Increased ROS and Persistent Pro-Inflammatory Responses in a Diabetic Wound Healing Model (db/db): Implications for Delayed Wound Healing
Abstract
1. Introduction
2. Results
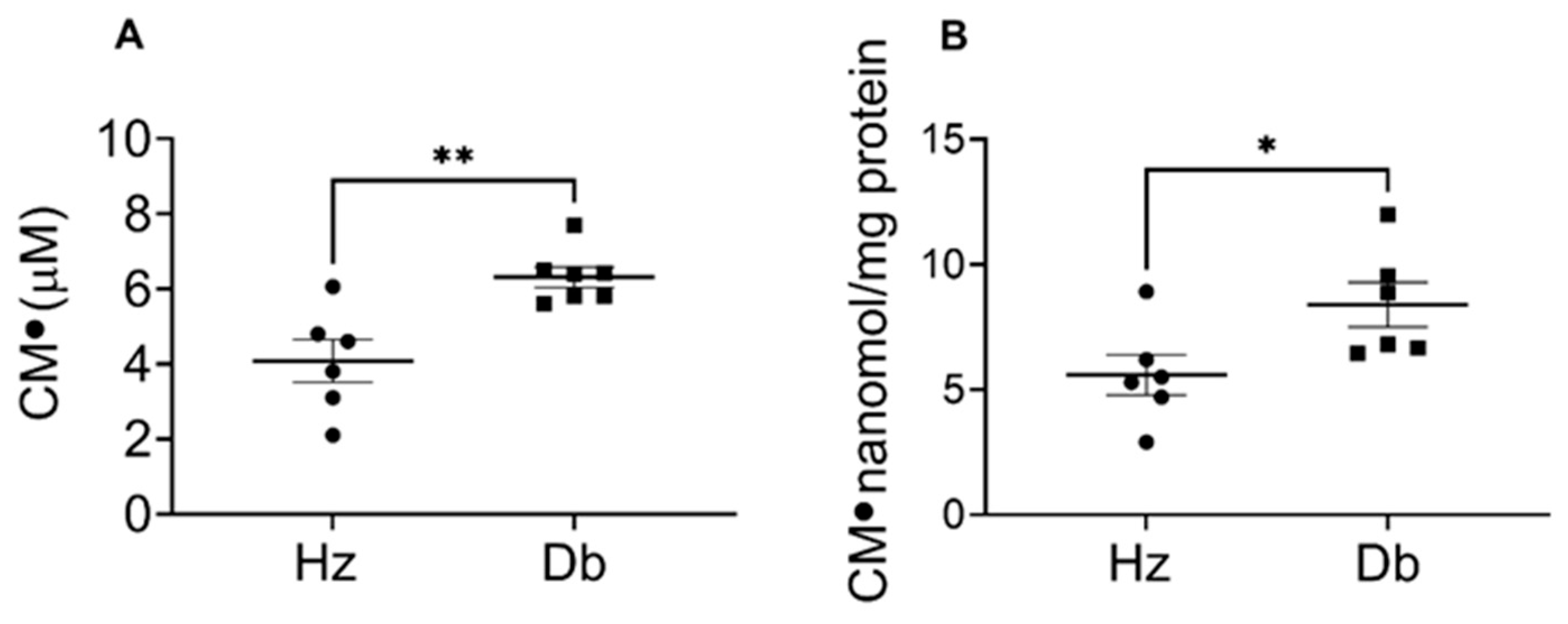
2.1. Superoxide Levels Are Elevated in Diabetic Blood and Dermal Fibroblasts
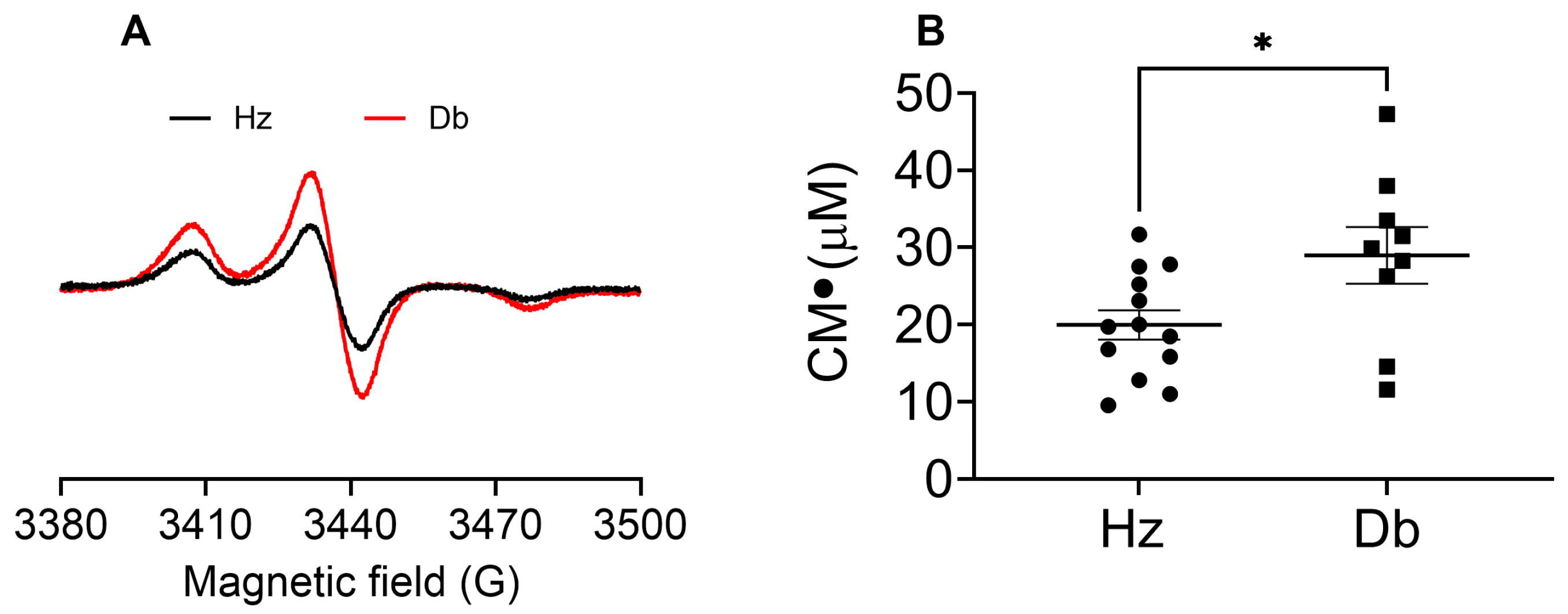
2.2. Superoxide Levels Are Elevated in Diabetic Wounds
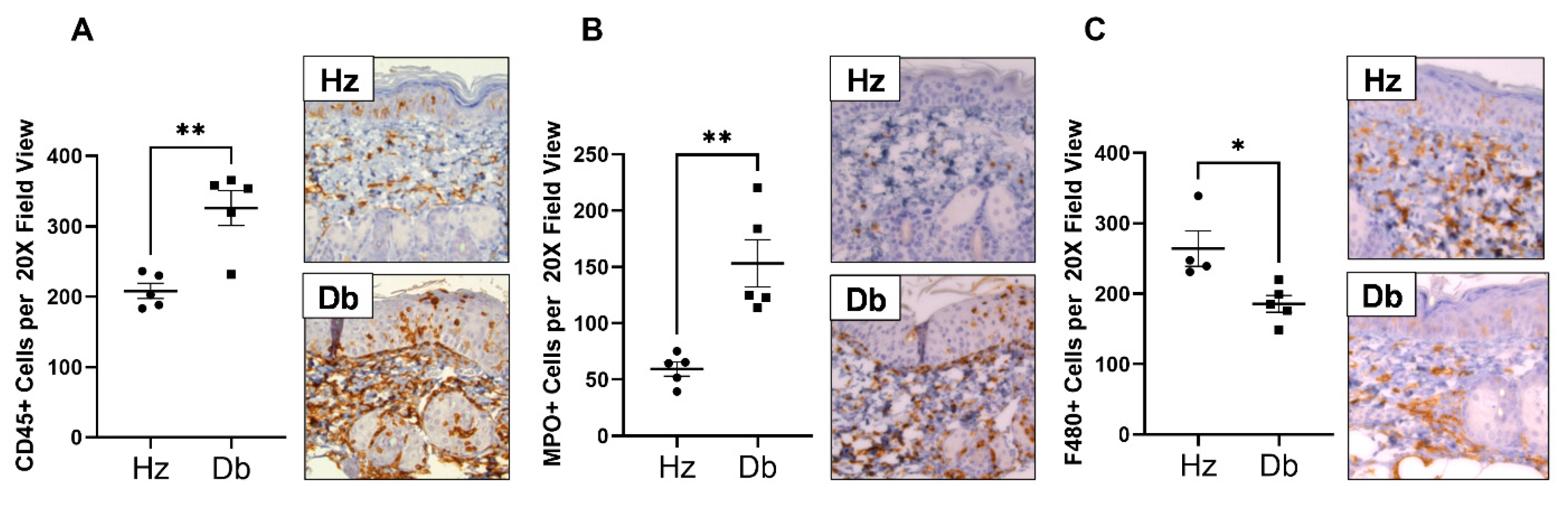
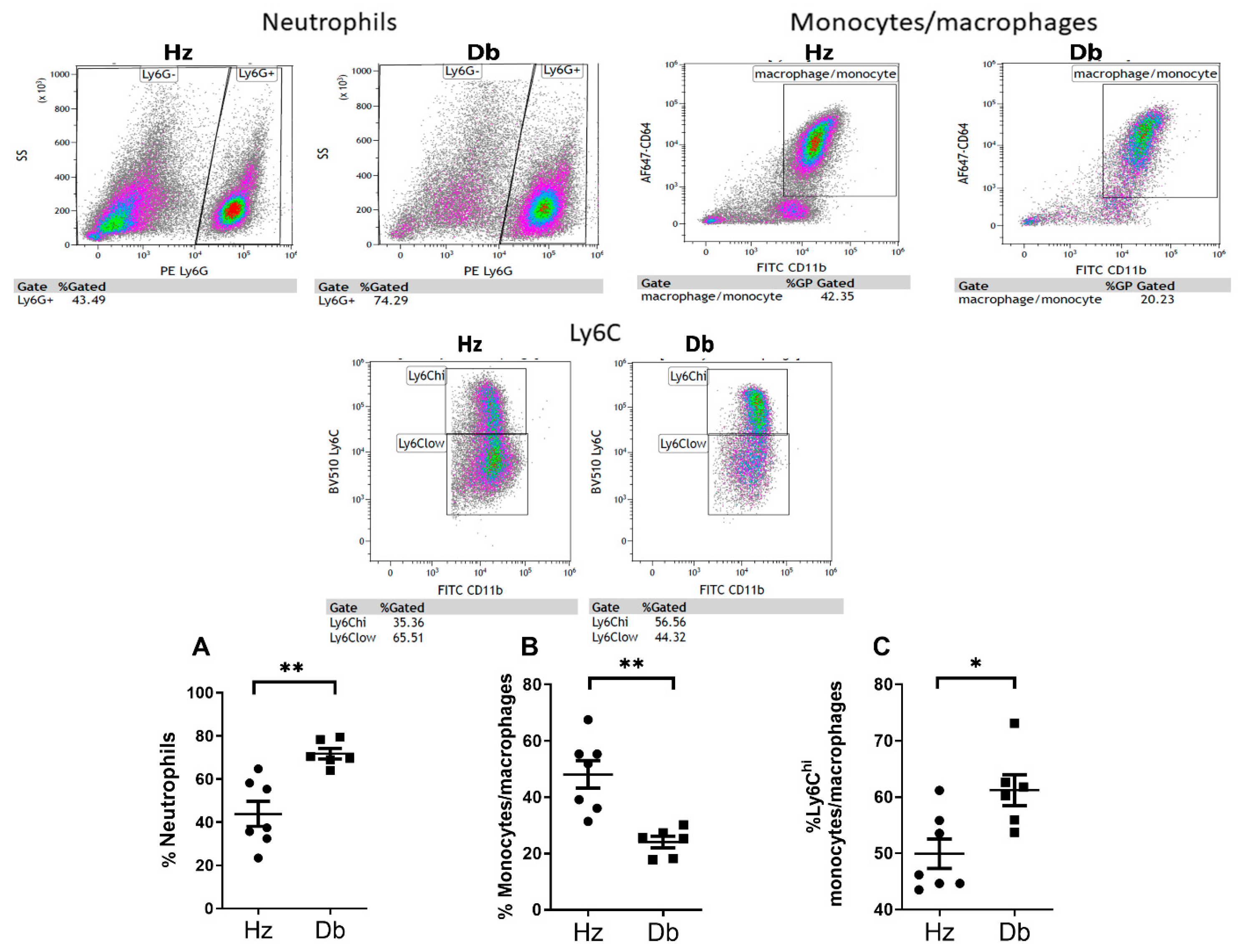
2.3. Diabetic Wounds Exhibit an Altered Immune Cell Repertoire Characterized by Increased Neutrophils, Decreased Total Macrophages, and an Increased Ratio of Pro-Inflammatory (Ly6Chi) Macrophages
3. Discussion
4. Materials and Methods
Animal Model of Diabetic Wound Healing
5. Methods
5.1. Electron Paramagnetic Resonance (EPR) Spectroscopy
5.1.1. Superoxide in the Blood
5.1.2. Superoxide in Dermal Fibroblasts
5.1.3. Superoxide in Wounds
5.1.4. Immunohistochemistry
5.1.5. Flow Cytometry
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hehenberger, K.; Kratz, G.; Hansson, A.; Brismar, K. Fibroblasts derived from human chronic diabetic wounds have a decreased proliferation rate, which is recovered by the addition of heparin. J. Dermatol. Sci. 1998, 16, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Loots, M.A.; Lamme, E.N.; Mekkes, J.R.; Bos, J.D.; Middelkoop, E. Cultured fibroblasts from chronic diabetic wounds on the lower extremity (non-insulin-dependent diabetes mellitus) show disturbed proliferation. Arch. Dermatol. Res. 1999, 291, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Seibold, J.R.; Uitto, J.; Dorwart, B.B.; Prockop, D.J. Collagen synthesis and collagenase activity in dermal fibroblasts from patients with diabetes and digital sclerosis. J. Lab. Clin. Med. 1985, 105, 664–667. [Google Scholar]
- Moura, J.; Børsheim, E.; Carvalho, E. The Role of MicroRNAs in Diabetic Complications-Special Emphasis on Wound Healing. Genes 2014, 5, 926–956. [Google Scholar] [CrossRef]
- Rafehi, H.; El-Osta, A.; Karagiannis, T.C. Epigenetic mechanisms in the pathogenesis of diabetic foot ulcers. J. Diabetes Complicat. 2012, 26, 554–561. [Google Scholar] [CrossRef]
- Blakytny, R.; Jude, E. The molecular biology of chronic wounds and delayed healing in diabetes. Diabet. Med. 2006, 23, 594–608. [Google Scholar] [CrossRef]
- Moura, J.; Madureira, P.; Leal, E.C.; Fonseca, A.C.; Carvalho, E. Immune aging in diabetes and its implications in wound healing. Clin. Immunol. 2019, 200, 43–54. [Google Scholar] [CrossRef]
- Sen, C.K.; Roy, S. Redox signals in wound healing. Biochim. Biophys. Acta 2008, 1780, 1348–1361. [Google Scholar] [CrossRef]
- Hunt, M.; Torres, M.; Bachar-Wikstrom, E.; Wikstrom, J.D. Cellular and molecular roles of reactive oxygen species in wound healing. Commun. Biol. 2024, 7, 1534. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, Z. ROS-Scavenging materials for skin wound healing: Advancements and applications. Front. Bioeng. Biotechnol. 2023, 11, 1304835. [Google Scholar] [CrossRef]
- Khorsandi, K.; Hosseinzadeh, R.; Esfahani, H.; Zandsalimi, K.; Shahidi, F.K.; Abrahamse, H. Accelerating skin regeneration and wound healing by controlled ROS from photodynamic treatment. Inflamm. Regen. 2022, 42, 40. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; He, W.; Mu, X.; Wu, X.; Deng, J.; Nie, X. Fibroblasts: Immunomodulatory factors in refractory diabetic wound healing. Front. Immunol. 2022, 13, 918223. [Google Scholar] [CrossRef]
- Martin, P.; Nunan, R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br. J. Dermatol. 2015, 173, 370–378. [Google Scholar] [CrossRef]
- Darenskaya, M.A.; Kolesnikova, L.I.; Kolesnikov, S.I. Oxidative Stress: Pathogenetic Role in Diabetes Mellitus and Its Complications and Therapeutic Approaches to Correction. Bull. Exp. Biol. Med. 2021, 171, 179–189. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M.; Schmidt, A.M. Oxidative Stress and Diabetic Complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Kimball, A.; Schaller, M.; Joshi, A.; Davis, F.M.; denDekker, A.; Boniakowski, A.; Bermick, J.; Obi, A.; Moore, B.; Henke, P.K.; et al. Ly6CHi Blood Monocyte/Macrophage Drive Chronic Inflammation and Impair Wound Healing in Diabetes Mellitus. Arter. Thromb. Vasc. Biol. 2018, 38, 1102–1114. [Google Scholar] [CrossRef]
- Bannon, P.; Wood, S.; Restivo, T.; Campbell, L.; Hardman, M.J.; Mace, K.A. Diabetes induces stable intrinsic changes to myeloid cells that contribute to chronic inflammation during wound healing in mice. Dis. Models Mech. 2013, 6, 1434–1447. [Google Scholar] [CrossRef]
- Barman, P.K.; Koh, T.J. Macrophage Dysregulation and Impaired Skin Wound Healing in Diabetes. Front. Cell Dev. Biol. 2020, 8, 528. [Google Scholar] [CrossRef]
- Mirza, R.; Koh, T.J. Dysregulation of monocyte/macrophage phenotype in wounds of diabetic mice. Cytokine 2011, 56, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, T.; Filgueiras, L.; Silva, I.A., Jr.; Pessoa, A.F.M.; Jancar, S. Impaired wound healing in type 1 diabetes is dependent on 5-lipoxygenase products. Sci. Rep. 2018, 8, 14164. [Google Scholar] [CrossRef]
- Pang, J.; Maienschein-Cline, M.; Koh, T.J. Enhanced Proliferation of Ly6C+ Monocytes/Macrophages Contributes to Chronic Inflammation in Skin Wounds of Diabetic Mice. J. Immunol. 2021, 206, 621–630. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elajaili, H.; Lyttle, B.D.; Lewis, C.V.; Bardill, J.R.; Dee, N.; Seal, S.; Nozik, E.S.; Liechty, K.W.; Zgheib, C. Increased ROS and Persistent Pro-Inflammatory Responses in a Diabetic Wound Healing Model (db/db): Implications for Delayed Wound Healing. Int. J. Mol. Sci. 2025, 26, 4884. https://doi.org/10.3390/ijms26104884
Elajaili H, Lyttle BD, Lewis CV, Bardill JR, Dee N, Seal S, Nozik ES, Liechty KW, Zgheib C. Increased ROS and Persistent Pro-Inflammatory Responses in a Diabetic Wound Healing Model (db/db): Implications for Delayed Wound Healing. International Journal of Molecular Sciences. 2025; 26(10):4884. https://doi.org/10.3390/ijms26104884
Chicago/Turabian StyleElajaili, Hanan, Bailey D. Lyttle, Caitlin V. Lewis, James R. Bardill, Nathan Dee, Sudipta Seal, Eva S. Nozik, Kenneth W. Liechty, and Carlos Zgheib. 2025. "Increased ROS and Persistent Pro-Inflammatory Responses in a Diabetic Wound Healing Model (db/db): Implications for Delayed Wound Healing" International Journal of Molecular Sciences 26, no. 10: 4884. https://doi.org/10.3390/ijms26104884
APA StyleElajaili, H., Lyttle, B. D., Lewis, C. V., Bardill, J. R., Dee, N., Seal, S., Nozik, E. S., Liechty, K. W., & Zgheib, C. (2025). Increased ROS and Persistent Pro-Inflammatory Responses in a Diabetic Wound Healing Model (db/db): Implications for Delayed Wound Healing. International Journal of Molecular Sciences, 26(10), 4884. https://doi.org/10.3390/ijms26104884







