Kisspeptin Receptor Agonists and Antagonists: Strategies for Discovery and Implications for Human Health and Disease
Abstract
1. Introduction
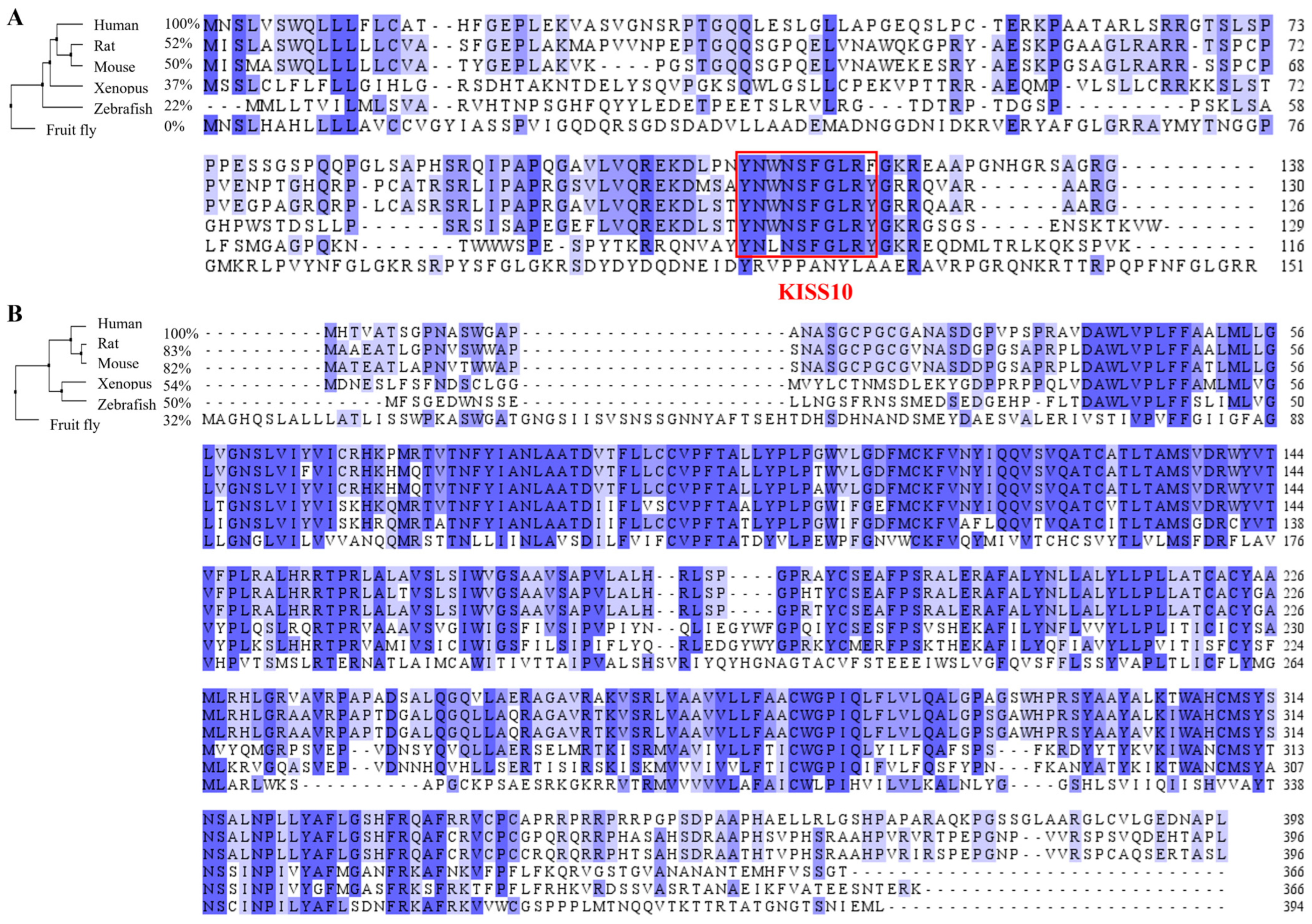
2. The Kisspeptin/Kisspeptin Receptor System
3. Role of the Kisspeptin System in Human Disease
3.1. Reproductive Disorders
3.2. Cancer
3.3. Diabetes and Metabolism
3.4. Cardiovascular Disease
4. Assay Technologies to Identify KISS1R Ligands in a High-Throughput Format
4.1. Radioligand Binding Assay
4.2. Calcium Flux Assay
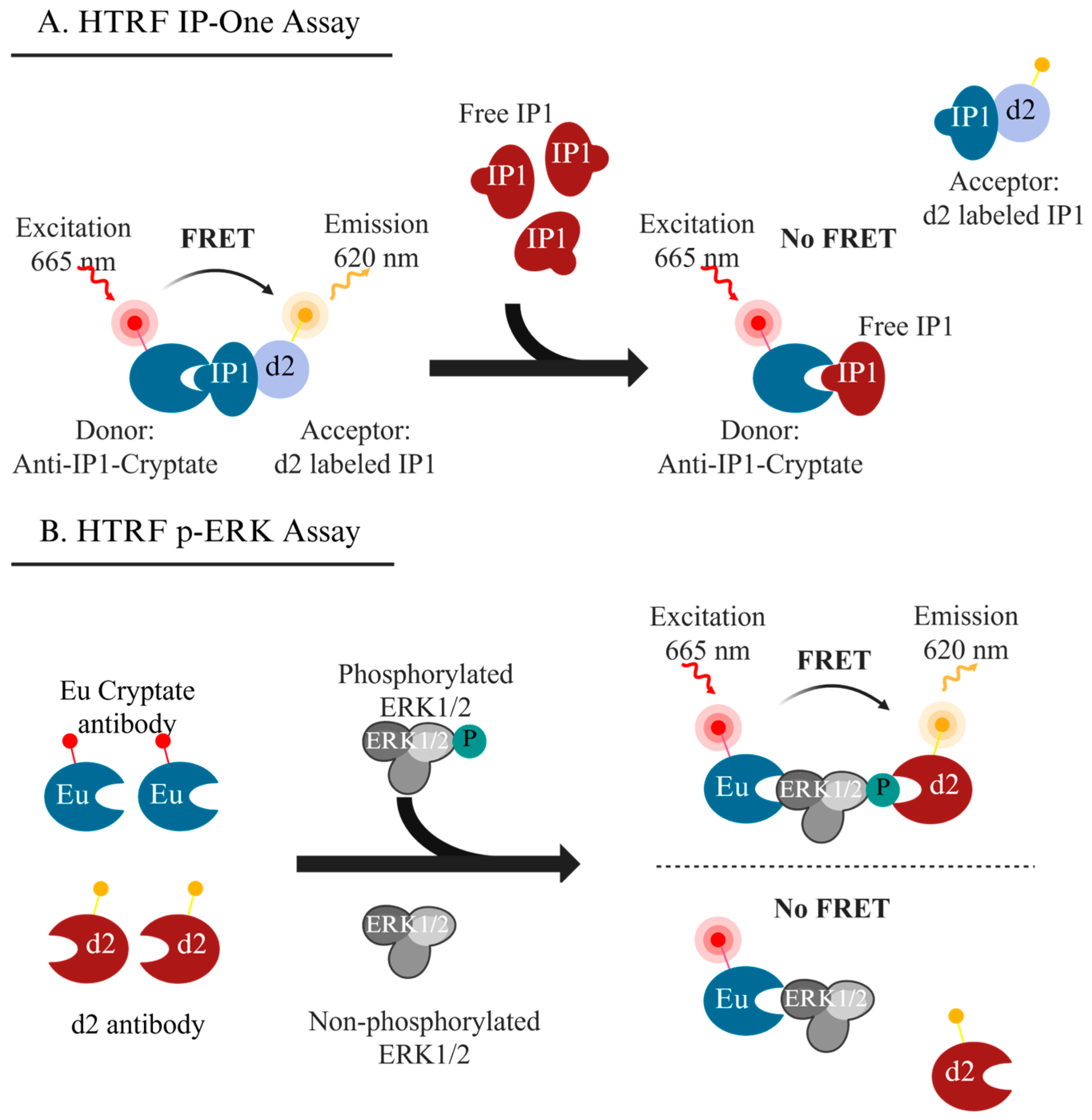
4.3. IP1 Formation Assay
4.4. Assays Targeting ERK Phosphorylation
4.5. qRT-PCR to Detect GnRH Expression
4.6. Potential AI-Based Virtual Screening Technology
5. Ligands of KISS1R
5.1. Agonists
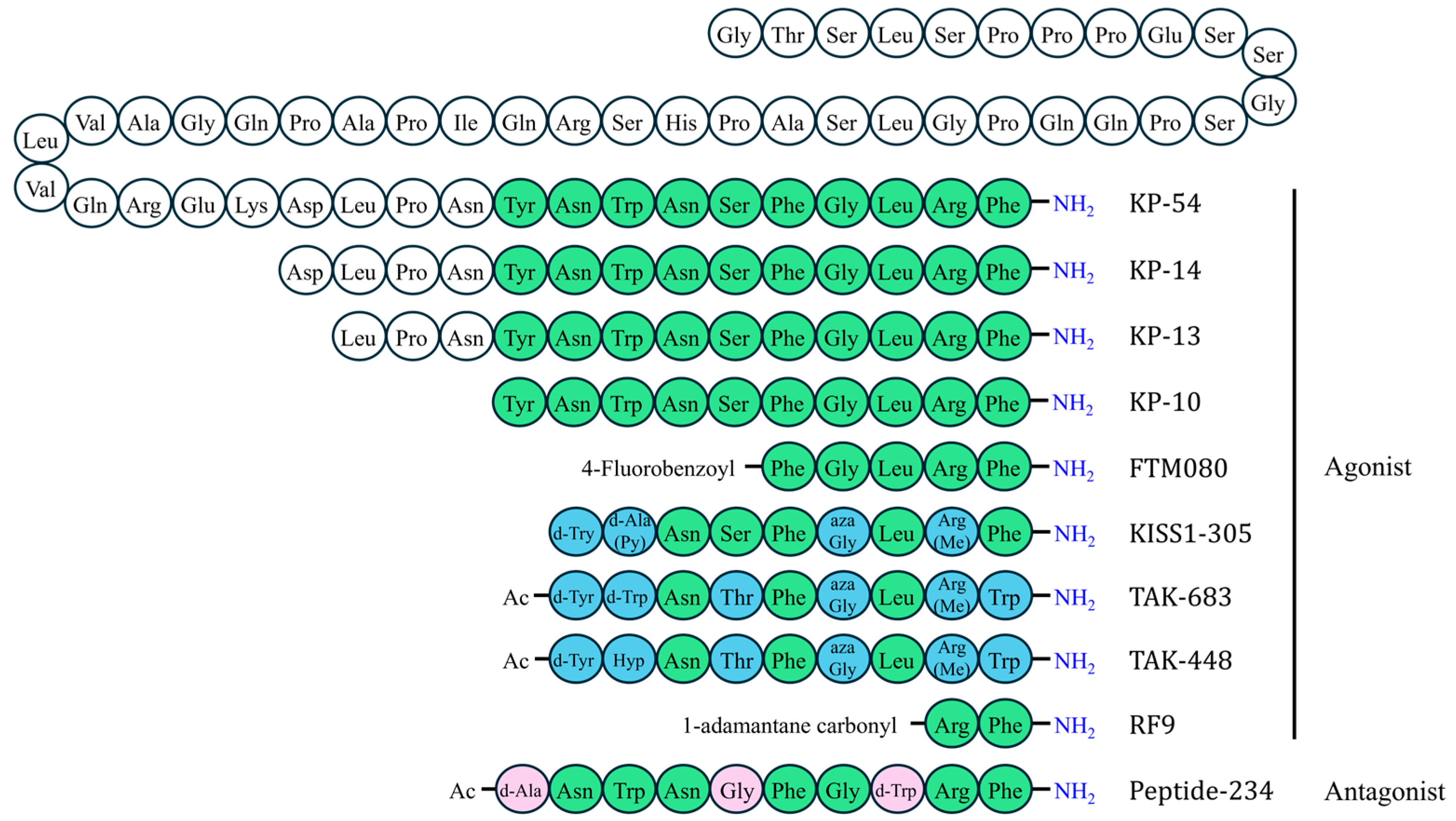
5.1.1. Kisspeptins (KP-54, KP-14, KP-13, KP-10)
5.1.2. FTM080
5.1.3. KISS1-305
5.1.4. TAK-683
5.1.5. TAK-448 (MVT-602)
5.1.6. RF9 (1-Adamantane Carbonyl-Arg-Phe-NH2)
5.1.7. Musk Ambrette
5.2. Antagonists
5.2.1. Peptide-234
5.2.2. 2-Acylamino-4,6-Diphenylpyridine Derivatives
| Compound | Object | Concentration | Measurement | Result | Reference |
|---|---|---|---|---|---|
| Peptide-234 | CHO cells expressing human KISS1R | 10 pM–10 μM | Whole-cell receptor binding assay | IC50 = 2.7 nM | Roseweir et al., 2009 [101] |
| CHO cells expressing human KISS1R | 100 pM–1 μM | IP1 | IC50 = 7 nM | Roseweir et al., 2009 [101] | |
| Female GnRH–GFP mice | 1–100 nM | Targeted extracellular recording | Blocked GnRH neuron firing by 1 nM KP-10 | Roseweir et al., 2009 [101] | |
| Female rhesus monkey | 10 nM | GnRH level | Inhibited pulsatile GnRH release | Roseweir et al., 2009 [101] | |
| Male rats and mice | 1/15 nM | LH level | Inhibited KP-10 stimulated LH | Roseweir et al., 2009 [101] | |
| Ovariectomized ewe | 40 μg | LH level | Inhibited LH secretory pulse | Roseweir et al., 2009 [101] | |
| Ovariectomized rats | 10/50 pM | LH level | Inhibited LH secretory pulse | Li et al., 2009 [105] | |
| 2-acylamino-4,6-diphenylpyridine | CHO cells expressing human KISS1R | NA | Binding assay | IC50 = 1.5 µM | Kobayashi et al., 2010 [103] |
| CHO cells expressing human KISS1R | 10 μM | Ca2+ assay | 58% inhibition | Kobayashi et al., 2010 [103] | |
| 2-acylamino-4,6-diphenylpyridine derivative, 9l | CHO cells expressing human KISS1R | NA | Binding assay | IC50 = 3.7 nM | Kobayashi et al., 2010 [103] |
| CHO cells expressing human KISS1R | NA | Ca2+ assay | IC50 = 0.46 µM | Kobayashi et al., 2010 [103] | |
| 2-acylamino-4,6-diphenylpyridine derivative, 15a | CHO cells expressing human KISS1R | NA | Binding assay | IC50 = 3.6 nM | Kobayashi et al., 2010 [104] |
| CHO cells expressing human KISS1R | 1 nM–100 μM | Ca2+ assay | IC50 = 0.93 µM | Kobayashi et al., 2010 [104] | |
| Castrated male rats | 0.22 mg/kg | LH level | Reduced plasma LH level | Kobayashi et al., 2010 [104] |
5.2.3. Other Studies
6. Future Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Kotani, M.; Detheux, M.; Vandenbogaerde, A.; Communi, D.; Vanderwinden, J.M.; Le Poul, E.; Brezillon, S.; Tyldesley, R.; Suarez-Huerta, N.; Vandeput, F.; et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J. Biol. Chem. 2001, 276, 34631–34636. [Google Scholar] [CrossRef] [PubMed]
- Navarro, V.M.; Castellano, J.M.; Fernandez-Fernandez, R.; Tovar, S.; Roa, J.; Mayen, A.; Barreiro, M.L.; Casanueva, F.F.; Aguilar, E.; Dieguez, C.; et al. Effects of KiSS-1 peptide, the natural ligand of GPR54, on follicle-stimulating hormone secretion in the rat. Endocrinology 2005, 146, 1689–1697. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Mechaly, A.S.; Somoza, G.M. Overview and New Insights Into the Diversity, Evolution, Role, and Regulation of Kisspeptins and Their Receptors in Teleost Fish. Front. Endocrinol. 2022, 13, 862614. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Babwah, A.V. Kisspeptin: Beyond the brain. Endocrinology 2015, 156, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Comninos, A.N.; Dhillo, W.S. Emerging Roles of Kisspeptin in Sexual and Emotional Brain Processing. Neuroendocrinology 2018, 106, 195–202. [Google Scholar] [CrossRef]
- Stathaki, M.; Stamatiou, M.E.; Magioris, G.; Simantiris, S.; Syrigos, N.; Dourakis, S.; Koutsilieris, M.; Armakolas, A. The role of kisspeptin system in cancer biology. Crit. Rev. Oncol. Hematol. 2019, 142, 130–140. [Google Scholar] [CrossRef]
- Mead, E.J.; Maguire, J.J.; Kuc, R.E.; Davenport, A.P. Kisspeptins: A multifunctional peptide system with a role in reproduction, cancer and the cardiovascular system. Br. J. Pharmacol. 2007, 151, 1143–1153. [Google Scholar] [CrossRef]
- Cvetkovic, D.; Babwah, A.V.; Bhattacharya, M. Kisspeptin/KISS1R System in Breast Cancer. J. Cancer 2013, 4, 653–661. [Google Scholar] [CrossRef]
- Makri, A.; Pissimissis, N.; Lembessis, P.; Polychronakos, C.; Koutsilieris, M. The kisspeptin (KiSS-1)/GPR54 system in cancer biology. Cancer Treat. Rev. 2008, 34, 682–692. [Google Scholar] [CrossRef]
- Ji, K.; Ye, L.; Mason, M.D.; Jiang, W.G. The Kiss-1/Kiss-1R complex as a negative regulator of cell motility and cancer metastasis (Review). Int. J. Mol. Med. 2013, 32, 747–754. [Google Scholar] [CrossRef]
- Teles, M.G.; Silveira, L.F.; Bianco, S.; Latronico, A.C. Human diseases associated with GPR54 mutations. Prog. Mol. Biol. Transl. Sci. 2009, 88, 33–56. [Google Scholar] [CrossRef]
- Lee, J.H.; Miele, M.E.; Hicks, D.J.; Phillips, K.K.; Trent, J.M.; Weissman, B.E.; Welch, D.R. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J. Natl. Cancer Inst. 1996, 88, 1731–1737. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Welch, D.R. Suppression of metastasis in human breast carcinoma MDA-MB-435 cells after transfection with the metastasis suppressor gene, KiSS-1. Cancer Res. 1997, 57, 2384–2387. [Google Scholar] [PubMed]
- Ohtaki, T.; Shintani, Y.; Honda, S.; Matsumoto, H.; Hori, A.; Kanehashi, K.; Terao, Y.; Kumano, S.; Takatsu, Y.; Masuda, Y.; et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature 2001, 411, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Bowe, J.E.; Hill, T.G.; Hunt, K.F.; Smith, L.I.; Simpson, S.J.; Amiel, S.A.; Jones, P.M. A role for placental kisspeptin in beta cell adaptation to pregnancy. JCI Insight 2019, 4, e124540. [Google Scholar] [CrossRef]
- Seminara, S.B.; Messager, S.; Chatzidaki, E.E.; Thresher, R.R.; Acierno, J.S., Jr.; Shagoury, J.K.; Bo-Abbas, Y.; Kuohung, W.; Schwinof, K.M.; Hendrick, A.G.; et al. The GPR54 gene as a regulator of puberty. N. Engl. J. Med. 2003, 349, 1614–1627. [Google Scholar] [CrossRef]
- De Roux, N.; Genin, E.; Carel, J.C.; Matsuda, F.; Chaussain, J.L.; Milgrom, E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc. Natl. Acad. Sci. USA 2003, 100, 10972–10976. [Google Scholar] [CrossRef]
- Teles, M.G.; Bianco, S.D.; Brito, V.N.; Trarbach, E.B.; Kuohung, W.; Xu, S.; Seminara, S.B.; Mendonca, B.B.; Kaiser, U.B.; Latronico, A.C. A GPR54-activating mutation in a patient with central precocious puberty. N. Engl. J. Med. 2008, 358, 709–715. [Google Scholar] [CrossRef]
- Silveira, L.G.; Noel, S.D.; Silveira-Neto, A.P.; Abreu, A.P.; Brito, V.N.; Santos, M.G.; Bianco, S.D.; Kuohung, W.; Xu, S.; Gryngarten, M.; et al. Mutations of the KISS1 gene in disorders of puberty. J. Clin. Endocrinol. Metab. 2010, 95, 2276–2280. [Google Scholar] [CrossRef]
- Lehman, M.N.; Coolen, L.M.; Goodman, R.L. Minireview: Kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: A central node in the control of gonadotropin-releasing hormone secretion. Endocrinology 2010, 151, 3479–3489. [Google Scholar] [CrossRef]
- Biran, J.; Ben-Dor, S.; Levavi-Sivan, B. Molecular identification and functional characterization of the kisspeptin/kisspeptin receptor system in lower vertebrates. Biol. Reprod. 2008, 79, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Terao, Y.; Kumano, S.; Takatsu, Y.; Hattori, M.; Nishimura, A.; Ohtaki, T.; Shintani, Y. Expression of KiSS-1, a metastasis suppressor gene, in trophoblast giant cells of the rat placenta. Biochim. Biophys. Acta 2004, 1678, 102–110. [Google Scholar] [CrossRef]
- Rhie, Y.J. Kisspeptin/G protein-coupled receptor-54 system as an essential gatekeeper of pubertal development. Ann. Pediatr. Endocrinol. Metab. 2013, 18, 55–59. [Google Scholar] [CrossRef]
- Wahab, F.; Atika, B.; Shahab, M.; Behr, R. Kisspeptin signalling in the physiology and pathophysiology of the urogenital system. Nat. Rev. Urol. 2016, 13, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, L.; Khan, K.; Travers, J.; Huang, R.; Jovanovic, V.M.; Veeramachaneni, R.; Sakamuru, S.; Tristan, C.A.; Davis, E.E.; et al. Identification of Environmental Compounds That May Trigger Early Female Puberty by Activating Human GnRHR and KISS1R. Endocrinology 2024, 165, bqae103. [Google Scholar] [CrossRef]
- Sukhbaatar, U.; Kanasaki, H.; Mijiddorj, T.; Oride, A.; Miyazaki, K. Kisspeptin induces expression of gonadotropin-releasing hormone receptor in GnRH-producing GT1-7 cells overexpressing G protein-coupled receptor 54. Gen. Comp. Endocrinol. 2013, 194, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Balraj, P.; Ambhore, N.S.; Ramakrishnan, Y.S.; Borkar, N.A.; Banerjee, P.; Reza, M.I.; Varadharajan, S.; Kumar, A.; Pabelick, C.M.; Prakash, Y.S.; et al. Kisspeptin/KISS1R Signaling Modulates Human Airway Smooth Muscle Cell Migration. Am. J. Respir. Cell Mol. Biol. 2024, 70, 507–518. [Google Scholar] [CrossRef]
- Parhar, I.S.; Ogawa, S.; Sakuma, Y. Laser-captured single digoxigenin-labeled neurons of gonadotropin-releasing hormone types reveal a novel g protein-coupled receptor (Gpr54) during maturation in cichlid fish. Endocrinology 2004, 145, 3613–3618. (In English) [Google Scholar] [CrossRef]
- Oakley, A.E.; Clifton, D.K.; Steiner, R.A. Kisspeptin Signaling in the Brain. Endocr. Rev. 2009, 30, 713–743. (In English) [Google Scholar] [CrossRef]
- Irwig, M.S.; Fraleyb, G.S.; Smith, J.T.; Acohido, B.V.; Popa, S.M.; Cunningham, M.J.; Gottsch, M.L.; Clifton, D.K.; Steiner, R.A. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology 2004, 80, 264–272. (In English) [Google Scholar] [CrossRef]
- Skorupskaite, K.; George, J.T.; Anderson, R.A. The kisspeptin-GnRH pathway in human reproductive health and disease. Human. Reprod. Update 2014, 20, 485–500. (In English) [Google Scholar] [CrossRef] [PubMed]
- Jayasena, C.N.; Abbara, A.; Comninos, A.N.; Nijher, G.M.; Christopoulos, G.; Narayanaswamy, S.; Izzi-Engbeaya, C.; Sridharan, M.; Mason, A.J.; Warwick, J.; et al. Kisspeptin-54 triggers egg maturation in women undergoing in vitro fertilization. J. Clin. Investig. 2014, 124, 3667–3677. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.H.; Koysombat, K.; Pierret, A.; Young, M.; Comninos, A.N.; Dhillo, W.S.; Abbara, A. Kisspeptin in functional hypothalamic amenorrhea: Pathophysiology and therapeutic potential. Ann. N. Y. Acad. Sci. 2024, 1540, 21–46. [Google Scholar] [CrossRef]
- Zhu, N.; Zhao, M.; Song, Y.; Ding, L.; Ni, Y. The KiSS-1/GPR54 system: Essential roles in physiological homeostasis and cancer biology. Genes. Dis. 2022, 9, 28–40. [Google Scholar] [CrossRef]
- Ji, K.; Ye, L.; Ruge, F.; Hargest, R.; Mason, M.D.; Jiang, W.G. Implication of metastasis suppressor gene, Kiss-1 and its receptor Kiss-1R in colorectal cancer. BMC Cancer 2014, 14, 723. (In English) [Google Scholar] [CrossRef] [PubMed]
- Goertzen, C.G.; Dragan, M.; Turley, E.; Babwah, A.V.; Bhattacharya, M. KISS1R signaling promotes invadopodia formation in human breast cancer cell via beta-arrestin2/ERK. Cell Signal 2016, 28, 165–176. [Google Scholar] [CrossRef]
- Cho, S.G.; Wang, Y.; Rodriguez, M.; Tan, K.; Zhang, W.; Luo, J.; Li, D.; Liu, M. Haploinsufficiency in the prometastasis Kiss1 receptor Gpr54 delays breast tumor initiation, progression, and lung metastasis. Cancer Res. 2011, 71, 6535–6546. [Google Scholar] [CrossRef]
- Teicher, B.A.; Fricker, S.P. CXCL12 (SDF-1)/CXCR4 Pathway in Cancer. Clin. Cancer Res. 2010, 16, 2927–2931. (In English) [Google Scholar] [CrossRef]
- Liang, Z.; Brooks, J.; Willard, M.; Liang, K.; Yoon, Y.; Kang, S.; Shim, H. CXCR4/CXCL12 axis promotes VEGF-mediated tumor angiogenesis through Akt signaling pathway. Biochem. Bioph Res. Co. 2007, 359, 716–722. (In English) [Google Scholar] [CrossRef]
- Liu, X.; Yu, J.H.; Song, S.J.; Yue, X.Q.; Li, Q. Protease-activated receptor-1 (PAR-1): A promising molecular target for cancer. Oncotarget 2017, 8, 107334–107345. (In English) [Google Scholar] [CrossRef]
- Hauge-Evans, A.C.; Richardson, C.C.; Milne, H.M.; Christie, M.R.; Persaud, S.J.; Jones, P.M. A role for kisspeptin in islet function. Diabetologia 2006, 49, 2131–2135. (In English) [Google Scholar] [CrossRef]
- Bowe, J.E.; King, A.J.; Kinsey-Jones, J.S.; Foot, V.L.; Li, X.F.; O’Byrne, K.T.; Persaud, S.J.; Jones, P.M. Kisspeptin stimulation of insulin secretion: Mechanisms of action in mouse islets and rats. Diabetologia 2009, 52, 855–862. (In English) [Google Scholar] [CrossRef] [PubMed]
- Smith, N.K.; Hackett, T.A.; Galli, A.; Flynn, C.R. GLP-1: Molecular mechanisms and outcomes of a complex signaling system. Neurochem. Int. 2019, 128, 94–105. (In English) [Google Scholar] [CrossRef] [PubMed]
- Song, W.J.; Mondal, P.; Wolfe, A.; Alonso, L.C.; Stamateris, R.; Ong, B.W.T.; Lim, O.C.; Yang, K.S.; Radovick, S.; Novaira, H.J.; et al. Glucagon Regulates Hepatic Kisspeptin to Impair Insulin Secretion. Cell Metab. 2014, 19, 667–681. (In English) [Google Scholar] [CrossRef]
- Dudek, M.; Kołodziejski, P.A.; Pruszyńska-Oszmałek, E.; Sassek, M.; Ziarniak, K.; Nowak, K.W.; Sliwowska, J.H. Effects of high-fat diet-induced obesity and diabetes on Kiss1 and GPR54 expression in the hypothalamic-pituitary-gonadal (HPG) axis and peripheral organs (fat, pancreas and liver) in male rats. Neuropeptides 2016, 56, 41–49. (In English) [Google Scholar] [CrossRef] [PubMed]
- Tolson, K.P.; Garcia, C.; Yen, S.; Simonds, S.; Stefanidis, A.; Lawrence, A.; Smith, J.T.; Kauffman, A.S. Impaired kisspeptin signaling decreases metabolism and promotes glucose intolerance and obesity. J. Clin. Investig. 2014, 124, 3075–3079. (In English) [Google Scholar] [CrossRef]
- Mead, E.J.; Maguire, J.J.; Kuc, R.E.; Davenport, A.P. Kisspeptins are novel potent vasoconstrictors in humans, with a discrete localization of their receptor, G protein-coupled receptor 54, to atherosclerosis-prone vessels. Endocrinology 2007, 148, 140–147. [Google Scholar] [CrossRef]
- Sato, K.; Shirai, R.; Hontani, M.; Shinooka, R.; Hasegawa, A.; Kichise, T.; Yamashita, T.; Yoshizawa, H.; Watanabe, R.; Matsuyama, T.A.; et al. Potent Vasoconstrictor Kisspeptin-10 Induces Atherosclerotic Plaque Progression and Instability: Reversal by its Receptor GPR54 Antagonist. J. Am. Heart Assoc. 2017, 6, e005790. [Google Scholar] [CrossRef]
- Radwanska, P.; Galdyszynska, M.; Piera, L.; Drobnik, J. Kisspeptin-10 increases collagen content in the myocardium by focal adhesion kinase activity. Sci. Rep. 2023, 13, 19977. [Google Scholar] [CrossRef]
- Nijher, G.M.; Chaudhri, O.B.; Ramachandran, R.; Murphy, K.G.; Zac-Varghese, S.E.; Fowler, A.; Chinthapalli, K.; Patterson, M.; Thompson, E.L.; Williamson, C.; et al. The effects of kisspeptin-54 on blood pressure in humans and plasma kisspeptin concentrations in hypertensive diseases of pregnancy. Br. J. Clin. Pharmacol. 2010, 70, 674–681. [Google Scholar] [CrossRef]
- Inglese, J.; Johnson, R.L.; Simeonov, A.; Xia, M.; Zheng, W.; Austin, C.P.; Auld, D.S. High-throughput screening assays for the identification of chemical probes. Nat. Chem. Biol. 2007, 3, 466–479. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Atienza, J.M.; Bernard, J.; Blanc, S.; Zhu, J.; Wang, X.; Xu, X.; Abassi, Y.A. Real-time monitoring of morphological changes in living cells by electronic cell sensor arrays: An approach to study G protein-coupled receptors. Anal. Chem. 2006, 78, 35–43. [Google Scholar] [CrossRef]
- Kuohung, W.; Burnett, M.; Mukhtyar, D.; Schuman, E.; Ni, J.; Crowley, W.F.; Glicksman, M.A.; Kaiser, U.B. A high-throughput small-molecule ligand screen targeted to agonists and antagonists of the G-protein-coupled receptor GPR54. J. Biomol. Screen. 2010, 15, 508–517. [Google Scholar] [CrossRef]
- Tomita, K.; Oishi, S.; Ohno, H.; Fujii, N. Structure-activity relationship study and NMR analysis of fluorobenzoyl pentapeptide GPR54 agonists. Biopolymers 2008, 90, 503–511. [Google Scholar] [CrossRef]
- Asami, T.; Nishizawa, N.; Matsui, H.; Nishibori, K.; Ishibashi, Y.; Horikoshi, Y.; Nakayama, M.; Matsumoto, S.; Tarui, N.; Yamaguchi, M.; et al. Design, synthesis, and biological evaluation of novel investigational nonapeptide KISS1R agonists with testosterone-suppressive activity. J. Med. Chem. 2013, 56, 8298–8307. [Google Scholar] [CrossRef]
- Dong, C.; Liu, Z.; Wang, F. Radioligand saturation binding for quantitative analysis of ligand-receptor interactions. Biophys. Rep. 2015, 1, 148–155. [Google Scholar] [CrossRef]
- Glickman, J.F.; Schmid, A.; Ferrand, S. Scintillation proximity assays in high-throughput screening. Assay. Drug Dev. Technol. 2008, 6, 433–455. [Google Scholar] [CrossRef]
- Chambers, C.; Smith, F.; Williams, C.; Marcos, S.; Liu, Z.H.; Hayter, P.; Ciaramella, G.; Keighley, W.; Gribbon, P.; Sewing, A. Measuring intracellular calcium fluxes in high throughput mode. Comb. Chem. High Throughput Screen. 2003, 6, 355–362. [Google Scholar] [CrossRef]
- Arkin, M.R.; Connor, P.R.; Emkey, R.; Garbison, K.E.; Heinz, B.A.; Wiernicki, T.R.; Johnston, P.A.; Kandasamy, R.A.; Rankl, N.B.; Sittampalam, S. FLIPR Assays for GPCR and Ion Channel Targets. In Assay Guidance Manual; Markossian, S., Grossman, A., Arkin, M., Auld, D., Austin, C., Baell, J., Brimacombe, K., Chung, T.D.Y., Coussens, N.P., Dahlin, J.L., et al., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004. [Google Scholar]
- Garbison, K.E.; Heinz, B.A.; Lajiness, M.E. IP-3/IP-1 Assays. In Assay Guidance Manual; Markossian, S., Grossman, A., Arkin, M., Auld, D., Austin, C., Baell, J., Brimacombe, K., Chung, T.D.Y., Coussens, N.P., Dahlin, J.L., et al., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004. [Google Scholar]
- Trinquet, E.; Fink, M.; Bazin, H.; Grillet, F.; Maurin, F.; Bourrier, E.; Ansanay, H.; Leroy, C.; Michaud, A.; Durroux, T.; et al. D-myo-inositol 1-phosphate as a surrogate of D-myo-inositol 1,4,5-tris phosphate to monitor G protein-coupled receptor activation. Anal. Biochem. 2006, 358, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Titus, S.; Southall, N.; Zhu, P.; Inglese, J.; Austin, C.P.; Zheng, W. Comparison on functional assays for Gq-coupled GPCRs by measuring inositol monophospate-1 and intracellular calcium in 1536-well plate format. Curr. Chem. Genom. 2008, 1, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Trinquet, E.; Bouhelal, R.; Dietz, M. Monitoring Gq-coupled receptor response through inositol phosphate quantification with the IP-One assay. Expert. Opin. Drug Discov. 2011, 6, 981–994. [Google Scholar] [CrossRef] [PubMed]
- Jamaluddin, A. Quantifying Gq Signaling Using the IP(1) Homogenous Time-Resolved Fluorescence (HTRF) Assay. Methods Mol. Biol. 2025, 2861, 23–32. [Google Scholar] [CrossRef]
- Novaira, H.J.; Ng, Y.; Wolfe, A.; Radovick, S. Kisspeptin increases GnRH mRNA expression and secretion in GnRH secreting neuronal cell lines. Mol. Cell Endocrinol. 2009, 311, 126–134. [Google Scholar] [CrossRef]
- Tumurbaatar, T.; Kanasaki, H.; Yacca, S.S.; Cairang, Z.; Tumurgan, Z.; Oride, A.; Okada, H.; Kyo, S. Kisspeptin induces Kiss-1 and GnRH gene expression in mHypoA-55 hypothalamic cell models: Involvement of the ERK and PKA signaling pathways. Gen. Comp. Endocrinol. 2023, 337, 114260. [Google Scholar] [CrossRef] [PubMed]
- Leister, K.P.; Huang, R.; Goodwin, B.L.; Chen, A.; Austin, C.P.; Xia, M. Two High Throughput Screen Assays for Measurement of TNF-alpha in THP-1 Cells. Curr. Chem. Genom. 2011, 5, 21–29. [Google Scholar] [CrossRef]
- Dehdashti, S.J.; Zheng, W.; Gever, J.R.; Wilhelm, R.; Nguyen, D.T.; Sittampalam, G.; McKew, J.C.; Austin, C.P.; Prusiner, S.B. A high-throughput screening assay for determining cellular levels of total tau protein. Curr. Alzheimer Res. 2013, 10, 679–687. [Google Scholar] [CrossRef][Green Version]
- Amendola, G.; Cosconati, S. PyRMD: A New Fully Automated AI-Powered Ligand-Based Virtual Screening Tool. J. Chem. Inf. Model. 2021, 61, 3835–3845. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Rusnac, D.V.; Park, H.; Canzani, D.; Nguyen, H.M.; Stewart, L.; Bush, M.F.; Nguyen, P.T.; Wulff, H.; Yarov-Yarovoy, V.; et al. An artificial intelligence accelerated virtual screening platform for drug discovery. Nat. Commun. 2024, 15, 7761. [Google Scholar] [CrossRef]
- Wu, Z.S.; Chen, G.; Qiu, C.; Yan, X.Y.; Xu, L.Z.; Jiang, S.R.; Xu, J.; Han, R.Y.; Shi, T.Y.; Liu, Y.M.; et al. Structural basis for the ligand recognition and G protein subtype selectivity of kisspeptin receptor. Sci. Adv. 2024, 10, eadn7771. (In English) [Google Scholar] [CrossRef]
- Shen, S.Y.; Wang, D.X.; Liu, H.; He, X.H.; Cao, Y.L.; Chen, J.H.; Li, S.J.; Cheng, X.; Xu, H.E.; Duan, J. Structural basis for hormone recognition and distinctive Gq protein coupling by the kisspeptin receptor. Cell Rep. 2024, 43, 114389. (In English) [Google Scholar] [CrossRef]
- GoBen, J.; Ribeiro, R.P.; Bier, D.; Neumaier, B.; Carloni, P.; Giorgetti, A.; Rossetti, G. AI-based identification of therapeutic agents targeting GPCRs: Introducing ligand type classifiers and systems biology. Chem. Sci. 2023, 14, 8651–8661. (In English) [Google Scholar] [CrossRef]
- Tsoutsouki, J.; Abbara, A.; Dhillo, W. Novel therapeutic avenues for kisspeptin. Curr. Opin. Pharmacol. 2022, 67, 102319. [Google Scholar] [CrossRef] [PubMed]
- Pampillo, M.; Camuso, N.; Taylor, J.E.; Szereszewski, J.M.; Ahow, M.R.; Zajac, M.; Millar, R.P.; Bhattacharya, M.; Babwah, A.V. Regulation of GPR54 signaling by GRK2 and beta-arrestin. Mol. Endocrinol. 2009, 23, 2060–2074. [Google Scholar] [CrossRef] [PubMed]
- Dhillo, W.S.; Chaudhri, O.B.; Thompson, E.L.; Murphy, K.G.; Patterson, M.; Ramachandran, R.; Nijher, G.K.; Amber, V.; Kokkinos, A.; Donaldson, M.; et al. Kisspeptin-54 stimulates gonadotropin release most potently during the preovulatory phase of the menstrual cycle in women. J. Clin. Endocrinol. Metab. 2007, 92, 3958–3966. [Google Scholar] [CrossRef] [PubMed]
- Jayasena, C.N.; Nijher, G.M.K.; Comninos, A.N.; Abbara, A.; Januszewki, A.; Vaal, M.L.; Sriskandarajah, L.; Murphy, K.G.; Farzad, Z.; Ghatei, M.A.; et al. The Effects of Kisspeptin-10 on Reproductive Hormone Release Show Sexual Dimorphism in Humans. J. Clin. Endocr. Metab. 2011, 96, E1963–E1972. (In English) [Google Scholar] [CrossRef]
- Tomita, K.; Oishi, S.; Cluzeau, J.; Ohno, H.; Navenot, J.M.; Wang, Z.X.; Peiper, S.C.; Akamatsu, M.; Fujii, N. SAR and QSAR studies on the n-terminally acylated pentapeptide agonists for GPR54. J. Med. Chem. 2007, 50, 3222–3228. (In English) [Google Scholar] [CrossRef]
- Tomita, K.; Oishi, S.; Ohno, H.; Peiper, S.C.; Fujii, N. Development of novel G-protein-coupled receptor 54 agonists with resistance to degradation by matrix metalloproteinase. J. Med. Chem. 2008, 51, 7645–7649. [Google Scholar] [CrossRef]
- Whitlock, B.K.; Daniel, J.A.; Amelse, L.L.; Tanco, V.M.; Chameroy, K.A.; Schrick, F.N. Kisspeptin receptor agonist (FTM080) increased plasma concentrations of luteinizing hormone in anestrous ewes. PeerJ 2015, 3, e1382. (In English) [Google Scholar] [CrossRef]
- Matsui, H.; Tanaka, A.; Yokoyama, K.; Takatsu, Y.; Ishikawa, K.; Asami, T.; Nishizawa, N.; Suzuki, A.; Kumano, S.; Terada, M.; et al. Chronic administration of the metastin/kisspeptin analog KISS1-305 or the investigational agent TAK-448 suppresses hypothalamic pituitary gonadal function and depletes plasma testosterone in adult male rats. Endocrinology 2012, 153, 5297–5308. [Google Scholar] [CrossRef]
- Matsui, H.; Masaki, T.; Akinaga, Y.; Kiba, A.; Takatsu, Y.; Nakata, D.; Tanaka, A.; Ban, J.K.; Matsumoto, S.; Kumano, S.; et al. Pharmacologic profiles of investigational kisspeptin/metastin analogues, TAK-448 and TAK-683, in adult male rats in comparison to the GnRH analogue leuprolide. Eur. J. Pharmacol. 2014, 735, 77–85. (In English) [Google Scholar] [CrossRef]
- MacLean, D.B.; Matsui, H.; Suri, A.; Neuwirth, R.; Colombel, M. Sustained exposure to the investigational Kisspeptin analog, TAK-448, down-regulates testosterone into the castration range in healthy males and in patients with prostate cancer: Results from two phase 1 studies. J. Clin. Endocrinol. Metab. 2014, 99, E1445–E1453. [Google Scholar] [CrossRef]
- Nishizawa, N.; Takatsu, Y.; Kumano, S.; Kiba, A.; Ban, J.; Tsutsumi, S.; Matsui, H.; Matsumoto, S.I.; Yamaguchi, M.; Ikeda, Y.; et al. Design and Synthesis of an Investigational Nonapeptide KISS1 Receptor (KISS1R) Agonist, Ac-d-Tyr-Hydroxyproline (Hyp)-Asn-Thr-Phe-azaGly-Leu-Arg(Me)-Trp-NH(2) (TAK-448), with Highly Potent Testosterone-Suppressive Activity and Excellent Water Solubility. J. Med. Chem. 2016, 59, 8804–8811. [Google Scholar] [CrossRef]
- Abbara, A.; Eng, P.C.; Phylactou, M.; Clarke, S.A.; Richardson, R.; Sykes, C.M.; Phumsatitpong, C.; Mills, E.; Modi, M.; Izzi-Engbeaya, C.; et al. Kisspeptin receptor agonist has therapeutic potential for female reproductive disorders. J. Clin. Investig. 2020, 130, 6739–6753. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.; Ahmad, I.; Howard, K.; MacLean, D.; Oliva, C.; Warrington, S.; Wilbraham, D.; Worthington, P. Double-blind, randomized, placebo-controlled study of safety, tolerability, pharmacokinetics and pharmacodynamics of TAK-683, an investigational metastin analogue in healthy men. Br. J. Clin. Pharmacol. 2013, 75, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Asami, T.; Nishizawa, N.; Matsui, H.; Takatsu, Y.; Suzuki, A.; Kiba, A.; Terada, M.; Nishibori, K.; Nakayama, M.; Ban, J.; et al. Physicochemically and pharmacokinetically stable nonapeptide KISS1 receptor agonists with highly potent testosterone-suppressive activity. J. Med. Chem. 2014, 57, 6105–6115. [Google Scholar] [CrossRef]
- Pineda, R.; Garcia-Galiano, D.; Sanchez-Garrido, M.A.; Romero, M.; Ruiz-Pino, F.; Aguilar, E.; Dijcks, F.A.; Blomenrohr, M.; Pinilla, L.; van Noort, P.I.; et al. Characterization of the potent gonadotropin-releasing activity of RF9, a selective antagonist of RF-amide-related peptides and neuropeptide FF receptors: Physiological and pharmacological implications. Endocrinology 2010, 151, 1902–1913. [Google Scholar] [CrossRef] [PubMed]
- Caraty, A.; Blomenrohr, M.; Vogel, G.M.; Lomet, D.; Briant, C.; Beltramo, M. RF9 powerfully stimulates gonadotrophin secretion in the ewe: Evidence for a seasonal threshold of sensitivity. J. Neuroendocrinol. 2012, 24, 725–736. [Google Scholar] [CrossRef]
- Liu, X.; Herbison, A.E. RF9 excitation of GnRH neurons is dependent upon Kiss1r in the adult male and female mouse. Endocrinology 2014, 155, 4915–4924. [Google Scholar] [CrossRef]
- Min, L.; Leon, S.; Li, H.; Pinilla, L.; Carroll, R.S.; Tena-Sempere, M.; Kaiser, U.B. RF9 Acts as a KISS1R Agonist In Vivo and In Vitro. Endocrinology 2015, 156, 4639–4648. [Google Scholar] [CrossRef]
- Sliwowska, J.H.; Woods, N.E.; Alzahrani, A.R.; Paspali, E.; Tate, R.J.; Ferro, V.A. Kisspeptin a potential therapeutic target in treatment of both metabolic and reproductive dysfunction. J. Diabetes 2024, 16, e13541. [Google Scholar] [CrossRef]
- Lippincott, M.F.; Leon, S.; Chan, Y.M.; Fergani, C.; Talbi, R.; Farooqi, I.S.; Jones, C.M.; Arlt, W.; Stewart, S.E.; Cole, T.R.; et al. Hypothalamic Reproductive Endocrine Pulse Generator Activity Independent of Neurokinin B and Dynorphin Signaling. J. Clin. Endocrinol. Metab. 2019, 104, 4304–4318. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.M.; Lippincott, M.F.; Butler, J.P.; Sidhoum, V.F.; Li, C.X.; Plummer, L.; Seminara, S.B. Exogenous kisspeptin administration as a probe of GnRH neuronal function in patients with idiopathic hypogonadotropic hypogonadism. J. Clin. Endocrinol. Metab. 2014, 99, E2762–E2771. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.M.; Lippincott, M.F.; Sales Barroso, P.; Alleyn, C.; Brodsky, J.; Granados, H.; Roberts, S.A.; Sandler, C.; Srivatsa, A.; Seminara, S.B. Using Kisspeptin to Predict Pubertal Outcomes for Youth With Pubertal Delay. J. Clin. Endocrinol. Metab. 2020, 105, e2717–e2725. [Google Scholar] [CrossRef]
- Chan, Y.M.; Lippincott, M.F.; Kusa, T.O.; Seminara, S.B. Divergent responses to kisspeptin in children with delayed puberty. JCI Insight 2018, 3, e99109. [Google Scholar] [CrossRef]
- Greenhill, C. Kisspeptin receptor agonist shows promise. Nat. Rev. Endocrinol. 2021, 17, 68. [Google Scholar] [CrossRef] [PubMed]
- Simonin, F.; Schmitt, M.; Laulin, J.P.; Laboureyras, E.; Jhamandas, J.H.; MacTavish, D.; Matifas, A.; Mollereau, C.; Laurent, P.; Parmentier, M.; et al. RF9, a potent and selective neuropeptide FF receptor antagonist, prevents opioid-induced tolerance associated with hyperalgesia. Proc. Natl. Acad. Sci. USA 2006, 103, 466–471. [Google Scholar] [CrossRef]
- Taylor, K.M.; Weisskopf, M.; Shine, J. Human exposure to nitro musks and the evaluation of their potential toxicity: An overview. Environ. Health-Glob. 2014, 13, 14. (In English) [Google Scholar] [CrossRef]
- Spencer, P.S.; Bischofffenton, M.C.; Moreno, O.M.; Opdyke, D.L.; Ford, R.A. Neurotoxic Properties of Musk Ambrette. Toxicol. Appl. Pharm. 1984, 75, 571–575. (In English) [Google Scholar] [CrossRef]
- Roseweir, A.K.; Kauffman, A.S.; Smith, J.T.; Guerriero, K.A.; Morgan, K.; Pielecka-Fortuna, J.; Pineda, R.; Gottsch, M.L.; Tena-Sempere, M.; Moenter, S.M.; et al. Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J. Neurosci. 2009, 29, 3920–3929. [Google Scholar] [CrossRef]
- Pineda, R.; Garcia-Galiano, D.; Roseweir, A.; Romero, M.; Sanchez-Garrido, M.A.; Ruiz-Pino, F.; Morgan, K.; Pinilla, L.; Millar, R.P.; Tena-Sempere, M. Critical roles of kisspeptins in female puberty and preovulatory gonadotropin surges as revealed by a novel antagonist. Endocrinology 2010, 151, 722–730. [Google Scholar] [CrossRef]
- Kobayashi, T.; Sasaki, S.; Tomita, N.; Fukui, S.; Kuroda, N.; Nakayama, M.; Kiba, A.; Takatsu, Y.; Ohtaki, T.; Itoh, F.; et al. Synthesis and structure-activity relationships of 2-acylamino-4,6-diphenylpyridine derivatives as novel antagonists of GPR54. Bioorganic Med. Chem. 2010, 18, 3841–3859. (In English) [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Sasaki, S.; Tomita, N.; Fukui, S.; Nakayama, M.; Kiba, A.; Kusaka, M.; Matsumoto, S.; Yamaguchi, M.; Itoh, F.; et al. 2-acylamino-4,6-diphenylpyridine derivatives as novel GPR54 antagonists with good brain exposure and in vivo efficacy for plasma LH level in male rats. Bioorganic Med. Chem. 2010, 18, 5157–5171. [Google Scholar] [CrossRef] [PubMed]
- Li, X.F.; Kinsey-Jones, J.S.; Cheng, Y.; Knox, A.M.; Lin, Y.; Petrou, N.A.; Roseweir, A.; Lightman, S.L.; Milligan, S.R.; Millar, R.P.; et al. Kisspeptin signalling in the hypothalamic arcuate nucleus regulates GnRH pulse generator frequency in the rat. PLoS ONE 2009, 4, e8334. [Google Scholar] [CrossRef] [PubMed]

| Compound | Object | Concentration | Measurement | Result | Reference |
|---|---|---|---|---|---|
| KP-54 | CHO-K1 cells expressing human or rat KISS1R | 10 pM–1 µM | Competitive binding assay | Human KISS1R: Ki = 1.45 ± 0.1 nM; rat KISS1R: Ki = 1.81 ± 0.05 nM | Kotani et al., 2001 [1] |
| CHO-K1 cells expressing human or rat KISS1R | 10 pM–1 µM | Aequorin-based functional assay to measure Ca2+ | Human KISS1R: EC50 = 5.47 ± 0.03 nM; rat KISS1R: EC50 = 1.39 ± 0.03 nM | Kotani et al., 2001 [1] | |
| Women | 0.2–6.4 nmol/kg | LH level | Increased | Dhillo et al., 2007 [76] | |
| Women | 1.6–12.8 nmol/kg | Egg maturation | Increased mature egg number | Jayasena et al., 2014 [32] | |
| KP-14 [1] | CHO-K1 cells expressing human or rat KISS1R | 10 pM–1 µM | Competitive binding assay | Human KISS1R: Ki = 1.65 ± 0.15 nM; rat KISS1R: Ki = 2.04 ± 0.03 nM | Kotani et al., 2001 [1] |
| CHO-K1 cells expressing human or rat KISS1R | 10 pM–1 µM | Aequorin-based functional assay to measure Ca2+ | Human KISS1R: EC50 = 7.22 ± 0.07 nM; rat KISS1R: EC50 = 1.33 ± 0.01 nM | Kotani et al., 2001 [1] | |
| KP-13 | CHO-K1 cells expressing human or rat KISS1R | 10 pM–1 µM | Competitive binding assay | Human KISS1R: Ki = 4.23 ± 0.10 nM; rat KISS1R: Ki = 2.08 ± 0.04 nM | Kotani et al., 2001 [1] |
| CHO-K1 cells expressing human or rat KISS1R | 10 pM–1 µM | Aequorin-based functional assay to measure Ca2+ | Human KISS1R: EC50= 4.62 ± 0.02 nM; rat KISS1R: EC50 = 1.38 ± 0.02 nM | Kotani et al., 2001 [1] | |
| KP-10 | CHO-K1 cells expressing human or rat KISS1R | 10 pM–1 µM | Saturation binding assay | Human KISS1R: Kd = 1.9 ± 0.4 nM; rat KISS1R: Kd = 1.0 ± 0.1 nM | Kotani et al., 2001 [1] |
| CHO-K1 cells expressing human or rat KISS1R | 10 pM–1 µM | Competitive binding assay | Human KISS1R: Ki = 2.33 ± 0.13 nM; rat KISS1R, Ki = 1.59 ± 0.07 nM | Kotani et al., 2001 [1] | |
| CHO-K1 cells expressing human or rat KISS1R | 10 pM–1 µM | Aequorin-based functional assay to measure Ca2+ | Human KISS1R: EC50= 4.13 ± 0.02 nM; rat KISS1R: EC50 = 1.17 ± 0.02 nM | Kotani et al., 2001 [1] | |
| Men and women | 0.3–32 nmol/kg | LH and FSH | Elevated | Jayasena et al., 2011 [77] | |
| FTM080 | CHO cells expressing KISS1R | NA | Ca2+ assay | EC50 = 0.45–0.69 nM | Tomita et al., 2007 [78]; Tomita et al., 2008 [79] |
| CHO cells expressing KISS1R | NA | Competitive binding assay | IC50 = 0.71 nM | Tomita et al., 2008 [79] | |
| Anestrous ewe | 500–5000 pmol/kg BW | LH | Elevated | Whitlock et al., 2015 [80] | |
| KISS1-305 | Male rats | 1–4 nmol/h | LH, testosterone, gene expression, and genital organ | Elevated plasma LH and testosterone; no alteration in gnrh expression; reduced genital organ weight. | Matsui et al., 2012 [81] |
| CHO cells expressing human KISS1R | NA | Ca2+ assay | EC50 = 4.8 nM | Asami et al., 2013 [55] | |
| CHO cells expressing human KISS1R | NA | Cell membrane binding assay | Human KISS1R: Ki = 0.089 nM; rat KISS1R: Ki = 0.10 nM | Asami et al., 2013 [55] | |
| TAK-448/MVT-602 | Male rats | 0.1 nmol/h | LH/FSH/testosterone/GnRH level and gene expression | Decreased plasma LH, FSH, testosterone, and hypothalamic GnRH | Matsui et al., 2012 [81] |
| CHO cells expressing rat KISS1R | NA | Ca2+ assay | EC50 = 632 pM | Matsui et al., 2014 [82] | |
| CHO cells expressing rat KISS1R | NA | Competitive binding assay | IC50 = 460 pM | Matsui et al., 2014 [82] | |
| Male rats | 0.008–8 µmol/kg/day | LH, testosterone, and genital organ weights | Elevated plasma LH and testosterone; reduced genital organ weights | Matsui et al., 2014 [82] | |
| Healthy men/patients with prostate cancer | 0.01–6 mg/day | Testosterone level | Healthy men: increased; patient: decreased | MacLean et al., 2014 [83] | |
| CHO cells expressing human or rat KISS1R | NA | Ca2+ assay | Human KISS1R: EC50 = 5.2 nM; rat KISS1R: EC50 = 36 nM | Nishizawa et al., 2016 [84] | |
| HEK293 cells expressing FLAG-KISS1R | 10 pM–1 µM | IP1 assay | EC50 = 10.71 | Abbara et al., 2020 [85] | |
| Women | 0.01/0.03 nmol/kg | LH | Elevated | Abbara et al., 2020 [85] | |
| TAK-683 | Healthy men | 0.01–2.0 mg/day | LH, FSH | Suppressed LH, FSH, and testosterone | Scott et al., 2013 [86] |
| CHO cells expressing human or rat KISS1R | NA | Ca2+ assay | Human KISS1R: EC50 = 0.33 nM; rat KISS1R: EC50 = 1.3 nM | Asami et al., 2014 [87] | |
| CHO cells expressing human KISS1R | NA | Cell membrane binding assay | Human KISS1R: Ki = 0.036 nM; rat KISS1R: Ki = 0.069 nM | Asami et al., 2014 [87] | |
| CHO cells expressing rat KISS1R | NA | Ca2+ assay | EC50 = 180 pM | Matsui et al., 2014 [82] | |
| CHO cells expressing rat KISS1R | NA | Competitive binding assay | IC50 = 170 pM | Matsui et al., 2014 [82] | |
| Male rats | 0.008–8 µmol/kg/day | LH, testosterone, and genital organ weights | Elevated plasma LH and testosterone; reduced genital organ weights | Matsui et al., 2014 [82] | |
| RF9 | Male and female rats | 0.01–20 nM | LH and FSH | Evoked a dose-dependent increase in LH and FSH levels | Pineda et al., 2010 [88] |
| Ewes | 2.1–18.6 μmol/h per ewe | LH | Induced plasma LH | Caraty et al., 2012 [89] | |
| GnRH-GFP or Kiss1r- null male and female rats | 0.05, 0.2, and 1μM | Cell-attached voltage of GnRH neuron | Generated an inward current in GnRH neurons | Liu et al., 2014 [90] | |
| CHO cells expressing human KISS1R | 10 pM–100 µM | Binding assay | Kd = 16 µM | Min et al., 2015 [91] | |
| CHO cells expressing human KISS1R | 1 nM–100 µM | Ca2+ assay | EC50 = 3 µM | Min et al., 2015 [91] | |
| CHO cells expressing human KISS1R | 1 nM–10 µM | IP1 | EC50 = 0.16 µM | Min et al., 2015 [91] | |
| NPFFR1−/−, KISS1R−/−, and NPFFR1−/−/KISS1R−/− mice | 5 nM/5 µL | LH | Stimulated a robust LH increase in Npffr1−/− mice | Min et al., 2015 [91] | |
| Musk ambrette | HEK293 cells expressing human KISS1R | 1 nM–66 µM | Ca2+ assay | EC50 = 16.71 µM | Yang et al., 2024 [25] |
| HEK293 cells expressing human KISS1R | 2 nM–115 µM | pERK assay | EC50 = 55.86 µM | Yang et al., 2024 [25] | |
| Murine hypothalamic cells | 6.25–50 µM | Gnrh1 expression | EC50 = 21.94 µM | Yang et al., 2024 [25] | |
| GnRH3-GFP zebrafish | 0.1–1 µg/mL | Gnrh3 expression | Expanded GnRH neuronal area | Yang et al., 2024 [25] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Yang, S.; Shaw, N.D.; Xia, M. Kisspeptin Receptor Agonists and Antagonists: Strategies for Discovery and Implications for Human Health and Disease. Int. J. Mol. Sci. 2025, 26, 4890. https://doi.org/10.3390/ijms26104890
Chen X, Yang S, Shaw ND, Xia M. Kisspeptin Receptor Agonists and Antagonists: Strategies for Discovery and Implications for Human Health and Disease. International Journal of Molecular Sciences. 2025; 26(10):4890. https://doi.org/10.3390/ijms26104890
Chicago/Turabian StyleChen, Xing, Shu Yang, Natalie D. Shaw, and Menghang Xia. 2025. "Kisspeptin Receptor Agonists and Antagonists: Strategies for Discovery and Implications for Human Health and Disease" International Journal of Molecular Sciences 26, no. 10: 4890. https://doi.org/10.3390/ijms26104890
APA StyleChen, X., Yang, S., Shaw, N. D., & Xia, M. (2025). Kisspeptin Receptor Agonists and Antagonists: Strategies for Discovery and Implications for Human Health and Disease. International Journal of Molecular Sciences, 26(10), 4890. https://doi.org/10.3390/ijms26104890






