Fisetin Attenuates Zinc Overload-Induced Hepatotoxicity in Mice via Autophagy-Dependent Nrf2 Activation
Abstract
1. Introduction
2. Results
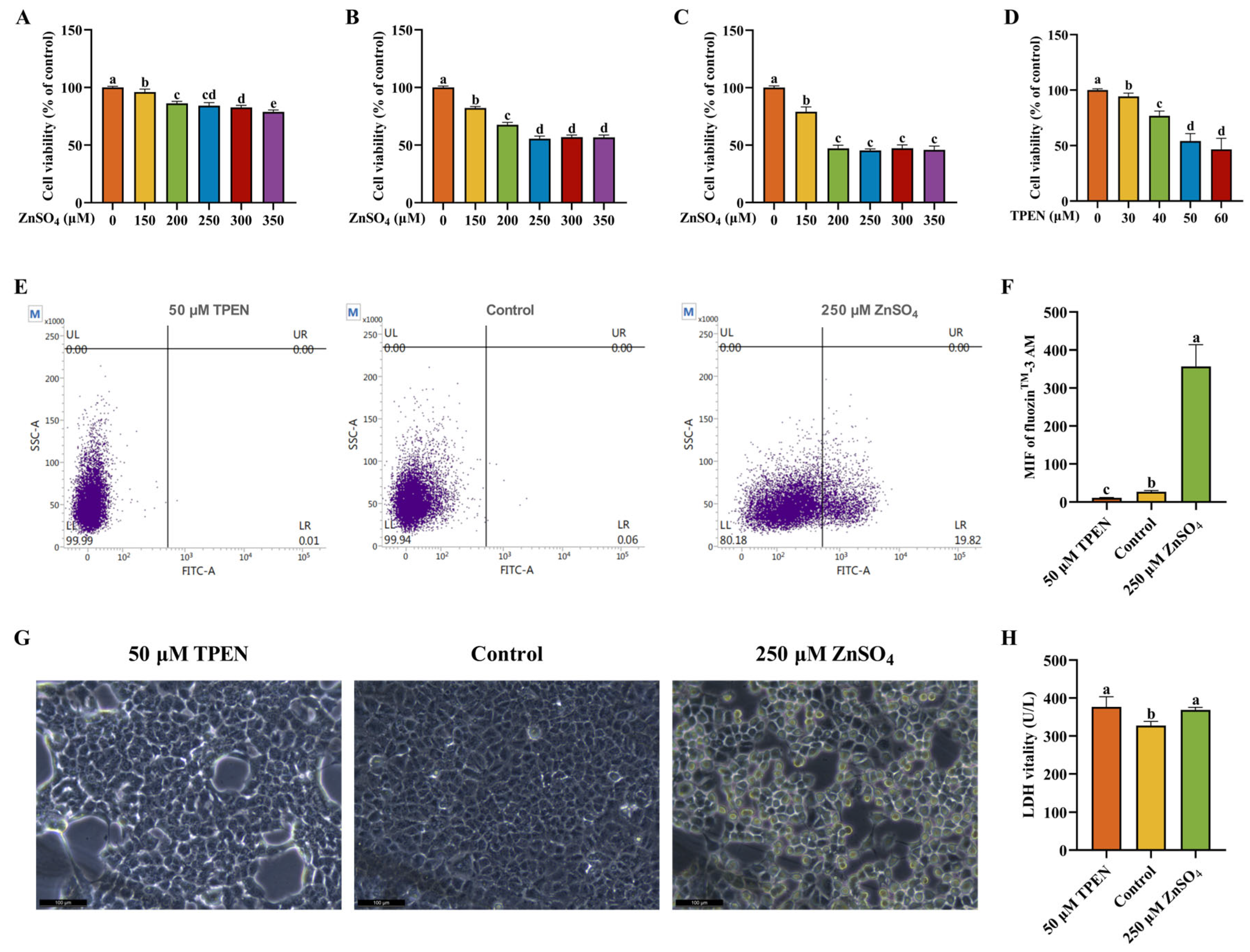
2.1. Establishment and Validation of Zn Imbalance Models on AML12 Cells
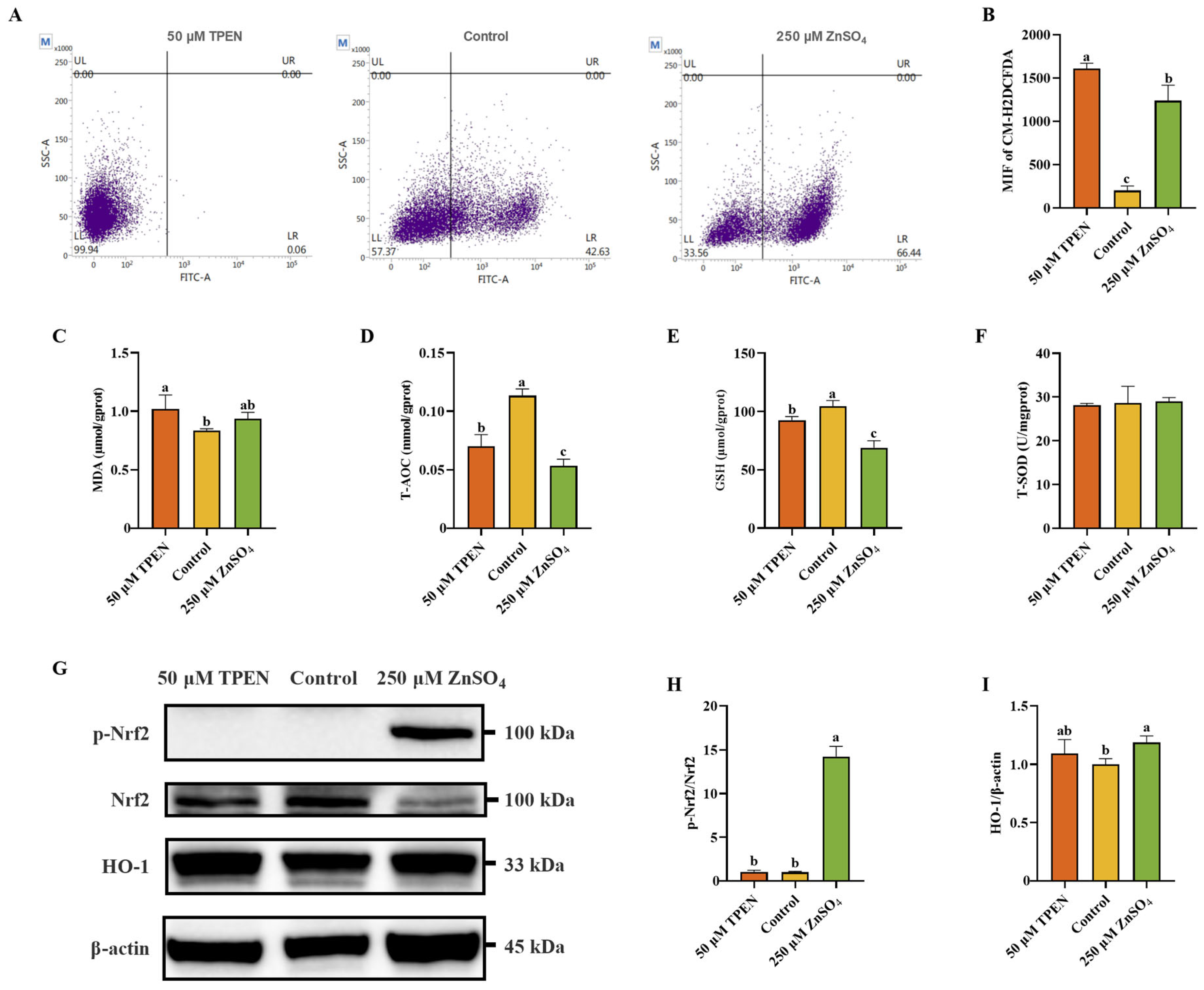
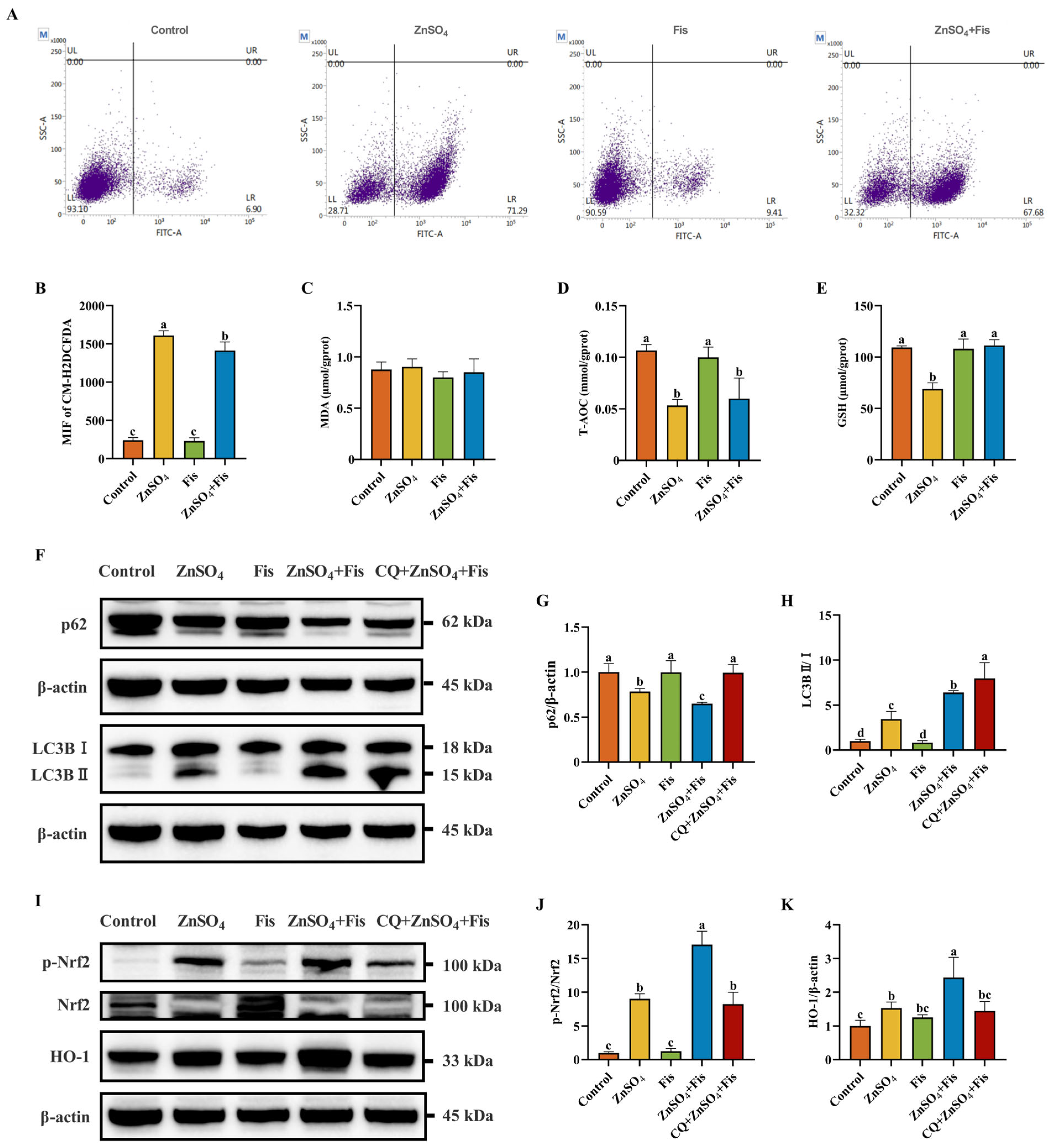
2.2. Zn Imbalance Induces Oxidative Stress in AML12 Cells
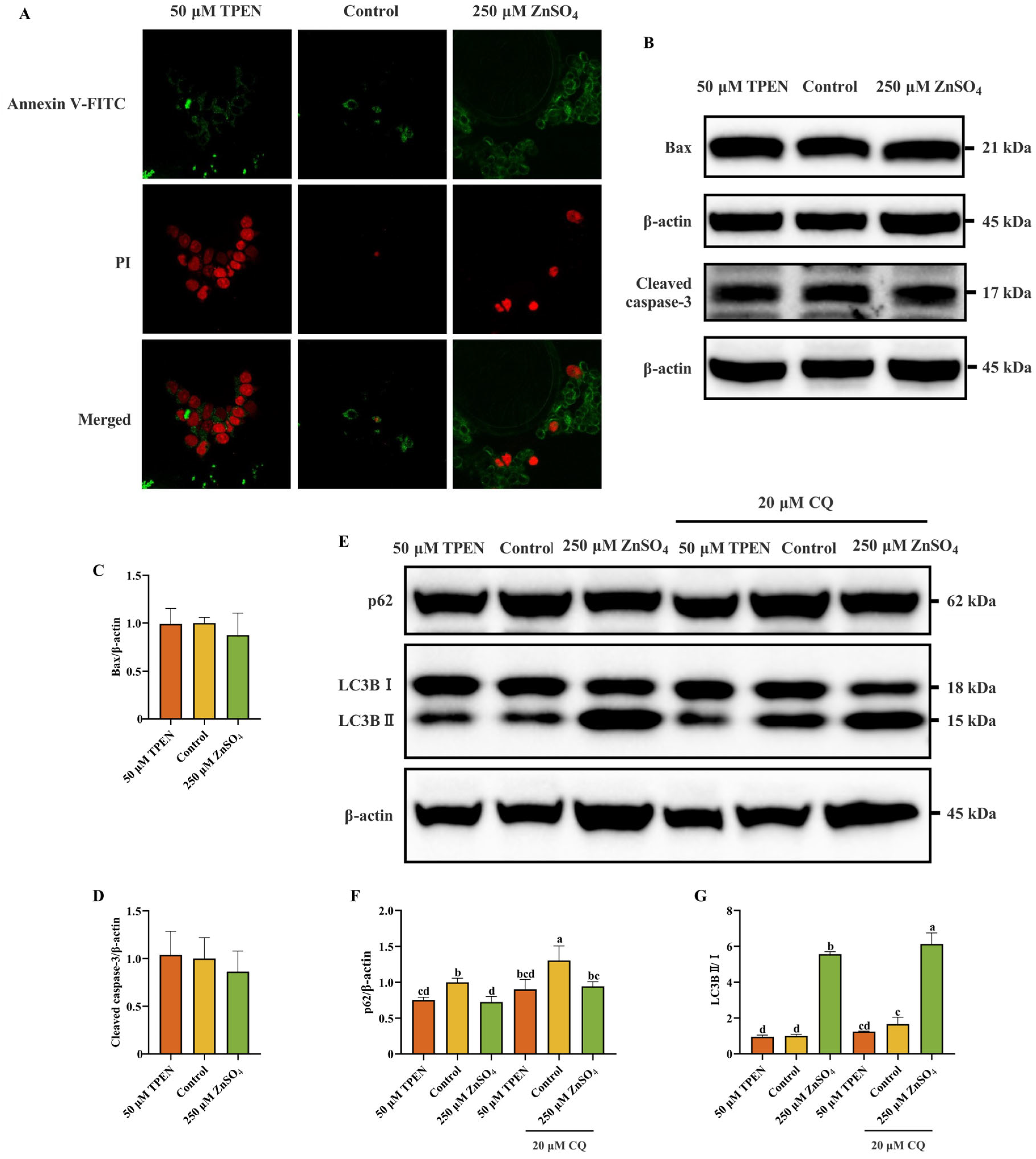
2.3. Effects of Zn Imbalance on Apoptosis and Autophagy in AML12 Cells
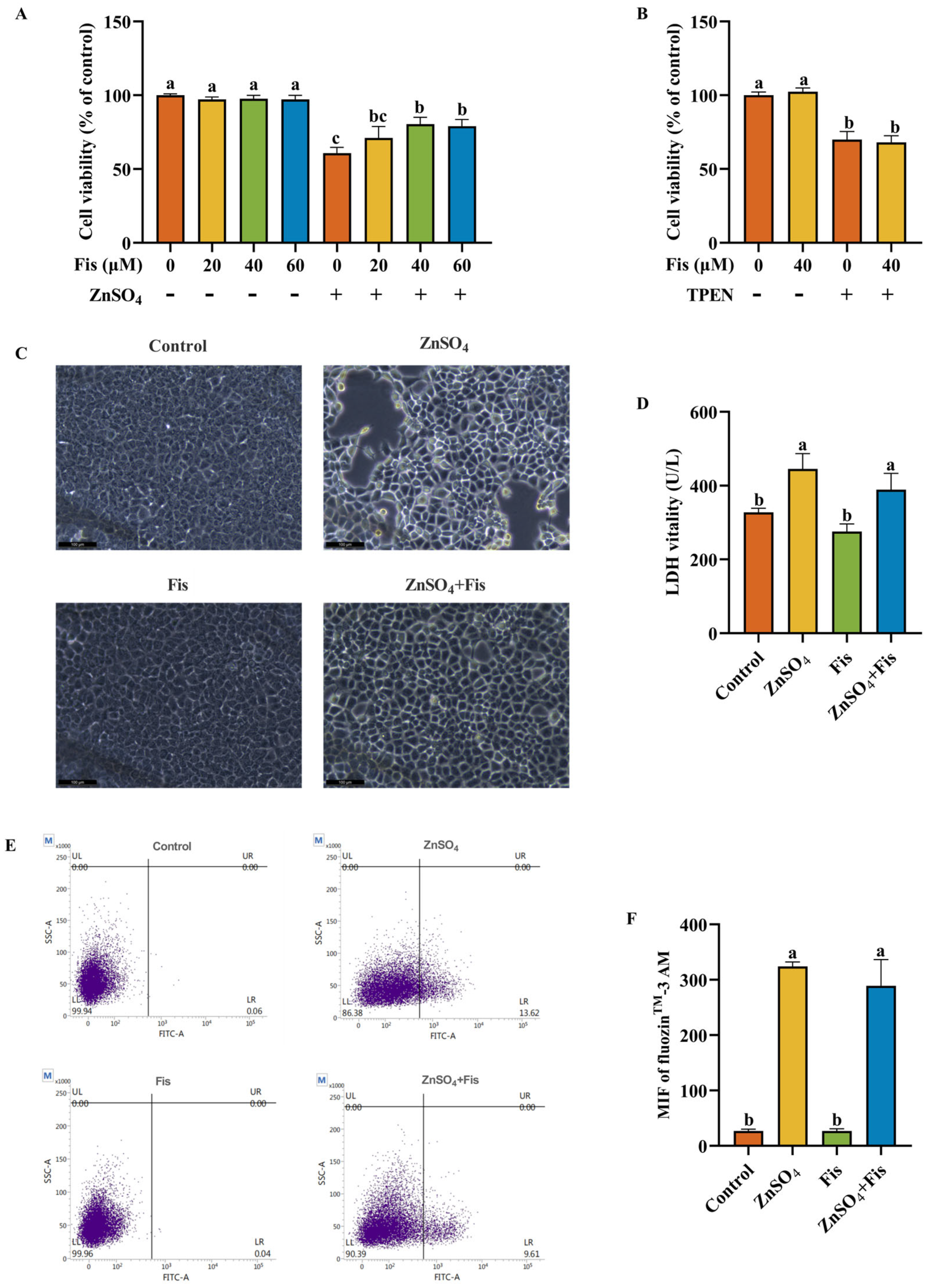
2.4. Fisetin Attenuates Zn Overload-Induced Cytotoxicity in AML12 Cells Through a Non-Chelating Way
2.5. Fisetin Attenuates Zn Overload-Induced Oxidative Stress by Activating the Nrf2 Signaling Pathway Through an Autophagy-Dependent Mechanism in AML12 Cells
2.6. Protective Effects of Fisetin Against Zn Overload-Induced Liver Damage In Vivo
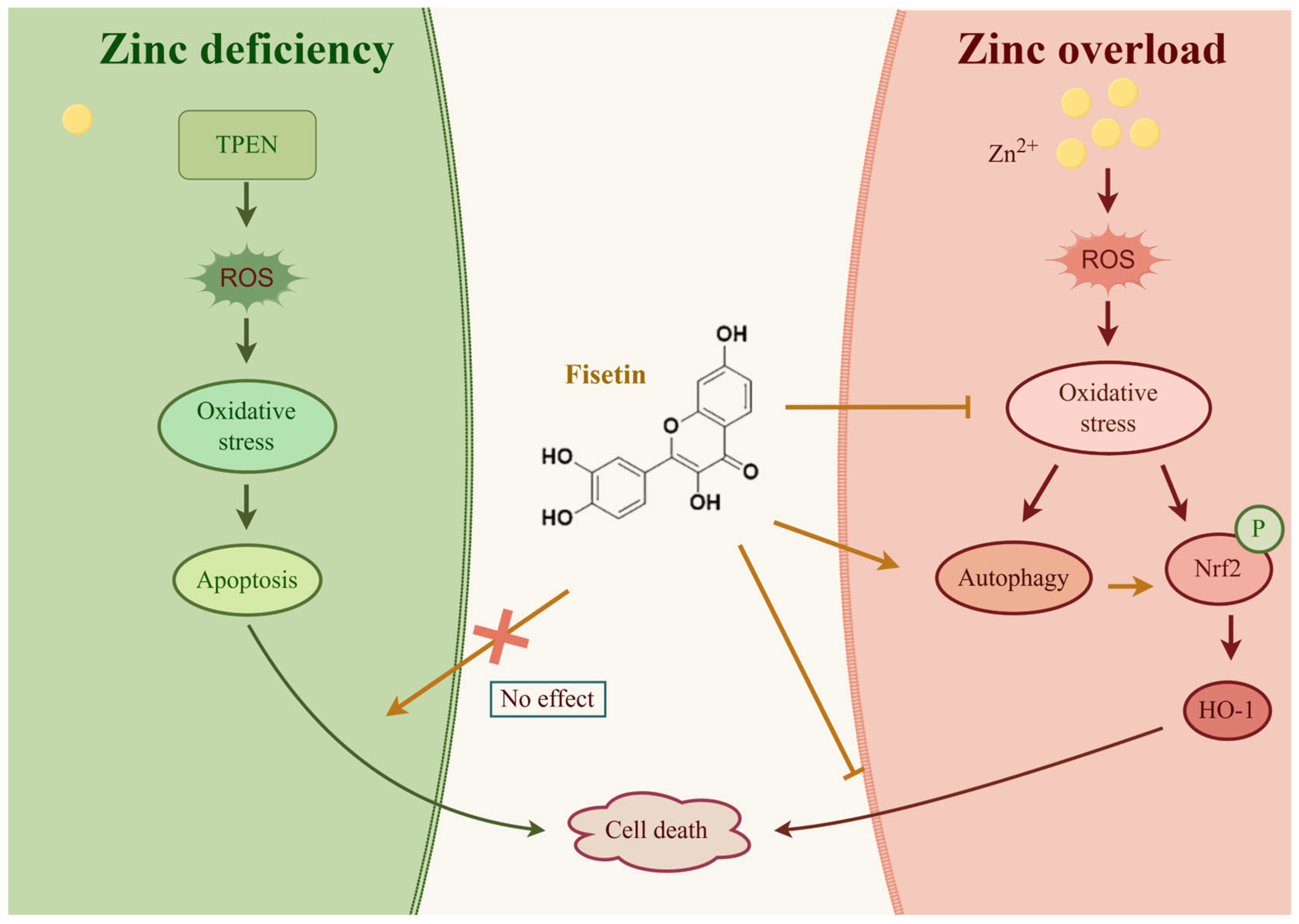
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatment
4.2. Cell Viability Assay
4.3. Measurement of Intracellular Zn2+ Level
4.4. Morphological Investigation and Quantification of Supernatant LDH Vitality
4.5. Determination of Intracellular Oxidative Stress
4.6. Apoptosis Detection
4.7. Western Blotting Analysis
4.8. Animal Experiments
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Bax | Bcl-2-associated X protein |
| CMC-Na | Carboxymethyl cellulose sodium |
| CM-H2DCFDA | 5-(and 6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate |
| CQ | Chloroquine |
| GSH | Glutathione |
| HO-1 | Heme oxygenase 1 |
| LDH | Lactate dehydrogenase |
| MDA | Malondialdehyde |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| PBS | Phosphate buffer saline |
| ROS | Reactive oxygen species |
| TPEN | N,N,N′,N′-Tetrakis-(2-pyridylmethyl)-ethylenediamine |
| T-AOC | Total antioxidant capability |
| T-SOD | Total superoxide dismutase |
| Zn | Zinc |
References
- Lotto, J.; Stephan, T.L.; Hoodless, P.A. Fetal liver development and implications for liver disease pathogenesis. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 561–581. [Google Scholar] [CrossRef]
- Robinson, M.W.; Harmon, C.; O’Farrelly, C. Liver immunology and its role in inflammation and homeostasis. Cell. Mol. Immunol. 2016, 13, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Driskill, J.H.; Pan, D. The Hippo pathway in liver homeostasis and pathophysiology. Annu. Rev. Pathol. 2021, 16, 299–322. [Google Scholar] [CrossRef] [PubMed]
- Arteel, G.E.; Gao, B. When alcohol and fat meet, neutrophil traps form to promote liver injury. Gut 2024, 73, 1778–1779. [Google Scholar] [CrossRef]
- Wong, M.C.S.; Huang, J.L.W.; George, J.; Huang, J.; Leung, C.; Eslam, M.; Chan, H.L.Y.; Ng, S.C. The changing epidemiology of liver diseases in the Asia-Pacific region. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Costello, E.; Rock, S.; Stratakis, N.; Eckel, S.P.; Walker, D.I.; Valvi, D.; Cserbik, D.; Jenkins, T.; Xanthakos, S.A.; Kohli, R.; et al. Exposure to per-and polyfluoroalkyl substances and markers of liver injury: A systematic review and meta-analysis. Environ. Health Perspect. 2022, 130, 046001. [Google Scholar] [CrossRef]
- Zou, Z.; Liu, X.; Yu, J.; Ban, T.; Zhang, Z.; Wang, P.; Huang, R.; Zheng, F.; Chang, Y.; Peng, W.; et al. Nuclear miR-204-3p mitigates metabolic dysfunction-associated steatotic liver disease in mice. J. Hepatol. 2024, 80, 834–845. [Google Scholar] [CrossRef]
- Devarbhavi, H.; Asrani, S.K.; Arab, J.P.; Nartey, Y.A.; Pose, E.; Kamath, P.S. Global burden of liver disease: 2023 update. J. Hepatol. 2023, 79, 516–537. [Google Scholar] [CrossRef]
- Li, D.; Wan, M.; Xue, L.; Zhang, Z.; Qiu, Y.; Mei, F.; Tang, N.; Yu, C.; Yu, Y.; Chen, T.; et al. Zinc promotes microbial p-coumaric acid production that protects against cholestatic liver injury. Cell Host Microbe 2024, 32, 2195–2211. [Google Scholar] [CrossRef]
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef]
- Iritani, S.; Kawamura, Y.; Muraishi, N.; Fujiyama, S.; Sezaki, H.; Hosaka, T.; Akuta, N.; Kobayashi, M.; Saitoh, S.; Suzuki, F.; et al. The useful predictors of zinc deficiency for the management of chronic liver disease. J. Gastroenterol. 2022, 57, 322–332. [Google Scholar] [CrossRef]
- Yang, D.; Tian, T.; Li, X.; Zhang, B.; Qi, L.; Zhang, F.; Han, M.; Wang, S.; Xiao, J.; Gou, Y.; et al. ZNT1 and Zn2+ control TLR4 and PD-L1 endocytosis in macrophages to improve chemotherapy efficacy against liver tumor. Hepatology 2024, 80, 312–329. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Jiang, H.; Xu, G.; Li, C.; Li, D.; Tang, S. Lethality of zinc oxide nanoparticles surpasses conventional zinc oxide via oxidative stress, mitochondrial damage and calcium overload: A comparative hepatotoxicity study. Int. J. Mol. Sci. 2022, 23, 6724. [Google Scholar] [CrossRef]
- Phillips, M.J.; Ackerley, C.A.; Superina, R.A.; Roberts, E.A.; Filler, R.M.; Levy, G.A. Excess zinc associated with severe progressive cholestasis in Cree and Ojibwa-Cree children. Lancet 1996, 347, 866–868. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qin, X.; Chen, T.; Chen, M.; Wu, L.; He, B. Exploring the interactions between metabolic dysfunction-associated fatty liver disease and micronutrients: From molecular mechanisms to clinical applications. Front. Nutr. 2024, 11, 1344924. [Google Scholar] [CrossRef]
- Teschke, R. Aluminum, arsenic, beryllium, cadmium, chromium, cobalt, copper, iron, lead, mercury, molybdenum, nickel, platinum, thallium, titanium, vanadium, and zinc: Molecular aspects in experimental liver injury. Int. J. Mol. Sci. 2022, 23, 12213. [Google Scholar] [CrossRef]
- Li, L.; Qin, Y.; Xin, X.; Wang, S.; Liu, Z.; Feng, X. The great potential of flavonoids as candidate drugs for NAFLD. Biomed. Pharmacother. 2023, 164, 114991. [Google Scholar] [CrossRef] [PubMed]
- Adeli, O.A.; Heidari-Soureshjani, S.; Rostamian, S.; Azadegan-Dehkordi, Z.; Khaghani, A. Effects and mechanisms of fisetin against ischemia-reperfusion injuries: A systematic review. Curr. Pharm. Biotechnol. 2024, 25, 2138–2153. [Google Scholar] [CrossRef]
- Liu, H.; Lu, Q. Fisetin alleviates inflammation and oxidative stress in deep vein thrombosis via MAPK and NRF2 signaling pathway. Int. J. Mol. Sci. 2024, 25, 3724. [Google Scholar] [CrossRef]
- Ren, Q.; Tao, S.; Guo, F.; Wang, B.; Yang, L.; Ma, L.; Fu, P. Natural flavonol fisetin attenuated hyperuricemic nephropathy via inhibiting IL-6/JAK2/STAT3 and TGF-β/SMAD3 signaling. Phytomedicine 2021, 87, 153552. [Google Scholar] [CrossRef]
- Ji, R.; Hao, Z.; Wang, H.; Su, Y.; Yang, W.; Li, X.; Duan, L.; Guan, F.; Ma, S. Fisetin promotes functional recovery after spinal cord injury by inhibiting microglia/macrophage M1 polarization and JAK2/STAT3 signaling pathway. J. Agric. Food Chem. 2024, 72, 17964–17976. [Google Scholar] [CrossRef] [PubMed]
- Ravula, A.R.; Teegala, S.B.; Kalakotla, S.; Pasangulapati, J.P.; Perumal, V.; Boyina, H.K. Fisetin, potential flavonoid with multifarious targets for treating neurological disorders: An updated review. Eur. J. Pharmacol. 2021, 910, 174492. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, A.A.; Naureen, H.; Zahid, R.; Youssef, L.; Attar, R.; Xu, B. Cancer chemopreventive role of fisetin: Regulation of cell signaling pathways in different cancers. Pharmacol. Res. 2021, 172, 105784. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, J.; Pan, L.; Chen, L.; Wang, Y.; Liu, X.; You, L.; Jia, Y.; Hu, C. Protective effect of 7,3′,4′-flavon-3-ol (fisetin) on acetaminophen-induced hepatotoxicity in vitro and in vivo. Phytomedicine 2019, 58, 152865. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Ge, C.; Qin, Y.; Gu, T.; Lv, J.; Wang, S.; Ma, Y.; Lou, D.; Li, Q.; Hu, L.; et al. Activated TNF-α/RIPK3 signaling is involved in prolonged high fat diet-stimulated hepatic inflammation and lipid accumulation: Inhibition by dietary fisetin intervention. Food Funct. 2019, 10, 1302–1316. [Google Scholar] [CrossRef]
- Zeng, Y.Q.; He, J.T.; Hu, B.Y.; Li, W.; Deng, J.; Lin, Q.L.; Fang, Y. Virgin coconut oil: A comprehensive review of antioxidant activity and mechanisms contributed by phenolic compounds. Crit. Rev. Food Sci. Nutr. 2024, 64, 1052–1075. [Google Scholar] [CrossRef]
- Ortuño-Costela, M.C.; Pinzani, M.; Vallier, L. Cell therapy for liver disorders: Past, present and future. Nat. Rev. Gastroenterol. Hepatol. 2025, 22, 329–342. [Google Scholar] [CrossRef]
- Lubna, S.; Ahmad, R. Clinical and biochemical understanding of Zinc interaction during liver diseases: A paradigm shift. J. Trace Elem. Med. Biol. 2023, 77, 127130. [Google Scholar] [CrossRef]
- Hao, Q.; Maret, W. Imbalance between pro-oxidant and pro-antioxidant functions of zinc in disease. J. Alzheimers. Dis. 2005, 8, 161–170, discussion 209–215. [Google Scholar] [CrossRef]
- Maret, W. The redox biology of redox-inert zinc ions. Free Radic. Biol. Med. 2019, 134, 311–326. [Google Scholar] [CrossRef]
- Rudolf, E.; Rudolf, K. Induced zinc loss produces heterogenous biological responses in melanoma cells. Int. J. Mol. Sci. 2022, 23, 8312. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, P.; Guo, J.; Ma, T.; Hu, Y.; Huang, L.; Xing, B.; He, Y.; Xi, J. Zinc overload induces damage to H9c2 cardiomyocyte through mitochondrial dysfunction and ROS-mediated mitophagy. Cardiovasc. Toxicol. 2023, 23, 388–405. [Google Scholar] [CrossRef]
- Chen, L.; Shen, Q.; Liu, Y.; Zhang, Y.; Sun, L.; Ma, X.; Song, N.; Xie, J. Homeostasis and metabolism of iron and other metal ions in neurodegenerative diseases. Signal Transduct. Target. Ther. 2025, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Jin, X.; Zhang, P.; Li, Q.; Lv, Z.; Ding, Y.; He, H.; Wang, Y.; He, Y.; Zhao, X.; et al. SPOP mutations promote p62/SQSTM1-dependent autophagy and Nrf2 activation in prostate cancer. Cell Death Differ. 2022, 29, 1228–1239. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Xu, J.; Gu, Y.; Wang, Y.; Wang, J.; Sun, F. Melatonin ameliorates 10-hydroxycamptothecin-induced oxidative stress and apoptosis via autophagy-regulated p62/Keap1/Nrf2 pathway in mouse testicular cells. J. Pineal Res. 2024, 76, e12959. [Google Scholar] [CrossRef]
- Hussein, R.M.; Sawy, D.M.; Kandeil, M.A.; Farghaly, H.S. Chlorogenic acid, quercetin, coenzyme Q10 and silymarin modulate Keap1-Nrf2/heme oxygenase-1 signaling in thioacetamide-induced acute liver toxicity. Life Sci. 2021, 277, 119460. [Google Scholar] [CrossRef]
- Janda, E.; Martino, C.; Riillo, C.; Parafati, M.; Lascala, A.; Mollace, V.; Boutin, J.A. Apigenin and luteolin regulate autophagy by targeting NRH-quinone oxidoreductase 2 in liver cells. Antioxidants 2021, 10, 776. [Google Scholar] [CrossRef]
- Deng, Z.; Lim, J.; Wang, Q.; Purtell, K.; Wu, S.; Palomo, G.M.; Tan, H.; Manfredi, G.; Zhao, Y.; Peng, J.; et al. ALS-FTLD-linked mutations of SQSTM1/p62 disrupt selective autophagy and NFE2L2/NRF2 anti-oxidative stress pathway. Autophagy 2020, 16, 917–931. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, B.; Fu, L.; Li, F. UBE2C regulates the KEAP1/NRF2 signaling pathway to promote the growth of gastric cancer by inhibiting autophagy. Int. J. Biol. Macromol. 2024, 276, 134011. [Google Scholar] [CrossRef]
- Lang, A.L.; Krueger, A.M.; Schnegelberger, R.D.; Kaelin, B.R.; Rakutt, M.J.; Chen, L.; Arteel, G.E.; Beier, J.I. Rapamycin attenuates liver injury caused by vinyl chloride metabolite chloroethanol and lipopolysaccharide in mice. Toxicol. Appl. Pharmacol. 2019, 382, 114745. [Google Scholar] [CrossRef]
- Neef, M.; Ledermann, M.; Saegesser, H.; Schneider, V.; Reichen, J. Low-dose oral rapamycin treatment reduces fibrogenesis, improves liver function, and prolongs survival in rats with established liver cirrhosis. J. Hepatol. 2006, 45, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Gabbia, D.; Pozzo, L.; Zigiotto, G.; Roverso, M.; Sacchi, D.; Dalla Pozza, A.; Carrara, M.; Bogialli, S.; Floreani, A.; Guido, M.; et al. Dexamethasone counteracts hepatic inflammation and oxidative stress in cholestatic rats via CAR activation. PLoS ONE 2018, 13, e0204336. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, Z.; Wang, P.; Yu, X.; Ding, H.; Wang, Z.; Feng, J. Effect of long-term and short-term imbalanced Zn manipulation on gut microbiota and screening for microbial markers sensitive to zinc status. Microbiol. Spectr. 2021, 9, e00483-21. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, F.; Wang, Z.; Zhou, M.; Zhang, Q.; Feng, J. Fisetin Attenuates Zinc Overload-Induced Hepatotoxicity in Mice via Autophagy-Dependent Nrf2 Activation. Int. J. Mol. Sci. 2025, 26, 4978. https://doi.org/10.3390/ijms26114978
Huang F, Wang Z, Zhou M, Zhang Q, Feng J. Fisetin Attenuates Zinc Overload-Induced Hepatotoxicity in Mice via Autophagy-Dependent Nrf2 Activation. International Journal of Molecular Sciences. 2025; 26(11):4978. https://doi.org/10.3390/ijms26114978
Chicago/Turabian StyleHuang, Feifei, Zhonghang Wang, Mohan Zhou, Qian Zhang, and Jie Feng. 2025. "Fisetin Attenuates Zinc Overload-Induced Hepatotoxicity in Mice via Autophagy-Dependent Nrf2 Activation" International Journal of Molecular Sciences 26, no. 11: 4978. https://doi.org/10.3390/ijms26114978
APA StyleHuang, F., Wang, Z., Zhou, M., Zhang, Q., & Feng, J. (2025). Fisetin Attenuates Zinc Overload-Induced Hepatotoxicity in Mice via Autophagy-Dependent Nrf2 Activation. International Journal of Molecular Sciences, 26(11), 4978. https://doi.org/10.3390/ijms26114978





