Fungal Bioactive Compounds as Emerging Therapeutic Options for Age-Related Neurodegenerative Disorders
Abstract
1. Introduction
2. Aging Mechanisms and Biomarkers
| Biological Mechanism | Biomarker | Reference |
|---|---|---|
| Genomic Instability | DNA damage accumulation, γ-H2AX foci, micronuclei formation | [11,12] |
| Telomere Attrition | Telomere length, TERT expression | [11,13] |
| Epigenetic Alterations | DNA methylation patterns (Epigenetic clocks), histone modifications | [14,15] |
| Mitochondrial Dysfunction | Mitochondrial DNA copy number, ATP production, ROS levels | [16,17,18,19] |
| Cellular Senescence | Senescence-associated β-gal, p16 INK4a, SASP factors | [3,20] |
| Chronic Inflammation | IL-6, TNF-α, CRP, NF-κB activation | [2,21,22,23] |
| Loss of Proteostasis | Amyloid-β (Aβ) aggregation, tau tangles, heat shock protein expression | [24,25] |
| Stem Cell Exhaustion | Decline in hematopoietic and mesenchymal stem cell markers (CD34+, CD133+) | [9,12,26] |
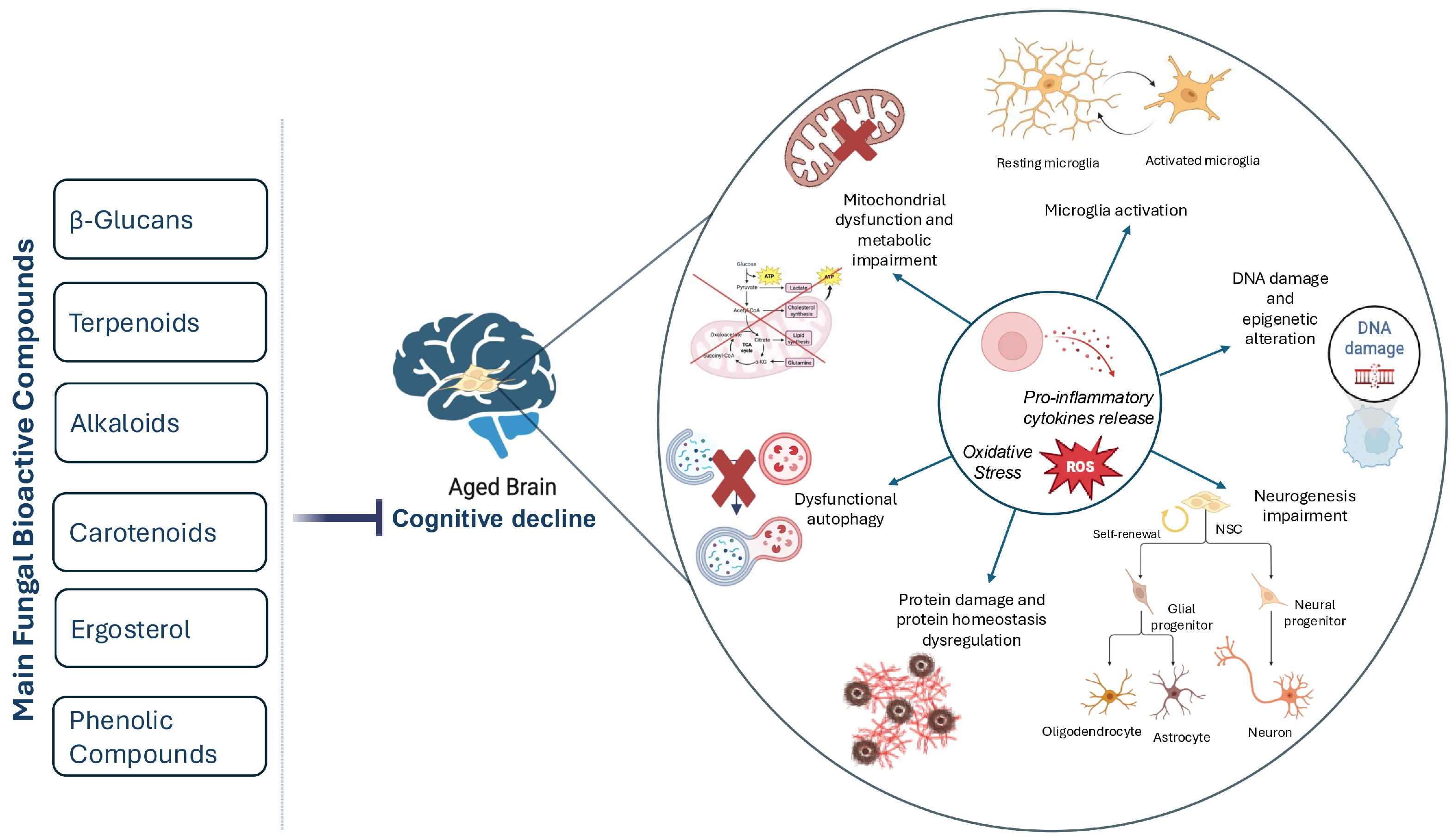
2.1. Neurodegenerative Process
2.2. Neural Stem Cells in Aging
2.3. Age-Related Neurodegenerative Diseases
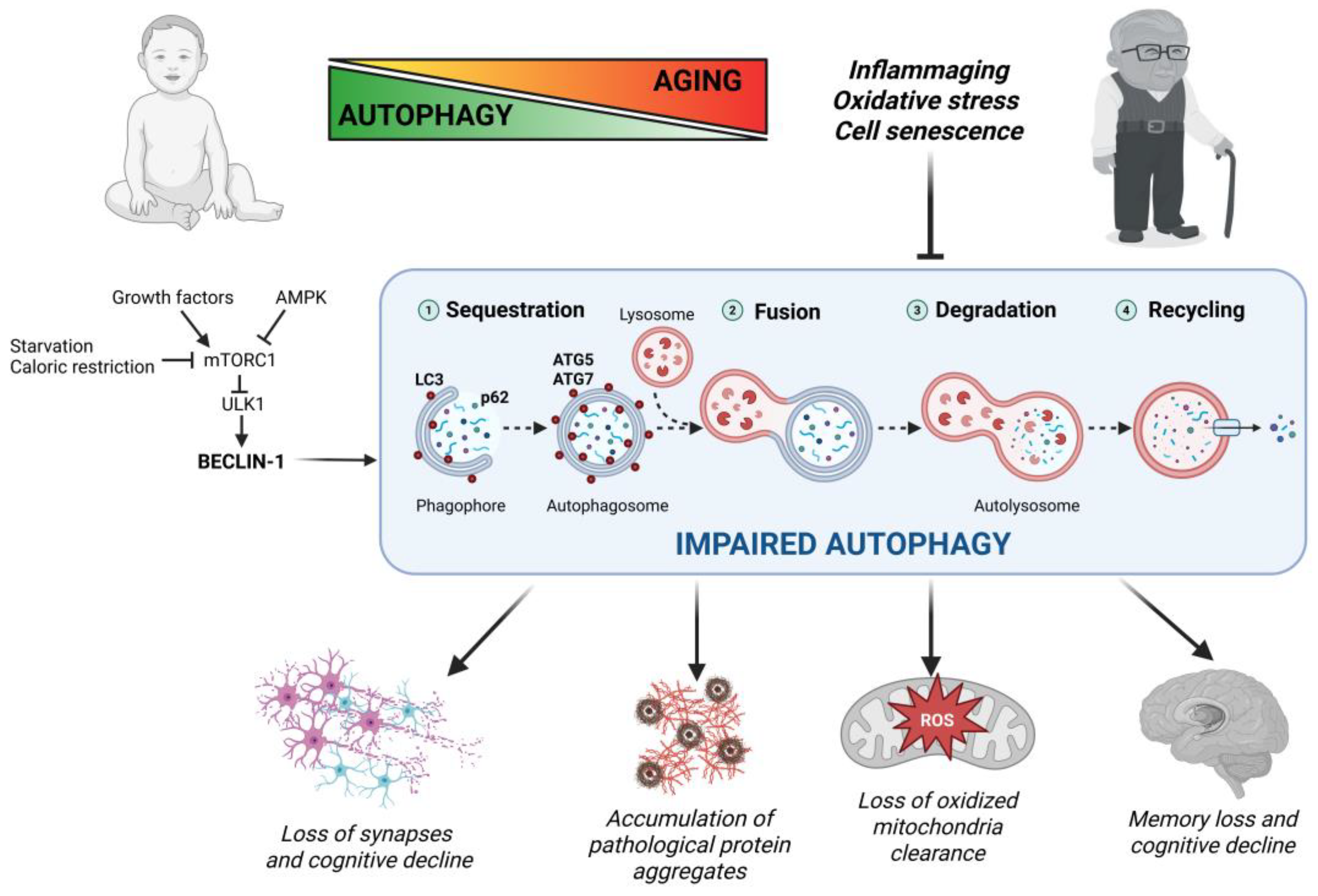
2.4. Autophagy in Aging and Age-Related Neurodegeneration
3. Fungal Bioactive Compound Involved in Neuroprotection
3.1. Antioxidant and Anti-Inflammatory Activity
3.2. Modulation of Neurogenic and Neurotransmitter Systems
3.3. Modulation of Mitochondrial Dynamics
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and aging-related diseases: From molecular mechanisms to interventions and treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Zhang, W.; Wang, Y.; Qian, P.; Huang, H. Inflammation and aging: Signaling pathways and intervention therapies. Signal Transduct. Target. Ther. 2023, 8, 239. [Google Scholar] [CrossRef]
- Liu, J.X.; Liu, X.G.; Wang, L.; Wu, F.; Yang, Z.W. Neuroprotective effects of ganoderic acid extract against epilepsy in primary hippocampal neurons. Res. Opin. Anim. Vet. Sci. 2013, 13, 420–425. [Google Scholar]
- Gao, Y.; Wei, Y.; Wang, Y.; Gao, F.; Chen, Z. Lycium barbarum: A traditional Chinese herb and a promising anti-aging agent. Aging Dis. 2017, 8, 778–791. [Google Scholar] [CrossRef] [PubMed]
- Kunugi, H.; Mohammed Ali, A. Royal jelly and its components promote healthy ageing and longevity: From animal models to humans. Int. J. Mol. Sci. 2019, 20, 4662. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Ganesan, K.; Xu, B. Unlocking the Power: New Insights into the Anti-Aging Properties of Mushrooms. J. Fungi 2024, 10, 215. [Google Scholar] [CrossRef]
- El Enshasy, H.A.; Hatti-Kaul, R. Mushroom immunomodulators: Unique molecules with unlimited applications. Trends Biotechnol. 2013, 31, 668–677. [Google Scholar] [CrossRef]
- Almadori, A.; Kalaskar, D.M. Stem Cell Applications in Rejuvenation. In Pancreas, Kidney and Skin Regeneration; Pham, P., Ed.; Stem Cells in Clinical Applications; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Blackburn, E.H.; Epel, E.S.; Lin, J. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science 2015, 350, 1193–1198. [Google Scholar] [CrossRef]
- Navarro Negredo, P.; Yeo, R.W.; Brunet, A. Aging and Rejuvenation of Neural Stem Cells and Their Niches. Cell Stem Cell 2020, 27, 202–223. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, A.L.; Kronmal, R.A.; Gardner, J.P.; Psaty, B.M.; Jenny, N.S.; Tracy, R.P.; Walston, J.; Kimura, M.; Aviv, A. Leukocyte telomere length and cardiovascular disease in the Cardiovascular Health Study. Am. J. Epidemiol. 2011, 170, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 2013, 14, R115. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, Z.; Wang, Z.; Shao, G.; Li, X. Epigenetic regulation in adult neural stem cells. Front. Cell Dev. Biol. 2024, 12, 1331074. [Google Scholar] [CrossRef]
- Bratic, A.; Larsson, N.G. The role of mitochondria in aging. J. Clin. Investig. 2013, 123, 951–957. [Google Scholar] [CrossRef]
- Hroudová, J.; Singh, N.; Fišar, Z. Mitochondrial dysfunctions in neurodegenerative diseases: Relevance to Alzheimer’s disease. Biomed. Res. Int. 2014, 2014, 175062. [Google Scholar] [CrossRef]
- Stoll, E.A.; Cheung, W.; Mikheev, A.M.; Sweet, I.R.; Bielas, J.H.; Zhang, J.; Rostomily, R.C.; Horner, P.J. Aging neural progenitor cells have decreased mitochondrial content and lower oxidative metabolism. J. Biol. Chem. 2011, 286, 38592–38601. [Google Scholar] [CrossRef]
- Coelho, P.; Fão, L.; Mota, S.; Rego, A.C. Mitochondrial function and dynamics in neural stem cells and neurogenesis: Implications for neurodegenerative diseases. Ageing Res. Rev. 2022, 80, 101667. [Google Scholar] [CrossRef]
- Sharpless, N.; Sherr, C. Erratum: Forging a signature of in vivo senescence. Nat. Rev. Cancer 2015, 15, 509. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune–metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- Jiménez Peinado, P.; Urbach, A. From Youthful Vigor to Aging Decline: Unravelling the Intrinsic and Extrinsic Determinants of Hippocampal Neural Stem Cell Aging. Cells 2023, 12, 2086. [Google Scholar] [CrossRef] [PubMed]
- Gaspar-Silva, F.; Trigo, D.; Magalhaes, J. Ageing in the brain: Mechanisms and rejuvenating strategies. Cell. Mol. Life Sci. 2023, 80, 190. [Google Scholar] [CrossRef] [PubMed]
- Hipp, M.S.; Kasturi, P.; Hartl, F.U. The proteostasis network and its decline in aging. Nat. Rev. Mol. Cell Biol. 2019, 20, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R.; Holtzman, D.M. Biomarker modeling of Alzheimer’s disease. Neuron 2019, 103, 191–203. [Google Scholar] [CrossRef]
- Goodell, M.A.; Rando, T.A. Stem cell aging: Mechanisms, regulators, and therapeutic opportunities. Cell Stem Cell 2015, 16, 601–612. [Google Scholar]
- Campisi, J.; Kapahi, P.; Lithgow, G.J.; Melov, S.; Newman, J.C.; Verdin, E. From discoveries in ageing research to therapeutics for healthy ageing. Nature 2019, 571, 183–192. [Google Scholar] [CrossRef]
- Palmer, J.E.; Wilson, N.; Son, S.M.; Obrocki, P.; Wrobel, L.; Rob, M.; Takla, M.; Korolchuk, V.I.; Rubinsztein, D.C. Autophagy, aging, and age-related neurodegeneration. Neuron 2025, 113, 29–48. [Google Scholar] [CrossRef]
- Bonetto, V.; Grilli, M. Neural stem cell-derived extracellular vesicles: Mini players with key roles in neurogenesis, immunomodulation, neuroprotection and aging. Front. Mol. Biosci. 2023, 10, 1187263. [Google Scholar] [CrossRef]
- Nicaise, A.M.; Willis, C.M.; Crocker, S.J.; Pluchino, S. Stem Cells of the Aging Brain. Front. Aging Neurosci. 2020, 12, 247. [Google Scholar] [CrossRef]
- Ruetz, T.J.; Pogson, A.N.; Kashiwagi, C.M.; Gagnon, S.D.; Morton, B.; Sun, E.D.; Na, J.; Yeo, R.W.; Leeman, D.S.; Morgens, D.W.; et al. CRISPR–Cas9 screens reveal regulators of ageing in neural stem cells. Nature 2024, 634, 1150–1159. [Google Scholar] [CrossRef]
- Youle, R.J.; van der Bliek, A.M. Mitochondrial fission, fusion, and stress. Science 2012, 337, 1062–1065. [Google Scholar] [CrossRef] [PubMed]
- Katsimpardi, L.; Litterman, N.K.; Schein, P.A.; Miller, C.M.; Loffredo, F.S.; Wojtkiewicz, G.R.; Chen, J.W.; Lee, R.T.; Wagers, A.J.; Rubin, L.L. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science 2014, 344, 630–634. [Google Scholar] [CrossRef]
- Somasundaram, I.; Jain, S.M.; Blot-Chabaud, M.; Pathak, S.; Banerjee, A.; Rawat, S.; Sharma, N.R.; Duttaroy, A.K. Mitochondrial dysfunction and its association with age-related disorders. Front. Physiol. 2024, 15, 1384966. [Google Scholar] [CrossRef]
- Hou, Y.; Dan, X.; Babbar, M.; Krishnamurthy, S.; Davila, A. Ageing as a risk factor for neurodegenerative diseases. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. Alzheimer’s disease: Genes, proteins, and therapy. Physiol. Rev. 2001, 81, 741–766. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef] [PubMed]
- Mosher, K.I.; Wyss-Coray, T. Microglial dysfunction in brain aging and Alzheimer’s disease. Biochem. Pharmacol. 2014, 88, 594–604. [Google Scholar] [CrossRef]
- Nelson, P.T.; Dickson, D.W.; Trojanowski, J.Q.; Jack, C.R.; Boyle, P.A.; Arfanakis, K.; Rademakers, R.; Alafuzoff, I.; Attems, J.; Brayne, C.; et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): Consensus working group report. Brain 2019, 142, 1503–1527, Erratum in Brain 2019, 142, e37. [Google Scholar] [CrossRef]
- Covarrubias, A.J.; Perrone, R.; Grozio, A.; Verdin, E. NAD+metabolism and its roles in cellular processes during ageing. Nat. Rev. Mol. Cell Biol. 2021, 22, 119–141. [Google Scholar] [CrossRef]
- Aman, Y.; Schmauck-Medina, T.; Hansen, M.; Morimoto, R.I.; Simon, A.K.; Bjedov, I.; Palikaras, K.; Simonsen, A.; Johansen, T.; Tavernarakis, N.; et al. Autophagy in healthy aging and disease. Nat. Aging 2021, 1, 634–650. [Google Scholar] [CrossRef]
- Magalhães, J.D.; Fão, L.; Vilaça, R.; Cardoso, S.M.; Rego, A.C. Macroautophagy and Mitophagy in Neurodegenerative Disorders: Focus on Therapeutic Interventions. Biomedicines 2021, 9, 1625. [Google Scholar] [CrossRef] [PubMed]
- Gerónimo-Olvera, C.; Massieu, L. Autophagy as a Homeostatic Mechanism in Response to Stress Conditions in the Central Nervous System. Mol. Neurobiol. 2019, 56, 6594–6608. [Google Scholar] [CrossRef] [PubMed]
- Leidal, A.M.; Levine, B.; Debnath, J. Autophagy and the cell biology of age-related disease. Nat. Cell Biol. 2018, 20, 1338–1348. [Google Scholar] [CrossRef]
- Cheon, S.Y.; Kim, H.; Rubinsztein, D.C.; Lee, J.E. Autophagy, cellular aging and age-related human diseases. Exp. Neurobiol. 2019, 28, 643–657. [Google Scholar] [CrossRef]
- Tóth, M.L.; Sigmond, T.; Borsos, E.; Barna, J.; Erdélyi, P.; Takács-Vellai, K.; Orosz, L.; Kovács, A.L.; Csikós, G.; Sass, M.; et al. Longevity pathways converge on autophagy genes to regulate life span in Caenorhabditis elegans. Autophagy 2008, 4, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Pyo, J.O.; Yoo, S.M.; Ahn, H.H.; Nah, J.; Hong, S.H.; Kam, T.I.; Jung, S.; Jung, Y.K. Overexpression of Atg5 in mice activates autophagy and extends lifespan. Nat. Commun. 2013, 4, 2300. [Google Scholar] [CrossRef]
- Fernandez, A.F.; Sebti, S.; Wei, Y.; Zou, Z.; Shi, M.; McMillan, K.L.; He, C.; Ting, T.; Liu, Y.; Chiang, W.C.; et al. Disruption of the beclin 1–BCL2 autophagy regulatory complex promotes longevity in mice. Nature 2018, 558, 136–140. [Google Scholar] [CrossRef]
- Liang, W.; Moyzis, A.G.; Lampert, M.A.; Diao, R.Y.; Najor, R.H.; Gustafsson, Å.B. Aging is associated with a decline in Atg9b-mediated autophagosome formation and appearance of enlarged mitochondria in the heart. Aging Cell 2020, 19, e13187. [Google Scholar] [CrossRef]
- Cuervo, A.M.; Dice, J.F. How do intracellular proteolytic systems change with age? Front. Biosci. 1998, 3, D25–D43. [Google Scholar]
- Burrinha, T.; Cunha, C.; Hall, M.J.; Lopes-da-Silva, M.; Seabra, M.C.; Guimas Almeida, C. Deacidification of endolysosomes by neuronal aging drives synapse loss. Traffic 2023, 24, 334–354. [Google Scholar] [CrossRef]
- Tsong, H.; Holzbaur, E.L.; Stavoe, A.K. Aging Differentially Affects Axonal Autophagosome Formation and Maturation. Autophagy 2023, 19, 3079–3095. [Google Scholar] [CrossRef]
- Ott, C.; Konig, J.; Hohn, A.; Jung, T.; Grune, T. Macroautophagy is impaired in old murine brain tissue as well as in senescent human fibroblasts. Redox Biol. 2016, 10, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Triplett, J.C.; Tramutola, A.; Swomley, A.; Kirk, J.; Grimes, K.; Lewis, K.; Orr, M.; Rodriguez, K.; Cai, J.; Klein, J.B.; et al. Age-related changes in the proteostasis network in the brain of the naked mole-rat: Implications promoting healthy longevity. Biochim. Biophys. Acta 2015, 1852, 2213–2224. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Feng, L.; Li, J.; Lan, X.; A, L.; Lv, X.; Zhang, M.; Chen, L. The alteration of autophagy and apoptosis in the hippocampus of rats with natural aging-dependent cognitive deficits. Behav. Brain Res. 2017, 334, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Pareja-Cajiao, M.; Gransee, H.M.; Stowe, J.M.; Rana, S.; Sieck, G.C.; Mantilla, C.B. Age-related impairment of autophagy in cervical motor neurons. Exp. Gerontol. 2021, 144, 111193. [Google Scholar] [CrossRef]
- De Risi, M.; Torromino, G.; Tufano, M.; Moriceau, S.; Pignataro, A.; Rivagorda, M.; Carrano, N.; Middei, S.; Settembre, C.; Ammassari-Teule, M.; et al. Mechanisms by which autophagy regulates memory capacity in ageing. Aging Cell 2020, 19, e13189. [Google Scholar] [CrossRef]
- Glatigny, M.; Moriceau, S.; Rivagorda, M.; Ramos-Brossier, M.; Nascimbeni, A.C.; Lante, F.; Shanley, M.R.; Boudarene, N.; Rousseaud, A.; Friedman, A.K.; et al. Autophagy Is Required for Memory Formation and Reverses Age-Related Memory Decline. Curr. Biol. 2019, 29, 435–448.e8. [Google Scholar] [CrossRef]
- Metaxakis, A.; Ploumi, C.; Tavernarakis, N. Autophagy in Age-Associated Neurodegeneration. Cells 2018, 7, 37. [Google Scholar] [CrossRef]
- Hamano, T.; Gendron, T.F.; Causevic, E.; Yen, S.H.; Lin, W.L.; Isidoro, C.; Deture, M.; Ko, L.W. Autophagic-lysosomal perturbation enhances tau aggregation in transfectants with induced wild-type tau expression. Eur. J. Neurosci. 2008, 27, 1119–1130. [Google Scholar] [CrossRef]
- Piras, A.; Collin, L.; Gruninger, F.; Graff, C.; Rönnbäck, A. Autophagic and lysosomal defects in human tauopathies: Analysis of postmortem brain from patients with familial Alzheimer disease, corticobasal degeneration and progressive supranuclear palsy. Acta Neuropathol. Commun. 2016, 4, 22. [Google Scholar] [CrossRef]
- Vidoni, C.; Castiglioni, A.; Seca, C.; Secomandi, E.; Melone, M.A.; Isidoro, C. Dopamine exacerbates mutant Huntingtin toxicity via oxidative-mediated inhibition of autophagy in SH-SY5Y neuroblastoma cells: Beneficial effects of anti-oxidant therapeutics. Neurochem. Int. 2016, 101, 132–143. [Google Scholar] [CrossRef]
- Vidoni, C.; Secomandi, E.; Castiglioni, A.; Melone, M.A.B.; Isidoro, C. Resveratrol protects neuronal-like cells expressing mutant Huntingtin from dopamine toxicity by rescuing ATG4-mediated autophagosome formation. Neurochem. Int. 2018, 117, 174–187. [Google Scholar] [CrossRef]
- Sakurai, M.; Kuwahara, T. Canonical and noncanonical autophagy: Involvement in Parkinson’s disease. Front. Cell Dev. Biol. 2025, 13, 1518991. [Google Scholar] [CrossRef]
- Isidoro, C.; Biagioni, F.; Giorgi, F.S.; Fulceri, F.; Paparelli, A.; Fornai, F. The role of autophagy on the survival of dopamine neurons. Curr. Top. Med. Chem. 2009, 9, 869–879. [Google Scholar] [PubMed]
- Shao, N.; Lu, Q.; Ouyang, Z.; Yang, P.; Wei, T.; Wang, J.; Cai, B. Ganoderic acid a alleviates Aβ25-35-induced HT22 cell apoptosis through the ERK/MAPK pathway: A system pharmacology and in vitro experimental validation. Metab. Brain Dis. 2024, 40, 51. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.F.; Liu, S.; Liu, Y.C.; Li, P.; Xu, X. Ganoderic Acid A Promotes Amyloid-β Clearance (In Vitro) and Ameliorates Cognitive Deficiency in Alzheimer’s Disease (Mouse Model) through Autophagy Induced by Activating Axl. Int. J. Mol. Sci. 2021, 22, 5559. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.L.; Wang, C.D.; Wang, T.; Ding, H.; Zhou, M.; Yang, N.; Liu, Y.Y.; Chan, P. Ganoderma lucidum extract ameliorates MPTP-induced parkinsonism and protects dopaminergic neurons from oxidative stress via regulating mitochondrial function, autophagy, and apoptosis. Acta Pharmacol. Sin. 2019, 40, 441–450. [Google Scholar] [CrossRef]
- Phan, C.W.; Lee, G.S.; Hong, S.L.; Wong, Y.T.; Brkljača, R.; Urban, S.; Abd Malek, S.N.; Sabaratnam, V. Hericium erinaceus (Bull.: Fr) Pers. cultivated under tropical conditions: Isolation of hericenones and demonstration of NGF-mediated neurite outgrowth in PC12 cells via MEK/ERK and PI3K-Akt signaling pathways. Food Funct. 2014, 5, 3160–3169. [Google Scholar] [CrossRef]
- Wu, Y.L.; Sun, H.L.; Chang, J.C.; Lin, W.Y.; Chen, P.Y.; Chen, C.C.; Lee, L.Y.; Li, C.C.; Hsieh, M.; Chen, H.W.; et al. Erinacine A-Enriched Hericium erinaceus Mycelium Ethanol Extract Lessens Cellular Damage in Cell and Drosophila Models of Spinocerebellar Ataxia Type 3 by Improvement of Nrf2 Activation. Antioxidants 2024, 13, 1495. [Google Scholar] [CrossRef]
- Tsai-Teng, T.; Chin-Chu, C.; Li-Ya, L.; Wan-Ping, C.; Chung-Kuang, L.; Chien-Chang, S.; Chi-Ying, H.F.; Chien-Chih, C.; Shiao, Y.J. Erinacine A-enriched Hericium erinaceus mycelium ameliorates Alzheimer’s disease-related pathologies in APPswe/PS1dE9 transgenic mice. J. Biomed. Sci. 2016, 23, 49. [Google Scholar] [CrossRef]
- Li, I.C.; Chang, H.H.; Lin, C.H.; Chen, W.P.; Lu, T.H.; Lee, L.Y.; Chen, Y.W.; Chen, Y.P.; Chen, C.C.; Lin, D.P. Prevention of Early Alzheimer’s Disease by Erinacine A-Enriched Hericium erinaceus Mycelia Pilot Double-Blind Placebo-Controlled Study. Front. Aging Neurosci. 2020, 12, 155. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Bai, M.; Xue, X.B.; Zou, C.X.; Huang, X.X.; Song, S.J. Isolation of chemical compositions as dietary antioxidant supplements and neuroprotectants from Chaga mushroom (Inonotus obliquus). Food Biosci. 2022, 47, 101623. [Google Scholar] [CrossRef]
- Xin, Y.; Zhang, Y.; Zhang, X. Protective Effects of Chaga Medicinal Mushroom, Inonotus obliquus (Agaricomycetes), Extract on β-Amyloid-Induced Neurotoxicity in PC12 Cells and Aging Rats: In Vitro and In Vivo Studies. Int. J. Med. Mushrooms 2021, 23, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Gong, S.; Meng, L.; Yao, W.; Du, K.; Jiao, L.; Ma, G.; Liang, J.; Wei, B.; Jin, X.; et al. Cordycepin Modulates Microglial M2 Polarization Coupled with Mitochondrial Metabolic Reprogramming by Targeting HKII and PDK2. Adv. Sci. 2024, 11, e2304687. [Google Scholar] [CrossRef]
- Cheng, C.; Zhu, X. Cordycepin mitigates MPTP-induced Parkinson’s disease through inhibiting TLR/NF-κB signaling pathway. Life Sci. 2019, 223, 120–127. [Google Scholar] [CrossRef]
- Li, W.; Wan, P.; Qiao, J.; Liu, Y.; Peng, Q.; Zhang, Z.; Shu, X.; Xia, Y.; Sun, B. Current and further outlook on the protective potential of Antrodia camphorata against neurological disorders. Front. Pharmacol. 2024, 15, 1372110. [Google Scholar] [CrossRef]
- Wang, L.C.; Wang, S.E.; Wang, J.J.; Tsai, T.Y.; Lin, C.H.; Pan, T.M.; Lee, C.L. In vitro and in vivo comparisons of the effects of the fruiting body and mycelium of Antrodia camphorata against amyloid β-protein-induced neurotoxicity and memory impairment. Appl. Microbiol. Biotechnol. 2012, 94, 1505–1519. [Google Scholar] [CrossRef]
- Francesca, F.; Caitlin, A.; Sarah, L.; Robyn, G.L. Antroquinonol administration in animal preclinical studies for Alzheimer’s disease (AD): A new avenue for modifying progression of AD pathophysiology. Brain Behav. Immun. Health 2022, 21, 100435. [Google Scholar] [CrossRef]
- Chang, W.H.; Chen, M.C.; Cheng, I.H. Antroquinonol Lowers Brain Amyloid-β Levels and Improves Spatial Learning and Memory in a Transgenic Mouse Model of Alzheimer’s Disease. Sci. Rep. 2015, 5, 15067. [Google Scholar] [CrossRef]
- Liu, L.; Shi, X.W.; Zong, S.C.; Tang, J.J.; Gao, J.M. Scabronine M, a novel inhibitor of NGF-induced neurite outgrowth from PC12 cells from the fungus Sarcodon scabrosus. Bioorg. Med. Chem. Lett. 2012, 22, 2401–2406. [Google Scholar] [CrossRef]
- Kou, R.W.; Du, S.T.; Li, Y.X.; Yan, X.T.; Zhang, Q.; Cao, C.Y.; Yin, X.; Gao, J.M. Cyathane diterpenoids and drimane sesquiterpenoids with neurotrophic activity from cultures of the fungus Cyathus africanus. J. Antibiot. 2019, 72, 15–21. [Google Scholar] [CrossRef]
- Wei, J.; Cheng, Y.; Guo, W.H.; Wang, D.C.; Zhang, Q.; Li, D.; Rong, J.; Gao, J.M. Molecular Diversity and Potential Anti-neuroinflammatory Activities of Cyathane Diterpenoids from the Basidiomycete Cyathus africanus. Sci. Rep. 2017, 7, 8883. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-W.; Lin, P.-Y.; Hsu, Y.-W.; Pan, T.-M.; Lee, C.-L. Monascus-Fermented Metabolites Repressed Amyloid β-Peptide-Induced Neurotoxicity and Inflammatory Response in In Vitro and In Vivo Studies. J. Funct. Foods 2023, 104, 105509. [Google Scholar] [CrossRef]
- Lee, C.-L.; Lin, P.-Y.; Hsu, Y.-W.; Pan, T.-M. Monascus-Fermented Monascin and Ankaflavin Improve the Memory and Learning Ability in Amyloid β-Protein Intracerebroventricular-Infused Rat via the Suppression of Alzheimer’s Disease Risk Factors. J. Funct. Foods 2015, 18 Pt A, 387–399. [Google Scholar] [CrossRef]
- Nallathamby, N.; Guan-Serm, L.; Vidyadaran, S.; Abd Malek, S.N.; Raman, J.; Sabaratnam, V. Ergosterol of Cordyceps militaris Attenuates LPS-Induced Inflammation in BV2 Microglia Cells. Nat. Prod. Commun. 2015, 10, 885–886. [Google Scholar] [CrossRef] [PubMed]
- Sillapachaiyaporn, C.; Wongwan, C.; Mongkolpobsin, K.; Nilkhet, S.; Isidoro, C.; Chuchawankul, S.; Tencomnao, T. Ergosterol promotes neurite outgrowth, inhibits amyloid-beta synthesis, and extends longevity: In vitro neuroblastoma and in vivo Caenorhabditis elegans evidence. Life Sci. 2024, 345, 122606. [Google Scholar] [CrossRef] [PubMed]
- Rangsinth, P.; Sharika, R.; Pattarachotanant, N.; Duangjan, C.; Wongwan, C.; Sillapachaiyaporn, C.; Nilkhet, S.; Wongsirojkul, N.; Prasansuklab, A.; Tencomnao, T.; et al. Potential Beneficial Effects and Pharmacological Properties of Ergosterol, a Common Bioactive Compound in Edible Mushrooms. Foods 2023, 12, 2529. [Google Scholar] [CrossRef]
- Miller, A.P.; Hornero-Méndez, D.; Bandara, S.; Parra-Rivero, O.; Limón, M.C.; Von Lintig, J.; Avalos, J.; Amengual, J. Bioavailability and Provitamin A Activity of Neurosporaxanthin in Mice. Commun. Biol. 2023, 6, 1068. [Google Scholar] [CrossRef]
- Lian, W.; Yang, X.; Duan, Q.; Li, J.; Zhao, Y.; Yu, C.; He, T.; Sun, T.; Zhao, Y.; Wang, W. The Biological Activity of Ganoderma lucidum on Neurodegenerative Diseases: The Interplay between Different Active Compounds and the Pathological Hallmarks. Molecules 2024, 29, 2516. [Google Scholar] [CrossRef]
- Gao, J.M. Neuroprotective effects of a new triterpenoid from edible mushroom on oxidative stress and apoptosis through the BDNF/TrkB/ERK/CREB and Nrf2 signaling pathway in vitro and in vivo. Food Funct. 2022, 13, 12121–12134. [Google Scholar] [CrossRef]
- Rousta, N.; Aslan, M.; Akbas, M.Y.; Ozcan, F.; Sar, T.; Taherzadeh, M.J. Effects of fungal-based bioactive compounds on human health: Review paper. Crit. Rev. Food Sci. Nutr. 2024, 64, 7004–7027. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Li, G.; Sun, Y.; Niu, Y.; Ma, L.; He, B.; Ai, M.; Han, J.; Zeng, B. Gene transcription profiling of Aspergillus oryzae 3.042 treated with ergosterol biosynthesis inhibitors. Braz. J. Microbiol. 2019, 50, 43–52. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Shi, W.; Liu, X.; Zhao, X.; Zhang, Z. Anticancer Action and Mechanism of Ergosterol Peroxide from Paecilomyces cicadae Fermentation Broth. Int. J. Mol. Sci. 2018, 19, 3935. [Google Scholar] [CrossRef]
- Nowak, R.; Nowacka-Jechalke, N.; Pietrzak, W.; Gawlik-Dziki, U. A new look at edible and medicinal mushrooms as a source of ergosterol and ergosterol peroxide—UHPLC-MS/MS analysis. Food Chem. 2022, 369, 130927. [Google Scholar] [CrossRef]
- Kobori, M.; Yoshida, M.; Ohnishi-Kameyama, M.; Shinmoto, H. Ergosterol peroxide from an edible mushroom suppresses inflammatory responses in RAW264.7 macrophages and growth of HT29 colon adenocarcinoma cells. Br. J. Pharmacol. 2007, 150, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Al-Rabia, M.W.; Mohamed, G.A.; Ibrahim, S.R.M.; Asfour, H.Z. Anti-inflammatory ergosterol derivatives from the endophytic fungus Fusarium chlamydosporum. Nat. Prod. Res. 2021, 35, 5011–5020. [Google Scholar] [CrossRef]
- Chen, S.; Yong, T.; Zhang, Y.; Su, J.; Jiao, C.; Xie, Y. Anti-tumor and Anti-angiogenic Ergosterols from Ganoderma lucidum. Front. Chem. 2017, 5, 85. [Google Scholar] [CrossRef]
- Xia, T.; Lei, H.; Wang, J.; He, Y.; Wang, H.; Gao, L.; Qi, T.; Xiong, X.; Liu, L.; Zhu, Y. Identification of an ergosterol derivative with anti-melanoma effect from the sponge-derived fungus Pestalotiopsis sp. XWS03F09. Front. Microbiol. 2022, 13, 1008053. [Google Scholar] [CrossRef]
- Ofir, R. Plants and fungi metabolites as novel autophagy inducers and senescence inhibitors. Explor. Drug Sci. 2024, 2, 361–368. [Google Scholar] [CrossRef]
- Liuzzi, G.M.; Petraglia, T.; Latronico, T.; Crescenzi, A.; Rossano, R. Antioxidant Compounds from Edible Mushrooms as Potential Candidates for Treating Age-Related Neurodegenerative Diseases. Nutrients 2023, 15, 1913. [Google Scholar] [CrossRef]
- Song, T.Y.; Lin, H.C.; Chen, C.L.; Wu, J.H.; Liao, J.W.; Hu, M.L. Ergothioneine and melatonin attenuate oxidative stress and protect against learning and memory deficits in C57BL/6J mice treated with D-galactose. Free Radic. Res. 2014, 48, 1049–1060. [Google Scholar] [CrossRef]
- Virmani, M.A.; Cirulli, M. The Role of l-Carnitine in Mitochondria, Prevention of Metabolic Inflexibility and Disease Initiation. Int. J. Mol. Sci. 2022, 23, 2717. [Google Scholar] [CrossRef] [PubMed]
- Ribas, G.S.; Vargas, C.R.; Wajner, M. L-carnitine supplementation as a potential antioxidant therapy for inherited neurometabolic disorders. Gene 2014, 533, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Yurchenko, E.A.; Menchinskaya, E.S.; Pislyagin, E.A.; Trinh, P.T.H.; Ivanets, E.V.; Smetanina, O.F.; Yurchenko, A.N. Neuroprotective Activity of Some Marine Fungal Metabolites in the 6-Hydroxydopamin- and Paraquat-Induced Parkinson’s Disease Models. Mar. Drugs 2018, 16, 457. [Google Scholar] [CrossRef]
- Sharma, H.; Sharma, N.; An, S.S.A. Unique Bioactives from Zombie Fungus (Cordyceps) as Promising Multitargeted Neuroprotective Agents. Nutrients 2024, 16, 102. [Google Scholar] [CrossRef]
- Shi, Y.; Shi, Y.; Jie, R.; He, J.; Luo, Z.; Li, J. Vitamin D: The crucial neuroprotective factor for nerve cells. Neuroscience 2024, 560, 272–285. [Google Scholar] [CrossRef]
- Ohta, T.; Kita, T.; Kobayashi, N.; Obara, Y.; Nakahata, N.; Ohizumi, Y.; Takaya, Y.; Oshima, Y. Scabronine A, a novel diterpenoid having potent inductive activity of the nerve growth factor synthesis, isolated from the mushroom, Sarcodon scabrosus. Tetrahedron Lett. 1998, 39, 6229–6232. [Google Scholar] [CrossRef]
- Alharbi, B.; Al-kuraishy, H.M.; Al-Gareeb, A.I.; Elekhnawy, E.; Alharbi, H.; Alexiou, A.; Papadakis, M.; Batiha, G.E. Role of GABA pathway in motor and non-motor symptoms in Parkinson’s disease: A bidirectional circuit. Eur. J. Med. Res. 2024, 29, 205. [Google Scholar] [CrossRef]
- Bai, X.; Tan, T.Y.; Li, Y.X.; Li, Y.; Chen, Y.F.; Ma, R.; Wang, S.Y.; Li, Q.; Liu, Z.Q. The protective effect of Cordyceps sinensis extract on cerebral ischemic injury via modulating the mitochondrial respiratory chain and inhibiting the mitochondrial apoptotic pathway. Biomed. Pharmacother. 2020, 124, 109834. [Google Scholar] [CrossRef]
- Jiang, Z.M.; Qiu, H.B.; Wang, S.Q.; Guo, J.; Yang, Z.W.; Zhou, S.B. Ganoderic acid A potentiates the antioxidant effect and protection of mitochondrial membranes and reduces the apoptosis rate in primary hippocampal neurons in magnesium free medium. Pharmazie 2018, 73, 87–91. [Google Scholar]
- Peng, H.H.; Wu, C.Y.; Hsiao, Y.C.; Martel, J.; Ke, P.Y.; Chiu, C.Y.; Liau, J.C.; Chang, I.T.; Su, Y.H.; Ko, Y.F.; et al. Ganoderma lucidum stimulates autophagy-dependent longevity pathways in Caenorhabditis elegans and human cells. Aging 2021, 13, 13474–13495. [Google Scholar] [CrossRef] [PubMed]
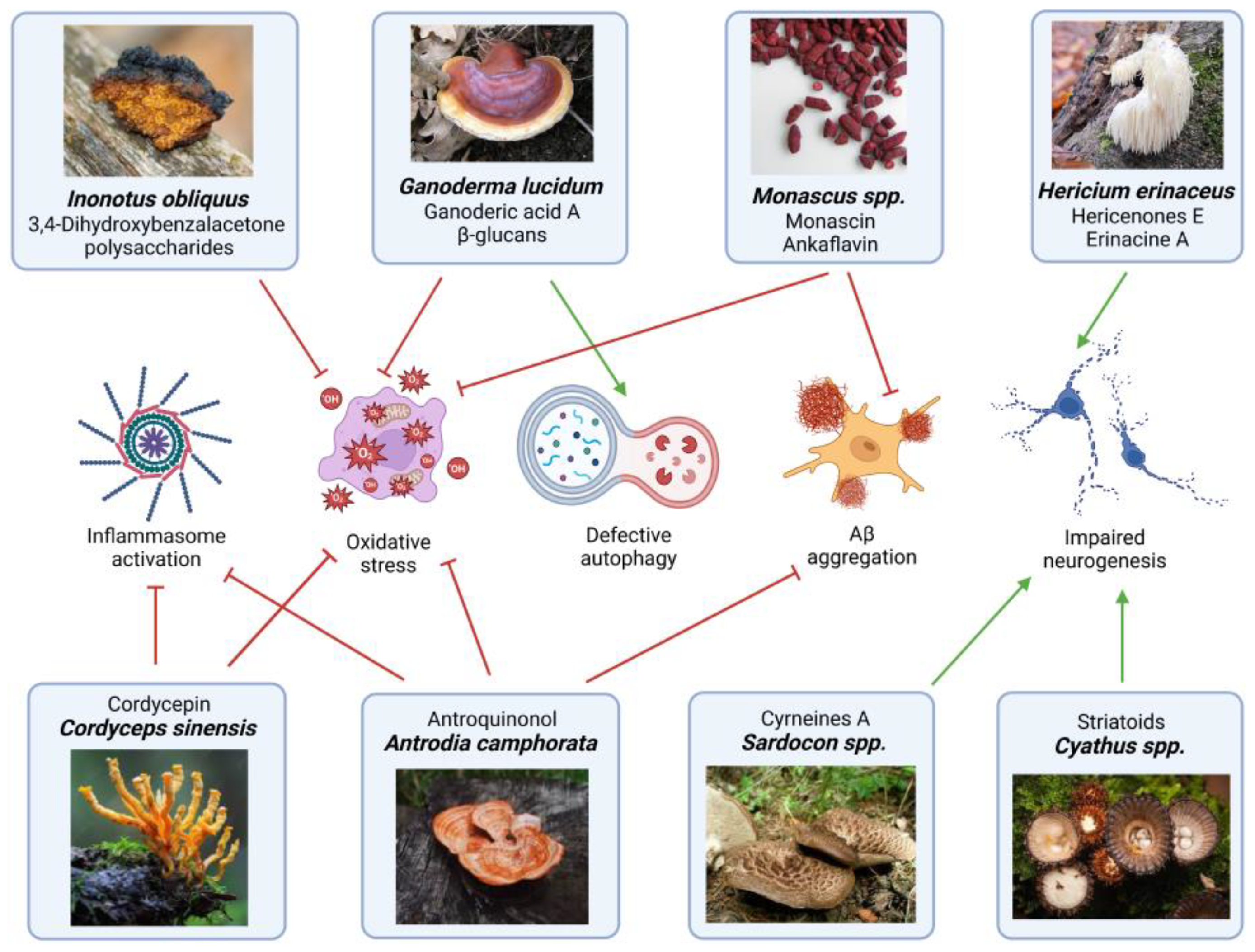
| Fungal Source | Main Molecules Extracted | Extraction Method | Effects and Bioactivities | Concentrations | Experimental Validation | References |
|---|---|---|---|---|---|---|
| Ganoderma lucidum (Curtis) P.Karst. known as Reishi | Ganoderic acid A; β-glucans | Ethanol extraction (basidiome); hot-water extraction (polysaccharides) |
| Ganoderic acid A: IC50 ≈ 25–35 μM; β-glucans EC50 ≈ 150 μg/mL | In vitro: HT22 BV2 In vivo: MPTP-lesioned mice, Aβ-injected C57BL/6 J mice | [66,67,68] |
| Hericium erinaceus (Bull.) Person, known as Lion’s Mane | Hericenones E; Erinacine A | Methanol extraction (basidiome); ethanol extraction (mycelium) |
| Hericenone E: active ≥ 10 μM; Erinacine A: EC50 ≈ 2.0 μg/mL | In vitro: PC12 cell line In vivo: rat model 3-AP–induced cerebellar ataxia; APP/PS1 mice Clinical: Mild AD trial with EAHE (NCT04065061) | [69,70,71,72] |
| Inonotus obliquus (Fr.) Pilat, known as Chaga | 3,4-Dihydroxybenzalacetone; polysaccharides | Ethyl acetate extraction; hot-water extraction |
| 3,4-Dihydroxybenzalacetone: IC50 ≈ 12.5 μM | In vitro: PC12; SH-SY5Y cell lines In vivo: Aβ-injected rats | [73,74] |
| Cordyceps sinensis (Bertk.) Sacc. known as Zombie Fungus | Cordycepin | Ethanol or Methanol extraction; silica-gel chromatography |
| Cordycepin: IC50 ≈ 20–30 μM | In vitro: BV2 and SH-SY5Y; PC12 In vivo: APP/PS1 mice, MPTP-lesioned mice | [75,76] |
| Antrodia camphorate (Chang and Chou) | Antroquinonol | Ethanol extraction; HPLC purification |
| Antroquinonol: IC50 ≈ 1.2 μM | In vitro: PC12 cell line In vivo: 3xTg mice; APP mice | [77,78,79,80] |
| Sarcodon spp. | Cyrneines A | Acetone/methanol extraction; silica-gel chromatography |
| Cyrneine A: EC50 ≈ 1.0 μM | In vitro: PC12 cell line | [81] |
| Cyathus spp. | Striatoids | Chloroform/methanol extraction; silica-gel purification |
| Striatoid EC50 ≈ 2.5–5 μM | In vitro: PC12 cell line; BV2 cells | [82,83] |
| Monascus spp. | Monascin; Ankaflavin | Solid-state fermentation on rice; ethyl acetate extraction |
| Monascin: IC50 ≈ 25 μM; Ankaflavin: IC50 value of 15 microg/mL | In vitro: PC-12 cells In vivo: Sprague Dawley rats | [84,85] |
| Various Fungi | Ergosterol | Lipid extraction (chloroform:methanol); crystallization |
| Ergosterol: active ≥ 20 μM | In vitro: BV2 cell line; Neuro2a cell line In vivo: Caenorhabditis elegans | [86,87,88] |
| Neurospora crassa (Shear & Dodge) and Fusarium fujikuroi (J. Shld.) | Neurosporaxanthin; β-carotene | Solvent extraction (acetone, ethanol); mechanical cell disruption; HPLC purification |
| EC50 ≈ 5–10 μM | In vivo: C57BL/6 wild-type | [89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonetto, V.; Ferraresi, A.; Sampò, S.; Isidoro, C. Fungal Bioactive Compounds as Emerging Therapeutic Options for Age-Related Neurodegenerative Disorders. Int. J. Mol. Sci. 2025, 26, 4800. https://doi.org/10.3390/ijms26104800
Bonetto V, Ferraresi A, Sampò S, Isidoro C. Fungal Bioactive Compounds as Emerging Therapeutic Options for Age-Related Neurodegenerative Disorders. International Journal of Molecular Sciences. 2025; 26(10):4800. https://doi.org/10.3390/ijms26104800
Chicago/Turabian StyleBonetto, Valentina, Alessandra Ferraresi, Simonetta Sampò, and Ciro Isidoro. 2025. "Fungal Bioactive Compounds as Emerging Therapeutic Options for Age-Related Neurodegenerative Disorders" International Journal of Molecular Sciences 26, no. 10: 4800. https://doi.org/10.3390/ijms26104800
APA StyleBonetto, V., Ferraresi, A., Sampò, S., & Isidoro, C. (2025). Fungal Bioactive Compounds as Emerging Therapeutic Options for Age-Related Neurodegenerative Disorders. International Journal of Molecular Sciences, 26(10), 4800. https://doi.org/10.3390/ijms26104800








