Effects of Resveratrol Derivatives on Melanogenesis and Antioxidant Activity in B16F10 Cells
Abstract
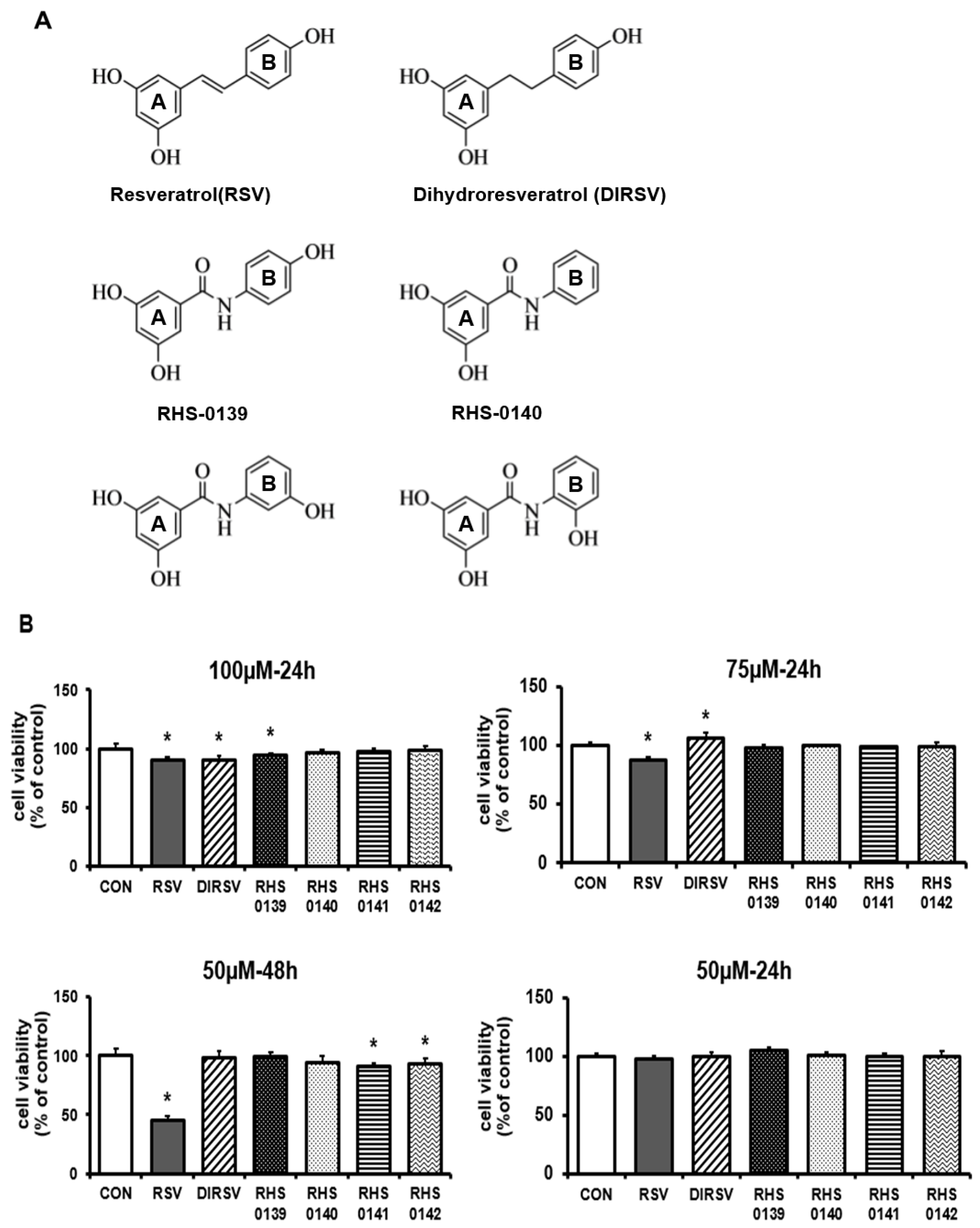
1. Introduction
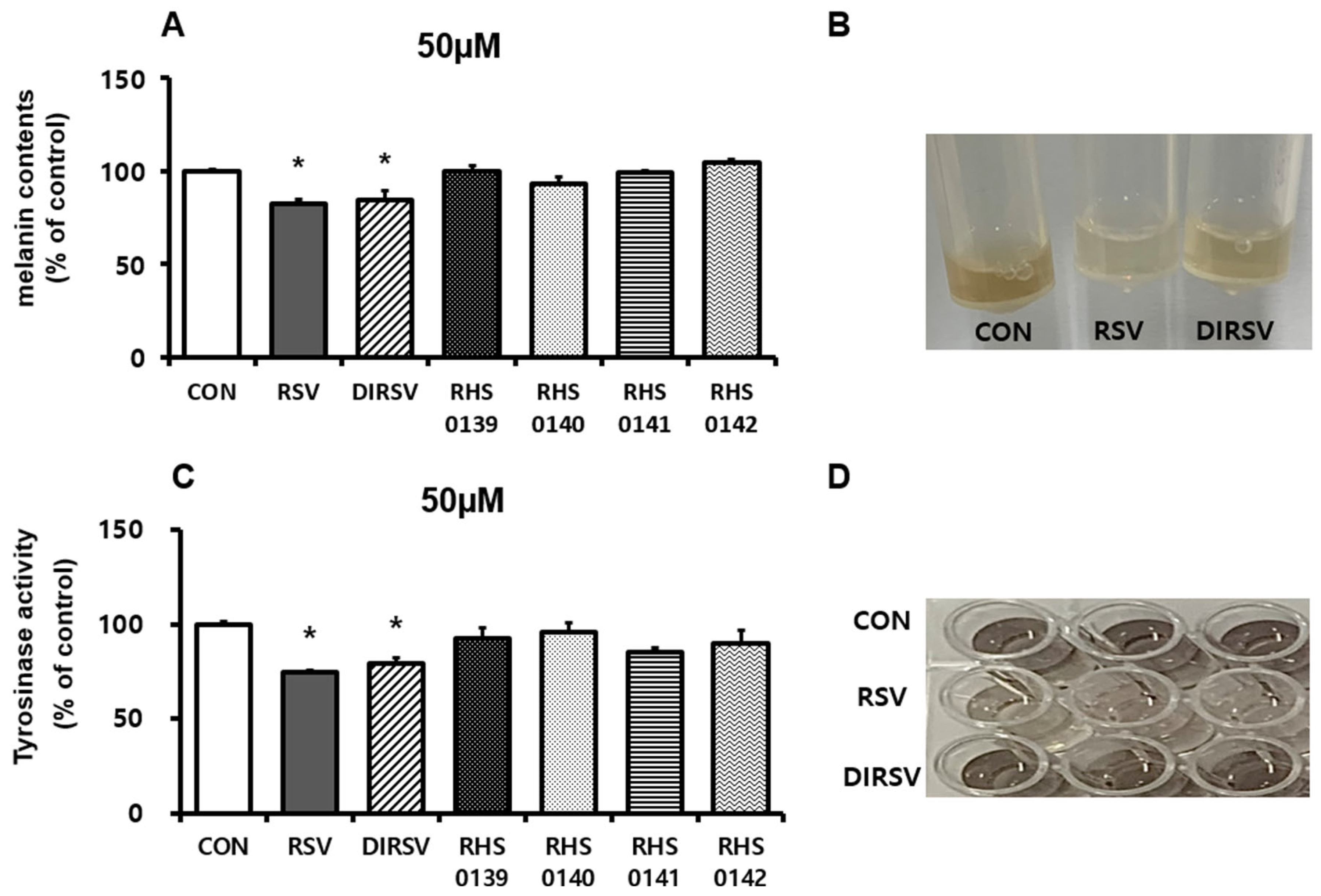
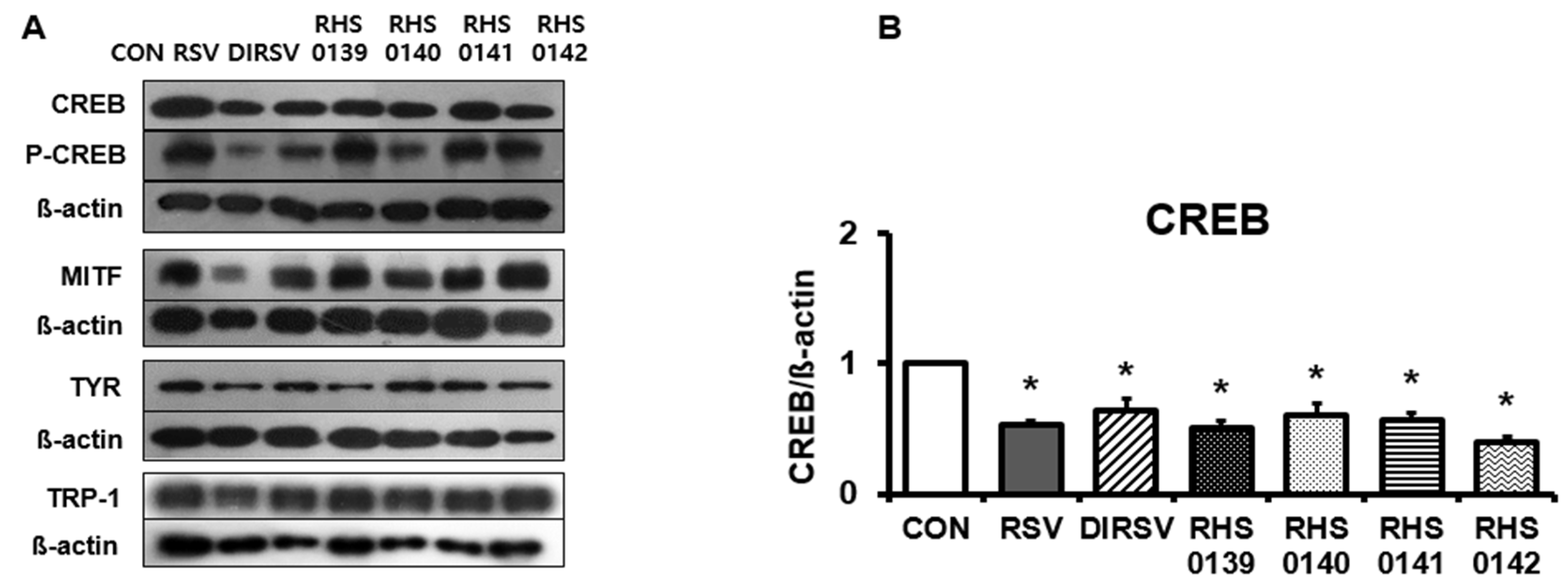
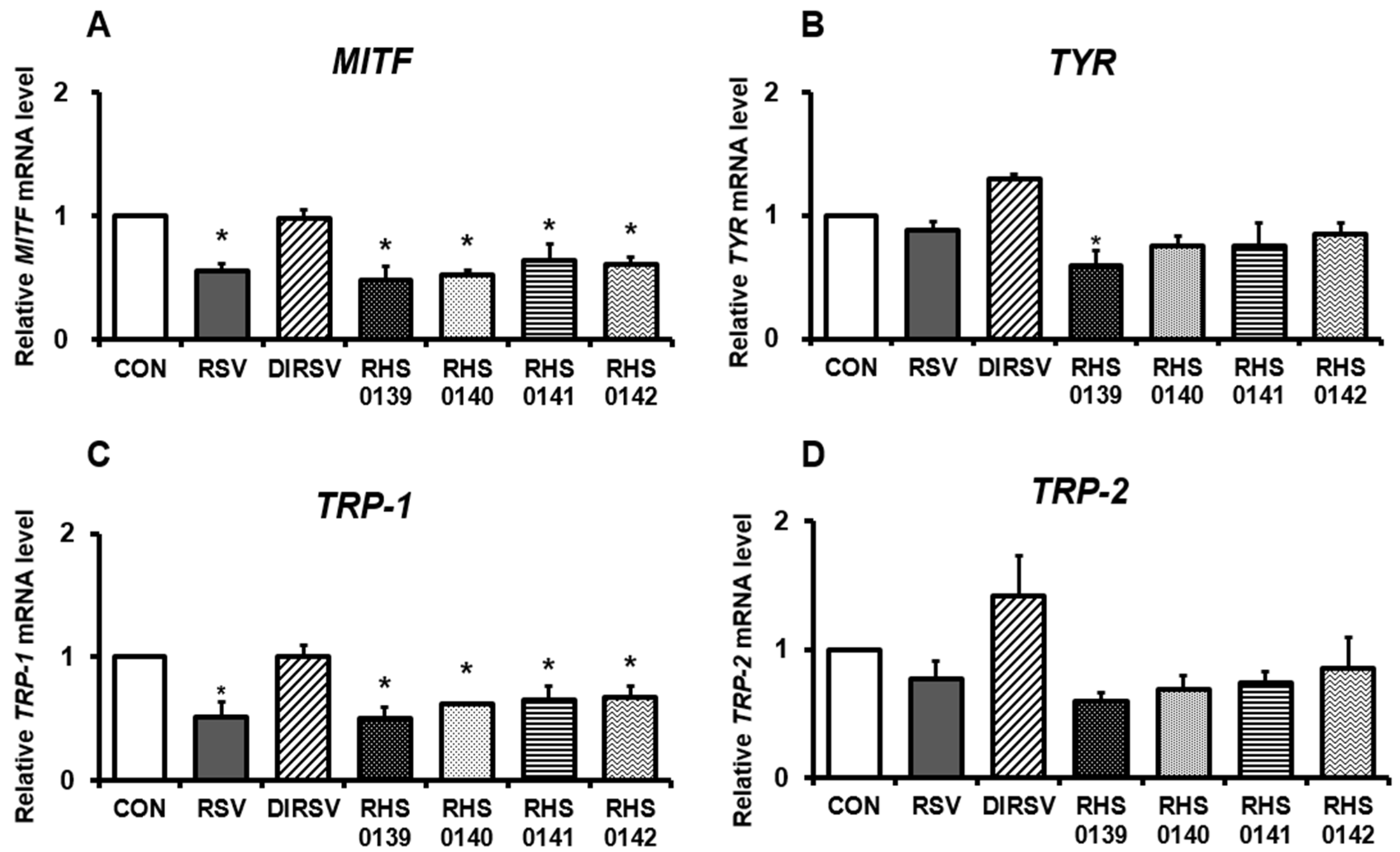
2. Results
3. Discussion
4. Materials and Methods
4.1. Preparation of RSV and Its Derivatives
4.2. Cell Culture and Sample Treatment
4.3. Cell Viability
4.4. Measurement of Melanin Content and Tyrosinase Activity
4.5. Molecular Docking
4.6. Western Blotting
4.7. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
4.8. Measurement of 1,1-Diphenyl-2-Picrylhydrazyl (DPPH) and 2,2′-Azino-Bis(3-Ethylbenzothiazoline-6-Sulfonic Acid (ABTS)) Radical Scavenging Activity
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulphonic acid) |
| cAMP | Cyclic Adenosine Monophosphate |
| CREB | cAMP response element-binding protein |
| DIRSV | Dihydroresveratrol |
| DMSO | Dimethyl sulfoxide |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| GCLC | Glutamate-cysteine ligase catalytic subunit |
| GCLM | Glutamate-cysteine ligase regulatory subunit |
| H2O2 | hydrogen peroxide |
| HO-1 | Heme Oxygenase-1 |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| MAPK | mitogen-activated protein kinase |
| MC1R | Melanocortin 1 receptor |
| MITF | Microphthalmia-associated transcription factor |
| NRF2 | Nuclear factor erythroid 2-related factor 2 |
| NQO1 | NAD(P)H dehydrogenase 1 |
| p-CREB | Phosphorylated CREB |
| PBS | Phosphate-buffered saline |
| PKA | Protein Kinase A |
| ROS | Reactive oxygen species |
| RSV | Resveratrol |
| SIRT1 | Sirtuin 1 |
| TRP-1 | Tyrosinase related protein-1 |
| TRP-2 | Tyrosinase related protein-2 |
| TRPs | Tyrosinase Related Proteins |
| UV | ultraviolet |
| Wnt/β-catenin | Wnt/β-catenin Signaling Pathway |
| α-MSH | α-Melanocyte-stimulating hormone |
| β-catenin | catenin beta-1 |
References
- Guo, L.; Li, W.; Gu, Z.; Wang, L.; Guo, L.; Ma, S.; Li, C.; Sun, J.; Han, B.; Chang, J. Recent Advances and Progress on Melanin: From Source to Application. Int. J. Mol. Sci. 2023, 24, 4360. [Google Scholar] [CrossRef] [PubMed]
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Hill, H.Z.; Li, W.; Xin, P.; Mitchell, D.L. Melanin: A two edged sword? Pigment Cell Res. 1997, 10, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Maranduca, M.A.; Branisteanu, D.; Serban, D.N.; Branisteanu, D.C.; Stoleriu, G.; Manolache, N.; Serban, I.L. Synthesis and physiological implications of melanic pigments. Oncol. Lett. 2019, 17, 4183–4187. [Google Scholar] [CrossRef]
- Hida, T.; Kamiya, T.; Kawakami, A.; Ogino, J.; Sohma, H.; Uhara, H.; Jimbow, K. Elucidation of Melanogenesis Cascade for Identifying Pathophysiology and Therapeutic Approach of Pigmentary Disorders and Melanoma. Int. J. Mol. Sci. 2020, 21, 6129. [Google Scholar] [CrossRef]
- Vachtenheim, J.; Borovanský, J. “Transcription physiology” of pigment formation in melanocytes: Central role of MITF. Exp. Dermatol. 2010, 19, 617–627. [Google Scholar] [CrossRef]
- Kim, K.; Yoon, J.; Lim, K.M. Syringaresinol Attenuates α-Melanocyte-Stimulating Hormone-Induced Reactive Oxygen Species Generation and Melanogenesis. Antioxidants 2024, 13, 876. [Google Scholar] [CrossRef]
- Searle, T.; Al-Niaimi, F.; Ali, F.R. The top 10 cosmeceuticals for facial hyperpigmentation. Dermatol. Ther. 2020, 33, e14095. [Google Scholar] [CrossRef]
- Kim, H.; Choi, H.R.; Kim, D.S.; Park, K.C. Topical hypopigmenting agents for pigmentary disorders and their mechanisms of action. Ann. Dermatol. 2012, 24, 1–6. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gover, M.D.; Nouri, K.; Taylor, S. The treatment of melasma: A review of clinical trials. J. Am. Acad. Dermatol. 2006, 55, 1048–1065. [Google Scholar] [CrossRef]
- Lee, T.H.; Seo, J.O.; Baek, S.H.; Kim, S.Y. Inhibitory effects of resveratrol on melanin synthesis in ultraviolet B-induced pigmentation in Guinea pig skin. Biomol. Ther. 2014, 22, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Denat, L.; Kadekaro, A.L.; Marrot, L.; Leachman, S.A.; Abdel-Malek, Z.A. Melanocytes as instigators and victims of oxidative stress. J. Investig. Dermatol. 2014, 134, 1512–1518. [Google Scholar] [CrossRef] [PubMed]
- Koga, S.; Nakano, M.; Tero-Kubota, S. Generation of superoxide during the enzymatic action of tyrosinase. Arch. Biochem. Biophys. 1992, 292, 570–575. [Google Scholar] [CrossRef]
- Tomita, Y.; Hariu, A.; Kato, C.; Seiji, M. Radical production during tyrosinase reaction, dopa-melanin formation, and photoirradiation of dopa-melanin. J. Investig. Dermatol. 1984, 82, 573–576. [Google Scholar] [CrossRef]
- Song, X.; Mosby, N.; Yang, J.; Xu, A.; Abdel-Malek, Z.; Kadekaro, A.L. alpha-MSH activates immediate defense responses to UV-induced oxidative stress in human melanocytes. Pigment Cell Melanoma Res. 2009, 22, 809–818. [Google Scholar] [CrossRef]
- Gęgotek, A.; Skrzydlewska, E. The role of transcription factor Nrf2 in skin cells metabolism. Arch. Dermatol. Res. 2015, 307, 385–396. [Google Scholar] [CrossRef]
- Zgorzynska, E.; Dziedzic, B.; Walczewska, A. An Overview of the Nrf2/ARE Pathway and Its Role in Neurodegenerative Diseases. Int. J. Mol. Sci. 2021, 22, 9592. [Google Scholar] [CrossRef] [PubMed]
- Rodboon, T.; Palipoch, S.; Okada, S.; Charoenchon, N.; Nakornpakdee, Y.; Suwannalert, P. Oxyresveratrol inhibits cellular tyrosinase-related oxidative stress-induced melanogenesis in B16 melanoma cells. J. Appl. Pharm. Sci. 2020, 10, 008–013. [Google Scholar]
- Athar, M.; Back, J.H.; Kopelovich, L.; Bickers, D.R.; Kim, A.L. Multiple molecular targets of resveratrol: Anti-carcinogenic mechanisms. Arch. Biochem. Biophys. 2009, 486, 95–102. [Google Scholar] [CrossRef]
- Zordoky, B.N.; Robertson, I.M.; Dyck, J.R. Preclinical and clinical evidence for the role of resveratrol in the treatment of cardiovascular diseases. Biochim. Biophys. Acta 2015, 1852, 1155–1177. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Wang, Y.; Chao, Y.; Zhang, J.; Jia, Y.; Tie, J.; Hu, D. Regulation of SIRT1 and Its Roles in Inflammation. Front. Immunol. 2022, 13, 831168. [Google Scholar] [CrossRef] [PubMed]
- Pyo, I.S.; Yun, S.; Yoon, Y.E.; Choi, J.W.; Lee, S.J. Mechanisms of Aging and the Preventive Effects of Resveratrol on Age-Related Diseases. Molecules 2020, 25, 4649. [Google Scholar] [CrossRef] [PubMed]
- Vidavalur, R.; Otani, H.; Singal, P.K.; Maulik, N. Significance of wine and resveratrol in cardiovascular disease: French paradox revisited. Exp. Clin. Cardiol. 2006, 11, 217–225. [Google Scholar] [PubMed]
- Chen, Y.J.; Chen, Y.Y.; Lin, Y.F.; Hu, H.Y.; Liao, H.F. Resveratrol inhibits alpha-melanocyte-stimulating hormone signaling, viability, and invasiveness in melanoma cells. Evid. Based Complement. Alternat. Med. 2013, 2013, 632121. [Google Scholar] [CrossRef]
- Yoon, H.S.; Hyun, C.G.; Lee, N.H.; Park, S.S.; Shin, D.B. Comparative Depigmentation Effects of Resveratrol and Its Two Methyl Analogues in α-Melanocyte Stimulating Hormone-Triggered B16/F10 Murine Melanoma Cells. Prev. Nutr. Food Sci. 2016, 21, 155–159. [Google Scholar] [CrossRef]
- Zhou, S.; Riadh, D.; Sakamoto, K. Grape Extract Promoted α-MSH-Induced Melanogenesis in B16F10 Melanoma Cells, Which Was Inverse to Resveratrol. Molecules 2021, 26, 5959. [Google Scholar] [CrossRef]
- Queiroz, A.N.; Gomes, B.A.; Moraes, W.M., Jr.; Borges, R.S. A theoretical antioxidant pharmacophore for resveratrol. Eur. J. Med. Chem. 2009, 44, 1644–1649. [Google Scholar] [CrossRef]
- Francioso, A.; Mastromarino, P.; Masci, A.; d’Erme, M.; Mosca, L. Chemistry, stability and bioavailability of resveratrol. Med. Chem. 2014, 10, 237–245. [Google Scholar] [CrossRef]
- Zhu, Y.; Pan, W.H.; Ku, C.F.; Zhang, H.J.; Tsang, S.W. Design, synthesis and evaluation of novel dihydrostilbene derivatives as potential anti-melanogenic skin-protecting agents. Eur. J. Med. Chem. 2018, 143, 1254–1260. [Google Scholar] [CrossRef]
- Oh, Y.S.; Shin, S.Y.; Kim, S.; Lee, K.H.; Shin, J.C.; Park, K.M. Comparison of antiaging, anti-melanogenesis effects, and active components of Raspberry (Rubus occidentalis L.) extracts according to maturity. J. Food Biochem. 2020, 44, e13464. [Google Scholar] [CrossRef]
- Promden, W.; Chanvorachote, P.; Viriyabancha, W.; Sintupachee, S.; De-Eknamkul, W. Maclura cochinchinensis (Lour.) Corner Heartwood Extracts Containing Resveratrol and Oxyresveratrol Inhibit Melanogenesis in B16F10 Melanoma Cells. Molecules 2024, 29, 2473. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.H.; Hung, C.F.; Sung, H.C.; Yang, S.C.; Yu, H.P.; Fang, J.Y. The bioactivities of resveratrol and its naturally occurring derivatives on skin. J. Food Drug Anal. 2021, 29, 15–38. [Google Scholar] [CrossRef]
- Faloon, P.W.; Bennion, M.; Weiner, W.S.; Smith, R.A.; Wurst, J.; Weiwer, M.; Hartland, C.; Mosher, C.M.; Johnston, S.; Porubsky, P.; et al. A Small Molecule Inhibitor of the MITF Molecular Pathway. In Probe Reports from the NIH Molecular Libraries Program; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2010. [Google Scholar]
- Best, J.L.; Amezcua, C.A.; Mayr, B.; Flechner, L.; Murawsky, C.M.; Emerson, B.; Zor, T.; Gardner, K.H.; Montminy, M. Identification of small-molecule antagonists that inhibit an activator: Coactivator interaction. Proc. Natl. Acad. Sci. USA 2004, 101, 17622–17627. [Google Scholar] [CrossRef]
- Varga, K.; Paszternák, A.; Kovács, V.; Guczogi, A.; Sikur, N.; Patakfalvi, D.; Bagaméry, F.; Szökő, É.; Tábi, T. Differential Cytoprotective Effect of Resveratrol and Its Derivatives: Focus on Antioxidant and Autophagy-Inducing Effects. Int. J. Mol. Sci. 2024, 25, 11274. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Yan, X. From resveratrol to its derivatives: New sources of natural antioxidant. Curr. Med. Chem. 2013, 20, 1005–1017. [Google Scholar]
- Xu, Y.; Yu, P.; Liang, J.; Chen, Y.; Yang, C.; Xia, C.; Deng, J.; Hai, L.; Chen, J.; Wu, Y. Synthesis and bioactivity evaluation of glycosylated resveratrol derivatives as antioxidative neuroprotection agents against cerebral Ischemia-Reperfusion injury. Bioorg. Chem. 2024, 153, 107791. [Google Scholar] [CrossRef]
- Kondratyuk, T.P.; Park, E.J.; Marler, L.E.; Ahn, S.; Yuan, Y.; Choi, Y.; Yu, R.; van Breemen, R.B.; Sun, B.; Hoshino, J.; et al. Resveratrol derivatives as promising chemopreventive agents with improved potency and selectivity. Mol. Nutr. Food Res. 2011, 55, 1249–1265. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.Y.; Shahidi, F. Antioxidant activity of resveratrol ester derivatives in food and biological model systems. Food Chem. 2018, 261, 267–273. [Google Scholar] [CrossRef]
- Seung Kug, C.; Hyun-Seuk, M. The Role of Resveratrol in Lipid Metabolism: A Systematic Review of Current Basic and Translational Evidence. J. Food Hyg. Saf. 2016, 31, 67–73. [Google Scholar]
- Wenzel, E.; Somoza, V. Metabolism and bioavailability of trans-resveratrol. Mol. Nutr. Food Res. 2005, 49, 472–481. [Google Scholar] [CrossRef]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E., Jr.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Kaldas, M.I.; Walle, U.K.; Walle, T. Resveratrol transport and metabolism by human intestinal Caco-2 cells. J. Pharm. Pharmacol. 2003, 55, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.G.; Ma, M.H.; Hu, X.M.; Zhang, Y.J.; Xu, X.H.; Nian, H. Detection and structural characterization of the metabolites of dihydroresveratrol in rats by liquid chromatography coupled to high-resolution tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2021, 35, e8991. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Malcangi, G.; Inchingolo, A.M.; Piras, F.; Settanni, V.; Garofoli, G.; Palmieri, G.; Ceci, S.; Patano, A.; De Leonardis, N.; et al. Benefits and Implications of Resveratrol Supplementation on Microbiota Modulations: A Systematic Review of the Literature. Int. J. Mol. Sci. 2022, 23, 4027. [Google Scholar] [CrossRef]
- Lam, C.S.; Xia, Y.X.; Chen, B.S.; Du, Y.X.; Liu, K.L.; Zhang, H.J. Dihydro-Resveratrol Attenuates Oxidative Stress, Adipogenesis and Insulin Resistance in In Vitro Models and High-Fat Diet-Induced Mouse Model via AMPK Activation. Nutrients 2023, 15, 3006. [Google Scholar] [CrossRef]
- Hassan, W.; Noreen, H.; Rehman, S.; Gul, S.; Kamal, M.A.; Kamdem, J.P.; Zaman, B.; da Rocha, J.B.T. Oxidative Stress and Antioxidant Potential of One Hundred Medicinal Plants. Curr. Top. Med. Chem. 2017, 17, 1336–1370. [Google Scholar] [CrossRef] [PubMed]
- Oode, C.; Shimada, W.; Izutsu, Y.; Yokota, M.; Iwadate, T.; Nihei, K. Synthesis of dihydroresveratrol glycosides and evaluation of their activity against melanogenesis in B16F0 melanoma cells. Eur. J. Med. Chem. 2014, 87, 862–867. [Google Scholar] [CrossRef]
- Kumar, K.J.S.; Vani, M.G.; Wu, P.C.; Lee, H.J.; Tseng, Y.H.; Wang, S.Y. Essential Oils of Alpinia nantoensis Retard Forskolin-Induced Melanogenesis via ERK1/2-Mediated Proteasomal Degradation of MITF. Plants 2020, 9, 1672. [Google Scholar] [CrossRef]
- Jeon, N.-J.; Kim, Y.-S.; Kim, E.-K.; Dong, X.; Lee, J.-W.; Park, J.-S.; Shin, W.-B.; Moon, S.-H.; Jeon, B.-T.; Park, P.-J. Inhibitory effect of carvacrol on melanin synthesis via suppression of tyrosinase expression. J. Funct. Foods 2018, 45, 199–205. [Google Scholar] [CrossRef]
- Yasumoto, K.; Takeda, K.; Saito, H.; Watanabe, K.; Takahashi, K.; Shibahara, S. Microphthalmia-associated transcription factor interacts with LEF-1, a mediator of Wnt signaling. Embo J. 2002, 21, 2703–2714. [Google Scholar] [CrossRef]
- Zeng, H.J.; Li, Q.Y.; Ma, J.; Yang, R.; Qu, L.B. A comparative study on the effects of resveratrol and oxyresveratrol against tyrosinase activity and their inhibitory mechanism. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 251, 119405. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishna, R.; Aguilar, J.; Oh, A.; Lee, E.; Hou, L.; Lee, T.; Xu, E.; Nguyen, J.; Mack, W.J. Resveratrol and its metabolites elicit neuroprotection via high-affinity binding to the laminin receptor at low nanomolar concentrations. FEBS Lett. 2024, 598, 995–1007. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Sun, P.; Li, X.; Wang, X.; Zhang, X.; Cui, H.; Yang, J. Investigating the Molecular Mechanisms of Resveratrol in Treating Cardiometabolic Multimorbidity: A Network Pharmacology and Bioinformatics Approach with Molecular Docking Validation. Nutrients 2024, 16, 2488. [Google Scholar] [CrossRef]
- Kim, Y.M.; Cho, S.E.; Seo, Y.K. The activation of melanogenesis by p-CREB and MITF signaling with extremely low-frequency electromagnetic fields on B16F10 melanoma. Life Sci. 2016, 162, 25–32. [Google Scholar] [CrossRef]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal 2018, 29, 1727–1745. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Daiber, A.; Förstermann, U.; Li, H. Antioxidant effects of resveratrol in the cardiovascular system. Br. J. Pharmacol. 2017, 174, 1633–1646. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Folgado, S.L.; Pourbagher-Shahri, A.M.; Ashrafizadeh, M.; Samarghandian, S. The therapeutic effect of resveratrol: Focusing on the Nrf2 signaling pathway. Biomed. Pharmacother. 2020, 127, 110234. [Google Scholar] [CrossRef]
- Krajka-Kuźniak, V.; Szaefer, H.; Stefański, T.; Sobiak, S.; Cichocki, M.; Baer-Dubowska, W. The effect of resveratrol and its methylthio-derivatives on the Nrf2-ARE pathway in mouse epidermis and HaCaT keratinocytes. Cell. Mol. Biol. Lett. 2014, 19, 500–516. [Google Scholar] [CrossRef]
- Kim, J.; Oh, J.; Averilla, J.N.; Kim, H.J.; Kim, J.S.; Kim, J.S. Grape Peel Extract and Resveratrol Inhibit Wrinkle Formation in Mice Model Through Activation of Nrf2/HO-1 Signaling Pathway. J. Food Sci. 2019, 84, 1600–1608. [Google Scholar] [CrossRef]
- Chaiprasongsuk, A.; Onkoksoong, T.; Pluemsamran, T.; Limsaengurai, S.; Panich, U. Photoprotection by dietary phenolics against melanogenesis induced by UVA through Nrf2-dependent antioxidant responses. Redox Biol. 2016, 8, 79–90. [Google Scholar] [CrossRef]
- Hu, Z.M.; Zhou, Q.; Lei, T.C.; Ding, S.F.; Xu, S.Z. Effects of hydroquinone and its glucoside derivatives on melanogenesis and antioxidation: Biosafety as skin whitening agents. J. Dermatol. Sci. 2009, 55, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Eskandani, M.; Golchai, J.; Pirooznia, N.; Hasannia, S. Oxidative stress level and tyrosinase activity in vitiligo patients. Indian J. Dermatol. 2010, 55, 15–19. [Google Scholar] [PubMed]
- Laajala, M.; Kalander, K.; Consalvi, S.; Amamuddy, O.S.; Bishop, Ö.T.; Biava, M.; Poce, G.; Marjomäki, V. Antiviral Mechanisms of N-Phenyl Benzamides on Coxsackie Virus A9. Pharmaceutics 2023, 15, 1028. [Google Scholar] [CrossRef] [PubMed]
- Perin, N.; Roškarić, P.; Sović, I.; Boček, I.; Starčević, K.; Hranjec, M.; Vianello, R. Amino-Substituted Benzamide Derivatives as Promising Antioxidant Agents: A Combined Experimental and Computational Study. Chem. Res. Toxicol. 2018, 31, 974–984. [Google Scholar] [CrossRef]
- Xin, M.; Wu, H.; Du, Y.; Liu, S.; Zhao, F.; Mou, X. Synthesis and biological evaluation of resveratrol amide derivatives as selective COX-2 inhibitors. Chem. Biol. Interact. 2023, 380, 110522. [Google Scholar] [CrossRef]
- Cunha, E.S.; Kawahara, R.; Kadowaki, M.K.; Amstalden, H.G.; Noleto, G.R.; Cadena, S.M.; Winnischofer, S.M.; Martinez, G.R. Melanogenesis stimulation in B16-F10 melanoma cells induces cell cycle alterations, increased ROS levels and a differential expression of proteins as revealed by proteomic analysis. Exp. Cell Res. 2012, 318, 1913–1925. [Google Scholar] [CrossRef]
- Chan, Y.Y.; Kim, K.H.; Cheah, S.H. Inhibitory effects of Sargassum polycystum on tyrosinase activity and melanin formation in B16F10 murine melanoma cells. J. Ethnopharmacol. 2011, 137, 1183–1188. [Google Scholar] [CrossRef]
- Wu, P.Y.; You, Y.J.; Liu, Y.J.; Hou, C.W.; Wu, C.S.; Wen, K.C.; Lin, C.Y.; Chiang, H.M. Sesamol Inhibited Melanogenesis by Regulating Melanin-Related Signal Transduction in B16F10 Cells. Int. J. Mol. Sci. 2018, 19, 1108. [Google Scholar] [CrossRef]
- OréMaldonado, K.A.; Cuesta, S.A.; Mora, J.R.; Loroño, M.A.; Paz, J.L. Discovering New Tyrosinase Inhibitors by Using In Silico Modelling, Molecular Docking, and Molecular Dynamics. Pharmaceuticals 2025, 18, 418. [Google Scholar] [CrossRef]
- Nguyen, B.C.; Tawata, S. Mimosine Dipeptide Enantiomsers: Improved Inhibitors against Melanogenesis and Cyclooxygenase. Molecules 2015, 20, 14334–14347. [Google Scholar] [CrossRef]



| Affinity with Protein (kcal/mol) | ||||
|---|---|---|---|---|
| Ligand | Tyrosinase | MITF | TRP-1 | CREB |
| Resveratrol | −7.3 | −5.1 | −7.5 | −5.2 |
| Dihydroresveratrol | −7.0 | −4.9 | −7.2 | −5.3 |
| RHS-0139 | −7.1 | −5.1 | −7.5 | −5.1 |
| RHS-0140 | −7.1 | −5.0 | −7.2 | −5.3 |
| RHS-0141 | −7.0 | −5.1 | −7.7 | −5.1 |
| RHS-0142 | −7.1 | −5.1 | −7.5 | −5.2 |
| Tropolone | −5.9 | - | - | - |
| ML329 | - | −5.8 | - | - |
| Mimosine | - | - | −6.2 | - |
| KG-501 | - | - | - | −6.1 |
| Gene | Forward Primer (5′→3′) | Reverse Primer (5′→3′) |
|---|---|---|
| Mouse MITF | TTCCGTTACCTTACCCAGAGG | AGACGCAGTGTTTTTGCTCAC |
| Mouse TYR | TTAGGATTTTCAGGGTGACGAC | TGGAGGGACATTGATTTTGC |
| Mouse TRP-1 | TATTGGCACACTCTCGTGGA | CATCTGAGCACCCCTGTCTT |
| Mouse TRP-2 | AAGTTGCTCTGCGGTTAGGA | AACGACCCTGTGTTTGTGGT |
| Mouse NRF2 | GTCACTGGGCTCTGCTATGAA | TCTCCTCGCTGGAAAAAGAA |
| Mouse HO-1 | GGTGAGGGAACTGTGTCAGG | CAGGGGCTGTGAACTCTGTC |
| Mouse GCLC | GAGAGCCTGATGTTCGCCTA | GAGAAGGGGGAGAGGACAAA |
| Mouse GCLM | TTGGAGTTGCACAGCTGGATT | TGGTTTTACCTGTGCCCACTG |
| Mouse NQO1 | GACCTTGCTTTCCATCACCACCGG | GTAGAGTGGTGACTCCTCCCAGAC |
| Mouse ß-actin | AATCGTGCGTGACATCAA | GCTCGTTGCCAATAGTGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Jhin, C.; Lee, S.; Rho, H.S.; Park, C.Y. Effects of Resveratrol Derivatives on Melanogenesis and Antioxidant Activity in B16F10 Cells. Int. J. Mol. Sci. 2025, 26, 4999. https://doi.org/10.3390/ijms26114999
Kim S, Jhin C, Lee S, Rho HS, Park CY. Effects of Resveratrol Derivatives on Melanogenesis and Antioxidant Activity in B16F10 Cells. International Journal of Molecular Sciences. 2025; 26(11):4999. https://doi.org/10.3390/ijms26114999
Chicago/Turabian StyleKim, Soyeon, Changho Jhin, Sullim Lee, Ho Sik Rho, and Chan Yoon Park. 2025. "Effects of Resveratrol Derivatives on Melanogenesis and Antioxidant Activity in B16F10 Cells" International Journal of Molecular Sciences 26, no. 11: 4999. https://doi.org/10.3390/ijms26114999
APA StyleKim, S., Jhin, C., Lee, S., Rho, H. S., & Park, C. Y. (2025). Effects of Resveratrol Derivatives on Melanogenesis and Antioxidant Activity in B16F10 Cells. International Journal of Molecular Sciences, 26(11), 4999. https://doi.org/10.3390/ijms26114999







