Exploring the Multifunctional Role of Alpha-Fetoprotein in Cancer Progression: Implications for Targeted Therapy in Hepatocellular Carcinoma and Beyond
Abstract
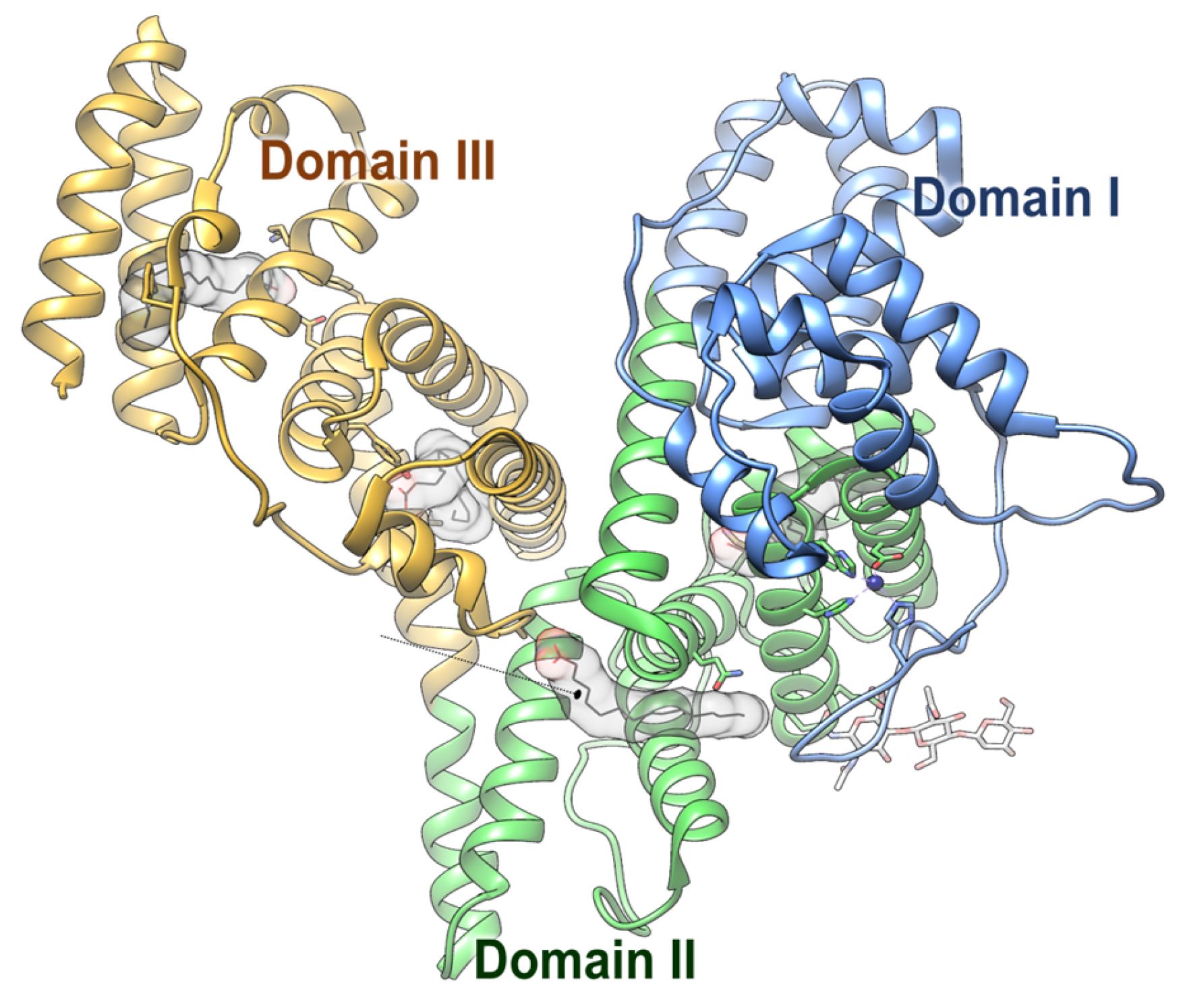
1. Introduction
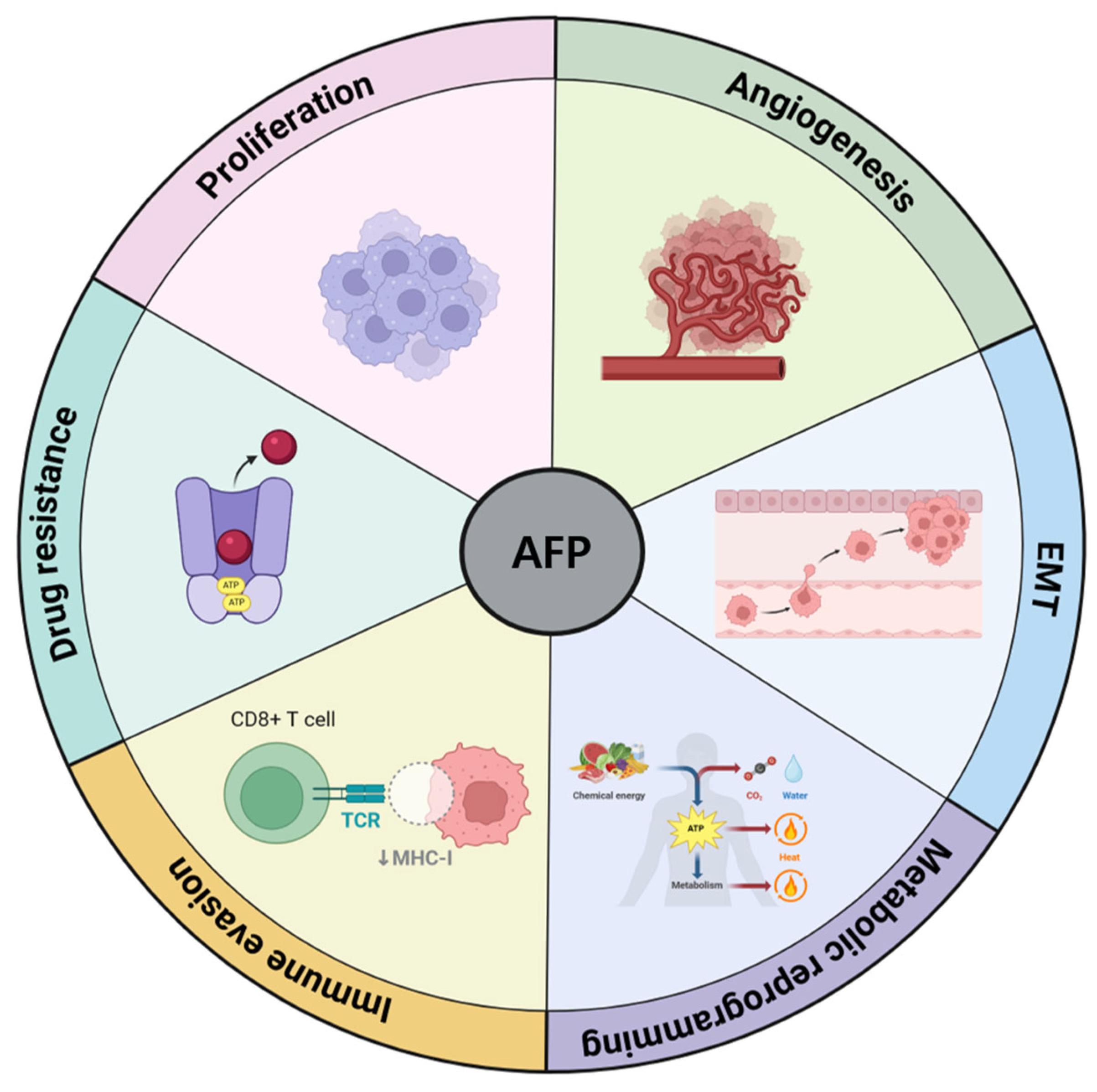
2. AFP as a Tumor Progression Factor in HCC
2.1. Tumor Growth and Proliferation
2.2. Angiogenesis
2.3. Epithelial-Mesenchymal Transition (EMT) and Invasion
2.4. Metabolic Reprogramming
2.4.1. Influence on Glycolysis and Gluconeogenesis
2.4.2. Regulation of Lipid and Energy Metabolism
2.4.3. Regulation of Amino Acid Metabolism
2.5. Immune Evasion
2.5.1. Effects on T Cells
2.5.2. Effects on Macrophages
2.5.3. Effects on DCs
2.5.4. Effects on Natural Killer (NK) Cells
2.6. Resistance to Therapy
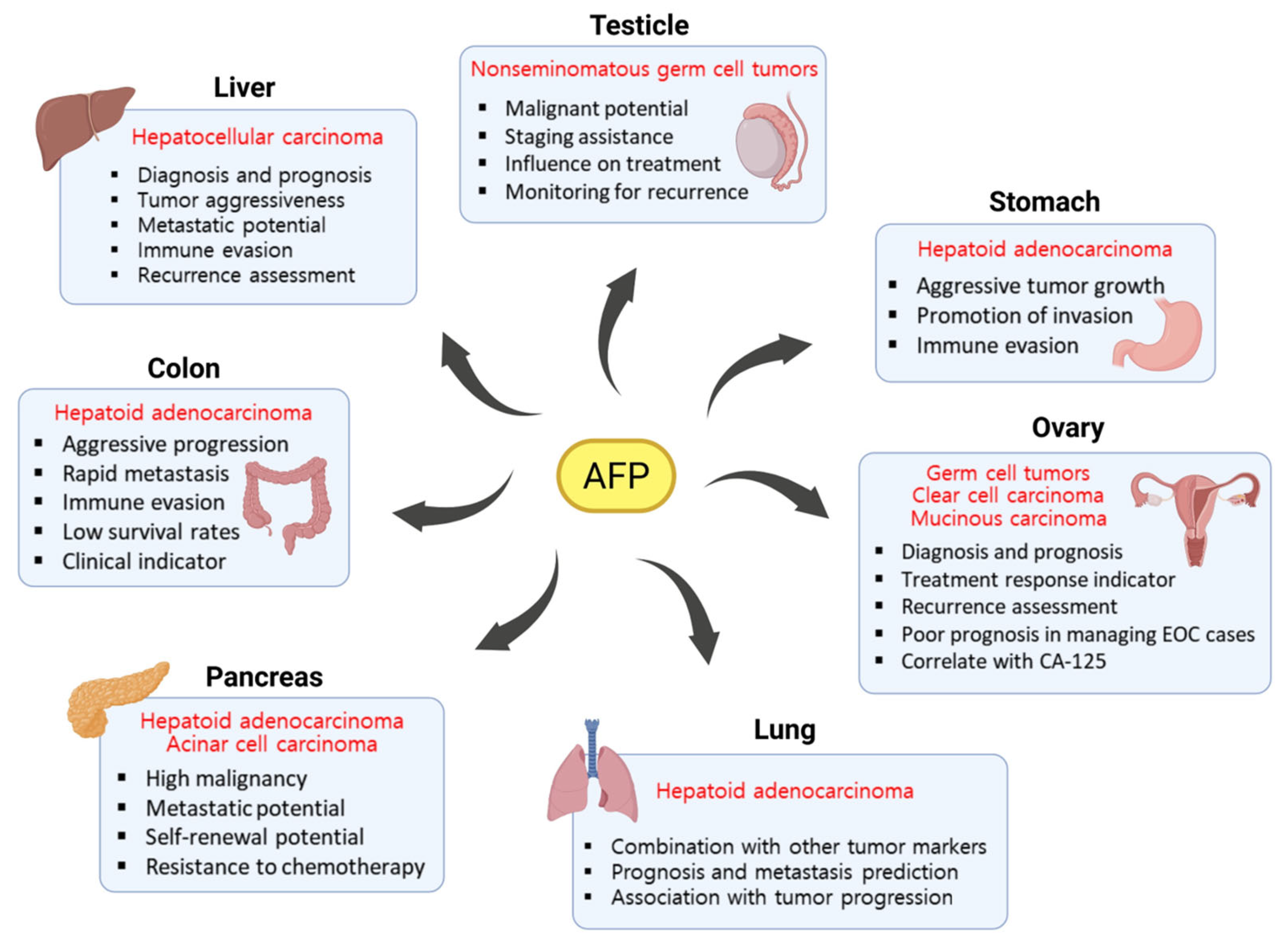
3. Role of AFP in Other Cancer Types
3.1. Testicular Cancer
3.2. Ovarian Cancer
3.3. Gastric Cancer
3.4. Colorectal Cancer
3.5. Pancreatic Cancer
3.6. Lung Cancer
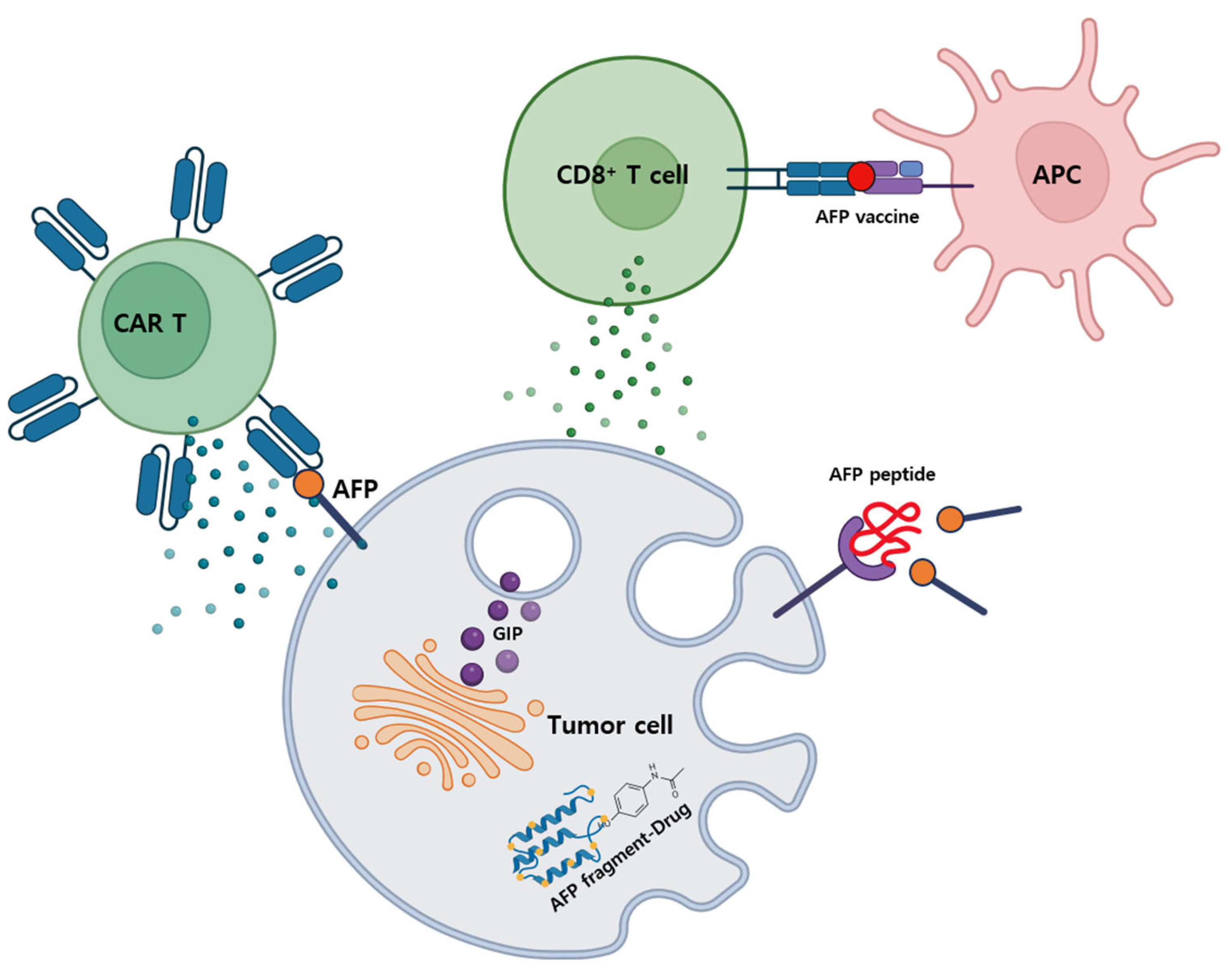
4. The Prospects of AFP-Targeted Anticancer Therapies
4.1. Current Status of AFP-Targeted Therapies
4.1.1. AFP-Inhibiting Fragments (AIFs)
4.1.2. AFP-Chimeric Antigen Receptor (CAR) T Cells
4.1.3. AFP Vaccine
4.2. Future Perspectives on AFP-Targeted Anticancer Therapies
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AIF | Alpha-fetoprotein-inhibiting fragments |
| ABCA12 | ATP-binding cassette, sub-family A, member 12 |
| ACLY | ATP citrate lyase |
| ACC | Acetyl-CoA carboxylase |
| AFP | Alpha-fetoprotein |
| APCs | Antigen-presenting cells |
| BCAAs | Branched-chain amino acids |
| cAMP | Cyclic adenosine monophosphate |
| CAR | Chimeric antigen receptor |
| CACT | Carnitine acylcarnitine translocase |
| COX | Cyclooxygenase |
| DCs | Dendritic cells |
| EMT | Epithelial–mesenchymal transition |
| EOC | Epithelial ovarian cancer |
| FASN | Fatty acid synthase |
| GLUTs | Glucose transporters |
| GIPs | Growth inhibitory peptides |
| HAL | Hepatoid adenocarcinoma of the lung |
| HCV | Hepatitis C virus |
| HSP | Heat shock protein |
| HuR | Human antigen R |
| IL | Interleukin |
| K19 | Keratin 19 |
| LMW | Low molecular weight |
| LPL | Lipoprotein lipase |
| MACC1 | Metastasis associated in colon cancer 1 |
| MDSCs | Myeloid-derived suppressor cells |
| MHC | Major histocompatibility complex |
| MMP | Matrix metalloproteinase |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NK | Natural killer |
| nAFP | Native alpha-fetoprotein |
| NSGCT | Non-seminomatous germ cell tumor |
| OXPHOS | Oxidative phosphorylation |
| PGF | Placental growth factor |
| PGC1-α | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PDH | Pyruvate dehydrogenase |
| PD-L1 | Programmed cell death ligand 1 |
| P-gp | P-glycoprotein |
| PKA | Protein kinase A |
| RAR | Retinoic acid receptor |
| TGF-β | Transforming growth factor beta |
| TAMs | Tumor-associated macrophages |
| tAFP | Tumor-derived alpha-fetoprotein |
| Treg | Regulatory T cells |
| VEGF | Vascular endothelial growth factor |
| ZEB1 | Zinc finger E-box binding homeobox 1 |
References
- Lu, Y.; Lin, B.; Li, M. The role of alpha-fetoprotein in the tumor microenvironment of hepatocellular carcinoma. Front. Oncol. 2024, 14, 1363695. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Wu, C.; Zhu, M.; Xu, J.; Lin, B.; Lin, H.; Liu, Z.; Li, M. Structural characteristics of alpha-fetoprotein, including N-glycosylation, metal ion and fatty acid binding sites. Commun. Biol. 2024, 7, 505. [Google Scholar] [CrossRef] [PubMed]
- Forner, A.; Bruix, J. Hepatocellular carcinoma–Authors’ reply. Lancet 2012, 380, 470–471. [Google Scholar] [CrossRef]
- Chan, Y.T.; Zhang, C.; Wu, J.; Lu, P.; Xu, L.; Yuan, H.; Feng, Y.; Chen, Z.S.; Wang, N. Biomarkers for diagnosis and therapeutic options in hepatocellular carcinoma. Mol. Cancer 2024, 23, 189. [Google Scholar] [CrossRef]
- Yao, M.; Zhao, J.; Lu, F. Alpha-fetoprotein still is a valuable diagnostic and prognosis predicting biomarker in hepatitis B virus infection-related hepatocellular carcinoma. Oncotarget 2016, 7, 3702–3708. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.X.; Yang, T.W.; Yin, J.M.; Yang, P.X.; Kou, B.X.; Chai, M.Y.; Liu, X.N.; Chen, D.X. Progress and prospects of biomarkers in primary liver cancer. Int. J. Oncol. 2020, 57, 54–66. [Google Scholar] [CrossRef]
- Yeo, Y.H.; Lee, Y.T.; Tseng, H.R.; Zhu, Y.; You, S.; Agopian, V.G.; Yang, J.D. Alpha-fetoprotein: Past, present, and future. Hepatol. Commun. 2024, 8, e0422. [Google Scholar] [CrossRef]
- Gabant, P.; Forrester, L.; Nichols, J.; Van Reeth, T.; De Mees, C.; Pajack, B.; Watt, A.; Smitz, J.; Alexandre, H.; Szpirer, C. Alpha-fetoprotein, the major fetal serum protein, is not essential for embryonic development but is required for female fertility. Proc. Natl. Acad. Sci. USA 2002, 99, 12865–12870. [Google Scholar] [CrossRef]
- Hanif, H.; Ali, M.J.; Susheela, A.T.; Khan, I.W.; Luna-Cuadros, M.A.; Khan, M.M.; Lau, D.T.Y. Update on the applications and limitations of alpha-fetoprotein for hepatocellular carcinoma. World J. Gastroenterol. 2022, 28, 216–229. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhu, M.; Li, M. Effects of alpha-fetoprotein on the occurrence and progression of hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2020, 146, 2439–2446. [Google Scholar] [CrossRef]
- Miyaaki, H.; Nakashima, O.; Kurogi, M.; Eguchi, K.; Kojiro, M. Lens culinaris agglutinin-reactive α-fetoprotein and protein induced by vitamin K absence II are potential indicators of a poor prognosis: A histopathological study of surgically resected hepatocellular carcinoma. J. Gastroenterol. 2007, 42, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Force, M.; Park, G.; Chalikonda, D.; Roth, C.; Cohen, M.; Halegoua-DeMarzio, D.; Hann, H.W. Alpha-fetoprotein (AFP) and AFP-L3 is most useful in detection of recurrence of hepatocellular carcinoma in patients after tumor ablation and with low AFP level. Viruses 2022, 14, 775. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Nakata, K.; Kato, Y.; Shima, M.; Ishii, N.; Koji, T.; Taketa, K.; Endo, Y.; Nagataki, S. Early recognition of hepatocellular carcinoma based on altered profiles of alpha-fetoprotein. N. Engl. J. Med. 1993, 328, 1802–1806. [Google Scholar] [CrossRef]
- Chen, T.; Dai, X.; Dai, J.; Ding, C.; Zhang, Z.; Lin, Z.; Hu, J.; Lu, M.; Wang, Z.; Qi, Y. AFP promotes HCC progression by suppressing the HuR-mediated Fas/FADD apoptotic pathway. Cell Death Dis. 2020, 11, 822. [Google Scholar] [CrossRef]
- Mitsuhashi, N.; Kobayashi, S.; Doki, T.; Kimura, F.; Shimizu, H.; Yoshidome, H.; Ohtsuka, M.; Kato, A.; Yoshitomi, H.; Nozawa, S. Clinical significance of α-fetoprotein: Involvement in proliferation, angiogenesis, and apoptosis of hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2008, 23, e189–e197. [Google Scholar] [CrossRef]
- Shan, Y.F.; Huang, Y.L.; Xie, Y.K.; Tan, Y.H.; Chen, B.C.; Zhou, M.T.; Shi, H.Q.; Yu, Z.P.; Song, Q.T.; Zhang, Q.Y. Angiogenesis and clinicopathologic characteristics in different hepatocellular carcinoma subtypes defined by EpCAM and α-fetoprotein expression status. Med. Oncol. 2011, 28, 1012–1016. [Google Scholar] [CrossRef]
- Santos, P.M.; Menk, A.V.; Shi, J.; Tsung, A.; Delgoffe, G.M.; Butterfield, L.H. Tumor-derived α-fetoprotein suppresses fatty acid metabolism and oxidative phosphorylation in dendritic cells. Cancer Immunol. Res. 2019, 7, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, K.; Chen, Y.; Zhu, M.; Li, M. Role of alpha-fetoprotein in hepatocellular carcinoma drug resistance. Curr. Med. Chem. 2021, 28, 1126–1142. [Google Scholar] [CrossRef]
- Ehrlich, Y.; Beck, S.D.; Foster, R.S.; Bihrle, R.; Einhorn, L.H. Serum tumor markers in testicular cancer. Urol. Oncol. 2013, 31, 17–23. [Google Scholar] [CrossRef]
- Chao, W.T.; Liu, C.H.; Lai, C.R.; Chen, Y.J.; Chuang, C.M.; Wang, P.H. Alpha-fetoprotein-producing ovarian clear cell adenocarcinoma with fetal gut differentiation: A rare case report and literature review. J. Ovarian Res. 2018, 11, 52. [Google Scholar] [CrossRef]
- Mao, X.; Wang, J.; Luo, F. Alpha-fetoprotein can promote gastric cancer progression via upregulation of metastasis-associated colon cancer 1. Oncol. Lett. 2022, 23, 84. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Xu, S.; Wang, K.; Wang, X.; Sun, G.; Li, X.; Ran, Y.; Sun, J. Alpha-fetoprotein-producing advanced colorectal cancer: A rare case report and literature review. J. Int. Med. Res. 2022, 50, 3000605221117218. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.J.; Wang, H.Y.; Teng, L.S. Correlation analysis of preoperative serum alpha-fetoprotein (AFP) level and prognosis of hepatocellular carcinoma (HCC) after hepatectomy. World J. Surg. Oncol. 2013, 11, 212. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.I.; Kim, H.S.; Park, J.W. Higher ratio of serum alpha-fetoprotein could predict outcomes in patients with hepatitis B virus-associated hepatocellular carcinoma and normal alanine aminotransferase. PLoS ONE 2016, 11, e0157299. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, W.; Chen, X.; Zhang, C.; Li, H.; Hou, W.; Liu, Z.; McNutt, M.A.; Lu, F.; Li, G. Alpha-fetoprotein acts as a novel signal molecule and mediates transcription of Fn14 in human hepatocellular carcinoma. J. Hepatol. 2012, 57, 322–329. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, M.; Wang, Q.; Hou, Y.; Li, L.; Weng, H.; Zhao, Y.; Chen, D.; Ding, H.; Guo, J. Alpha-fetoprotein inhibits autophagy to promote malignant behaviour in hepatocellular carcinoma cells by activating PI3K/AKT/mTOR signalling. Cell Death Dis. 2018, 9, 1027. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, J.; Wang, J.; Yan, Y.; Zhang, C. Alpha-fetoprotein accelerates the progression of hepatocellular carcinoma by promoting Bcl-2 gene expression through an RA-RAR signalling pathway. J. Cell. Mol. Med. 2020, 24, 13804–13812. [Google Scholar] [CrossRef]
- Mizejewski, G.J. Does alpha-fetoprotein contribute to the mortality and morbidity of human hepatocellular carcinoma? A commentary. J. Hepatocell. Carcinoma 2016, 3, 37–40. [Google Scholar] [CrossRef]
- Li, M.; Zhou, S.; Liu, X.; Li, P.; McNutt, M.A.; Li, G. α-Fetoprotein shields hepatocellular carcinoma cells from apoptosis induced by tumor necrosis factor-related apoptosis-inducing ligand. Cancer lett. 2007, 249, 227–234. [Google Scholar] [CrossRef]
- Li, M.; Liu, X.; Zhou, S.; Li, P.; Li, G. Effects of alpha fetoprotein on escape of Bel 7402 cells from attack of lymphocytes. BMC Cancer 2005, 5, 96. [Google Scholar] [CrossRef]
- Li, M.; Li, H.; Li, C.; Zhou, S.; Guo, L.; Liu, H.; Jiang, W.; Liu, X.; Li, P.; McNutt, M.A. Alpha fetoprotein is a novel protein-binding partner for caspase-3 and blocks the apoptotic signaling pathway in human hepatoma cells. Int. J. Cancer 2009, 124, 2845–2854. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Folkman, J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996, 86, 353–364. [Google Scholar] [CrossRef]
- Masood, R.; Cai, J.; Zheng, T.; Smith, D.L.; Hinton, D.R.; Gill, P.S. Vascular endothelial growth factor (VEGF) is an autocrine growth factor for VEGF receptor–positive human tumors. Blood 2001, 98, 1904–1913. [Google Scholar] [CrossRef]
- Risau, W. Mechanisms of angiogenesis. Nature 1997, 386, 671–674. [Google Scholar] [CrossRef]
- Montal, R.; Andreu-Oller, C.; Bassaganyas, L.; Esteban-Fabró, R.; Moran, S.; Montironi, C.; Moeini, A.; Pinyol, R.; Peix, J.; Cabellos, L. Molecular portrait of high alpha-fetoprotein in hepatocellular carcinoma: Implications for biomarker-driven clinical trials. Br. J. Cancer 2019, 121, 340–343. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhu, M.; Li, W.; Lin, B.; Dong, X.; Chen, Y.; Xie, X.; Guo, J.; Li, M. Alpha fetoprotein plays a critical role in promoting metastasis of hepatocellular carcinoma cells. J. Cell. Mol. Med. 2016, 20, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Wang, A.J.; Zhang, T.T.; Chen, S.H. Association of alpha-fetoprotein and metastasis for small hepatocellular carcinoma: A propensity-matched analysis. Sci. Rep. 2022, 12, 15676. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- Ren, Y.J.; Huang, T.; Yu, H.L.; Zhang, L.; He, Q.J.; Xiong, Z.F.; Peng, H. Expression of β-catenin protein in hepatocellular carcinoma and its relationship with alpha-fetoprotein. J. Huazhong Univ. Sci. Technolog. Med. Sci. 2016, 36, 846–851. [Google Scholar] [CrossRef]
- Kim, E.; Lisby, A.; Ma, C.; Lo, N.; Ehmer, U.; Hayer, K.E.; Furth, E.E.; Viatour, P. Promotion of growth factor signaling as a critical function of β-catenin during HCC progression. Nat. Commun. 2019, 10, 1909. [Google Scholar] [CrossRef]
- Deldar Abad Paskeh, M.; Mirzaei, S.; Ashrafizadeh, M.; Zarrabi, A.; Sethi, G. Wnt/β-catenin signaling as a driver of hepatocellular carcinoma progression: An emphasis on molecular pathways. J. Hepatocell. Carcinoma 2021, 8, 1415–1444. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Xie, K.; Lan, T.; Xu, L.; Chen, X.; Li, X.; Liao, M.; Li, J.; Huang, J.; Zeng, Y. TXNDC12 promotes EMT and metastasis of hepatocellular carcinoma cells via activation of β-catenin. Cell Death Differ. 2020, 27, 1355–1368. [Google Scholar] [CrossRef]
- Lu, W.; Kang, Y. Epithelial-mesenchymal plasticity in cancer progression and metastasis. Dev. Cell 2019, 49, 361–374. [Google Scholar] [CrossRef]
- Debnath, P.; Huirem, R.S.; Dutta, P.; Palchaudhuri, S. Epithelial–mesenchymal transition and its transcription factors. Biosci. Rep. 2022, 42, BSR20211754. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Wong, Y.S.; Goh, B.K.; Chan, C.Y.; Cheow, P.C.; Chow, P.K.; Lim, T.K.; Goh, G.B.; Krishnamoorthy, T.L.; Kumar, R. Circulating microRNAs as potential diagnostic and prognostic biomarkers in hepatocellular carcinoma. Sci. Rep. 2019, 9, 10464. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef]
- Yang, J.; Shay, C.; Saba, N.F.; Teng, Y. Cancer metabolism and carcinogenesis. Exp. Hematol. Oncol. 2024, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Shiau, J.P.; Chuang, Y.T.; Cheng, Y.B.; Tang, J.Y.; Hou, M.F.; Yen, C.Y.; Chang, H.W. Impacts of oxidative stress and PI3K/AKT/mTOR on metabolism and the future direction of investigating fucoidan-modulated metabolism. Antioxidants 2022, 11, 911. [Google Scholar] [CrossRef]
- Ward, P.S.; Thompson, C.B. Signaling in control of cell growth and metabolism. Cold Spring Harb. Perspect. Biol. 2012, 4, a006783. [Google Scholar] [CrossRef]
- Fan, H.; Wu, Y.; Yu, S.; Li, X.; Wang, A.; Wang, S.; Chen, W.; Lu, Y. Critical role of mTOR in regulating aerobic glycolysis in carcinogenesis. Int. J. Oncol. 2020, 58, 9–19. [Google Scholar] [CrossRef]
- Ruan, Z.; Wang, Y.; Shi, L.; Yang, X.J. Progress of research on glucose transporter proteins in hepatocellular carcinoma. World J. Hepatol. 2025, 17, 104715. [Google Scholar] [CrossRef] [PubMed]
- Munson, P.V.; Adamik, J.; Hartmann, F.J.; Favaro, P.M.; Ho, D.; Bendall, S.C.; Combes, A.J.; Krummel, M.F.; Zhang, K.; Kelley, R.K. Polyunsaturated Fatty Acid–Bound α-Fetoprotein Promotes Immune Suppression by Altering Human Dendritic Cell Metabolism. Cancer Res. 2023, 83, 1543–1557. [Google Scholar] [CrossRef] [PubMed]
- Röhrig, F.; Schulze, A. The multifaceted roles of fatty acid synthesis in cancer. Nat. Rev. Cancer 2016, 16, 732–749. [Google Scholar] [CrossRef]
- Park, S.; Hall, M.N. Metabolic reprogramming in hepatocellular carcinoma: Mechanisms and therapeutic implications. Exp. Mol. Med. 2025, 57, 515–523. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Nagao, Y.; Abe, K.; Imazeki, F.; Honda, K.; Yamasaki, K.; Miyanishi, K.; Taniguchi, E.; Kakuma, T.; Kato, J. Effects of branched-chain amino acids and zinc-enriched nutrients on prognosticators in HCV-infected patients: A multicenter randomized controlled trial. Mol. Med. Rep. 2015, 11, 2159–2166. [Google Scholar] [CrossRef]
- Alisa, A.; Ives, A.; Pathan, A.A.; Navarrete, C.V.; Williams, R.; Bertoletti, A.; Behboudi, S. Analysis of CD4+ T-cell responses to a novel α-fetoprotein-derived epitope in hepatocellular carcinoma patients. Clin. Cancer Res. 2005, 11, 6686–6694. [Google Scholar] [CrossRef] [PubMed]
- Alisa, A.; Boswell, S.; Pathan, A.A.; Ayaru, L.; Williams, R.; Behboudi, S. Human CD4+ T cells recognize an epitope within α-fetoprotein sequence and develop into TGF-β-producing CD4+ T cells. J. Immunol. 2008, 180, 5109–5117. [Google Scholar] [CrossRef]
- Shi, X.; Yang, J.; Deng, S.; Xu, H.; Wu, D.; Zeng, Q.; Wang, S.; Hu, T.; Wu, F.; Zhou, H. TGF-β signaling in the tumor metabolic microenvironment and targeted therapies. J. Hematol. Oncol. 2022, 15, 135. [Google Scholar] [CrossRef]
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumor-associated macrophages in tumor immunity. Front. Immunol. 2020, 11, 583084. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Chen, Z.; Luo, J.; Guo, W.; Sun, L.; Lin, L. Targeting M2-like tumor-associated macrophages is a potential therapeutic approach to overcome antitumor drug resistance. NPJ precis. Oncol. 2024, 8, 31. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, K.; Zhang, Q.; Xu, J.; Liu, J.; Lin, H.; Lin, B.; Zhu, M.; Li, M. Alpha fetoprotein promotes polarization of macrophages towards M2-like phenotype and inhibits macrophages to phagocytize hepatoma cells. Front. Immunol. 2023, 14, 1081572. [Google Scholar] [CrossRef]
- Suzuki, Y.; Zeng, C.; Alpert, E. Isolation and partial characterization of a specific alpha-fetoprotein receptor on human monocytes. J. Clin. Invest. 1992, 90, 1530–1536. [Google Scholar] [CrossRef]
- Pan, Y.; Yin, Q.; Wang, Z.; Wu, G.; Liu, K.; Li, X.; Liu, J.; Zeng, J.; Lin, B.; Li, W. AFP shields hepatocellular carcinoma from macrophage phagocytosis by regulating HuR-mediated CD47 translocation in cellular membrane. Transl. Oncol. 2025, 52, 102240. [Google Scholar] [CrossRef]
- Suryatenggara, J.; Wibowo, H.; Atmodjo, W.L.; Mathew, G. Characterization of alpha-fetoprotein effects on dendritic cell and its function as effector immune response activator. J. Hepatocell. Carcinoma 2017, 4, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Vujanovic, L.; Stahl, E.C.; Pardee, A.D.; Geller, D.A.; Tsung, A.; Watkins, S.C.; Gibson, G.A.; Storkus, W.J.; Butterfield, L.H. Tumor-derived α-fetoprotein directly drives human natural killer–cell activation and subsequent cell death. Cancer Immunol. Res. 2017, 5, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Tatsumi, T.; Miyagi, T.; Tsunematsu, H.; Aketa, H.; Hosui, A.; Kanto, T.; Hiramatsu, N.; Hayashi, N.; Takehara, T. α-Fetoprotein impairs activation of natural killer cells by inhibiting the function of dendritic cells. Clin. Exp. Immunol. 2011, 165, 211–219. [Google Scholar] [CrossRef]
- Son, B.; Lee, S.; Youn, H.; Kim, E.; Kim, W.; Youn, B. The role of tumor microenvironment in therapeutic resistance. Oncotarget 2016, 8, 3933. [Google Scholar] [CrossRef] [PubMed]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug resistance in cancer: An overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef]
- Sun, Y. Tumor microenvironment and cancer therapy resistance. Cancer Lett. 2016, 380, 205–215. [Google Scholar] [CrossRef]
- Dhar, D.K.; Nagasue, N.; Yoshimura, H.; Tachibana, M.; Tahara, H.; Matsuura, H.; Abe, S.I.; Chang, Y.C.; Nakamura, T. Overexpression of P-glycoprotein in untreated AFP-producing gastric carcinoma. J. Surg. Oncol. 1995, 60, 50–54. [Google Scholar] [CrossRef]
- Samban, S.S.; Hari, A.; Nair, B.; Kumar, A.R.; Meyer, B.S.; Valsan, A.; Vijayakurup, V.; Nath, L.R. An insight into the role of alpha-fetoprotein (AFP) in the development and progression of hepatocellular carcinoma. Mol. Biotechnol. 2024, 66, 2697–2709. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.Q.; Wang, X.Q.; Fan, G.; Wang, C.Y.; Zheng, Y.W.; Song, X.; Pan, C.C.; Chu, F.L.; Liu, Z.F.; Lu, B.R. Value of AFP and PIVKA-II in diagnosis of HBV-related hepatocellular carcinoma and prediction of vascular invasion and tumor differentiation. Infect. Agents Cancer 2020, 15, 70. [Google Scholar] [CrossRef]
- Galle, P.R.; Foerster, F.; Kudo, M.; Chan, S.L.; Llovet, J.M.; Qin, S.; Schelman, W.R.; Chintharlapalli, S.; Abada, P.B.; Sherman, M. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver Int. 2019, 39, 2214–2229. [Google Scholar] [CrossRef] [PubMed]
- Yazdanpanah, F.; Al-Daoud, O.; Moradpour, M.; Hunt, S. Measures for response assessment in HCC treatment. Hepatoma Res. 2024, 10, 27. [Google Scholar] [CrossRef]
- Milose, J.C.; Filson, C.P.; Weizer, A.Z.; Hafez, K.S.; Montgomery, J.S. Role of biochemical markers in testicular cancer: Diagnosis, staging, and surveillance. Res. Rep. Urol. 2011, 4, 1–8. [Google Scholar]
- Sykes, J.; Kaldany, A.; Jang, T.L. Current and Evolving Biomarkers in the Diagnosis and Management of Testicular Germ Cell Tumors. J. Clin. Med. 2024, 13, 7448. [Google Scholar] [CrossRef]
- Nicholson, B.D.; Jones, N.R.; Protheroe, A.; Joseph, J.; Roberts, N.W.; Van den Bruel, A.; Fanshawe, T.R. The diagnostic performance of current tumour markers in surveillance for recurrent testicular cancer: A diagnostic test accuracy systematic review. Cancer Epidemiol. 2019, 59, 15–21. [Google Scholar] [CrossRef]
- Leão, R.; Ahmad, A.E.; Hamilton, R.J. Testicular cancer biomarkers: A role for precision medicine in testicular cancer. Clin. Genitourin. Cancer 2019, 17, e176–e183. [Google Scholar] [CrossRef]
- Talerman, A.; Haije, W.; Baggerman, L. Serum alphafetoprotein (AFP) in patients with germ cell tumors of the gonads and extragonadal sites: Correlation between endodermal sinus (yolk sac) tumor and raised serum AFP. Cancer 1980, 46, 380–385. [Google Scholar] [CrossRef]
- Zong, X.; Yang, J.X.; Zhang, Y. Persistently elevated alpha-fetoprotein associated with chronic hepatitis B during chemotherapy for malignant ovarian germ cell tumors: A case series and a review of the literature. J. Ovarian Res. 2019, 12, 124. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Cao, D.; Yang, J.; Shen, K.; Huang, H.; Shi, X. Alpha-fetoprotein (AFP)-producing epithelial ovarian carcinoma (EOC): A retrospective study of 27 cases. Arch. Gynecol. Obstet. 2021, 304, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, H.; Tageldin, O.; Hasak, S.; Lee, H. AFP-producing gastric carcinoma. Hum. Pathol. Rep. 2022, 28, 300640. [Google Scholar] [CrossRef]
- Chun, H.; Kwon, S.J. Clinicopathological characteristics of alpha-fetoprotein-producing gastric cancer. J. Gastric Cancer 2011, 11, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lin, Y.; Zhao, L.; Yang, C.; Wang, B.; Gao, Z.; Ye, Y.; Wang, S.; Shen, Z. Characteristics of alpha-fetoprotein-positive gastric cancer revealed by analysis of cancer databases and transcriptome sequencing data. Transl. Oncol. 2023, 36, 101737. [Google Scholar] [CrossRef]
- Abada, E.; Anaya, I.C.; Abada, O.; Lebbos, A.; Beydoun, R. Colorectal adenocarcinoma with enteroblastic differentiation: Diagnostic challenges of a rare case encountered in clinical practice. J. Pathol. Transl. Med. 2022, 56, 97–102. [Google Scholar] [CrossRef]
- Karakuchi, N.; Toyota, K.; Kajikawa, R.; Watanabe, A.; Saito, Y.; Inoue, M.; Ohmori, I.; Miyamoto, K.; Ikeda, M.; Sadamoto, S. Alpha-fetoprotein-producing Colon Carcinoma with Rapidly Increasing Liver Metastases That Resulted in Death: A Case Report. J. Anus Rectum Colon 2021, 5, 100–106. [Google Scholar] [CrossRef]
- Banga, R.; Sonpal, N.; Thelmo, W.; Arya, Y.; Arya, M. Agressive AFP Producing Colorectal Cancer in a 40 Year Old Male: 946. Am. J. Gastroenterol. 2009, 104, S348–S349. [Google Scholar] [CrossRef]
- Ren, F.; Weng, W.; Zhang, Q.; Tan, C.; Xu, M.; Zhang, M.; Wang, L.; Sheng, W.; Ni, S.; Huang, D. Clinicopathological features and prognosis of AFP-producing colorectal cancer: A single-center analysis of 20 cases. Cancer Manag. Res. 2019, 11, 4557–4567. [Google Scholar] [CrossRef]
- Gvajaia, A.; Imeh, M.; Raza, A. An Interesting Case of Alpha-Fetoprotein (AFP)-Producing Pancreaticoduodenal Tumor. Cureus 2024, 16, e59384. [Google Scholar] [CrossRef]
- Sasaki, N.; Ishii, T.; Kamimura, R.; Kajiwara, M.; Machimoto, T.; Nakatsuji, N.; Suemori, H.; Ikai, I.; Yasuchika, K.; Uemoto, S. Alpha-fetoprotein-producing pancreatic cancer cells possess cancer stem cell characteristics. Cancer lett. 2011, 308, 152–161. [Google Scholar] [CrossRef]
- Cai, C.; Zhang, N.; Wang, M.; Wang, L.; Zhao, H.; Zhang, X.; Li, B.; Wang, J. Primary hepatoid adenocarcinoma of the lung with extremely elevated serum AFP: A case report and literature review. Front. Oncol. 2024, 14, 1448219. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Ge, Q.M.; Huang, R.; Shu, H.Y.; Su, T.; Wu, J.L.; Pan, Y.C.; Liang, R.B.; Zhang, L.J.; Shao, Y. Clinical significance of CYFRA21-1, AFP, CA-153, CEA, and CA-199 in the diagnosis of lung cancer ocular metastasis in hypertension population. Front. Cardiovasc. Med. 2021, 8, 670594. [Google Scholar] [CrossRef]
- Yang, W.; Liu, L.; Li, J.; Liu, Y.; Rong, L.; Ma, J.; Li, R.; Zhang, Q.; Liu, Y. Abnormal expression of miR-668-3p in non-small cell lung cancer patients and its correlation with serum-related tumor markers. J. Cardiothorac. Surg. 2025, 20, 58. [Google Scholar] [CrossRef]
- Zitvogel, L.; Apetoh, L.; Ghiringhelli, F.; Kroemer, G. Immunological aspects of cancer chemotherapy. Nat. Rev. Immunol. 2008, 8, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef] [PubMed]
- Bentzen, S.M. Preventing or reducing late side effects of radiation therapy: Radiobiology meets molecular pathology. Nat. Rev. Cancer 2006, 6, 702–713. [Google Scholar] [CrossRef]
- Lin, B.; Dong, X.; Wang, Q.; Li, W.; Zhu, M.; Li, M. AFP-inhibiting fragments for drug delivery: The promise and challenges of targeting therapeutics to cancers. Front. Cell Dev. Biol. 2021, 9, 635476. [Google Scholar] [CrossRef]
- Mizejewski, G.J.; Butterstein, G. Survey of functional activities of alpha-fetoprotein derived growth inhibitory peptides: Review and prospects. Curr. Protein Pept. Sci. 2006, 7, 73–100. [Google Scholar] [CrossRef]
- Mizejewski, G.; Mirowski, M.; Garnuszek, P.; Maurin, M.; Cohen, B.; Poiesz, B.; Posypanova, G.; Makarov, V.; Severin, E.; Severin, S. Targeted delivery of anti-cancer growth inhibitory peptides derived from human α-fetoprotein: Review of an International Multi-Center Collaborative Study. J. Drug Target. 2010, 18, 575–588. [Google Scholar] [CrossRef]
- Głowska-Ciemny, J.; Szymański, M.; Kuszerska, A.; Malewski, Z.; von Kaisenberg, C.; Kocyłowski, R. The role of alpha-fetoprotein (AFP) in contemporary oncology: The path from a diagnostic biomarker to an anticancer drug. Int. J. Mol. Sci. 2023, 24, 2539. [Google Scholar] [CrossRef]
- Pak, V.N. Alpha-Fetoprotein: A Revolutionary Anti-Cancer Drug. Med. Res. Arch. 2023, 11, n.7.1. [Google Scholar] [CrossRef]
- June, C.H.; O’Connor, R.S.; Kawalekar, O.U.; Ghassemi, S.; Milone, M.C. CAR T cell immunotherapy for human cancer. Science 2018, 359, 1361–1365. [Google Scholar] [CrossRef]
- Sterner, R.C.; Sterner, R.M. CAR-T cell therapy: Current limitations and potential strategies. Blood Cancer J. 2021, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, Y.; Xiang, J.; Long, L.; Green, S.; Yang, Z.; Zimdahl, B.; Lu, J.; Cheng, N.; Horan, L.H. Targeting alpha-fetoprotein (AFP)–MHC complex with CAR T-cell therapy for liver cancer. Clin. Cancer Res. 2017, 23, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Bachiller, M.; Perez-Amill, L.; Battram, A.M.; Carné, S.C.; Najjar, A.; Verhoeyen, E.; Juan, M.; Urbano-Ispizua, A.; Martin-Antonio, B. NK cells enhance CAR-T cell antitumor efficacy by enhancing immune/tumor cells cluster formation and improving CAR-T cell fitness. J. Immunother. Cancer 2021, 9, e002866. [Google Scholar] [CrossRef]
- Mizukoshi, E.; Nakamoto, Y.; Tsuji, H.; Yamashita, T.; Kaneko, S. Identification of α-fetoprotein-derived peptides recognized by cytotoxic T lymphocytes in HLA-A24+ patients with hepatocellular carcinoma. Int. J. Cancer 2006, 118, 1194–1204. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, C.M., Jr.; Eilber, F.C.; Butterfield, L.H.; Ribas, A.; Dissette, V.B.; Koh, A.; Montejo, L.D.; Lee, M.C.; Andrews, K.J.; McBride, W.H. α-Fetoprotein-specific genetic immunotherapy for hepatocellular carcinoma. Cancer Res. 1999, 59, 3064–3067. [Google Scholar]
- Hong, Y.; Peng, Y.; Guo, Z.S.; Guevara-Patino, J.; Pang, J.; Butterfield, L.H.; Mivechi, N.F.; Munn, D.H.; Bartlett, D.L.; He, Y. Epitope-optimized alpha-fetoprotein genetic vaccines prevent carcinogen-induced murine autochthonous hepatocellular carcinoma. Hepatology 2014, 59, 1448–1458. [Google Scholar] [CrossRef]
- Bei, R.; Mizejewski, G. Alpha fetoprotein is more than a hepatocellular cancer biomarker: From spontaneous immune response in cancer patients to the development of an AFP-based cancer vaccine. Curr. Mol. Med. 2011, 11, 564–581. [Google Scholar] [CrossRef]
- Lu, X.; Deng, S.; Xu, J.; Green, B.L.; Zhang, H.; Cui, G.; Zhou, Y.; Zhang, Y.; Xu, H.; Zhang, F. Combination of AFP vaccine and immune checkpoint inhibitors slows hepatocellular carcinoma progression in preclinical models. J. Clin. Invest. 2023, 133, e163291. [Google Scholar] [CrossRef]
- Lan, Y.H.; Li, Y.G.; Liang, Z.W.; Chen, M.; Peng, M.L.; Tang, L.; Hu, H.D.; Ren, H. A DNA vaccine against chimeric AFP enhanced by HSP70 suppresses growth of hepatocellular carcinoma. Cancer Immunol. Immunother. 2007, 56, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Pak, V.N. Selective targeting of myeloid-derived suppressor cells in cancer patients through AFP-binding receptors. Future Sci. OA. 2019, 5, FSO321. [Google Scholar] [CrossRef] [PubMed]
- Houwert, A.; Giltay, J.; Lentjes, E.; Lock, M. Hereditary persistence of alpha-fetoprotein (HPAF P): Review of the literature. Neth. J. Med. 2010, 68, 354–358. [Google Scholar] [PubMed]
- Chan, L.K.; Tsui, Y.M.; Ho, D.W.H.; Ng, I.O.L. Cellular heterogeneity and plasticity in liver cancer. Semin. Cancer Biol. 2022, 82, 134–149. [Google Scholar] [CrossRef]
- Safri, F.; Nguyen, R.; Zerehpooshnesfchi, S.; George, J.; Qiao, L. Heterogeneity of hepatocellular carcinoma: From mechanisms to clinical implications. Cancer Gene Ther. 2024, 31, 1105–1112. [Google Scholar] [CrossRef]
- Wong, R.J.; Ahmed, A.; Gish, R.G. Elevated alpha-fetoprotein: Differential diagnosis-hepatocellular carcinoma and other disorders. Clin. Liver Dis. 2015, 19, 309–323. [Google Scholar] [CrossRef]
- Shah, P.A.; Onken, A.; Ishtiaq, R.; Maqsood, M.H.; Patel, S.S.; Campoverde Reyes, K.J.; Herskovits, A.Z.; Lau, D.T. A challenging case of alpha-fetoprotein-result discrepancies in a patient with chronic hepatitis B. Gastroenterol. Rep. 2020, 8, 484–486. [Google Scholar] [CrossRef]
| Cell Type | Mechanism | Effect | Outcome |
|---|---|---|---|
| T cells |
|
|
|
| Macrophages |
|
|
|
| DCs |
|
|
|
| NK cells |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Jang, M.; Kim, E. Exploring the Multifunctional Role of Alpha-Fetoprotein in Cancer Progression: Implications for Targeted Therapy in Hepatocellular Carcinoma and Beyond. Int. J. Mol. Sci. 2025, 26, 4863. https://doi.org/10.3390/ijms26104863
Kim H, Jang M, Kim E. Exploring the Multifunctional Role of Alpha-Fetoprotein in Cancer Progression: Implications for Targeted Therapy in Hepatocellular Carcinoma and Beyond. International Journal of Molecular Sciences. 2025; 26(10):4863. https://doi.org/10.3390/ijms26104863
Chicago/Turabian StyleKim, Hyunjung, Minji Jang, and Eunmi Kim. 2025. "Exploring the Multifunctional Role of Alpha-Fetoprotein in Cancer Progression: Implications for Targeted Therapy in Hepatocellular Carcinoma and Beyond" International Journal of Molecular Sciences 26, no. 10: 4863. https://doi.org/10.3390/ijms26104863
APA StyleKim, H., Jang, M., & Kim, E. (2025). Exploring the Multifunctional Role of Alpha-Fetoprotein in Cancer Progression: Implications for Targeted Therapy in Hepatocellular Carcinoma and Beyond. International Journal of Molecular Sciences, 26(10), 4863. https://doi.org/10.3390/ijms26104863




