Anti-Tumor Activities of Anti-Siglec-15 Chimeric Heavy-Chain Antibodies
Abstract
1. Introduction
2. Results
2.1. Construction and Screening of the Phage Display Nanobody Library
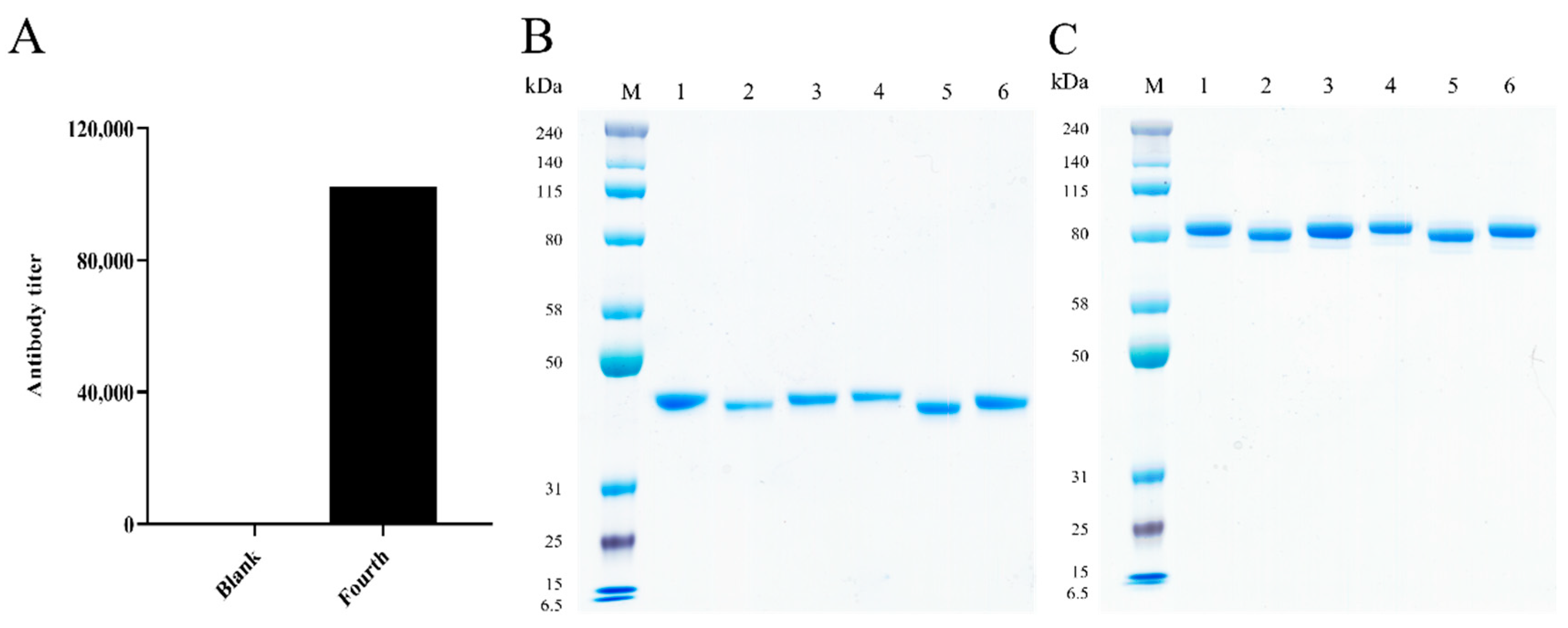
2.2. Generation of the Chimeric Heavy-Chain Antibodies
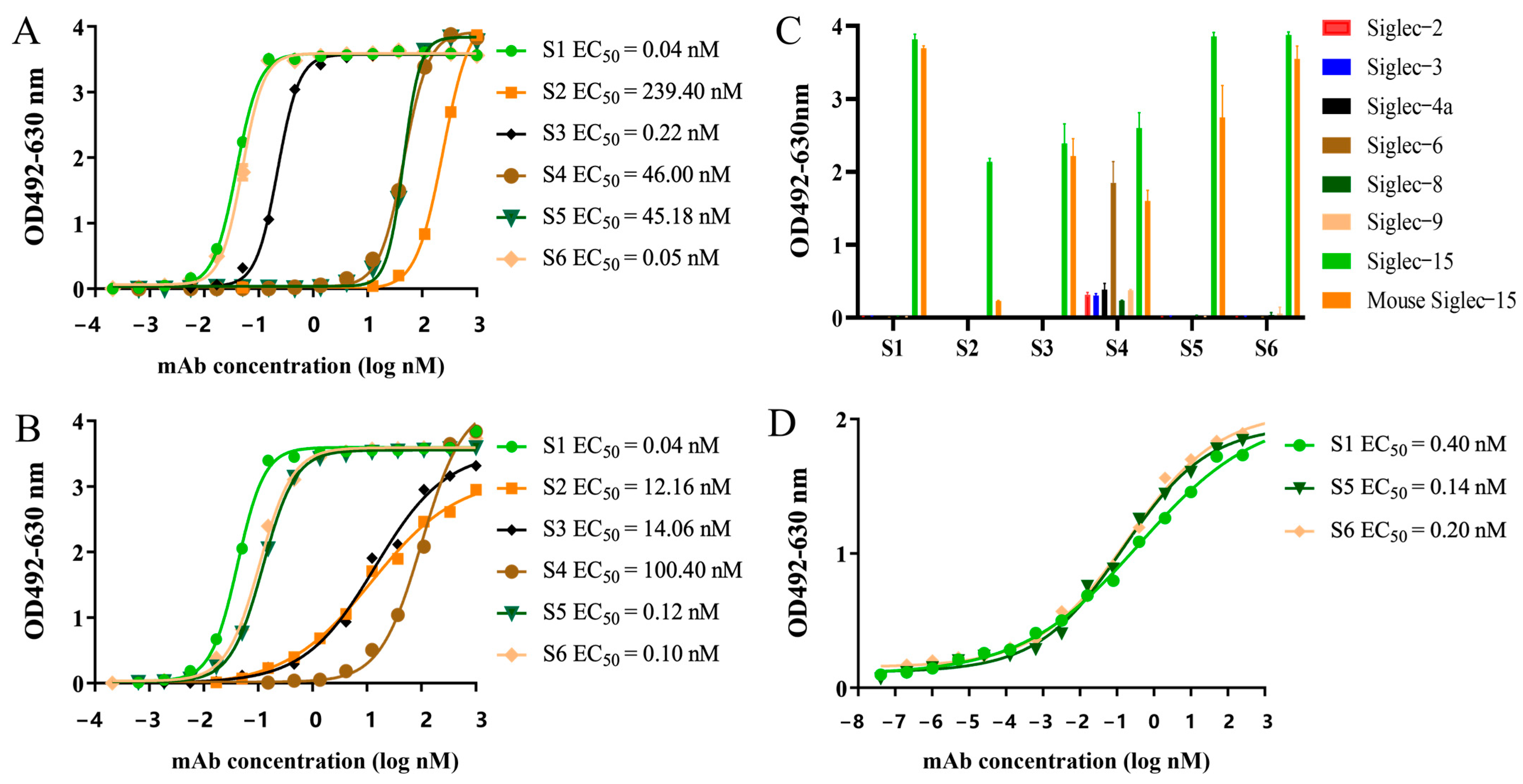
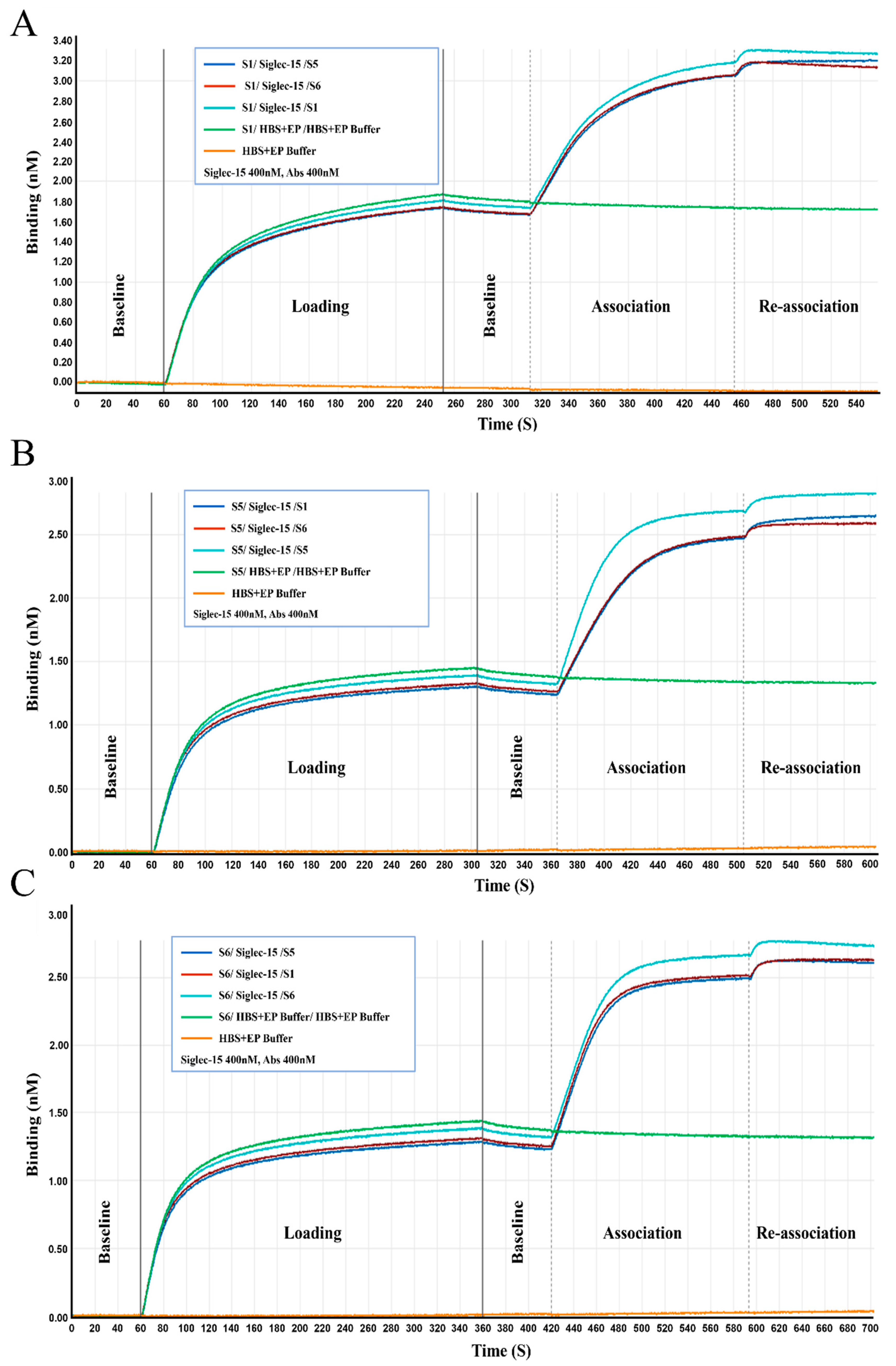
2.3. Characterization of the Chimeric Heavy-Chain Antibodies
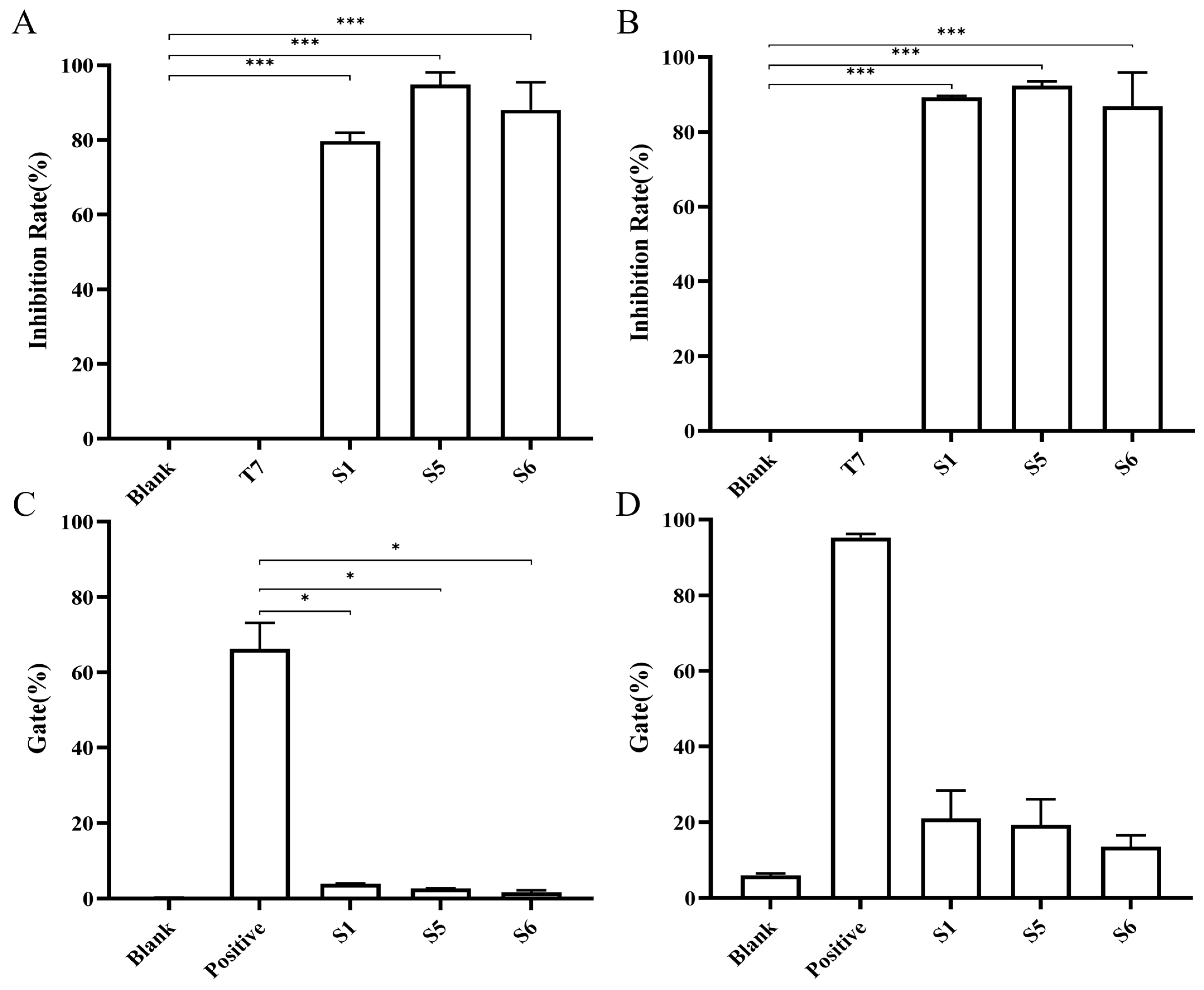
2.4. Chimeric Heavy-Chain Antibodies’ Blocking Activity
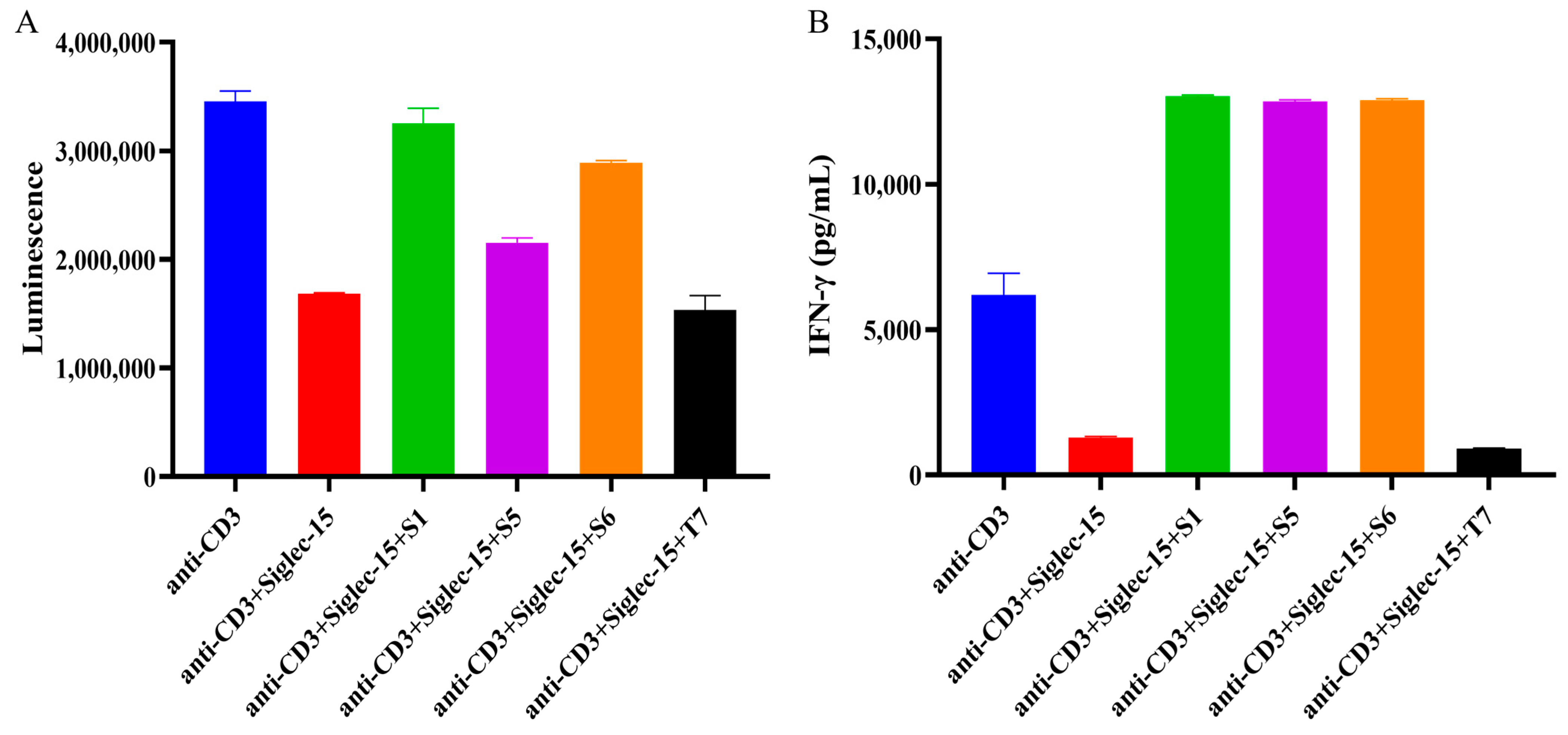
2.5. Evaluation of Chimeric Heavy Chain Antibodies Mediating T Cell Proliferation and IFN-γ Secretion
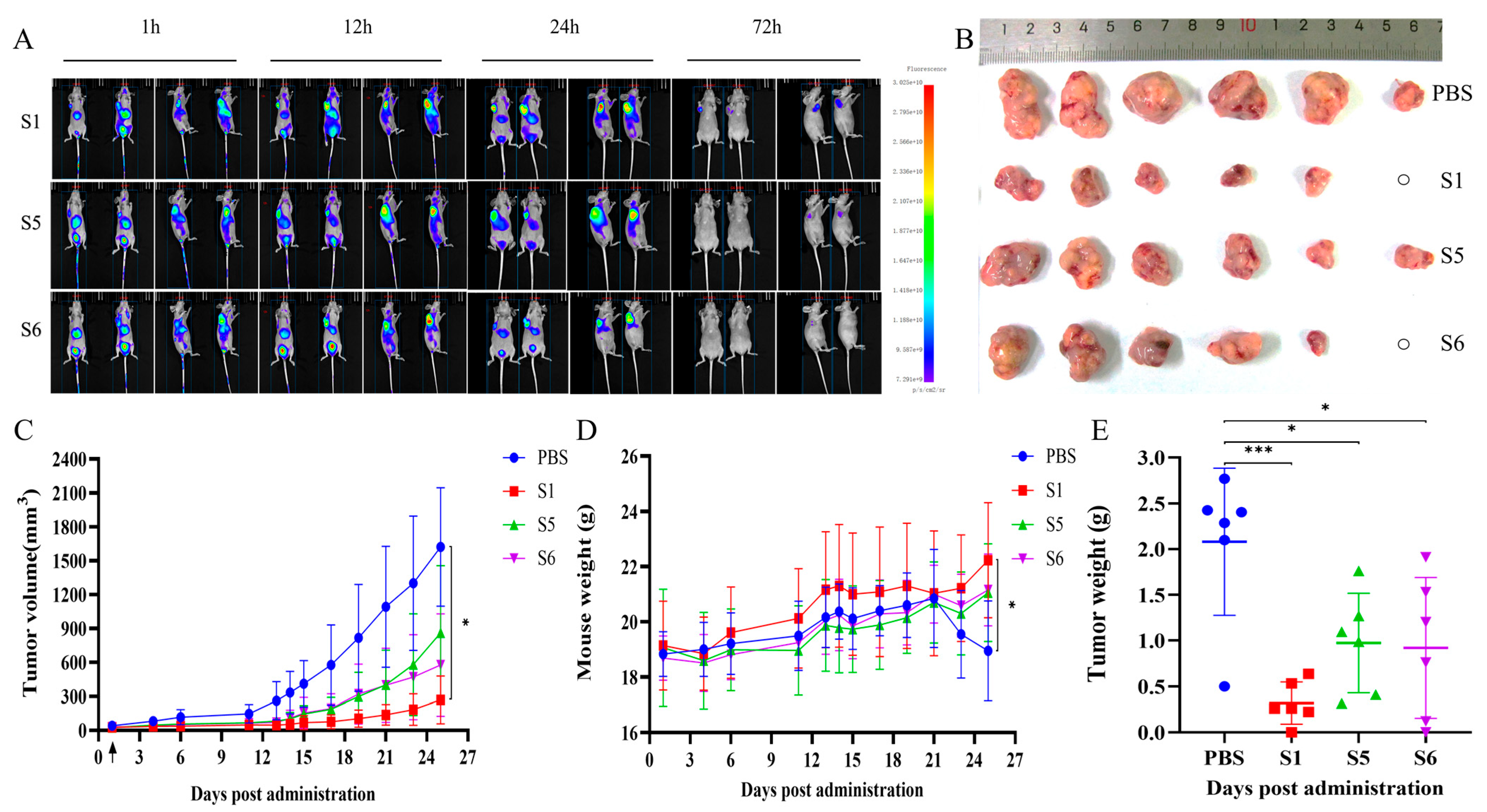
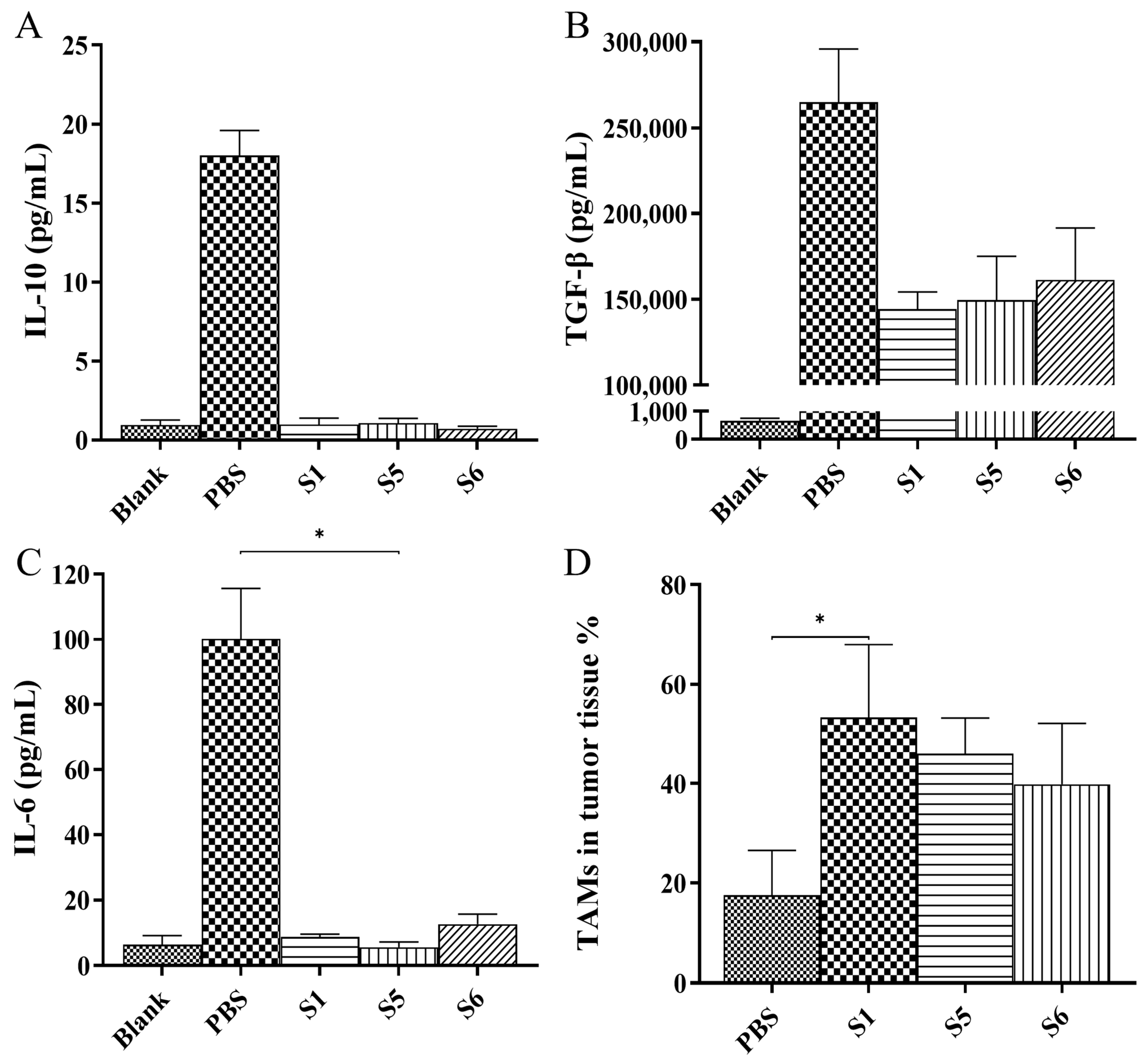
2.6. Evaluation of the Anti-Tumor Activity Mediated by the Chimeric Heavy-Chain Antibodies In Vivo
3. Discussion
4. Materials and Methods
4.1. Animals and Cell Lines
4.2. Immunization of the Camel
4.3. Construction of the Phage Display Nanobody Library
4.4. Panning and Screening of the Phage Display Nanobody Library
4.5. Construction, Expression and Purification of the Chimeric Heavy-Chain Antibodies
4.6. Binding Activity and Specificity Assays
4.7. Competitive Binding Assay
4.8. Blocking Activity Assay
4.9. T-Cell Response Assays
4.10. Evaluation of Anti-Tumor Activity In Vivo
4.11. Statistical Considerations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, A.; Maji, A.; Potdar, P.D.; Singh, N.; Parikh, P.; Bisht, B.; Mukherjee, A.; Paul, M.K. Lung cancer immunotherapy: Progress, pitfalls, and promises. Mol. Cancer. 2023, 22, 40. [Google Scholar] [CrossRef]
- Bagchi, S.; Yuan, R.; Engleman, E.G. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu. Rev. Pathol. 2021, 16, 223–249. [Google Scholar] [CrossRef]
- Sun, J.; Lu, Q.; Sanmamed, M.F.; Wang, J. Siglec-15 as an Emerging Target for Next-generation Cancer Immunotherapy. Clin. Cancer Res. 2021, 27, 680–688. [Google Scholar] [CrossRef]
- Suda, T.; Takahashi, N.; Udagawa, N.; Jimi, E.; Gillespie, M.T.; Martin, T.J. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr. Rev. 1999, 20, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Yu, A.; Othmane, B.; Qiu, D.; Li, H.; Li, C.; Liu, P.; Ren, W.; Chen, M.; Gong, G.; et al. Siglec15 shapes a non-inflamed tumor microenvironment and predicts the molecular subtype in bladder cancer. Theranostics 2021, 11, 3089–3108. [Google Scholar] [CrossRef] [PubMed]
- Korn, M.A.; Schmitt, H.; Angermüller, S.; Chambers, D.; Seeling, M.; Lux, U.T.; Brey, S.; Royzman, D.; Brückner, C.; Popp, V.; et al. Siglec-15 on Osteoclasts Is Crucial for Bone Erosion in Serum-Transfer Arthritis. J. Immunol. 2020, 205, 2595–2605. [Google Scholar] [CrossRef]
- Cao, G.; Xiao, Z.; Yin, Z. Normalization cancer immunotherapy: Blocking Siglec-15! Signal Transduct. Target Ther. 2019, 4, 10. [Google Scholar] [CrossRef]
- Okazaki, T.; Chikuma, S.; Iwai, Y.; Fagarasan, S.; Honjo, T. A rheostat for immune responses: The unique properties of PD-1 and their advantages for clinical application. Nat. Immunol. 2013, 14, 1212–1218. [Google Scholar] [CrossRef]
- Wang, J.; Sun, J.; Liu, L.N.; Flies, D.B.; Nie, X.; Toki, M.; Zhang, J.; Song, C.; Zarr, M.; Zhou, X.; et al. Siglec-15 as an immune suppressor and potential target for normalization cancer immunotherapy. Nat. Med. 2019, 25, 656–666. [Google Scholar] [CrossRef]
- Takamiya, R.; Ohtsubo, K.; Takamatsu, S.; Taniguchi, N.; Angata, T. The interaction between Siglec-15 and tumor-associated sialyl-Tn antigen enhances TGF-beta secretion from monocytes/macrophages through the DAP12-Syk pathway. Glycobiology 2013, 23, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ji, Z.; Wu, B.; Huang, S.; Chen, Q.; Chen, X.; Wei, Y.; Jiang, J. Siglec-15 promotes the migration of liver cancer cells by repressing lysosomal degradation of CD44. FEBS Lett. 2021, 595, 2290–2302. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Wang, Q.; An, J.; Chen, J.; Li, X.; Long, Q.; Xiao, L.; Guan, X.; Liu, J. CD44 Glycosylation as a Therapeutic Target in Oncology. Front. Oncol. 2022, 12, 883831. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, G.; Correia, V.G.; Palma, A.S.; Chai, W.; Li, C.; Feizi, T.; Martin, E.; Laux, B.; Franz, A.; Fuchs, K.; et al. Siglec-15 recognition of sialoglycans on tumor cell lines can occur independently of sialyl Tn antigen expression. Glycobiology 2021, 31, 44–54. [Google Scholar] [CrossRef]
- Lenza, M.P.; Egia-Mendikute, L.; Antoñana-Vildosola, A.; Soares, C.O.; Coelho, H.; Corzana, F.; Bosch, A.; Manisha, P.; Quintana, J.I.; Oyenarte, I.; et al. Structural insights into Siglec-15 reveal glycosylation dependency for its interaction with T cells through integrin CD11b. Nat. Commun. 2023, 14, 3496. [Google Scholar] [CrossRef]
- Wu, J.; Peng, J.; Zhou, Y.; Zhang, R.; Wang, Z.; Hu, N.; Zhang, D.; Quan, G.; Wu, Y.; Feng, J.; et al. Screening and Identification of a Novel Anti-Siglec-15 Human Antibody 3F1 and Relevant Antitumor Activity. Mol. Pharmacol. 2022, 102, 161–171. [Google Scholar] [CrossRef]
- Clevers, H.; Alarcon, B.; Wileman, T.; Terhorst, C. The T cell receptor/CD3 complex: A dynamic protein ensemble. Annu. Rev. Immunol. 1988, 6, 629–662. [Google Scholar] [CrossRef]
- Geisler, C.; Kuhlmann, J.; Rubin, B. Assembly, intracellular processing, and expression at the cell surface of the human alpha beta T cell receptor/CD3 complex. Function of the CD3-zeta chain. J. Immunol. 1989, 143, 4069–4077. [Google Scholar] [CrossRef]
- Gettinger, S.; Horn, L.; Jackman, D.; Spigel, D.; Antonia, S.; Hellmann, M.; Powderly, J.; Heist, R.; Sequist, L.V.; Smith, D.C.; et al. Five-Year Follow-Up of Nivolumab in Previously Treated Advanced Non-Small-Cell Lung Cancer: Results from the CA209-003 Study. J. Clin. Oncol. 2018, 36, 1675–1684. [Google Scholar] [CrossRef]
- Grinda, T.; Antoine, A.; Jacot, W.; Blaye, C.; Cottu, P.-H.; Diéras, V.; Dalenc, F.; Gonçalves, A.; Debled, M.; Patsouris, A.; et al. Evolution of overall survival and receipt of new therapies by subtype among 20 446 metastatic breast cancer patients in the 2008–2017 ESME cohort. ESMO Open 2021, 6, 100114. [Google Scholar] [CrossRef]
- Gu, R.; Liu, F.; Zou, D.; Xu, Y.; Lu, Y.; Liu, B.; Liu, W.; Chen, X.; Liu, K.; Guo, Y.; et al. Efficacy and safety of CD19 CAR T constructed with a new anti-CD19 chimeric antigen receptor in relapsed or refractory acute lymphoblastic leukemia. J. Hematol. Oncol. 2020, 13, 122. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.V.; Reck, M.; Mansfield, A.S.; Mok, T.; Scherpereel, A.; Reinmuth, N.; Garassino, M.C.; De Castro Carpeno, J.; Califano, R.; Nishio, M.; et al. Updated Overall Survival and PD-L1 Subgroup Analysis of Patients with Extensive-Stage Small-Cell Lung Cancer Treated with Atezolizumab, Carboplatin, and Etoposide (IMpower133). J. Clin. Oncol. 2021, 39, 619–630. [Google Scholar] [CrossRef]
- Reuss, J.E.; Anagnostou, V.; Cottrell, T.R.; Smith, K.N.; Verde, F.; Zahurak, M.; Lanis, M.; Murray, J.C.; Chan, H.Y.; McCarthy, C.; et al. Neoadjuvant nivolumab plus ipilimumab in resectable non-small cell lung cancer. J. Immunother. Cancer. 2020, 8, e001282. [Google Scholar] [CrossRef]
- Tanyi, J.L.; Bobisse, S.; Ophir, E.; Tuyaerts, S.; Roberti, A.; Genolet, R.; Baumgartner, P.; Stevenson, B.J.; Iseli, C.; Dangaj, D.; et al. Personalized cancer vaccine effectively mobilizes antitumor T cell immunity in ovarian cancer. Sci. Transl. Med. 2018, 10, eaao5931. [Google Scholar] [CrossRef] [PubMed]
- Derksen, P.W.B.; de Gorter, D.J.J.; Meijer, H.P.; Bende, R.J.; van Dijk, M.; Lokhorst, H.M.; Bloem, A.C.; Spaargaren, M.; Pals, S.T. The hepatocyte growth factor/Met pathway controls proliferation and apoptosis in multiple myeloma. Leukemia 2003, 17, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Sanmamed, M.F.; Chen, L. A Paradigm Shift in Cancer Immunotherapy: From Enhancement to Normalization. Cell 2018, 175, 313–326. [Google Scholar] [CrossRef]
- Arora, S.; Velichinskii, R.; Lesh, R.W.; Ali, U.; Kubiak, M.; Bansal, P.; Borghaei, H.; Edelman, M.J.; Boumber, Y. Existing and Emerging Biomarkers for Immune Checkpoint Immunotherapy in Solid Tumors. Adv. Ther. 2019, 36, 2638–2678. [Google Scholar] [CrossRef]
- Ren, X. Immunosuppressive checkpoint Siglec-15: A vital new piece of the cancer immunotherapy jigsaw puzzle. Cancer Biol. Med. 2019, 16, 205–210. [Google Scholar]
- He, F.; Wang, N.; Li, J.; He, L.; Yang, Z.; Lu, J.; Xiong, G.; Yu, C.; Wang, S. High affinity monoclonal antibody targeting Siglec-15 for cancer immunotherapy. J. Clin. Transl. Res. 2021, 7, 739–749. [Google Scholar]
- NextCure. A Phase 2 Study of Anti-Siglec-15 Antibody, NC318, in Combination with Pembrolizumab (NCT04699123) Demonstrates Clinical Activity in Patients with Advanced PD-1 Axis Inhibitor Refractory NSCLC. 2023. Available online: https://www.globenewswire.com/news-release/2023/09/12/2741628/0/en/A-Phase-2-Study-of-Anti-Siglec-15-Antibody-NC318-in-Combination-with-Pembrolizumab-NCT04699123-Demonstrates-Clinical-Activity-in-Patients-with-Advanced-PD-1-Axis-Inhibitor-Refracto.html (accessed on 14 September 2023).
- Jiang, Y.; Wang, R.; Guo, J.; Cheng, K.; Chen, L.; Wang, X.; Li, Y.; Du, P.; Gao, C.; Lu, J.; et al. Isolation and characterization of Hc-targeting chimeric heavy chain antibodies neutralizing botulinum neurotoxin type B. Front. Immunol. 2024, 15, 1380694. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, J.; Du, P.; Lu, J.; Chen, L.; Huang, Y.; Yu, Y.; Xie, Q.; Wang, R.; Yang, Z. Generation and characterization of humanized synergistic neutralizing antibodies against SARS-CoV-2. J. Med. Virol. 2022, 94, 3791–3800. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Lu, L.; Deng, S.; Meng, J.; Wan, C.; Huang, J.; Sun, Y.; Hu, Y.; Wu, B.; Wu, G.; et al. USP7 targeting modulates anti-tumor immune response by reprogramming Tumor-associated Macrophages in Lung Cancer. Theranostics 2020, 10, 9332–9347. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.N.; Wang, Y.Y.; Wang, J.; Bai, W.J.; Miao, N.J.; Wang, J. Vinblastine resets tumor-associated macrophages toward M1 phenotype and promotes antitumor immune response. J. Immunother. Cancer 2023, 11, e007253. [Google Scholar] [CrossRef] [PubMed]
| Round of Panning | Input (pfu) | Output (pfu) | Output/Input |
|---|---|---|---|
| Round 1 | 5.00 × 1011 | 2.80 × 105 | 5.60 × 10−5 |
| Round 2 | 3.00 × 1011 | 2.70 × 106 | 9.00 × 10−4 |
| Round 3 | 1.00 × 1011 | 1.60 × 108 | 1.60 × 10−3 |
| Antibody | Mean | |||
|---|---|---|---|---|
| Kon (104 Ms−1) | Kdis (10−4 s−1) | KD (10−9 M) | R2 | |
| S1 | 4.20 | 5.33 | 0.13 | 0.99 |
| S5 | 5.73 | 8.92 | 0.16 | 0.99 |
| S6 | 6.89 | 14.30 | 0.21 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, K.; Guo, J.; Li, Y.; Kang, Q.; Wang, R.; Luo, L.; Wang, W.; Lu, J. Anti-Tumor Activities of Anti-Siglec-15 Chimeric Heavy-Chain Antibodies. Int. J. Mol. Sci. 2025, 26, 5068. https://doi.org/10.3390/ijms26115068
Cheng K, Guo J, Li Y, Kang Q, Wang R, Luo L, Wang W, Lu J. Anti-Tumor Activities of Anti-Siglec-15 Chimeric Heavy-Chain Antibodies. International Journal of Molecular Sciences. 2025; 26(11):5068. https://doi.org/10.3390/ijms26115068
Chicago/Turabian StyleCheng, Kexuan, Jiazheng Guo, Yating Li, Qinglin Kang, Rong Wang, Longlong Luo, Wei Wang, and Jiansheng Lu. 2025. "Anti-Tumor Activities of Anti-Siglec-15 Chimeric Heavy-Chain Antibodies" International Journal of Molecular Sciences 26, no. 11: 5068. https://doi.org/10.3390/ijms26115068
APA StyleCheng, K., Guo, J., Li, Y., Kang, Q., Wang, R., Luo, L., Wang, W., & Lu, J. (2025). Anti-Tumor Activities of Anti-Siglec-15 Chimeric Heavy-Chain Antibodies. International Journal of Molecular Sciences, 26(11), 5068. https://doi.org/10.3390/ijms26115068





