LEF1-AS1 Deregulation in the Peripheral Blood of Patients with Persistent Post-COVID Symptoms
Abstract
1. Introduction
2. Results
2.1. Long COVID Patient Characteristics
2.2. No Difference in Plasma miR-144-3p Levels in MPS or MNS Long COVID Patients
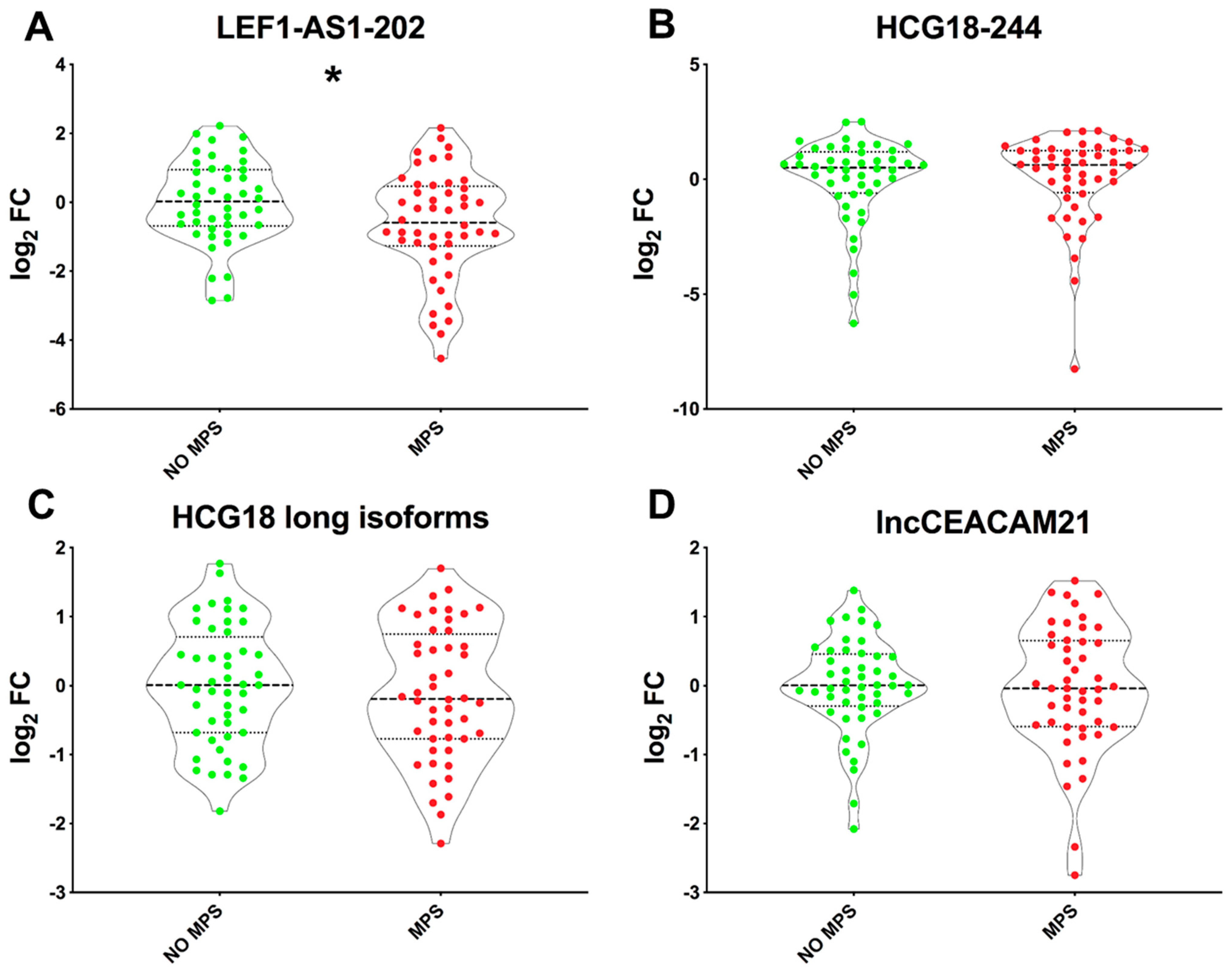
2.3. Lower LEF1-AS1 Expression Levels in the PBMC of MPS Patients
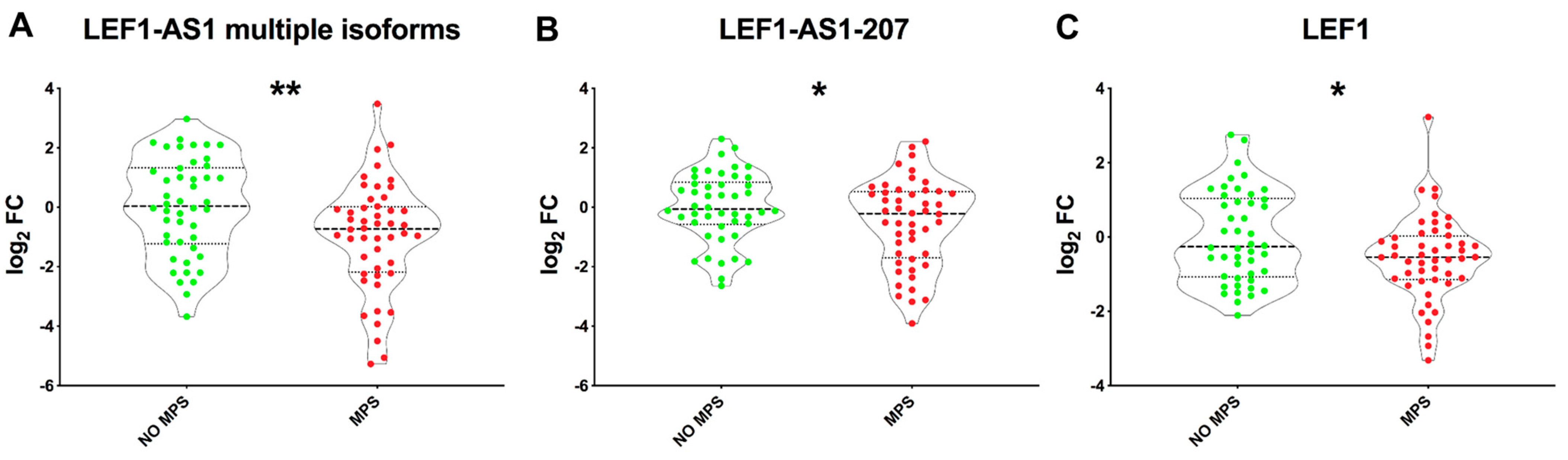
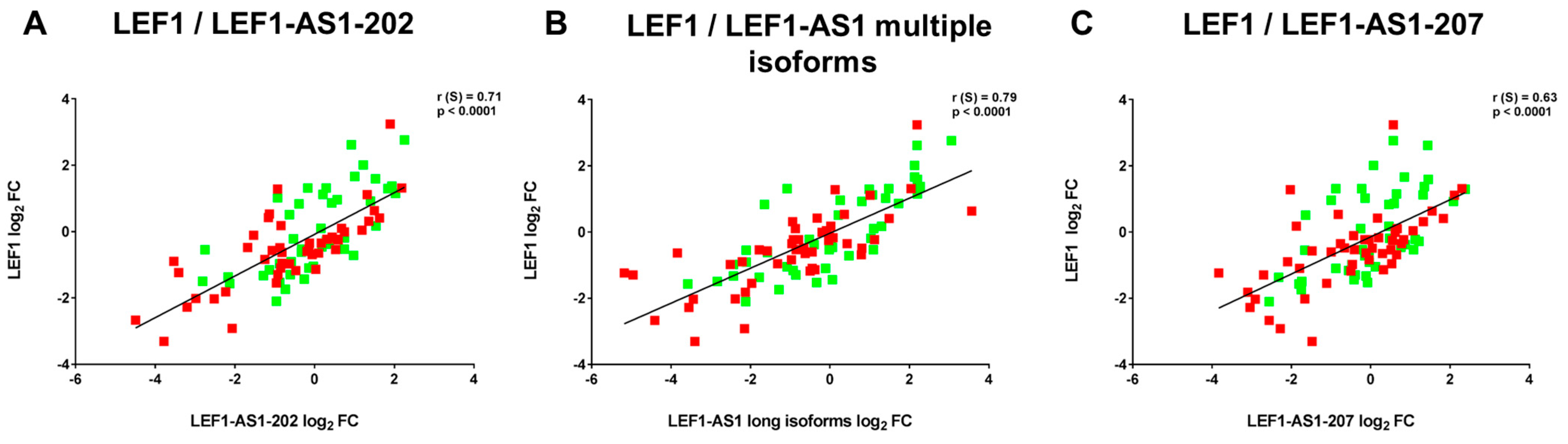
2.4. LEF1-AS1 Isoforms and LEF1 Are Similarly Deregulated in PBMCs of Long COVID Patients
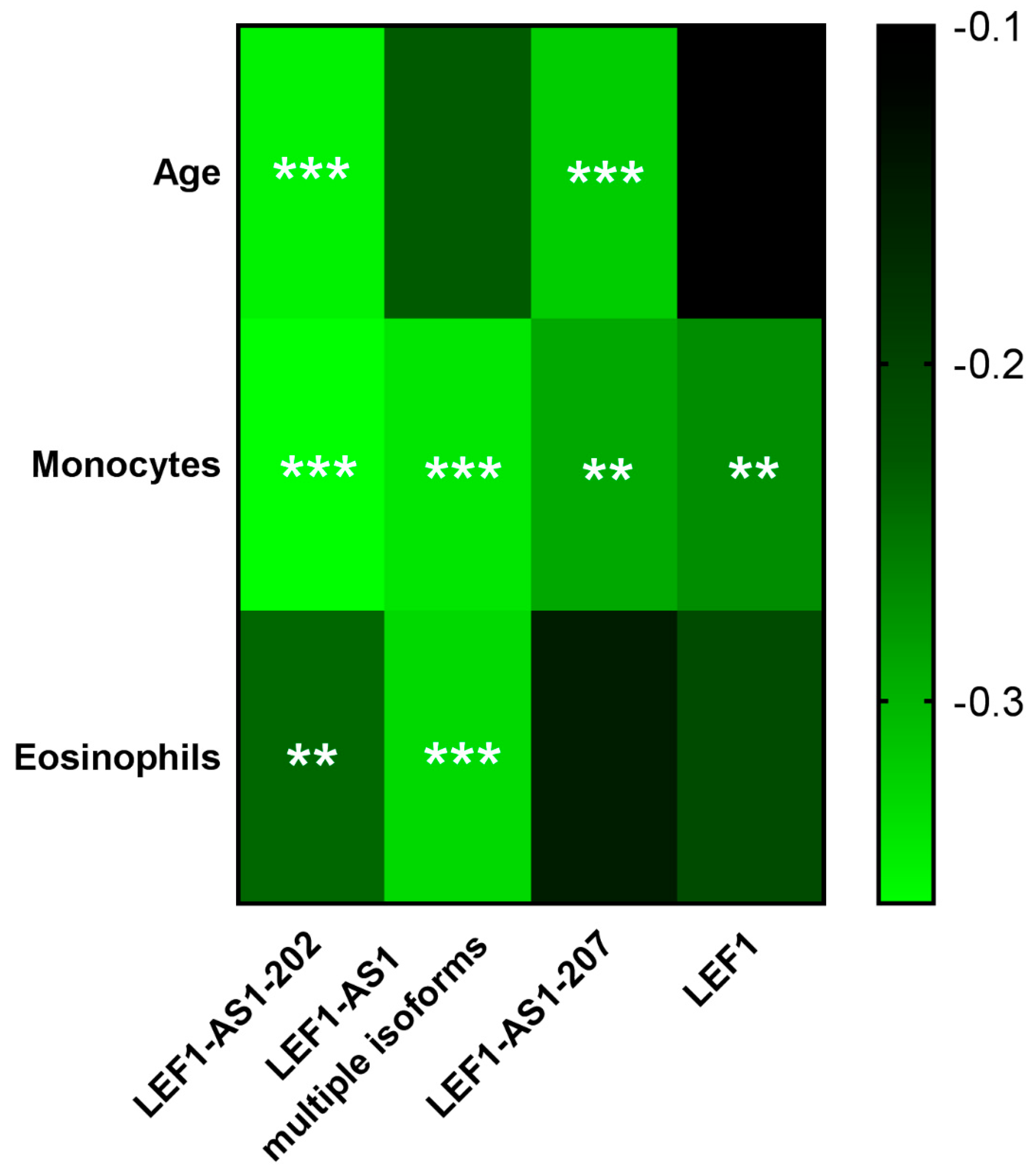
2.5. LEF1-AS1 Isoforms and LEF1 Correlated with Relevant Clinical Parameters
3. Discussion
4. Materials and Methods
4.1. Ethics Approval and Consent to Participate
4.2. Patient Selection and Sample Collection
4.3. RNA Isolation and RT-qPCR Quantification
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Post COVID-19 Condition (Long COVID). Available online: https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-condition#:~:text=Definition,months%20with%20no%20other%20explanation (accessed on 15 October 2024).
- WHO Coronavirus Disease (COVID-19): Post COVID-19 Condition. Available online: https://www.who.int/news-room/questions-and-answers/item/coronavirus-disease-(covid-19)-post-covid-19-condition (accessed on 15 October 2024).
- Català, M.; Mercadé-Besora, N.; Kolde, R.; Trinh, N.T.H.; Roel, E.; Burn, E.; Rathod-Mistry, T.; Kostka, K.; Man, W.Y.; Delmestri, A.; et al. The Effectiveness of COVID-19 Vaccines to Prevent Long COVID Symptoms: Staggered Cohort Study of Data from the UK, Spain, and Estonia. Lancet Respir. Med. 2024, 12, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Trinh, N.T.; Jödicke, A.M.; Català, M.; Mercadé-Besora, N.; Hayati, S.; Lupattelli, A.; Prieto-Alhambra, D.; Nordeng, H.M. Effectiveness of COVID-19 Vaccines to Prevent Long COVID: Data from Norway. Lancet Respir. Med. 2024, 12, e33–e34. [Google Scholar] [CrossRef] [PubMed]
- Ranucci, M.; Baryshnikova, E.; Anguissola, M.; Pugliese, S.; Ranucci, L.; Falco, M.; Menicanti, L. The Very Long COVID: Persistence of Symptoms after 12–18 Months from the Onset of Infection and Hospitalization. J. Clin. Med. 2023, 12, 1915. [Google Scholar] [CrossRef]
- Ramonfaur, D.; Ayad, N.; Liu, P.H.Z.; Zhou, J.; Wu, Y.; Li, J.; Chen, G. The Global Clinical Studies of Long COVID. Int. J. Infect. Dis. 2024, 146, 107105. [Google Scholar] [CrossRef]
- Marjenberg, Z.; Leng, S.; Tascini, C.; Garg, M.; Misso, K.; El Guerche Seblain, C.; Shaikh, N. Risk of Long COVID Main Symptoms after SARS-CoV-2 Infection: A Systematic Review and Meta-Analysis. Sci. Rep. 2023, 13, 15332. [Google Scholar] [CrossRef]
- Al-Aly, Z.; Bowe, B.; Xie, Y. Long COVID after Breakthrough SARS-CoV-2 Infection. Nat. Med. 2022, 28, 1461–1467. [Google Scholar] [CrossRef]
- Quaranta, V.N.; Portacci, A.; Dragonieri, S.; Locorotondo, C.; Buonamico, E.; Diaferia, F.; Iorillo, I.; Quaranta, S.; Carpagnano, G.E. The Predictors of Long COVID in Southeastern Italy. J. Clin. Med. 2023, 12, 6303. [Google Scholar] [CrossRef]
- Antoniou, K.M.; Vasarmidi, E.; Russell, A.-M.; Andrejak, C.; Crestani, B.; Delcroix, M.; Dinh-Xuan, A.T.; Poletti, V.; Sverzellati, N.; Vitacca, M.; et al. European Respiratory Society Statement on Long COVID Follow-Up. Eur. Respir. J. 2022, 60, 2102174. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major Findings, Mechanisms and Recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Gusev, E.; Sarapultsev, A. Exploring the Pathophysiology of Long COVID: The Central Role of Low-Grade Inflammation and Multisystem Involvement. Int. J. Mol. Sci. 2024, 25, 6389. [Google Scholar] [CrossRef]
- Huang, L.; Yao, Q.; Gu, X.; Wang, Q.; Ren, L.; Wang, Y.; Hu, P.; Guo, L.; Liu, M.; Xu, J.; et al. 1-Year Outcomes in Hospital Survivors with COVID-19: A Longitudinal Cohort Study. Lancet 2021, 398, 747–758. [Google Scholar] [CrossRef]
- Watanabe, A.; So, M.; Iwagami, M.; Fukunaga, K.; Takagi, H.; Kabata, H.; Kuno, T. One-year Follow-up CT Findings in COVID-19 Patients: A Systematic Review and Meta-analysis. Respirology 2022, 27, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Huang, H.; Wang, C.; Jin, Z.; Zhang, Z.; He, J.; Yin, S.; Fan, M.; Huang, J.; Chen, F.; et al. Follow-up Study of Pulmonary Function among COVID-19 Survivors 1 Year after Recovery. J. Infect. 2021, 83, 381–412. [Google Scholar] [CrossRef]
- Wu, X.; Liu, X.; Zhou, Y.; Yu, H.; Li, R.; Zhan, Q.; Ni, F.; Fang, S.; Lu, Y.; Ding, X.; et al. 3-Month, 6-Month, 9-Month, and 12-Month Respiratory Outcomes in Patients Following COVID-19-Related Hospitalisation: A Prospective Study. Lancet Respir. Med. 2021, 9, 747–754. [Google Scholar] [CrossRef]
- Sobrino-Relaño, S.; Balboa-Bandeira, Y.; Peña, J.; Ibarretxe-Bilbao, N.; Zubiaurre-Elorza, L.; Ojeda, N. Neuropsychological Deficits in Patients with Persistent COVID-19 Symptoms: A Systematic Review and Meta-Analysis. Sci. Rep. 2023, 13, 10309. [Google Scholar] [CrossRef]
- Demko, Z.O.; Yu, T.; Mullapudi, S.K.; Varela Heslin, M.G.; Dorsey, C.A.; Payton, C.B.; Tornheim, J.A.; Blair, P.W.; Mehta, S.H.; Thomas, D.L.; et al. Post-Acute Sequelae of SARS-CoV-2 (PASC) Impact Quality of Life at 6, 12 and 18 Months Post-Infection. MedRxiv 2022, 2022:22278543. [Google Scholar] [CrossRef]
- Hastie, C.E.; Lowe, D.J.; McAuley, A.; Mills, N.L.; Winter, A.J.; Black, C.; Scott, J.T.; O’Donnell, C.A.; Blane, D.N.; Browne, S.; et al. True Prevalence of Long-COVID in a Nationwide, Population Cohort Study. Nat. Commun. 2023, 14, 7892. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Bae, S.; Chang, H.-H.; Kim, S.-W. Author Correction: Long COVID Prevalence and Impact on Quality of Life 2 Years after Acute COVID-19. Sci. Rep. 2023, 13, 11960. [Google Scholar] [CrossRef]
- López-Hernández, Y.; Monárrez-Espino, J.; López, D.A.G.; Zheng, J.; Borrego, J.C.; Torres-Calzada, C.; Elizalde-Díaz, J.P.; Mandal, R.; Berjanskii, M.; Martínez-Martínez, E.; et al. The Plasma Metabolome of Long COVID Patients Two Years after Infection. Sci. Rep. 2023, 13, 12420. [Google Scholar] [CrossRef]
- Greco, S.; Madè, A.; Gaetano, C.; Devaux, Y.; Emanueli, C.; Martelli, F. Noncoding RNAs Implication in Cardiovascular Diseases in the COVID-19 Era. J. Transl. Med. 2020, 18, 408. [Google Scholar] [CrossRef]
- Madè, A.; Greco, S.; Vausort, M.; Miliotis, M.; Schordan, E.; Baksi, S.; Zhang, L.; Baryshnikova, E.; Ranucci, M.; Cardani, R.; et al. Association of miR-144 Levels in the Peripheral Blood with COVID-19 Severity and Mortality. Sci. Rep. 2022, 12, 20048. [Google Scholar] [CrossRef]
- Greco, S.; Made’, A.; Mutoli, M.; Zhang, L.; Piella, S.N.; Vausort, M.; Lumley, A.I.; Beltrami, A.P.; Srivastava, P.K.; Milani, V.; et al. HCG18, LEF1AS1 and lncCEACAM21 as Biomarkers of Disease Severity in the Peripheral Blood Mononuclear Cells of COVID-19 Patients. J. Transl. Med. 2023, 21, 758. [Google Scholar] [CrossRef]
- Devaux, Y.; Zhang, L.; Lumley, A.I.; Karaduzovic-Hadziabdic, K.; Mooser, V.; Rousseau, S.; Shoaib, M.; Satagopam, V.; Adilovic, M.; Srivastava, P.K.; et al. Development of a Long Noncoding RNA-Based Machine Learning Model to Predict COVID-19 in-Hospital Mortality. Nat. Commun. 2024, 15, 4259. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, M.E.B.; Bloemsma, L.D.; Vaes, A.W.; Baalbaki, N.; Deng, Q.; Beijers, R.J.H.C.G.; Noij, L.C.E.; Houweling, L.; Bazdar, S.; Spruit, M.A.; et al. Fatigue and Symptom-Based Clusters in Post COVID-19 Patients: A Multicentre, Prospective, Observational Cohort Study. J. Transl. Med. 2024, 22, 191. [Google Scholar] [CrossRef]
- López-Hernández, Y.; Oropeza-Valdez, J.J.; García Lopez, D.A.; Borrego, J.C.; Murgu, M.; Valdez, J.; López, J.A.; Monárrez-Espino, J. Untargeted Analysis in Post-COVID-19 Patients Reveals Dysregulated Lipid Pathways Two Years after Recovery. Front. Mol. Biosci. 2023, 10, 1100486. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Choi, Y.; Joung, J.-Y.; Son, C.-G. Clinical and Laboratory Characteristics of Fatigue-Dominant Long-COVID Subjects: A Cross-Sectional Study. Am. J. Med. 2024, 138, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Wahlgren, C.; Forsberg, G.; Divanoglou, A.; Östholm Balkhed, Å.; Niward, K.; Berg, S.; Levi, R. Two-Year Follow-up of Patients with Post-COVID-19 Condition in Sweden: A Prospective Cohort Study. Lancet Reg. Health-Eur. 2023, 28, 100595. [Google Scholar] [CrossRef]
- Mansell, V.; Hall Dykgraaf, S.; Kidd, M.; Goodyear-Smith, F. Long COVID and Older People. Lancet Healthy Longev. 2022, 3, e849–e854. [Google Scholar] [CrossRef]
- Ravkov, E.V.; Williams, E.S.C.P.; Elgort, M.; Barker, A.P.; Planelles, V.; Spivak, A.M.; Delgado, J.C.; Lin, L.; Hanley, T.M. Reduced Monocyte Proportions and Responsiveness in Convalescent COVID-19 Patients. Front. Immunol. 2024, 14, 1329026. [Google Scholar] [CrossRef]
- Jukema, B.N.; Smit, K.; Hopman, M.T.E.; Bongers, C.C.W.G.; Pelgrim, T.C.; Rijk, M.H.; Platteel, T.N.; Venekamp, R.P.; Zwart, D.L.M.; Rutten, F.H.; et al. Neutrophil and Eosinophil Responses Remain Abnormal for Several Months in Primary Care Patients With COVID-19 Disease. Front. Allergy 2022, 3, 942699. [Google Scholar] [CrossRef]
- Cheng, Z.; Wang, G.; Zhu, W.; Luo, C.; Guo, Z. LEF1-AS1 Accelerates Tumorigenesis in Glioma by Sponging miR-489-3p to Enhance HIGD1A. Cell Death Dis. 2020, 11, 690. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lin, X.; Jiang, W.; Wu, J.; Lin, L. lncRNA LEF1-AS1 Promotes Malignancy in Non-Small-Cell Lung Cancer by Modulating the miR-489/SOX4 Axis. DNA Cell Biol. 2019, 38, 1013–1021. [Google Scholar] [CrossRef]
- Vausort, M.; Lumley, A.I.; Boubakeur, H.; Zhang, L.; Hefeng, F.Q.; Ollert, M.; Wilmes, P.; Fagherazzi, G.; Devaux, Y. Association of LEF1-AS1 with Cardiovascular and Neurological Complications of COVID-19. J. Mol. Cell. Cardiol. Plus 2025, 11, 100280. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Xue, H.-H. Cutting Edge: Generation of Memory Precursors and Functional Memory CD8+ T Cells Depends on T Cell Factor-1 and Lymphoid Enhancer-Binding Factor-1. J. Immunol. 2012, 189, 2722–2726. [Google Scholar] [CrossRef]
- Qi, Y.; Shan, Y.; Li, S.; Huang, Y.; Guo, Y.; Huang, T.; Zhao, X.; Jia, L. LncRNA LEF1-AS1/LEF1/FUT8 Axis Mediates Colorectal Cancer Progression by Regulating A1, 6-Fucosylationvia Wnt/β-Catenin Pathway. Dig. Dis. Sci. 2022, 67, 2182–2194. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Qiao, L.; Liu, Y. Long Noncoding RNA LEF1-AS1 Binds with HNRNPL to Boost the Proliferation, Migration, and Invasion in Osteosarcoma by Enhancing the mRNA Stability of LEF1. J. Cell. Biochem. 2020, 121, 4064–4073. [Google Scholar] [CrossRef]
- Greco, S.; Zaccagnini, G.; Perfetti, A.; Fuschi, P.; Valaperta, R.; Voellenkle, C.; Castelvecchio, S.; Gaetano, C.; Finato, N.; Beltrami, A.P.; et al. Long Noncoding RNA Dysregulation in Ischemic Heart Failure. J. Transl. Med. 2016, 14, 183. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative CT Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
| ALL (N = 98) | MPS (N = 48) | NO MPS (N = 50) | Odds Ratio (95% CI) | p Value | MNS (N = 39) | NO MNS (N = 59) | Odd Ratio (95% CI) | p Value | |
|---|---|---|---|---|---|---|---|---|---|
| Age, median (range) | 66 (55–74) | 71 (60–77) | 58 (49–67) | 1.06 (1.02–1.10) | 0.0011 | 71 (61–78) | 59 (52–71) | 1.05 (1.02–1.09) | 0.0047 |
| Gender, male, n (%) | 67 (68.37) | 29 (60.42) | 38 (76.00) | 2.08 (0.87–4.95) | 0.0999 | 21 (53.85) | 46 (77.97) | 3.03 (1.26–7.32) | 0.0135 |
| Medical history/ comorbidities, n (%) | |||||||||
| Current smoker | 5 (5.10) | 2 (4.17) | 3 (6.00) | 0.68 (0.10–4.27) | 0.6817 | 1 (2.56) | 4 (6.78) | 0.36 (0.04–3.37) | 0.3716 |
| Former smoker | 43 (43.88) | 28 (58.33) | 15 (30.00) | 3.27 (1.42–7.52) | 0.0054 | 20 (51.28) | 23 (38.98) | 1.65 (0.73–3.73) | 0.2312 |
| Hypertension | 46 (46.94) | 28 (58.33) | 19 (36.00) | 2.49 (1.10–5.62) | 0.0281 | 21 (53.85) | 25 (42.37) | 1.58 (0.70–3.58) | 0.2665 |
| Diabetes | 16 (16.33) | 10 (20.85) | 6 (12.00) | 1.93 (0.0.64–5.81) | 0.2420 | 11 (28.21) | 5 (8.47) | 4.24 (1.34–13.42) | 0.0139 |
| Obesity | 24 (24.49) | 13 (27.08) | 11 (22.00) | 1.32 (0.52–3.32) | 0.5596 | 11 (28.21) | 13 (22.03) | 1.39 (0.55–3.52) | 0.4877 |
| Ischemic cardiomyopathy | 17 (17.35) | 8 (16.67) | 9 (18.00) | 0.91 (0.32–2.60) | 0.8618 | 9 (23.08) | 8 (13.56) | 1.91 (0.67–5.49) | 0.2278 |
| Chronic heart failure | 7 (7.14) | 4 (8.33) | 3 (6.00) | 1.42 (0.30–6.73) | 0.6552 | 3 (7.69) | 4 (6.78) | 1.15 (0.24–5.43) | 0.8637 |
| Atrial fibrillation | 9 (9.18) | 8 (16.67) | 1 (2.00) | 9.80 (1.18–81.68) | 0.0349 | 5 (12.82) | 4 (6.78) | 2.02 (0.51–8.06) | 0.3182 |
| Left ventricular disfunction | 1 (1.02) | 0 (0.00) | 1 (2.00) | - | - | 0 (0.00) | 1 (1.69) | - | - |
| BPCO | 5 (5.10) | 3 (6.25) | 2 (4.00) | 1.60 (0.26–10.03) | 0.6156 | 2 (5.13) | 3 (5.08) | 1.00 (0.16–6.33) | 0.9924 |
| Asthma | 4 (4.08) | 3 (6.25) | 1 (2.00) | 3.28 (0.33–32.55) | 0.3129 | 2 (5.13) | 2 (3.39) | 1.54 (0.21–11.42) | 0.6724 |
| Cancer | 9 (9.18) | 3 (6.25) | 6 (12.00) | 0.49 (0.12–2.08) | 0.3324 | 6 (15.38) | 3 (5.08) | 3.39 (0.79–14.48) | 0.0988 |
| Pre-existing stroke | 5 (5.10) | 3 (6.25) | 2 (4.00) | 1.60 (0.26–10.02) | 0.6256 | 1 (2.56) | 4 (6.78) | 0.36 (0.04–3.37) | 0.3716 |
| Chronic neurological disorders | 3 (3.06) | 3 (6.25) | 0 (0.00) | - | - | 1 (2.56) | 2 (3.39) | 0.75 (0.07–8.56) | 0.8169 |
| Chronic kidney disorders | 7 (7.14) | 3 (6.25) | 4 (8.00) | 0.77 (0.16–3.62) | 0.7373 | 3 (7.69) | 4 (6.78) | 1.15 (0.24–5.43) | 0.8637 |
| Liver disorders | 3 (3.06) | 1 (2.08) | 2 (4.00) | 0.51 (0.05–5.82) | 0.5884 | 2 (5.13) | 1 (1.69) | 3.14 (0.27–35.81) | 0.3578 |
| Chronic gut inflammation | 3 (3.06) | 3 (6.25) | 0 (0.00) | - | - | 2 (5.13) | 1 (1.69) | 3.14 (0.28–35.81) | 0.3578 |
| Anxiety | 11 (11.22) | 8 (16.67) | 3 (6.00) | 3.13 (0.78–12.61) | 0.1079 | 8 (20.51) | 3 (5.08) | 4.82 (1.91–19.49) | 0.0275 |
| Depression | 11 (11.22) | 7 (14.59) | 4 (8.00) | 1.96 (0.54–7.19) | 0.3086 | 8 (20.51) | 3 (5.08) | 4.82 (1.91–19.49) | 0.0275 |
| COVID-19 disease | |||||||||
| Days of hospitalization, median (range) | 14 (10–22) | 14 (10–22) | 15 (10–25) | 0.99 (0.96–1.02) | 0.4097 | 14 (9–23) | 14 (10–22) | 1.01 (0.98–1.04) | 0.7099 |
| No oxygen therapy, n (%) | 17 (17) | 3 (6) | 14 (28) | 0.17 (0.05–0.66) | 0.01 | 5 (12) | 12 (20) | 0.56 (0.18–1.75) | ns |
| Oxygen therapy, n (%) | 51 (52.04) | 27 (56.25) | 24 (48.00) | 1.39 (0.63–3.07) | 0.4143 | 21 (53.85) | 30 (50.85) | 1.13 (0.50–2.54) | 0.7712 |
| CPAP therapy, n (%) | 27 (27.55) | 17 (35.42) | 10 (20.00) | 2.19 (0.88–5.46) | 0.0911 | 11 (28.21) | 16 (27.12) | 1.06 (0.43–2.61) | 0.9060 |
| Admission to ICU, n (%) | 3 (3.06) | 1 (2.08) | 2 (4.00) | 0.51(0.05–5.82) | 0.5884 | 1 (2.56) | 2 (3.39) | 0.75 (0.07–8.56) | 0.8169 |
| ALL (N = 98) | MPS (N = 48) | NO MPS (N = 50) | p Value | MNS (N = 39) | NO MNS (N = 59) | p Value | |
|---|---|---|---|---|---|---|---|
| Months from COVID-19 disease, median (range) | 18 (16–19) | 18 (17–19) | 17 (12–19) | 0.2356 | 18 (17–19) | 18 (16–19) | 0.8543 |
| Symptoms developed after COVID-19 disease, number of patients (%) | |||||||
| Abdominal pain/diarrhea | 1 (1.02) | 1 (2.08) | 0 (0.00) | 0.4898 # | 1 (2.56) | 0 (0.00) | 0.3980 # |
| Anxiety | 15 (15.31) | 13 (27.08) | 2 (4.00) | 0.0015 | 15 (38.46) | 0 (0.00) | <0.0001 |
| Arthralgia | 26 (26.53) | 20 (41.67) | 6 (12.00) | 0.0009 | 17 (43.59) | 9 (15.25) | 0.0019 |
| Brain fog | 9 (9.18) | 6 (12.50) | 3 (6.00) | 0.3128 # | 9 (23.08) | 0 (0.00) | 0.0001 # |
| Chest pain | 2 (2.04) | 2 (4.17) | 0 (0.00) | 0.2373 # | 0 (0.00) | 2 (3.39) | 0.5159 # |
| Decreased appetite | 4 (4.08) | 4 (8.33) | 0 (0.00) | 0.0539 # | 3 (7.69) | 1 (1.69) | 0.2980 # |
| Delirium | 4 (4.08) | 4 (8.33) | 0 (0.00) | 0.0539 | 3 (7.69) | 1(1.69) | 0.2980 # |
| Depression | 15 (15.31) | 12 (25.00) | 3 (6.00) | 0.0090 | 15 (38.46) | 0 (0.00) | <0.0001 |
| Difficulties in concentrating | 17 (17.35) | 13 (27.08) | 4 (8.00) | 0.0126 | 16 (41.03) | 1 (1.69) | <0.0001 |
| Dizziness and balance disorders | 23 (23.47) | 21 (43.75) | 2 (4.00) | <0.0001 | 16 (41.03) | 7 (11.86) | 0.0009 |
| Dyspnoea/shortness of breath | 34 (34.69) | 34 (70.83) | 0 (0.00) | <0.0001 | 20 (51.28) | 14 (23.73) | 0.0050 |
| Headache | 6 (6.12) | 4 (8.33) | 2 (4.00) | 0.412 | 3 (7.69) | 3 (5.08) | 0.6796 # |
| High temperature | 0 (0.00) | 0 (0.00) | 0 (0.00) | - | 0 (0.00) | 0 (0.00) | - |
| Memory dysfunction | 30 (30.61) | 22 (45.83) | 8 (16.00) | 0.0014 | 30 (76.92) | 0 (0.00) | <0.0001 |
| Myalgia | 19 (19.39) | 14 (29.17) | 5 (10.00) | 0.0164 | 11 (28.21) | 8 (13.56) | 0.0726 |
| Nausea/vomiting | 1 (1.02) | 1 (2.08) | 0 (0.00) | 0.4898 # | 1 (2.56) | 0 (0.00) | 0.3980 # |
| Palpitations/tachycardia | 3 (3.06) | 3 (6.25) | 0 (0.00) | 0.1137 # | 3 (7.69) | 0 (0.00) | 0.0601 # |
| Persistent cough | 10 (10.20) | 10 (20.83) | 0 (0.00) | 0.0005 # | 3 (7.69) | 7 (11.86) | 0.7354 # |
| Persistent fatigue | 36 (36.73) | 38 (75.00) | 0 (0.00) | <0.0001 | 22 (56.41) | 14 (23.73) | 0.0010 |
| Sleep disorders | 22 (22.45) | 18 (37.5) | 4 (8.00) | 0.0005 | 15 (38.46) | 7 (11.86) | 0.0020 |
| Tingling and numbness | 13 (13.27) | 11 (22.92) | 2 (4.00) | 0.0058 | 10 (25.64) | 3 (5.08) | 0.0033 |
| Weight loss | 1 (1.02) | 1 (2.08) | 0 (0.00) | 0.4898 # | 0 (0.00) | 1 (1.69) | 1.000 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madè, A.; Piella, S.N.; Ranucci, M.; Gaetano, C.; Renna, L.V.; Cardani, R.; Spinetti, G.; Milani, V.; Martelli, F. LEF1-AS1 Deregulation in the Peripheral Blood of Patients with Persistent Post-COVID Symptoms. Int. J. Mol. Sci. 2025, 26, 4806. https://doi.org/10.3390/ijms26104806
Madè A, Piella SN, Ranucci M, Gaetano C, Renna LV, Cardani R, Spinetti G, Milani V, Martelli F. LEF1-AS1 Deregulation in the Peripheral Blood of Patients with Persistent Post-COVID Symptoms. International Journal of Molecular Sciences. 2025; 26(10):4806. https://doi.org/10.3390/ijms26104806
Chicago/Turabian StyleMadè, Alisia, Santiago Nicolas Piella, Marco Ranucci, Carlo Gaetano, Laura Valentina Renna, Rosanna Cardani, Gaia Spinetti, Valentina Milani, and Fabio Martelli. 2025. "LEF1-AS1 Deregulation in the Peripheral Blood of Patients with Persistent Post-COVID Symptoms" International Journal of Molecular Sciences 26, no. 10: 4806. https://doi.org/10.3390/ijms26104806
APA StyleMadè, A., Piella, S. N., Ranucci, M., Gaetano, C., Renna, L. V., Cardani, R., Spinetti, G., Milani, V., & Martelli, F. (2025). LEF1-AS1 Deregulation in the Peripheral Blood of Patients with Persistent Post-COVID Symptoms. International Journal of Molecular Sciences, 26(10), 4806. https://doi.org/10.3390/ijms26104806









