The Role of Helicobacter pylori Heat Shock Proteins in Gastric Diseases’ Pathogenesis
Abstract
1. Introduction
2. The Evolution of Heat Shock Proteins: From Very Ancestral Forms of Life to Humans
3. Bacterial Heat Shock Proteins
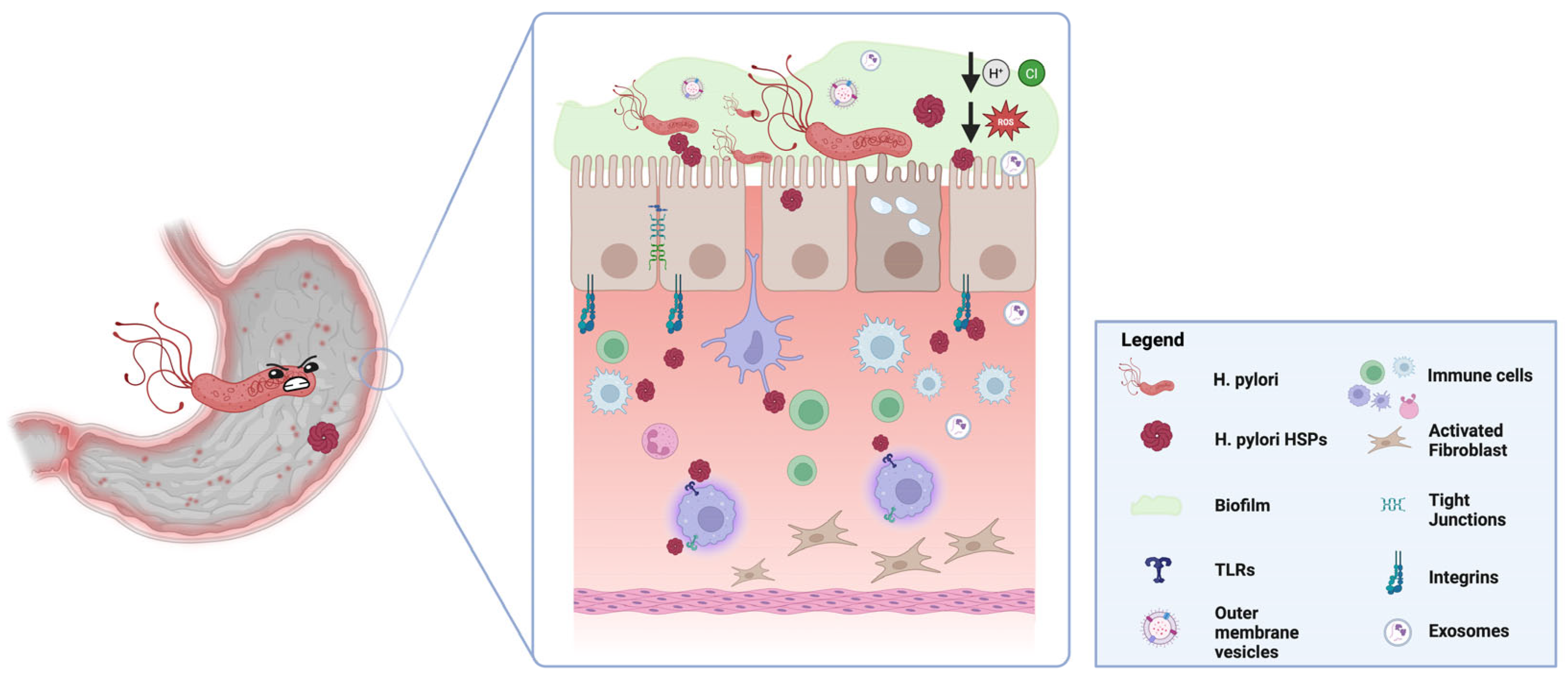
4. Heat Shock Proteins in H. pylori
| Key Aspects | Human HSPs | H. pylori HSP | References |
|---|---|---|---|
| Origin and evolution | Human HSPs are eukaryotic proteins, encoded by the human genome, and have evolved within the context of complex, compartmentalized cells and multicellular organisms. | H. pylori HSPs are prokaryotic, adapted to the bacterium’s specific intracellular and extracellular environments, including the highly acidic gastric niche. | [18,19,30,31] |
| Sequence homology with divergence | Despite structural conservation (e.g., GroEL–DnaK in H. pylori vs. Hsp60–Hsp70 in humans), there are significant sequence differences that distinguish the bacterial from the human homologs. These differences are critical for pathogen-specific targeting by the immune system and for the development of therapeutic interventions. | [39] | |
| Function and cellular context | While both human and bacterial HSPs assist in protein folding, stress response, and prevention of protein aggregation, human HSPs also have broader roles in apoptosis and cancer biology. | In contrast, H. pylori HSPs contribute to bacterial survival under stress, colonization, immune evasion, and biofilm formation. | [34,35,37,38] |
| HSP Family | Bacterial Protein | Main Function | Main Role in H. pylori Pathogenesis | References |
|---|---|---|---|---|
| Hsp10 | GroES | Regulates urease activity and epithelial cell adhesion | Adhesion of H. pylori to primary human gastric epithelial cells facilitates colonization of stomach | [30] |
| Hsp60 | GroEL | Facilitates protein folding and refolding of denatured proteins | Involved in host cell adhesion and immune modulation, stimulates inflammatory responses, and contributes to virulence | [35,36] |
| Hsp70 | DnaK | Assists in protein folding, stabilizes denatured proteins, and regulates stress responses | Supports bacterial survival under stress, regulates enzymatic activity, and modulates metabolic mechanisms | [37,38] |
5. Role of Heat Shock Proteins in H. pylori Pathogenesis
5.1. Adhesion and Colonization
5.2. Resistance to Environmental Stress
5.3. Modulation of the Immune Response
6. HSP-Mediated Crosstalk Between H. pylori and Gastric Mucosa: The Role of Extracellular Vesicles
7. HSPs and Antibiotic Resistance
8. Clinical Implications and Future Perspectives
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Manna, O.M.; Bavisotto, C.C.; Gratie, M.I.; Damiani, P.; Bonaventura, G.; Cappello, F.; Tomasello, G.; D’Andrea, V. Targeting Helicobacter pylori Through the “Muco-Microbiotic Layer” Lens: The Challenge of Probiotics and Microbiota Nanovesicles. Nutrients 2025, 17, 569. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kansau, I.; Labigne, A. Heat shock proteins of Helicobacter pylori. Aliment. Pharmacol. Ther. 1996, 10 (Suppl. S1), 51–56. [Google Scholar] [CrossRef] [PubMed]
- de Macario, E.C.; Yohda, M.; Macario, A.J.L.; Robb, F.T. Bridging human chaperonopathies and microbial chaperonins. Commun. Biol. 2019, 2, 103. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Macario, A.J.L.; Conway de Macario, E. Sick chaperones, cellular stress, and disease. N. Engl. J. Med. 2005, 353, 1489–1501. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, P.S.; Garduno, R.A. Surface-associated heat shock proteins of Legionella pneumophila and Helicobacter pylori: Roles in pathogenesis and immunity. Infect. Dis. Obstet. Gynecol. 1999, 7, 58–63. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Asante, M.A.; Mendall, M.A.; Ballam, L.; Morris, J.; Northfield, T.C. Relationship between Helicobacter pylori, gastric parietal cell antibodies and heat shock proteins. Eur. J. Gastroenterol. Hepatol. 1999, 11, 1365–1370. [Google Scholar] [CrossRef] [PubMed]
- Ritossa, F. A new puffing pattern induced by temperature shock and DNP in drosophila. Experientia 1962, 18, 571–573. [Google Scholar] [CrossRef]
- Macario, A.J.L.; Malz, M.; de Macario, E.C. Evolution of assisted protein folding: The distribution of the main chaperoning systems within the phylogenetic domain archaea. Front. Biosci. 2004, 9, 1318–1332. [Google Scholar] [CrossRef] [PubMed]
- Macario, A.J.L.; de Macario, E.C. The archaeal molecular chaperone machine: Peculiarities and paradoxes. Genetics 1999, 152, 1277–1283. [Google Scholar] [CrossRef]
- Cappello, F.; Conway de Macario, E.; Macario, A.J.L. HSP60: A Story as Long as Life on the Earth. In Heat Shock Protein 60 in Human Diseases and Disorders; Springer International Publishing: Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Macario, A.J.; Lange, M.; Ahring, B.K.; de Macario, E.C. Stress genes and proteins in the archaea. Microbiol. Mol. Biol. Rev. 1999, 63, 923–967. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kobayashi, Y.; Ohtsu, I.; Fujimura, M.; Fukumori, F. A mutation in dnaK causes stabilization of the heat shock sigma factor σ32, accumulation of heat shock proteins and increase in toluene-resistance in Pseudomonas putida. Environ. Microbiol. 2011, 13, 2007–2017. [Google Scholar] [CrossRef] [PubMed]
- Nakahigashi, K.; Ron, E.Z.; Yanagi, H.; Yura, T. Differential and independent roles of a sigma(32) homolog (RpoH) and an HrcA repressor in the heat shock response of Agrobacterium tumefaciens. J. Bacteriol. 1999, 181, 7509–7515. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheng, M.Y.; Hartl, F.U.; Horwich, A.L. The mitochondrial chaperonin hsp60 is required for its own assembly. Nature 1990, 348, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Hao, X.; Wang, Q.; Hou, J.; Lai, X.; Dong, Z.; Shao, C. Genome-wide identification and characterization of heat shock protein family 70 provides insight into its divergent functions on immune response and development of Paralichthys olivaceus. PeerJ 2019, 7, e7781. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zuñiga-Hernandez, J.; Meneses, C.; Bastias, M.; Allende, M.L.; Glavic, A. Drosophila DAxud1 Has a Repressive Transcription Activity on Hsp70 and Other Heat Shock Genes. Int. J. Mol. Sci. 2023, 24, 7485. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sollars, V.; Lu, X.; Xiao, L.; Wang, X.; Garfinkel, M.D.; Ruden, D.M. Evidence for an epigenetic mechanism by which Hsp90 acts as a capacitor for morphological evolution. Nat. Genet. 2003, 33, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sedlacek, A.L.; Pawaria, S.; Xu, H.; Scott, M.J.; Binder, R.J. Cutting Edge: The Heat Shock Protein gp96 Activates Inflammasome-Signaling Platforms in APCs. J. Immunol. 2018, 201, 2209–2214. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bavisotto, C.C.; Cappello, F.; de Macario, E.C.; Macario, A.J.L.; Rappa, F. Immunohistochemistry of Human Hsp60 in Health and Disease: Recent Advances in Immunomorphology and Methods for Assessing the Chaperonin in Extracellular Vesicles. Methods Mol. Biol. 2023, 2693, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Gammazza, A.M.; Bavisotto, C.C.; Macario, A.J.L. Editorial: Physiology and Pathophysiology of Heat Shock Protein 60. Front. Mol. Biosci. 2020, 7, 604476. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bavisotto, C.C.; Alberti, G.; Vitale, A.M.; Paladino, L.; Campanella, C.; Rappa, F.; Gorska, M.; Conway de Macario, E.; Cappello, F.; Macario, A.J.L.; et al. Hsp60 Post-translational Modifications: Functional and Pathological Consequences. Front. Mol. Biosci. 2020, 7, 95. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Macario, A.J.L.; Conway de Macario, E. Molecular mechanisms in chaperonopathies: Clues to understanding the histopathological abnormalities and developing novel therapies. J. Pathol. 2020, 250, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.; Ron, E.Z. Proteome analysis in the study of the bacterial heat-shock response. Mass. Spectrom. Rev. 2002, 21, 244–265. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H.; Yuzawa, H.; Kanemori, M.; Yura, T. A distinct segment of the sigma 32 polypeptide is involved in DnaK-mediated negative control of the heat shock response in Escherichia coli. Proc. Natl. Acad. Sci. USA 1994, 91, 10280–10284. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vilasi, S.; Bulone, D.; Bavisotto, C.C.; Campanella, C.; Gammazza, A.M.; San Biagio, P.L.; Cappello, F.; de Macario, E.C.; Macario, A.J.L. Chaperonin of Group I: Oligomeric Spectrum and Biochemical and Biological Implications. Front. Mol. Biosci. 2018, 4, 99. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morita, M.T.; Tanaka, Y.; Kodama, T.S.; Kyogoku, Y.; Yanagi, H.; Yura, T. Translational induction of heat shock transcription factor sigma32: Evidence for a built-in RNA thermosensor. Genes Dev. 1999, 13, 655–665. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hagemann, L.; Gründel, A.; Jacobs, E.; Dumke, R. The surface-displayed chaperones GroEL and DnaK of Mycoplasma pneumoniae interact with human plasminogen and components of the extracellular matrix. Pathog. Dis. 2017, 75, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bolhassani, A.; Rafati, S. Heat-shock proteins as powerful weapons in vaccine development. Expert Rev. Vaccines 2008, 7, 1185–1199. [Google Scholar] [CrossRef]
- Coenye, T. Response of sessile cells to stress: From changes in gene expression to phenotypic adaptation. FEMS Immunol. Med. Microbiol. 2010, 59, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Homuth, G.; Domm, S.; Kleiner, D.; Schumann, W. Transcriptional analysis of major heat shock genes of Helicobacter pylori. J. Bacteriol. 2000, 182, 4257–4263. [Google Scholar] [CrossRef]
- Pepe, S.; Scarlato, V.; Roncarati, D. The Helicobacter pylori HspR-Modulator CbpA Is a Multifunctional Heat-Shock Protein. Microorganisms 2020, 8, 251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pierzchalski, P.; Jastrzebska, M.; Link-Lenczowski, P.; Leja-Szpak, A.; Bonior, J.; Jaworek, J.; Okon, K.; Wojcik, P. The dynamics of heat shock system activation in Monomac-6 cells upon Helicobacter pylori infection. J. Physiol. Pharmacol. 2014, 65, 791–800. [Google Scholar] [PubMed]
- Pepe, S.; Pinatel, E.; Fiore, E.; Puccio, S.; Peano, C.; Brignoli, T.; Vannini, A.; Danielli, A.; Scarlato, V.; Roncarati, D. The Helicobacter pylori Heat-Shock Repressor HspR: Definition of Its Direct Regulon and Characterization of the Cooperative DNA-Binding Mechanism on Its Own Promoter. Front. Microbiol. 2018, 9, 1887. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- González-López, M.A.; Velázquez-Guadarrama, N.; Romero-Espejel, M.E.; Olivares-Trejo, J.d.J. Helicobacter pylori secretes the chaperonin GroEL (HSP60), which binds iron. FEBS Lett. 2013, 587, 1823–1828. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Huang, Y.S.; Li, C.H.; Hsieh, Y.T.; Tsai, N.M.; He, P.J.; Hsu, W.T.; Yeh, Y.C.; Chiang, F.H.; Wu, M.S.; et al. Characterizing the polymeric status of Helicobacter pylori heat shock protein 60. Biochem. Biophys. Res. Commun. 2009, 388, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, R.; Yokota, K.; Okada, H.; Hayashi, S.; Mizuno, M.; Yoshino, T.; Hirai, Y.; Saitou, D.; Akagi, T.; Oguma, K. Immune response in Helicobacter pylori-induced low-grade gastric-mucosa-associated lymphoid tissue (MALT) lymphoma. J. Med. Microbiol. 2004, 53 Pt 1, 21–29. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nurgalieva, Z.Z.; Conner, M.E.; Opekun, A.R.; Zheng, C.Q.; Elliott, S.N.; Ernst, P.B.; Osato, M.; Estes, M.K.; Graham, D.Y. B-cell and T-cell immune responses to experimental Helicobacter pylori infection in humans. Infect. Immun. 2005, 73, 2999–3006. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grave, E.; Yokota, S.; Yamamoto, S.; Tamura, A.; Ohtaki-Mizoguchi, T.; Yokota, K.; Oguma, K.; Fujiwara, K.; Ogawa, N.; Okamoto, T.; et al. Geranylgeranylacetone selectively binds to the HSP70 of Helicobacter pylori and alters its coccoid morphology. Sci. Rep. 2015, 5, 13738. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karlin, S.; Brocchieri, L. Heat shock protein 60 sequence comparisons: Duplications, lateral transfer, and mitochondrial evolution. Proc. Natl. Acad. Sci. USA 2000, 97, 11348–11353. [Google Scholar] [CrossRef] [PubMed Central]
- Fregonezi, N.F.; Oliveira, L.T.; Singulani, J.L.; Marcos, C.M.; Dos Santos, C.T.; Taylor, M.L.; Mendes-Giannini, M.J.S.; de Oliveira, H.C.; Fusco-Almeida, A.M. Heat Shock Protein 60, Insights to Its Importance in Histoplasma capsulatum: From Biofilm Formation to Host-Interaction. Front. Cell Infect. Microbiol. 2021, 10, 591950. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, K.J.; Jung, Y.C.; Park, S.J.; Lee, K.H. Role of Heat Shock Proteases in Quorum-Sensing-Mediated Regulation of Biofilm Formation by Vibrio Species. mBio 2018, 9, e02086-17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Becherelli, M.; Tao, J.; Ryder, N.S. Involvement of heat shock proteins in Candida albicans biofilm formation. J. Mol. Microbiol. Biotechnol. 2013, 23, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Roilides, E.; Simitsopoulou, M.; Katragkou, A.; Walsh, T.J. How Biofilms Evade Host Defenses. Microbiol. Spectr. 2015, 3, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Gondil, V.S.; Subhadra, B. Biofilms and their role on diseases. BMC Microbiol. 2023, 23, 203. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Balcázar, J.L.; Subirats, J.; Borrego, C.M. The role of biofilms as environmental reservoirs of antibiotic resistance. Front. Microbiol. 2015, 6, 1216. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kao, C.Y.; Sheu, B.S.; Wu, J.J. Helicobacter pylori infection: An overview of bacterial virulence factors and pathogenesis. Biomed. J. 2016, 39, 14–23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rather, M.A.; Gupta, K.; Mandal, M. Microbial biofilm: Formation, architecture, antibiotic resistance, and control strategies. Braz. J. Microbiol. 2021, 52, 1701–1718. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gupta, N.; Kumar, A.; Verma, V.K. Strategies adopted by gastric pathogen Helicobacter pylori for a mature biofilm formation: Antimicrobial peptides as a visionary treatment. Microbiol. Res. 2023, 273, 127417. [Google Scholar] [CrossRef] [PubMed]
- Yonezawa, H.; Osaki, T.; Kamiya, S. Biofilm Formation by Helicobacter pylori and Its Involvement for Antibiotic Resistance. Biomed Res. Int. 2015, 2015, 914791. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hathroubi, S.; Zerebinski, J.; Clarke, A.; Ottemann, K.M. Helicobacter pylori Biofilm Confers Antibiotic Tolerance in Part via A Protein-Dependent Mechanism. Antibiotics 2020, 9, 355. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roncarati, D.; Danielli, A.; Spohn, G.; Delany, I.; Scarlato, V. Transcriptional regulation of stress response and motility functions in Helicobacter pylori is mediated by HspR and HrcA. J. Bacteriol. 2007, 189, 7234–7243. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roncarati, D.; Pinatel, E.; Fiore, E.; Peano, C.; Loibman, S.; Scarlato, V. Helicobacter pylori Stress-Response: Definition of the HrcA Regulon. Microorganisms 2019, 7, 436. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hathroubi, S.; Zerebinski, J.; Ottemann, K.M. Helicobacter pylori Biofilm Involves a Multigene Stress-Biased Response, Including a Structural Role for Flagella. mBio 2018, 9, e01973-18. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fourie, K.R.; Wilson, H.L. Understanding GroEL and DnaK Stress Response Proteins as Antigens for Bacterial Diseases. Vaccines 2020, 8, 773. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- García-Muñoz, A.M.; Victoria-Montesinos, D.; Ballester, P.; Cerdá, B.; Zafrilla, P. A Descriptive Review of the Antioxidant Effects and Mechanisms of Action of Berberine and Silymarin. Molecules 2024, 29, 4576. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fan, J.; Zhu, J.; Xu, H. Strategies of Helicobacter pylori in evading host innate and adaptive immunity: Insights and prospects for therapeutic targeting. Front. Cell Infect. Microbiol. 2024, 14, 1342913. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baj, J.; Forma, A.; Sitarz, M.; Portincasa, P.; Garruti, G.; Krasowska, D.; Maciejewski, R. Helicobacter pylori Virulence Factors-Mechanisms of Bacterial Pathogenicity in the Gastric Microenvironment. Cells 2020, 10, 27. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karkhah, A.; Ebrahimpour, S.; Rostamtabar, M.; Koppolu, V.; Darvish, S.; Vasigala, V.K.R.; Validi, M.; Nouri, H.R. Helicobacter pylori evasion strategies of the host innate and adaptive immune responses to survive and develop gastrointestinal diseases. Microbiol. Res. 2019, 218, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Yang, J.; Qi, Z.; Wu, H.; Wang, B.; Zou, F.; Mei, H.; Liu, J.; Wang, W.; Liu, Q. Heat shock proteins: Biological functions, pathological roles, and therapeutic opportunities. MedComm 2022, 3, e161. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, L.; Duan, M.; Pu, X.; Zheng, H.; Ning, X.; Tu, Y.; Xu, C.; Zhang, D.; Liu, C.; Xie, J. GroEL triggers NLRP3 inflammasome activation through the TLR/NF-κB p-p65 axis in human periodontal ligament stem cells. Acta Biochim. Biophys. Sin. 2024, 56, 1340–1351. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sangiorgi, C.; Vallese, D.; Gnemmi, I.; Bucchieri, F.; Balbi, B.; Brun, P.; Leone, A.; Giordano, A.; Conway de Macario, E.; Macario, A.J.; et al. HSP60 activity on human bronchial epithelial cells. Int. J. Immunopathol. Pharmacol. 2017, 30, 333–340. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gammazza, A.M.; Bucchieri, F.; Grimaldi, L.M.; Benigno, A.; de Macario, E.C.; Macario, A.J.; Zummo, G.; Cappello, F. The molecular anatomy of human Hsp60 and its similarity with that of bacterial orthologs and acetylcholine receptor reveal a potential pathogenetic role of anti-chaperonin immunity in myasthenia gravis. Cell Mol. Neurobiol. 2012, 32, 943–947. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Campanella, C.; Gammazza, A.M.; Mularoni, L.; Cappello, F.; Zummo, G.; Di Felice, V. A comparative analysis of the products of GROEL-1 gene from Chlamydia trachomatis serovar D and the HSP60 var1 transcript from Homo sapiens suggests a possible autoimmune response. Int. J. Immunogenet. 2009, 36, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Bavisotto, C.C.; Provenzano, A.; Passantino, R.; Gammazza, A.M.; Cappello, F.; San Biagio, P.L.; Bulone, D. Oligomeric State and Holding Activity of Hsp60. Int. J. Mol. Sci. 2023, 24, 7847. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chmiela, M.; Gonciarz, W. Molecular mimicry in Helicobacter pylori infections. World J. Gastroenterol. 2017, 23, 3964–3977. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fucarino, A.; Burgio, S.; Paladino, L.; Caruso Bavisotto, C.; Pitruzzella, A.; Bucchieri, F.; Cappello, F. The Microbiota Is Not an Organ: Introducing the Muco-Microbiotic Layer as a Novel Morphofunctional Structure. Anatomia 2022, 1, 186–203. [Google Scholar] [CrossRef]
- Cappello, F.; Saguto, D.; Burgio, S.; Paladino, L.; Bucchieri, F. Does Intestine Morphology Still Have Secrets to Reveal? A Proposal about the “Ghost” Layer of the Bowel. Appl. Biosci. 2022, 1, 95–100. [Google Scholar] [CrossRef]
- Cappello, F.; Rappa, F.; Canepa, F.; Carini, F.; Mazzola, M.; Tomasello, G.; Bonaventura, G.; Giuliana, G.; Leone, A.; Saguto, D.; et al. Probiotics Can Cure Oral Aphthous-Like Ulcers in Inflammatory Bowel Disease Patients: A Review of the Literature and a Working Hypothesis. Int. J. Mol. Sci. 2019, 20, 5026. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alberti, G.; Paladino, L.; Vitale, A.M.; Bavisotto, C.C.; de Macario, E.C.; Campanella, C.; Macario, A.J.L.; Gammazza, A.M. Functions and Therapeutic Potential of Extracellular Hsp60, Hsp70, and Hsp90 in Neuroinflammatory Disorders. Appl. Sci. 2021, 11, 736. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Luo, H.; Xie, Y.; Cao, H.; Mao, L.; Liu, T.; Yue, Y.; Qian, H. Extracellular vesicles in Helicobacter pylori-mediated diseases: Mechanisms and therapeutic potential. Cell Commun. Signal. 2025, 23, 79. [Google Scholar] [CrossRef]
- Li, Y.; Cao, H.; Qiu, D.; Wang, N.; Wang, Y.; Wen, T.; Wang, J.; Zhu, H. The proteomics analysis of extracellular vesicles revealed the possible function of heat shock protein 60 in Helicobacter pylori infection. Cancer Cell Int. 2023, 23, 272. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Lei, Q.; Zou, X.; Ma, D. The role and mechanisms of gram-negative bacterial outer membrane vesicles in inflammatory diseases. Front. Immunol. 2023, 14, 1157813. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Muralidharan, S.; Mandrekar, P. Cellular stress response and innate immune signaling: Integrating pathways in host defense and inflammation. J. Leukoc. Biol. 2013, 94, 1167–1184. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Balhuizen, M.D.; van Dijk, A.; Jansen, J.W.A.; van de Lest, C.H.A.; Veldhuizen, E.J.A.; Haagsman, H.P. Outer Membrane Vesicles Protect Gram-Negative Bacteria against Host Defense Peptides. mSphere 2021, 6, e0052321. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schorey, J.S.; Cheng, Y.; Singh, P.P.; Smith, V.L. Exosomes and other extracellular vesicles in host-pathogen interactions. EMBO Rep. 2015, 16, 24–43. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dudeja, V.; Vickers, S.M.; Saluja, A.K. The role of heat shock proteins in gastrointestinal diseases. Gut 2009, 58, 1000–1009. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Linder, M.; von Strandmann, E.P. The Role of Extracellular HSP70 in the Function of Tumor-Associated Immune Cells. Cancers 2021, 13, 4721. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kumar, S.; Mohan, V.; Singh, R.K.; Gautam, P.K.; Kumar, S.; Shukla, A.; Patel, A.K.; Yadav, L.; Acharya, A. Tumor-derived Hsp70-CD14 interaction enhances the antitumor potential of cytotoxic T cells by activating tumor-associated macrophages to express CC chemokines and CD40 costimulatory molecules. Int. Immunopharmacol. 2024, 138, 112584. [Google Scholar] [CrossRef]
- O’Donoghue, E.J.; Krachler, A.M. Mechanisms of outer membrane vesicle entry into host cells. Cell Microbiol. 2016, 18, 1508–1517. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dalbanjan, N.P.; Kadapure, A.J.; Kumar, S.K.P. A comprehensive review on latent role of stress proteins in antibiotic resistance. Microbe 2024, 4, 100151. [Google Scholar] [CrossRef]
- Brand, C.; Newton-Foot, M.; Grobbelaar, M.; Whitelaw, A. Antibiotic-induced stress responses in Gram-negative bacteria and their role in antibiotic resistance. J. Antimicrob. Chemother. 2025, 80, 1165–1184. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Cao, Y.; Cao, L. Novel therapeutic regimens against Helicobacter pylori: An updated systematic review. Front. Microbiol. 2024, 15, 1418129. [Google Scholar] [CrossRef] [PubMed]
- Zügel, U.; Kaufmann, S.H. Role of heat shock proteins in protection from and pathogenesis of infectious diseases. Clin. Microbiol. Rev. 1999, 12, 19–39. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maleki, F.; Khosravi, A.; Nasser, A.; Taghinejad, H.; Azizian, M. Bacterial Heat Shock Protein Activity. J. Clin. Diagn. Res. 2016, 10, BE01–BE03. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arita-Morioka, K.; Yamanaka, K.; Mizunoe, Y.; Ogura, T.; Sugimoto, S. Novel strategy for biofilm inhibition by using small molecules targeting molecular chaperone DnaK. Antimicrob. Agents Chemother. 2015, 59, 633–641. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Figaj, D. The Role of Heat Shock Protein (Hsp) Chaperones in Environmental Stress Adaptation and Virulence of Plant Pathogenic Bacteria. Int. J. Mol. Sci. 2025, 26, 528. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pot, R.G.; Kusters, J.G.; Smeets, L.C.; Van Tongeren, W.; Vandenbroucke-Grauls, C.M.; Bart, A. Interspecies transfer of antibiotic resistance between Helicobacter pylori and Helicobacter acinonychis. Antimicrob. Agents Chemother. 2001, 45, 2975–2976. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lv, B.; Huang, X.; Lijia, C.; Ma, Y.; Bian, M.; Li, Z.; Duan, J.; Zhou, F.; Yang, B.; Qie, X.; et al. Heat shock potentiates aminoglycosides against gram-negative bacteria by enhancing antibiotic uptake, protein aggregation, and ROS. Proc. Natl. Acad. Sci. USA 2023, 120, e2217254120. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Murshid, A.; Gong, J.; Stevenson, M.A.; Calderwood, S.K. Heat shock proteins and cancer vaccines: Developments in the past decade and chaperoning in the decade to come. Expert Rev. Vaccines 2011, 10, 1553–1568. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hussain, T.; Wang, J.; Murtaza, G.; Metwally, E.; Yang, H.; Kalhoro, M.S.; Kalhoro, D.H.; Rahu, B.A.; Tan, B.; Sahito, R.G.A.; et al. The Role of Polyphenols in Regulation of Heat Shock Proteins and Gut Microbiota in Weaning Stress. Oxidative Med. Cell Longev. 2021, 2021, 6676444. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Role in H. pylori Pathogenesis | Involved HSP(s) | Putative Mechanism of Action | Putative Effects | References |
|---|---|---|---|---|
| Acid stress resistance | GroEL, DnaK | Maintains protein stability, interacts with urease | Neutralizes gastric acidity, promotes survival | [54] |
| Adaptation to temperature variations | HSPs (various) | Maintains protein homeostasis | Ensures bacterial survival under environmental stress | [59] |
| Adhesion and colonization | GroEL | Interaction with host cell integrins | Facilitates bacterial adhesion and colonization | [46] |
| Biofilm formation | GroEL, other chaperonins | Protein stabilization, regulation of biofilm-related genes | Enhances persistence and antibiotic resistance | [47,48,51,52,53] |
| Immune evasion | HSPs (various) | Apoptosis modulation, macrophage inhibition | Promotes bacterial persistence | [65] |
| Immune system modulation | GroEL | Activation of TLR2/TLR4 | Induces chronic inflammation | [60,61] |
| Molecular mimicry | GroEL, GroES | Structural similarity with human HSPs | May trigger autoimmune responses | [62,63,64,65] |
| Oxidative stress resistance | HSPs (various) | Protein stabilization, enhances antioxidant enzyme activity | Protects against ROS, increases bacterial survival | [55,56,57,58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manna, O.M.; Caruso Bavisotto, C.; Gratie, M.I.; Damiani, P.; Tomasello, G.; Cappello, F. The Role of Helicobacter pylori Heat Shock Proteins in Gastric Diseases’ Pathogenesis. Int. J. Mol. Sci. 2025, 26, 5065. https://doi.org/10.3390/ijms26115065
Manna OM, Caruso Bavisotto C, Gratie MI, Damiani P, Tomasello G, Cappello F. The Role of Helicobacter pylori Heat Shock Proteins in Gastric Diseases’ Pathogenesis. International Journal of Molecular Sciences. 2025; 26(11):5065. https://doi.org/10.3390/ijms26115065
Chicago/Turabian StyleManna, Olga Maria, Celeste Caruso Bavisotto, Melania Ionelia Gratie, Provvidenza Damiani, Giovanni Tomasello, and Francesco Cappello. 2025. "The Role of Helicobacter pylori Heat Shock Proteins in Gastric Diseases’ Pathogenesis" International Journal of Molecular Sciences 26, no. 11: 5065. https://doi.org/10.3390/ijms26115065
APA StyleManna, O. M., Caruso Bavisotto, C., Gratie, M. I., Damiani, P., Tomasello, G., & Cappello, F. (2025). The Role of Helicobacter pylori Heat Shock Proteins in Gastric Diseases’ Pathogenesis. International Journal of Molecular Sciences, 26(11), 5065. https://doi.org/10.3390/ijms26115065









