Serum RNA Profile Reflects Fluid Status and Atrophic Retinal Changes in Neovascular Age-Related Macular Degeneration
Abstract
1. Introduction
2. Results
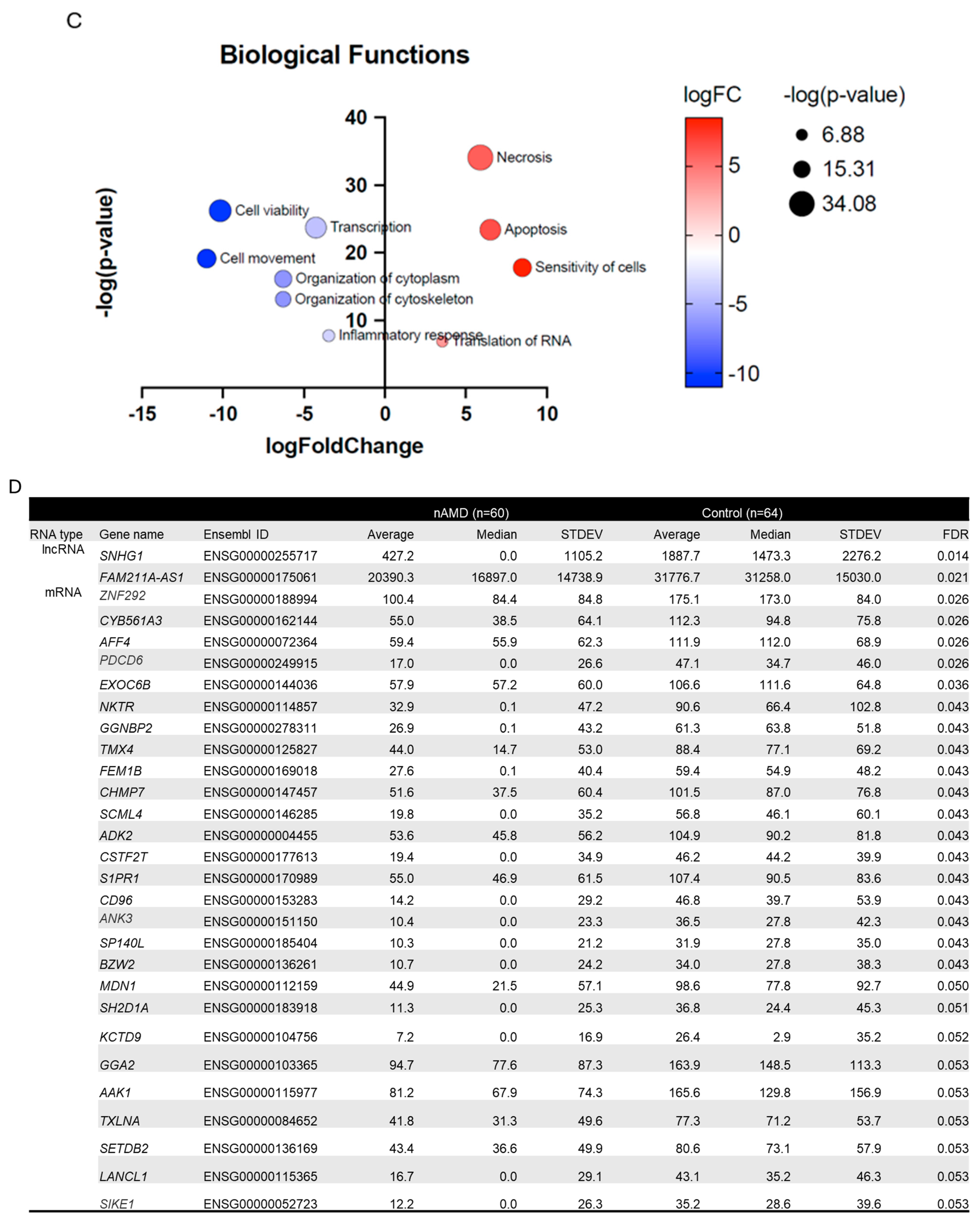
2.1. Differentially Expressed Serum RNAs in nAMD vs. Controls
2.2. RNA Profile Linked to nAMD Progression
| A | ||||||||||
| Characteristic | nAMD. N = 60 | |||||||||
| Sampling at baseline. n (treatment-naïve) | 23 | |||||||||
| Sampling after beginning of anti-VEGF treatment. n | 37 | |||||||||
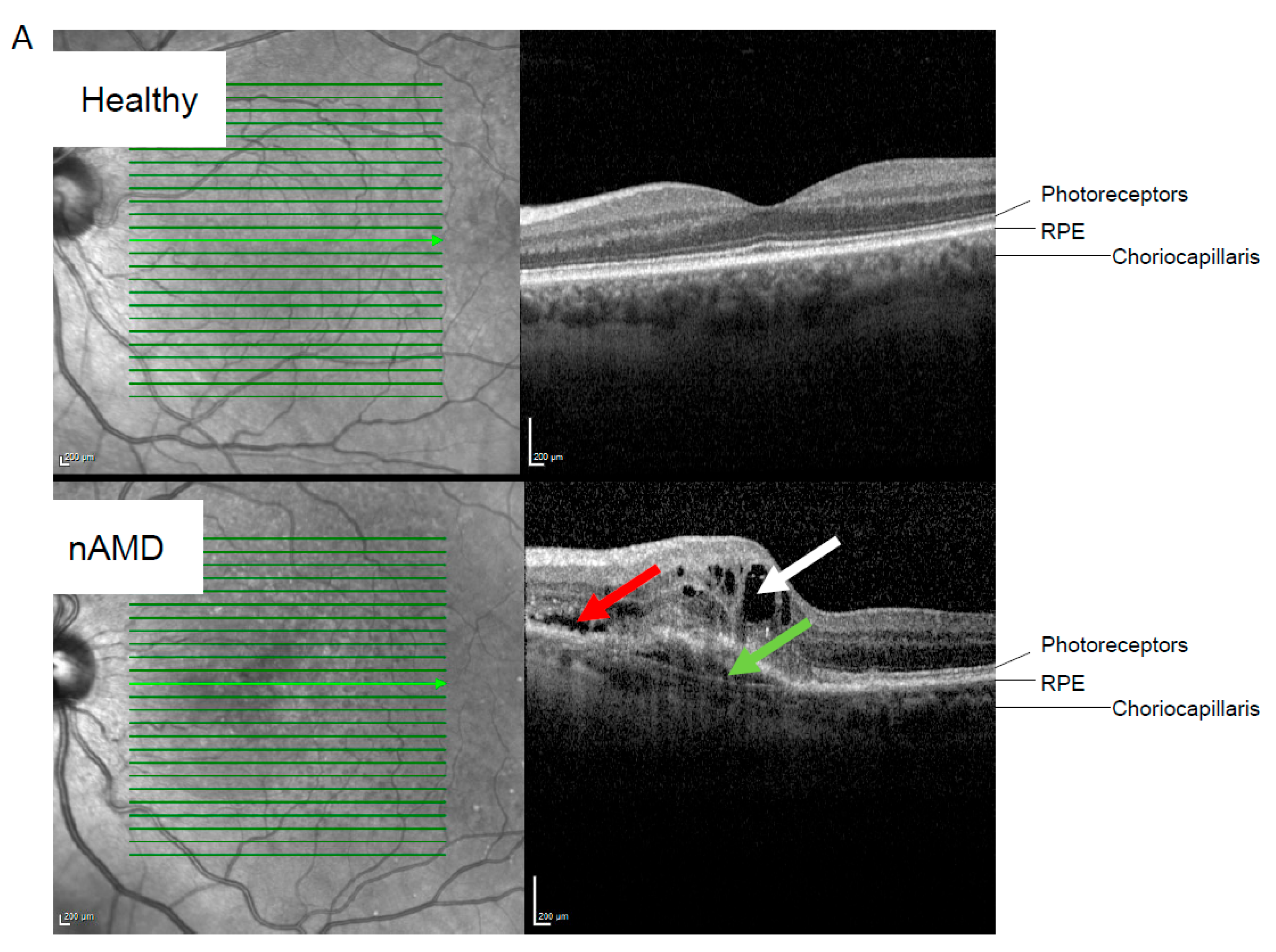
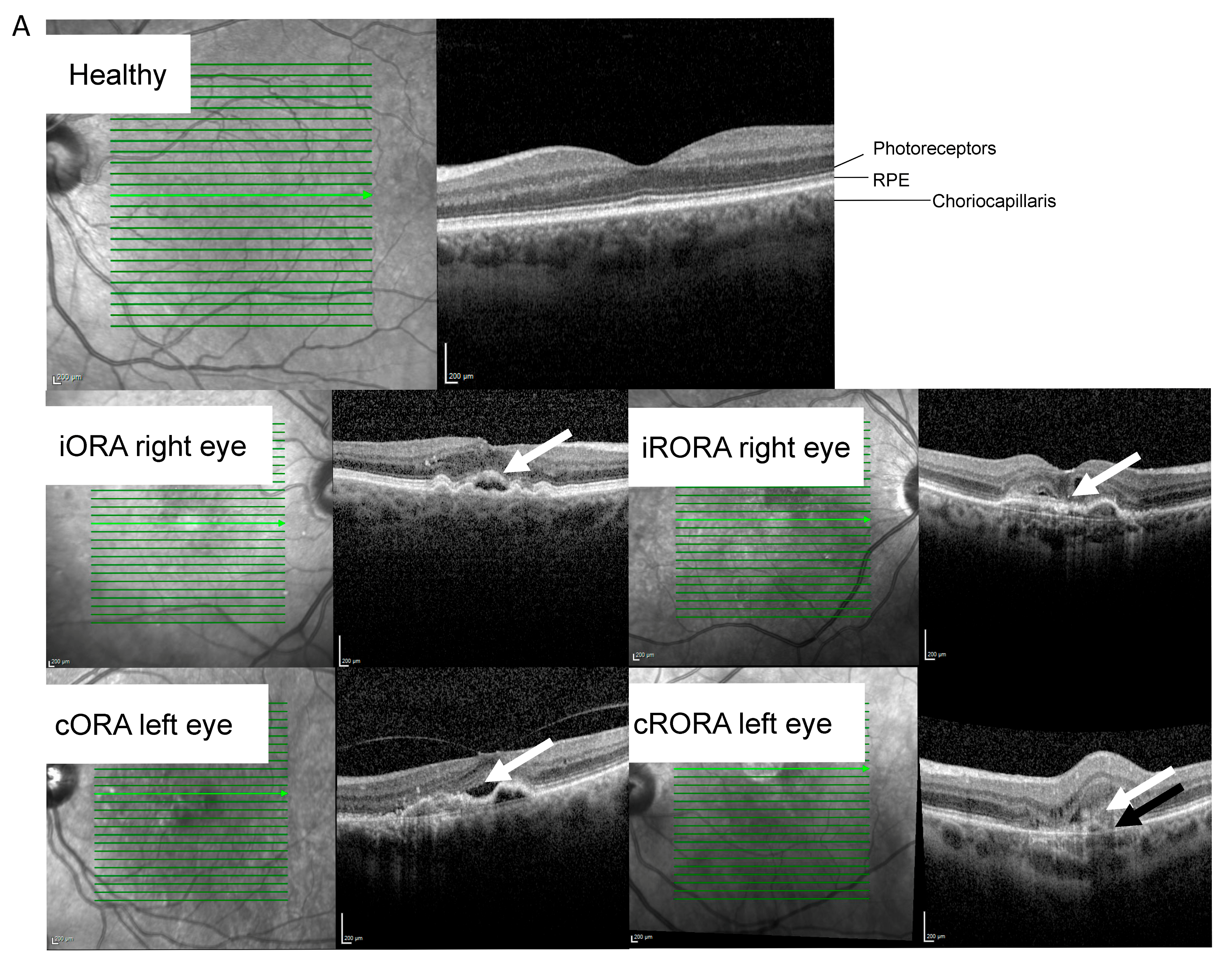
| Retinal atrophy at sampling. n (iORA/cORA/iRORA/cRORA/RPE tear/unknown) | 11/12/12/17/6/2 | |||||||||
| Fluid status at sampling. n (IRF/SRF/IRF & SRF/unknown) | 18/21/18/3 | |||||||||
| B | ||||||||||
| Adjusted means | Adjusted means. nAMD | Direction of change | ||||||||
| RNA type | Gene name | Ensembl ID | Control. N = 64 | nAMD. N = 59 | Adjusted p-value. Control vs. nAMD | Baseline | After anti-VEGF | Adjusted p-value. Baseline vs. anti-VEGF | in nAMD | after anti-VEGF |
| lncRNA | SNHG29 | ENSG00000175061 | 34,896.0 | 20,597.8 | 4.0 × 10−6 | 23,379.0 | 20,046.7 | 0.008 | Down | Down |
| mRNA | SKAP1 | ENSG00000141293 | 52.3 | 24.9 | 0.029 | 48.0 | 18.9 | 3.4 × 10−6 | ||
| PAG1 | ENSG00000076641 | 81.0 | 41.8 | 0.003 | 57.2 | 41.1 | 0.016 | |||
| C | ||||||||||
| Protein/lncRNA Name | Function | References | ||||||||
| SNHG29 | Inhibits vascular smooth muscle cell calcification. In glioblastoma cells accelerated cell proliferation, migration and EMT process. Enhanced cellular senescence during premature birth. | [19,20,21] | ||||||||
| SKAP1 | An immune cell adaptor that regulates T-cell adhesion and optimal cell growth. mRNA downregulated in blood monocytes of nAMD patients. | [25,26] | ||||||||
| PAG1 | Hypoxia induced. Interacts with with C-terminal of Src kinase. | [27] | ||||||||
| GRSF1 | Regulates cellular senescence during aging. Antagonizes age-related hypercoagulability. | [22,28] | ||||||||
| CDK5RAP2 | Involved in cell cycle regulation, cell cycle checkpoint control and DNA repair. Role in development of the eye and retina. | [23,24] | ||||||||
2.3. Analysis for Covariance
2.4. Staining of TUBGCP3 and CHMP6 in the Human Retina, RPE, and Choroid
2.5. RNAs Enriched After Both Approaches
3. Discussion
4. Materials and Methods
4.1. Patient Blood Sample and Data Collection
4.2. RNA Sequencing
4.3. Data Processing and Statistical Analysis
4.4. Staining of Cadaver Samples
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaarniranta, K.; Blasiak, J.; Liton, P.; Boulton, M.; Klionsky, D.J.; Sinha, D. Autophagy in age-related macular degeneration. Autophagy 2023, 19, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Fleckenstein, M.; Schmitz-Valckenberg, S.; Chakravarthy, U. Age-Related Macular Degeneration: A Review. JAMA 2024, 331, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Boopathiraj, N.; Wagner, I.V.; Dorairaj, S.K.; Miller, D.D.; Stewart, M.W. Recent Updates on the Diagnosis and Management of Age-Related Macular Degeneration. Mayo Clin. Proc. Innov. Qual. Outcomes 2024, 8, 364–374. [Google Scholar] [CrossRef]
- Helotera, H.; Kaarniranta, K. A Linkage between Angiogenesis and Inflammation in Neovascular Age-Related Macular Degeneration. Cells 2022, 11, 3453. [Google Scholar] [CrossRef]
- Helotera, H.; Siintamo, L.; Kivinen, N.; Abrahamsson, N.; Aaltonen, V.; Kaarniranta, K. Analysis of prognostic and predictive factors in neovascular age-related macular degeneration Kuopio cohort. Acta Ophthalmol. 2024, 102, 703–713. [Google Scholar] [CrossRef]
- Spaide, R.F.; Jaffe, G.J.; Sarraf, D.; Freund, K.B.; Sadda, S.R.; Staurenghi, G.; Waheed, N.K.; Chakravarthy, U.; Rosenfeld, P.J.; Holz, F.G.; et al. Consensus Nomenclature for Reporting Neovascular Age-Related Macular Degeneration Data: Consensus on Neovascular Age-Related Macular Degeneration Nomenclature Study Group. Ophthalmology 2020, 127, 616–636. [Google Scholar] [CrossRef]
- Mathis, T.; Kodjikian, L. Age-Related Macular Degeneration: New Insights in Diagnosis, Treatment, and Prevention. J. Clin. Med. 2022, 11, 1064. [Google Scholar] [CrossRef]
- Abd El-Khalek, A.A.; Balaha, H.M.; Alghamdi, N.S.; Ghazal, M.; Khalil, A.T.; Abo-Elsoud, M.E.A.; El-Baz, A. A concentrated machine learning-based classification system for age-related macular degeneration (AMD) diagnosis using fundus images. Sci. Rep. 2024, 14, 2434. [Google Scholar] [CrossRef]
- Sarkar, A.; Dyawanapelly, S. Nanodiagnostics and Nanotherapeutics for age-related macular degeneration. J. Control Release 2021, 329, 1262–1282. [Google Scholar] [CrossRef]
- Blasiak, J.; Watala, C.; Tuuminen, R.; Kivinen, N.; Koskela, A.; Uusitalo-Jarvinen, H.; Tuulonen, A.; Winiarczyk, M.; Mackiewicz, J.; Zmorzynski, S.; et al. Expression of VEGFA-regulating miRNAs and mortality in wet AMD. J. Cell Mol. Med. 2019, 23, 8464–8471. [Google Scholar] [CrossRef]
- Rozing, M.P.; Durhuus, J.A.; Krogh Nielsen, M.; Subhi, Y.; Kirkwood, T.B.; Westendorp, R.G.; Sorensen, T.L. Age-related macular degeneration: A two-level model hypothesis. Prog. Retin. Eye Res. 2020, 76, 100825. [Google Scholar] [CrossRef] [PubMed]
- Hyttinen, J.M.T.; Blasiak, J.; Felszeghy, S.; Kaarniranta, K. MicroRNAs in the regulation of autophagy and their possible use in age-related macular degeneration therapy. Ageing Res. Rev. 2021, 67, 101260. [Google Scholar] [CrossRef]
- Lad, E.M.; Finger, R.P.; Guymer, R. Biomarkers for the Progression of Intermediate Age-Related Macular Degeneration. Ophthalmol. Ther. 2023, 12, 2917–2941. [Google Scholar] [CrossRef]
- ElShelmani, H.; Wride, M.A.; Saad, T.; Rani, S.; Kelly, D.J.; Keegan, D. Identification of Novel Serum MicroRNAs in Age-Related Macular Degeneration. Transl. Vis. Sci. Technol. 2020, 9, 28. [Google Scholar] [CrossRef]
- Yen, C.Y.; Chiu, C.M.; Fang, I.M. MicroRNA expression profiling in tears and blood as predictive biomarkers for anti-VEGF treatment response in wet age-related macular degeneration. Graefes Arch. Clin. Exp. Ophthalmol. 2024, 262, 2875–2884. [Google Scholar] [CrossRef]
- Cheng, K.C.; Hsu, Y.T.; Liu, W.; Huang, H.L.; Chen, L.Y.; He, C.X.; Sheu, S.J.; Chen, K.J.; Lee, P.Y.; Lin, Y.H.; et al. The Role of Oxidative Stress and Autophagy in Blue-Light-Induced Damage to the Retinal Pigment Epithelium in Zebrafish In Vitro and In Vivo. Int. J. Mol. Sci. 2021, 22, 1338. [Google Scholar] [CrossRef]
- Campillo, S.; Gutierrez-Calabres, E.; Garcia-Miranda, S.; Griera, M.; Fernandez Rodriguez, L.; de Frutos, S.; Rodriguez-Puyol, D.; Calleros, L. Integrin-linked kinase mRNA expression in circulating mononuclear cells as a biomarker of kidney and vascular damage in experimental chronic kidney disease. Cell Commun. Signal 2024, 22, 264. [Google Scholar] [CrossRef]
- Liukkonen, M.; Heloterä, H.; Siintamo, L.; Ghimire, B.; Mattila, P.; Kivinen, N.; Kostanek, J.; Watala, C.; Hytti, M.; Hyttinen, J.; et al. Oxidative Stress and Inflammation-Related mRNAs Are Elevated in Serum of a Finnish Wet Age-Related Macular Degeneration Cohort. Investig. Ophthalmol. Vis. Sci. 2024, 65, 30. [Google Scholar] [CrossRef]
- Huang, C.; Zhan, J.F.; Chen, Y.X.; Xu, C.Y.; Chen, Y. LncRNA-SNHG29 inhibits vascular smooth muscle cell calcification by downregulating miR-200b-3p to activate the alpha-Klotho/FGFR1/FGF23 axis. Cytokine 2020, 136, 155243. [Google Scholar] [CrossRef]
- Han, L.; Li, Z.; Jiang, Y.; Jiang, Z.; Tang, L. SNHG29 regulates miR-223-3p/CTNND1 axis to promote glioblastoma progression via Wnt/beta-catenin signaling pathway. Cancer Cell Int. 2019, 19, 345. [Google Scholar] [CrossRef]
- Jiang, J.; Hu, H.; Chen, Q.; Zhang, Y.; Chen, W.; Huang, Q.; Chen, X.; Li, J.; Zhong, M. Long non-coding RNA SNHG29 regulates cell senescence via p53/p21 signaling in spontaneous preterm birth. Placenta 2021, 103, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Xu, C.; Gong, Z.; Zhao, Y.; Fang, Z.; Rao, X.; Chen, Q.; Li, G.; Kong, W.; Chen, J. GRSF1 antagonizes age-associated hypercoagulability via modulation of fibrinogen mRNA stability. Cell Death Dis. 2023, 14, 717. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, N.; Issa, L.; Hauck, S.C.; Mani, S.; Ninnemann, O.; Kaindl, A.M. What’s the hype about CDK5RAP2? Cell Mol. Life Sci. 2011, 68, 1719–1736. [Google Scholar] [CrossRef]
- Zaqout, S.; Ravindran, E.; Stoltenburg-Didinger, G.; Kaindl, A.M. Congenital microcephaly-linked CDK5RAP2 affects eye development. Ann. Hum. Genet. 2020, 84, 87–91. [Google Scholar] [CrossRef]
- Liu, C.; Raab, M.; Gui, Y.; Rudd, C.E. Multi-functional adaptor SKAP1: Regulator of integrin activation, the stop-signal, and the proliferation of T cells. Front. Immunol. 2023, 14, 1192838. [Google Scholar] [CrossRef]
- Grunin, M.; Hagbi-Levi, S.; Rinsky, B.; Smith, Y.; Chowers, I. Transcriptome Analysis on Monocytes from Patients with Neovascular Age-Related Macular Degeneration. Sci. Rep. 2016, 6, 29046. [Google Scholar] [CrossRef]
- Schorg, A.; Santambrogio, S.; Platt, J.L.; Schodel, J.; Lindenmeyer, M.T.; Cohen, C.D.; Schrodter, K.; Mole, D.R.; Wenger, R.H.; Hoogewijs, D. Destruction of a distal hypoxia response element abolishes trans-activation of the PAG1 gene mediated by HIF-independent chromatin looping. Nucleic Acids Res. 2015, 43, 5810–5823. [Google Scholar] [CrossRef]
- Kim, S.J.; Chun, M.; Wan, J.; Lee, C.; Yen, K.; Cohen, P. GRSF1 is an age-related regulator of senescence. Sci. Rep. 2019, 9, 5546. [Google Scholar] [CrossRef]
- Yang, J.; Qu, T.; Li, Y.; Ma, J.; Yu, H. Biological role of long non-coding RNA FTX in cancer progression. Biomed. Pharmacother. 2022, 153, 113446. [Google Scholar] [CrossRef]
- Hosoi, Y.; Soma, M.; Shiura, H.; Sado, T.; Hasuwa, H.; Abe, K.; Kohda, T.; Ishino, F.; Kobayashi, S. Female mice lacking Ftx lncRNA exhibit impaired X-chromosome inactivation and a microphthalmia-like phenotype. Nat. Commun. 2018, 9, 3829. [Google Scholar] [CrossRef]
- Peng, H.; Yu, Y.; Wang, P.; Yao, Y.; Wu, X.; Zheng, Q.; Wang, J.; Tian, B.; Wang, Y.; Ke, T.; et al. NINJ2 deficiency inhibits preadipocyte differentiation and promotes insulin resistance through regulating insulin signaling. Obesity 2023, 31, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fa, J.; Wang, P.; Jia, X.; Peng, H.; Chen, J.; Wang, Y.; Wang, C.; Chen, Q.; Tu, X.; et al. NINJ2- A novel regulator of endothelial inflammation and activation. Cell Signal 2017, 35, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Telegina, D.V.; Korbolina, E.E.; Ershov, N.I.; Kolosova, N.G.; Kozhevnikova, O.S. Identification of functional networks associated with cell death in the retina of OXYS rats during the development of retinopathy. Cell Cycle 2015, 14, 3544–3556. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Stohl, L.L.; Xu, L.; Zhou, X.K.; Manni, M.; Wagner, J.A.; Granstein, R.D. Calcitonin Gene-Related Peptide-Exposed Endothelial Cells Bias Antigen Presentation to CD4+ T Cells toward a Th17 Response. J. Immunol. 2016, 196, 2181–2194. [Google Scholar] [CrossRef]
- Guo, X.; Chen, D.; An, S.; Wang, Z. ChIP-seq Profiling Identifies Histone Deacetylase 2 Targeting Genes Involved in Immune and Inflammatory Regulation Induced by Calcitonin Gene-Related Peptide in Microglial Cells. J. Immunol. Res. 2020, 2020, 4384696. [Google Scholar] [CrossRef]
- Li, G.; Jin, D.; Zhong, T.P. Tubgcp3 Is Required for Retinal Progenitor Cell Proliferation During Zebrafish Development. Front. Mol. Neurosci. 2019, 12, 126. [Google Scholar] [CrossRef]
- Jeon, H.; Byun, J.; Kang, H.; Kim, K.; Lee, E.; Kim, J.H.; Hong, C.K.; Song, S.W.; Kim, Y.H.; Chong, S.; et al. Proteomic analysis predicts anti-angiogenic resistance in recurred glioblastoma. J. Transl. Med. 2023, 21, 69. [Google Scholar] [CrossRef]
- Hung, C.Y.; Volkmar, B.; Baker, J.D.; Bauer, J.W.; Gussoni, E.; Hainzl, S.; Klausegger, A.; Lorenzo, J.; Mihalek, I.; Rittinger, O.; et al. A defect in the inner kinetochore protein CENPT causes a new syndrome of severe growth failure. PLoS ONE 2017, 12, e0189324. [Google Scholar] [CrossRef]
- Tatsukawa, H.; Hitomi, K. Role of Transglutaminase 2 in Cell Death, Survival, and Fibrosis. Cells 2021, 10, 1842. [Google Scholar] [CrossRef]
- Macchioni, L.; Chiasserini, D.; Mezzasoma, L.; Davidescu, M.; Orvietani, P.L.; Fettucciari, K.; Salviati, L.; Cellini, B.; Bellezza, I. Crosstalk between Long-Term Sublethal Oxidative Stress and Detrimental Inflammation as Potential Drivers for Age-Related Retinal Degeneration. Antioxidants 2020, 10, 25. [Google Scholar] [CrossRef]
- Lei, Z.; Chai, N.; Tian, M.; Zhang, Y.; Wang, G.; Liu, J.; Tian, Z.; Yi, X.; Chen, D.; Li, X.; et al. Novel peptide GX1 inhibits angiogenesis by specifically binding to transglutaminase-2 in the tumorous endothelial cells of gastric cancer. Cell Death Dis. 2018, 9, 579. [Google Scholar] [CrossRef] [PubMed]
- Jeansson, M.; Gawlik, A.; Anderson, G.; Li, C.; Kerjaschki, D.; Henkelman, M.; Quaggin, S.E. Angiopoietin-1 is essential in mouse vasculature during development and in response to injury. J. Clin. Investig. 2011, 121, 2278–2289. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Lavine, J.A.; Fawzi, A.; Quaggin, S.E.; Thomson, B.R. Angiopoietin-1 Is Required for Vortex Vein and Choriocapillaris Development in Mice. Arter. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 1413–1427. [Google Scholar] [CrossRef] [PubMed]
- Cano, M.; Wang, L.; Wan, J.; Barnett, B.P.; Ebrahimi, K.; Qian, J.; Handa, J.T. Oxidative stress induces mitochondrial dysfunction and a protective unfolded protein response in RPE cells. Free Radic. Biol. Med. 2014, 69, 1–14. [Google Scholar] [CrossRef]
- Nuzhat, N.; Van Schil, K.; Liakopoulos, S.; Bauwens, M.; Rey, A.D.; Kaseberg, S.; Jager, M.; Willer, J.R.; Winter, J.; Truong, H.M.; et al. CEP162 deficiency causes human retinal degeneration and reveals a dual role in ciliogenesis and neurogenesis. J. Clin. Invest. 2023, 133, e161156. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, M.; Zhang, Y.Y.; Zhao, S.Z.; Gu, S. The endosomal sorting complex required for transport repairs the membrane to delay cell death. Front. Oncol. 2022, 12, 1007446. [Google Scholar] [CrossRef]
- Whitehead, M.; Yusoff, S.; Ahmad, S.; Schmidt, L.; Mayr, M.; Madine, J.; Middleton, D.; Shanahan, C.M. Vascular smooth muscle cell senescence accelerates medin aggregation via small extracellular vesicle secretion and extracellular matrix reorganization. Aging Cell 2023, 22, e13746. [Google Scholar] [CrossRef]
- Wang, L.; Sun, L.; Sun, H.; Xing, Y.; Zhou, S.; An, G.; Li, J.; Ren, K.; Sun, J. GPR65 as a potential immune checkpoint regulates the immune microenvironment according to pan-cancer analysis. Heliyon 2023, 9, e13617. [Google Scholar] [CrossRef]
- Mercier, V.; Boucher, G.; Devost, D.; Bourque, K.; Alikashani, A.; Beauchamp, C.; Bitton, A.; Foisy, S.; Goyette, P.; Charron, G.; et al. IBD-associated G protein-coupled receptor 65 variant compromises signalling and impairs key functions involved in inflammation. Cell Signal 2022, 93, 110294. [Google Scholar] [CrossRef]
- Ail, D.; Rufenacht, V.; Caprara, C.; Samardzija, M.; Kast, B.; Grimm, C. Increased expression of the proton-sensing G protein-coupled receptor Gpr65 during retinal degeneration. Neuroscience 2015, 301, 496–507. [Google Scholar] [CrossRef]
- Hackler, L., Jr.; Wan, J.; Swaroop, A.; Qian, J.; Zack, D.J. MicroRNA profile of the developing mouse retina. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1823–1831. [Google Scholar] [CrossRef] [PubMed]
- Korucu, A.N.; Inandiklioglu, N. Is STARD3 A New Biomarker for Breast Cancer? Eur. J. Breast Health 2024, 20, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Almarhoun, M.; Biswas, L.; Alhasani, R.H.; Wong, A.; Tchivelekete, G.M.; Zhou, X.; Patterson, S.; Bartholomew, C.; Shu, X. Overexpression of STARD3 attenuates oxidized LDL-induced oxidative stress and inflammation in retinal pigment epithelial cells. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158927. [Google Scholar] [CrossRef]
- Jeong, B.C.; Kim, T.S.; Kim, H.S.; Lee, S.H.; Choi, Y. Transmembrane protein 64 reciprocally regulates osteoblast and adipocyte differentiation by modulating Wnt/beta-catenin signaling. Bone 2015, 78, 165–173. [Google Scholar] [CrossRef]
- Rosier, K.; McDevitt, M.T.; Smet, J.; Floyd, B.J.; Verschoore, M.; Marcaida, M.J.; Bingman, C.A.; Lemmens, I.; Dal Peraro, M.; Tavernier, J.; et al. Prolyl endopeptidase-like is a (thio)esterase involved in mitochondrial respiratory chain function. iScience 2021, 24, 103460. [Google Scholar] [CrossRef]
- Grimsey, N.J.; Lin, Y.; Narala, R.; Rada, C.C.; Mejia-Pena, H.; Trejo, J. G protein-coupled receptors activate p38 MAPK via a non-canonical TAB1-TAB2- and TAB1-TAB3-dependent pathway in endothelial cells. J. Biol. Chem. 2019, 294, 5867–5878. [Google Scholar] [CrossRef]
- Cai, H.; Fields, M.A.; Hoshino, R.; Priore, L.V. Effects of aging and anatomic location on gene expression in human retina. Front. Aging Neurosci. 2012, 4, 8. [Google Scholar] [CrossRef]
- Kushner, E.J.; Ferro, L.S.; Liu, J.Y.; Durrant, J.R.; Rogers, S.L.; Dudley, A.C.; Bautch, V.L. Excess centrosomes disrupt endothelial cell migration via centrosome scattering. J. Cell Biol. 2014, 206, 257–272. [Google Scholar] [CrossRef]
- Ruscetta, V.M.; Seaton, T.J.; Shakeel, A.; Vasconcelos, S.N.S.; Viirre, R.D.; Adler, M.J.; Olson, M.F. Opportunities and Challenges for the Development of MRCK Kinases Inhibitors as Potential Cancer Chemotherapeutics. Cells 2023, 12, 534. [Google Scholar] [CrossRef]
- Zhang, P.; Dufresne, C.; Turner, R.; Ferri, S.; Venkatraman, V.; Karani, R.; Lutty, G.A.; Van Eyk, J.E.; Semba, R.D. The proteome of human retina. Proteomics 2015, 15, 836–840. [Google Scholar] [CrossRef]
- Mirra, S.; Ulloa, F.; Gutierrez-Vallejo, I.; Marti, E.; Soriano, E. Function of Armcx3 and Armc10/SVH Genes in the Regulation of Progenitor Proliferation and Neural Differentiation in the Chicken Spinal Cord. Front. Cell Neurosci. 2016, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Gavalda-Navarro, A.; Mirra, S.; Manso, Y.; Sanchez-Infantes, D.; Giralt, M.; Soriano, E.; Villarroya, F. The armadillo-repeat containing X-linked protein 3, ARMCX3, is a negative regulator of the browning of adipose tissue associated with obesity. Int. J. Obes. 2022, 46, 1652–1661. [Google Scholar] [CrossRef] [PubMed]
- Beghi, S.; Furmanik, M.; Jaminon, A.; Veltrop, R.; Rapp, N.; Wichapong, K.; Bidar, E.; Buschini, A.; Schurgers, L.J. Calcium Signalling in Heart and Vessels: Role of Calmodulin and Downstream Calmodulin-Dependent Protein Kinases. Int. J. Mol. Sci. 2022, 23, 16139. [Google Scholar] [CrossRef]
- Shukal, D.K.; Malaviya, P.B.; Sharma, T. Role of the AMPK signalling pathway in the aetiopathogenesis of ocular diseases. Hum. Exp. Toxicol. 2022, 41, 9603271211063165. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Singh, A.K.; Prajapati, K.S.; Kushwaha, P.P.; Shuaib, M.; Kumar, S. Emerging role of ZBTB7A as an oncogenic driver and transcriptional repressor. Cancer Lett. 2020, 483, 22–34. [Google Scholar] [CrossRef]
- Cheong, K.X.; Li, H.; Tham, Y.C.; Teo, K.Y.C.; Tan, A.C.S.; Schmetterer, L.; Wong, T.Y.; Cheung, C.M.G.; Cheng, C.Y.; Fan, Q. Relationship Between Retinal Layer Thickness and Genetic Susceptibility to Age-Related Macular Degeneration in Asian Populations. Ophthalmol. Sci. 2023, 3, 100396. [Google Scholar] [CrossRef]
- Williams, G.D.; Gokhale, N.S.; Snider, D.L.; Horner, S.M. The mRNA Cap 2’-O-Methyltransferase CMTR1 Regulates the Expression of Certain Interferon-Stimulated Genes. mSphere 2020, 5, 10–1128. [Google Scholar] [CrossRef]
- Porciello, N.; Tuosto, L. CD28 costimulatory signals in T lymphocyte activation: Emerging functions beyond a qualitative and quantitative support to TCR signalling. Cytokine Growth Factor. Rev. 2016, 28, 11–19. [Google Scholar] [CrossRef]
- Rodriguez, I.J.; Lalinde Ruiz, N.; Llano Leon, M.; Martinez Enriquez, L.; Montilla Velasquez, M.D.P.; Ortiz Aguirre, J.P.; Rodriguez Bohorquez, O.M.; Velandia Vargas, E.A.; Hernandez, E.D.; Parra Lopez, C.A. Immunosenescence Study of T Cells: A Systematic Review. Front. Immunol. 2020, 11, 604591. [Google Scholar] [CrossRef]
- Brina, D.; Miluzio, A.; Ricciardi, S.; Clarke, K.; Davidsen, P.K.; Viero, G.; Tebaldi, T.; Offenhauser, N.; Rozman, J.; Rathkolb, B.; et al. eIF6 coordinates insulin sensitivity and lipid metabolism by coupling translation to transcription. Nat. Commun. 2015, 6, 8261. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, X.; Ying, X.; Xie, W.; Yin, Y.; Wang, X. CEBPG suppresses ferroptosis through transcriptional control of SLC7A11 in ovarian cancer. J. Transl. Med. 2023, 21, 334. [Google Scholar] [CrossRef] [PubMed]
- Del Valle-Perez, B.; Martinez, V.G.; Lacasa-Salavert, C.; Figueras, A.; Shapiro, S.S.; Takafuta, T.; Casanovas, O.; Capella, G.; Ventura, F.; Vinals, F. Filamin B plays a key role in vascular endothelial growth factor-induced endothelial cell motility through its interaction with Rac-1 and Vav-2. J. Biol. Chem. 2010, 285, 10748–10760. [Google Scholar] [CrossRef] [PubMed]
- Henri, J.; Chagot, M.E.; Bourguet, M.; Abel, Y.; Terral, G.; Maurizy, C.; Aigueperse, C.; Georgescauld, F.; Vandermoere, F.; Saint-Fort, R.; et al. Deep Structural Analysis of RPAP3 and PIH1D1, Two Components of the HSP90 Co-chaperone R2TP Complex. Structure 2018, 26, 1196–1209.e8. [Google Scholar] [CrossRef]
- Low, H.B.; Wong, Z.L.; Wu, B.; Kong, L.R.; Png, C.W.; Cho, Y.L.; Li, C.W.; Xiao, F.; Xin, X.; Yang, H.; et al. DUSP16 promotes cancer chemoresistance through regulation of mitochondria-mediated cell death. Nat. Commun. 2021, 12, 2284. [Google Scholar] [CrossRef]
- Muraleva, N.A.; Kolosova, N.G. P38 MAPK Signaling in the Retina: Effects of Aging and Age-Related Macular Degeneration. Int. J. Mol. Sci. 2023, 24, 11586. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, H.; Mu, W.; He, Z.; Yang, B.; Ji, Y.; Hui, L. DUSP16 ablation arrests the cell cycle and induces cellular senescence. FEBS J. 2015, 282, 4580–4594. [Google Scholar] [CrossRef]
- Lin, H.H.; Kuo, M.W.; Fan, T.C.; Yu, A.L.; Yu, J. YULINK regulates vascular formation in zebrafish and HUVECs. Biol. Res. 2023, 56, 7. [Google Scholar] [CrossRef]
- Lin, X.; Zhou, L.; Zhong, J.; Zhong, L.; Zhang, R.; Kang, T.; Wu, Y. RNA-binding protein RBM28 can translocate from the nucleolus to the nucleoplasm to inhibit the transcriptional activity of p53. J. Biol. Chem. 2022, 298, 101524. [Google Scholar] [CrossRef]
- Thapar, R. Roles of Prolyl Isomerases in RNA-Mediated Gene Expression. Biomolecules 2015, 5, 974–999. [Google Scholar] [CrossRef]
- Sehrawat, U.; Koning, F.; Ashkenazi, S.; Stelzer, G.; Leshkowitz, D.; Dikstein, R. Cancer-Associated Eukaryotic Translation Initiation Factor 1A Mutants Impair Rps3 and Rps10 Binding and Enhance Scanning of Cell Cycle Genes. Mol. Cell Biol. 2019, 39, e00441-18. [Google Scholar] [CrossRef]
- Shin, E.Y.; Soung, N.K.; Schwartz, M.A.; Kim, E.G. Altered endocytosis in cellular senescence. Ageing Res. Rev. 2021, 68, 101332. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gong, B.; Bushel, P.R.; Thierry-Mieg, J.; Thierry-Mieg, D.; Xu, J.; Fang, H.; Hong, H.; Shen, J.; Su, Z.; et al. The concordance between RNA-seq and microarray data depends on chemical treatment and transcript abundance. Nat. Biotechnol. 2014, 32, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, P.; Wang, H.; Yu, Y. Genes expressed at low levels raise false discovery rates in RNA samples contaminated with genomic DNA. BMC Genom. 2022, 23, 554. [Google Scholar] [CrossRef] [PubMed]
- Oca, A.I.; Perez-Sala, A.; Pariente, A.; Ochoa, R.; Velilla, S.; Pelaez, R.; Larrayoz, I.M. Predictive Biomarkers of Age-Related Macular Degeneration Response to Anti-VEGF Treatment. J. Pers. Med. 2021, 11, 1329. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Chong, V.; Loewenstein, A.; Larsen, M.; Souied, E.; Schlingemann, R.; Eldem, B.; Mones, J.; Richard, G.; Bandello, F.; et al. Guidelines for the management of neovascular age-related macular degeneration by the European Society of Retina Specialists (EURETINA). Br. J. Ophthalmol. 2014, 98, 1144–1167. [Google Scholar] [CrossRef]
- Eissing, T.; Stewart, M.W.; Qian, C.X.; Rittenhouse, K.D. Durability of VEGF Suppression With Intravitreal Aflibercept and Brolucizumab: Using Pharmacokinetic Modeling to Understand Clinical Outcomes. Transl. Vis. Sci. Technol. 2021, 10, 9. [Google Scholar] [CrossRef]
- Gurubaran, I.S. Mitochondrial damage and clearance in retinal pigment epithelial cells. Acta Ophthalmol. 2024, 102 (Suppl. 282), 3–53. [Google Scholar] [CrossRef]
- Tokarz, P.; Kaarniranta, K.; Blasiak, J. Role of the Cell Cycle Re-Initiation in DNA Damage Response of Post-Mitotic Cells and Its Implication in the Pathogenesis of Neurodegenerative Diseases. Rejuvenation Res. 2016, 19, 131–139. [Google Scholar] [CrossRef]
- Patil, N.S.; Mihalache, A.; Dhoot, A.S.; Popovic, M.M.; Muni, R.H.; Kertes, P.J. Association Between Visual Acuity and Residual Retinal Fluid Following Intravitreal Anti-Vascular Endothelial Growth Factor Treatment for Neovascular Age-Related Macular Degeneration: A Systematic Review and Meta-analysis. JAMA Ophthalmol. 2022, 140, 611–622. [Google Scholar] [CrossRef]
- Blasiak, J.; Piechota, M.; Pawlowska, E.; Szatkowska, M.; Sikora, E.; Kaarniranta, K. Cellular Senescence in Age-Related Macular Degeneration: Can Autophagy and DNA Damage Response Play a Role? Oxid. Med. Cell Longev. 2017, 2017, 5293258. [Google Scholar] [CrossRef]
- Cheung, C.M.; Wong, T.Y. Is age-related macular degeneration a manifestation of systemic disease? New prospects for early intervention and treatment. J. Intern. Med. 2014, 276, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Chernykh, V.; Shevchenko, A.; Konenkov, V.; Prokofiev, V.; Eremina, A.; Trunov, A. TNF-alpha gene polymorphisms: Association with age-related macular degeneration in Russian population. Int. J. Ophthalmol. 2019, 12, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Velilla, S.; Garcia-Medina, J.J.; Garcia-Layana, A.; Dolz-Marco, R.; Pons-Vazquez, S.; Pinazo-Duran, M.D.; Gomez-Ulla, F.; Arevalo, J.F.; Diaz-Llopis, M.; Gallego-Pinazo, R. Smoking and age-related macular degeneration: Review and update. J. Ophthalmol. 2013, 2013, 895147. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Tie, L.J.; Wu, S.S.; Lv, P.L.; Huang, H.W.; Wang, W.Q.; Wang, H.; Ma, L. Overweight, Obesity, and Risk of Age-Related Macular Degeneration. Invest. Ophthalmol. Vis. Sci. 2016, 57, 1276–1283. [Google Scholar] [CrossRef]
- Xu, X.; Ritz, B.; Coleman, A.; Liew, Z.; Deapen, D.; Lee, E.; Bernstein, L.; Pinder, R.; Marshall, S.; Heck, J.E. Hypertension, antihypertensive medications use and risk of age-related macular degeneration in California Teachers Cohort. J. Hum. Hypertens. 2020, 34, 568–576. [Google Scholar] [CrossRef]
- Moir, J.; Hyman, M.J.; Wang, J.; Shah, A.; Maatouk, C.; Flores, A.; Skondra, D. Associations Between Autoimmune Disease and the Development of Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2023, 64, 45. [Google Scholar] [CrossRef]
- Chen, X.; Rong, S.S.; Xu, Q.; Tang, F.Y.; Liu, Y.; Gu, H.; Tam, P.O.; Chen, L.J.; Brelen, M.E.; Pang, C.P.; et al. Diabetes mellitus and risk of age-related macular degeneration: A systematic review and meta-analysis. PLoS ONE 2014, 9, e108196. [Google Scholar] [CrossRef]
- Nahavandipour, A.; Krogh Nielsen, M.; Sorensen, T.L.; Subhi, Y. Systemic levels of interleukin-6 in patients with age-related macular degeneration: A systematic review and meta-analysis. Acta Ophthalmol. 2020, 98, 434–444. [Google Scholar] [CrossRef]
- Khan, A.H.; Pierce, C.O.; De Salvo, G.; Griffiths, H.; Nelson, M.; Cree, A.J.; Menon, G.; Lotery, A.J. The effect of systemic levels of TNF-alpha and complement pathway activity on outcomes of VEGF inhibition in neovascular AMD. Eye 2022, 36, 2192–2199. [Google Scholar] [CrossRef]
- Akter, T.; Annamalai, B.; Obert, E.; Simpson, K.N.; Rohrer, B. Dabigatran and Wet AMD, Results From Retinal Pigment Epithelial Cell Monolayers, the Mouse Model of Choroidal Neovascularization, and Patients From the Medicare Data Base. Front. Immunol. 2022, 13, 896274. [Google Scholar] [CrossRef]
- Sugiura, Y.; Araki, K.; Iemura, S.; Natsume, T.; Hoseki, J.; Nagata, K. Novel thioredoxin-related transmembrane protein TMX4 has reductase activity. J. Biol. Chem. 2010, 285, 7135–7142. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, E.E.; Hardie, R.C.; Colley, N.J. Calnexin is essential for rhodopsin maturation, Ca2+ regulation, and photoreceptor cell survival. Neuron 2006, 49, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Kaarniranta, K.; Uusitalo, H.; Blasiak, J.; Felszeghy, S.; Kannan, R.; Kauppinen, A.; Salminen, A.; Sinha, D.; Ferrington, D. Mechanisms of mitochondrial dysfunction and their impact on age-related macular degeneration. Prog. Retin. Eye Res. 2020, 79, 100858. [Google Scholar] [CrossRef]
- Kangas, S.M.; Teppo, J.; Lahtinen, M.J.; Suoranta, A.; Ghimire, B.; Mattila, P.; Uusimaa, J.; Varjosalo, M.; Katisko, J.; Hinttala, R. Analysis of human brain tissue derived from DBS surgery. Transl. Neurodegener. 2022, 11, 22. [Google Scholar] [CrossRef]
- Edgar, R.; Domrachev, M.; Lash, A.E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002, 30, 207–210. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Durinck, S.; Moreau, Y.; Kasprzyk, A.; Davis, S.; De Moor, B.; Brazma, A.; Huber, W. BioMart and Bioconductor: A powerful link between biological databases and microarray data analysis. Bioinformatics 2005, 21, 3439–3440. [Google Scholar] [CrossRef]
- Durinck, S.; Spellman, P.T.; Birney, E.; Huber, W. Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nat. Protoc. 2009, 4, 1184–1191. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]




| A | B | C | ||||||
|---|---|---|---|---|---|---|---|---|
| Confounding Variable | Number of RNAs Affected | Confounding Variable | Number of RNAs Affected | Confounding Variable | Number of RNAs Affected | |||
| Control vs. nAMD | Baseline vs. anti-VEGF | Control vs. nAMD | Atrophy status | Control vs. nAMD | SRF vs. IRF | |||
| Gender | 0/5 | 1/5 | Gender | 1/22 | 3/22 | Gender | 1/12 | 3/12 |
| BMI | 2/5 | 1/5 | BMI | 2/22 | 3/22 | BMI | 1/12 | 2/12 |
| Smoking | 0/5 | 1/5 | Smoking | 1/22 | 2/22 | Smoking | 0/12 | 4/12 |
| Blood pressure | 1/5 | 1/5 | Blood pressure | 1/22 | 1/22 | Blood pressure | 1/12 | 2/12 |
| Anti-cholesterol | 0/5 | 3/5 | Anti-cholesterol | 1/22 | 1/22 | Anti-cholesterol | 3/12 | 0/12 |
| Anti-coagulants | 2/5 | 3/5 | Anti-coagulants | 1/22 | 1/22 | Anti-coagulants | 0/12 | 1/12 |
| Anti-aggregation | 0/5 | 2/5 | Anti-aggregation | 0/22 | 0/22 | Anti-aggregation | 2/12 | 1/12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heloterä, H.; Kostanek, J.; Liukkonen, M.; Siintamo, L.; Linna-Kuosmanen, S.; Watala, C.; Blasiak, J.; Kaarniranta, K. Serum RNA Profile Reflects Fluid Status and Atrophic Retinal Changes in Neovascular Age-Related Macular Degeneration. Int. J. Mol. Sci. 2025, 26, 4852. https://doi.org/10.3390/ijms26104852
Heloterä H, Kostanek J, Liukkonen M, Siintamo L, Linna-Kuosmanen S, Watala C, Blasiak J, Kaarniranta K. Serum RNA Profile Reflects Fluid Status and Atrophic Retinal Changes in Neovascular Age-Related Macular Degeneration. International Journal of Molecular Sciences. 2025; 26(10):4852. https://doi.org/10.3390/ijms26104852
Chicago/Turabian StyleHeloterä, Hanna, Joanna Kostanek, Mikko Liukkonen, Leea Siintamo, Suvi Linna-Kuosmanen, Cezary Watala, Janusz Blasiak, and Kai Kaarniranta. 2025. "Serum RNA Profile Reflects Fluid Status and Atrophic Retinal Changes in Neovascular Age-Related Macular Degeneration" International Journal of Molecular Sciences 26, no. 10: 4852. https://doi.org/10.3390/ijms26104852
APA StyleHeloterä, H., Kostanek, J., Liukkonen, M., Siintamo, L., Linna-Kuosmanen, S., Watala, C., Blasiak, J., & Kaarniranta, K. (2025). Serum RNA Profile Reflects Fluid Status and Atrophic Retinal Changes in Neovascular Age-Related Macular Degeneration. International Journal of Molecular Sciences, 26(10), 4852. https://doi.org/10.3390/ijms26104852










