Aerobic Exercise and PI3K Inhibitor Ameliorate Obesity Cardiomyopathy by Alleviating Pyroptosis in Middle-Aged Mice
Abstract
1. Introduction
2. Results
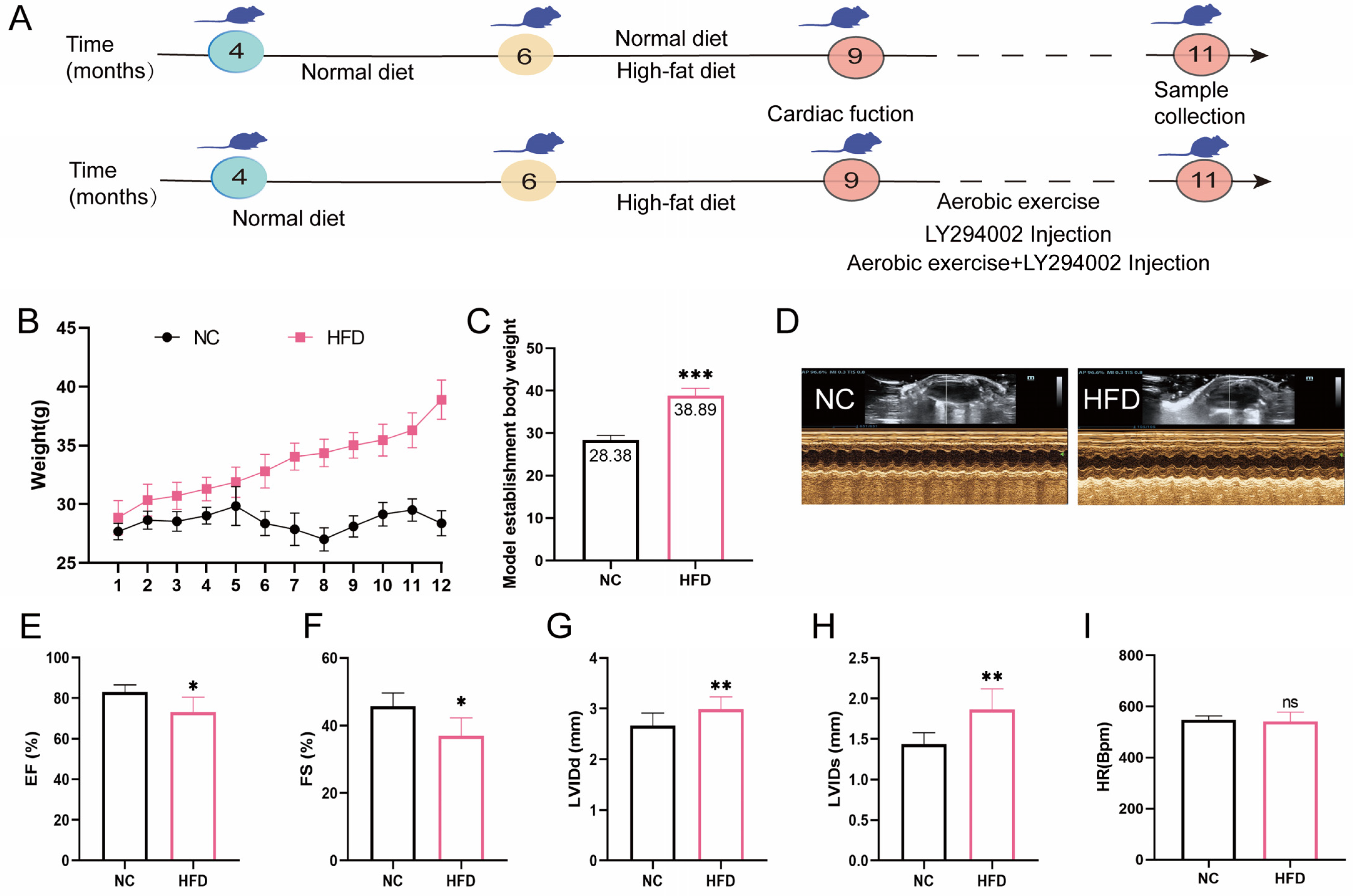
2.1. High-Fat Diet Feed Established Middle-Aged OCM Mice
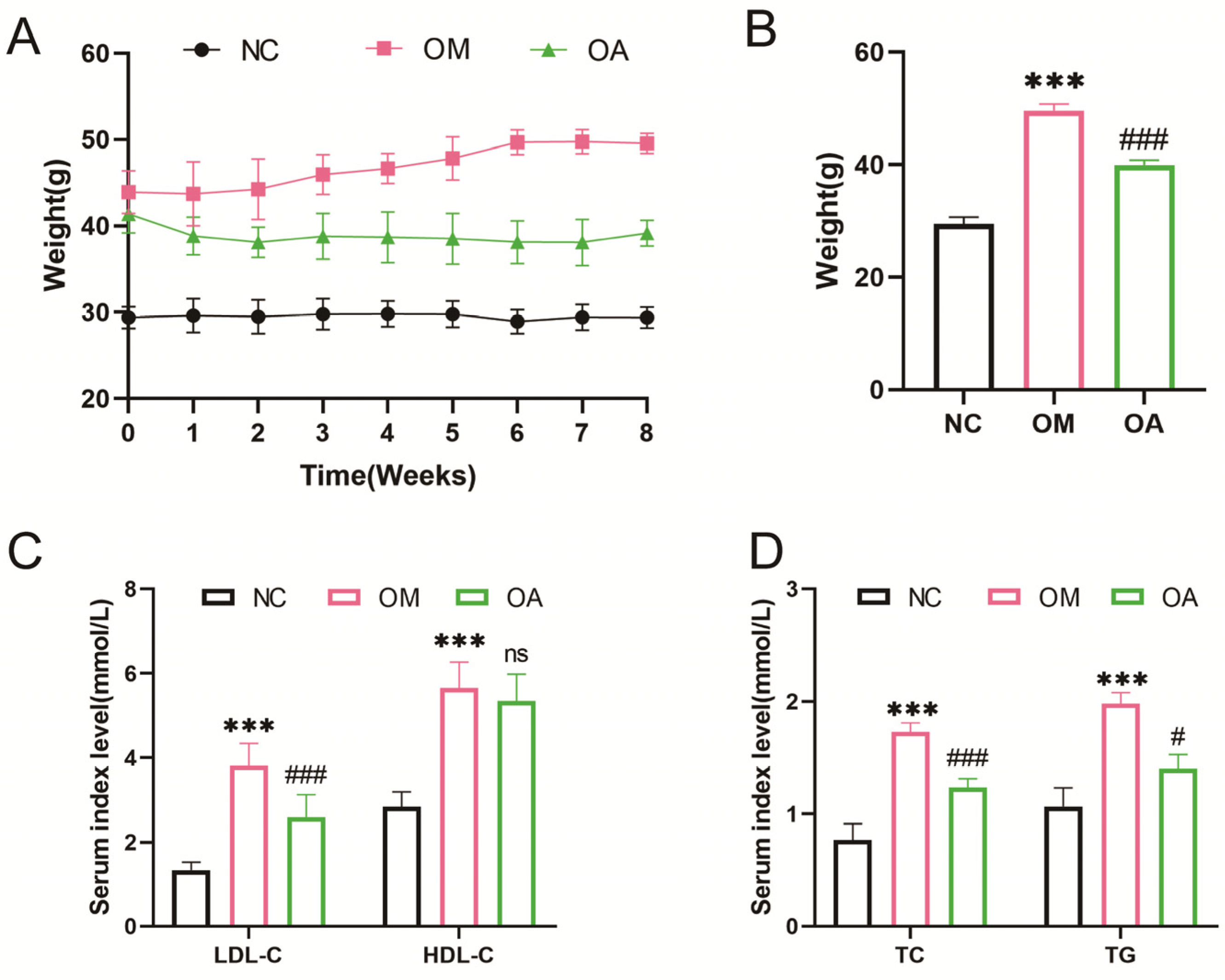
2.2. Aerobic Exercise Alleviated Weight and Blood Lipid Level in Middle-Aged OCM Mice
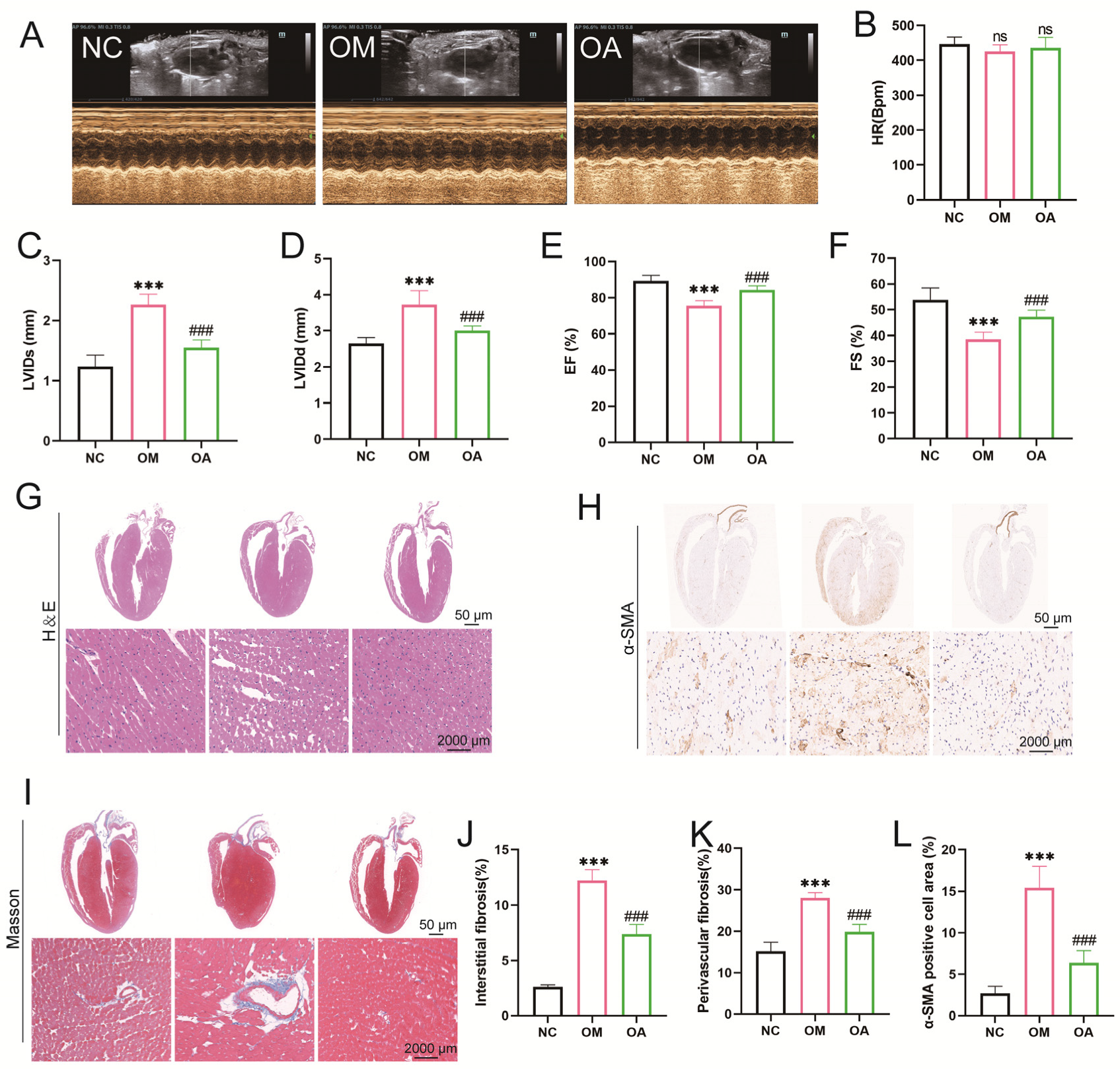
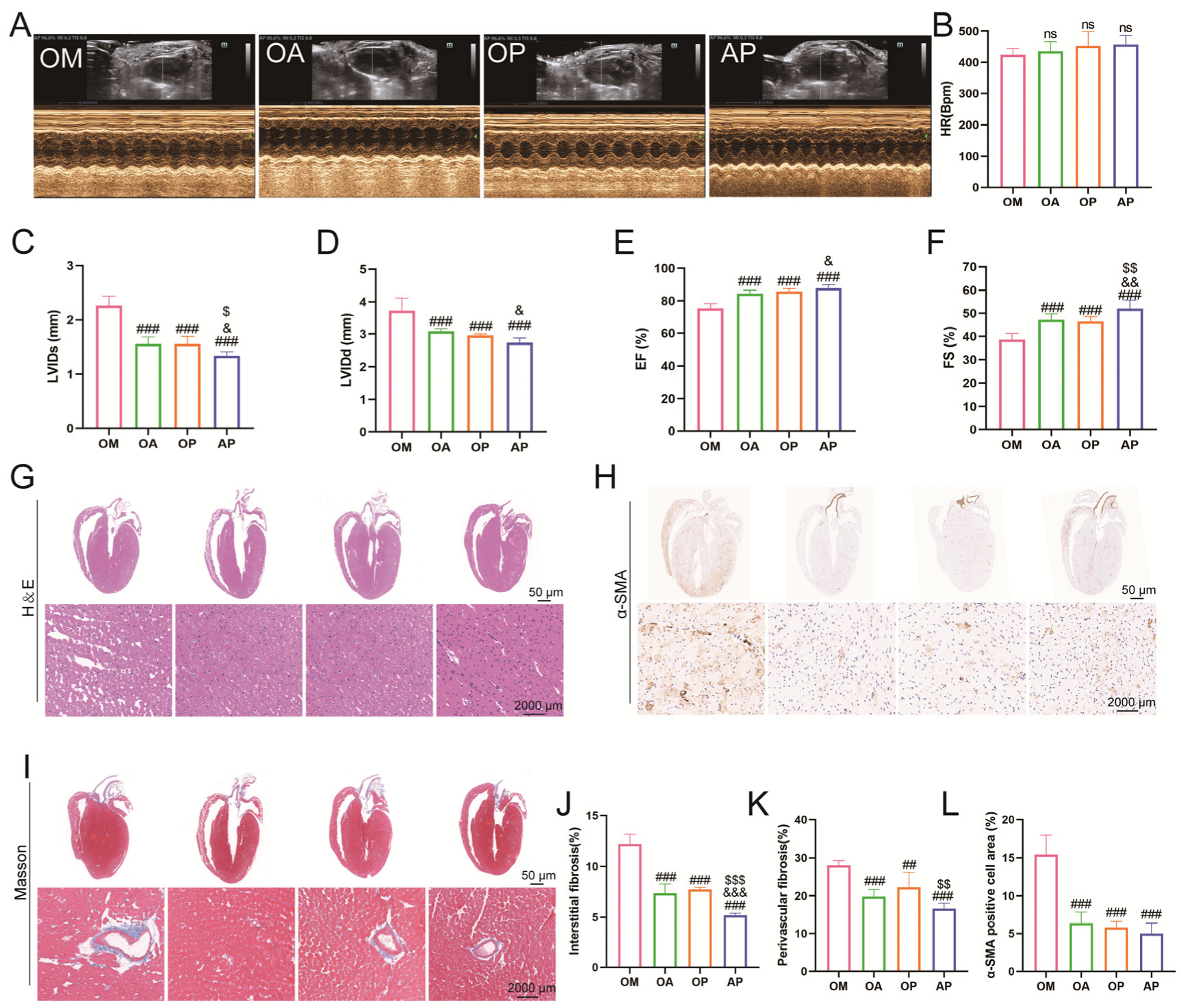
2.3. Aerobic Exercise Ameliorated Myocardial Fibrosis and Cardiac Dysfunction in Middle-Aged OCM Mice
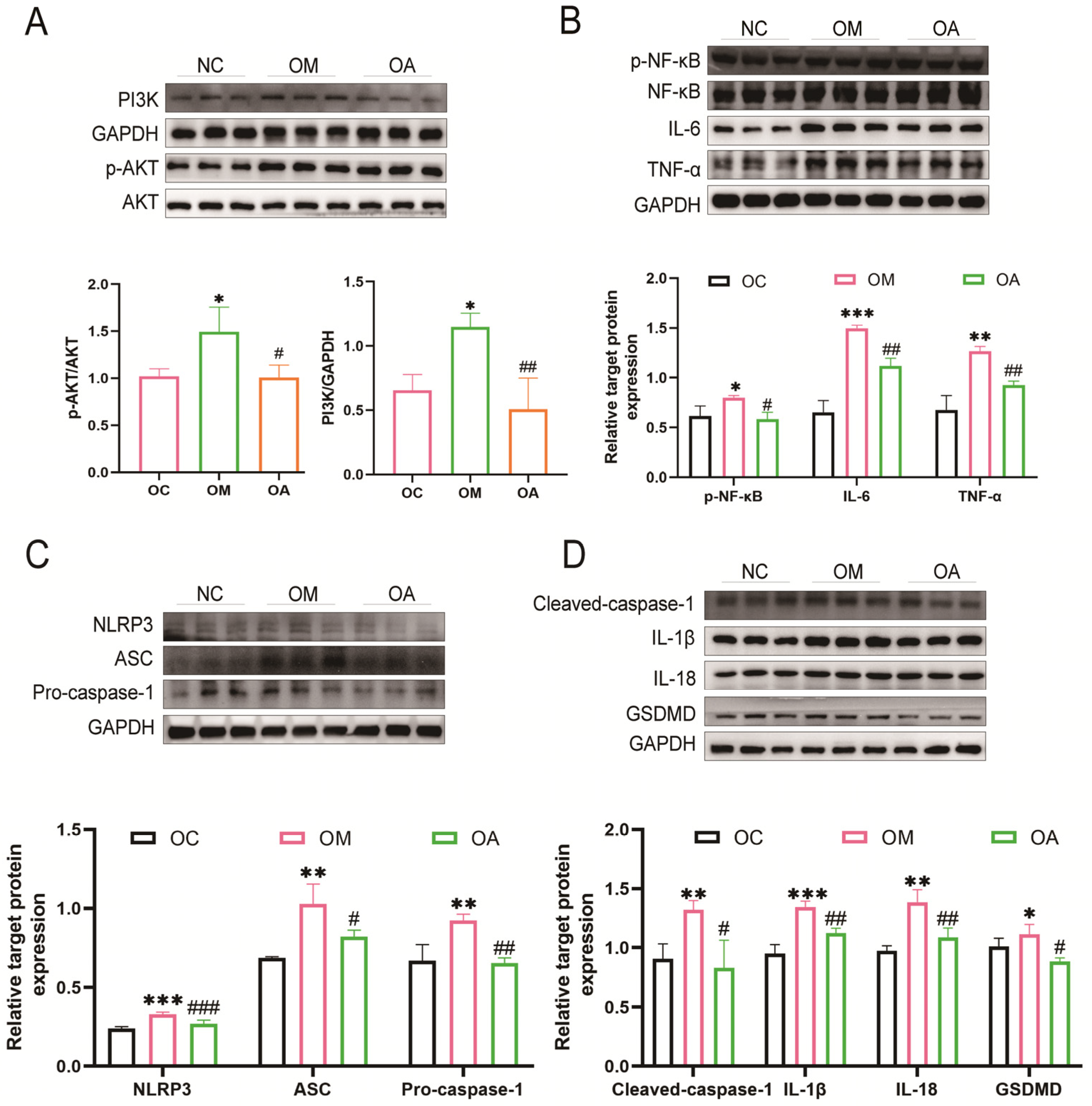
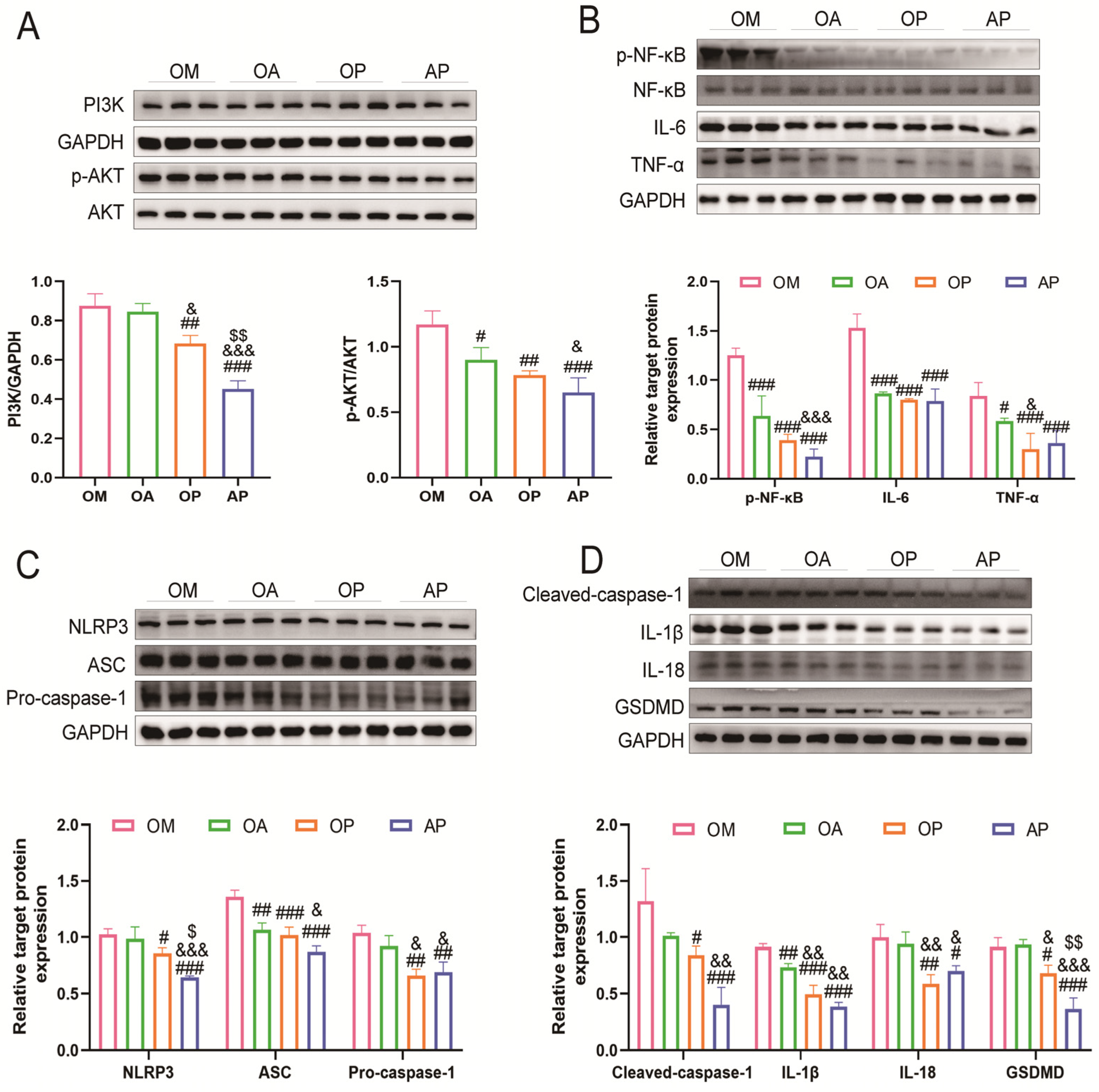
2.4. Aerobic Exercise Suppressed PI3K/AKT Activation and Activation of Pyroptosis
2.5. Inhibition of PI3K/AKT Signaling Pathway Attenuates Lipid Deposition in Middle-Aged Obese Cardiomyopathy Mice
2.6. Inhibition of PI3K Signaling Alleviates Impaired Myocardial Structure and Function in Middle-Aged OCM Mice
2.7. Exercise Synergises with PI3K Inhibitors to Inhibit Pyroptosis in Middle-Aged OCM Mice
3. Discussion
Limitations
4. Materials and Methods
4.1. Animal Groupings and Intervention Programs
4.2. Lipid Level Detection
4.3. Echocardiography
4.4. Histopathology
4.5. Immunohistochemistry
4.6. Western Blot
4.7. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kivimäki, M.; Strandberg, T.; Pentti, J.; Nyberg, S.T.; Frank, P.; Jokela, M.; Ervasti, J.; Suominen, S.B.; Vahtera, J.; Sipilä, P.N.; et al. Body-mass index and risk of obesity-related complex multimorbidity: An observational multicohort study. Lancet Diabetes Endocrinol. 2022, 10, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Lavie, C.J.; Arena, R.; Alpert, M.A.; Milani, R.V.; Ventura, H.O. Management of cardiovascular diseases in patients with obesity. Nat. Rev. Cardiol. 2018, 15, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, J.; Zhang, S.; Zhu, X.; Hu, J.; Liu, C.; Liu, L. Epigenetic regulation of diverse regulated cell death modalities in cardiovascular disease: Insights into necroptosis, pyroptosis, ferroptosis, and cuproptosis. Redox Biol. 2024, 76, 103321. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Liu, X.; Xie, M.; Zhao, R.; Ji, L.; Tang, K.; Yang, W.; Ou, W.; Xie, M.; Li, T. Obesity Enables NLRP3 Activation and Induces Myocardial Fibrosis via Hyperacetylation of HADHa. Diabetes 2023, 72, 1597–1608. [Google Scholar] [CrossRef]
- Zhou, C.; Yin, X. Wogonin Ameliorated Obesity-Induced Lipid Metabolism Disorders and Cardiac Injury via Suppressing Pyroptosis and Deactivating IL-17 Signaling Pathway. Am. J. Chin. Med. 2022, 50, 1553–1564. [Google Scholar] [CrossRef]
- Leemans, J.C.; Cassel, S.L.; Sutterwala, F.S. Sensing damage by the NLRP3 inflammasome. Immunol. Rev. 2011, 243, 152–162. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, X.; Liu, N.; Tang, L.; Peng, C.; Chen, X. Pyroptosis: Mechanisms and diseases. Signal Transduct. Target. Ther. 2021, 6, 128. [Google Scholar] [CrossRef]
- Jafari, M.; Ghadami, E.; Dadkhah, T.; Akhavan-Niaki, H. PI3k/AKT signaling pathway: Erythropoiesis and beyond. J. Cell. Physiol. 2019, 234, 2373–2385. [Google Scholar] [CrossRef]
- Xi, X.; Zhang, R.; Chi, Y.; Zhu, Z.; Sun, R.; Gong, W. TXNIP Regulates NLRP3 Inflammasome-Induced Pyroptosis Related to Aging via cAMP/PKA and PI3K/Akt Signaling Pathways. Mol. Neurobiol. 2024, 61, 8051–8068. [Google Scholar] [CrossRef]
- Perdomo, C.M.; Cohen, R.V.; Sumithran, P.; Clément, K.; Frühbeck, G. Contemporary medical, device, and surgical therapies for obesity in adults. Lancet 2023, 401, 1116–1130. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qin, S.; Tang, J.; Wang, T.; Ren, W.; Di, L.; Bo, W.; Ma, Y.; Wu, F.; Xu, Z.; et al. Resistance exercise upregulates Irisin expression and suppresses myocardial fibrosis following myocardial infarction via activating AMPK-Sirt1 and inactivating TGFβ1-Smad2/3. Acta Physiol. 2024, 240, e14163. [Google Scholar] [CrossRef]
- Kar, S.; Shahshahan, H.R.; Hackfort, B.T.; Yadav, S.K.; Yadav, R.; Kambis, T.N.; Lefer, D.J.; Mishra, P.K. Exercise Training Promotes Cardiac Hydrogen Sulfide Biosynthesis and Mitigates Pyroptosis to Prevent High-Fat Diet-Induced Diabetic Cardiomyopathy. Antioxidants 2019, 8, 638. [Google Scholar] [CrossRef]
- Suthivanich, P.; Boonhoh, W.; Sumneang, N.; Punsawad, C.; Cheng, Z.; Phungphong, S. Aerobic Exercise Attenuates Doxorubicin-Induced Cardiomyopathy by Suppressing NLRP3 Inflammasome Activation in a Rat Model. Int. J. Mol. Sci. 2024, 25, 9692. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, J. Epigenetics and obesity cardiomyopathy: From pathophysiology to prevention and management. Pharmacol. Ther. 2016, 161, 52–66. [Google Scholar] [CrossRef]
- Samson, W.K.; Yosten, G.L.C.; Remme, C.A. A primer on obesity-related cardiomyopathy. Physiol. Rev. 2022, 102, 1–6. [Google Scholar] [CrossRef]
- Wong, N.D.; Karthikeyan, H.; Fan, W. US Population Eligibility and Estimated Impact of Semaglutide Treatment on Obesity Prevalence and Cardiovascular Disease Events. Cardiovasc. Drugs Ther. 2023, 39, 75–84. [Google Scholar] [CrossRef]
- Jyothidasan, A.; Sunny, S.; Devarajan, A.; Sayed, A.; Afortude, J.K.; Dalley, B.; Nanda, V.; Pogwizd, S.; Litovsky, S.H.; Trinity, J.D.; et al. Exercise mitigates reductive stress-induced cardiac remodeling in mice. Redox Biol. 2024, 75, 103263. [Google Scholar] [CrossRef]
- Xu, Z.; Ma, Z.; Zhao, X.; Zhang, B. Aerobic exercise mitigates high-fat diet-induced cardiac dysfunction, pyroptosis, and inflammation by inhibiting STING-NLRP3 signaling pathway. Mol. Cell. Biochem. 2024, 479, 3459–3470. [Google Scholar] [CrossRef]
- Li, S.; Qian, X.; Gong, J.; Chen, J.; Tu, W.; Chen, X.; Chu, M.; Yang, G.; Li, L.; Jiang, S. Exercise Training Reverses Lipotoxicity-induced Cardiomyopathy by Inhibiting HMGCS2. Med. Sci. Sports Exerc. 2021, 53, 47–57. [Google Scholar] [CrossRef]
- Ren, J.; Wu, N.N.; Wang, S.; Sowers, J.R.; Zhang, Y. Obesity cardiomyopathy: Evidence, mechanisms, and therapeutic implications. Physiol. Rev. 2021, 101, 1745–1807. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.; Farb, M.G.; Gokce, N. Cardiometabolic implications of adipose tissue aging. Obes. Rev. 2024, 25, e13806. [Google Scholar] [CrossRef]
- Qian, C.; Xu, D.; Wang, J.; Luo, Y.; Jin, T.; Huang, L.; Zhou, Y.; Cai, Z.; Jin, B.; Bao, H.; et al. Toll-like receptor 2 deficiency ameliorates obesity-induced cardiomyopathy via inhibiting NF-κB signaling pathway. Int. Immunopharmacol. 2024, 128, 111551. [Google Scholar] [CrossRef]
- Scott, L., Jr.; Fender, A.C.; Saljic, A.; Li, L.; Chen, X.; Wang, X.; Linz, D.; Lang, J.; Hohl, M.; Twomey, D.; et al. NLRP3 inflammasome is a key driver of obesity-induced atrial arrhythmias. Cardiovasc. Res. 2021, 117, 1746–1759. [Google Scholar] [CrossRef]
- Lu, L.; Shao, Y.; Xiong, X.; Ma, J.; Zhai, M.; Lu, G.; Jiang, L.; Jin, P.; Tang, J.; Yang, J.; et al. Irisin improves diabetic cardiomyopathy-induced cardiac remodeling by regulating GSDMD-mediated pyroptosis through MITOL/STING signaling. Biomed. Pharmacother. 2024, 171, 116007. [Google Scholar] [CrossRef]
- Vandanmagsar, B.; Youm, Y.H.; Ravussin, A.; Galgani, J.E.; Stadler, K.; Mynatt, R.L.; Ravussin, E.; Stephens, J.M.; Dixit, V.D. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 2011, 17, 179–188. [Google Scholar] [CrossRef]
- Wang, J.; An, Z.; Wu, Z.; Zhou, W.; Sun, P.; Wu, P.; Dang, S.; Xue, R.; Bai, X.; Du, Y.; et al. Spatial organization of PI3K-PI(3,4,5)P(3)-AKT signaling by focal adhesions. Mol. Cell 2024, 84, 4401–4418.e9. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Liu, Y.Y.; Li, J.; Zhang, Y.Y.; Ding, Y.F.; Peng, Y.R. Gualou xiebai decoction ameliorates cardiorenal syndrome type II by regulation of PI3K/AKT/NF-κB signalling pathway. Phytomedicine 2024, 123, 155172. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Xu, H.; Liu, M.; Qian, L.; Yan, J.; Yang, G.; Chen, L. Postnatal Deletion of Bmal1 in Cardiomyocyte Promotes Pressure Overload Induced Cardiac Remodeling in Mice. J. Am. Heart Assoc. 2022, 11, e025021. [Google Scholar] [CrossRef]
- Wang, X.; Yi, X.; Tang, D. Regular aerobic exercise activates PDGF-BB/PDGFR-β signaling and modulates the inflammatory-anti-inflammatory balance in diet-induced obese mice. Obes. Res. Clin. Pract. 2021, 15, 387–394. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, J.; Liu, Y.; Li, J.; Liu, B.; Li, M.; Lou, S. Aerobic Exercise Improves Synaptic-Related Proteins of Diabetic Rats by Inhibiting FOXO1/NF-κB/NLRP3 Inflammatory Signaling Pathway and Ameliorating PI3K/Akt Insulin Signaling Pathway. J. Mol. Neurosci. 2019, 69, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Zhang, X.; Guo, Y.; Wen, H.; Guo, D.; Liang, Q.; Pu, S.; Wang, Y.; Liu, M.; Li, Z.; et al. Moderate-intensity interval exercise exacerbates cardiac lipotoxicity in high-fat, high-calories diet-fed mice. Nat. Commun. 2025, 16, 613. [Google Scholar] [CrossRef]
- Ayalasomayajula, Y.; Hesaraghatta, A.; Dantuluri, N.; Yassine, J.; Saleem, F.; Mansour, H.; Chayawatto, C.; Rangarajan, N.; Rangarajan, S.; Krishnan, S.; et al. Influence of age and sex on physical, cardiac electrical and functional alterations in progressive hyperoxia treatment: A time course study in a murine model. Exp. Gerontol. 2024, 191, 112435. [Google Scholar] [CrossRef]
- Han, Y.S.; Pakkam, M.; Fogarty, M.J.; Sieck, G.C.; Brozovich, F.V. Alterations in cardiac contractile and regulatory proteins contribute to age-related cardiac dysfunction in male rats. Physiol. Rep. 2024, 12, e70012. [Google Scholar] [CrossRef]
- Kang, R.; Laborde, C.; Savchenko, L.; Swiader, A.; Pizzinat, N.; Marsal, D.; Sainte-Marie, Y.; Boal, F.; Tronchere, H.; Roncalli, J.; et al. Age-Related Shift in Cardiac and Metabolic Phenotyping Linked to Inflammatory Cytokines and Antioxidant Status in Mice. Int. J. Mol. Sci. 2023, 24, 15841. [Google Scholar] [CrossRef]
- McMullen, J.R.; Amirahmadi, F.; Woodcock, E.A.; Schinke-Braun, M.; Bouwman, R.D.; Hewitt, K.A.; Mollica, J.P.; Zhang, L.; Zhang, Y.; Shioi, T.; et al. Protective effects of exercise and phosphoinositide 3-kinase(p110alpha) signaling in dilated and hypertrophic cardiomyopathy. Proc. Natl. Acad. Sci. USA 2007, 104, 612–617. [Google Scholar] [CrossRef]
- Liu, F.; Zhao, Y.; Pei, Y.; Lian, F.; Lin, H. Role of the NF-kB signalling pathway in heterotopic ossification: Biological and therapeutic significance. Cell Commun. Signal. 2024, 22, 159. [Google Scholar] [CrossRef]
- Alsereidi, F.R.; Khashim, Z.; Marzook, H.; Al-Rawi, A.M.; Salomon, T.; Almansoori, M.K.; Madkour, M.M.; Hamam, A.M.; Ramadan, M.M.; Peterson, Q.P.; et al. Dapagliflozin mitigates cellular stress and inflammation through PI3K/AKT pathway modulation in cardiomyocytes, aortic endothelial cells, and stem cell-derived β cells. Cardiovasc. Diabetol. 2024, 23, 388. [Google Scholar] [CrossRef]
- Wang, F.; Wang, J.; Liang, X.; Wu, Z.; Xue, J.; Yin, L.; Wei, L.; Zhang, X. Ghrelin inhibits myocardial pyroptosis in diabetic cardiomyopathy by regulating ERS and NLRP3 inflammasome crosstalk through the PI3K/AKT pathway. J. Drug Target. 2024, 32, 148–158. [Google Scholar] [CrossRef]
- Zhang, F.; Meng, T.; Feng, R.; Jin, C.; Zhang, S.; Meng, J.; Zhang, M.; Liang, C. MIF aggravates experimental autoimmune prostatitis through activation of the NLRP3 inflammasome via the PI3K/AKT pathway. Int. Immunopharmacol. 2024, 141, 112891. [Google Scholar] [CrossRef]
- Li, J.; Dong, S.; Quan, S.; Ding, S.; Zhou, X.; Yu, Y.; Wu, Y.; Huang, W.; Shi, Q.; Li, Q. Nuciferine reduces inflammation induced by cerebral ischemia-reperfusion injury through the PI3K/Akt/NF-κB pathway. Phytomedicine 2024, 125, 155312. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tu, H.; Zhang, S.; Ding, Z.; Wu, G.; Piao, J.; Lv, D.; Hu, L.; Li, F.; Wang, Q. P2Y6 Receptor Activation Aggravates NLRP3-dependent Microglial Pyroptosis via Downregulation of the PI3K/AKT Pathway in a Mouse Model of Intracerebral Hemorrhage. Mol. Neurobiol. 2024, 61, 4259–4277. [Google Scholar] [CrossRef]
- Zhu, W.; Zhu, F.; Zhang, B.; Liu, W.; Xiao, J. Effect of LY249002 on myocardial structure and cardiac function in rats with dilated cardiomyopathy. J. Cent. South Univ. Med. Sci. 2018, 43, 35–40. [Google Scholar] [CrossRef]
- Liu, J.; Jia, S.; Yang, Y.; Piao, L.; Wang, Z.; Jin, Z.; Bai, L. Exercise induced meteorin-like protects chondrocytes against inflammation and pyroptosis in osteoarthritis by inhibiting PI3K/Akt/NF-κB and NLRP3/caspase-1/GSDMD signaling. Biomed. Pharmacother. 2023, 158, 114118. [Google Scholar] [CrossRef]
- Vanhaesebroeck, B.; Perry, M.W.D.; Brown, J.R.; André, F.; Okkenhaug, K. PI3K inhibitors are finally coming of age. Nat. Rev. Drug Discov. 2021, 20, 741–769. [Google Scholar] [CrossRef]
- Gong, G.Q.; Bilanges, B.; Allsop, B.; Masson, G.R.; Roberton, V.; Askwith, T.; Oxenford, S.; Madsen, R.R.; Conduit, S.E.; Bellini, D.; et al. A small-molecule PI3Kα activator for cardioprotection and neuroregeneration. Nature 2023, 618, 159–168. [Google Scholar] [CrossRef]
- Kelly, L.M.; Rutter, J.C.; Lin, K.H.; Ling, F.; Duchmann, M.; Latour, E.; Arang, N.; Pasquer, H.; Ho Nhat, D.; Charles, J.; et al. Targeting a lineage-specific PI3Kɣ-Akt signaling module in acute myeloid leukemia using a heterobifunctional degrader molecule. Nat. Cancer 2024, 5, 1082–1101. [Google Scholar] [CrossRef]
- Hou, D.; Yu, T.; Lu, X.; Hong, J.Y.; Yang, M.; Zi, Y.; Ho, T.T.; Lin, H. Sirt2 inhibition improves gut epithelial barrier integrity and protects mice from colitis. Proc. Natl. Acad. Sci. USA 2024, 121, e2319833121. [Google Scholar] [CrossRef]
- Tong, M.; Saito, T.; Zhai, P.; Oka, S.I.; Mizushima, W.; Nakamura, M.; Ikeda, S.; Shirakabe, A.; Sadoshima, J. Alternative Mitophagy Protects the Heart Against Obesity-Associated Cardiomyopathy. Circ. Res. 2021, 129, 1105–1121. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Xu, J.; Dao, X.; Huang, Y.; Liang, J.; Huang, J.; Gou, B.; Yan, H.; Chen, N.; Fan, J. Aerobic Exercise and PI3K Inhibitor Ameliorate Obesity Cardiomyopathy by Alleviating Pyroptosis in Middle-Aged Mice. Int. J. Mol. Sci. 2025, 26, 4935. https://doi.org/10.3390/ijms26104935
Yang B, Xu J, Dao X, Huang Y, Liang J, Huang J, Gou B, Yan H, Chen N, Fan J. Aerobic Exercise and PI3K Inhibitor Ameliorate Obesity Cardiomyopathy by Alleviating Pyroptosis in Middle-Aged Mice. International Journal of Molecular Sciences. 2025; 26(10):4935. https://doi.org/10.3390/ijms26104935
Chicago/Turabian StyleYang, Bojun, Jiahao Xu, Xiaoyan Dao, Yu Huang, Jiling Liang, Jielun Huang, Bo Gou, Hanyu Yan, Ning Chen, and Jingjing Fan. 2025. "Aerobic Exercise and PI3K Inhibitor Ameliorate Obesity Cardiomyopathy by Alleviating Pyroptosis in Middle-Aged Mice" International Journal of Molecular Sciences 26, no. 10: 4935. https://doi.org/10.3390/ijms26104935
APA StyleYang, B., Xu, J., Dao, X., Huang, Y., Liang, J., Huang, J., Gou, B., Yan, H., Chen, N., & Fan, J. (2025). Aerobic Exercise and PI3K Inhibitor Ameliorate Obesity Cardiomyopathy by Alleviating Pyroptosis in Middle-Aged Mice. International Journal of Molecular Sciences, 26(10), 4935. https://doi.org/10.3390/ijms26104935





