbuy, H.; Camus, F.; Graignic, R.; Kermarrec, S.; Fougerou, C.; Cohen, D.; et al. Day and nighttime excretion of 6-sulphatoxymelatonin in adolescents and young adults with autistic disorder. Psychoneuroendocrinology 2012, 37, 1990–1997. [Google Scholar] [CrossRef]
- Tordjman, S.; Anderson, G.M.; Pichard, N.; Charbuy, H.; Touitou, Y. Nocturnal excretion of 6-sulphatoxymelatonin in children and adolescents with autistic disorder. Biol. Psychiatry 2005, 57, 134–138. [Google Scholar] [CrossRef]
- Nogueira, H.A.; de Castro, C.T.; da Silva, D.C.G.; Pereira, M. Melatonin for sleep disorders in people with autism: Systematic review and meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2023, 123, 110695. [Google Scholar] [CrossRef] [PubMed]
- Arendt, J.; Aulinas, A. Physiology of the Pineal Gland and Melatonin. In Endotext [Internet]; Feingold, K.R., Feingold, K.R., Ahmed, S.F., Anawalt, B., Blackman, M.R., Boyce, A., Eds.; MDTextcom, Inc.: South Dartmouth, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK550972/ (accessed on 13 January 2025).
- Guerini, F.R.; Bolognesi, E.; Chiappedi, M.; Mensi, M.M.; Fumagalli, O.; Rogantini, C.; Zanzottera, M.; Ghezzo, A.; Zanette, M.; Agliardi, C.; et al. Vitamin D Receptor Polymorphisms Associated with Autism Spectrum Disorder. Autism Res. 2020, 13, 680–690. [Google Scholar] [CrossRef]
- Bolognesi, E.; Guerini, F.R.; Sotgiu, S.; Chiappedi, M.; Carta, A.; Mensi, M.M.; Agliardi, C.; Zanzottera, M.; Clerici, M. GC1f Vitamin D Binding Protein Isoform as a Marker of Severity in Autism Spectrum Disorders. Nutrients 2022, 14, 5153. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Riestra, A.; Estrada-Reyes, R.; Torres-Sanchez, E.D.; Carreño-García, S.; Ortiz, G.G.; Benítez-King, G. Melatonin: A Neurotrophic Factor? Molecules 2022, 27, 7742. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.B.; Ali, A.; Bilal, M.; Rashid, S.M.; Wani, A.B.; Bhat, R.R.; Rehman, M.U. Melatonin and Health: Insights of Melatonin Action, Biological Functions, and Associated Disorders. Cell. Mol. Neurobiol. 2023, 43, 2437–2458. [Google Scholar] [CrossRef]
- Starnes, A.N.; Jones, J.R. Inputs and Outputs of the Mammalian Circadian Clock. Biology 2023, 12, 508. [Google Scholar] [CrossRef]
- Gobbi, G.; Comai, S. Differential Function of Melatonin MT1 and MT2 Receptors in REM and NREM Sleep. Front. Endocrinol. 2019, 10, 87. [Google Scholar] [CrossRef]
- Waly, N.E.; Hallworth, R. Circadian Pattern of Melatonin MT1 and MT2 Receptor Localization in the Rat Suprachiasmatic Nucleus. J. Circadian Rhythms. 2015, 13, 1. [Google Scholar] [CrossRef]
- Park, H.J.; Park, J.K.; Kim, S.K.; Cho, A.R.; Kim, J.W.; Yim, S.V.; Chung, J.H. Association of polymorphism in the promoter of the melatonin receptor 1A gene with schizophrenia and with insomnia symptoms in schizophrenia patients. J. Mol. Neurosci. 2011, 45, 304–308. [Google Scholar] [CrossRef]
- Mulayim, E.; Karababa, İ.F.; Akbaş, H.; Bayazıt, H.; Selek, S. Melatonin Receptor Gene Polymorphism in Bipolar-I Disorder. Arch. Med. Res. 2021, 52, 523–528. [Google Scholar] [CrossRef]
- Ebisawa, T.; Kajimura, N.; Uchiyama, M.; Katoh, M.; Sekimoto, M.; Watanabe, T.; Ozeki, Y.; Ikeda, M.; Jodoi, T.; Sugishita, M.; et al. Allelic variants of human melatonin 1a receptor: Function and prevalence in subjects with circadian rhythm sleep disorders. Biochem. Biophys. Res. Commun. 1999, 262, 832–837. [Google Scholar] [CrossRef]
- Chang, A.M.; Bjonnes, A.C.; Aeschbach, D.; Buxton, O.M.; Gooley, J.J.; Anderson, C.; Van Reen, E.; Cain, S.W.; Czeisler, C.A.; Duffy, J.F.; et al. Circadian gene variants influence sleep and the sleep electroencephalogram in humans. Chronobiol. Int. 2016, 33, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Bruni, O.; Ottaviano, S.; Guidetti, V.; Romoli, M.; Innocenzi, M.; Cortesi, F.; Giannotti, F. The Sleep Disturbance Scale for Children (SDSC) Construction and validation of an instrument to evaluate sleep disturbances in childhood and adolescence. J. Sleep Res. 1996, 5, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Feige, B.; Baglioni, C.; Spiegelhalder, K.; Hirscher, V.; Nissen, C.; Riemann, D. The microstructure of sleep in primary insomnia: An overview and extension. Int. J. Psychophysiol. 2013, 89, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Pérusse, A.D.; Pedneault-Drolet, M.; Rancourt, C.; Turcotte, I.; St-Jean, G.; Bastien, C.H. REM sleep as a potential indicator of hyperarousal in psychophysiological and paradoxical insomnia sufferers. Int. J. Psychophysiol. 2015, 95, 372–378. [Google Scholar] [CrossRef]
- Romeo, D.M.; Brogna, C.; Belli, A.; Lucibello, S.; Cutrona, C.; Apicella, M.; Mercuri, E.; Mariotti, P. Sleep Disorders in Autism Spectrum Disorder Pre-School Children: An Evaluation Using the Sleep Disturbance Scale for Children. Medicina 2021, 57, 95. [Google Scholar] [CrossRef]
- Scarpelli, S.; Menghini, D.; Alfonsi, V.; Giumello, F.; Annarumma, L.; Gorgoni, M.; Valeri, G.; Pazzaglia, M.; De Gennaro, L.; Vicari, S. Sleep Disturbances and Co-sleeping in Italian Children and Adolescents with Autism Spectrum Disorder. J. Autism Dev. Disord. 2024. [Google Scholar] [CrossRef]
- Emet, M.; Ozcan, H.; Ozel, L.; Yayla, M.; Halici, Z.; Hacimuftuoglu, A. A Review of Melatonin, Its Receptors and Drugs. Eurasian J. Med. 2016, 48, 135–141. [Google Scholar] [CrossRef]
- Muller, M.D.; Sauder, C.L.; Ray, C.A. Melatonin attenuates the skin sympathetic nerve response to mental stress. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1382–H1386. [Google Scholar] [CrossRef]
- Karamitri, A.; Jockers, R. Melatonin in type 2 diabetes mellitus and obesity. Nat. Rev. Endocrinol. 2019, 15, 105–125. [Google Scholar] [CrossRef]
- Tuomi, T.; Nagorny, C.L.F.; Singh, P.; Bennet, H.; Yu, Q.; Alenkvist, I.; Isomaa, B.; Östman, B.; Söderström, J.; Pesonen, A.-K.; et al. Increased Melatonin Signaling Is a Risk Factor for Type 2 Diabetes. Cell Metab. 2016, 23, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Lane, J.M.; Chang, A.M.; Bjonnes, A.C.; Aeschbach, D.; Anderson, C.; Cade, B.E.; Cain, S.W.; Czeisler, C.A.; Gharib, S.A.; Gooley, J.J.; et al. Impact of Common Diabetes Risk Variant in MTNR1B on Sleep, Circadian, and Melatonin Physiology. Diabetes 2016, 65, 1741–1751. [Google Scholar] [CrossRef]
- Terrelonge, M.; LaHue, S.C.; Tang, C.; Movsesyan, I.; Pullinger, C.R.; Dubal, D.B.; Leung, J.; Douglas, V.C. KIBRA, MTNR1B, and FKBP5 genotypes are associated with decreased odds of incident delirium in elderly post-surgical patients. Sci. Rep. 2022, 12, 556. [Google Scholar] [CrossRef] [PubMed]
- Mahanna-Gabrielli, E.; Miano, T.A.; Augoustides, J.G.; Kim, C.; Bavaria, J.E.; Kofke, W.A. Does the melatonin receptor 1B gene polymorphism have a role in postoperative delirium? PLoS ONE 2018, 13, e0207941. [Google Scholar] [CrossRef]
- Vejrazkova, D.; Vankova, M.; Vcelak, J.; Krejci, H.; Anderlova, K.; Tura, A.; Pacini, G.; Sumova, A.; Sladek, M.; Bendlova, B. The rs10830963 Polymorphism of the MTNR1B Gene: Association with Abnormal Glucose, Insulin and C-peptide Kinetics. Front. Endocrinol. 2022, 13, 868364. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kuang, A.; Bain, J.R.; Hayes, M.G.; Muehlbauer, M.J.; Ilkayeva, O.R.; Newgard, C.B.; Powe, C.E.; Hivert, M.-F.; Scholtens, D.M.; et al. Metabolomic and genetic architecture of gestational diabetes subtypes. Diabetologia 2024, 67, 895–907. [Google Scholar] [CrossRef]
- Arnoriaga-Rodríguez, M.; Serrano, I.; Paz, M.; Barabash, A.; Valerio, J.; Del Valle, L.; O’connors, R.; Melero, V.; de Miguel, P.; Diaz, Á.; et al. A Simplified Screening Model to Predict the Risk of Gestational Diabetes Mellitus in Caucasian and Latin American Pregnant Women. Genes 2024, 15, 482. [Google Scholar] [CrossRef]
- Kotagal, S.; Broomall, E. Sleep in children with autism spectrum disorder. Pediatr. Neurol. 2012, 47, 242–251. [Google Scholar] [CrossRef]
- Galli, J.; Loi, E.; Visconti, L.M.; Mattei, P.; Eusebi, A.; Calza, S.; Fazzi, E.; ASD Collaborative Group. Sleep Disturbances in Children Affected by Autism Spectrum Disorder. Front. Psychiatry 2022, 13, 736696. [Google Scholar] [CrossRef]
- Miano, S.; Paolino, M.C.; Urbano, A.; Parisi, P.; Massolo, A.C.; Castaldo, R.; Villa, M.P. Neurocognitive assessment and sleep analysis in children with sleep-disordered breathing. Clin. Neurophysiol. 2011, 122, 311–319. [Google Scholar] [CrossRef]
- Fleetham, J.A.; Fleming, J.A. Parasomnias. CMAJ 2014, 186, E273–E280. [Google Scholar] [CrossRef] [PubMed]
- Zaidman-Zait, A.; Zwaigenbaum, L.; Duku, E.; Bennett, T.; Szatmari, P.; Mirenda, P.; Smith, I.; Vaillancourt, T.; Volden, J.; Waddell, C.; et al. Factor analysis of the children’s sleep habits questionnaire among preschool children with autism spectrum disorder. Res. Dev. Disabil. 2020, 97, 103548. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, L.; Postorino, V.; Siracusano, M.; Riccioni, A.; Curatolo, P. The Relationship between Sleep Problems, Neurobiological Alterations, Core Symptoms of Autism Spectrum Disorder, and Psychiatric Comorbidities. J. Clin. Med. 2018, 7, 102. [Google Scholar] [CrossRef] [PubMed]
- Carmassi, C.; Palagini, L.; Caruso, D.; Masci, I.; Nobili, L.; Vita, A.; Dell’Osso, L. Systematic Review of Sleep Disturbances and Circadian Sleep Desynchronization in Autism Spectrum Disorder: Toward an Integrative Model of a Self-Reinforcing Loop. Front. Psychiatry 2019, 10, 366. [Google Scholar] [CrossRef]
- Roid, G.H.; Miller, L.J.; Pomplun, M.; Koch, C. (Leiter-3) Leiter International Performance Scale, 3rd ed.; Cornoldi, C., Giofrè, D., Belacchi, C., Eds.; Western Psychological Services: Los Angeles, CA, USA, 2013. [Google Scholar]
- Wechsler, D. Wechsler Intelligence Scale for Children, 4th ed.; Harcourt Assessment: San Antonio, TX, USA, 2003. [Google Scholar]
- Raven, J.C.; Court, J.H.; Raven, J. Manual for Raven’s Progressive Matrices and Vocabulary Scales Section 2: Coloured Progressive Matrices; Belacchi, C., Scalisi, T.G., Cannoni, E., Cornoldi, C., Eds.; H. K. Lewis & Co. Ltd.: London, UK, 1984. [Google Scholar]
- Lord, C.; Rutter, M.; DiLavore, P.C.; Risi, S.; Gotham, K.; Bishop, S. Autism Diagnostic Observation Schedule–Second Edition (ADOS-2); Colombi, C., Tancredi, R., Persico, A., Faggioli, A., Eds.; Western Psychological Services: Los Angeles, CA, USA, 2012. [Google Scholar]
- Lord, C.; Rutter, M.; Le Couteur, A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 1994, 24, 659–685. [Google Scholar] [CrossRef]
- Schopler, E.; Reichler, R.J.; Renner, B.R. The Childhood Autism Rating Scale (CARS), for Diagnostic Screening and Classification in Autism; Irvington: New York, NY, USA, 1986; 63p. [Google Scholar]

| Sardinian ASD | ASD vs. SIB | ASD vs. HC | Sardinian SIBS | SIB vs. HC | Sardinian Healthy Controls | ||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | p | p | N | % | p | N | % | |
| MT1 rs6847693 | |||||||||
| T | 250 | 90.0 | 148 | 90.0 | 95 | 89.6 | |||
| C | 28 | 10.0 | 0.9 | 0.9 | 16 | 10.0 | 0.8 | 11 | 10.3 |
| MT1 rs2119882 | |||||||||
| C | 160 | 58.0 | 95 | 58.0 | 54 | 50.9 | |||
| T | 118 | 42.0 | 0.9 | 0.2 | 69 | 42.0 | 0.3 | 52 | 49.6 |
| MT1 rs6553010 | |||||||||
| G | 241 | 87.0 | 140 | 85.0 | 91 | 85.9 | |||
| A | 37 | 13.0 | 0.7 | 0.8 | 24 | 15.0 | 0.9 | 15 | 14.1 |
| MT2 rs4753426 | |||||||||
| C | 139 | 50.0 | 89 | 54.0 | 47 | 44.4 | |||
| T | 139 | 50.0 | 0.4 | 0.3 | 75 | 46.0 | 0.1 | 59 | 55.6 |
| MT2 rs10830963 | |||||||||
| C | 209 | 75.0 | 122 | 74.0 | 91 | 85.9 | |||
| G | 69 | 25.0 | 0.8 | 0.02 | 42 | 26.0 | 0.02 | 15 | 14.1 |
| MT2 rs1562444 | |||||||||
| A | 144 | 52.0 | 80 | 49.0 | 54 | 50.9 | |||
| G | 134 | 48.0 | 0.5 | 0.8 | 84 | 51.0 | 0.7 | 52 | 49.6 |
| N | 278 | 164 | 106 | ||||||
| MT1 rs6847693 | |||||||||
| C/C | 1 | 1.0 | 1 | 1.0 | 0 | 0.0 | |||
| T/C | 26 | 19.0 | 14 | 17.0 | 11 | 20.8 | |||
| T/T | 112 | 80.0 | 0.8 | 0.8 | 67 | 82.0 | 0.8 | 42 | 79.2 |
| MT1 rs2119882 | |||||||||
| C/C | 51 | 37.0 | 28 | 34.0 | 15 | 28.3 | |||
| C/T | 58 | 41.0 | 39 | 48.0 | 24 | 45.3 | |||
| T/T | 30 | 22.0 | 0.7 | 0.5 | 15 | 18.0 | 0.5 | 14 | 26.4 |
| MT1 rs6553010 | |||||||||
| A/A | 2 | 1.0 | 1 | 1.0 | 0 | 0.0 | |||
| G/A | 33 | 24.0 | 22 | 29.0 | 15 | 28.3 | |||
| G/G | 104 | 75.0 | 0.9 | 0.6 | 59 | 72.0 | 0.7 | 38 | 71.7 |
| MT2 rs4753426 | |||||||||
| C/C | 37 | 27.0 | 23 | 28.0 | 9 | 17.0 | |||
| C/T | 65 | 46.0 | 43 | 52.0 | 29 | 54.7 | |||
| T/T | 37 | 27.0 | 0.5 | 0.4 | 16 | 20.0 | 0.2 | 15 | 28.3 |
| MT2 rs10830963 | |||||||||
| C/C | 77 | 55.0 | 46 | 56.0 | 39 | 73.6 | |||
| C/G | 55 | 40.0 | 30 | 37.0 | 13 | 24.5 | |||
| G/G | 7 | 5.0 | 0.7 | 0.06 | 6 | 7.0 | 0.08 | 1 | 1.9 |
| MT2 rs1562444 | |||||||||
| A/A | 39 | 28.0 | 21 | 26.0 | 13 | 24.5 | |||
| A/G | 66 | 47.0 | 38 | 46.0 | 28 | 52.9 | |||
| G/G | 34 | 25.0 | 0.8 | 0.8 | 23 | 28.0 | 0.7 | 12 | 22.6 |
| N | 139 | 82 | 53 | ||||||
| ASD | SIBS | ||
|---|---|---|---|
| N = 38 | N = 15 | p-Value | |
| AGE [years] * | 13.3 ± 1.6 | 13.3 ± 2.3 | 0.5 |
| SEX (% males) | 65.7 | 26.7 | 0.15 |
| ADOS * | 11.47 ± 4.8 | N.T. | - |
| CARS * | 39.6 ± 7.9 | N.T. | - |
| TOTAL SDSC * | 79.24 ± 17.7 | 42.5 ± 3.3 | <0.001 |
| DIMS * | 77.5 ± 22.9 | 41.5 ± 1.8 | <0.001 |
| SBD * | 60.24 ± 20.0 | 45.5 ± 1.9 | 0.009 |
| DA * | 58.53 ± 16.7 | 47.0 ± 0.0 | 0.017 |
| SWTD * | 74.0 ± 16.6 | 45.8 ± 5.6 | <0.001 |
| DOES * | 62.9 ± 16.4 | 44.5 ± 3.2 | <0.001 |
| SHY * | 60.6 ± 18.7 | 45.6 ± 1.7 | 0.006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolognesi, E.; Carta, A.; Guerini, F.R.; Sotgiu, S.; Agliardi, C.; Dettori, C.; Zanzottera, M.; Clerici, M. Outcome of Sleep Rehabilitation in Autistic Children with Sleep Disorders Is Linked to Melatonin Receptor Genes SNPs. Int. J. Mol. Sci. 2025, 26, 5198. https://doi.org/10.3390/ijms26115198
Bolognesi E, Carta A, Guerini FR, Sotgiu S, Agliardi C, Dettori C, Zanzottera M, Clerici M. Outcome of Sleep Rehabilitation in Autistic Children with Sleep Disorders Is Linked to Melatonin Receptor Genes SNPs. International Journal of Molecular Sciences. 2025; 26(11):5198. https://doi.org/10.3390/ijms26115198
Chicago/Turabian StyleBolognesi, Elisabetta, Alessandra Carta, Franca Rosa Guerini, Stefano Sotgiu, Cristina Agliardi, Chiara Dettori, Milena Zanzottera, and Mario Clerici. 2025. "Outcome of Sleep Rehabilitation in Autistic Children with Sleep Disorders Is Linked to Melatonin Receptor Genes SNPs" International Journal of Molecular Sciences 26, no. 11: 5198. https://doi.org/10.3390/ijms26115198
APA StyleBolognesi, E., Carta, A., Guerini, F. R., Sotgiu, S., Agliardi, C., Dettori, C., Zanzottera, M., & Clerici, M. (2025). Outcome of Sleep Rehabilitation in Autistic Children with Sleep Disorders Is Linked to Melatonin Receptor Genes SNPs. International Journal of Molecular Sciences, 26(11), 5198. https://doi.org/10.3390/ijms26115198







