Uncovering Hidden Gluten Exposure in Celiac Patients: A Case Study in Family-Based Management and the Role of Point-of-Care Urine Testing and Psychological Assessment
Abstract
1. Introduction
2. Results
2.1. Patients
2.2. Clinical Follow-Up and Monitoring During CD Follow-Up Visit
2.2.1. Biochemical Evaluation
2.2.2. CD Antibodies and Duodenal Histology
2.2.3. Celiac Dietary Adherence Test (CDAT)
2.2.4. Symptomatology
2.2.5. Psychological Assessment of Adherence to the GFD
2.2.6. POCT-Based GIP Determination in Primary Care
3. Discussion
4. Materials and Methods
4.1. Study Design and Population
4.2. Urine and Blood Collection
4.3. CD Antibodies
4.4. Duodenal Histology
4.5. Celiac Dietary Adherence Test (CDAT)
4.6. Symptoms Questionnaire
4.7. The Hospital Anxiety Scale (HAS)
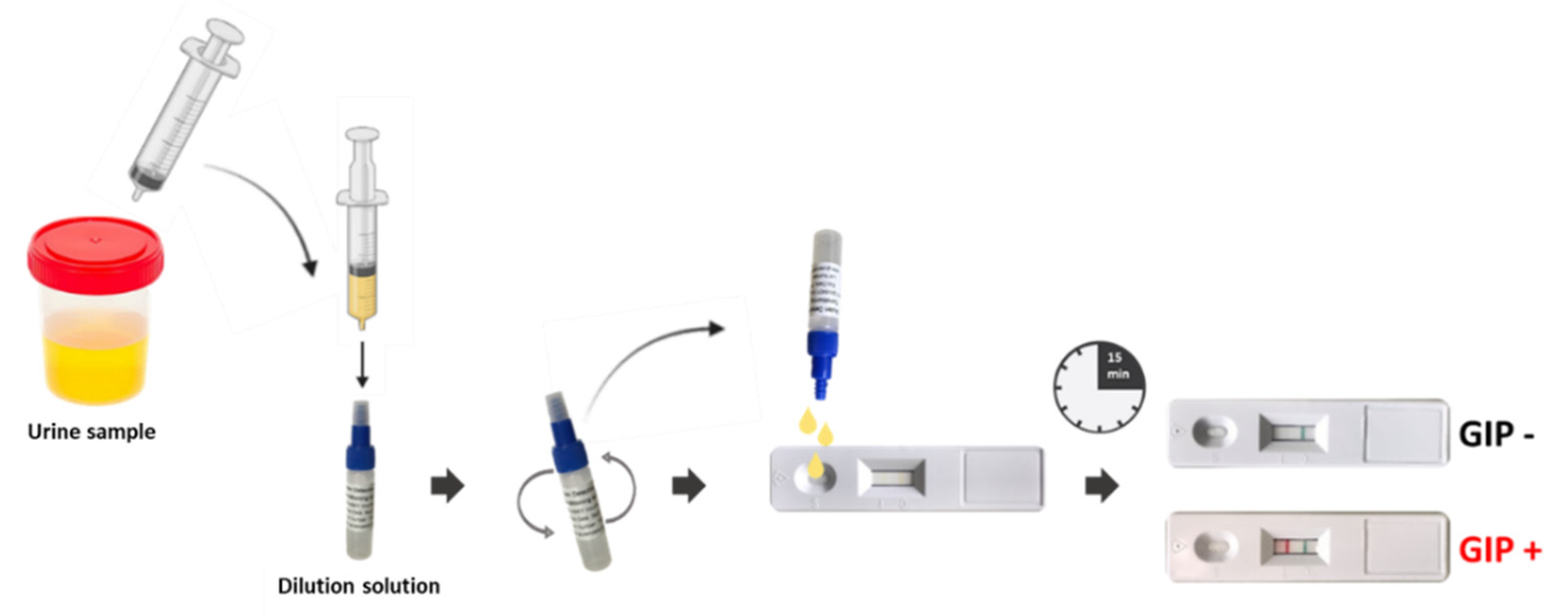
4.8. POCT-Based GIP Determination in Urine Samples
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AGAs | Anti-gliadin antibodies |
| Anti-DGP | Anti-deamidated gliadin antibodies |
| anti-tTG | Tissue transglutaminase antibodies |
| CD | Celiac disease |
| CDAT | Celiac dietary adherence test |
| GIP | Gluten immunogenic peptides |
| GFD | Gluten-free diet |
| HAS | Hospital Anxiety Scale |
| POCT | Point-of-care testing |
References
- Besser, H.A.; Khosla, C. Celiac disease: Mechanisms and emerging therapeutics. Trends Pharmacol. Sci. 2023, 44, 949–962. [Google Scholar] [CrossRef] [PubMed]
- Olmstead, J. Celiac disease: Guideline update overview. Nurse Pract. 2024, 49, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Arora, A.; Strand, T.A.; Leffler, D.A.; Catassi, C.; Green, P.H.; Kelly, C.P.; Ahuja, V.; Makharia, G.K. Global Prevalence of Celiac Disease: Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2018, 16, 823–836.e2. [Google Scholar] [CrossRef]
- Lebwohl, B.; Rubio-Tapia, A. Epidemiology, Presentation, and Diagnosis of Celiac Disease. Gastroenterology 2021, 160, 63–75. [Google Scholar] [CrossRef]
- Mazzola, A.M.; Zammarchi, I.; Valerii, M.C.; Spisni, E.; Saracino, I.M.; Lanzarotto, F.; Ricci, C. Gluten-Free Diet and Other Celiac Disease Therapies: Current Understanding and Emerging Strategies. Nutrients 2024, 16, 1006. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.R.; Zybert, P.; Chen, Z.; Lebovits, J.; Wolf, R.L.; Lebwohl, B.; Green, P.H.R. Food Avoidance beyond the Gluten-Free Diet and the Association with Quality of Life and Eating Attitudes and Behaviors in Adults with Celiac Disease. Nutrients 2024, 6, 3411. [Google Scholar] [CrossRef]
- Ciacci, C.; Iavarone, A.; Siniscalchi, M.; Romano, R.; De Rosa, A. Psychological dimensions of celiac disease: Toward an integrated approach. Dig. Dis. Sci. 2002, 47, 2082–2087. [Google Scholar] [CrossRef]
- Valero-Moreno, S.; Lacomba-Trejo, L.; Casaña-Granell, S.; Prado-Gascó, V.J.; Montoya-Castilla, I.; Pérez-Marín, M. Factor structure of the Hospital Anxiety and Depression Scale in adolescent patients with chronic disease. Arch. Argent Pediatr. 2019, 117, 252–258. (In Spanish) [Google Scholar] [CrossRef]
- Di Tola, M.; Bontkes, H.J.; Irure-Ventura, J.; López-Hoyos, M.; Bizzaro, N. The follow-up of patients with celiac disease. J. Transl. Autoimmun. 2025, 10, 100278. [Google Scholar] [CrossRef]
- Segura, V.; Ruiz-Carnicer, Á.; Mendía, I.; Garzón-Benavides, M.; Pizarro, Á.E.; Comino, I.; Sousa, C. Evaluation of the Usefulness of an Automatable Immunoassay for Monitoring Celiac Disease by Quantification of Immunogenic Gluten Peptides in Urine. Nutrients 2023, 15, 1730. [Google Scholar] [CrossRef]
- Ruiz-Carnicer, Á.; Garzón-Benavides, M.; Fombuena, B.; Segura, V.; García-Fernández, F.; Sobrino-Rodríguez, S.; Gómez-Izquierdo, L.; Montes-Cano, M.A.; Rodríguez-Herrera, A.; Millán, R.; et al. Negative predictive value of the repeated absence of gluten immunogenic peptides in the urine of treated celiac patients in predicting mucosal healing: New proposals for follow-up in celiac disease. Am. J. Clin. Nutr. 2020, 112, 1240–1251. [Google Scholar] [CrossRef] [PubMed]
- Soler, M.; Estevez, M.C.; de Lourdes Moreno, M.; Cebolla, A.; Lechuga, L.M. Label-free SPR detection of gluten peptides in urine for non-invasive celiac disease follow-up. Biosens. Bioelectron. 2016, 79, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Skodje, G.I.; van Megen, F.; Stendahl, M.; Henriksen, C.; Lundin, K.E.A.; Veierød, M.B. Detection of gluten immunogenic peptides and the Celiac Disease Adherence Test to monitor gluten-free diet: A pilot study. Eur. J. Clin. Nutr. 2022, 76, 902–903. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Carnicer, Á.; Segura, V.; Moreno, M.L.; Coronel-Rodríguez, C.; Sousa, C.; Comino, I. Transfer of celiac disease-associated immunogenic gluten peptides in breast milk: Variability in kinetics of secretion. Front. Immunol. 2024, 15, 1405344. [Google Scholar] [CrossRef]
- Moreno, M.L.; Sánchez-Muñoz, D.; Sanders, D.; Rodríguez-Herrera, A.; Sousa, C. Verifying Diagnosis of Refractory Celiac Disease with Urine Gluten Immunogenic Peptides as Biomarker. Front. Med. 2021, 7, 601854. [Google Scholar] [CrossRef]
- Stefanolo, J.P.; Tálamo, M.; Dodds, S.; de la Paz Temprano, M.; Costa, A.F.; Moreno, M.L.; Pinto-Sánchez, M.I.; Smecuol, E.; Vázquez, H.; Gonzalez, A.; et al. Real-World Gluten Exposure in Patients with Celiac Disease on Gluten-Free Diets, Determined From Gliadin Immunogenic Peptides in Urine and Fecal Samples. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2021, 19, 484–491.e1. [Google Scholar] [CrossRef]
- Mello, C.R.L.E.; da Silva, G.A.P.; Lins, M.T.C.; Antunes, M.M.C. Gluten-free diet adherence in pediatric celiac patients: Evaluating CDAT questionnaire and test for gluten detection in urine. J. Pediatr. Gastroenterol. Nutr. 2024, 78, 1310–1316. [Google Scholar] [CrossRef]
- Syage, J.A.; Kelly, C.P.; Dickason, M.A.; Ramirez, A.C.; Leon, F.; Dominguez, R.; Sealey-Voyksner, J.A. Determination of gluten consumption in celiac disease patients on a gluten-free diet. Am. J. Clin. Nutr. 2018, 107, 201–207. [Google Scholar] [CrossRef]
- Segura, V.; Ruiz-Carnicer, Á.; Sousa, C.; Moreno, M.L. New Insights into Non-Dietary Treatment in Celiac Disease: Emerging Therapeutic Options. Nutrients 2021, 13, 2146. [Google Scholar] [CrossRef]
- Shushari, M.K.; Wei, T.; Zhang, J.; Tidwell, D.K.; Conard, A.; Tolar-Peterson, T. Adherence to a gluten-free diet, depression, and nutrient distribution in participants with celiac disease. Nutrition 2025, 132, 112676. [Google Scholar] [CrossRef]
- Hoteit, M.; Chamas, Z.; Assaf, S.; Bouhairie, M.M.; Bahr, A.; Daccache, R.; Matar, R.; Hallal, M.; Hotayt, S.; Hotayt, B. Nutritional status, nutrient imbalances, food-related behaviors and dietary supplements use among patients with celiac disease on a gluten free diet in Lebanon: A national cross-sectional study. F1000Research 2022, 11, 725. [Google Scholar] [CrossRef] [PubMed]
- Trasciatti, S.; Grizzi, F. Vitamin D and celiac disease. Adv. Food Nutr. Res. 2024, 109, 249–270. [Google Scholar] [CrossRef] [PubMed]
- Lamjadli, S.; Oujamaa, I.; Souli, I.; Eddehbi, F.E.; Lakhouaja, N.; M’raouni, B.; Salami, A.; Guennouni, M.; Belghali, M.Y.; Hazime, R.; et al. Micronutrient deficiencies in patients with celiac disease: A systematic review and meta-analysis. Int. J. Immunopathol. Pharmacol. 2025, 39, 3946320241313426. [Google Scholar] [CrossRef] [PubMed]
- Marsh, M.N. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’). Gastroenterology 1992, 102, 330–354. [Google Scholar] [CrossRef]
- Oberhuber, G.; Granditsch, G.; Vogelsang, H. The histopathology of coeliac disease: Time for a standardized report scheme for pathologists. Eur. J. Gastroenterol. Hepatol. 1999, 11, 1185–1194. [Google Scholar] [CrossRef]
- Mubarak, A.; Nikkels, P.; Houwen, R.; Ten Kate, F. Reproducibility of the histological diagnosis of celiac disease. Scand. J. Gastroenterol. 2011, 46, 1065–1073. [Google Scholar] [CrossRef]
- Arguelles-Grande, C.; Tennyson, C.A.; Lewis, S.K.; Green, P.H.; Bhagat, G. Variability in small bowel histopathology reporting between different pathology practice settings: Impact on the diagnosis of coeliac disease. J. Clin. Pathol. 2012, 65, 242–247. [Google Scholar] [CrossRef]
- Leffler, D.A.; Dennis, M.; Edwards George, J.B.; Jamma, S.; Magge, S.; Cook, E.F.; Schuppan, D.; Kelly, C.P. A simple validated gluten-free diet adherence survey for adults with celiac disease. Clin. Gastroenterol. Hepatol. 2009, 7, 530–536.e2. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Carnicer, Á.; Coronel-Rodríguez, C.; Guisado-Rasco, M.C.; Comino, I.; Sousa, C.; Segura, V. Uncovering Hidden Gluten Exposure in Celiac Patients: A Case Study in Family-Based Management and the Role of Point-of-Care Urine Testing and Psychological Assessment. Int. J. Mol. Sci. 2025, 26, 5135. https://doi.org/10.3390/ijms26115135
Ruiz-Carnicer Á, Coronel-Rodríguez C, Guisado-Rasco MC, Comino I, Sousa C, Segura V. Uncovering Hidden Gluten Exposure in Celiac Patients: A Case Study in Family-Based Management and the Role of Point-of-Care Urine Testing and Psychological Assessment. International Journal of Molecular Sciences. 2025; 26(11):5135. https://doi.org/10.3390/ijms26115135
Chicago/Turabian StyleRuiz-Carnicer, Ángela, Cristóbal Coronel-Rodríguez, María Cinta Guisado-Rasco, Isabel Comino, Carolina Sousa, and Verónica Segura. 2025. "Uncovering Hidden Gluten Exposure in Celiac Patients: A Case Study in Family-Based Management and the Role of Point-of-Care Urine Testing and Psychological Assessment" International Journal of Molecular Sciences 26, no. 11: 5135. https://doi.org/10.3390/ijms26115135
APA StyleRuiz-Carnicer, Á., Coronel-Rodríguez, C., Guisado-Rasco, M. C., Comino, I., Sousa, C., & Segura, V. (2025). Uncovering Hidden Gluten Exposure in Celiac Patients: A Case Study in Family-Based Management and the Role of Point-of-Care Urine Testing and Psychological Assessment. International Journal of Molecular Sciences, 26(11), 5135. https://doi.org/10.3390/ijms26115135





