Opposing Calcium-Dependent Effects of GsMTx4 in Acute Lymphoblastic Leukemia: In Vitro Proliferation vs. In Vivo Survival Advantage
Abstract
1. Introduction
2. Results
2.1. MG Ion-Channel Inhibitor GsMTx4 Enhances Basal Ca2+ Levels in ALL Cell Lines
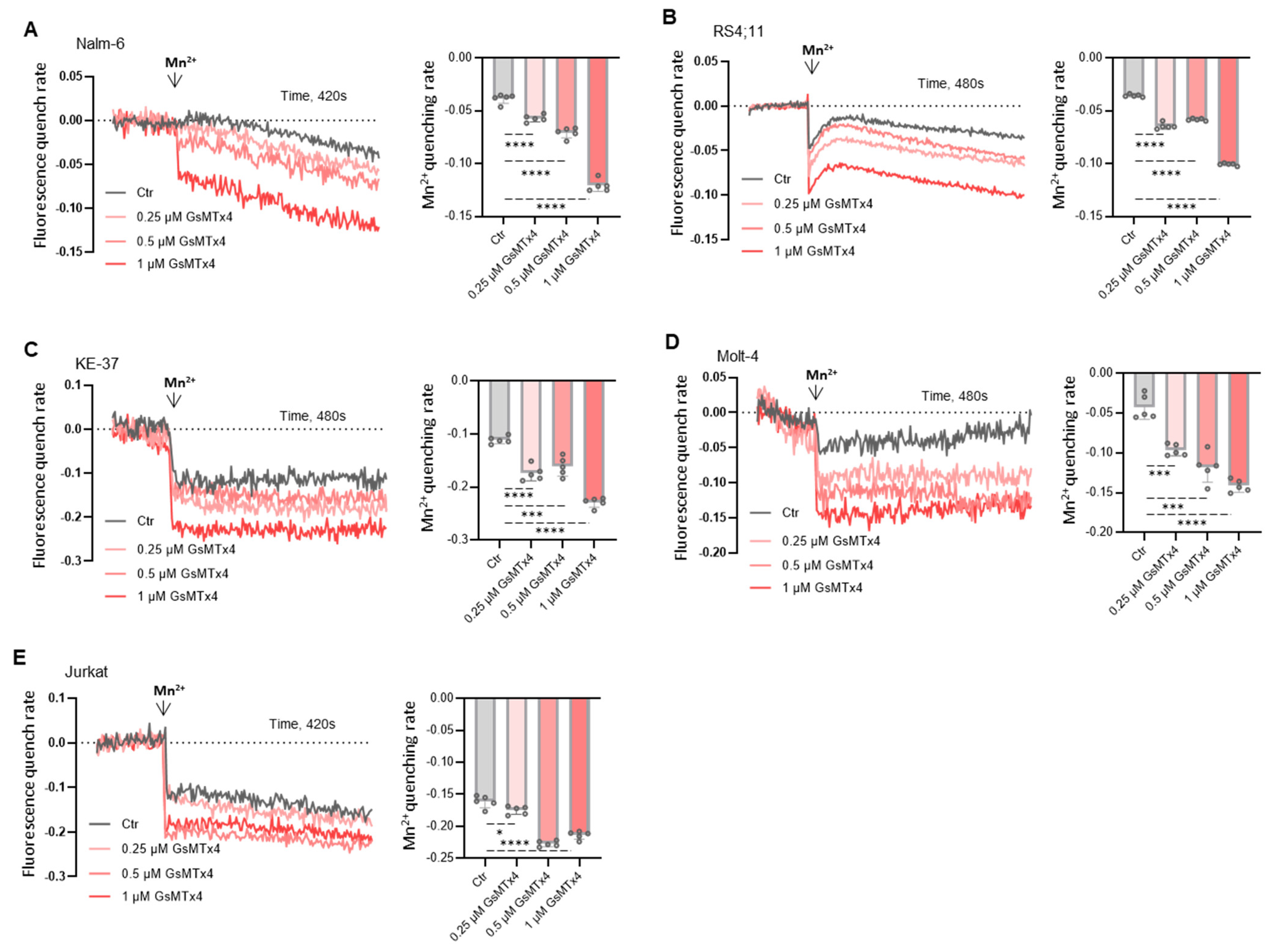
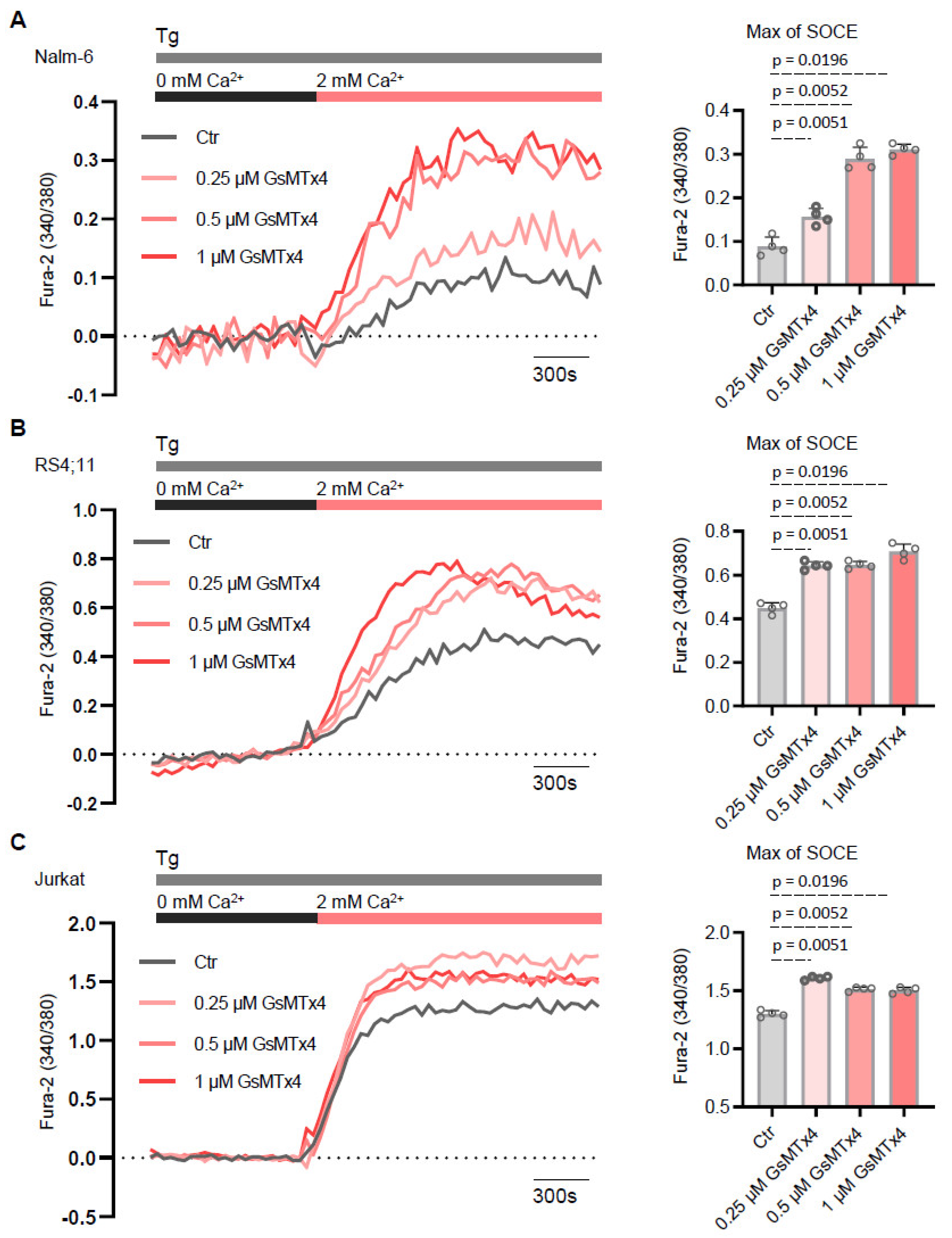
2.2. GsMTx4 Increases Cytosolic Ca2+ Levels Through Activation of Constitutive Ca2+ Entry in ALL Cell Lines
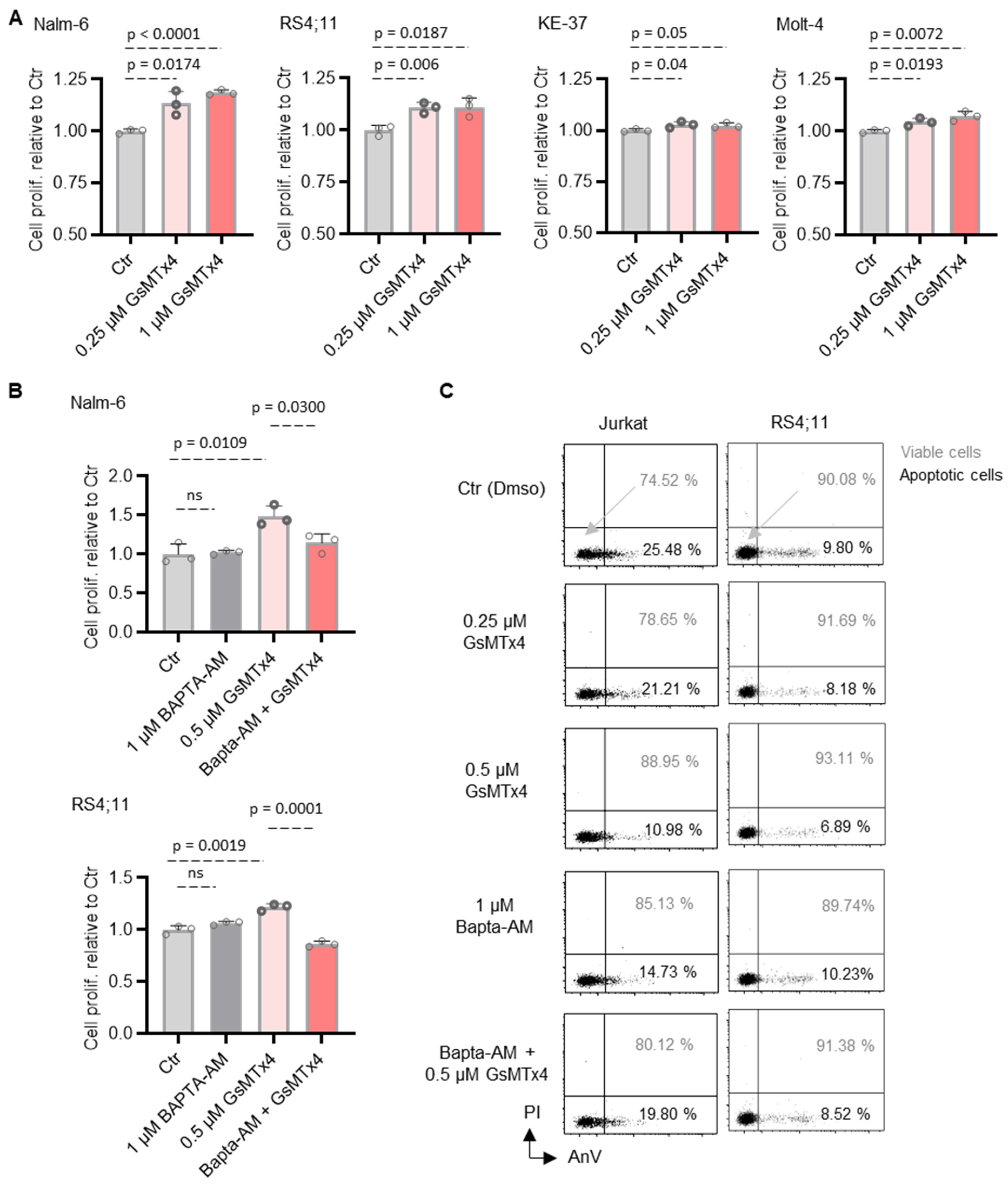
2.3. GsMTx4-Mediated Intracellular Ca2+ Signaling Positively Regulates ALL Cell Proliferation and Viability
2.4. GsMTx4 Prolongs Overall Survival of NSG Mice by Decreasing Cytosolic Ca2+ Levels of ALL Cells In Vivo
3. Discussion
4. Materials and Methods
4.1. Cell Lines, Cell Culture, and Reagents
4.2. Animal Experiments
4.3. Intracellular Ca2+ Measurement
4.4. Basal Cytosolic Ca2+ Measurements
4.5. Mn2+ Quenching
4.6. CCK-8 Cell Proliferation Assay
4.7. Bioluminescent Imaging
4.8. Flow Cytometry Ca2+ Measurement
4.9. Apoptosis Assay
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Conter, V.; Aricò, M.; Basso, G.; Biondi, A.; Barisone, E.; Messina, C.; Parasole, R.; De Rossi, G.; Locatelli, F.; Pession, A.; et al. EDUCATIONAL REPORT Long-Term Results of the Italian Association of Pediatric Hematology and Oncology (AIEOP) Studies 82, 87, 88, 91 and 95 for Childhood Acute Lymphoblastic Leukemia. Leukemia 2010, 24, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Hefazi, M.; Litzow, M.R. Recent Advances in the Biology and Treatment of B-Cell Acute Lymphoblastic Leukemia. Blood Lymphat. Cancer Targets Ther. 2018, 8, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Serafin, V.; Capuzzo, G.; Milani, G.; Minuzzo, S.A.; Pinazza, M.; Bortolozzi, R.; Bresolin, S.; Porcù, E.; Frasson, C.; Indraccolo, S.; et al. Glucocorticoid Resistance Is Reverted by LCK Inhibition in Pediatric T-Cell Acute Lymphoblastic Leukemia. Blood 2017, 130, 2750–2761. [Google Scholar] [CrossRef]
- Graff, Z.; Burke, M.J.; Gossai, N. Novel Therapies for Pediatric Acute Lymphoblastic Leukemia. Curr. Opin. Pediatr. 2024, 36, 64–70. [Google Scholar] [CrossRef]
- Oskarsson, T.; Söderhäll, S.; Arvidson, J.; Forestier, E.; Frandsen, T.L.; Hellebostad, M.; Lähteenmäki, P.; Jónsson, Ó.G.; Myrberg, I.H.; Heyman, M.; et al. Treatment-Related Mortality in Relapsed Childhood Acute Lymphoblastic Leukemia. Pediatr. Blood Cancer 2018, 65, e26909. [Google Scholar] [CrossRef]
- Nguyen, K.; Devidas, M.; Cheng, S.-C.; La, M.; Raetz, E.A.; Carroll, W.L.; Winick, N.J.; Hunger, S.P.; Gaynon, P.S.; Loh, M.L.; et al. Factors Influencing Survival after Relapse from Acute Lymphoblastic Leukemia: A Children’s Oncology Group Study. Leukemia 2008, 22, 2142–2150. [Google Scholar] [CrossRef] [PubMed]
- Abdoul-Azize, S.; Dubus, I.; Vannier, J.P. Improvement of Dexamethasone Sensitivity by Chelation of Intracellular Ca2+ in Pediatric Acute Lymphoblastic Leukemia Cells through the Prosurvival Kinase ERK1/2 Deactivation. Oncotarget 2017, 8, 27339–27352. [Google Scholar] [CrossRef]
- Monteith, G.R.; McAndrew, D.; Faddy, H.M.; Roberts-Thomson, S.J. Calcium and Cancer: Targeting Ca2+ Transport. Nat. Rev. Cancer 2007, 7, 519–530. [Google Scholar] [CrossRef]
- Roderick, H.L.; Cook, S.J. Ca2+ Signalling Checkpoints in Cancer: Remodelling Ca2+ for Cancer Cell Proliferation and Survival. Nat. Rev. Cancer 2008, 8, 361–375. [Google Scholar] [CrossRef]
- Prevarskaya, N.; Skryma, R.; Shuba, Y. Calcium in Tumour Metastasis: New Roles for Known Actors. Nat. Rev. Cancer 2011, 11, 609–618. [Google Scholar] [CrossRef]
- Sachs, F.; Morris, C.E. Mechanosensitive Ion Channels in Nonspecialized Cells. Rev. Physiol. Biochem. Pharmacol. 1998, 132, 1–77. [Google Scholar] [CrossRef] [PubMed]
- Vanoye, C.G.; Reuss, L. Stretch-Activated Single K+ Channels Account for Whole-Cell Currents Elicited by Swelling. Proc. Natl. Acad. Sci. USA 1999, 96, 6511–6516. [Google Scholar] [CrossRef]
- Du, H.; Bartleson, J.M.; Butenko, S.; Alonso, V.; Liu, W.F.; Winer, D.A.; Butte, M.J. Tuning Immunity through Tissue Mechanotransduction. Nat. Rev. Immunol. 2023, 23, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Thien, N.D.; Hai-Nam, N.; Anh, D.T.; Baecker, D. Piezo1 and Its Inhibitors: Overview and Perspectives. Eur. J. Med. Chem. 2024, 273, 116502. [Google Scholar] [CrossRef]
- Velasco-Estevez, M.; Gadalla, K.K.E.; Liñan-Barba, N.; Cobb, S.; Dev, K.K.; Sheridan, G.K. Inhibition of Piezo1 Attenuates Demyelination in the Central Nervous System. Glia 2020, 68, 356–375. [Google Scholar] [CrossRef] [PubMed]
- Hope, J.M.; Lopez-Cavestany, M.; Wang, W.; Reinhart-King, C.A.; King, M.R. Activation of Piezo1 Sensitizes Cells to TRAIL-Mediated Apoptosis through Mitochondrial Outer Membrane Permeability. Cell Death Dis. 2019, 10, 837. [Google Scholar] [CrossRef]
- Pathak, M.M.; Nourse, J.L.; Tran, T.; Hwe, J.; Arulmoli, J.; Le, D.T.T.; Bernardis, E.; Flanagan, L.A.; Tombola, F. Stretch-Activated Ion Channel Piezo1 Directs Lineage Choice in Human Neural Stem Cells. Proc. Natl. Acad. Sci. USA 2014, 111, 16148–16153. [Google Scholar] [CrossRef]
- Acheva, A.; Kärki, T.; Schaible, N.; Krishnan, R.; Tojkander, S. Adipokine Leptin Co-Operates With Mechanosensitive Ca2+-Channels and Triggers Actomyosin-Mediated Motility of Breast Epithelial Cells. Front. Cell Dev. Biol. 2020, 8, 607038. [Google Scholar] [CrossRef]
- Kwak, K.; Sohn, H.; George, R.; Torgbor, C.; Manzella-Lapeira, J.; Brzostowski, J.; Pierce, S.K. B Cell Responses to Membrane-Presented Antigens Require the Function of the Mechanosensitive Cation Channel Piezo1. Sci. Signal. 2023, 16, eabq5096. [Google Scholar] [CrossRef]
- Maroto, R.; Kurosky, A.; Hamill, O.P. Mechanosensitive Ca2+ Permeant Cation Channels in Human Prostate Tumor Cells. Channels 2012, 6, 290–307. [Google Scholar] [CrossRef]
- Suchyna, T.M.; Tape, S.E.; Koeppe, R.E.; Andersen, O.S.; Sachs, F.; Gottlieb, P.A. Bilayer-Dependent Inhibition of Mechanosensitive Channels by Neuroactive Peptide Enantiomers. Nature 2004, 430, 235–240. [Google Scholar] [CrossRef]
- Scaviner, J.; Bagacean, C.; Christian, B.; Renaudineau, Y.; Mignen, O.; Abdoul-Azize, S. Blocking Orai1 Constitutive Activity Inhibits B-Cell Cancer Migration and Synergistically Acts with Drugs to Reduce B-CLL Cell Survival. Eur. J. Pharmacol. 2024, 971, 176515. [Google Scholar] [CrossRef] [PubMed]
- Abdoul-Azize, S.; Hami, R.; Riou, G.; Derambure, C.; Charbonnier, C.; Vannier, J.-P.; Guzman, M.L.; Schneider, P.; Boyer, O. Glucocorticoids Paradoxically Promote Steroid Resistance in B Cell Acute Lymphoblastic Leukemia through CXCR4/PLC Signaling. Nat. Commun. 2024, 15, 4557. [Google Scholar] [CrossRef] [PubMed]
- Bowman, C.L.; Gottlieb, P.A.; Suchyna, T.M.; Murphy, Y.K.; Sachs, F. Mechanosensitive Ion Channels and the Peptide Inhibitor GsMTx-4: History, Properties, Mechanisms and Pharmacology. Toxicon 2007, 49, 249–270. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, X.; Chen, S.; Hu, C.; Guo, R.; Wu, Y.; Liu, Z.; Shu, X.; Jiang, M. Examination of the Mechanism of Piezo Ion Channel in 5-HT Synthesis in the Enterochromaffin Cell and Its Association with Gut Motility. Front. Endocrinol. 2023, 14, 1193556. [Google Scholar] [CrossRef]
- Alcaino, C.; Knutson, K.R.; Treichel, A.J.; Yildiz, G.; Strege, P.R.; Linden, D.R.; Li, J.H.; Leiter, A.B.; Szurszewski, J.H.; Farrugia, G.; et al. A Population of Gut Epithelial Enterochromaffin Cells Is Mechanosensitive and Requires Piezo2 to Convert Force into Serotonin Release. Proc. Natl. Acad. Sci. USA 2018, 115, E7632–E7641. [Google Scholar] [CrossRef] [PubMed]
- Jairaman, A.; Othy, S.; Dynes, J.L.; Yeromin, A.V.; Zavala, A.; Greenberg, M.L.; Nourse, J.L.; Holt, J.R.; Cahalan, S.M.; Marangoni, F.; et al. Piezo1 Channels Restrain Regulatory T Cells but Are Dispensable for Effector CD4+ T Cell Responses. Sci. Adv. 2021, 7, eabg5859. [Google Scholar] [CrossRef]
- Ren, X.; Zhuang, H.; Li, B.; Jiang, F.; Zhang, Y.; Zhou, P. Gsmtx4 Alleviated Osteoarthritis through Piezo1/Calcineurin/NFAT1 Signaling Axis under Excessive Mechanical Strain. Int. J. Mol. Sci. 2023, 24, 4022. [Google Scholar] [CrossRef]
- Liu, C.S.C.; Raychaudhuri, D.; Paul, B.; Chakrabarty, Y.; Ghosh, A.R.; Rahaman, O.; Talukdar, A.; Ganguly, D. Cutting Edge: Piezo1 Mechanosensors Optimize Human T Cell Activation. J. Immunol. 2018, 200, 1255–1260. [Google Scholar] [CrossRef]
- Peng, H.; Chao, Z.; Wang, Z.; Hao, X.; Xi, Z.; Ma, S.; Guo, X.; Zhang, J.; Zhou, Q.; Qu, G.; et al. Biomechanics in the Tumor Microenvironment: From Biological Functions to Potential Clinical Applications. Exp. Hematol. Oncol. 2025, 14, 4. [Google Scholar] [CrossRef]
- Suchyna, T.M. Piezo Channels and GsMTx4: Two Milestones in Our Understanding of Excitatory Mechanosensitive Channels and Their Role in Pathology. Prog. Biophys. Mol. Biol. 2017, 130, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, J.; Babicheva, A.; Jain, P.P.; Rodriguez, M.; Ayon, R.J.; Ravellette, K.S.; Wu, L.; Balistrieri, F.; Tang, H.; et al. Endothelial Upregulation of Mechanosensitive Channel Piezo1 in Pulmonary Hypertension. Am. J. Physiol. Cell Physiol. 2021, 321, C1010–C1027. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, L.; Huang, T.; Lu, D.; Song, Y.; Wang, L.; Gao, J. Mechanosensitive Cation Channel Piezo1 Contributes to Ventilator-Induced Lung Injury by Activating RhoA/ROCK1 in Rats. Respir. Res. 2021, 22, 250. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdoul-Azize, S.; Zoubairi, R.; Boyer, O. Opposing Calcium-Dependent Effects of GsMTx4 in Acute Lymphoblastic Leukemia: In Vitro Proliferation vs. In Vivo Survival Advantage. Int. J. Mol. Sci. 2025, 26, 4822. https://doi.org/10.3390/ijms26104822
Abdoul-Azize S, Zoubairi R, Boyer O. Opposing Calcium-Dependent Effects of GsMTx4 in Acute Lymphoblastic Leukemia: In Vitro Proliferation vs. In Vivo Survival Advantage. International Journal of Molecular Sciences. 2025; 26(10):4822. https://doi.org/10.3390/ijms26104822
Chicago/Turabian StyleAbdoul-Azize, Souleymane, Rachid Zoubairi, and Olivier Boyer. 2025. "Opposing Calcium-Dependent Effects of GsMTx4 in Acute Lymphoblastic Leukemia: In Vitro Proliferation vs. In Vivo Survival Advantage" International Journal of Molecular Sciences 26, no. 10: 4822. https://doi.org/10.3390/ijms26104822
APA StyleAbdoul-Azize, S., Zoubairi, R., & Boyer, O. (2025). Opposing Calcium-Dependent Effects of GsMTx4 in Acute Lymphoblastic Leukemia: In Vitro Proliferation vs. In Vivo Survival Advantage. International Journal of Molecular Sciences, 26(10), 4822. https://doi.org/10.3390/ijms26104822





