Bioimaging and Sensing Properties of Curcumin and Derivatives
Abstract
1. Introduction
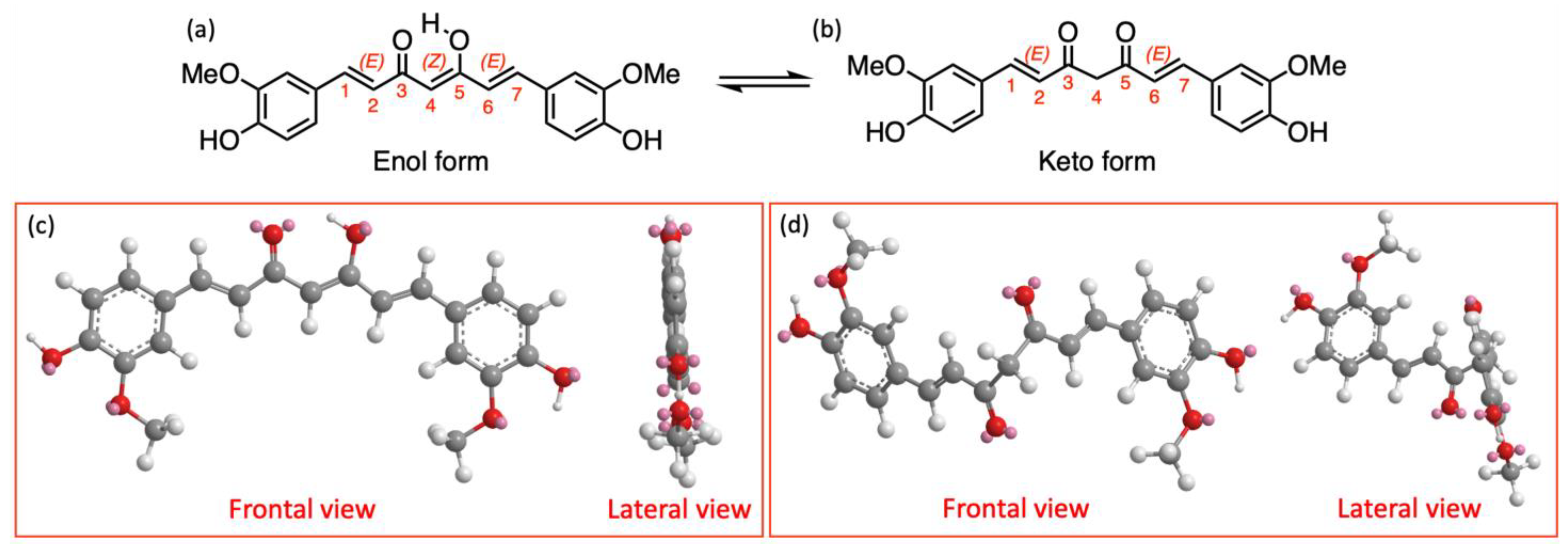
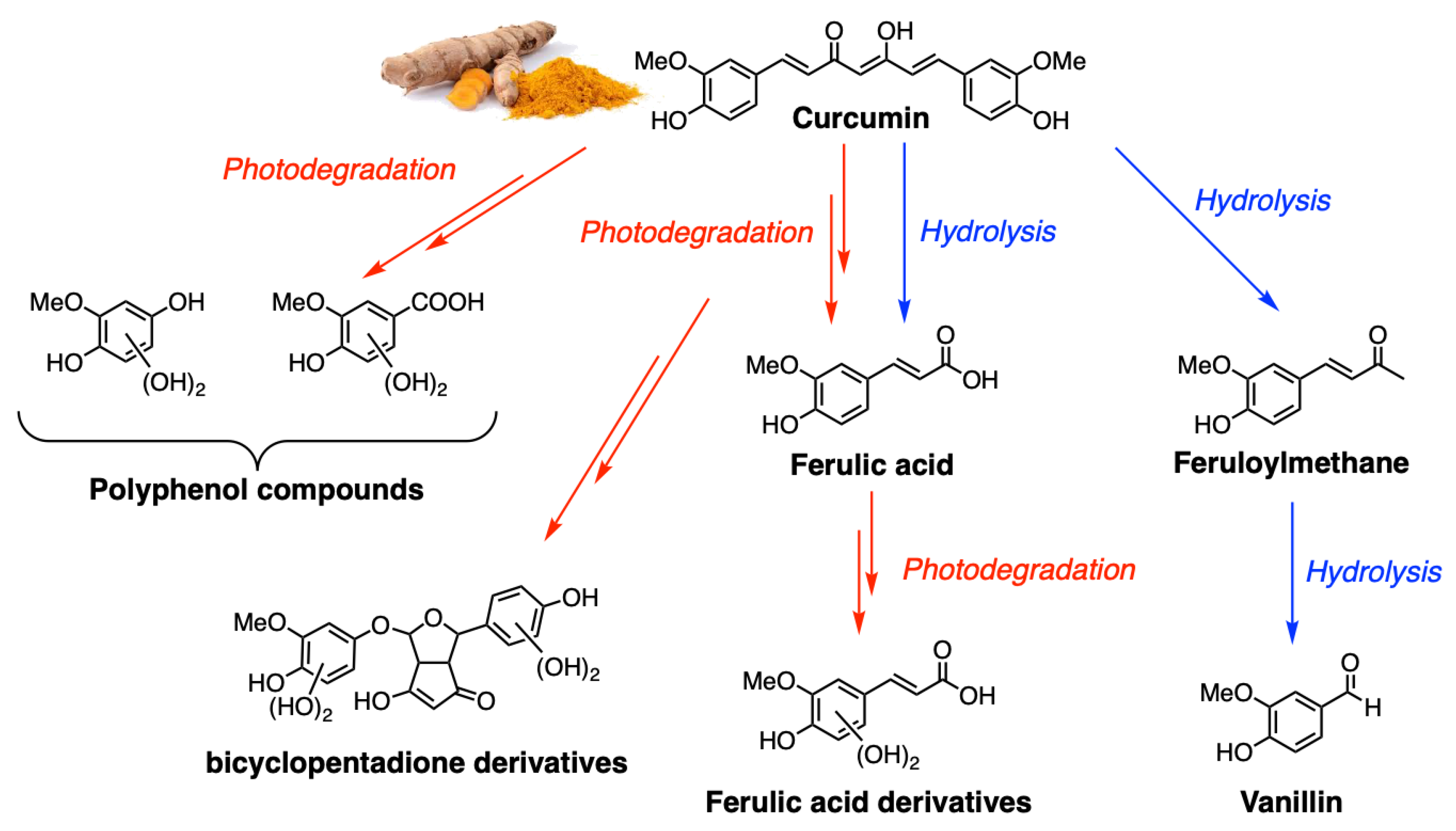
2. Photophysical Properties of Curcumin
3. Curcumin-Based Bioimaging and Sensing
3.1. Bioimaging
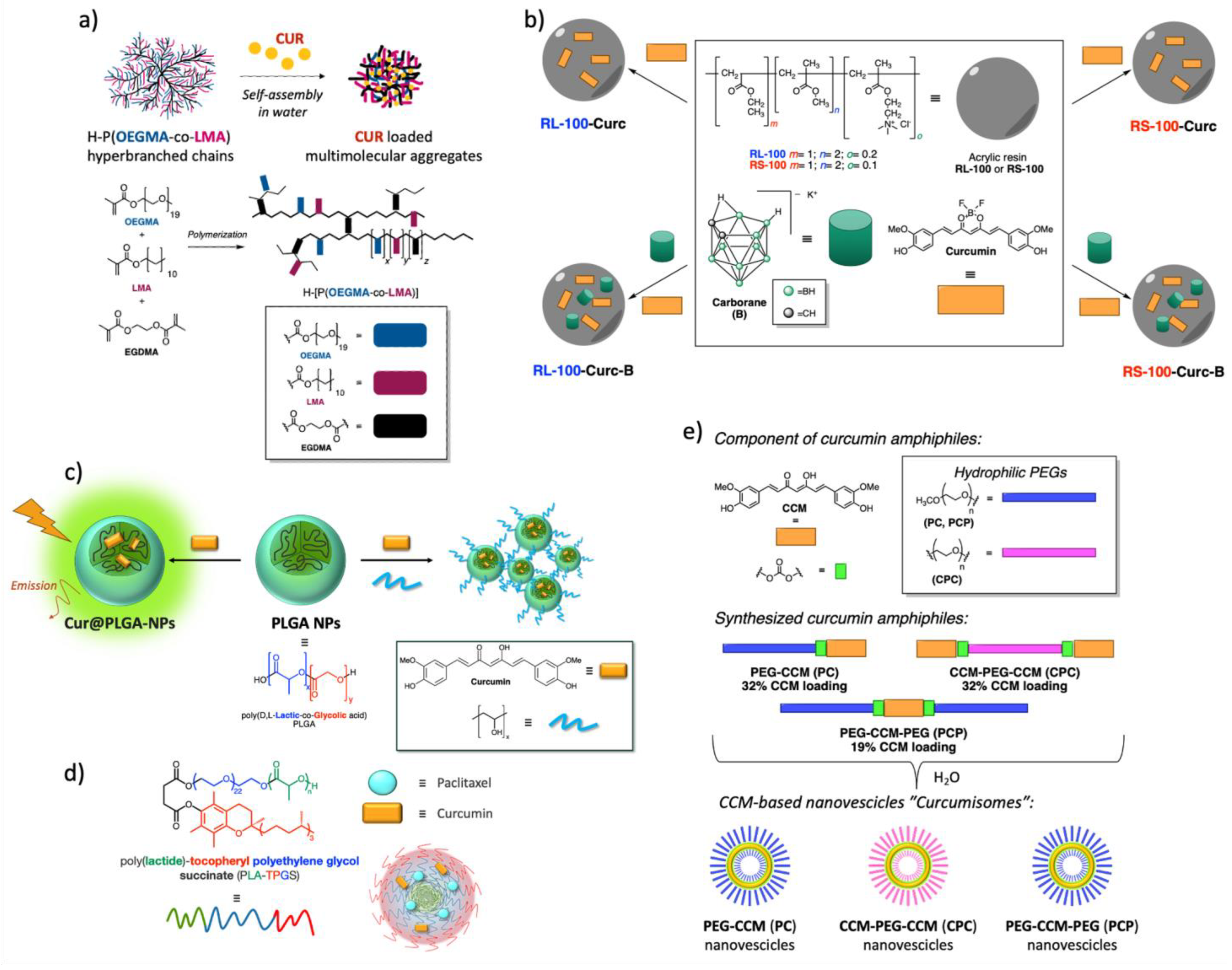
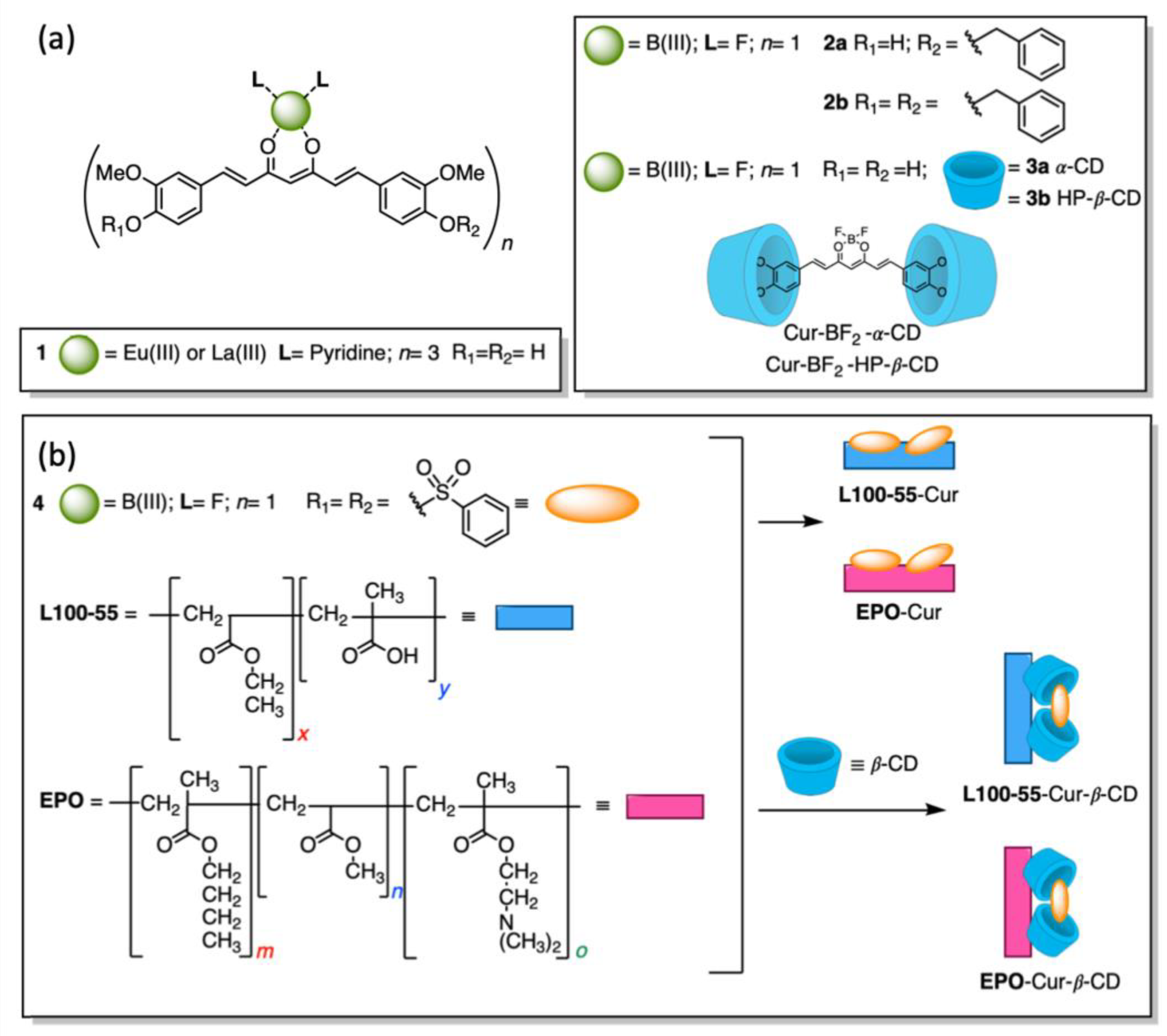
3.1.1. Polymeric Curcumin-Based Nanoformulations
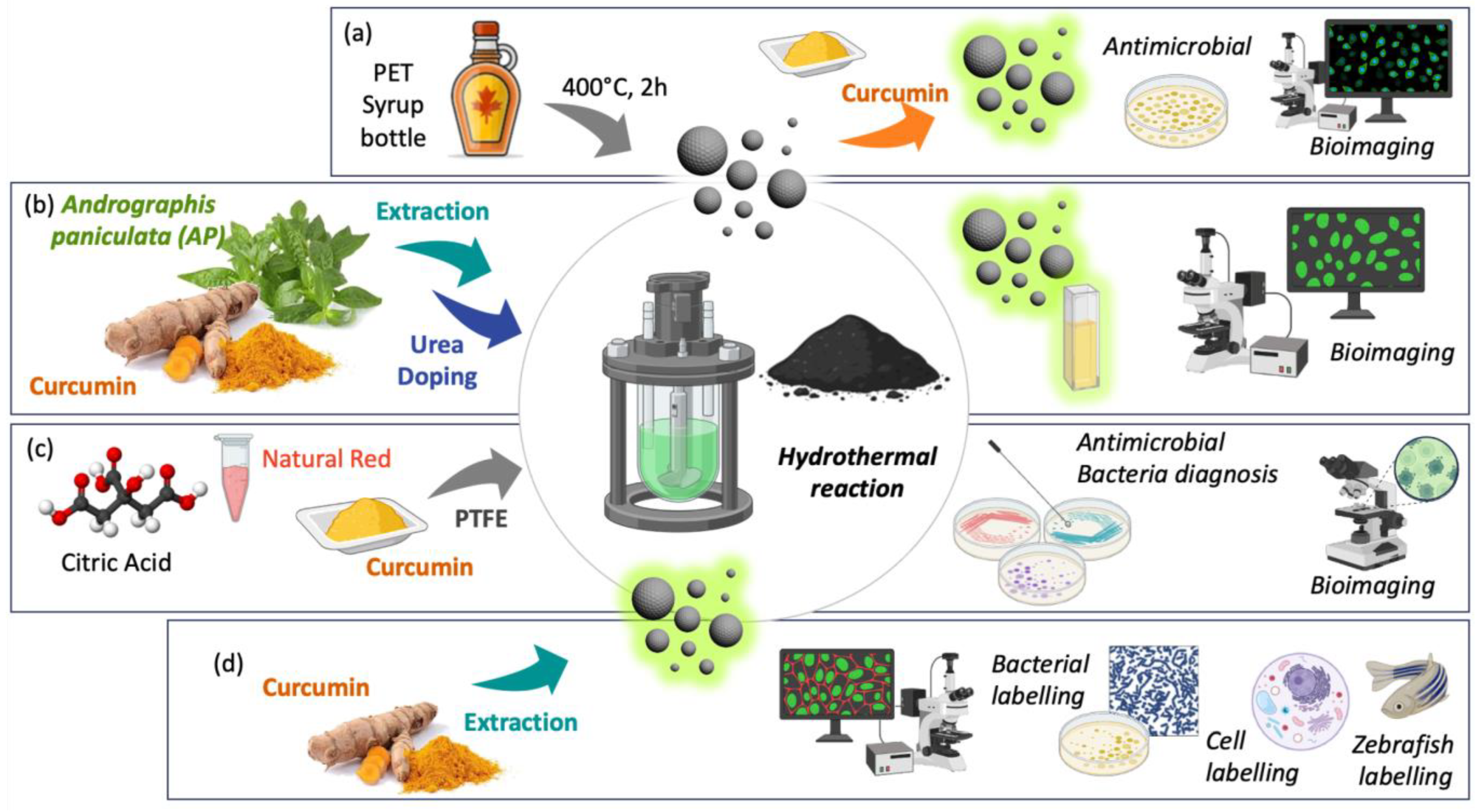
3.1.2. Carbon Dot Curcumin-Based Nanoformulations
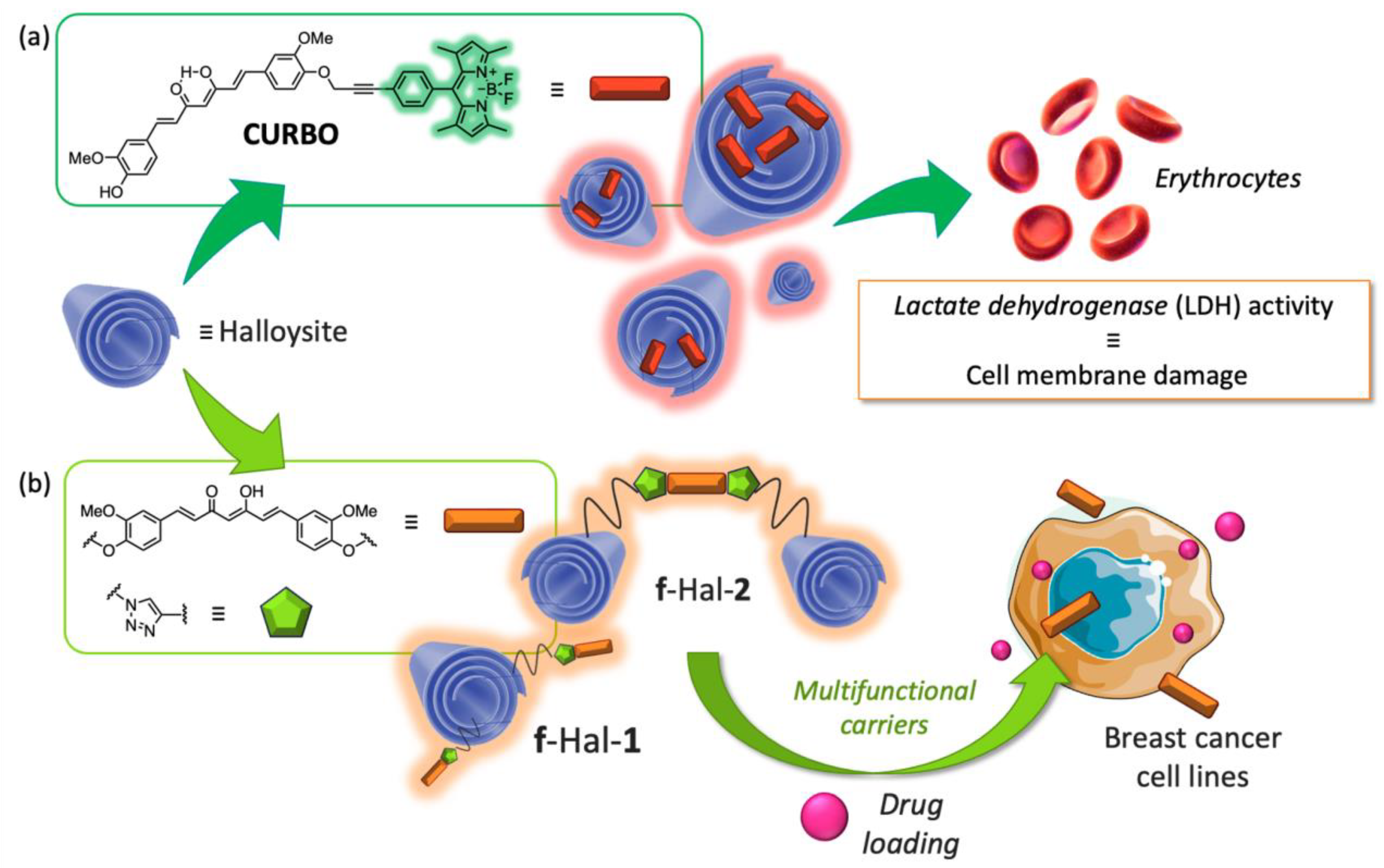
3.1.3. Curcumin-Based Inorganic Nanoformulations
3.1.4. Curcumin-Based Liposomes
3.1.5. Boron Difluoride Curcumin-Based Nanoformulations
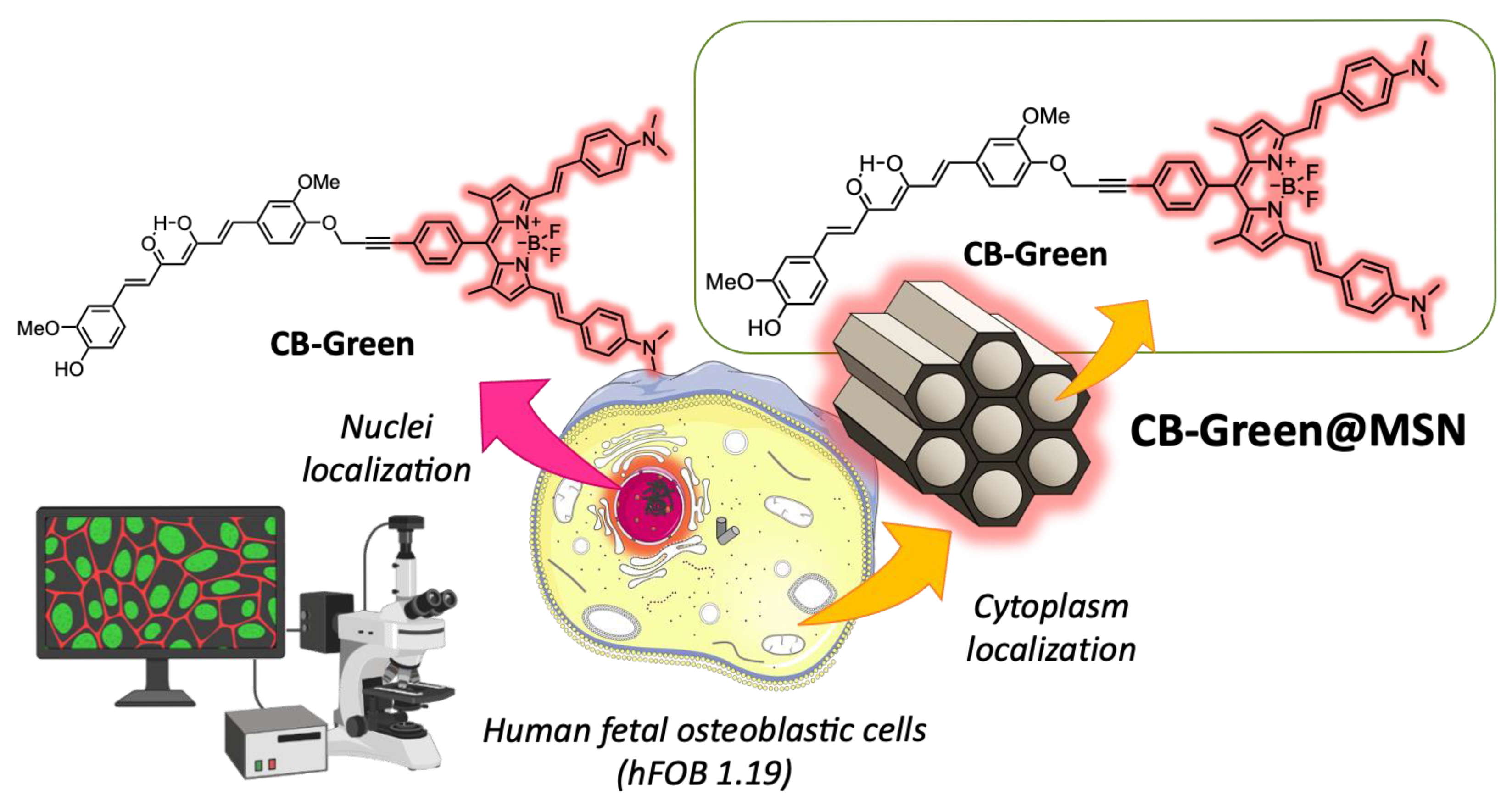
3.1.6. Mesoporous Curcumin-Based Nanoformulations
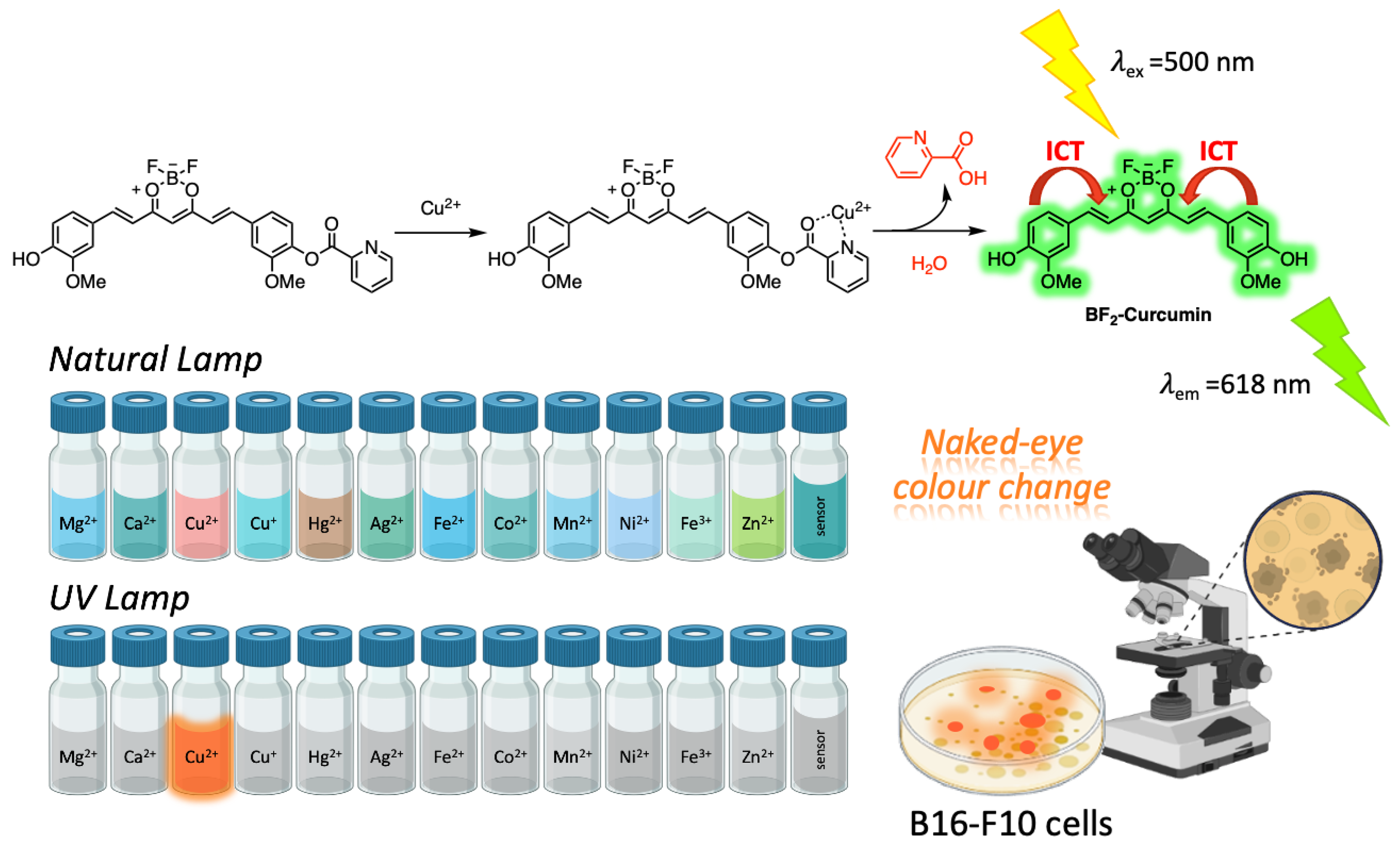
3.2. Sensing
3.2.1. Curcumin-Based Sensing in Food and Environment
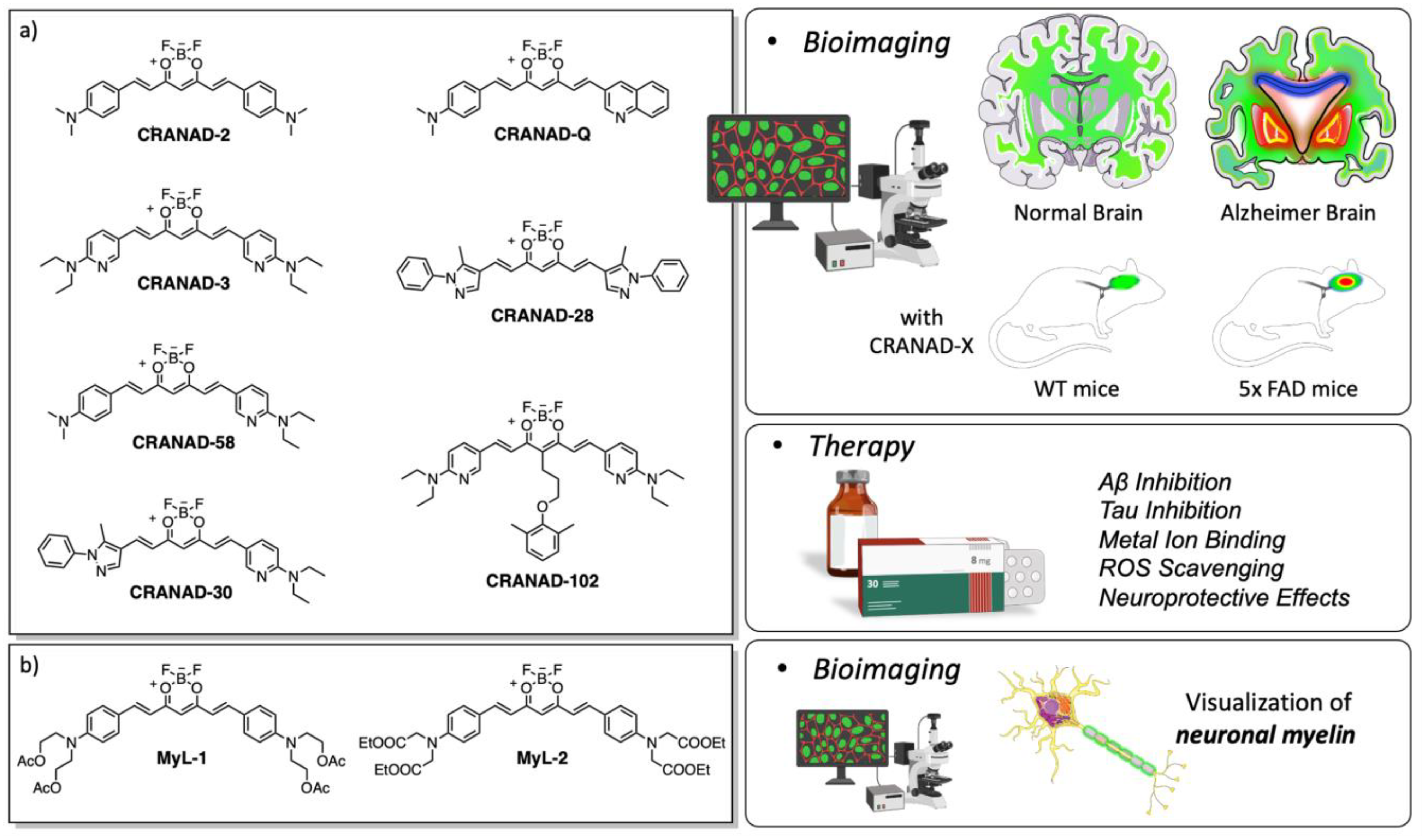
3.2.2. Curcumin-Based Sensing in Neurodegenerative Diseases
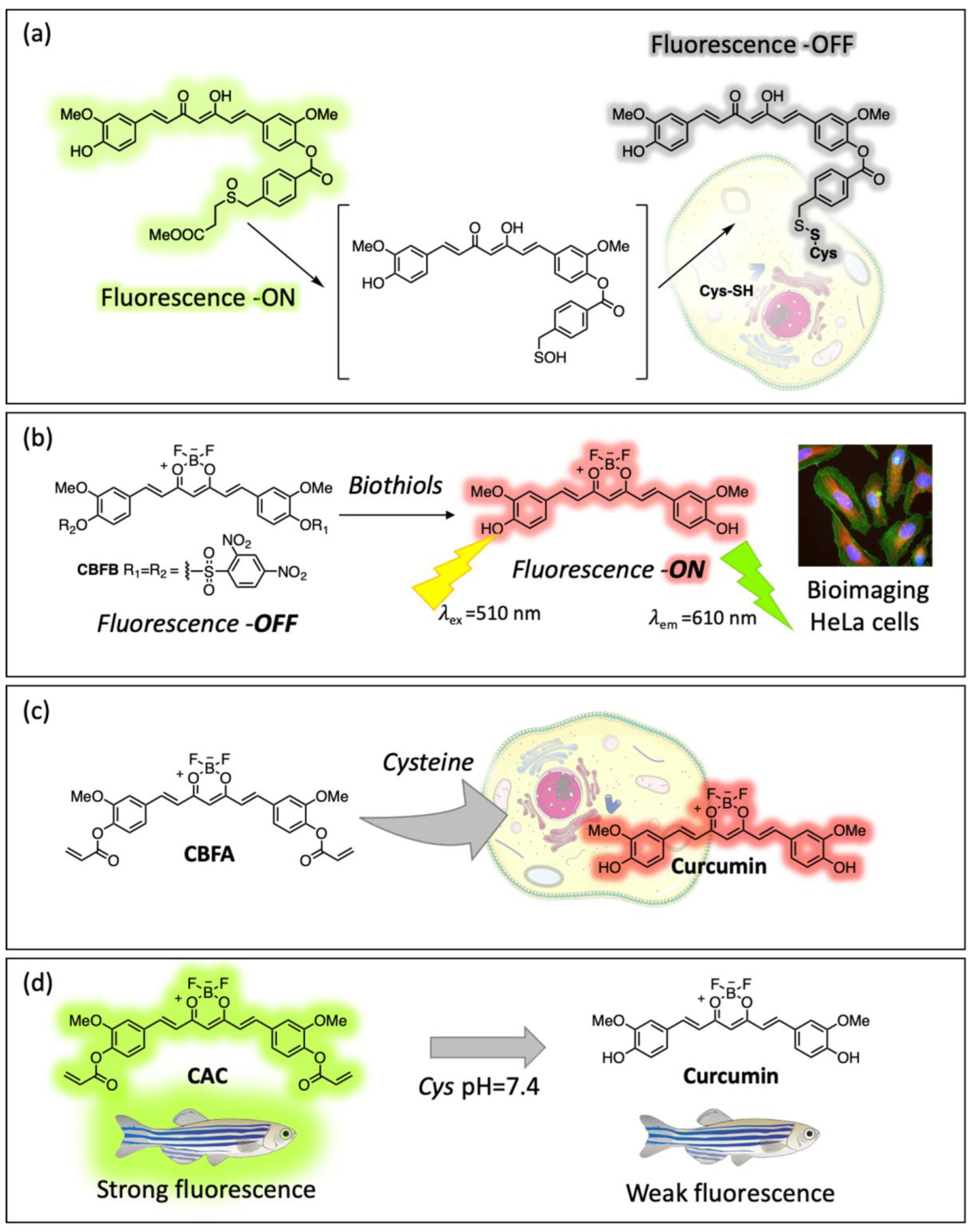
3.2.3. Curcumin-Based Sensing of Biothiols
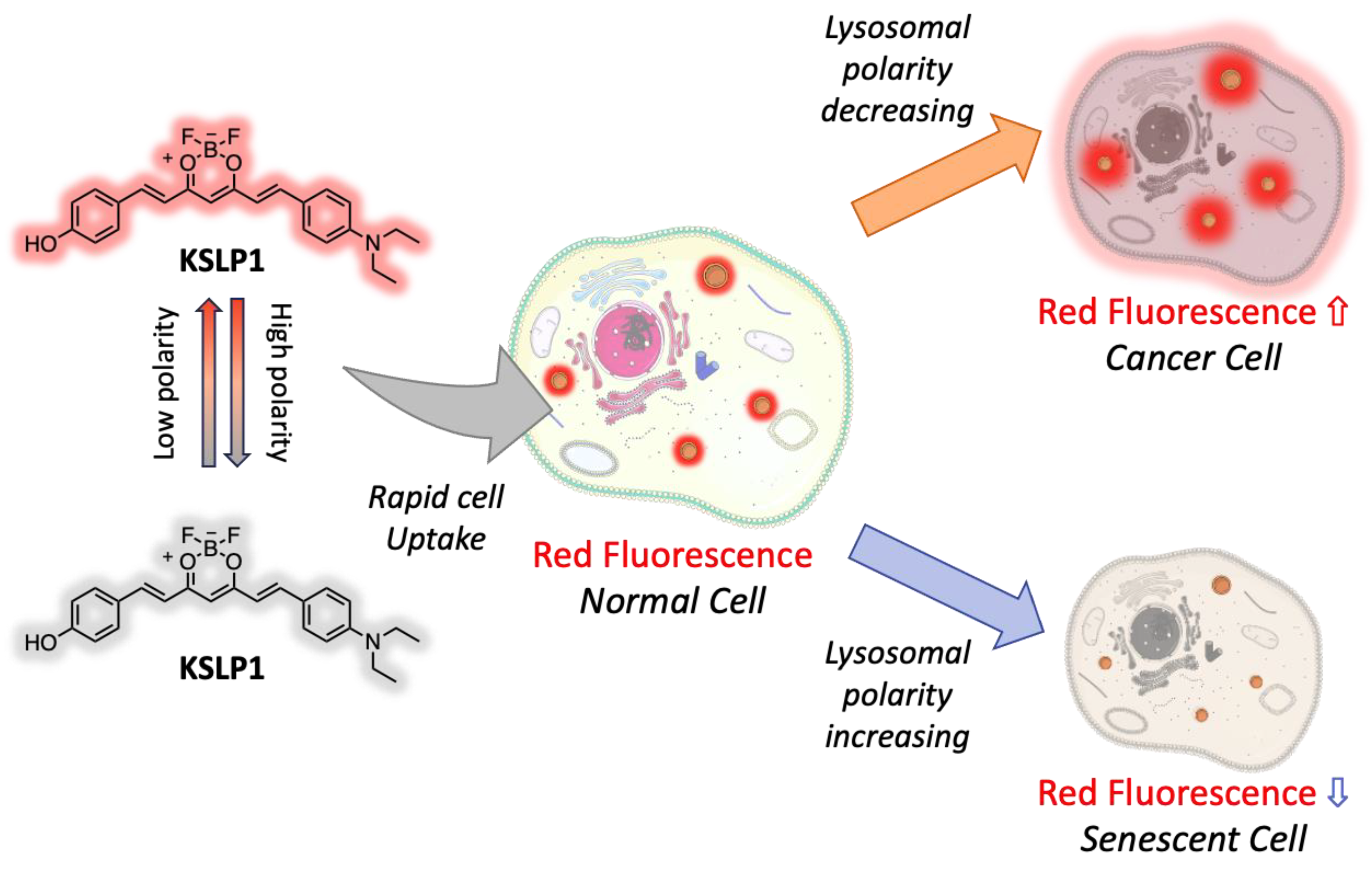
3.2.4. Curcumin-Based Sensing for Improving Human Health
4. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jyotirmayee, B.; Mahalik, G. A review on selected pharmacological activities of Curcuma longa L. Int. J. Food Prop. 2022, 25, 1377–1398. [Google Scholar] [CrossRef]
- Kah, G.; Chandran, R.; Abrahamse, H. Curcumin a Natural Phenol and Its Therapeutic Role in Cancer and Photodynamic Therapy: A Review. Pharmaceutics 2023, 15, 639–714. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorsi, P.M.; Labbozzetta, M.; Barattucci, A.; Salerno, T.M.G.; Poma, P.; Notarbartolo, M. Synthesis of Curcumin Derivatives and Analysis of Their Antitumor Effects in Triple Negative Breast Cancer (TNBC) Cell Lines. Pharmaceuticals 2019, 12, 161–180. [Google Scholar] [CrossRef]
- Ai, S.; Li, Y.; Zheng, H.; Zhang, M.; Tao, J.; Liu, W.; Peng, L.; Wang, Z.; Wang, Y. Collision of herbal medicine and nanotechnology: A bibliometric analysis of herbal nanoparticles from 2004 to 2023. J. Nanobiotechnol. 2024, 22, 140. [Google Scholar] [CrossRef]
- Ghosh, M.; Sarkar, N. Exploring the World of Curcumin: Photophysics, Photochemistry, and Applications in Nanoscience and Biology. ChemBioChem 2024, 25, e202400335. [Google Scholar] [CrossRef]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: The golden pigment from golden spice. Cancer Res. Treat. 2014, 46, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Moro, E.; Calderòn-Montaño, J.M.; Salvador, J.; Robles, A.; Lòpez-Làzaro, M. The dark side of curcumin. Int. J. Cancer 2010, 126, 1771–1775. [Google Scholar] [CrossRef]
- Fu, Y.S.; Chen, T.H.; Weng, L.; Huang, L.; Lai, D.; Weng, C.F. Pharmacological properties and underlying mechanisms of curcumin and prospects in medicinal potential. Biomed. Pharmacother. 2021, 141, 111888. [Google Scholar] [CrossRef]
- Hegde, M.; Girisa, S.; BharathwajChetty, B.; Vishwa, R.; Kunnumakkara, A.B. Curcumin Formulations for Better Bioavailability: What We Learned from Clinical Trials Thus Far? ACS Omega 2023, 8, 10713–10746. [Google Scholar] [CrossRef]
- Ghoran, S.H.; Calcaterra, A.; Abbasi, M.; Taktaz, F.; Nieselt, K.; Babaei, E. Curcumin-Based Nanoformulations: A Promising Adjuvant towards Cancer Treatment. Molecules 2022, 27, 5236. [Google Scholar] [CrossRef]
- Gayathri, K.; Bhaskaran, M.; Selvam, C.; Thilagavathi, R. Nano formulation approaches for curcumin delivery-A review. J. Drug Deliv. Sci. Technol. 2023, 82, 104326. [Google Scholar] [CrossRef]
- Govindammal, M.; Prasath, M.; Kamaraj, S.; Muthu, S.; Selvapandiyan, M. Exploring the molecular structure, vibrational spectroscopic, quantum chemical calculation and molecular docking studies of curcumin: A potential PI3K/AKT uptake inhibitor. Heliyon 2021, 7, e06646. [Google Scholar] [CrossRef]
- Hansen, P.E. Structural Studies of β-Diketones and Their Implications on Biological Effects. Pharmaceuticals 2021, 14, 1189–1207. [Google Scholar] [CrossRef]
- Margar, S.N.; Sekar, N. Nonlinear optical properties of curcumin: Solvatochromism-based approach and computational study. Mol. Phys. 2016, 114, 1867–1879. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. Photophysics, photochemistry and photobiology of curcumin: Studies from organic solutions, bio-mimetics and living cells. J. Photochem. Photobiol. C Photochem. Rev. 2009, 10, 81–95. [Google Scholar] [CrossRef]
- Patra, D.; Barakat, C. Synchronous fluorescence spectroscopic study of solvatochromic curcumin dye. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 79, 1034–1041. [Google Scholar] [CrossRef]
- Khopde, S.M.; Priyadarsini, K.I.; Palit, D.K.; Mukherjee, T. Effect of Solvent on the Excited-state Photophysical Properties of Curcumin. Photochem. Photobiol. 2000, 72, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.R.; Silva, C.M.; da Silva, L.M.; Machulek, A., Jr.; de Souza, A.P.; de Oliveira, K.T.; Souza, L.M.; Inada, N.M.; Bagnato, V.S.; Oliveira, S.L.; et al. Environmentally Safe Photodynamic Control of Aedes aegypti Using Sunlight-Activated Synthetic Curcumin: Photodegradation, Aquatic Ecotoxicity, and Field Trial. Molecules 2022, 27, 5699. [Google Scholar] [CrossRef]
- Das, K.C.; Das, C.K. Curcumin (diferuloylmethane), a singlet oxygen 1O2 quencher. Biochem. Biophys. Res. Commun. 2002, 295, 62–66. [Google Scholar] [CrossRef]
- Kee, T.W.; Adhikary, R.; Carlson, P.J.; Mukherjee, P.; Petrich, J.W. Femtosecond Fluorescence Upconversion Investigations on the Excited-State Photophysics of Curcumin. Aust. J. Chem. 2011, 64, 23–30. [Google Scholar] [CrossRef]
- Soham, S.; Kaitao, L.; Feihu, W.; Yingchao, L.; Songtao, C.; Xusan, Y.; Junle, Q.; Zhigang, Y. Xanthene, cyanine, oxazine and BODIPY: The four pillars of the fluorophore empire for super-resolution bioimaging. Chem. Soc. Rev. 2023, 52, 7197. [Google Scholar]
- Kunwar, A.; Barik, A.; Mishra, B.; Rathinasamy, K.; Pandey, R.; Priyadarsini, K.I. Quantitative cellular uptake, localization and cytotoxicity of curcumin in normal and tumor cells. Biochim. Biophys. Acta 2008, 1780, 673–679. [Google Scholar] [CrossRef]
- D’Acunto, M.; Cioni, P.; Gabellieri, E.; Presciuttin, G. Exploiting gold nanoparticles for diagnosis and cancer treatments. Nanotechnology 2021, 32, 192001. [Google Scholar] [CrossRef] [PubMed]
- Kaurav, H.; Verma, D.; Bansal, A.; Kapoor, D.N.; Sheth, S. Progress in drug delivery and diagnostic applications of carbon dots: A systematic review. Front. Chem. 2023, 11, 1227843. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Dong, X.; Li, J.; Wei, J. A short review on NIR-II organic small molecule dyes. Dye. Pig. 2020, 183, 108756. [Google Scholar] [CrossRef]
- Li, C.; Pang, Y.; Xu, Y.; Lu, M.; Tu, L.; Li, Q.; Sharma, A.; Guo, Z.; Li, X.; Sun, Y. Near-infrared metal agents assisting precision medicine: From strategic design to bioimaging and therapeutic applications. Chem. Soc. Rev. 2023, 52, 4392–4442. [Google Scholar] [CrossRef]
- Dhupal, M.; Chowdhury, D. Phytochemical-Based Nanomedicine for Advanced Cancer Theranostics: Perspectives on Clinical Trials to Clinical Use. Int. J. Nanomed. 2020, 15, 9125–9157. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, C.; Liu, D.B.; Yan, J.; Liang, H.P. The Clinical Applications of Curcumin: Current State and the Future. Curr. Pharm. Des. 2013, 19, 2011–2031. [Google Scholar]
- Ji, J.; Ma, Z.; Wang, Y. Advancing Gastrointestinal Health: Curcumin’s Efficacy and Nanopreparations. Molecules 2024, 29, 1659. [Google Scholar] [CrossRef]
- Zagami, R.; Barattucci, A.; Monsù Scolaro, L.; Viale, M.; Raffaini, G.; Bonaccorsi, P.M.; Mazzaglia, A. Curcumin/amphiphilic cyclodextrin nanoassemblies: Theoretical and spectroscopic studies to address their debut in anticancer therapy. J. Mol. Liquids 2023, 389, 122841. [Google Scholar] [CrossRef]
- Aborode, A.T.; Oluwajoba, A.S.; Ibrahim, A.M.; Ahmad, S.; Mehta, A.; Osayawe, O.J.-K.; Oyebode, D.; Akinsola, O.; Osinuga, A.; Onifade, I.A.; et al. Nanomedicine in cancer therapy: Advancing precision treatments. ABST 2024, 6, 105–119. [Google Scholar] [CrossRef]
- Trigo-Gutierrez, J.K.; Vega-Chacón, Y.; Brandão Soares, A.; de Oliveira Mima, E.G. Antimicrobial Activity of Curcumin in Nanoformulations: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 7130. [Google Scholar] [CrossRef]
- Al-Shehri, A.H.; Kabel, A.M.; Elmaaboud, M.A.A. The Nanoformulations of Curcumin for Cancer Therapy: New Perspectives. J. Cancer Res. Treat. 2019, 7, 21–26. [Google Scholar]
- Gerardos, A.M.; Balafouti, A.; Pispas, S. Mixed Hyperbranched/Triblock Copolymer Micelle Assemblies: Physicochemical Properties and Potential for Drug Encapsulation. Macromol. Chem. Phys. 2023, 224, 2300109. [Google Scholar] [CrossRef]
- Lu, C.; Yao, Z.; Feng, J.; Mao, B.; Jin, G. Oil-in-water strategy coating Curcumin-nido-Carborane fluorescent complex with acrylic resins for cell imaging. Arab. J. Chem. 2023, 16, 104876. [Google Scholar] [CrossRef]
- Wang, R.; Zou, L.; Yi, Z.; Zhang, Z.; Zhao, M.; Shi, S. PLGA nanoparticles loaded with curcumin produced luminescence for cell bioimaging. Int. J. Pharm. 2023, 639, 122944. [Google Scholar] [CrossRef]
- Liu, M.; Teng, C.P.; Win, K.Y.; Chen, Y.; Zhang, X.; Yang, D.P.; Li, Z.; Ye, E. Polymeric Encapsulation of Turmeric Extract for Bioimaging and Antimicrobial Applications. Macromol. Rapid Commun. 2019, 40, e1800216. [Google Scholar] [CrossRef]
- Nguyen, H.N.; Ha, P.T.; Nguyen, A.S.; Nguyen, D.T.; Do, H.D.; Thi, Q.N.; Thi, M.N.H. Curcumin as fluorescent probe for directly monitoring in vitro uptake of curcumin combined paclitaxel loaded PLA-TPGS nanoparticles. Adv. Nat. Sci Nanosci. Nanotechnol. 2016, 7, 025001. [Google Scholar] [CrossRef]
- Nagahama, K.; Kumano, T.; Oyama, N.; Kawakami, J. Curcumisome nanovesicles generated by self-assembly of curcumin amphiphiles toward cancer theranostics. Biomater. Sci. 2015, 3, 1566–1578. [Google Scholar] [CrossRef]
- Thirumalaivasan, N.; Kanagaraj, K.; Logesh, K.; Chandrasekaran, S.; Kumar, S.; Subramanian, R.; Senthilkumar, N.; Kumar, A.; Angadi, V.J.; Al-Kahtani, A. Exploring luminescent carbon dots derived from syrup bottle waste and curcumin for potential antimicrobial and bioimaging applications. Chemosphere 2024, 354, 141592. [Google Scholar] [CrossRef]
- Annisa, W.D.; Permatasari, F.A.; Iskandar, F.; Rachmawati, H. Functionalized Phytochemicals-Embedded Carbon Dots Derived from Medicinal Plant for Bioimaging Application. ACS Appl. Bio Mater. 2024, 7, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Yan, H.; Jiang, X.; Zhang, Y.; Li, P.; Su, W. Orange-red to NIR emissive carbon dots for antimicrobial, bioimaging and bacteria diagnosis. J. Mater. Chem. B 2022, 10, 1250–1264. [Google Scholar] [CrossRef] [PubMed]
- Pal, T.; Mohiyuddin, S.; Packirisamy, G. Facile and Green Synthesis of Multicolor Fluorescence Carbon Dots from Curcumin: In Vitro and in Vivo Bioimaging and Other Applications. ACS Omega 2018, 3, 831–843. [Google Scholar] [CrossRef]
- Chandran, N.; Ramachandran, V.K.J.; Janardhanan, P.; Deepak, N.K.; Pilankatta, R.; Nair, S.S. Plasmonic CuS 2D nanosheets through biologically and environmentally benign template free one-pot synthesis. Colloids Surf. A Physicochem. Eng. Asp. 2023, 678, 132429. [Google Scholar] [CrossRef]
- Xu, G.; Wang, J.; Si, G.; Wang, M.; Cheng, H.; Chen, B.; Zhou, S. Preparation, photoluminescence properties and application for in vivo tumor imaging of curcumin derivative-functionalized graphene oxide composite. Dye. Pigm. 2017, 141, 470–478. [Google Scholar] [CrossRef]
- Zheng, P.; Ding, B.; Shi, R.; Jiang, Z.; Xu, W.; Li, G.; Ding, J.; Chen, X. A Multichannel Ca2+ Nanomodulator for Multilevel Mitochondrial Destruction-Mediated Cancer Therapy. Adv. Mater. 2021, 33, e2007426. [Google Scholar] [CrossRef]
- Govindaraju, S.; Rengaraj, A.; Arivazhagan, R.; Huh, Y.-S.; Yun, K. Curcumin-Conjugated Gold Clusters for Bioimaging and Anticancer Applications. Bioconjug. Chem. 2018, 29, 363–370. [Google Scholar] [CrossRef]
- Demir, B.; Moulahoum, H.; Ghorbanizamani, F.; Barlas, F.B.; Yesiltepe, O.; Gumus, Z.P.; Meral, K.; Demirkol, D.O.; Timur, S. Carbon dots and curcumin-loaded CD44-Targeted liposomes for imaging and tracking cancer chemotherapy: A multi-purpose tool for theranostics. J. Drug. Deliv. Sci. Technol. 2021, 62, 102363. [Google Scholar] [CrossRef]
- Sadeghi Mohammadi, S.; Vaezi, Z.; Shojaedin-Givi, B.; Naderi-Manesh, H. Chemiluminescent liposomes as a theranostic carrier for detection of tumor cells under oxidative stress. Anal. Chim. Acta. 2019, 1059, 113–123. [Google Scholar] [CrossRef]
- Aromí, G.; Gamez, P.; Reedijk, J. Poly beta-diketones: Prime ligands to generate supramolecular metalloclusters. Coord. Chem. Rev. 2008, 252, 964–989. [Google Scholar] [CrossRef]
- Zhou, S.-S.; Xue, X.; Wang, J.-F.; Dong, Y.; Jiang, B.; Wei, D.; Wan, M.-L.; Jia, Y. Synthesis, optical properties and biological imaging of the rare earth complexes with curcumin and pyridine. J. Mater. Chem. 2012, 22, 22774–22780. [Google Scholar] [CrossRef]
- Delgado, D.; Abonia, R. Synthetic approaches for BF2-containing adducts of outstanding biological potential. A review. Arab. J. Chem. 2022, 15, 103528. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.; Pan, H.; Li, L.; Yu, Y.; Liu, B. An insight into the in vivo imaging potential of curcumin analogues as fluorescence probes. Asian J. Pharm. Sci. 2021, 16, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Du, Z.-Y.; Zheng, X.; Li, D.-L.; Zhou, R.-P.; Zhang, K. Use of curcumin in diagnosis, prevention, and treatment of Alzheimer’s disease. Neural Regen. Res. 2018, 13, 742–752. [Google Scholar] [PubMed]
- Obregón-Mendoza, M.A.; Arias-Olguín, I.I.; Estévez-Carmona, M.M.; Meza-Morales, W.; Alvarez-Ricardo, Y.; Toscano, R.A.; Arenas-Huertero, F.; Cassani, J.; Enríquez, R.G. Non-Cytotoxic Dibenzyl and Difluoroborate Curcuminoid Fluorophores Allow Visualization of Nucleus or Cytoplasm in Bioimaging. Molecules 2020, 25, 3205. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, K.; Lian, G.; Zhou, M.; Lu, C.; Jin, G. Bioactivity and Cell Imaging of Antitumor Fluorescent Agents (Curcumin Derivatives) Coated by Two-Way Embedded Cyclodextrin Strategy. Chem. Biodivers. 2022, 19, e202200644. [Google Scholar] [CrossRef]
- Shao, C.; Zhang, X.; Shao, S.; Jin, F. Application study of curcumin fluorescent complex coated with pharmaceutical excipients for cell imaging. Front. Chem. 2023, 11, 1153729. [Google Scholar] [CrossRef]
- Duan, L.; Wang, C.; Zhang, W.; Ma, B.; Deng, Y.; Li, W.; Zhao, D. Interfacial Assembly and Applications of Functional Mesoporous Materials. Chem. Rev. 2021, 121, 14349–14429. [Google Scholar] [CrossRef]
- Maleki Dizaj, S.; Sharifi, S.; Tavakoli, F.; Hussain, Y.; Forouhandeh, H.; Hosseiniyan Khatibi, S.M.; Memar, M.Y.; Yekani, M.; Khan, H.; Goh, K.W.; et al. Curcumin-Loaded Silica Nanoparticles: Applications in Infectious Disease and Food Industry. Nanomaterials 2022, 12, 2848. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, C.; Feng, L.; Yang, K.; Liu, Z. Functional Nanomaterials for Phototherapies of Cancer. Chem. Rev. 2014, 114, 10869–10939. [Google Scholar] [CrossRef]
- Gangemi, C.M.A.; Salerno, T.M.G.; Barattucci, A.; Cucinotta, F.; Bonaccorsi, P.; Calabrese, G.; Poma, P.; Rizzo, M.G.; Campagna, S.; Puntoriero, F. A Curcumin-BODIPY Dyad and Its Silica Hybrid as NIR Bioimaging Probes. Int. J. Mol. Sci. 2022, 23, 9542. [Google Scholar] [CrossRef]
- Riela, S.; Barattucci, A.; Barreca, D.; Campagna, S.; Cavallaro, G.; Lazzara, G.; Massaro, M.; Pizzolanti, G.; Salerno, T.M.G.; Bonaccorsi, P.; et al. Boosting the properties of a fluorescent dye by encapsulation into halloysite nanotubes. Dye. Pigm. 2021, 187, 109094. [Google Scholar] [CrossRef]
- Massaro, M.; Poma, P.; Colletti, C.G.; Barattucci, A.; Bonaccorsi, P.M.; Lazzara, G.; Nicotra, G.; Parisi, F.; Salerno, T.M.G.; Spinella, C.; et al. Chemical and biological evaluation of cross-linked halloysite-curcumin derivatives. Appl. Clay Sci. 2020, 184, 105400. [Google Scholar] [CrossRef]
- Biddeci, G.; Spinelli, G.; Massaro, M.; Riela, S.; Bonaccorsi, P.; Barattucci, A.; Di Blasi, F. Study of Uptake Mechanisms of Halloysite Nanotubes in Different Cell Lines. Int. J. Nanomed. 2021, 16, 4755–4768. [Google Scholar] [CrossRef] [PubMed]
- Chiorcea-Paquim, A.-M. Electrochemical Sensing of Curcumin: A Review. Antioxidants 2023, 12, 2029. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Liu, Y.; Wang, L.; Wang, H.; Hu, Z.; Dong, H.; Yu, Z.; Yuan, Y. A review of curcumin in food preservation: Delivery system and photosensitization. Food Chem. 2023, 424, 136464. [Google Scholar] [CrossRef]
- Khorasani, M.Y.; Langari, H.; Sany, S.B.T.; Rezayi, M.; Sahebkar, A. The role of curcumin and its derivatives in sensory applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109792. [Google Scholar] [CrossRef]
- Devasena, T.; Balasubramanian, N.; Muninathan, N.; Baskaran, K.; John, S.T. Curcumin Is an Iconic Ligand for Detecting Environmental Pollutants. Bioinorg. Chem. Appl. 2022, 2022, 9248988. [Google Scholar] [CrossRef]
- Shi, Y.; Li, C.; Li, W.; Hu, X.; Zhang, X.; Li, Z.; Huang, X.; Zhang, H.; Shi, J.; Zou, X. Visualization of salt diffusion in beef curing process using Smartphone-Assisted ratiometric fluorescent sensor. Microchem. J. 2024, 200, 110382. [Google Scholar] [CrossRef]
- Shi, Y.; Li, W.; Hu, X.; Zhang, X.; Huang, X.; Li, Z.; Zhai, X.; Shen, T.; Shi, J.; He, Y.; et al. A novel sustainable biomass-based fluorescent probe for sensitive detection of salicylic acid in rice. Food Chem. 2024, 434, 137260. [Google Scholar] [CrossRef]
- Abdul-Rahman, T.; Awuah, W.A.; Mikhailova, T.; Kalmanovich, J.; Mehta, A.; Ng, J.C.; Coghlan, M.A.; Zivcevska, M.; Tedeschi, A.J.; de Oliveira, E.C.; et al. Antioxidant, anti-inflammatory and epigenetic potential of curcumin in Alzheimer’s disease. Biofactors 2024, 50, 693–708. [Google Scholar] [CrossRef] [PubMed]
- Zang, W.-B.; Wie, H.-L.; Zhang, W.-W.; Ma, W.; Li, J.; Yao, Y. Curcumin hybrid molecules for the treatment of Alzheimer’s disease: Structure and pharmacological activities. Eur. J. Med. Chem. 2024, 265, 116070. [Google Scholar] [CrossRef] [PubMed]
- Ran, C.; Xu, X.; Raymond, S.B.; Ferrara, B.J.; Neal, K.; Bacskai, B.J.; Medarova, Z.; Moore, A. Design, Synthesis, and Testing of Difluoroboron-Derivatized Curcumins as Near-Infrared Probes for in Vivo Detection of Amyloid-β Deposits. J. Am. Chem. Soc. 2009, 131, 15257–15261. [Google Scholar] [CrossRef]
- Yang, H.; Zeng, F.; Luo, Y.; Zheng, C.; Ran, C.; Yang, J. Curcumin Scaffold as a Multifunctional Tool for Alzheimer’s Disease Research. Molecules 2022, 27, 3879. [Google Scholar] [CrossRef]
- Chen, J.; Yu, Y.; Wang, S.; Shen, Y.; Tian, Y.; Rizzello, L.; Luo, K.; Tian, X.; Wang, T.; Xiong, L. Nanoscale myelinogenesis image in developing brain via super-resolution nanoscopy by near-infrared emissive curcumin-BODIPY derivatives. J. Nanobiotechnol. 2024, 22, 106. [Google Scholar] [CrossRef] [PubMed]
- Papalia, T.; Barattucci, A.; Barreca, D.; Bellocco, E.; Bonaccorsi, P.; Minuti, L.; Nicolò, M.S.; Temperini, A.; Foti, C. Sequestering ability to Cu2+ of a new bodipy-based dye and its behavior as in vitro fluorescent sensor. J. Inorg. Biochem. 2017, 167, 116–123. [Google Scholar] [CrossRef]
- Xu, G.; Wang, J.; Si, G.; Wang, M.; Xue, X.; Wu, B.; Zhou, S. A novel highly selective chemosensor based on curcumin for detection of Cu2+ and its application for bioimaging. Sens. Actuators B Chem. 2016, 230, 684–689. [Google Scholar] [CrossRef]
- Li, Y.; Ji, Y.X.; Song, L.J.; Zhang, Y.; Li, Z.-C.; Yang, L.; Huang, W.-C. A novel BF2–curcumin-based fluorescent chemosensor for detection of Cu2+ in aqueous solution and living cells. Res. Chem. Intermed. 2018, 44, 5169–5180. [Google Scholar] [CrossRef]
- Hu, J.; Wang, Y.; Shao, T.; Lian, G.; Hu, K.; Liu, Y.; Zhou, M.; Wang, X.; Huang, L.; Meng, X.; et al. Simple and practical, highly sensitive and responsive recognition of cysteine: Design, synthesis and mechanism study of a novel curcumin fluorescent probe. Arab. J. Chem. 2022, 15, 104087. [Google Scholar] [CrossRef]
- Aversa, M.C.; Barattucci, A.; Bonaccorsi, P.; Temperini, A. Regio-and stereocontrolled synthesis of (Z)-α-(phenylseleno)sulfinyl and-sulfonyl alkenes via sulfenic acids, and a study of their reactivity. Eur. J. Org. Chem. 2011, 2011, 5668–5673. [Google Scholar] [CrossRef]
- Aversa, M.C.; Barattucci, A.; Bonaccorsi, P.; Faggi, C.; Papalia, T. Thiacyclophane Cages and Related Bi- and Tripodal Molecules via Transient Polysulfenic Acids. J. Org. Chem. 2007, 72, 4486–4496. [Google Scholar] [CrossRef]
- Barattucci, A.; Salerno, T.M.G.; Kohnke, F.H.; Papalia, T.; Puntoriero, F.; Bonaccorsi, P. Curcumin-based sulfenic acid as a light switch for the binding of biothiols. New J. Chem. 2020, 44, 19508–19514. [Google Scholar] [CrossRef]
- Chen, D.; Yang, J.; Dai, J.; Lou, X.; Zhong, C.; Yu, X.; Xia, F. A low background D–A–D type fluorescent probe for imaging of biothiols in living cells. J. Mater. Chem. B 2018, 6, 5248–5255. [Google Scholar] [CrossRef]
- Chen, D.; Long, Z.; Dang, Y.; Chen, L. A new fluorescent probe for specific detection of cysteine with facile preparation and living cell imaging. Dye. Pigm. 2019, 166, 266–271. [Google Scholar] [CrossRef]
- Pang, L.; Zhou, Y.; Gao, W.; Zhang, J.; Song, H.; Wang, X.; Wang, Y.; Peng, X. Curcumin-Based Fluorescent and Colorimetric Probe for Detecting Cysteine in Living Cells and Zebrafish. Ind. Eng. Chem. Res. 2017, 56, 7650–7655. [Google Scholar] [CrossRef]
- Rai, A.; Jha, N.S.; Sharma, P.; Tiwari, S.; Subramanian, R. Curcumin-derivatives as fluorescence-electrochemical dual probe for ultrasensitive detections of picric acid in aqueous media. Talanta 2024, 275, 126113. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Hu, L.; Li, X.; Liu, W.; Gao, Y.; Li, X.; Jiang, B.; Xia, C.; Guo, Y.; Li, J. Lysosomal polarity increases with aging as revealed by a lysosome-targetable near-infrared fluorescent probe. Sens. Actuators B Chem. 2020, 319, 128302. [Google Scholar] [CrossRef]
- Shao, K.; Guo, L.; Zhong, Y.; Zhang, L.; Lu, Z.; Wang, D. Carbon Quantum Dots for Rapid and Ratiometric Fluorescence Determination of Hypochlorite. ACS Appl. Nano Mater. 2024, 7, 8645–8654. [Google Scholar] [CrossRef]
- Zhang, Y.; Tu, L.; Lu, L.; Li, Y.; Song, L.; Qi, Q.; Song, H.; Li, Z.; Huang, W. Screening and application of boron difluoride complexes of curcumin as colorimetric and ratiometric fluorescent probes for bisulfite. Anal. Methods 2020, 12, 1514–1521. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gangemi, C.M.A.; Mirabile, S.; Monforte, M.; Barattucci, A.; Bonaccorsi, P.M. Bioimaging and Sensing Properties of Curcumin and Derivatives. Int. J. Mol. Sci. 2025, 26, 4871. https://doi.org/10.3390/ijms26104871
Gangemi CMA, Mirabile S, Monforte M, Barattucci A, Bonaccorsi PM. Bioimaging and Sensing Properties of Curcumin and Derivatives. International Journal of Molecular Sciences. 2025; 26(10):4871. https://doi.org/10.3390/ijms26104871
Chicago/Turabian StyleGangemi, Chiara Maria Antonietta, Salvatore Mirabile, Maura Monforte, Anna Barattucci, and Paola Maria Bonaccorsi. 2025. "Bioimaging and Sensing Properties of Curcumin and Derivatives" International Journal of Molecular Sciences 26, no. 10: 4871. https://doi.org/10.3390/ijms26104871
APA StyleGangemi, C. M. A., Mirabile, S., Monforte, M., Barattucci, A., & Bonaccorsi, P. M. (2025). Bioimaging and Sensing Properties of Curcumin and Derivatives. International Journal of Molecular Sciences, 26(10), 4871. https://doi.org/10.3390/ijms26104871









