Using an Innovative Bifunctional Siloxane to Protect Cement Composite Surfaces from Biological Corrosion
Abstract
1. Introduction
2. Results
2.1. Synthesis Results
- Product characterization:
- 1H NMR (CDCl3) δ (ppm): −0.08 (6H, (Si(CH2)CH3); −0.04 (18H Si(CH3)3); 0.40 (4H, SiCH2); 1.49 (4H, SiCH2CH2); 3.31 (4H, SiCH2CH2CH2); 3.51 (18H, OCH2CH2), 3.66 (18H OCH2CH2), 6.01 (1H, OH); 7.26 (1H, CF2H)
- 13C NMR (CDCl3) δ (ppm): −0.05 (Si(CH2)CH3); 0.88 (Si(CH3)2); 13.94 (SiCH2CH2CH2), 23.06 (SiCH2CH2CH2); 61.39 (OCH2CH2) 64.15 (CH2O(CF2)3CF2H); 70.33 (OCH2CH2); 105.78 (OCF2CF2CF2CF2H); 107.46 (OCF2CF2CF2CF2H); 109.14 (OCF2CF2CF2CF2H); 115.29 (OCF2CF2CF2CF2H);
- 29Si NMR (CDCl3) δ (ppm): 7.67 (Si(CH3)3); −20.87 (Si(CH3)2);
2.2. Physical and Mechanical Properties of Coated Cement Samples
2.2.1. Density
2.2.2. Water Absorption
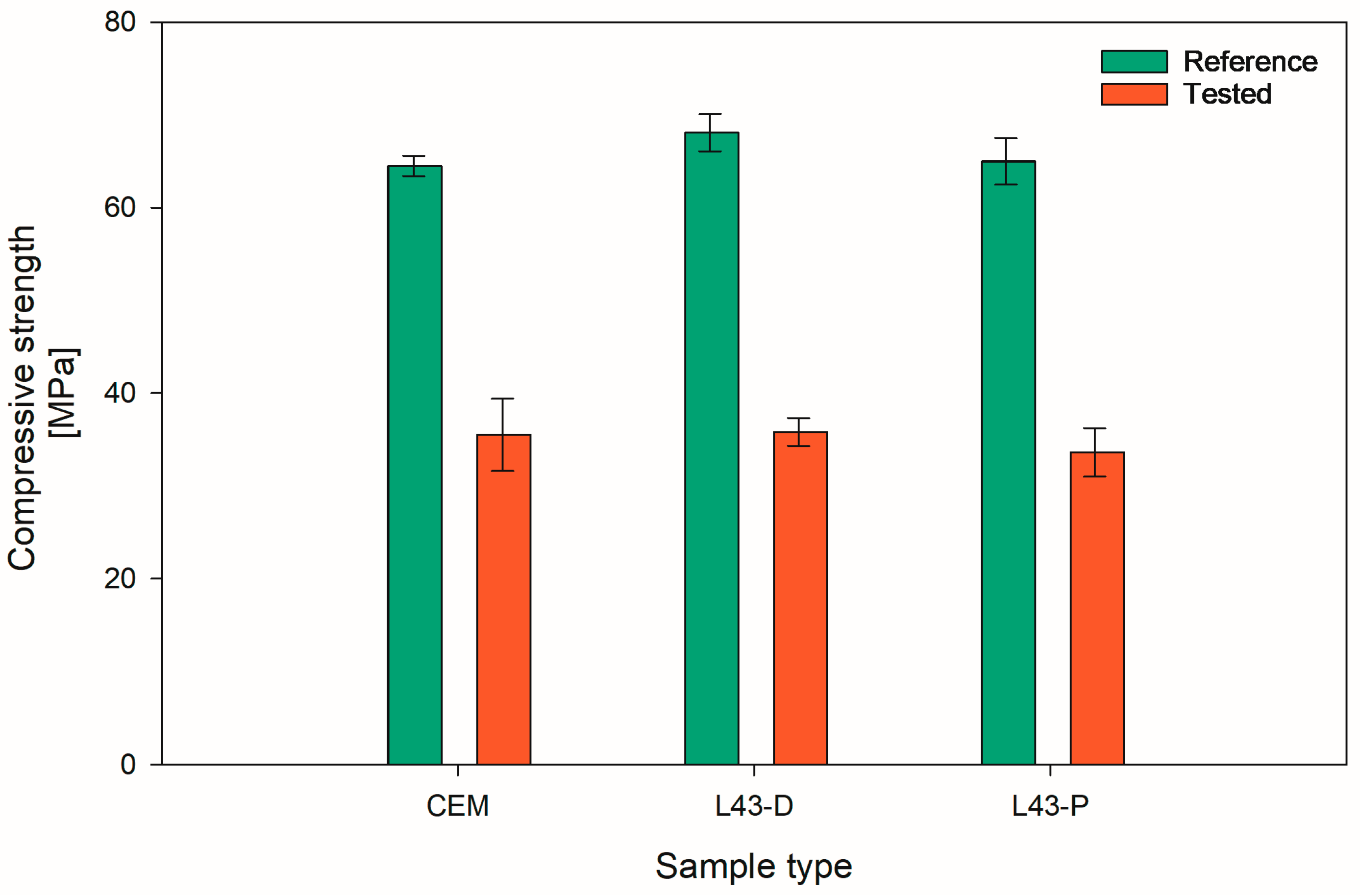
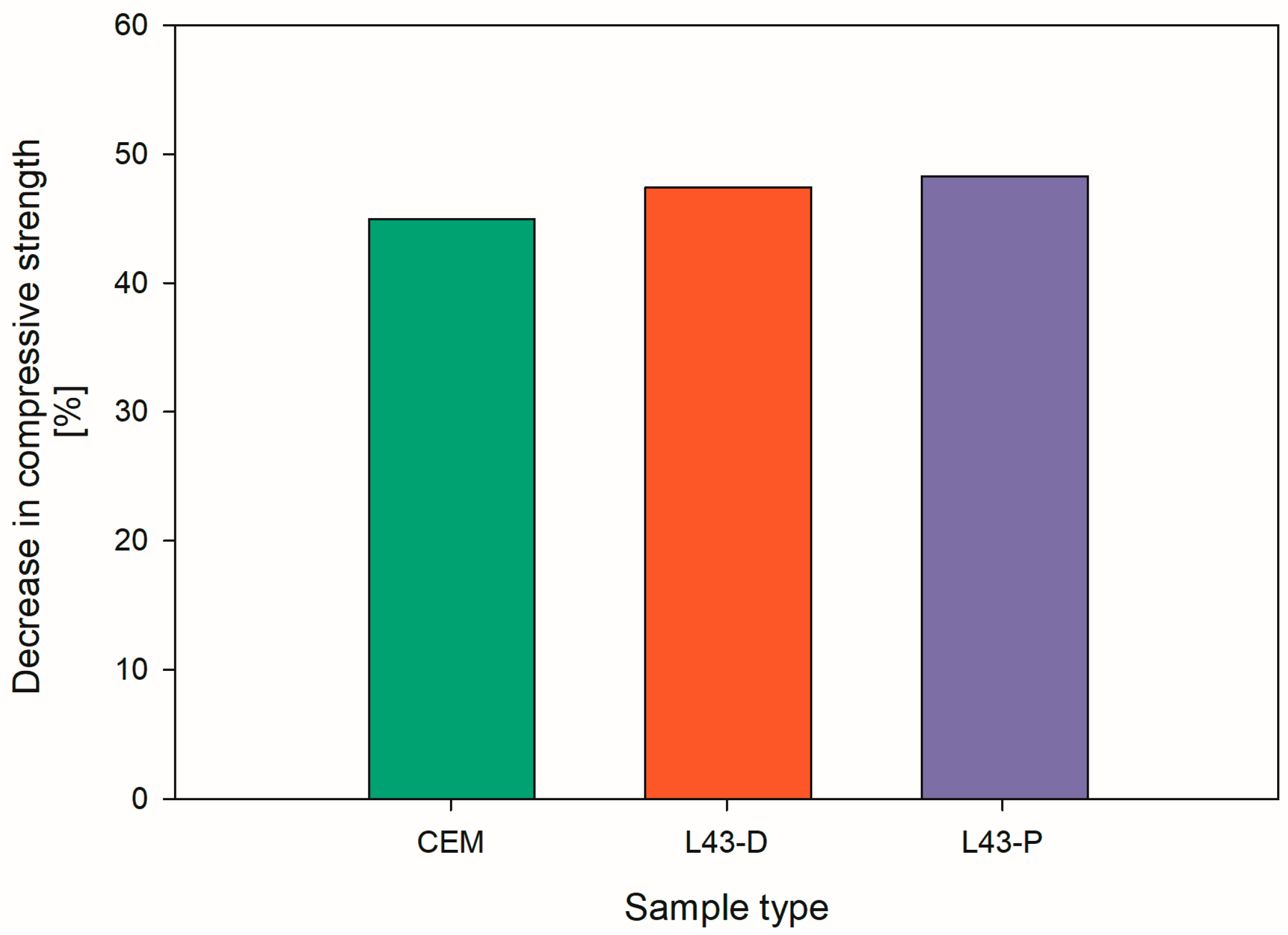
2.2.3. Compressive Strength
2.2.4. Test of Resistance to Cyclic Freezing and Thawing
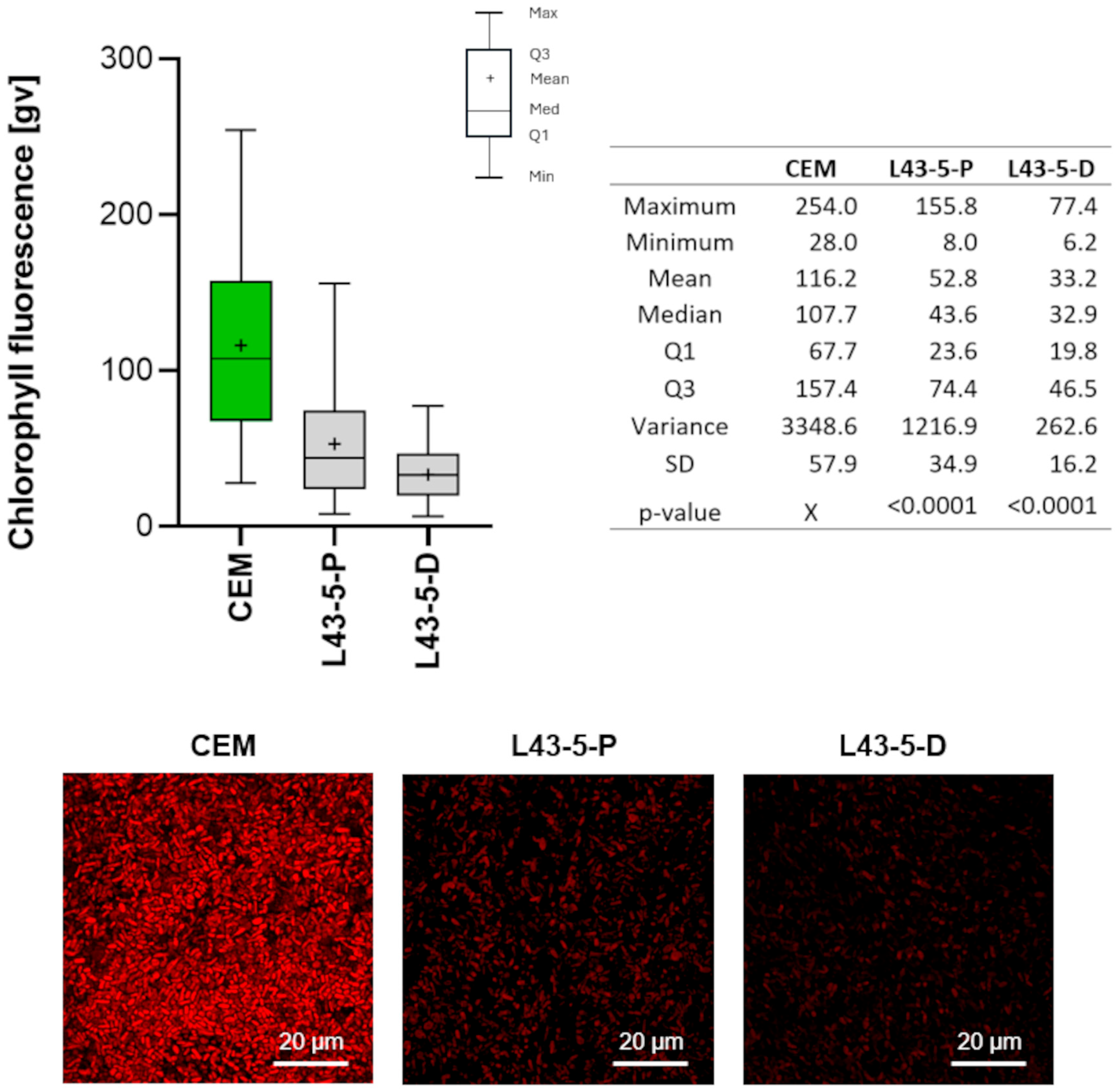
2.2.5. Biological Testing
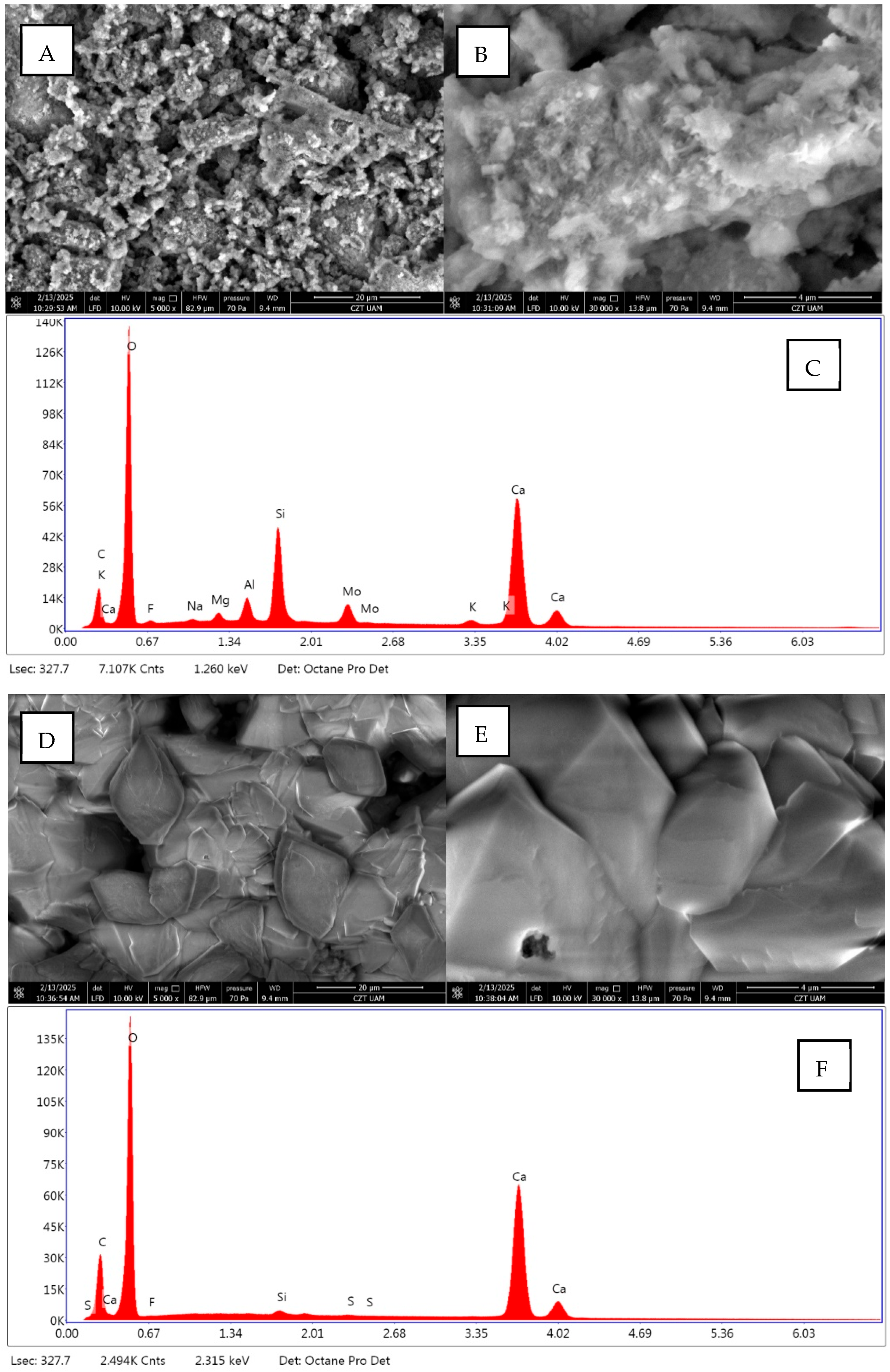
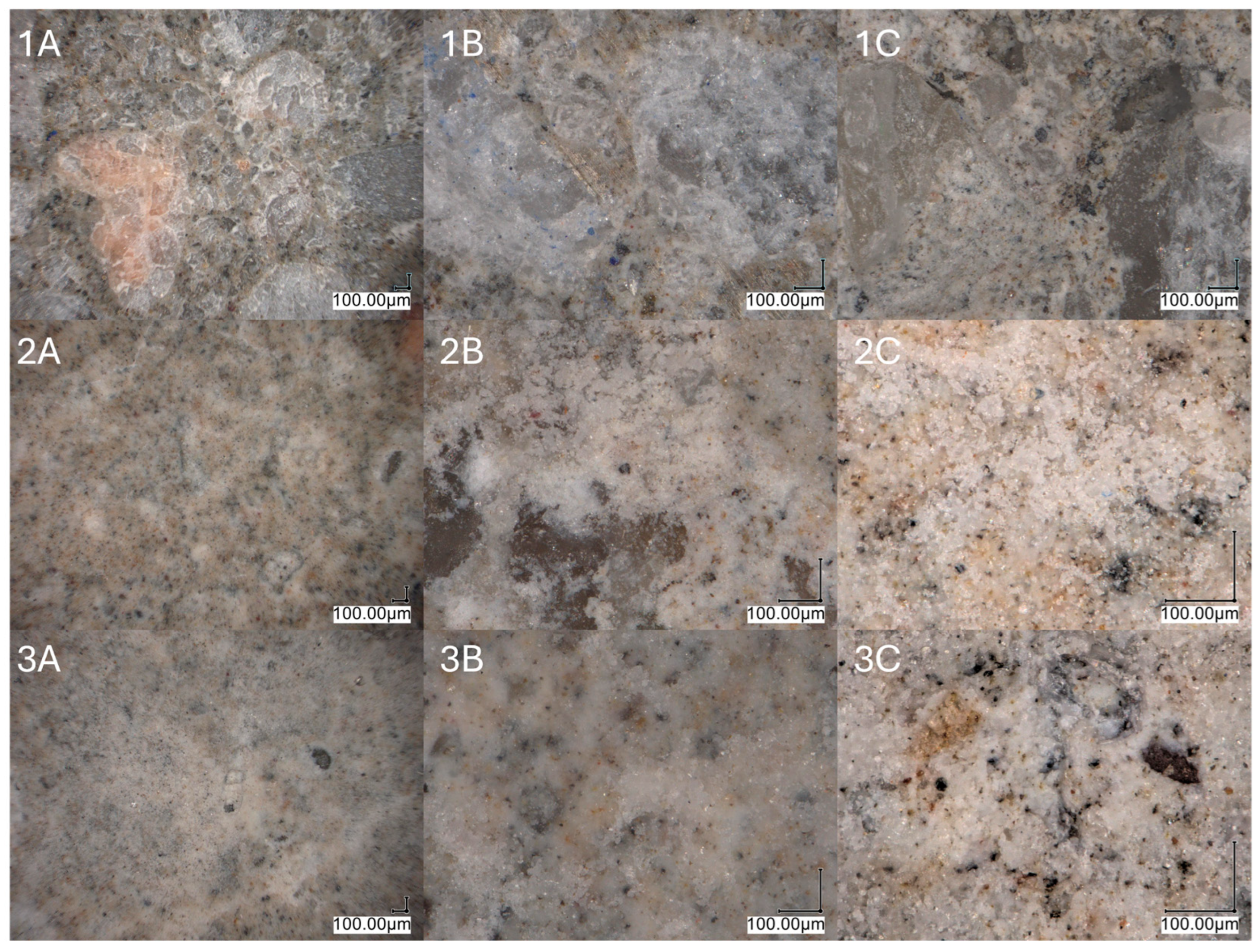
2.2.6. Surface Properties
3. Discussion
4. Materials and Methods
4.1. Materials
4.1.1. Bifunctional Siloxane L43
4.1.2. Mortar Composition
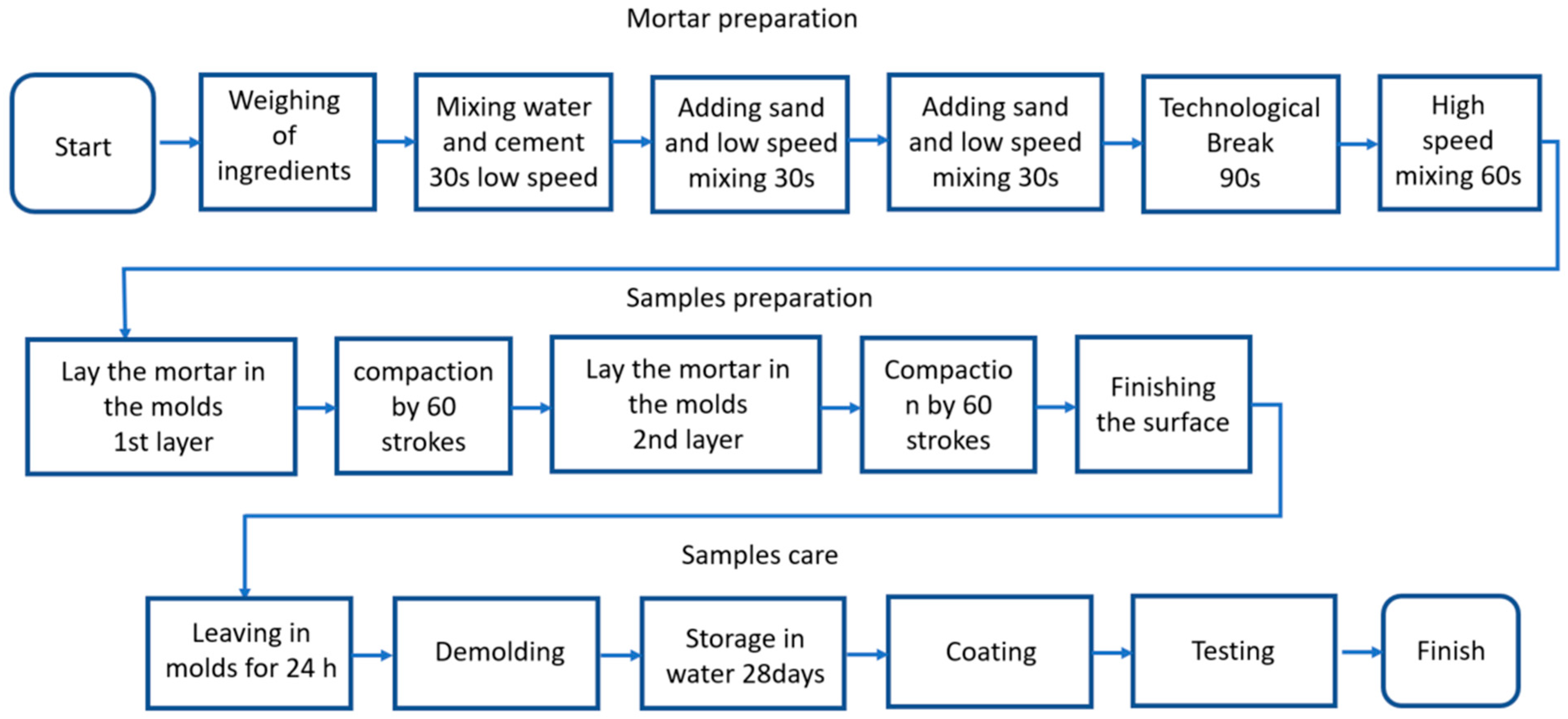
4.1.3. Mortar Preparation
4.1.4. Modification of Specimens with L43 Compound
4.2. Methods
4.2.1. Synthesis of Bifunctional Siloxane L43
4.2.2. Water Absorption
4.2.3. Density
4.2.4. Compressive Strength
4.2.5. Frost Resistance Test According to PN-B-06256 [41]
4.3. Physicochemical Characterization of Bifunctional Siloxane L43
4.3.1. Magnetic Nuclear Resonance Spectra
4.3.2. Mid-Infrared FT-IR Spectrometer
4.3.3. Thermogravimetric Analysis
4.3.4. Differential Scanning Calorimetry
- (1)
- Isotherm at −80 °C for 5 min;
- (2)
- Heating to 200 °C at a rate of 10 °C/min;
- (3)
- Isotherm at 200 °C for 5 min;
- (4)
- Cooling to −80 °C at a rate of −10 °C/min;
4.3.5. Scanning Electron Microscopy (SEM) Analysis
4.3.6. Surface Structures Analysis
4.3.7. Biological Testing
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Erich, S.J.F.; Mendoza, S.M.; Floor, W.; Hermanns, S.P.M.; Homan, W.J.; Adan, O.C.G. Decreased Bio-Inhibition of Building Materials Due to Transport of Biocides. Heron 2011, 56, 93–106. [Google Scholar]
- Graziani, L.; Quagliarini, E.; Osimani, A.; Aquilanti, L.; Clementi, F.; Yéprémian, C.; Lariccia, V.; Amoroso, S.; D’Orazio, M. Evaluation of Inhibitory Effect of TiO2 Nanocoatings against Microalgal Growth on Clay Brick Façades under Weak UV Exposure Conditions. Build. Environ. 2013, 64, 38–45. [Google Scholar] [CrossRef]
- Martinez, T.; Bertron, A.; Escadeillas, G.; Ringot, E. Algal Growth Inhibition on Cement Mortar: Efficiency of Water Repellent and Photocatalytic Treatments under UV/VIS Illumination. Int. Biodeterior. Biodegrad. 2014, 89, 115–1125. [Google Scholar] [CrossRef]
- Cutler, N.A.; Viles, H.A.; Ahmad, S.; McCabe, S.; Smith, B.J. Algal “greening” and the Conservation of Stone Heritage Structures. Sci. Total Environ. 2013, 442, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, W.K. Toxic or Otherwise Harmful Algae and the Built Environment. Toxins 2021, 13, 465. [Google Scholar] [CrossRef]
- Bertron, A. Understanding Interactions between Cementitious Materials and Microorganisms: A Key to Sustainable and Safe Concrete Structures in Various Contexts. Mater. Struct./Mater. Constr. 2014, 47, 1787–1806. [Google Scholar] [CrossRef]
- Edyvean, R.G.J.; Videla, H.A. Biological Corrosion. Interdiscip. Sci. Rev. 1991, 16, 267–282. [Google Scholar] [CrossRef]
- Javaherdashti, R.; Nikraz, H.; Borowitzka, M.A.; Moheimani, N.R.; Olivia, M. On the Impact of Algae on Accelerating the Biodeterioration/Biocorrosion of Reinforced Concrete: A Mechanistic Review. Eur. J. Sci. Res. 2009, 36, 394–406. [Google Scholar]
- Wang, D.; Guan, F.; Feng, C.; Mathivanan, K.; Zhang, R.; Sand, W. Review on Microbially Influenced Concrete Corrosion. Microorganisms 2023, 11, 2076. [Google Scholar] [CrossRef]
- Jayakumar, S.; Saravanane, R. Detrimental Effects on Coastal Concrete by Ulva fasciata. Proc. Inst. Civ. Eng. Constr. Mater. 2010, 163, 239–246. [Google Scholar] [CrossRef]
- Kukletová, I.; Chromková, I. Testing of Algae Colonization Growth Risk on Building Materials. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Blansko-Češkovice, Czech Republic, 29–31 May 2018; Volume 379. [Google Scholar]
- Jayakumar, S.; Saravanane, R. Biodeterioration of Coastal Concrete Structures by Marine Green Algae. Int. J. Civ. Eng. 2010, 8, 352–361. [Google Scholar]
- Nowicka-Krawczyk, P.; Komar, M.; Gutarowska, B. Towards Understanding the Link between the Deterioration of Building Materials and the Nature of Aerophytic Green Algae. Sci. Total Environ. 2022, 802, 149856. [Google Scholar] [CrossRef] [PubMed]
- Rendon Diaz Miron, L.E.; Lara, M.R. The Effect of Microorganisms on Concrete Weathering; Springer: Cham, Switzerland, 2017; ISBN 9783319554631. [Google Scholar]
- Rendon Diaz Miron, L.E.; Lara Magaña, M.E.; Lara, M.R. Microorganisms Concrete Interactions. In Proceedings of the Materials Research Society Symposium Proceedings, Boston, MA, USA, 29 November–4 December 2015; Volume 1768, pp. 25–33. [Google Scholar]
- Fytianos, G.; Banti, D.; Dushku, E.; Papastergiadis, E.; Yiangou, M.; Samaras, P. Novel Approaches for Biocorrosion Mitigation in Sewer Systems. Chemistry 2021, 3, 1166–1177. [Google Scholar] [CrossRef]
- Harilal, M.; Uthaman, S.; George, R.P.; Anandkumar, B.; Thinaharan, C.; Philip, J.; Kamachi Mudali, U. Enhanced Anti-microbial Activity in Green Concrete Specimens Containing Fly Ash, Nanophase Modifiers, and Corrosion Inhibitor. Environ. Prog. Sustain. Energy 2019, 38, 13102. [Google Scholar] [CrossRef]
- Shkromada, O.; Fotina, T.; Dudnyk, Y.; Petrov, R.; Levytska, V.; Chivanov, V.; Bogatko, N.; Pikhtirova, A.; Bordun, O. Reducing the Biogenic Corrosion of Concrete in a Pigsty by Using Disinfectants. East.-Eur. J. Enterp. Technol. 2022, 4, 57–66. [Google Scholar] [CrossRef]
- Alum, A.; Rashid, A.; Mobasher, B.; Abbaszadegan, M. Cement-Based Biocide Coatings for Controlling Algal Growth in Water Distribution Canals. Cem. Concr. Compos. 2008, 30, 839–847. [Google Scholar] [CrossRef]
- Kohzadi, S.; Müller, A.; Österlund, H.; Viklander, M. Building Surface Materials as Potential Sources of Biocides: Insights from Laboratory Leaching Investigations of Different Material Types. Chemosphere 2024, 368, 143741. [Google Scholar] [CrossRef]
- Lidén, C. Pesticides; Springer: Berlin/Heidelberg, Germany, 2011; ISBN 9783642038266. [Google Scholar]
- Hurraß, J.; Messal, C.; Bonner, A. Application of Biocides for Mold Damages|Biozidanwendung Bei Schimmelpilzschäden. Gefahrstoffe Reinhalt. Luft 2018, 78, 31–42. [Google Scholar]
- Nistor, A. Silicones Involved in Surface Modification of Materials Based on Organic Polymers; Nova Science Pub Inc.: Hauppauge, NY, USA, 2010; ISBN 9781616686246. [Google Scholar]
- Wang, H.S.; Li, L.; Ni, Z.W.; Tian, Q. Water Absorption and Resistance to Chloride Ion Performance of Concrete Waterproof Silicone Material. Appl. Mech. Mater. 2013, 357–360, 809–812. [Google Scholar] [CrossRef]
- Wang, H.S.; Wang, W.; Wang, R.; Wang, W.B.; Li, L.; Tian, Q. The Concrete Protective Effect of Silane Impregnated Materials with Different Molecular Chain Structure. Adv. Mater. Res. 2014, 919–921, 439–442. [Google Scholar] [CrossRef]
- Wang, H.S.; Li, L.; Wang, R.; Tian, Q. An Efficient Environmentally-Friendly Materials for Concrete Building Protection. Appl. Mech. Mater. 2013, 368–370, 901–904. [Google Scholar] [CrossRef]
- Wiśniewska, K.A.; Śliwińska-Wilczewska, S.; Lewandowska, A.U. Airborne Microalgal and Cyanobacterial Diversity and Composition during Rain Events in the Southern Baltic Sea Region. Sci. Rep. 2022, 12, 2029. [Google Scholar] [CrossRef] [PubMed]
- Sahu, N.; Tangutur, A.D. Airborne Algae: Overview of the Current Status and Its Implications on the Environment. Aerobiologia 2015, 31, 89–97. [Google Scholar] [CrossRef]
- Hobley, L.; Harkins, C.; MacPhee, C.E.; Stanley-Wall, N.R. Giving Structure to the Biofilm Matrix: An Overview of Individual Strategies and Emerging Common Themes. FEMS Microbiol. Rev. 2015, 39, 649–669. [Google Scholar] [CrossRef]
- Qiu, H.; Feng, K.; Gapeeva, A.; Meurisch, K.; Kaps, S.; Li, X.; Yu, L.; Mishra, Y.K.; Adelung, R.; Baum, M. Functional Polymer Materials for Modern Marine Biofouling Control. Prog. Polym. Sci. 2022, 127, 101516. [Google Scholar] [CrossRef]
- Callow, J.A.; Callow, M.E. Trends in the Development of Environmentally Friendly Fouling-Resistant Marine Coatings. Nat. Commun. 2011, 2, 244. [Google Scholar] [CrossRef]
- Wang, Y.; Pitet, L.M.; Finlay, J.A.; Brewer, L.H.; Cone, G.; Betts, D.E.; Callow, M.E.; Callow, J.A.; Wendt, D.E.; Hillmyer, M.A.; et al. Investigation of the Role of Hydrophilic Chain Length in Amphiphilic Perfluoropolyether/Poly(Ethylene Glycol) Networks: Towards High-Performance Antifouling Coatings. Biofouling 2011, 27, 1139–1150. [Google Scholar] [CrossRef]
- Karasiewicz, J.; Olszyński, R.M.; Nowicka-Krawczyk, P.; Krawczyk, J.; Majchrzycki, Ł. Siloxane Containing Polyether Groups—Synthesis Use as an Anti-Biocorrosion Coating. Int. J. Mol. Sci. 2024, 25, 6801. [Google Scholar] [CrossRef]
- PN-EN 197-1; Cement—Part 1: Composition, Specifications and Conformity Criteria for Common Cements. Polski Komitet Normalizacyjny: Warszawa, Poland, 2013.
- PN-EN 196-2; Method of Testing Cement—Part 2: Chemical Analysis of Cement. Polski Komitet Normalizacyjny: Warszawa, Poland, 2013.
- PN-EN 196-1; Methods of Testing Cement—Part 1: Determination of Strength. Polski Komitet Normalizacyjny: Warszawa, Poland, 2016.
- PN-EN 1008:2004; Mixing Water for Concrete—Specification for Sampling, Testing and Assessing the Suitability of Water, Including Water Recovered from Processes in the Concrete Industry, as Mixing Water for Concrete. Polski Komitet Normalizacyjny: Warszawa, Poland, 2004.
- Karasiewicz, J.; Ślosarczyk, A.; Thomas, M.; Olszyński, R. Component Based on Bifunctional Siloxane and Isopropanol, How to Produce the Component and How to Use It for Surface Impregnation. P.451000, 2025. [Google Scholar]
- PN-B-04500; Building Mortars: Testing of Physical and Mechanical Properties. Polski Komitet Normalizacji, Miar i Jakości: Warszawa, Poland, 1985.
- PN-EN 1015; Methods of Test for Mortar for Masonry—Part 10: Determination of Dry Bulk Density of Hardened Mortar. Polski Komitet Normalizacyjny: Warszawa, Poland, 2001.
- PN-B-06256; Abrasion Resistant Concrete. Polski Komitet Normalizacji, Miar i Jakości: Warszawa, Poland, 1983.
- Nowicka-Krawczyk, P.; Żelazna-Wieczorek, J.; Koziróg, A.; Otlewska, A.; Rajkowska, K.; Piotrowska, M.; Gutarowska, B.; Brycki, B. Multistep Approach to Control Microbial Fouling of Historic Building Materials by Aerial Phototrophs. Biofouling 2019, 35, 284–298. [Google Scholar] [CrossRef]

| Type of Sample | Chemical Properties | ||||
|---|---|---|---|---|---|
| SO3 [%] | Cl− [%] | Roasting Loss [%] | Insoluble Residues [%] | NaOeq [%] | |
| CEM I 42.5 R | 2.63 | 0.040 | 2.48 | 0.68 | 0.61 |
| Type of Sample | Mechanical Properties | Physical Properties | ||||
|---|---|---|---|---|---|---|
| Compressive Strength After 2 Days [MPa] | Compressive Strength After 28 Days [MPa] | Setting Time [min] | Water to Normal Consistency [%] | Volume Constancy [mm] | Specific Surface [cm2/g] | |
| CEM I 42.5 R | 25.4 | 59.3 | 204 | 27.9 | 0.8 | 3713 |
| Type of Sample | Chemical Components [%] | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Roasting Loss | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | NaO2 | K2O | |
| CEM I 42.5 R | <1.0 | 17.6 | 4.3 | 3.9 | 67.2 | 1.5 | 3.8 | 0.2 | 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, M.; Karasiewicz, J.; Nowicka-Krawczyk, P.; Olszyński, R.M.; Balcerzak, Ł.; Frydrych, M.; Sztorch, B.; Ślosarczyk, A. Using an Innovative Bifunctional Siloxane to Protect Cement Composite Surfaces from Biological Corrosion. Int. J. Mol. Sci. 2025, 26, 5052. https://doi.org/10.3390/ijms26115052
Thomas M, Karasiewicz J, Nowicka-Krawczyk P, Olszyński RM, Balcerzak Ł, Frydrych M, Sztorch B, Ślosarczyk A. Using an Innovative Bifunctional Siloxane to Protect Cement Composite Surfaces from Biological Corrosion. International Journal of Molecular Sciences. 2025; 26(11):5052. https://doi.org/10.3390/ijms26115052
Chicago/Turabian StyleThomas, Marta, Joanna Karasiewicz, Paulina Nowicka-Krawczyk, Rafał M. Olszyński, Łucja Balcerzak, Miłosz Frydrych, Bogna Sztorch, and Agnieszka Ślosarczyk. 2025. "Using an Innovative Bifunctional Siloxane to Protect Cement Composite Surfaces from Biological Corrosion" International Journal of Molecular Sciences 26, no. 11: 5052. https://doi.org/10.3390/ijms26115052
APA StyleThomas, M., Karasiewicz, J., Nowicka-Krawczyk, P., Olszyński, R. M., Balcerzak, Ł., Frydrych, M., Sztorch, B., & Ślosarczyk, A. (2025). Using an Innovative Bifunctional Siloxane to Protect Cement Composite Surfaces from Biological Corrosion. International Journal of Molecular Sciences, 26(11), 5052. https://doi.org/10.3390/ijms26115052








