Human Herpes Virus Genotype and Immunological Gene Expression Profile in Prostate Cancer with Prominent Inflammation
Abstract
1. Introduction
2. Results
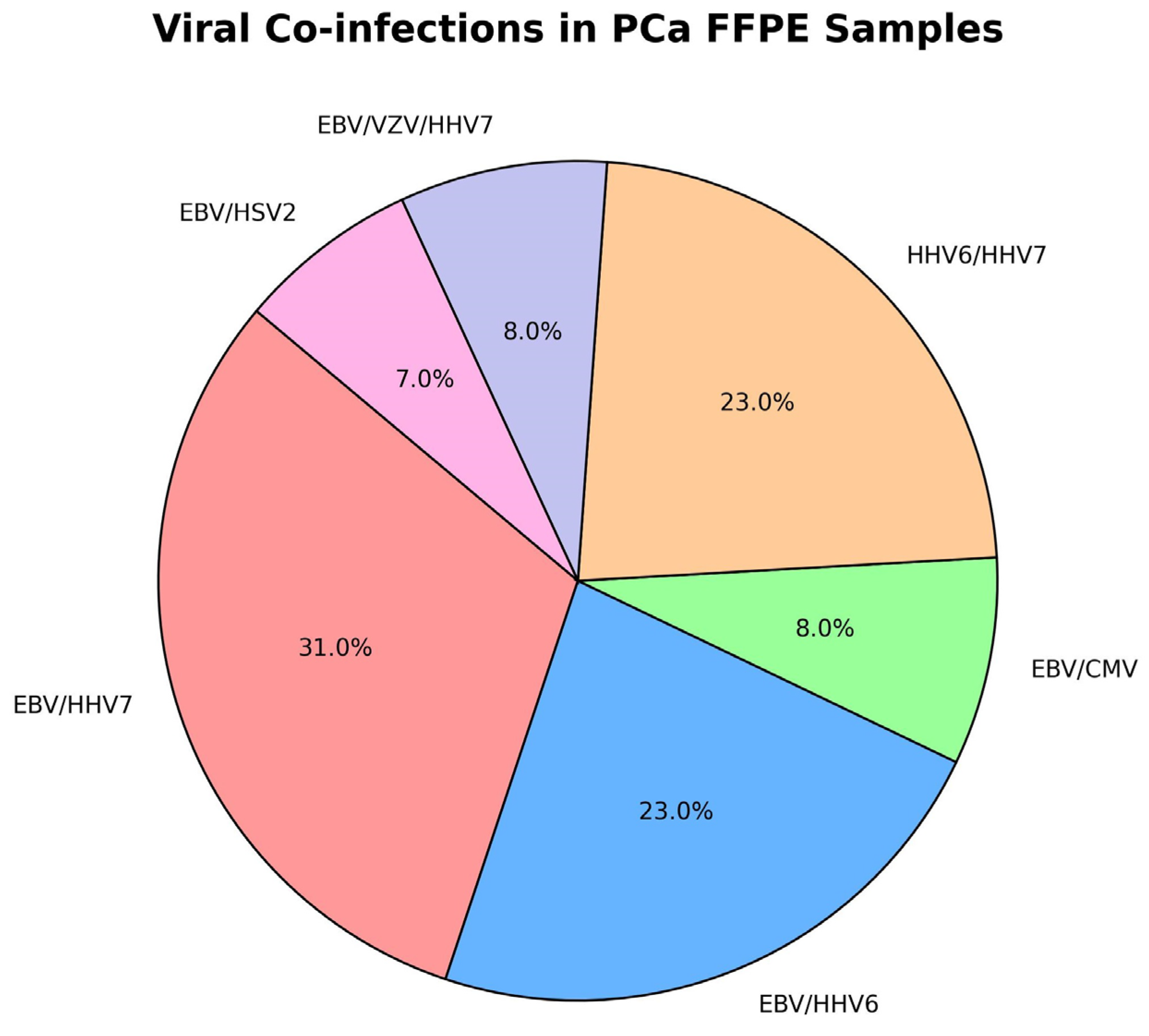
2.1. Molecular Virological Results
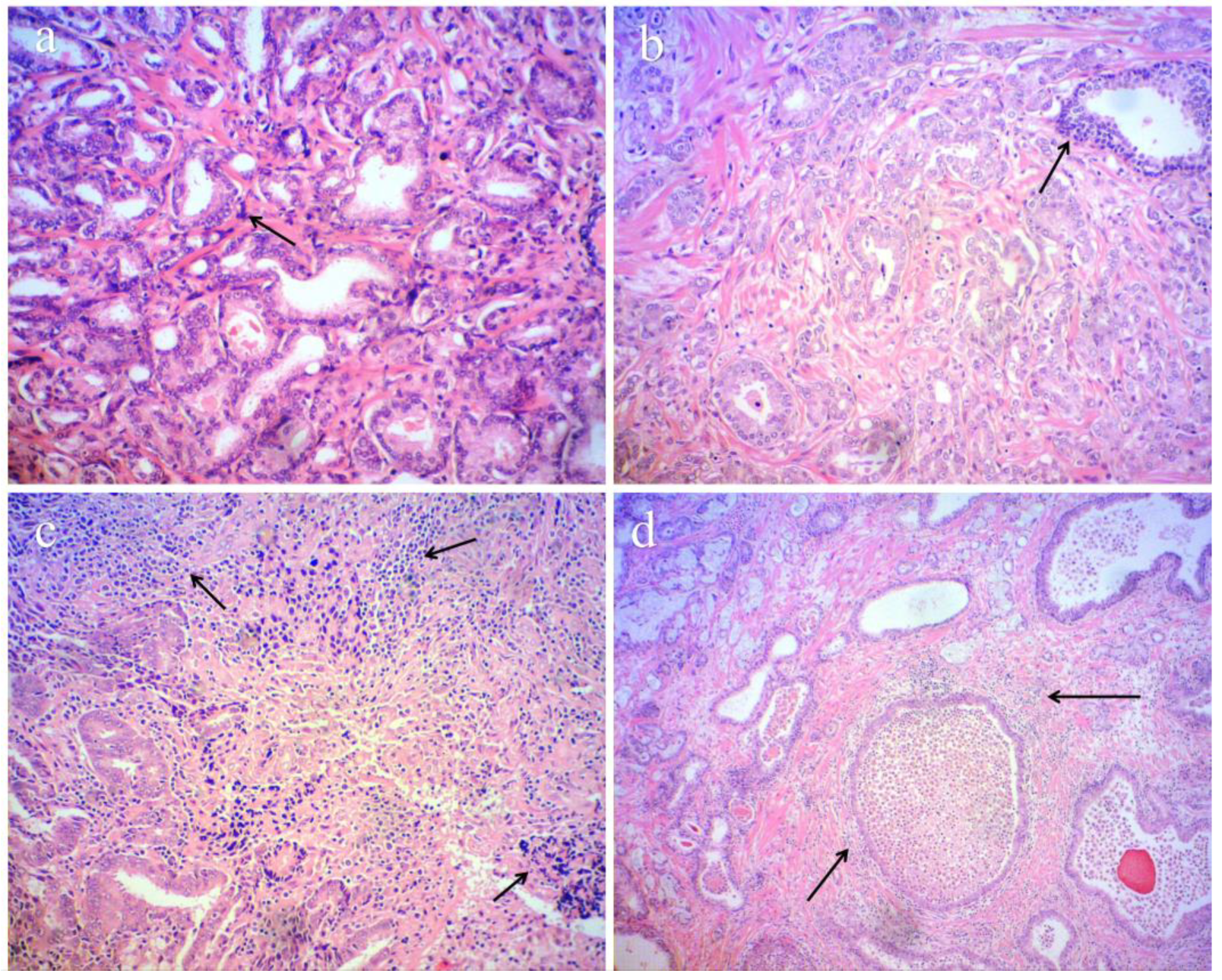
2.2. Histopathological Results
2.3. Pilot Immunological Results
3. Discussion
4. Materials and Methods
4.1. Patients and Samples
4.2. Nucleic Acid Extraction
4.3. Real-Time PCR
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morka, N.; Norris, J.M.; Emberton, M.; Kelly, D. Prostate Cancer and the Human Papilloma Virus: Causative Association, Role of Vaccines, and the Impact of the COVID-19 Pandemic. Prostate Cancer Prostatic Dis. 2022, 25, 55–57. [Google Scholar] [CrossRef] [PubMed]
- Bulgaria Cancer Incidence 2016–2017|GHDx. Available online: https://ghdx.healthdata.org/record/bulgaria-cancer-incidence-2016-2017 (accessed on 17 February 2025).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Patel, S.K.; Bellavia, D.; Messina, E.; Palermo, R.; Ceccarelli, S.; Marchese, C.; Anastasiadou, E.; Minter, L.M.; Felli, M.P. When Viruses Cross Developmental Pathways. Front. Cell Dev. Biol. 2021, 9, 691644. [Google Scholar] [CrossRef]
- Sugar, L.M. Inflammation and Prostate Cancer. Can. J. Urol. 2006, 13 (Suppl. 1), 46–47. [Google Scholar]
- Abidi, S.H.; Bilwani, F.; Ghias, K.; Abbas, F. Viral Etiology of Prostate Cancer: Genetic Alterations and Immune Response. A Literature Review. Int. J. Surg. 2018, 52, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Palapattu, G.S.; Sutcliffe, S.; Bastian, P.J.; Platz, E.A.; De Marzo, A.M.; Isaacs, W.B.; Nelson, W.G. Prostate Carcinogenesis and Inflammation: Emerging Insights. Carcinogenesis 2005, 26, 1170–1181. [Google Scholar] [CrossRef]
- Taverna, G.; Pedretti, E.; Di Caro, G.; Borroni, E.M.; Marchesi, F.; Grizzi, F. Inflammation and Prostate Cancer: Friends or Foe? Inflamm. Res. 2015, 64, 275–286. [Google Scholar] [CrossRef]
- Alibek, K.; Baiken, Y.; Kakpenova, A.; Mussabekova, A.; Zhussupbekova, S.; Akan, M.; Sultankulov, B. Implication of Human Herpesviruses in Oncogenesis through Immune Evasion and Supression. Infect. Agents Cancer 2014, 9, 3. [Google Scholar] [CrossRef]
- Moore, P.S.; Chang, Y. Why Do Viruses Cause Cancer? Highlights of the First Century of Human Tumour Virology. Nat. Rev. Cancer 2010, 10, 878–889. [Google Scholar] [CrossRef]
- Henning, J.D.; Bunker, C.H.; Patrick, A.L.; Jenkins, F.J. Human Herpesvirus 8 Establishes a Latent Infection in Prostates of Tobago Men Resulting in Increased Macrophage Infiltration. Prostate 2016, 76, 735–743. [Google Scholar] [CrossRef]
- Valverde, A.; Seal, A.; Nares, S.; Shukla, D.; Naqvi, A.R. Human Herpesvirus-Encoded MicroRNA in Host-Pathogen Interaction. Adv. Biol. Regul. 2021, 82, 100829. [Google Scholar] [CrossRef] [PubMed]
- Weaver, G.C.; Schneider, C.L.; Becerra-Artiles, A.; Clayton, K.L.; Hudson, A.W.; Stern, L.J. The HHV-6B U20 Glycoprotein Binds ULBP1, Masking It from Recognition by NKG2D and Interfering with Natural Killer Cell Activation. Front. Immunol. 2024, 15, 1363156. [Google Scholar] [CrossRef] [PubMed]
- Solares, S.; León, J.; García-Gutiérrez, L. The Functional Interaction Between Epstein–Barr Virus and MYC in the Pathogenesis of Burkitt Lymphoma. Cancers 2024, 16, 4212. [Google Scholar] [CrossRef]
- Yu, C.J.; Damania, B. Molecular Mechanisms of Kaposi Sarcoma-Associated Herpesvirus (HHV8)-Related Lymphomagenesis. Cancers 2024, 16, 3693. [Google Scholar] [CrossRef]
- Jenkins, F.J.; Minas, T.Z.; Tang, W.; Dorsey, T.H.; Ambs, S. Human Herpesvirus 8 Infection Is Associated with Prostate Cancer among IFNL4-ΔG Carriers. Prostate Cancer Prostatic Dis. 2023, 26, 338–346. [Google Scholar] [CrossRef]
- Ding, X.-B.; Ren, S.-Y.; Wen, H.-Z.; Zhang, Z.-B.; Ye, J.-A.; Pan, W.-K.; Ye, J.-Q. A Bidirectional Mendelian Randomization Study on the Causal Relationship Between Epstein-Barr Virus Antibodies and Prostate Cancer Risk. Cancer Control 2025, 32, 10732748251320842. [Google Scholar] [CrossRef]
- Kiś, J.; Góralczyk, M.; Sikora, D.; Stępień, E.; Drop, B.; Polz-Dacewicz, M. Can the Epstein-Barr Virus Play a Role in the Development of Prostate Cancer? Cancers 2024, 16, 328. [Google Scholar] [CrossRef]
- Suthaus, J.; Stuhlmann-Laeisz, C.; Tompkins, V.S.; Rosean, T.R.; Klapper, W.; Tosato, G.; Janz, S.; Scheller, J.; Rose-John, S. HHV-8–Encoded Viral IL-6 Collaborates with Mouse IL-6 in the Development of Multicentric Castleman Disease in Mice. Blood 2012, 119, 5173–5181. [Google Scholar] [CrossRef]
- Suthaus, J.; Adam, N.; Grötzinger, J.; Scheller, J.; Rose-John, S. Viral Interleukin-6: Structure, Pathophysiology and Strategies of Neutralization. European Journal of Cell Biology 2011, 90, 495–504. [Google Scholar] [CrossRef]
- Torti, N.; Oxenius, A. T Cell Memory in the Context of Persistent Herpes Viral Infections. Viruses 2012, 4, 1116–1143. [Google Scholar] [CrossRef]
- Seckert, C.K.; Griessl, M.; Büttner, J.K.; Scheller, S.; Simon, C.O.; Kropp, K.A.; Renzaho, A.; Kühnapfel, B.; Grzimek, N.K.A.; Reddehase, M.J. Viral Latency Drives “Memory Inflation”: A Unifying Hypothesis Linking Two Hallmarks of Cytomegalovirus Infection. Med. Microbiol. Immunol. 2012, 201, 551–566. [Google Scholar] [CrossRef]
- Oseni, S.O.; Naar, C.; Pavlović, M.; Asghar, W.; Hartmann, J.X.; Fields, G.B.; Esiobu, N.; Kumi-Diaka, J. The Molecular Basis and Clinical Consequences of Chronic Inflammation in Prostatic Diseases: Prostatitis, Benign Prostatic Hyperplasia, and Prostate Cancer. Cancers 2023, 15, 3110. [Google Scholar] [CrossRef] [PubMed]
- Grinstein, S.; Preciado, M.V.; Gattuso, P.; Chabay, P.A.; Warren, W.H.; De Matteo, E.; Gould, V.E. Demonstration of Epstein-Barr Virus in Carcinomas of Various Sites. Cancer Res. 2002, 62, 4876–4878. [Google Scholar]
- Bergh, J.; Marklund, I.; Gustavsson, C.; Wiklund, F.; Grönberg, H.; Allard, A.; Alexeyev, O.; Elgh, F. No Link between Viral Findings in the Prostate and Subsequent Cancer Development. Br. J. Cancer 2007, 96, 137–139. [Google Scholar] [CrossRef]
- Sfanos, K.S.; Sauvageot, J.; Fedor, H.L.; Dick, J.D.; De Marzo, A.M.; Isaacs, W.B. A Molecular Analysis of Prokaryotic and Viral DNA Sequences in Prostate Tissue from Patients with Prostate Cancer Indicates the Presence of Multiple and Diverse Microorganisms. Prostate 2008, 68, 306–320. [Google Scholar] [CrossRef]
- Eliassen, E.; Lum, E.; Pritchett, J.; Ongradi, J.; Krueger, G.; Crawford, J.R.; Phan, T.L.; Ablashi, D.; Hudnall, S.D. Human Herpesvirus 6 and Malignancy: A Review. Front. Oncol. 2018, 8, 512. [Google Scholar] [CrossRef] [PubMed]
- Rickinson, A. Concluding Overview: Looking Back, Looking Forward. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001, 356, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Khan, G. Epstein-Barr Virus, Cytokines, and Inflammation: A Cocktail for the Pathogenesis of Hodgkin’s Lymphoma? Exp. Hematol. 2006, 34, 399–406. [Google Scholar] [CrossRef]
- Kashanchi, F.; Araujo, J.; Doniger, J.; Muralidhar, S.; Hoch, R.; Khleif, S.; Mendelson, E.; Thompson, J.; Azumi, N.; Brady, J.N.; et al. Human Herpesvirus 6 (HHV-6) ORF-1 Transactivating Gene Exhibits Malignant Transforming Activity and Its Protein Binds to P53. Oncogene 1997, 14, 359–367. [Google Scholar] [CrossRef]
- Castillo, J.P.; Kowalik, T.F. Human Cytomegalovirus Immediate Early Proteins and Cell Growth Control. Gene 2002, 290, 19–34. [Google Scholar] [CrossRef]
- Doniger, J.; Muralidhar, S.; Rosenthal, L.J. Human Cytomegalovirus and Human Herpesvirus 6 Genes That Transform and Transactivate. Clin. Microbiol. Rev. 1999, 12, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, M.; Doerr, H.W.; Cinatl, J. The Story of Human Cytomegalovirus and Cancer: Increasing Evidence and Open Questions. Neoplasia 2009, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Samanta, M.; Harkins, L.; Klemm, K.; Britt, W.J.; Cobbs, C.S. High Prevalence of Human Cytomegalovirus in Prostatic Intraepithelial Neoplasia and Prostatic Carcinoma. J. Urol. 2003, 170, 998–1002. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.J.; Jeong, P.; Kang, H.W.; Shinn, H.K.; Kim, Y.-H.; Yan, C.; Choi, Y.-K.; Kim, D.; Ryu, D.H.; Ha, Y.-S.; et al. Increased Expression of Herpes Virus-Encoded Hsv1-miR-H18 and Hsv2-miR-H9-5p in Cancer-Containing Prostate Tissue Compared to That in Benign Prostate Hyperplasia Tissue. Int. Neurourol. J. 2016, 20, 122–130. [Google Scholar] [CrossRef]
- GE, X.; WANG, X.; SHEN, P. Herpes Simplex Virus Type 2 or Human Herpesvirus 8 Infection and Prostate Cancer Risk: A Meta-Analysis. Biomed. Rep. 2013, 1, 433–439. [Google Scholar] [CrossRef]
- Thomas, F.; Elguero, E.; Brodeur, J.; Le Goff, J.; Missé, D. Herpes Simplex Virus Type 2 and Cancer: A Medical Geography Approach. Infect. Genet. Evol. 2011, 11, 1239–1242. [Google Scholar] [CrossRef]
- Tsao, Y.-H.; Hsieh, C.-J.; Juan, Y.-S.; Lee, Y.-C.; Shen, J.-T.; Wang, H.-S.; Jhan, J.-H.; Geng, J.-H. Herpes Zoster and the Subsequent Risk of Prostate Cancer in an Asian Population: A Nationwide Population-Based Cohort Study. Medicine 2020, 99, e22441. [Google Scholar] [CrossRef]
- Ali, S.H.M.; Al-Alwany, S.H.M. Molecular Localization of Epstein Barr Virus and Rb Tumor Suppressor Gene Expression in Tissues from Prostatic Adenocarcinoma and Benign Prostatic Hyperplasia. Iraqi J. Biotechnol. 2014, 13. [Google Scholar]
- Berrington de González, A.; Urban, M.; Sitas, F.; Blackburn, N.; Hale, M.; Patel, M.; Ruff, P.; Sur, R.; Newton, R.; Beral, V. Antibodies against Six Human Herpesviruses in Relation to Seven Cancers in Black South Africans: A Case Control Study. Infect. Agents Cancer 2006, 1, 2. [Google Scholar] [CrossRef][Green Version]
- Kamalov, A.A.; Mikhaleva, L.M.; Matveev, V.B.; Karpov, V.K.; Okhobotov, D.A.; Osmanov, O.A.; Akopyan, E.P.; Shaparov, B.M. Viral infections in prostate carcinogenesis: Literature review. Cancer Urol. 2022, 18, 182–189. [Google Scholar] [CrossRef]
- Ahmed, K.; Sheikh, A.; Fatima, S.; Ghulam, T.; Haider, G.; Abbas, F.; Sarria-Santamera, A.; Ghias, K.; Mughal, N.; Abidi, S.H. Differential Analysis of Histopathological and Genetic Markers of Cancer Aggressiveness, and Survival Difference in EBV-Positive and EBV-Negative Prostate Carcinoma. Sci. Rep. 2024, 14, 10315. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.; Ibrahim, M.A.A.; Abbas, F.; Sidhom, P.A.; Hegazy, M.-E.F.; Mughal, N.; Sayed, S.R.M.; Ghias, K.; Abidi, S.H. In Silico Characterization of Putative Ebv Receptor(S) on Prostate Cells and Repurposing Clinically Approved Drugs as Ebv Entry Inhibitors. Heliyon 2023. [Google Scholar] [CrossRef]
- Ferrer, F.A.; Miller, L.J.; Andrawis, R.I.; Kurtzman, S.H.; Albertsen, P.C.; Laudone, V.P.; Kreutzer, D.L. Vascular Endothelial Growth Factor (VEGF) Expression in Human Prostate Cancer: In Situ and in Vitro Expression of VEGF by Human Prostate Cancer Cells. J. Urol. 1997, 157, 2329–2333. [Google Scholar] [CrossRef] [PubMed]
- Michalaki, V.; Syrigos, K.; Charles, P.; Waxman, J. Serum Levels of IL-6 and TNF-Alpha Correlate with Clinicopathological Features and Patient Survival in Patients with Prostate Cancer. Br. J. Cancer 2004, 90, 2312–2316. [Google Scholar] [CrossRef]
- Baker, K.J.; Houston, A.; Brint, E. IL-1 Family Members in Cancer; Two Sides to Every Story. Front. Immunol. 2019, 10, 1197. [Google Scholar] [CrossRef]
- Thomas-Jardin, S.E.; Kanchwala, M.S.; Jacob, J.; Merchant, S.; Meade, R.K.; Gahnim, N.M.; Nawas, A.F.; Xing, C.; Delk, N.A. Identification of an IL-1-Induced Gene Expression Pattern in AR+ PCa Cells That Mimics the Molecular Phenotype of AR- PCa Cells. Prostate 2018, 78, 595–606. [Google Scholar] [CrossRef]
- Hirooka, Y.; Nozaki, Y. Interleukin-18 in Inflammatory Kidney Disease. Front. Med. 2021, 8, 639103. [Google Scholar] [CrossRef]
- Dwivedi, S.; Goel, A.; Natu, S.M.; Mandhani, A.; Khattri, S.; Pant, K.K. Diagnostic and Prognostic Significance of Prostate Specific Antigen and Serum Interleukin 18 and 10 in Patients with Locally Advanced Prostate Cancer: A Prospective Study. Asian Pac. J. Cancer Prev. 2011, 12, 1843–1848. [Google Scholar] [PubMed]
- Wang, X.; Breeze, A.; Kulka, M. N-3 Polyunsaturated Fatty Acids Inhibit IFN-γ-Induced IL-18 Binding Protein Production by Prostate Cancer Cells. Cancer Immunol. Immunother. 2015, 64, 249–258. [Google Scholar] [CrossRef]
- Tong, Y.; Cao, Y.; Jin, T.; Huang, Z.; He, Q.; Mao, M. Role of Interleukin-1 Family in Bone Metastasis of Prostate Cancer. Front. Oncol. 2022, 12, 951167. [Google Scholar] [CrossRef]
- Maolake, A.; Izumi, K.; Natsagdorj, A.; Iwamoto, H.; Kadomoto, S.; Makino, T.; Naito, R.; Shigehara, K.; Kadono, Y.; Hiratsuka, K.; et al. Tumor Necrosis Factor-α Induces Prostate Cancer Cell Migration in Lymphatic Metastasis through CCR7 Upregulation. Cancer Sci. 2018, 109, 1524–1531. [Google Scholar] [CrossRef] [PubMed]
- Hessels, D.; Klein Gunnewiek, J.M.T.; van Oort, I.; Karthaus, H.F.M.; van Leenders, G.J.L.; van Balken, B.; Kiemeney, L.A.; Witjes, J.A.; Schalken, J.A. DD3(PCA3)-Based Molecular Urine Analysis for the Diagnosis of Prostate Cancer. Eur. Urol. 2003, 44, 8–15; discussion 15–16. [Google Scholar] [CrossRef] [PubMed]
| Group | Number | Sample Type | HHV + | HHV − | Group Information |
|---|---|---|---|---|---|
| Prostate Cancer | 58 | Tru-cut Biopsies | 4/6.9% | 54/93.1% | Randomly selected biopsies (from core samples) |
| Benign Prostatic Hyperplasia | 58 | Tru-cut Biopsies | 15/25.9% | 43/74.1 | Randomly selected biopsies (from core samples) |
| Prostate Cancer | 49 | FFPE Tissue (Tumour-Targeted) | 34/69.4% | 15/30.6% | Areas pre-identified as tumour target zones |
| Healthy Controls (Clinically PCa-Free) | 100 | Semen fluid | 6/6% | 94/94% | Clinically healthy subjects were tested for HHV status |
| PCa + ve FFPE Samples | Inflammation Index <50% | Inflammation Index >50% and <80% | Inflammation Index >80% |
|---|---|---|---|
| HHVs positive | 13/34 | 3/34 | 18/34 |
| HHVs negative | 15/15 | 0/15 | 0/15 |
| Group | Group Information | Number | Age (Years) | PSA (ng/mL) | Origin/Ethnic Race |
|---|---|---|---|---|---|
| Prostate Cancer | Randomly selected biopsies (from core samples) | 58 | 68.7 ± 5.09 | 17.06 ± 15.84 | Bulgarian/Caucasian |
| Benign Prostatic Hyperplasia | Randomly selected biopsies (from core samples) | 58 | 64.6 ± 6.9 | 9.3 ± 1.9 | Bulgarian/Caucasian |
| Prostate Cancer (FFPE) | Areas pre-identified as tumour target zones | 49 | 66.6 ± 9.6 | 63.3 ± 35.6 | Bulgarian/Caucasian |
| Healthy Controls (Clinically PCa-Free) | Clinically healthy subjects were tested for HHV status | 100 | 60.1 ± 7.7 | All below <3 ng/mL | Bulgarian/Caucasian |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todorova, E.; Kavrakova, A.; Derimachkovski, G.; Georgieva, B.; Odzhakov, F.; Bachurska, S.; Terziev, I.; Boyadzhieva, M.-E.; Valkov, T.; Popov, E.; et al. Human Herpes Virus Genotype and Immunological Gene Expression Profile in Prostate Cancer with Prominent Inflammation. Int. J. Mol. Sci. 2025, 26, 4945. https://doi.org/10.3390/ijms26104945
Todorova E, Kavrakova A, Derimachkovski G, Georgieva B, Odzhakov F, Bachurska S, Terziev I, Boyadzhieva M-E, Valkov T, Popov E, et al. Human Herpes Virus Genotype and Immunological Gene Expression Profile in Prostate Cancer with Prominent Inflammation. International Journal of Molecular Sciences. 2025; 26(10):4945. https://doi.org/10.3390/ijms26104945
Chicago/Turabian StyleTodorova, Elena, Anita Kavrakova, Goran Derimachkovski, Bilyana Georgieva, Feodor Odzhakov, Svitlana Bachurska, Ivan Terziev, Maria-Elena Boyadzhieva, Trifon Valkov, Elenko Popov, and et al. 2025. "Human Herpes Virus Genotype and Immunological Gene Expression Profile in Prostate Cancer with Prominent Inflammation" International Journal of Molecular Sciences 26, no. 10: 4945. https://doi.org/10.3390/ijms26104945
APA StyleTodorova, E., Kavrakova, A., Derimachkovski, G., Georgieva, B., Odzhakov, F., Bachurska, S., Terziev, I., Boyadzhieva, M.-E., Valkov, T., Popov, E., Slavov, C., Tourtourikov, I., Mitev, V., & Todorova, A. (2025). Human Herpes Virus Genotype and Immunological Gene Expression Profile in Prostate Cancer with Prominent Inflammation. International Journal of Molecular Sciences, 26(10), 4945. https://doi.org/10.3390/ijms26104945







