Metabolomic Analysis Provides Insights into Bud Paradormancy in Camellia sinensis cv. Huangdan
Abstract
1. Introduction
2. Results
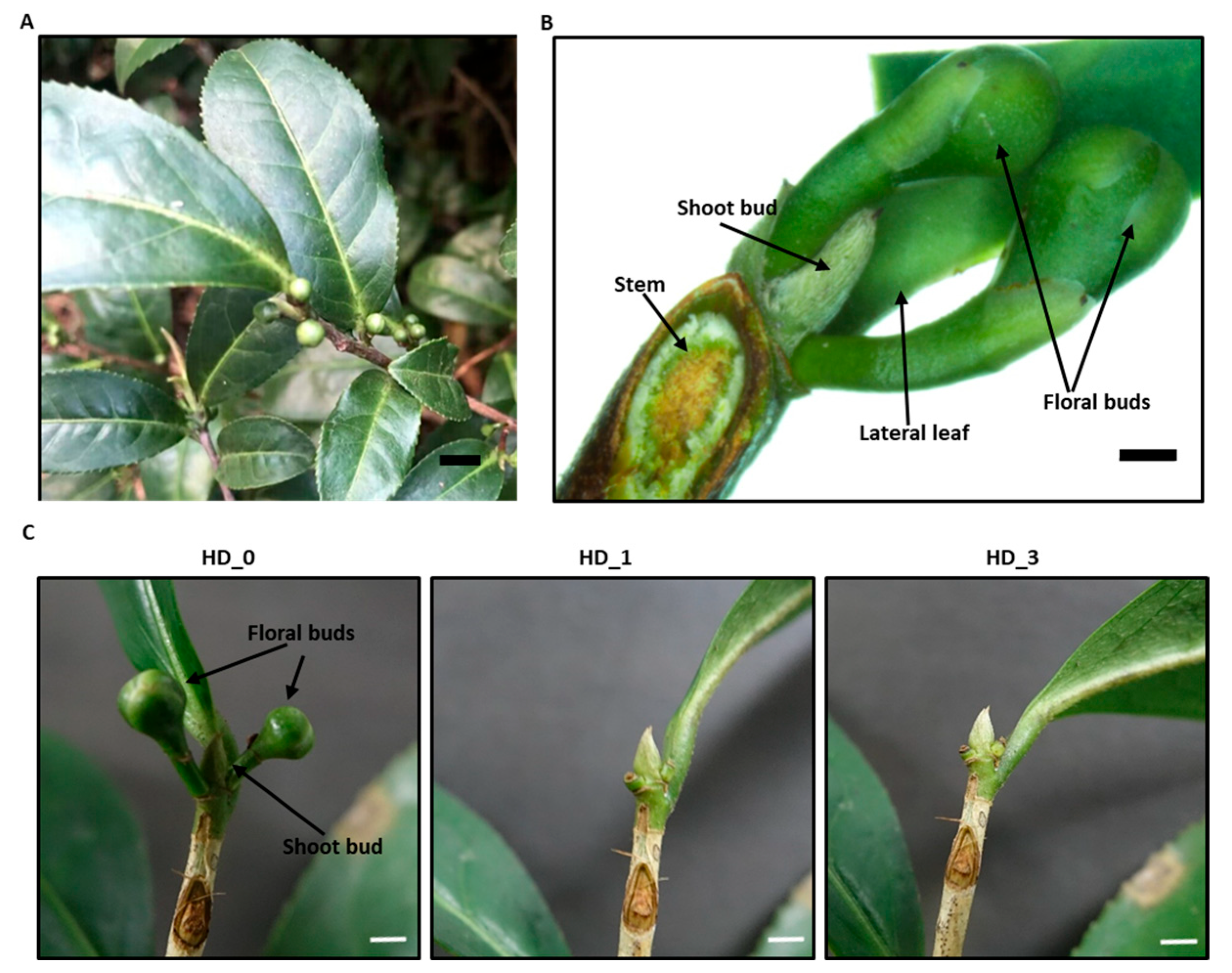
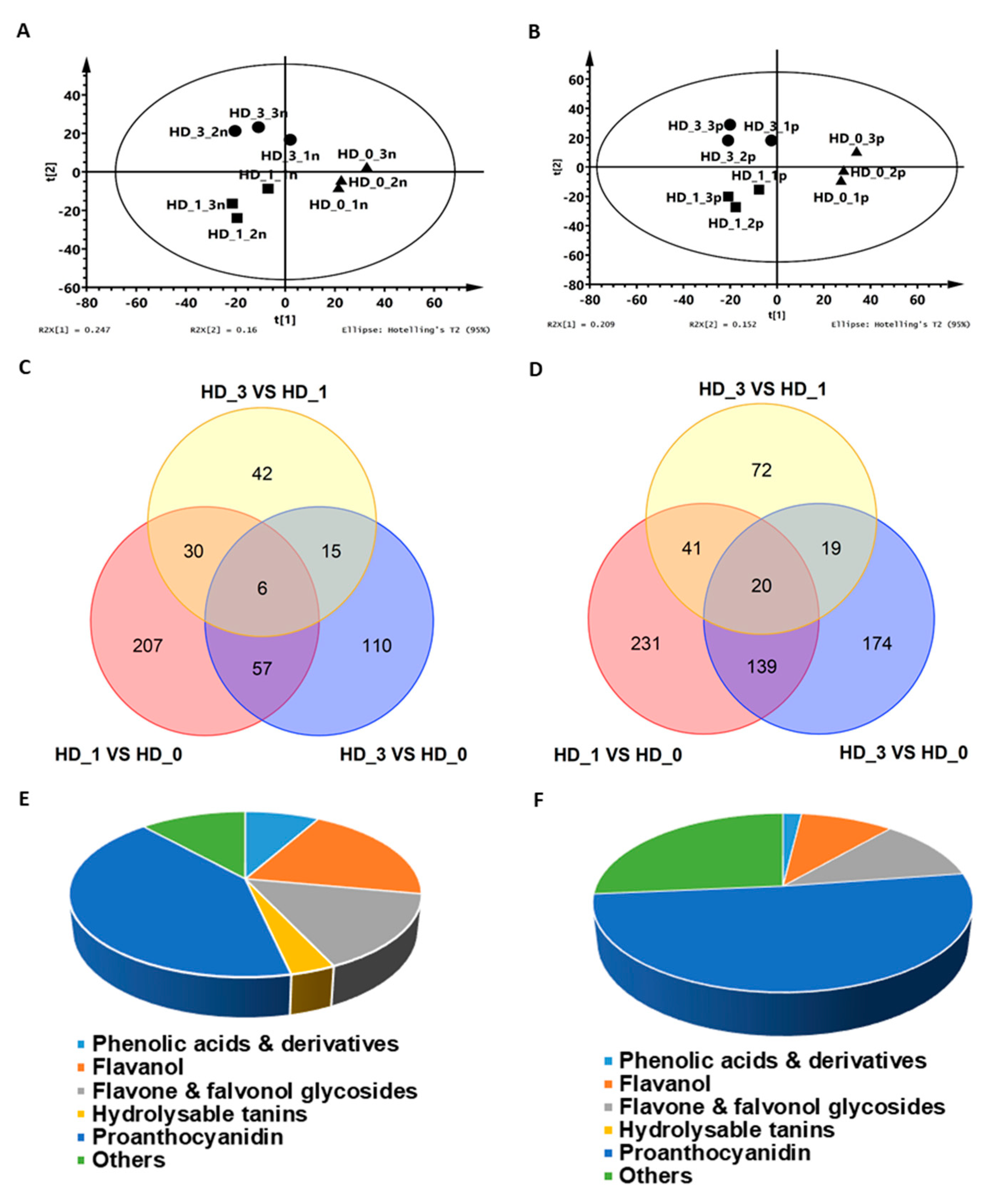
2.1. The Axillary Shoot Bud Metabolome Was Significantly Modified One Day After Removing the Local Axillary Floral Buds
2.2. Sugars and Sugar Alcohols
2.3. Amino Acids and Amins
2.4. Organic Acids and Phenolic Acids
2.5. Flavones and Flavone Glycosides
2.6. Flavonol Glycosides and Falvanols
2.7. Proanthocyanidins
2.8. Antioxidants
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Metabolite Extraction
4.3. UPLC-QTOF MS Analysis
4.4. GC-TOF MS Analysis
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garrison, R. Studies in the development of axillary buds. Am. J. Bot. 1955, 42, 257–266. [Google Scholar] [CrossRef]
- Barua, D.N. Seasonal dormancy in tea (Camellia sinensis L.). Nature 1969, 224, 514. [Google Scholar] [CrossRef]
- Cooke, J.E.K.; Eriksson, M.E.; Junttila, O. The dynamic nature of bud dormancy in trees: Environmental control and molecular mechanisms. Plant Cell Environ. 2012, 35, 1707–1728. [Google Scholar] [CrossRef] [PubMed]
- Horvath, D.P.; Anderson, J.V.; Chao, W.S.; Foley, M.E. Knowing when to grow: Signals regulating bud dormancy. Trends Plant Sci. 2003, 8, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Kumar, S. Responses to winter dormancy, temperature, and plant hormones share gene networks. Funct. Integr. Genomic. 2011, 11, 659–664. [Google Scholar] [CrossRef]
- Lang, G.A.; Early, J.D.; Martin, G.C.; Darnell, R.L. Endo-, para-, and ecodormancy: Physiological terminology and classification for dormancy research. HortScience 1987, 22, 371–377. [Google Scholar] [CrossRef]
- Rinne, P.L.H.; Paul, L.K.; Vahala, J.; Kangasjärvi, J.; van der Schoot, C. Axillary buds are dwarfed shoots that tightly regulate GA pathway and GA-inducible 1,3-β-glucanase genes during branching in hybrid aspen. J. Exp. Bot. 2016, 67, 5975–5991. [Google Scholar] [CrossRef]
- Wei, J.; Yang, Q.; Ni, J.; Gao, Y.; Tang, Y.; Bai, S.; Teng, Y. Early defoliation induces auxin redistribution, promoting paradormancy release in pear buds. Plant Physiol. 2022, 190, 2739–2756. [Google Scholar] [CrossRef]
- Ruttink, T.; Arend, M.; Morreel, K.; Storme, V.; Rombauts, S.; Fromm, J.; Bhalerao, R.P.; Boerjan, W.; Rohde, A. A molecular timetable for apical bud formation and dormancy induction in Poplar. Plant Cell 2007, 19, 2370–2390. [Google Scholar] [CrossRef]
- Anderson, J.V.; Gesch, R.W.; Jia, Y.; Chao, W.S.; Horvath, D.P. Seasonal shifts in dormancy status, carbohydrate metabolism, and related gene expression in crown buds of leafy spurge. Plant Cell Environ. 2005, 28, 1567–1578. [Google Scholar] [CrossRef]
- Guillamón, J.G.; Prudencio, Á.S.; Yuste, J.E.; Dicenta, F.; Sánchez-Pérez, R. Ascorbic acid and prunasin, two candidate biomarkers for endodormancy release in almond flower buds identified by a nontargeted metabolomic study. Hortic. Res. 2020, 7, 203. [Google Scholar] [CrossRef] [PubMed]
- Horvath, D.P.; Chao, W.S.; Suttle, J.C.; Thimmapuram, J.; Anderson, J.V. Transcriptome analysis identifies novel responses and potential regulatory genes involved in seasonal dormancy transitions of leafy spurge (Euphorbia esula L.). BMC Genom. 2008, 9, 536. [Google Scholar] [CrossRef]
- Ibáñez, C.; Kozarewa, I.; Johansson, M.; Ögren, E.; Rohde, A.; Eriksson, M.E. Circadian clock components regulate entry and affect exit of seasonal dormancy as well as winter hardiness in Populus trees. Plant Physiol. 2010, 153, 1823–1833. [Google Scholar] [CrossRef]
- Wu, R.M.; Walton, E.F.; Richardson, A.C.; Wood, M.; Hellens, R.P.; Varkonyi-Gasic, E. Conservation and divergence of four kiwifruit SVP-like MADS-box genes suggest distinct roles in kiwifruit bud dormancy and flowering. J. Exp. Bot. 2012, 63, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Gao, Z.; Zhuang, W.; Shi, T.; Zhang, Z.; Ni, Z. Genome-wide expression profiles of seasonal bud dormancy at four critical stages in Japanese apricot. Plant Mol. Biol. 2013, 83, 247–264. [Google Scholar] [CrossRef]
- Tarancón, C.; González-Grandío, E.; Oliveros, J.C.; Nicolas, M.; Cubas, P. A conserved carbon starvation response underlies bud dormancy in woody and herbaceous species. Front. Plant Sci. 2017, 8, 788. [Google Scholar] [CrossRef]
- Paul, M.J.; Primavesi, L.F.; Jhurreea, D.; Zhang, Y. Trehalose metabolism and signaling. Annu. Rev. Plant Biol. 2008, 59, 417–441. [Google Scholar] [CrossRef] [PubMed]
- Tanton, T.W. The banjhi (dormancy) cycle in tea (Camellia sinensis). Exp. Agric. 1981, 17, 149–156. [Google Scholar] [CrossRef]
- Li, N.-N.; Qian, W.-J.; Wang, L.; Cao, H.-L.; Hao, X.-Y.; Yang, Y.-J.; Wang, X.-C. Isolation and expression features of hexose kinase genes under various abiotic stresses in the tea plant (Camellia sinensis). J. Plant Physiol. 2017, 209, 95–104. [Google Scholar] [CrossRef]
- Qian, W.; Yue, C.; Wang, Y.; Cao, H.; Li, N.; Wang, L.; Hao, X.; Wang, X.C.; Xiao, B.; Yang, Y. Identification of the invertase gene family (INVs) in tea plant and their expression analysis under abiotic stress. Plant Cell Rep. 2016, 35, 2269–2283. [Google Scholar] [CrossRef]
- Yue, C.; Cao, H.-L.; Wang, L.; Zhou, Y.-H.; Huang, Y.-T.; Hao, X.-Y.; Wang, Y.-C.; Wang, B.; Yang, Y.-J.; Wang, X.-C. Effects of cold acclimation on sugar metabolism and sugar-related gene expression in tea plant during the winter season. Plant Mol. Biol. 2015, 88, 591–608. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.J. Trehalose 6-phosphate. Curr. Opin. Plant Biol. 2007, 10, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, A.; Yabuta, Y.; Shigeoka, S. Galactinol and raffinose constitute a novel function to protect plants from oxidative damage. Plant Physiol. 2008, 147, 1251–1263. [Google Scholar] [CrossRef]
- Van den Ende, W. Multifunctional fructans and raffinose family oligosaccharides. Front. Plant Sci. 2013, 4, 247. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, Y.; Zhang, Y.; Dong, Y.; Liu, Y.; Liu, L.; Wan, S.; He, J.; Yu, Y. Accumulation of galactinol and ABA is involved in exogenous EBR-induced drought tolerance in tea plants. J. Agric. Food Chem. 2022, 70, 13391–13403. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Alexander, D.; Wulff, J.; Olsen, J.E. Changes in metabolite profiles in Norway spruce shoot tips during short-day induced winter bud development and long-day induced bud flush. Metabolomics 2014, 10, 842–858. [Google Scholar] [CrossRef]
- Dhuli, P.; Rohloff, J.; Strimbeck, G.R. Metabolite changes in conifer buds and needles during forced bud break in Norway spruce (Picea abies) and European silver fir (Abies alba). Front. Plant Sci. 2014, 5, 706. [Google Scholar] [CrossRef]
- Moreira, A.S.P.; da Costa, E.V.; Evtuguin, D.V.; Coimbra, M.A.; Nunes, F.M.; Domingues, M.R.M. Neutral and acidic products derived from hydroxyl radical-induced oxidation of arabinotriose assessed by electrospray ionisation mass spectrometry. J. Mass Spectrom. 2014, 49, 280–290. [Google Scholar] [CrossRef]
- Dixon, R.A.; Sarnala, S. Proanthocyanidin biosynthesis—A matter of protection. Plant Physiol. 2020, 184, 579–591. [Google Scholar] [CrossRef]
- Barbier, F.; Péron, T.; Lecerf, M.; Perez-Garcia, M.-D.; Barrière, Q.; Rolčík, J.; Boutet-Mercey, S.; Citerne, S.; Lemoine, R.; Porcheron, B.; et al. Sucrose is an early modulator of the key hormonal mechanisms controlling bud outgrowth in Rosa hybrida. J. Exp. Bot. 2015, 66, 2569–2582. [Google Scholar] [CrossRef]
- Mason, M.G.; Ross, J.J.; Babst, B.A.; Wienclaw, B.N.; Beveridge, C.A. Sugar demand, not auxin, is the initial regulator of apical dominance. Proc. Natl. Acad. Sci. USA 2014, 111, 6092–6097. [Google Scholar] [CrossRef]
- Fennell, A.Y.; Schlauch, K.A.; Gouthu, S.; Deluc, L.G.; Khadka, V.; Sreekantan, L.; Grimplet, J.; Cramer, G.R.; Mathiason, K.L. Short day transcriptomic programming during induction of dormancy in grapevine. Front. Plant Sci. 2015, 6, 834. [Google Scholar] [CrossRef]
- Tylewicz, S.; Tsuji, H.; Miskolczi, P.; Petterle, A.; Azeez, A.; Jonsson, K.; Shimamoto, K.; Bhalerao, R.P. Dual role of tree florigen activation complex component FD in photoperiodic growth control and adaptive response pathways. Proc. Natl. Acad. Sci. USA 2015, 112, 3140–3145. [Google Scholar] [CrossRef]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef]
- Conrad, A.; Yu, J.; Staton, M.; Audergon, J.-M.; Roch, G.; Decroocq, V.; Knagge, K.; Chen, H.; Zhebentyayeva, T.; Liu, Z.; et al. Association of the phenylpropanoid pathway with dormancy and adaptive trait variation in apricot (Prunus armeniaca). Tree Physiol. 2019, 39, 1136–1148. [Google Scholar] [CrossRef]
- Vagiri, M.; Ekholm, A.; Johansson, E.; Andersson, S.; Rumpunen, K. Major phenolic compounds in black currant (Ribes nigrum L.) buds: Variation due to genotype, ontogenetic stage and location. LWT-Food Sci. Technol. 2015, 63, 1274–1280. [Google Scholar] [CrossRef]
- Wang, S.Y.; Jiao, H.J.; Faust, M. Changes in the activities of catalase, peroxidase, and polyphenol oxidase in apple buds during bud break induced by thidiazuron. J. Plant Growth Regul. 1991, 10, 33–39. [Google Scholar] [CrossRef]
- Yin, Y.; Nie, W.; Tang, Z.-Q.; Zhu, S.-J. Flavonoid-rich extracts from Chuju (Asteraceae Chrysanthemum L.) alleviate the disturbance of glycolipid metabolism on type2 diabetic mice via modulating the gut microbiota. Foods 2025, 14, 765. [Google Scholar] [CrossRef]
- Idris, S.; Mishra, A.; Khushtar, M. Recent therapeutic interventions of Fenugreek Seed: A mechanistic approach. Drug Res. 2021, 71, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-L.; Quan, Q.; Ji, R.; Guo, X.-Y.; Zhang, J.-M.; Li, X.; Liu, Y.-G. Isorhamnetin suppresses PANC-1 pancreatic cancer cell proliferation through S phase arrest. Biomed. Pharmacother. 2018, 108, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Wang, H.-J.; Yu, L.-H.; Guan, Z.-R.; Jiang, Y.-P.; Hu, J.-H.; Yan, Y.-X.; Zhou, Z.-H.; Lou, J.-S. The role of Ginkgo Folium on antitumor: Bioactive constituents and the potential mechanism. J. Ethnopharmacol. 2024, 321, 117202. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, J.; Chen, Y.; Tang, H.; Wang, Y.; He, Y.; Ou, Y.; Sun, X.; Wang, S.; Yao, Y. Tomato SlAN11 regulates flavonoid biosynthesis and seed dormancy by interaction with bHLH proteins but not with MYB proteins. Hortic. Res. 2018, 5, 27. [Google Scholar] [CrossRef]
- Debeaujon, I.; Peeters, A.J.M.; Leon-Kloosterziel, K.M.; Koornneef, M. The TRANSPARENT TESTA12 gene of Arabidopsis encodes a multidrug secondary transporter-like protein required for flavonoid sequestration in vacuoles of the seed coat endothelium. Plant Cell 2001, 13, 853–871. [Google Scholar] [CrossRef]
- Buer, C.S.; Muday, G.K. The transparent testa4 mutation prevents flavonoid synthesis and alters auxin transport and the response of Arabidopsis roots to gravity and light. Plant Cell 2004, 16, 1191–1205. [Google Scholar] [CrossRef]
- Scalbert, A. Antimicrobial properties of tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- Harding, S.A. Condensed tannins: Arbiters of abiotic stress tolerance? Tree Physiol. 2019, 39, 341–344. [Google Scholar] [CrossRef]
- Buer, C.S.; Muday, G.K.; Djordjevic, M.A. Flavonoids are differentially taken up and transported long distances in Arabidopsis. Plant Physiol. 2007, 145, 478–490. [Google Scholar] [CrossRef]
- Csepregi, K.; Hideg, E. Phenolic compound diversity explored in the context of photo-oxidative stress protection. Phytochem. Anal. 2018, 29, 129–136. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Salvi, P.; Varshney, V.; Majee, M. Raffinose family oligosaccharides (RFOs): Role in seed vigor and longevity. Biosci. Rep. 2022, 42, BSR20220198. [Google Scholar] [CrossRef]
- Iqbal, S.; Andrabi, S.M.H.; Riaz, A.; Durrani, A.Z.; Ahmad, N. Trehalose improves semen antioxidant enzymes activity, post-thaw quality, and fertility in Nili Ravi buffaloes (Bubalus bubalis). Theriogenology 2016, 85, 954–959. [Google Scholar] [CrossRef]
- Smirnoff, N.; Cumbes, Q.J. Hydroxyl radical scavenging activity of compatible solutes. Phytochemrstry 1989, 28, 1057–1060. [Google Scholar] [CrossRef]
- Azam, S.; Hadi, N.; Khan, N.U.; Hadi, S.M. Antioxidant and prooxidant properties of caffeine, theobromine and xanthine. Med. Sci. Monit. 2003, 9, BR325-30. [Google Scholar]
- Qin, Q. ROS: Important factor in plant stem cell fate regulation. J. Plant Physiol. 2023, 289, 154082. [Google Scholar] [CrossRef]
- Tsukagoshi, H.; Busch, W.; Benfey, P.N. Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell 2010, 143, 606–616. [Google Scholar] [CrossRef]
- Yang, S.; Yu, Q.; Zhang, Y.; Jia, Y.; Wan, S.; Kong, X.; Ding, Z. ROS: The fine-tuner of plant stem cell fate. Trends Plant Sci. 2018, 23, 850–853. [Google Scholar] [CrossRef]
- Zeng, J.; Dong, Z.; Wu, H.; Tian, Z.; Zhao, Z. Redox regulation of plant stem cell fate. EMBO J. 2017, 36, 2844–2855. [Google Scholar] [CrossRef]
- Chen, M.J.; Thelen, J.J. Plastid uridine salvage activity is required for photoassimilate allocation and partitioning in Arabidopsis. Plant Cell 2011, 23, 2991–3006. [Google Scholar] [CrossRef]
- Yue, W.J.; Sun, W.J.; Rao, R.S.P.; Ye, N.X.; Yang, Z.B.; Chen, M.J. Non-targeted metabolomics reveals distinct chemical compositions among different grades of Bai Mudan white tea. Food Chem. 2019, 277, 289–297. [Google Scholar] [CrossRef]
- Tautenhahn, R.; Cho, K.; Uritboonthai, W.; Zhu, Z.J.; Patti, G.J.; Siuzdak, G. An accelerated workflow for untargeted metabolomics using the METLIN database. Nat. Biotechnol. 2012, 30, 826–828. [Google Scholar] [CrossRef]
- Horai, H.; Arita, M.; Kanaya, S.; Nihei, Y.; Ikeda, T.; Suwa, K.; Ojima, Y.; Tanaka, K.; Tanaka, S.; Aoshima, K.; et al. MassBank: A public repository for sharing mass spectral data for life sciences. J. Mass Spectrom. 2010, 45, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Jewison, T.; Guo, A.C.; Wilson, M.; Knox, C.; Liu, Y.; Djoumbou, Y.; Mandal, R.; Aziat, F.; Dong, E.; et al. HMDB 3.0–the human metabolome database in 2013. Nucleic Acids Res. 2013, 41, D801–D807. [Google Scholar] [CrossRef] [PubMed]
- Afendi, F.M.; Okada, T.; Yamazaki, M.; Hirai-Morita, A.; Nakamura, Y.; Nakamura, K.; Ikeda, S.; Takahashi, H.; Amin, A.U.; Darusman, L.K.; et al. KNApSAcK family databases: Integrated metabolite-plant species databases for multifaceted plant research. Plant Cell Physiol. 2012, 53, e1. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Nakabayashi, R.; Yamada, Y.; Suzuki, M.; Sato, M.; Sakata, A.; Akiyama, K.; Sakurai, T.; Matsuda, F.; Aoki, T.; et al. RIKEN tandem mass spectral database (ReSpect) for phytochemicals: A plant-specific MS/MS-based data resource and database. Phytochemistry 2012, 82, 38–45. [Google Scholar] [CrossRef]
| Compounds | Platform | Peak Area | ||
|---|---|---|---|---|
| HD_0 | HD_1 | HD_3 | ||
| Sugars and sugar alcohols | ||||
| Glucose-1-phosphate | GC–MS | 4735 ± 1167 b | 15,016 ± 2191 a | 16,453 ± 1388 a |
| Fructose 1 | GC–MS | ND b | 37,175 ± 5760 a | 26,315 ± 6877 a |
| D-(-)-Tagatofuranose | GC–MS | 109,341 ± 19,127 b | 213,012 ± 3111 a | 191,165 ± 11,631 a |
| Trehalose | GC–MS | 143,896 ± 16,715 a | 99,136 ± 20,320 ab | 56,627 ± 21,021 b |
| Raffinose | GC–MS | 15,683,254 ± 1,873,902 a | 7,252,704 ± 317,388 b | 8,417,784 ± 3,488,121 ab |
| Galactinol | GC–MS | 2,887,986 ± 325,887 a | 2,465,573 ± 563,001 ab | 1,242,611 ± 394,221 b |
| α-D-Xylopyranose | GC–MS | 16,952 ± 111 a | ND b | 15,533 ± 3206 a |
| Amino acids and amins | ||||
| Asparagine 4 | GC–MS | 572,463 ± 112,880 a | 308,529 ± 113,548 ab | 104,202 ± 48,827 b |
| N,N,O-Triacetylhydroxylamine | GC–MS | ND c | 7545 ± 52 b | 14,886 ± 21 a |
| Benzenamine | GC–MS | 10,604 ± 70 b | 12,449 ± 85 b | 18,189 ± 2730 a |
| Organic acids and phenolic acids | ||||
| Arabinonic acid | [M + H]+ | 1448 ± 36 b | 588 ± 53 a | 703 ± 112 a |
| Malonic acid | GC–MS | 46,286 ± 9803 b | 84,527 ± 13361 b | 149,950 ± 19002 a |
| 5-p-coumaroylquinic acid | [M − H]− | 237 ± 15 b | 491 ± 84 ab | 1289 ± 447 a |
| Benzoic acid 1 | GC–MS | 13,645 ± 1392 b | 15,410 ± 2558 b | 23,169 ± 1098 a |
| Benzoic acid 2 | GC–MS | 262,832 ± 4059 a | 194,220 ± 26,305 b | 265,754 ± 379 a |
| 5-Ethoxy-3,4-dihydro-2H-pyrrole-2-carboxylic acid | GC–MS | ND b | ND b | 7572 ± 825 a |
| Flavones and flavone glycosides | ||||
| Tricetin | [M + H]+ | 1402 ± 170 b | 2101 ± 124 a | 1607 ± 129 b |
| C-diglucosylapigenin | [M − H]− | 748 ± 79 a | 513 ± 55 b | 513 ± 11 b |
| C-diglucosylapigenin | [M + H]+ | 990 ± 748 a | 680 ± 61 b | 636 ± 8 b |
| Apigenin 6-C-glucoside 8-C-arabinoside | [M − H]− | 1952 ± 116 a | 1515 ± 139 b | 1193 ± 116 b |
| Apigenin 6-C-glucoside 8-C-arabinoside | [M + H]+ | 2889 ± 300 a | 2159 ± 270 ab | 1786 ± 176 b |
| Prunin | GC–MS | 14,725 ± 14,420 a | 10,156 ± 1027 b | 11,844 ± 659 ab |
| Flavonol glycosides | ||||
| Rutin | [M − H]− | 2142 ± 58 b | 3779 ± 261 a | 2536 ± 111 b |
| Rutin | [M + H]+ | 893 ± 25 b | 1619 ± 141 a | 1157 ± 48 b |
| Quercetin 3-O-glucosyl rutinoside | [M − H]− | 1153 ± 173 b | 2138 ± 179 a | 1419 ± 183 b |
| Flavanols | ||||
| Gallocatechin 3′-O-gallate | [M − H]− | 252 ± 74 b | 713 ± 112 a | 1306 ± 325 b |
| Epigallocatechin gallate | [M − H]− | 1,102,389 ± 25132 b | 1,259,731 ± 30219 a | 1,131,323 ± 43874 b |
| Epigallocatechin gallate | [M + H]+ | 160,946 ± 3510 b | 179,918 ± 1830 a | 166,984 ± 7311 b |
| Epigallocatechin 1 | GC–MS | 74,737 ± 10,588 b | 132,233 ± 10,310 a | 93,694 ± 19,126 b |
| Proanthocyanidins | ||||
| Procyanidin trimer isomer 1 | [M − H]− | 1674 ± 87 a | 962 ± 105 b | 1710 ± 257 a |
| Procyanidin trimer isomer 1 | [M + H]+ | 2774 ± 218 a | 1803 ± 214 b | 3029 ± 245 a |
| Galloylprocyanidin dimer | [M − H]− | 51,022 ± 3318 a | 42,665 ± 827 b | 43,313 ± 1823 ab |
| Procyanidin trimer isomer 3 | [M − H]− | 511 ± 37 a | 262 ± 48 b | 542 ± 83 a |
| Galloylated trimeric proanthocyanidin | [M − H]− | 3320 ± 34 a | 2672 ± 137 b | 2349 ± 62 c |
| Galloylprodelphinidin dimer | [M − H]− | 75,967 ± 3931 a | 61,363 ± 2054 ab | 53,936 ± 8451 a |
| Procyanidin trimer isomer 2 | [M − H]− | 5246 ± 296 ab | 3623 ± 461 b | 5696 ± 694 a |
| (E)GC-(E)CG dimer | [M − H]− | 50,924 ± 1577 a | 44,062 ± 1442 ab | 37,029 ± 3672 b |
| EC-EGCG dimer | [M + H]+ | 30,461 ± 1037 a | 26,529 ± 1349 ab | 22,612 ± 1547 b |
| Procyanidin trimer isomer 4 | [M + H]+ | 2484 ± 218 ab | 1759 ± 197 b | 2727 ± 254 a |
| Prodelphinidin A2 3′-gallate | [M + H]+ | 1909 ± 133 b | 2002 ± 122 b | 2528 ± 163 a |
| Antioxidants | ||||
| α-Tocopherol | GC–MS | 102,347 ± 19,412 b | 91,070 ± 22,521 b | 178,108 ± 975 a |
| Theobromine | [M + H]+ | 6751 ± 1740 b | 28,939 ± 2641 a | 42,349 ± 7566 a |
| Volatile glycosides | ||||
| Linalool primeveroside isomer 1 | [M − H]− | 75 ± 15 b | 737 ± 310 a | 1124 ± 301 a |
| Phenylethyl primeveroside isomer 1 | [M + Na]+ | 360 ± 21 b | 593 ± 12 a | 621 ± 48 a |
| Others | ||||
| 1,3-Dioxolane 1 | GC–MS | 34,645 ± 228 a | 14,556 ± 1088 b | 20,223 ± 4971 b |
| 1,3-Dioxolane 3 | GC–MS | 33,013 ± 2378 b | 88,752 ± 3702 a | 122,253 ± 23,023 a |
| 2-Methyl-3-buten-2-ol | GC–MS | 105,417 ± 14,456 b | 228,198 ± 14,914 b | 430,873 ± 84,003 a |
| 2-hydroxypyridine | GC–MS | 917,334 ± 112,468 a | 795,023 ± 73,921 b | 884,118 ± 123,235 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Du, Z.; Yue, W.; Kong, X.; Xu, Q.; Fang, D.; Chen, C. Metabolomic Analysis Provides Insights into Bud Paradormancy in Camellia sinensis cv. Huangdan. Int. J. Mol. Sci. 2025, 26, 5094. https://doi.org/10.3390/ijms26115094
Chen M, Du Z, Yue W, Kong X, Xu Q, Fang D, Chen C. Metabolomic Analysis Provides Insights into Bud Paradormancy in Camellia sinensis cv. Huangdan. International Journal of Molecular Sciences. 2025; 26(11):5094. https://doi.org/10.3390/ijms26115094
Chicago/Turabian StyleChen, Mingjie, Zhenghua Du, Wenjie Yue, Xiangrui Kong, Quanming Xu, Dongsheng Fang, and Changsong Chen. 2025. "Metabolomic Analysis Provides Insights into Bud Paradormancy in Camellia sinensis cv. Huangdan" International Journal of Molecular Sciences 26, no. 11: 5094. https://doi.org/10.3390/ijms26115094
APA StyleChen, M., Du, Z., Yue, W., Kong, X., Xu, Q., Fang, D., & Chen, C. (2025). Metabolomic Analysis Provides Insights into Bud Paradormancy in Camellia sinensis cv. Huangdan. International Journal of Molecular Sciences, 26(11), 5094. https://doi.org/10.3390/ijms26115094








