Potential Role of SESN3 in Linking Heart Failure with Preserved Ejection Fraction and Chronic Obstructive Pulmonary Disease via Autophagy Dysregulation
Abstract
1. Introduction
2. Results
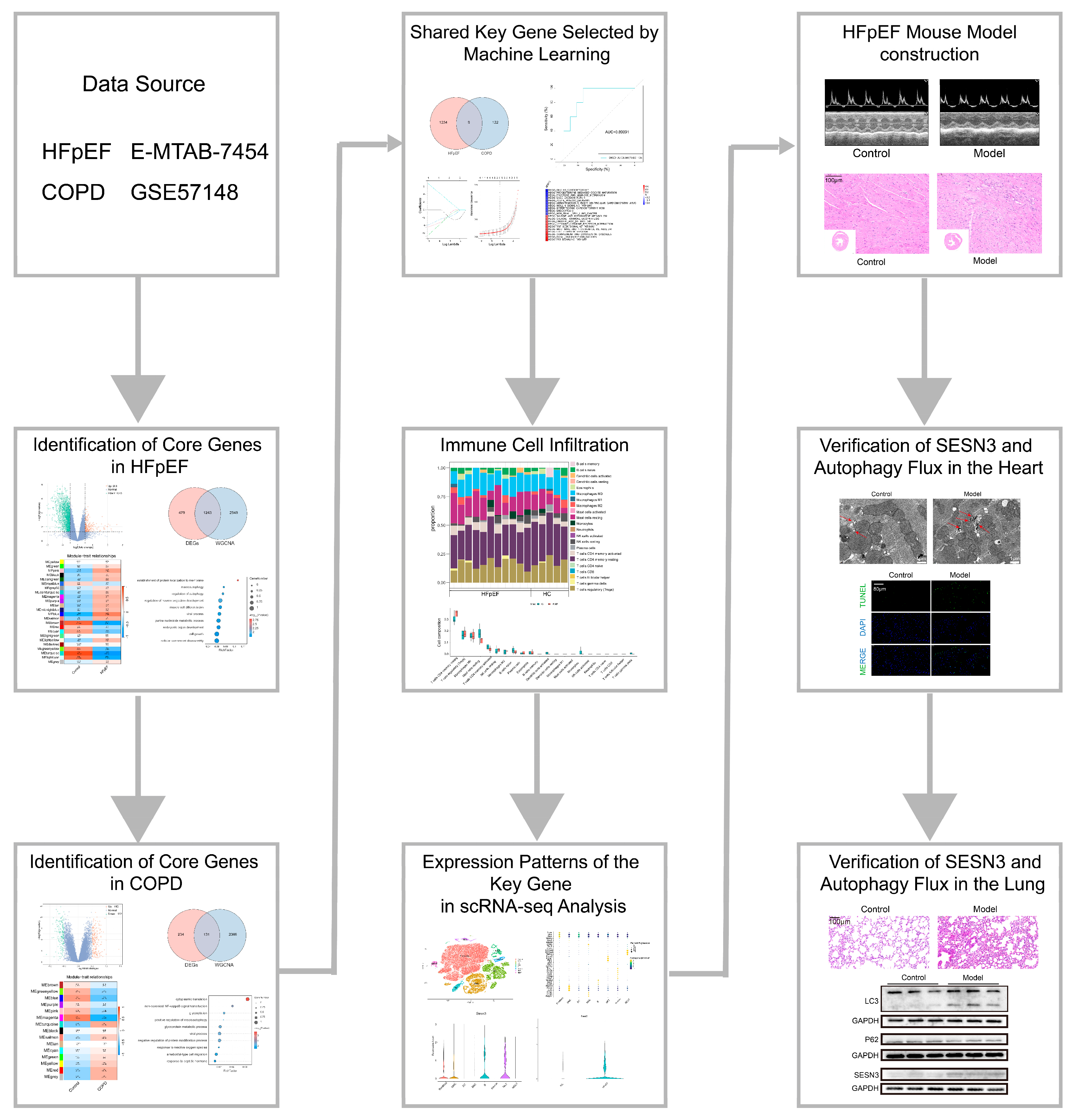
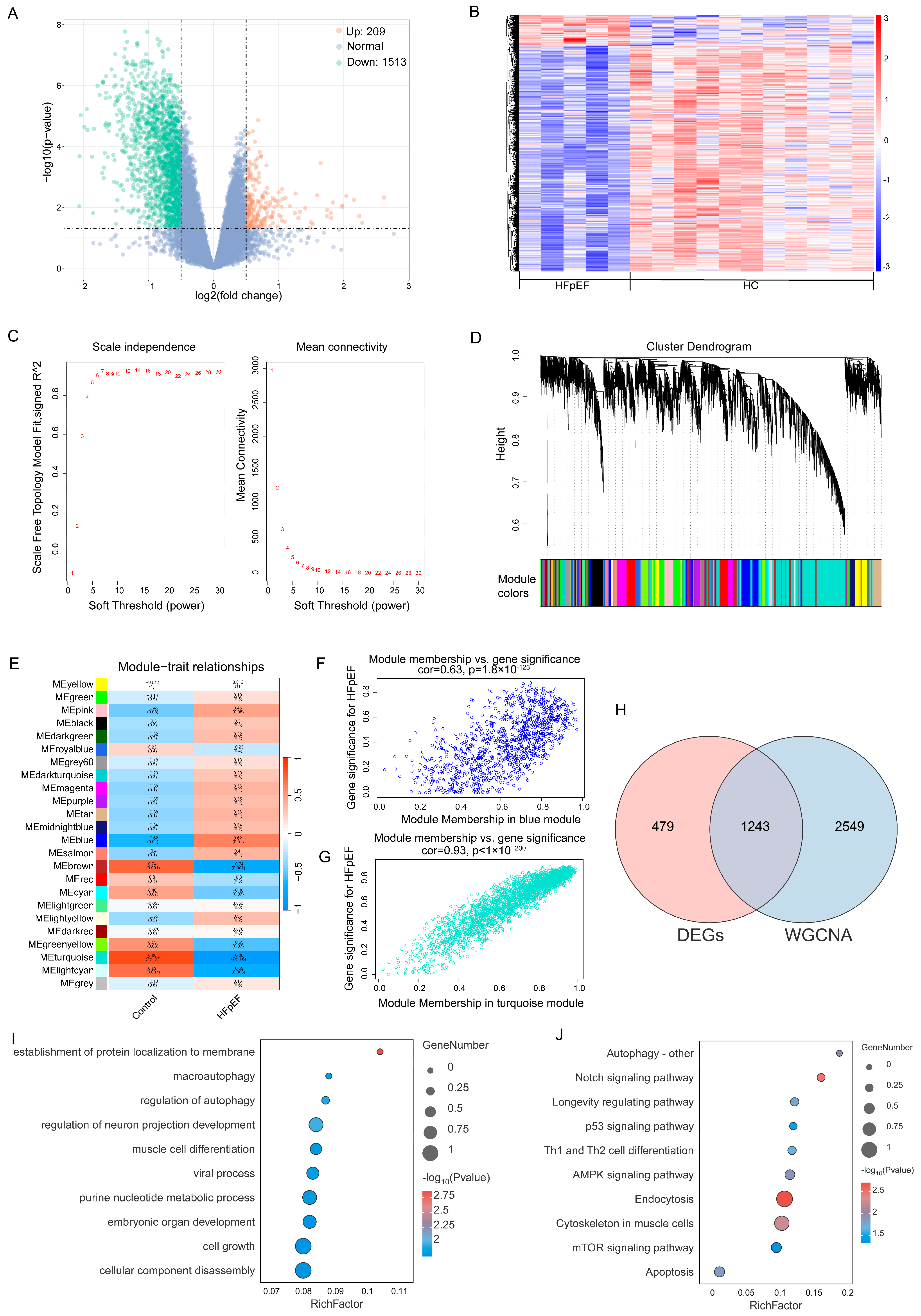
2.1. Identification of Core Genes in HFpEF and Functional Enrichment Analysis
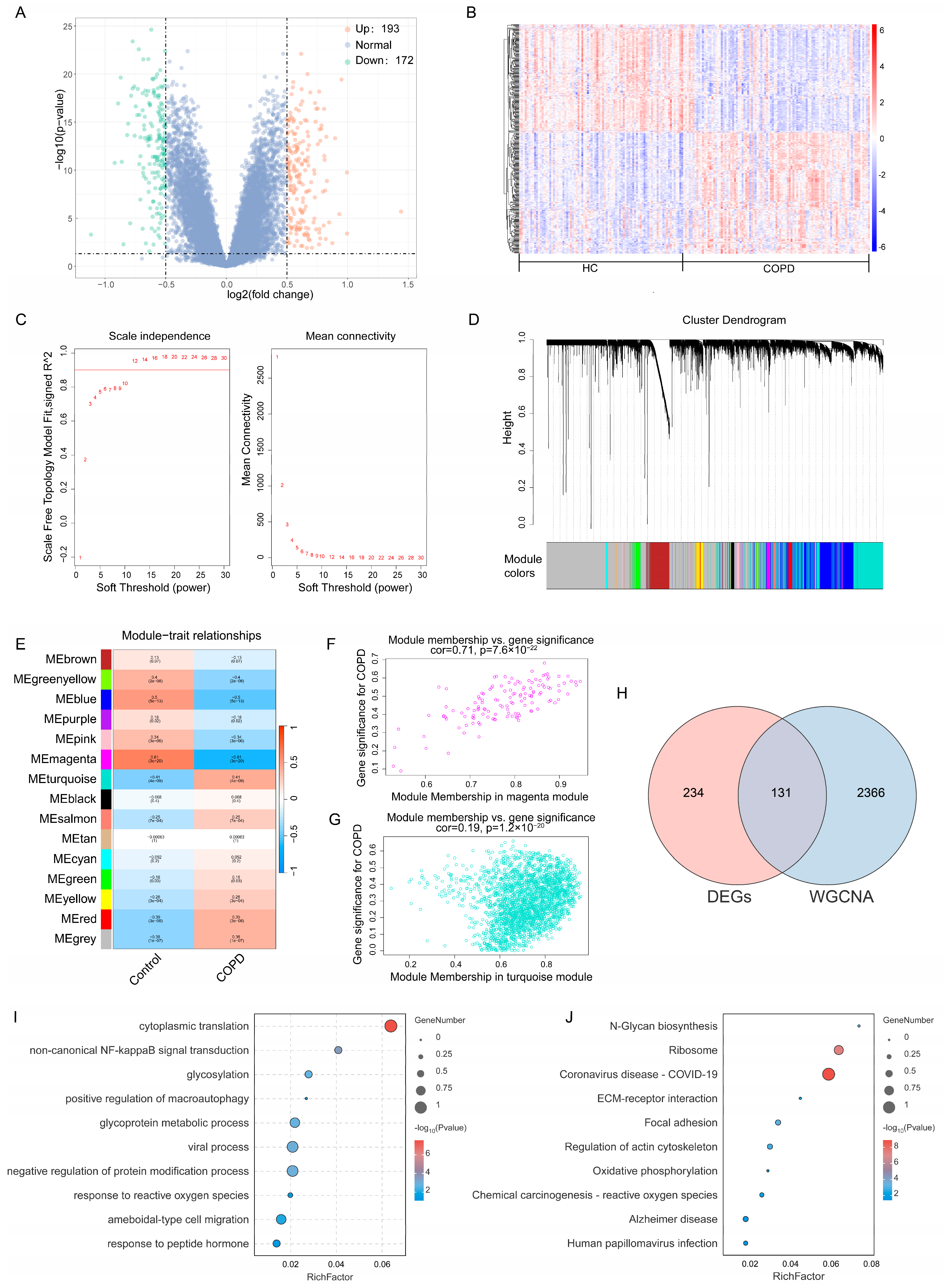
2.2. Identification of Core Genes in COPD and Functional Enrichment Analysis
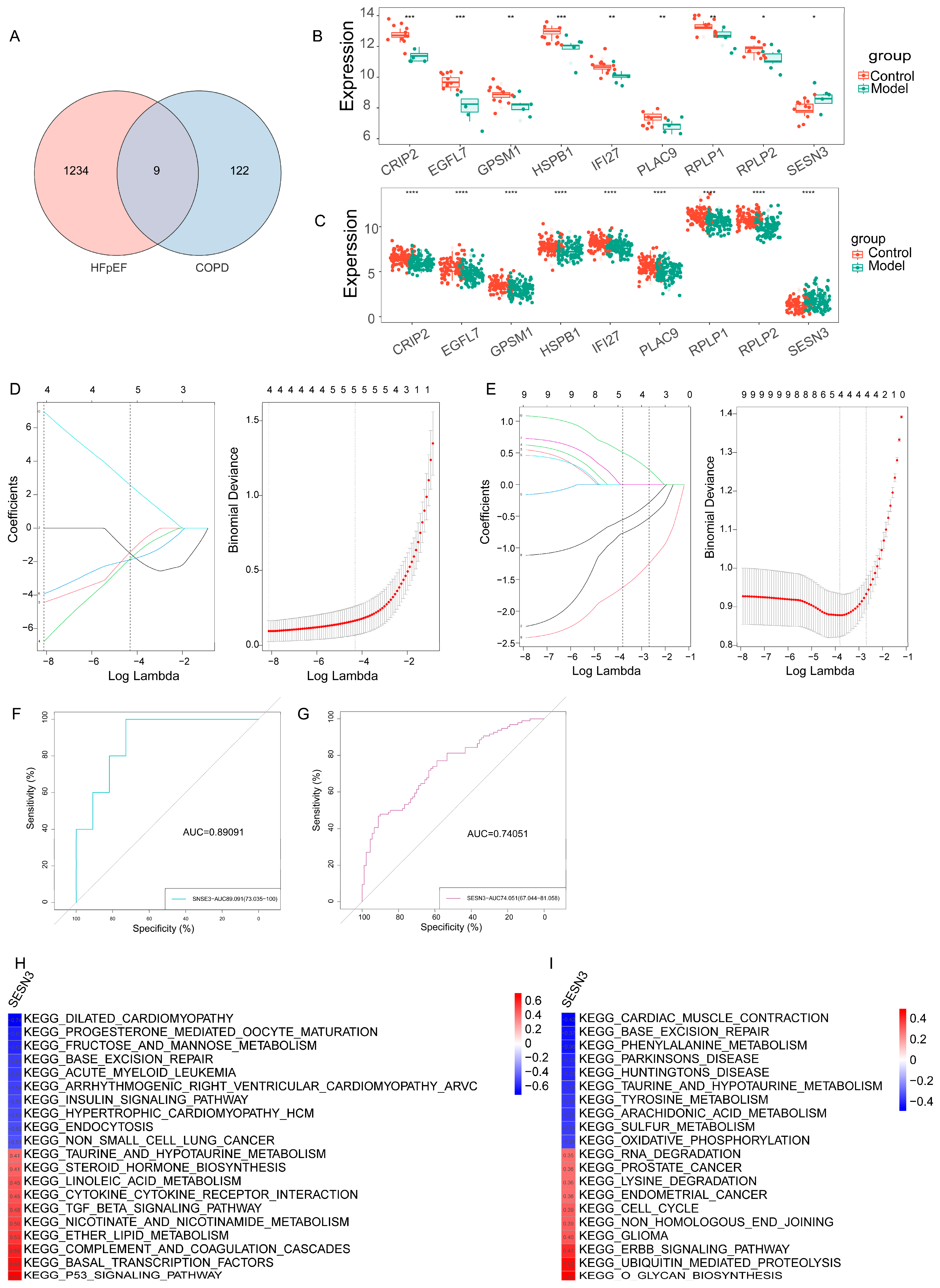
2.3. Identification of Shared Key Gene in HFpEF and COPD via Machine Learning
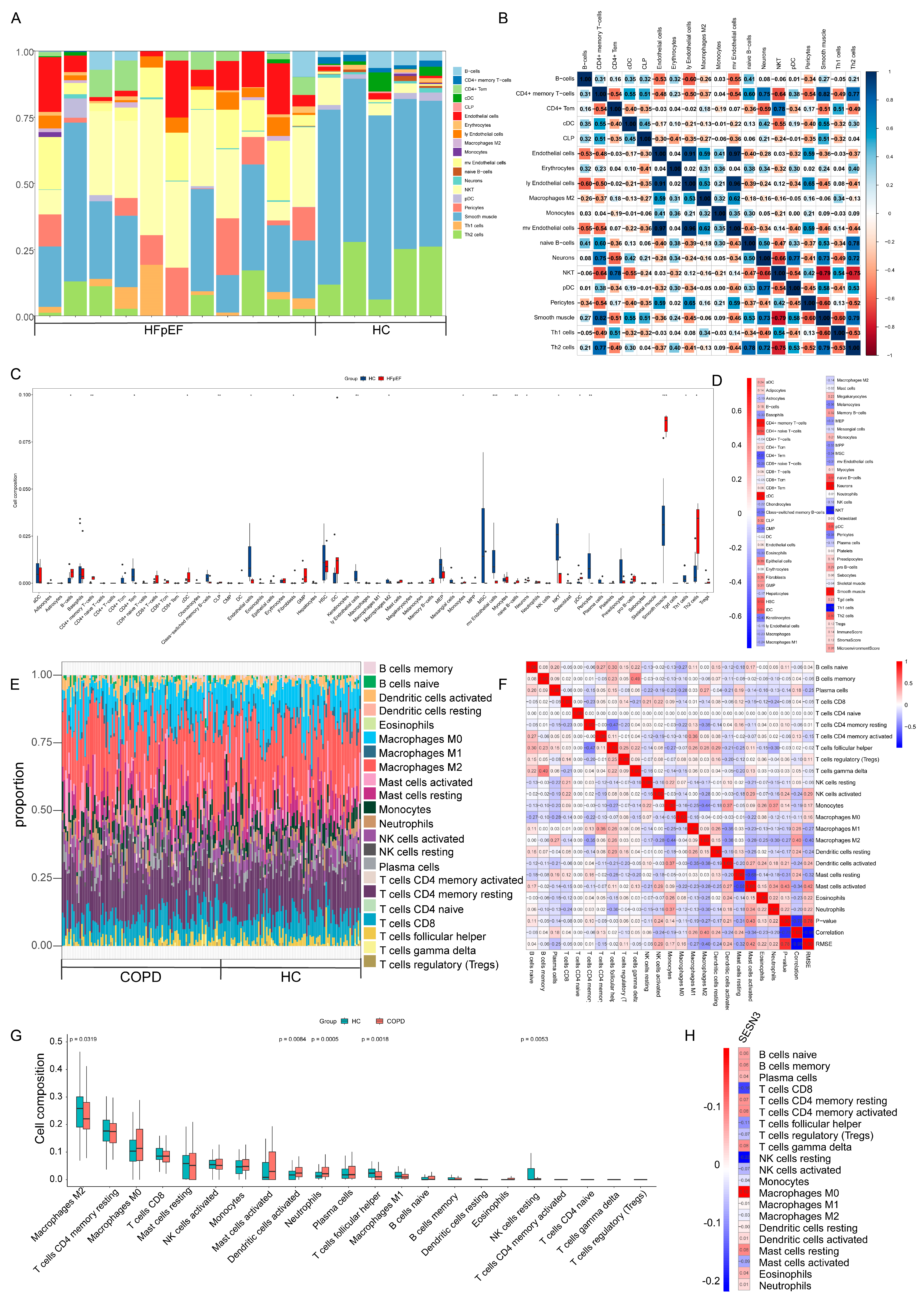
2.4. Immune Cell Infiltration and Its Correlation with Key Genes
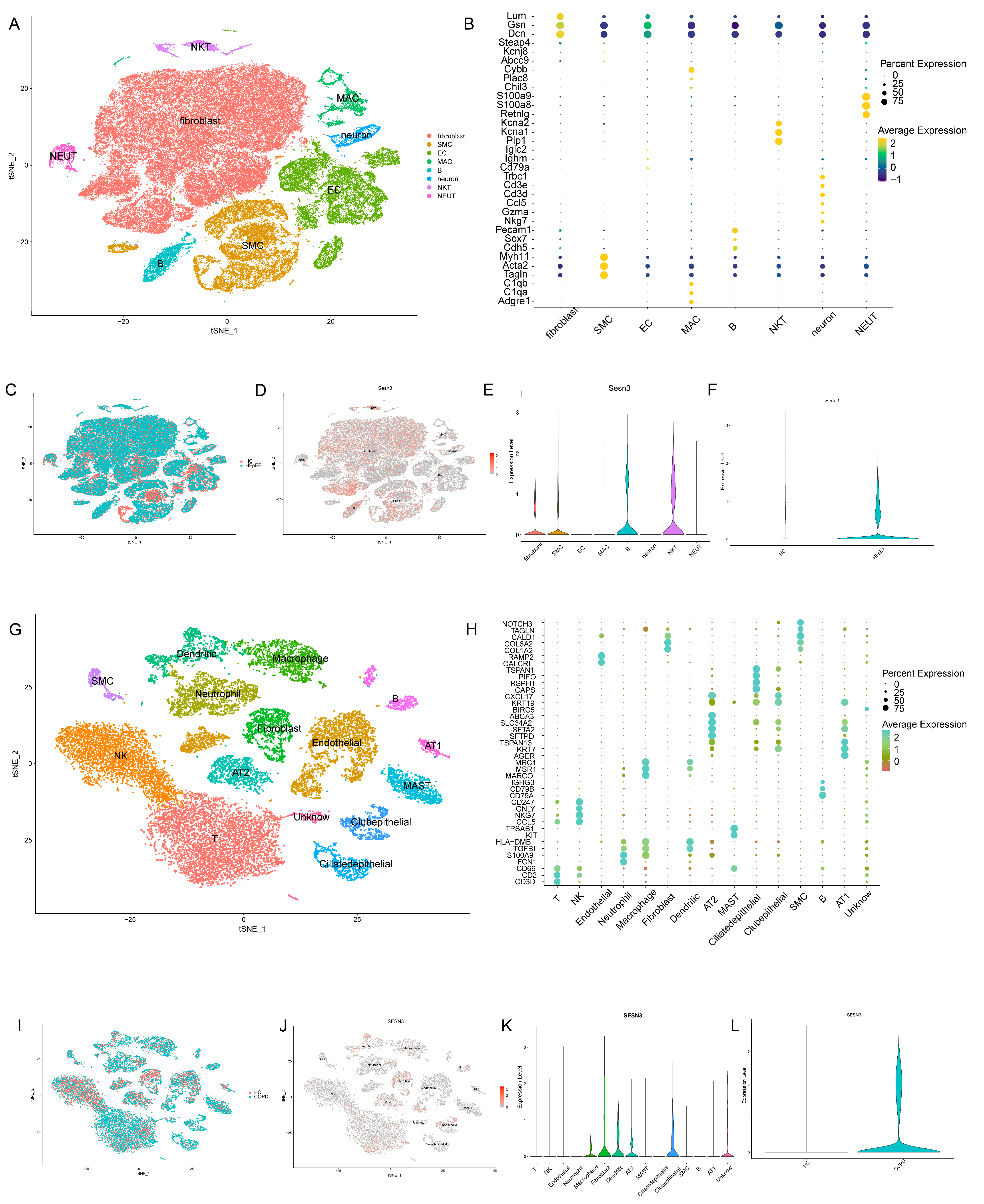
2.5. Expression Patterns of the Key Gene in scRNA-Seq Analysis
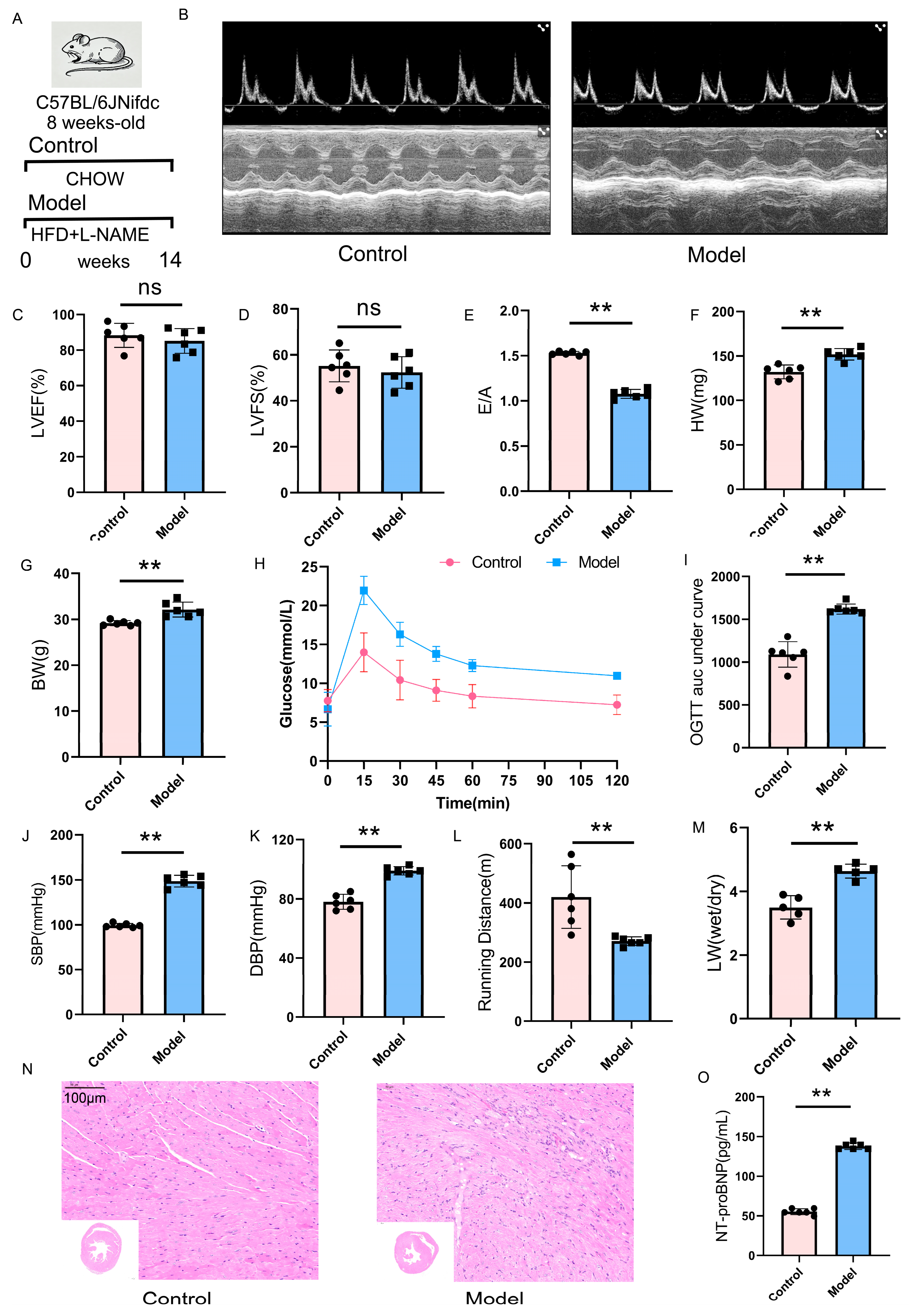
2.6. Validation of the HFpEF Mouse Model: Cardiac Function and Metabolic Alterations
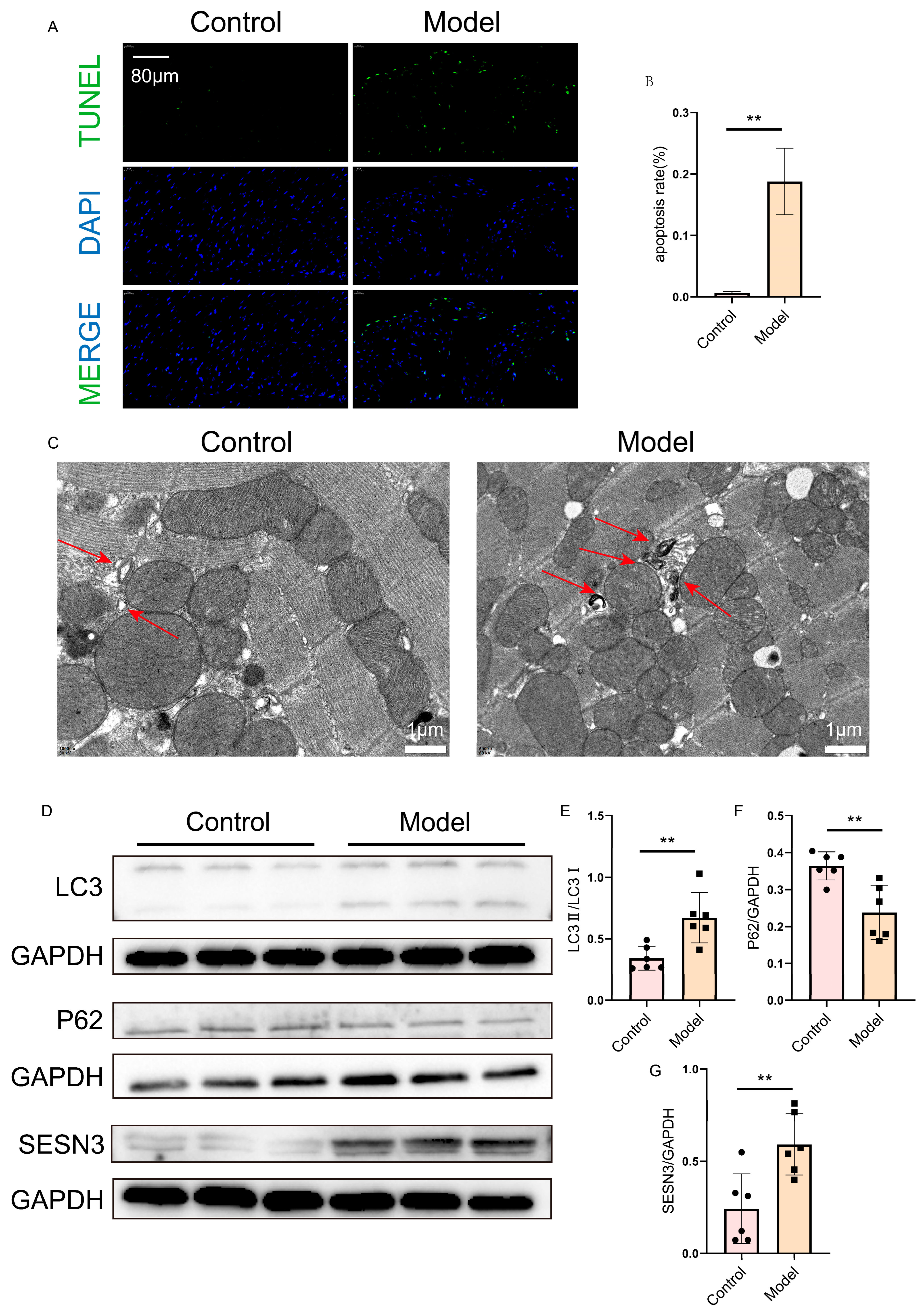
2.7. Verification of SESN3 and Autophagy Flux in the Heart
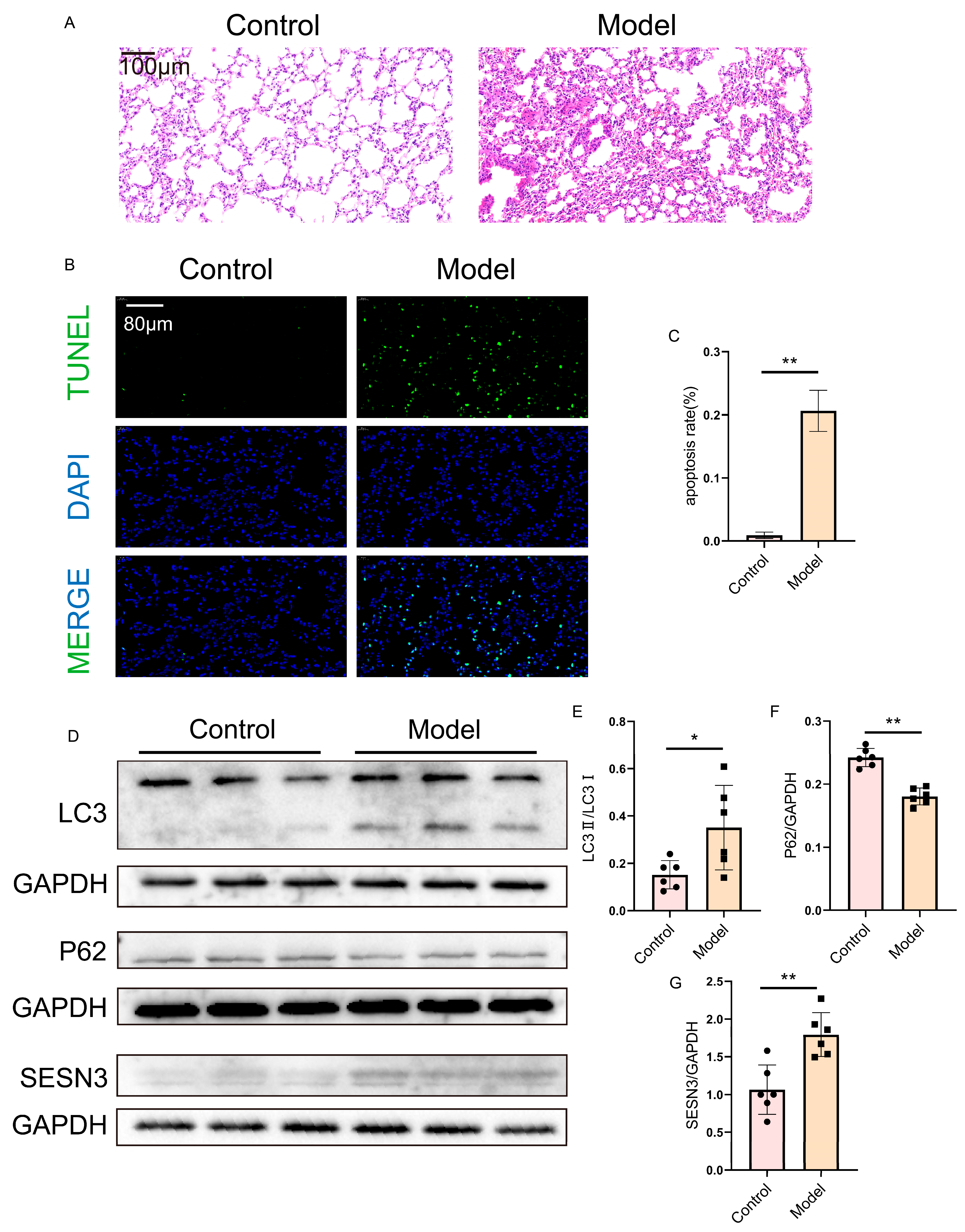
2.8. Verification of SESN3 and Autophagy Flux in the Lung
3. Discussion
4. Materials and Methods
4.1. Data Collection and Processing
4.2. Identification of Differentially Expressed Genes (DEGs)
4.3. Weighted Gene Co-Expression Network Analysis
4.4. Functional Enrichment Analysis
4.5. Core Gene Selection via LASSO
4.6. Gene Set Variation Analysis (GSVA)
4.7. Immune Infiltration and Correlation Analysis
4.8. Single-Cell Data Processing and Gene Expression Analysis
4.9. Experimental Animals
4.10. Echocardiographic Assessment
4.11. Blood Pressure Measurement
4.12. Glucose Tolerance Test
4.13. Exercise Exhaustion Test
4.14. Histological Analysis
4.15. Transmission Electron Microscopy (TEM)
4.16. Western Blot Analysis
4.17. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heart Failure Society of America. Evaluation and management of patients with heart failure and preserved left ventricular ejection fraction. J. Card. Fail 2006, 12, e80–e85. [Google Scholar] [CrossRef]
- Upadhya, B.; Kitzman, D.W. Heart failure with preserved ejection fraction: New approaches to diagnosis and management. Clin. Cardiol. 2020, 43, 145–155. [Google Scholar] [CrossRef]
- Xu, J.; Zeng, Q.; Li, S.; Su, Q.; Fan, H. Inflammation mechanism and research progress of COPD. Front. Immunol. 2024, 15, 1404615. [Google Scholar] [CrossRef]
- Patel, N. An update on COPD prevention, diagnosis, and management: The 2024 GOLD Report. Nurse Pract. 2024, 49, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Deng, M.; Gao, Q.; Zhang, Q.; Bian, Y.; Wang, Z.; Li, J.; Xu, W.; Li, C.; Wang, K.; et al. Predictive and therapeutic value of lipoprotein-associated phospholipaseA2 in sarcopenia in chronic obstructive pulmonary disease. Int. J. Biol. Macromol. 2024, 275, 133741. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, N.M.; Petrie, M.C.; Jhund, P.S.; Chalmers, G.W.; Dunn, F.G.; McMurray, J.J. Heart failure and chronic obstructive pulmonary disease: Diagnostic pitfalls and epidemiology. Eur. J. Heart Fail. 2009, 11, 130–139. [Google Scholar] [CrossRef]
- Berry, C.; Hogg, K.; Norrie, J.; Stevenson, K.; Brett, M.; McMurray, J. Heart failure with preserved left ventricular systolic function: A hospital cohort study. Heart 2005, 91, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Mentz, R.J.; Fiuzat, M.; Wojdyla, D.M.; Chiswell, K.; Gheorghiade, M.; Fonarow, G.C.; O’Connor, C.M. Clinical characteristics and outcomes of hospitalized heart failure patients with systolic dysfunction and chronic obstructive pulmonary disease: Findings from OPTIMIZE-HF. Eur. J. Heart Fail. 2021, 14, 395–403. [Google Scholar] [CrossRef]
- Paulus, W.J.; Tschöpe, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef]
- Reddy, Y.N.V.; Frantz, R.P.; Hassoun, P.M.; Hemnes, A.R.; Horn, E.; Leopold, J.A.; Rischard, F.; Rosenzweig, E.B.; Hill, N.S.; Erzurum, S.C.; et al. Clinical Implications of Pretest Probability of HFpEF on Outcomes in Precapillary Pulmonary Hypertension. J. Am. Coll. Cardiol. 2024, 84, 2196–2210. [Google Scholar] [CrossRef]
- Thorp, E.B.; Filipp, M. Contributions of Inflammation to Cardiometabolic Heart Failure with Preserved Ejection Fraction. Annu. Rev. Pathol. 2025, 20, 143–167. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, N.; Matsumoto, H.; Morimoto, C.; Hirai, T. Sputum short-chain fatty acids, microbiome, inflammation, and mucus plugging in obstructive airway disease. J. Allergy Clin. Immunol. 2025, 155, 1675–1680. [Google Scholar] [CrossRef]
- Ather, S.; Chan, W.; Bozkurt, B.; Aguilar, D.; Ramasubbu, K.; Zachariah, A.A.; Wehrens, X.H.; Deswal, A. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J. Am. Coll. Cardiol. 2012, 59, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.M.; Prince, M.R.; Hoffman, E.A.; Bluemke, D.A.; Liu, C.Y.; Rabinowitz, D.; Hueper, K.; Parikh, M.A.; Gomes, A.S.; Michos, E.D.; et al. Impaired left ventricular filling in COPD and emphysema: Is it the heart or the lungs? The Multi-Ethnic Study of Atherosclerosis COPD Study. Chest 2013, 144, 1143–1151. [Google Scholar] [CrossRef]
- Mentz, R.J.; Kelly, J.P.; von Lueder, T.G.; Voors, A.A.; Lam, C.S.; Cowie, M.R.; Kjeldsen, K.; Jankowska, E.A.; Atar, D.; Butler, J.; et al. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J. Am. Coll. Cardiol. 2014, 64, 2281–2293. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Miguel, S.; Buckbinder, L.; Jean, P.; Gelbert, L.; Talbott, R.; Laidlaw, J.; Seizinger, B.; Kley, N. PA26, a novel target of the p53 tumor suppressor and member of the GADD family of DNA damage and growth arrest inducible genes. Oncogene 1999, 18, 127–137. [Google Scholar] [CrossRef]
- Peeters, H.; Debeer, P.; Bairoch, A.; Wilquet, V.; Huysmans, C.; Parthoens, E.; Fryns, J.P.; Gewillig, M.; Nakamura, Y.; Niikawa, N.; et al. PA26 is a candidate gene for heterotaxia in humans: Identification of a novel PA26-related gene family in human and mouse. Hum. Genet. 2003, 112, 573–580. [Google Scholar] [CrossRef]
- Tao, R.; Xiong, X.; Liangpunsakul, S.; Dong, X.C. Sestrin 3 protein enhances hepatic insulin sensitivity by direct activation of the mTORC2-Akt signaling. Diabetes. 2015, 64, 1211–1223. [Google Scholar] [CrossRef]
- Cordani, M.; Butera, G.; Dando, I.; Torrens-Mas, M.; Butturini, E.; Pacchiana, R.; Oppici, E.; Cavallini, C.; Gasperini, S.; Tamassia, N.; et al. Mutant p53 blocks SESN1/AMPK/PGC-1α/UCP2 axis increasing mitochondrial O2− production in cancer cells. Br. J. Cancer 2018, 119, 994–1008. [Google Scholar] [CrossRef]
- Deng, W.; Cha, J.; Yuan, J.; Haraguchi, H.; Bartos, A.; Leishman, E.; Viollet, B.; Bradshaw, H.B.; Hirota, Y.; Dey, S.K. p53 coordinates decidual sestrin 2/AMPK/mTORC1 signaling to govern parturition timing. J. Clin. Investig. 2016, 126, 2941–2954. [Google Scholar] [CrossRef]
- Morsch, A.L.B.C.; Wisniewski, E.; Luciano, T.F.; Comin, V.H.; Silveira, G.B.; Marques, S.O.; Thirupathi, A.; Silveira Lock, P.C.; De Souza, C.T. Cigarette smoke exposure induces ROS-mediated autophagy by regulating sestrin, AMPK, and mTOR level in mice. Redox Rep. 2019, 24, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Zeng, J.; Chen, Y.; Chen, C.; Tan, W.; Zhao, J.; Dong, B.; Sun, Y.; Dong, Y.; Liu, C. Sestrin 1 ameliorates cardiac hypertrophy via autophagy activation. J. Cell Mol. Med. 2017, 21, 1193–1205. [Google Scholar] [CrossRef] [PubMed]
- Lear, T.B.; Lockwood, K.C.; Ouyang, Y.; Evankovich, J.W.; Larsen, M.B.; Lin, B.; Liu, Y.; Chen, B.B. The RING-type E3 ligase RNF186 ubiquitinates Sestrin-2 and thereby controls nutrient sensing. J. Biol. Chem. 2019, 294, 16527–16534. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ng, S.; Wang, J.; Zhou, J.; Tan, S.H.; Yang, N.; Lin, Q.; Xia, D.; Shen, H.M. Histone deacetylase inhibitors induce autophagy through FOXO1-dependent pathways. Autophagy 2015, 11, 629–642. [Google Scholar] [CrossRef]
- He, Y.; Cao, X.; Guo, P.; Li, X.; Shang, H.; Liu, J.; Xie, M.; Xu, Y.; Liu, X. Quercetin induces autophagy via FOXO1-dependent pathways and autophagy suppression enhances quercetin-induced apoptosis in PASMCs in hypoxia. Free Radic. Biol. Med. 2017, 103, 165–176. [Google Scholar] [CrossRef]
- Budanov, A.V. Stress-responsive sestrins link p53 with redox regulation and mammalian target of rapamycin signaling. Antioxid. Redox Signal. 2011, 15, 1679–1690. [Google Scholar] [CrossRef]
- Abdellatif, M.; Vasques-Nóvoa, F.; Trummer-Herbst, V.; Durand, S.; Koser, F.; Islam, M.; Nah, J.; Sung, E.A.; Feng, R.; Aprahamian, F.; et al. Autophagy is required for the therapeutic effects of the NAD+ precursor nicotinamide in obesity-related heart failure with preserved ejection fraction. Eur. Heart J. 2025, 25, ehaf062. [Google Scholar] [CrossRef]
- Lin, L.; Lin, Y.; Han, Z.; Wang, K.; Zhou, S.; Wang, Z.; Wang, S.; Chen, H. Understanding the molecular regulatory mechanisms of autophagy in lung disease pathogenesis. Front. Immunol. 2024, 15, 1460023. [Google Scholar] [CrossRef]
- Agrawal, V.; Kropski, J.A.; Gokey, J.J.; Kobeck, E.; Murphy, M.B.; Murray, K.T.; Fortune, N.L.; Moore, C.S.; Meoli, D.F.; Monahan, K.; et al. Myeloid Cell Derived IL1β Contributes to Pulmonary Hypertension in HFpEF. Circ. Res. 2023, 133, 885–898. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, L.; Zhao, Y.; Geng, Y.; Liu, R.; Ma, Y.; Bo, W.; Xi, Y.; Tian, Z. Lack of ALCAT1 enhances the protective effects of aerobic exercise on kidney in HFpEF mice. Life Sci. 2025, 366–367, 123500. [Google Scholar] [CrossRef]
- Sharif, S.M.; Smith, J.R.; Borlaug, B.A.; Olson, T.P. Association between locomotor muscle quality and cardiac function during exercise in heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2024, ejhf.3528. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, M.; Dhabalia, S.; Dash, T.; Sarkar, A.; Mondal, S. A comprehensive multi-omics study reveals potential prognostic and diagnostic biomarkers for colorectal cancer. Int. J. Biol. Macromol. 2025, 303, 140443. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yuan, A.; Liu, C.; Liu, Y.; Qiao, L.; Xu, Z.; Bi, S.; Tian, J.; Yu, B.; Lin, Z.; et al. Identification of key antifibrotic targets FPR1, TAS2R5, and LRP2BP of valsartan in diabetic nephropathy: A transcriptomics-driven study integrating machine learning, molecular docking, and dynamics simulations. Int. J. Biol. Macromol. 2025, 297, 139842. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Y.; Zhi, L.; Yu, L.; Hu, X.; Shen, Y.; Du, W. SDC4 protein action and related key genes in nonhealing diabetic foot ulcers based on bioinformatics analysis and machine learning. Int. J. Biol. Macromol. 2024, 283, 137789. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhao, H.; Cheng, Z.; Liu, J.; Li, G.; Guo, Y. Single-cell transcriptomic profiling of heart reveals ANGPTL4 linking fibroblasts and angiogenesis in heart failure with preserved ejection fraction. J. Adv. Res. 2025, 68, 215–230. [Google Scholar] [CrossRef]
- Huang, Y.; Niu, Y.; Wang, X.; Li, X.; He, Y.; Liu, X. Identification of novel biomarkers related to neutrophilic inflammation in COPD. Front. Immunol. 2024, 30, 1410158. [Google Scholar] [CrossRef]
- Schiattarella, G.G.; Altamirano, F.; Tong, D.; French, K.M.; Villalobos, E.; Kim, S.Y.; Luo, X.; Jiang, N.; May, H.I.; Wang, Z.V.; et al. Nitrosative stress drives heart failure with preserved ejection fraction. Nature 2019, 568, 351–356. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, R.; Yuan, B.; Li, Y.; Liu, X.; Huang, M.; Jiao, B.; Sun, Y.; Gao, S.; Sun, X.; Liu, T.; et al. Potential Role of SESN3 in Linking Heart Failure with Preserved Ejection Fraction and Chronic Obstructive Pulmonary Disease via Autophagy Dysregulation. Int. J. Mol. Sci. 2025, 26, 5174. https://doi.org/10.3390/ijms26115174
Zhu R, Yuan B, Li Y, Liu X, Huang M, Jiao B, Sun Y, Gao S, Sun X, Liu T, et al. Potential Role of SESN3 in Linking Heart Failure with Preserved Ejection Fraction and Chronic Obstructive Pulmonary Disease via Autophagy Dysregulation. International Journal of Molecular Sciences. 2025; 26(11):5174. https://doi.org/10.3390/ijms26115174
Chicago/Turabian StyleZhu, Rongxin, Binhua Yuan, Yunlin Li, Xiangning Liu, Mingyue Huang, Boyang Jiao, Ying Sun, Sheng Gao, Xiaoqian Sun, Tianhua Liu, and et al. 2025. "Potential Role of SESN3 in Linking Heart Failure with Preserved Ejection Fraction and Chronic Obstructive Pulmonary Disease via Autophagy Dysregulation" International Journal of Molecular Sciences 26, no. 11: 5174. https://doi.org/10.3390/ijms26115174
APA StyleZhu, R., Yuan, B., Li, Y., Liu, X., Huang, M., Jiao, B., Sun, Y., Gao, S., Sun, X., Liu, T., Wu, Y., & Li, C. (2025). Potential Role of SESN3 in Linking Heart Failure with Preserved Ejection Fraction and Chronic Obstructive Pulmonary Disease via Autophagy Dysregulation. International Journal of Molecular Sciences, 26(11), 5174. https://doi.org/10.3390/ijms26115174





