Network Pharmacology-Guided Evaluation of Ginger and Cornelian Cherry Extracts Against Depression and Metabolic Dysfunction in Estrogen-Deficient Chronic Stressed Rats
Abstract
1. Introduction
2. Results
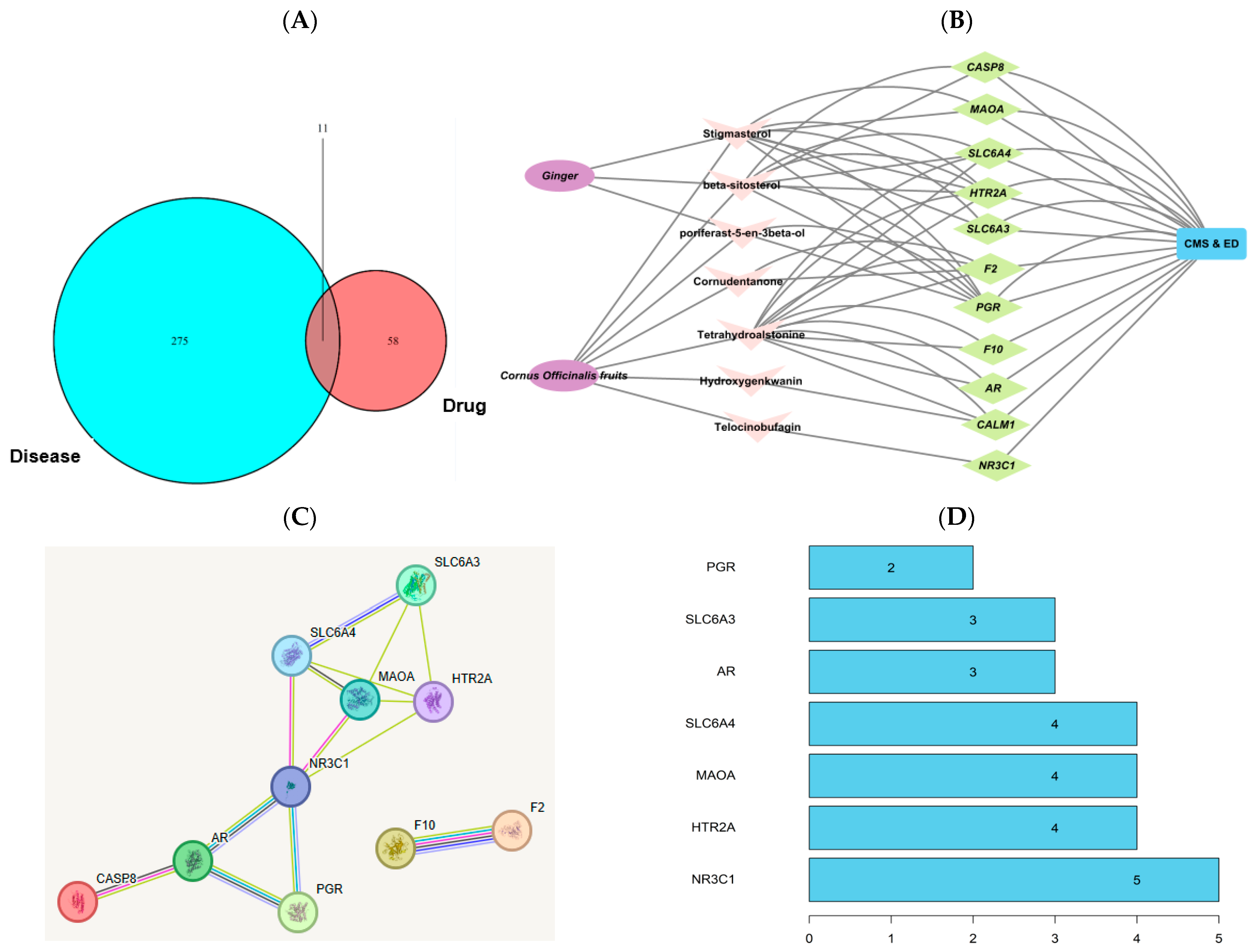
2.1. Network Pharmacology-Based Analysis
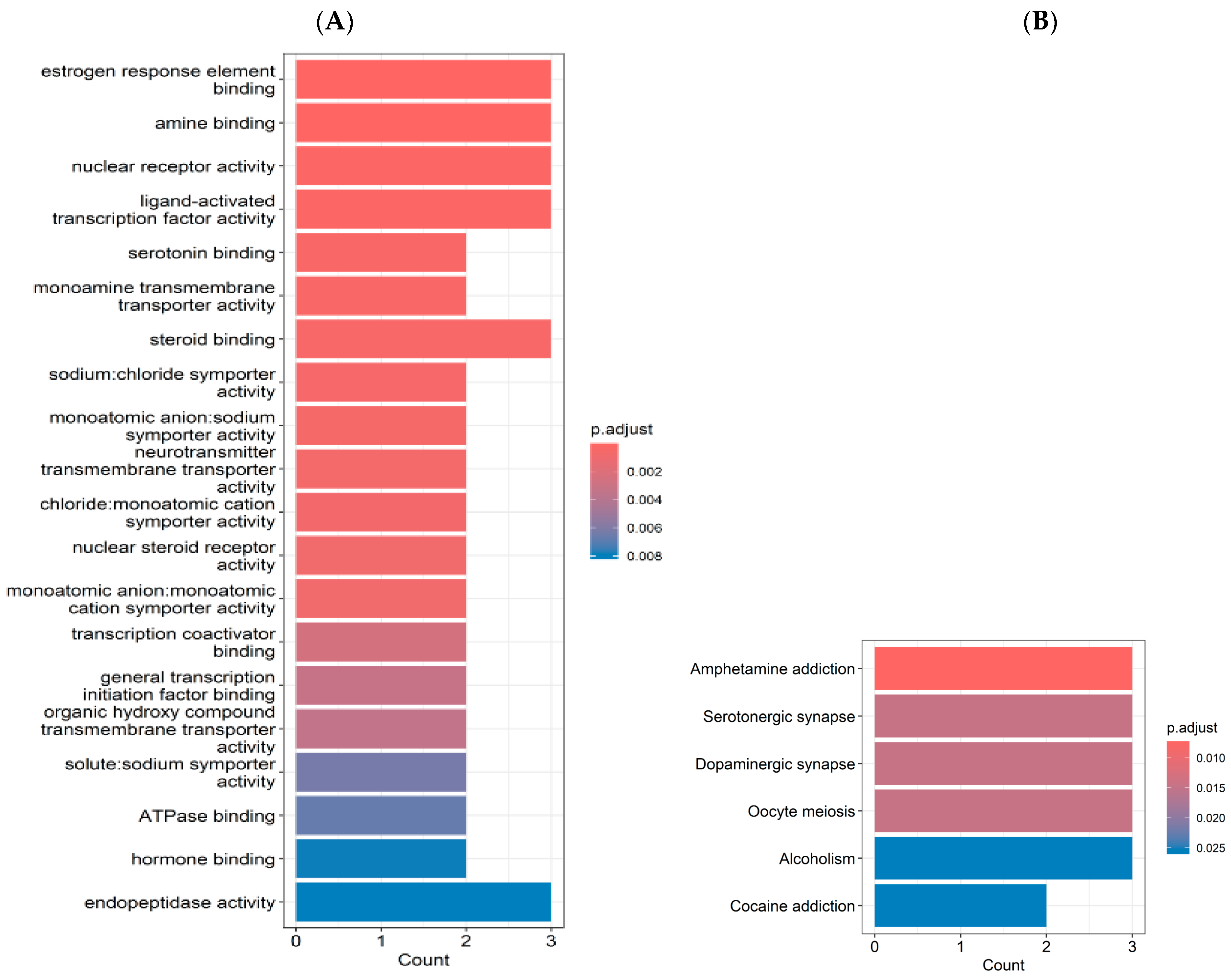
2.2. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Enrichment Analyses
2.3. The Contents of Index Compounds in Ginger and COF
2.4. Energy and Glucose Metabolism
2.5. Impact of Metabolic and Inflammatory Biomarkers on Neurochemical Signaling in CMS
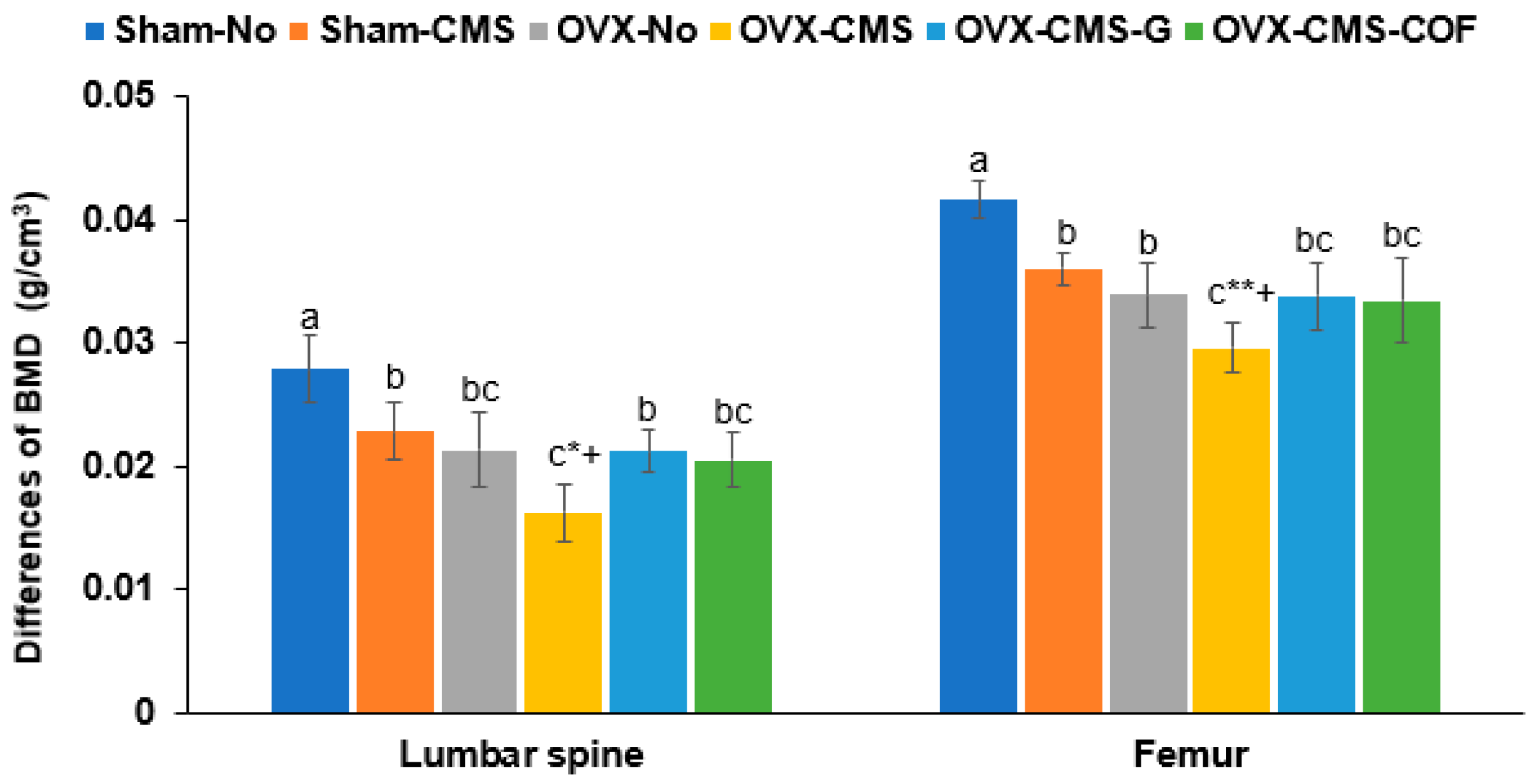
2.6. Bone Mineral Density (BMD)
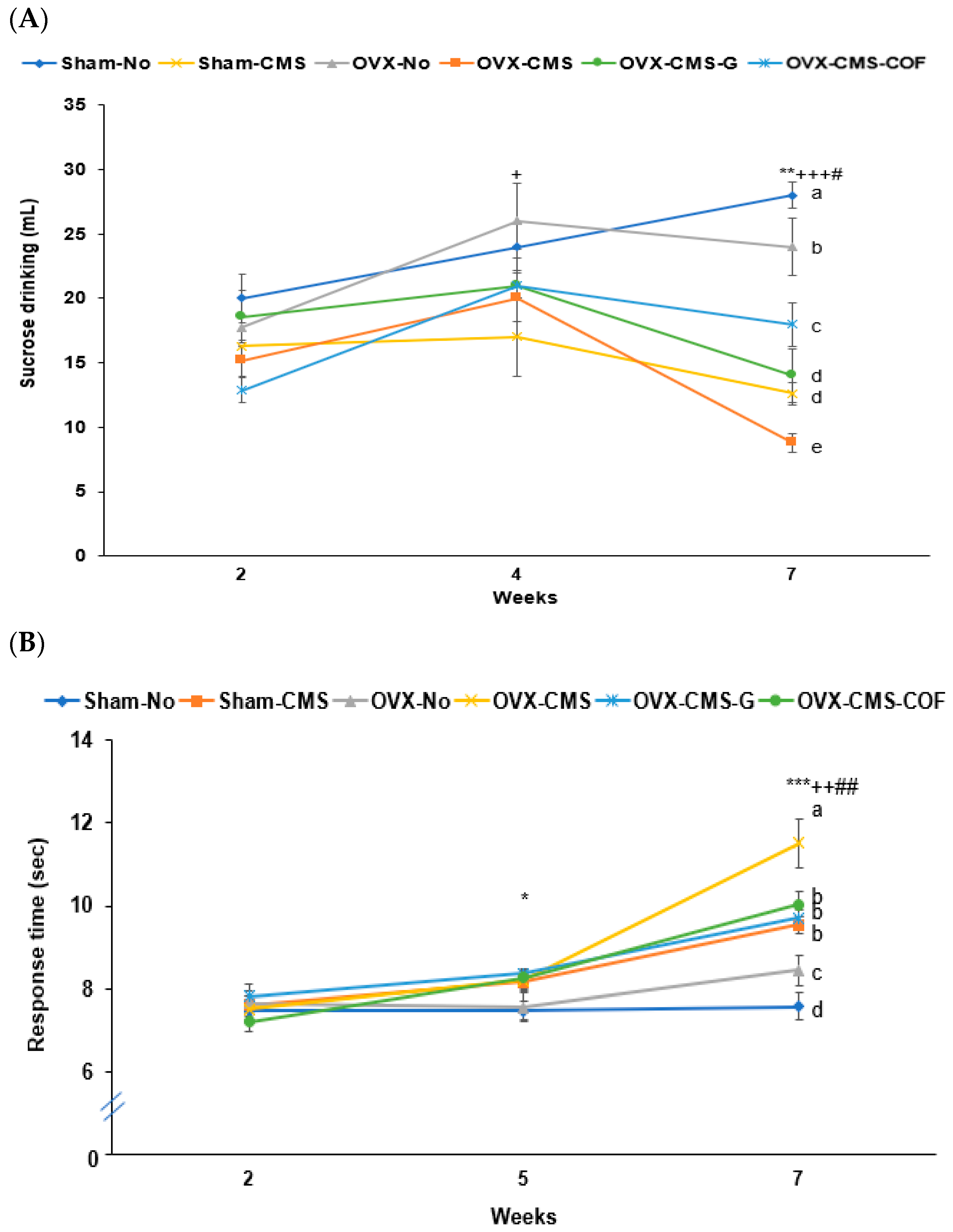
2.7. Depression-like Behaviors by Sucrose Preference, Y-Maze, Forced Swimming, and Planar Tests
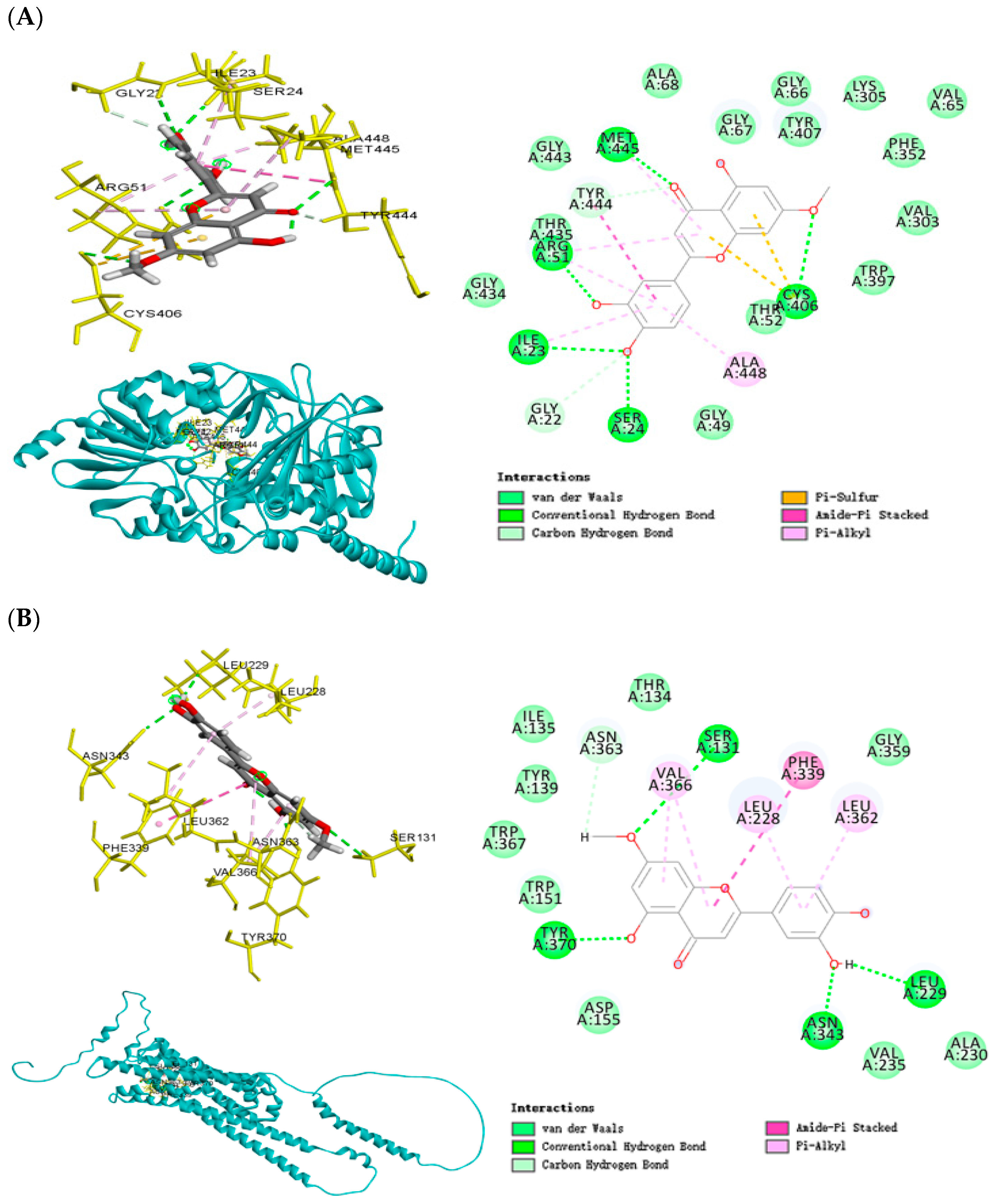
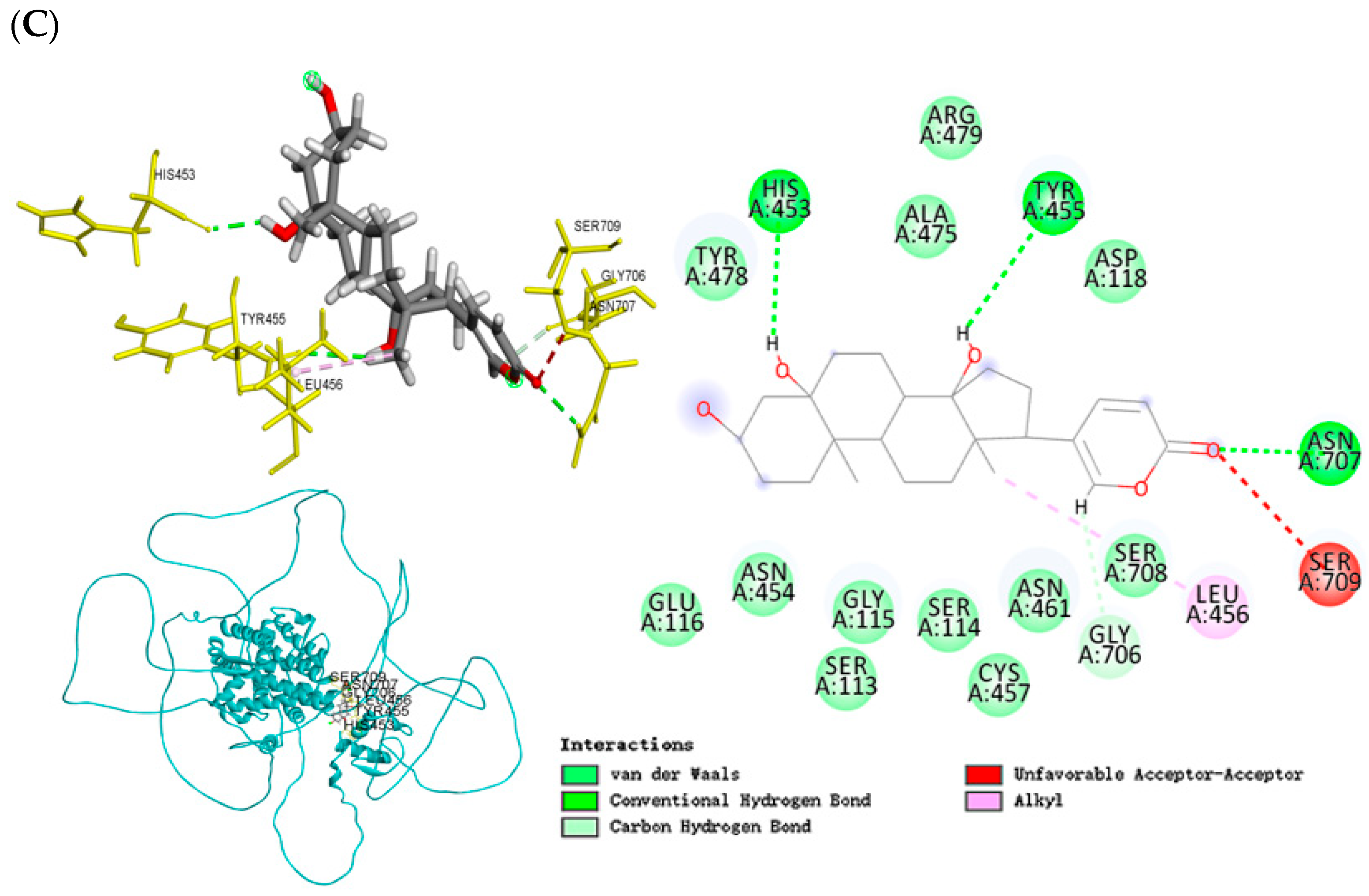
2.8. Molecular Docking Analysis of Co-Crystallized Protein and Ligand (Redocking Validation)
2.9. Molecular Docking Analysis
3. Discussion
Limitations and Future Directions
4. Materials and Methods
4.1. Network Pharmacology-Based Analysis
4.2. Molecular Docking Analysis
4.3. Preparation of Ginger and COF Water Extracts and Quantification of Index Compounds
4.4. Animals and Experimental Design
4.5. CMS Induction Protocol
4.6. Behavioral Assessments
4.7. Glucose and Insulin Tolerance Tests
4.8. BMD Measurement
4.9. Tissue Collection and Biochemical Assays
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Albert, K.M.; Newhouse, P.A. Estrogen, Stress, and Depression: Cognitive and Biological Interactions. Annu. Rev. Clin. Psychol. 2019, 15, 399–423. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, E.; Nenic, K.; Milanovic, V.; Knezevic, N.N. The Role of Cortisol in Chronic Stress, Neurodegenerative Diseases, and Psychological Disorders. Cells 2023, 12, 2726. [Google Scholar] [CrossRef] [PubMed]
- Bucklin, M.A.; Gehrke, E.C.; Westrick, J.C.; Gottlieb, M.; Martin, J.T. Depression predicts decreased lumbar bone mineral density: A scoping review of chronic psychological stress and spinal tissue pathology. Osteoarthr. Cartil. Open 2024, 6, 100529. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Liu, Y.; Chen, S.; Dai, Z.; Yang, D.; Gao, D.; Shao, J.; Wang, Y.; Wang, T.; Zhang, Z.; et al. A GABAergic neural circuit in the ventromedial hypothalamus mediates chronic stress-induced bone loss. J. Clin. Investig. 2020, 130, 6539–6554. [Google Scholar] [CrossRef]
- Sun, Q.; Li, G.; Zhao, F.; Dong, M.; Xie, W.; Liu, Q.; Yang, W.; Cui, R. Role of estrogen in treatment of female depression. Aging 2024, 16, 3021–3042. [Google Scholar] [CrossRef]
- Tongta, S.; Daendee, S.; Kalandakanond-Thongsong, S. Anxiety-like behavior and GABAergic system in ovariectomized rats exposed to chronic mild stress. Physiol. Behav. 2023, 258, 114014. [Google Scholar] [CrossRef]
- Hou, H.; Adzika, G.K.; Wu, Q.; Ma, T.; Ma, Y.; Geng, J.; Shi, M.; Fu, L.; Rizvi, R.; Gong, Z.; et al. Estrogen Attenuates Chronic Stress-Induced Cardiomyopathy by Adaptively Regulating Macrophage Polarizations via β2-Adrenergic Receptor Modulation. Front. Cell Dev. Biol. 2021, 9, 737003. [Google Scholar] [CrossRef]
- Ayustaningwarno, F.; Anjani, G.; Ayu, A.M.; Fogliano, V. A critical review of Ginger’s (Zingiber officinale) antioxidant, anti-inflammatory, and immunomodulatory activities. Front. Nutr. 2024, 11, 1364836. [Google Scholar] [CrossRef]
- Gao, X.; Liu, Y.; An, Z.; Ni, J. Active Components and Pharmacological Effects of Cornus officinalis: Literature Review. Front. Pharmacol. 2021, 12, 633447. [Google Scholar] [CrossRef]
- Tian, W.; Zhao, J.; Lee, J.H.; Akanda, M.R.; Cho, J.H.; Kim, S.K.; Choi, Y.J.; Park, B.Y. Neuroprotective Effects of Cornus officinalis on Stress-Induced Hippocampal Deficits in Rats and H2O2-Induced Neurotoxicity in SH-SY5Y Neuroblastoma Cells. Antioxidants 2019, 9, 27. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, P.-P.; Li, P.-Y.; Zheng, Y.-J.; Li, S.-F.; Zhao, L.-R.; Ma, Q.Y.; Cheng, J.L.; Ma, J.S.; Feng, W.S.; et al. Investigating the possible mechanism of Cornus officinalis in the therapy of ischemic stroke by UHPLC-Q-TOF-MS, network pharmacology, molecular docking, and experimental verification. J. Ethnopharmacol. 2025, 338, 119072. [Google Scholar] [CrossRef] [PubMed]
- Bakusic, J.; Vrieze, E.; Ghosh, M.; Bekaert, B.; Claes, S.; Godderis, L. Increased methylation of NR3C1 and SLC6A4 is associated with blunted cortisol reactivity to stress in major depression. Neurobiol. Stress 2020, 13, 100272. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Liu, H. The estrogen receptor and metabolism. Women’s Health 2024, 20, 17455057241227362. [Google Scholar] [CrossRef]
- Qiu, W.; Cai, X.; Zheng, C.; Qiu, S.; Ke, H.; Huang, Y. Update on the Relationship Between Depression and Neuroendocrine Metabolism. Front. Neurosci. 2021, 15, 728810. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zou, D.; Li, Y.; Gu, S.; Dong, J.; Ma, X.; Xu, S.; Wang, F.; Huang, J.H. Monoamine Neurotransmitters Control Basic Emotions and Affect Major Depressive Disorders. Pharmaceuticals 2022, 15, 1203. [Google Scholar] [CrossRef]
- Razak, A.M.; Tan, J.K.; Mohd Said, M.; Makpol, S. Modulating Effects of Zingiberaceae Phenolic Compounds on Neurotrophic Factors and Their Potential as Neuroprotectants in Brain Disorders and Age-Associated Neurodegenerative Disorders: A Review. Nutrients 2023, 15, 2564. [Google Scholar] [CrossRef]
- Giannini, A.; Caretto, M.; Genazzani, A.R.; Simoncini, T. Neuroendocrine Changes during Menopausal Transition. Endocrines 2021, 2, 405–416. [Google Scholar] [CrossRef]
- Turek, J.; Gąsior, Ł. Estrogen fluctuations during the menopausal transition are a risk factor for depressive disorders. Pharmacol. Rep. 2023, 75, 32–43. [Google Scholar] [CrossRef]
- Zhang, Z.; He, Z.; Pan, J.; Yuan, M.; Lang, Y.; Wei, X.; Zhang, C. The interaction of BDNF with estrogen in the development of hypertension and obesity, particularly during menopause. Front. Endocrinol. 2024, 15, 1384159. [Google Scholar] [CrossRef]
- Corrigan, M.; O’Rourke, A.M.; Moran, B.; Fletcher, J.M.; Harkin, A. Inflammation in the pathogenesis of depression: A disorder of neuroimmune origin. Neuronal Signal. 2023, 7, Ns20220054. [Google Scholar] [CrossRef]
- Tongta, S.; Daendee, S.; Kalandakanond-Thongsong, S. Effects of estrogen receptor β or G protein-coupled receptor 30 activation on anxiety-like behaviors in relation to GABAergic transmission in stress-ovariectomized rats. Neurosci. Lett. 2022, 789, 136885. [Google Scholar] [CrossRef] [PubMed]
- Khaleghi, M.; Rajizadeh, M.A.; Bashiri, H.; Kohlmeier, K.A.; Mohammadi, F.; Khaksari, M.; Shabani, M. Estrogen attenuates physical and psychological stress-induced cognitive impairments in ovariectomized rats. Brain Behav. 2021, 11, e02139. [Google Scholar] [CrossRef] [PubMed]
- Brann, D.W.; Lu, Y.; Wang, J.; Zhang, Q.; Thakkar, R.; Sareddy, G.R.; Pratap, U.P.; Tekmal, R.R.; Vadlamudi, R.K. Brain-derived estrogen and neural function. Neurosci. Biobehav. Rev. 2022, 132, 793–817. [Google Scholar] [CrossRef] [PubMed]
- Green, M.R.; Marcolin, M.L.; McCormick, C.M. The effects of ovarian hormones on stressor-induced hormonal responses, glucocorticoid receptor expression and translocation, and genes related to receptor signaling in adult female rats. Stress 2018, 21, 90–100. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Chakraborty, M.; Chanda, A.; Alqahtani, T.; Kumer, A.; Dhara, B.; Chattopadhyay, M. Neuroendocrine and cellular mechanisms in stress resilience: From hormonal influence in the CNS to mitochondrial dysfunction and oxidative stress. J. Cell. Mol. Med. 2024, 28, e18220. [Google Scholar] [CrossRef]
- Hei, M.; Chen, P.; Wang, S.; Li, X.; Xu, M.; Zhu, X.; Wang, Y.; Duan, J.; Huang, Y.; Zhao, S. Effects of chronic mild stress induced depression on synaptic plasticity in mouse hippocampus. Behav. Brain Res. 2019, 365, 26–35. [Google Scholar] [CrossRef]
- Chaurasiya, N.D.; Leon, F.; Muhammad, I.; Tekwani, B.L. Natural Products Inhibitors of Monoamine Oxidases-Potential New Drug Leads for Neuroprotection, Neurological Disorders, and Neuroblastoma. Molecules 2022, 27, 4297. [Google Scholar] [CrossRef]
- Shaikh, A.; Ahmad, F.; Teoh, S.L.; Kumar, J.; Yahaya, M.F. Targeting dopamine transporter to ameliorate cognitive deficits in Alzheimer’s disease. Front. Cell. Neurosci. 2023, 17, 1292858. [Google Scholar] [CrossRef]
- Boyle, C.C.; Bower, J.E.; Eisenberger, N.I.; Irwin, M.R. Stress to inflammation and anhedonia: Mechanistic insights from preclinical and clinical models. Neurosci. Biobehav. Rev. 2023, 152, 105307. [Google Scholar] [CrossRef]
- Ruggiero, R.N.; Rossignoli, M.T.; Marques, D.B.; de Sousa, B.M.; Romcy-Pereira, R.N.; Lopes-Aguiar, C.; Leite, J.P. Neuromodulation of Hippocampal-Prefrontal Cortical Synaptic Plasticity and Functional Connectivity: Implications for Neuropsychiatric Disorders. Front. Cell. Neurosci. 2021, 15, 732360. [Google Scholar] [CrossRef]
- Rong, J.; Wang, Y.; Liu, N.; Shen, L.; Ma, Q.; Wang, M.; Han, B. Chronic stress induces insulin resistance and enhances cognitive impairment in AD. Brain Res. Bull. 2024, 217, 111083. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Hong, S.M.; Ahn, I.L.; Kim, D.S.; Kim, S.H. Estrogen replacement reverses olanzapine-induced weight gain and hepatic insulin resistance in ovariectomized diabetic rats. Neuropsychobiology 2010, 61, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Beaupere, C.; Liboz, A.; Fève, B.; Blondeau, B.; Guillemain, G. Molecular Mechanisms of Glucocorticoid-Induced Insulin Resistance. Int. J. Mol. Sci. 2021, 22, 623. [Google Scholar] [CrossRef]
- Daily, J.W.; Zhang, X.; Kim, D.S.; Park, S. Efficacy of Ginger for Alleviating the Symptoms of Primary Dysmenorrhea: A Systematic Review and Meta-analysis of Randomized Clinical Trials. Pain Med. 2015, 16, 2243–2255. [Google Scholar] [CrossRef] [PubMed]
- Cozma, D.; Siatra, P.; Oikonomakos, I.; Kalra, D.; Friedrich, U.A.; Dahl, A.; Bornstein, S.R.; Zeissig, S.; Andoniadou, C.L.; Steenblock, C. SAT272 Insulin Signaling in Hyperactivation of the HPA Axis in Metabolic Diseases. J. Endocr. Soc. 2023, 7, bvad114-277. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, B.H.; Nayak, R.; Pandey, S.; Kumar, N.; Pai, K.S.R. Computational screening and molecular dynamics of natural compounds targeting the SH2 domain of STAT3: A multitarget approach using network pharmacology. Mol. Divers. 2025. [Google Scholar] [CrossRef]
- Geha, R.M.; Chen, K.; Wouters, J.; Ooms, F.; Shih, J.C. Analysis of conserved active site residues in monoamine oxidase A and B and their three-dimensional molecular modeling. J. Biol. Chem. 2002, 277, 17209–17216. [Google Scholar] [CrossRef]
- Kim, K.; Che, T.; Panova, O.; DiBerto, J.F.; Lyu, J.; Krumm, B.E.; Wacker, D.; Robertson, M.J.; Seven, A.B.; Nichols, D.E.; et al. Structure of a Hallucinogen-Activated Gq-Coupled 5-HT2A Serotonin Receptor. Cell 2020, 182, 1574–1588. [Google Scholar] [CrossRef]
- Shi, Y.; Cao, S.; Ni, D.; Fan, J.; Lu, S.; Xue, M. The Role of Conformational Dynamics and Allostery in the Control of Distinct Efficacies of Agonists to the Glucocorticoid Receptor. Front. Mol. Biosci. 2022, 9, 933676. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, H.; Song, Z.; Wang, X.; Sun, Z. Effects of Ginger (Zingiber officinale Roscoe) on Type 2 Diabetes Mellitus and Components of the Metabolic Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Evid. Based Complement. Altern. Med. 2018, 2018, 5692962. [Google Scholar] [CrossRef]
- Simon, A.; Darcsi, A.; Kéry, Á.; Riethmüller, E. Blood-brain barrier permeability study of ginger constituents. J. Pharm. Biomed. Anal. 2020, 177, 112820. [Google Scholar] [CrossRef] [PubMed]
- Andrei, C.; Zanfirescu, A.; Nițulescu, G.M.; Negreș, S. Understanding the Molecular Mechanisms Underlying the Analgesic Effect of Ginger. Nutraceuticals 2022, 2, 384–403. [Google Scholar] [CrossRef]
- Yang, H.J.; Zhang, T.; Kim, M.J.; Hur, H.J.; Wu, X.; Jang, D.J.; Park, S. Efficacy and Mechanism of Schisandra chinensis Fructus Water Extract in Alzheimer’s Disease: Insights from Network Pharmacology and Validation in an Amyloid-β Infused Animal Model. Nutrients 2024, 16, 3751. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Kim, Y.K.; Li, C.; Park, S. Natural compounds for Alzheimer’s prevention and treatment: Integrating SELFormer-based computational screening with experimental validation. Comput. Biol. Med. 2025, 185, 109523. [Google Scholar] [CrossRef]
- Liu, M.; Park, S. The Role of PNPLA3_rs738409 Gene Variant, Lifestyle Factors, and Bioactive Compounds in Nonalcoholic Fatty Liver Disease: A Population-Based and Molecular Approach towards Healthy Nutrition. Nutrients 2024, 16, 1239. [Google Scholar] [CrossRef]
- Pavlova, I.V.; Broshevitskaya, N.D.; Zaichenko, M.I.; Grigoryan, G.A. Effects of Ovariectomy on Anxious-Depressive Behavior in Female Rats in Normal Conditions and after Early Proinflammatory Stress. Neurosci. Behav. Physiol. 2022, 52, 1287–1298. [Google Scholar] [CrossRef]
- Santos, J.M.; Deshmukh, H.; Elmassry, M.M.; Yakhnitsa, V.; Ji, G.; Kiritoshi, T.; Presto, P.; Antenucci, N.; Liu, X.; Neugebauer, V.; et al. Beneficial Effects of Ginger Root Extract on Pain Behaviors, Inflammation, and Mitochondrial Function in the Colon and Different Brain Regions of Male and Female Neuropathic Rats: A Gut-Brain Axis Study. Nutrients 2024, 16, 3563. [Google Scholar] [CrossRef]
- Park, E.; Lim, E.; Yeo, S.; Yong, Y.; Yang, J.; Jeong, S.Y. Anti-Menopausal Effects of Cornus officinalis and Ribes fasciculatum Extract In Vitro and In Vivo. Nutrients 2020, 12, 369. [Google Scholar] [CrossRef]
- Ju, C.G.; Zhu, L.; Wang, W.; Gao, H.; Xu, Y.B.; Jia, T.Z. Cornus officinalis prior and post-processing: Regulatory effects on intestinal flora of diabetic nephropathy rats. Front. Pharmacol. 2022, 13, 1039711. [Google Scholar] [CrossRef]
- Seo-Ah, S.; Sung-Chul, K.; Bo-Ram, J.; Min-jeong, K.; Sujung, Y.; In-hwa, P.; Hyo-Jin, A. A 13-Week Repeated Oral Dose Toxicity Test and a 4-Week Recovery Test of Standardized Cornus officinalis and Psoralea corylifolia L. in Sprague-Dawley Rats. Korea J. Herbol. 2021, 36, 27–37. [Google Scholar]
- Willner, P. The chronic mild stress (CMS) model of depression: History, evaluation and usage. Neurobiol. Stress 2017, 6, 78–93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Kim, M.J.; Kim, M.J.; Wu, X.; Yang, H.J.; Yuan, H.; Huang, S.; Yoon, S.M.; Kim, K.N.; Park, S. Long-Term Effect of Porcine Brain Enzyme Hydrolysate Intake on Scopolamine-Induced Memory Impairment in Rats. Int. J. Mol. Sci. 2022, 23, 3361. [Google Scholar] [CrossRef] [PubMed]
- Markov, D.D. Sucrose Preference Test as a Measure of Anhedonic Behavior in a Chronic Unpredictable Mild Stress Model of Depression: Outstanding Issues. Brain Sci. 2022, 12, 1287. [Google Scholar] [CrossRef] [PubMed]
- Cheah, M.; Fawcett, J.W.; Andrews, M.R. Assessment of Thermal Pain Sensation in Rats and Mice Using the Hargreaves Test. Bio Protoc. 2017, 7, e2506. [Google Scholar] [CrossRef]
- Park, S.; Kang, S.; Kim, D.S. Severe calcium deficiency increased visceral fat accumulation, down-regulating genes associated with fat oxidation, and increased insulin resistance while elevating serum parathyroid hormone in estrogen-deficient rats. Nutr. Res. 2020, 73, 48–57. [Google Scholar] [CrossRef]
- Peinnequin, A.; Mouret, C.; Birot, O.; Alonso, A.; Mathieu, J.; Clarençon, D.; Agay, D.; Chancerelle, Y.; Multon, E. Rat pro-inflammatory cytokine and cytokine-related mRNA quantification by real-time polymerase chain reaction using SYBR green. BMC Immunol. 2004, 5, 3. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]



| Retention Time | Ginger Water Extract | Cornelian Cherry Water Extract | |
|---|---|---|---|
| 6-gingerol (mg/g) | 6.502 | 6.25 | |
| 8-gingerol A (mg/g) | 11.780 | 1.36 | |
| 8-gingerol B (mg/g) | 18.507 | 1.31 | |
| 6-gingerdiol (mg/g) | 20.557 | 0.09 | |
| 6-gingerdione (mg/g) | 21.270 | 0.40 | |
| 10-gingerol (mg/g) | 27.578 | 0.03 | |
| 8-gingerdione (mg/g) | 41.705 | 0.61 | |
| Morroniside (mg/g) | 9.523 | 5.695 | |
| Loganin (mg/g) | 23.258 | 3.476 |
| Sham-No | Sham-CMS | OVX-No | OVX-CMS | OVX-CMS-G | OVX-CMS-COF | |
|---|---|---|---|---|---|---|
| Serum 17β-estradiol (pg/mL) | 6.42 ± 0.56 a | 5.39 ± 0.75 b | 1.42 ± 0.13 d | 1.35 ± 0.16 d,***+## | 1.72 ± 0.12 c | 1.79 ± 0.19 c |
| Uterine mass (g) | 0.84 ± 0.06 a | 0.84 ± 0.02 a | 0.16 ± 0.02 c | 0.16 ± 0.01 c,*** | 0.20 ± 0.01 b | 0.21 ± 0.01 b |
| Final BW (g) | 264 ± 10.2 b | 243 ± 8.2 c | 286 ± 12.1 a | 261 ± 12.7 b,**++ | 251 ± 15.1 b | 259 ± 12.6 b |
| Weight gain (g/5 weeks) | 103 ± 6.55 b | 88.5 ± 6.18 c | 130 ± 6.52 a | 101 ± 6.12 b,**++ | 95.6 ± 6.01 b | 99.3 ± 4.97 b |
| Food intake (g/day) | 17.2 ± 1.39 b | 15.5 ± 1.11 c | 19.8 ± 1.52 a | 16.7 ± 1.13 b,*+ | 16.3 ± 1.49 bc | 16.5 ± 1.23 bc |
| Uterine fat (g) | 3.49 ± 0.30 b | 2.64 ± 0.54 c | 7.27 ± 0.44 a | 3.07 ± 0.32 c,***++## | 3.11 ± 0.23 bc | 3.20 ± 0.39 bc |
| Retroperitoneal fat (g) | 2.35 ± 0.36 b | 2.0 ± 0.20 bc | 3.85 ± 0.56 a | 2.52 ± 0.26 b,**++## | 1.79 ± 0.20 c | 2.41 ± 0.51 b |
| Visceral fat (BW %) | 2.22 ± 0.20 b | 1.35 ± 0.12 d | 3.69 ± 0.25 a | 2.04 ± 0.16 b,**++# | 1.75 ± 0.10 c | 1.94 ± 0.24 bc |
| Fasting blood glucose (mg/dL) | 98.1 ± 6.11 c | 104 ± 4.06 bc | 107 ± 3.03 b | 120 ± 4.09 a,*+# | 103 ± 5.73 bc | 102 ± 4.45 bc |
| Serum insulin (ng/mL) | 0.52 ± 0.02 c | 0.52 ± 0.01 c | 0.58 ± 0.01 a | 0.60 ± 0.02 a,*# | 0.55 ± 0.01 b | 0.54 ± 0.02 b |
| HOMA-IR (µU/mL) | 6.80 ± 0.47 c | 7.21 ± 0.49 bc | 8.28 ± 0.24 b | 9.60 ± 0.48 a,***+## | 7.54 ± 045 b | 7.34 ± 0.53 bc |
| Serum AST (U/L) | 24.4 ± 1.43 c | 25.9 ± 0.63 bc | 26.4 ± 1.33 b | 30.9 ± 1.15 a,*+## | 18.8 ± 1.15 d | 20.5 ± 1.56 d |
| Serum ALT (U/L) | 19.2 ± 2.36 b | 18.3 ± 2.27 b | 21.8 ± 2.02 a | 24.9 ± 3.33 a,**# | 17.7 ± 3.77 b | 16.5 ± 2.22 b |
| Sham-No | Sham-CMS | OVX-No | OVX-CMS | OVX-CMS-G | OVX-CMS-COF | |
|---|---|---|---|---|---|---|
| Serum | ||||||
| TG (mg/dL) | 80.3 ± 4.55 bc | 84.7 ± 4.69 b | 84.1 ± 3.58 b | 94.6 ± 5 a,*+# | 77.8 ± 4.44 c | 75.2 ± 5.41 c |
| Total cholesterol (mg/dL) | 130 ± 9.42 c | 128 ± 9.32 c | 147 ± 12.2 b | 166 ± 8.23 a,**++## | 128 ± 5.32 c | 147 ± 14.1 b |
| HDL (mg/dL) | 33.7 ± 2.64 a | 29.9 ± 3.11 b | 26 ± 2.47 c | 22.3 ± 1.56 d,**++ | 27 ± 1.18 c | 29.1 ± 2.15 b |
| TNF-α (ng/mL) | 85.6 ± 7.21 d | 124 ± 10.2 c | 138 ± 11.6 b | 162 ± 13.8 a,**++ | 136 ± 11.8 b | 131 ± 11.2 b |
| IL-1β (ng/mL) | 68.7 ± 7.21 c | 85.6 ± 8.14 b | 82.6 ± 7.91 b | 102 ± 10.8 a,**++ | 89.6 ± 7.99 b | 87.6 ± 8.36 b |
| Corticosterone (ng/mL) | 78 ± 7.02 d | 103.5 ± 6.1 b | 94.2 ± 2.7 c | 118.7 ± 3.83 a,**+++ | 103.2 ± 5.01 b | 104.2 ± 4.76 b |
| ACTH (ng/mL) | 3.23 ± 0.42 d | 6.35 ± 0.73 b | 4.93 ± 0.56 c | 8.52 ± 0.94 a,***+++ | 6.23 ± 0.73 b | 6.31 ± 0.76 b |
| Hippocampus | ||||||
| Serotonin (ng/mg protein) | 15.4 ± 2.05 a | 8.26 ± 0.71 b | 9.24 ± 1.02 b | 4.48 ± 0.29 d,***+++ | 7.58 ± 1.02 c | 7.43 ± 0.94 c |
| Dopamine (pg/mg protein) | 7.81 ± 0.97 a | 5.83 ± 0.74 b | 6.48 ± 0.71 b | 4.54 ± 0.67 c,**++ | 6.61 ± 0.42 b | 6.58 ± 0.53 b |
| MDA (nmol/mg protein) | 1.62 ± 0.24 c | 2.43 ± 0.28 b | 2.35 ± 0.28 b | 3.52 ± 0.32 a,**++ | 2.16 ± 0.38 b | 2.39 ± 0.37 b |
| SOD (U/mg protein) | 54.3 ± 6.5 a | 47.9 ± 4.22 b | 46.5 ± 4.34 b | 40.4 ± 4.37 c,**+ | 48.5 ± 4.31 b | 47.4 ± 4.42 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, N.; Zhang, T.; Joe, H.; Park, S. Network Pharmacology-Guided Evaluation of Ginger and Cornelian Cherry Extracts Against Depression and Metabolic Dysfunction in Estrogen-Deficient Chronic Stressed Rats. Int. J. Mol. Sci. 2025, 26, 4829. https://doi.org/10.3390/ijms26104829
Lee N, Zhang T, Joe H, Park S. Network Pharmacology-Guided Evaluation of Ginger and Cornelian Cherry Extracts Against Depression and Metabolic Dysfunction in Estrogen-Deficient Chronic Stressed Rats. International Journal of Molecular Sciences. 2025; 26(10):4829. https://doi.org/10.3390/ijms26104829
Chicago/Turabian StyleLee, Nara, Ting Zhang, Hanbin Joe, and Sunmin Park. 2025. "Network Pharmacology-Guided Evaluation of Ginger and Cornelian Cherry Extracts Against Depression and Metabolic Dysfunction in Estrogen-Deficient Chronic Stressed Rats" International Journal of Molecular Sciences 26, no. 10: 4829. https://doi.org/10.3390/ijms26104829
APA StyleLee, N., Zhang, T., Joe, H., & Park, S. (2025). Network Pharmacology-Guided Evaluation of Ginger and Cornelian Cherry Extracts Against Depression and Metabolic Dysfunction in Estrogen-Deficient Chronic Stressed Rats. International Journal of Molecular Sciences, 26(10), 4829. https://doi.org/10.3390/ijms26104829







