Fatty Acid Metabolism Regulators Have Pivotal Roles in the Pathogenesis of Ovarian Carcinoma
Abstract
1. Introduction
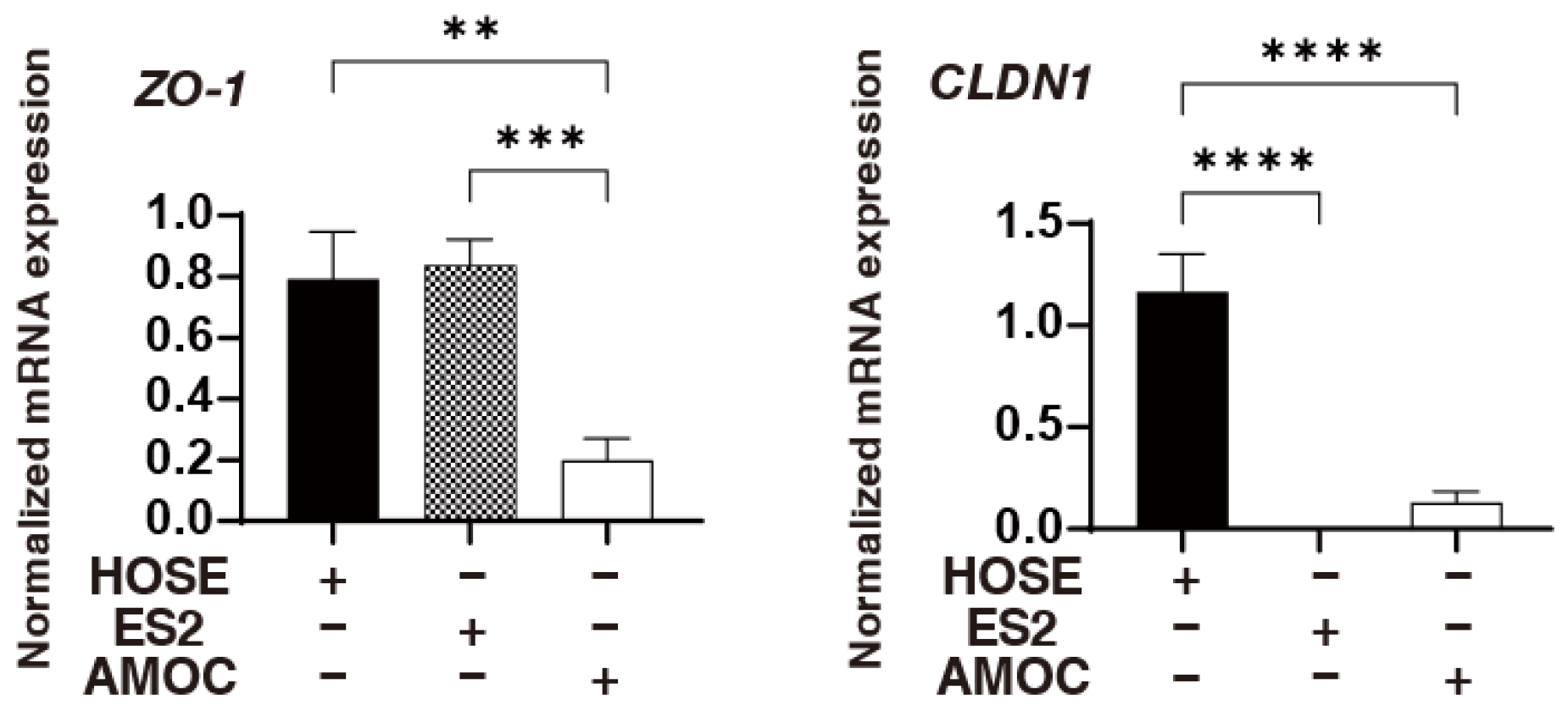
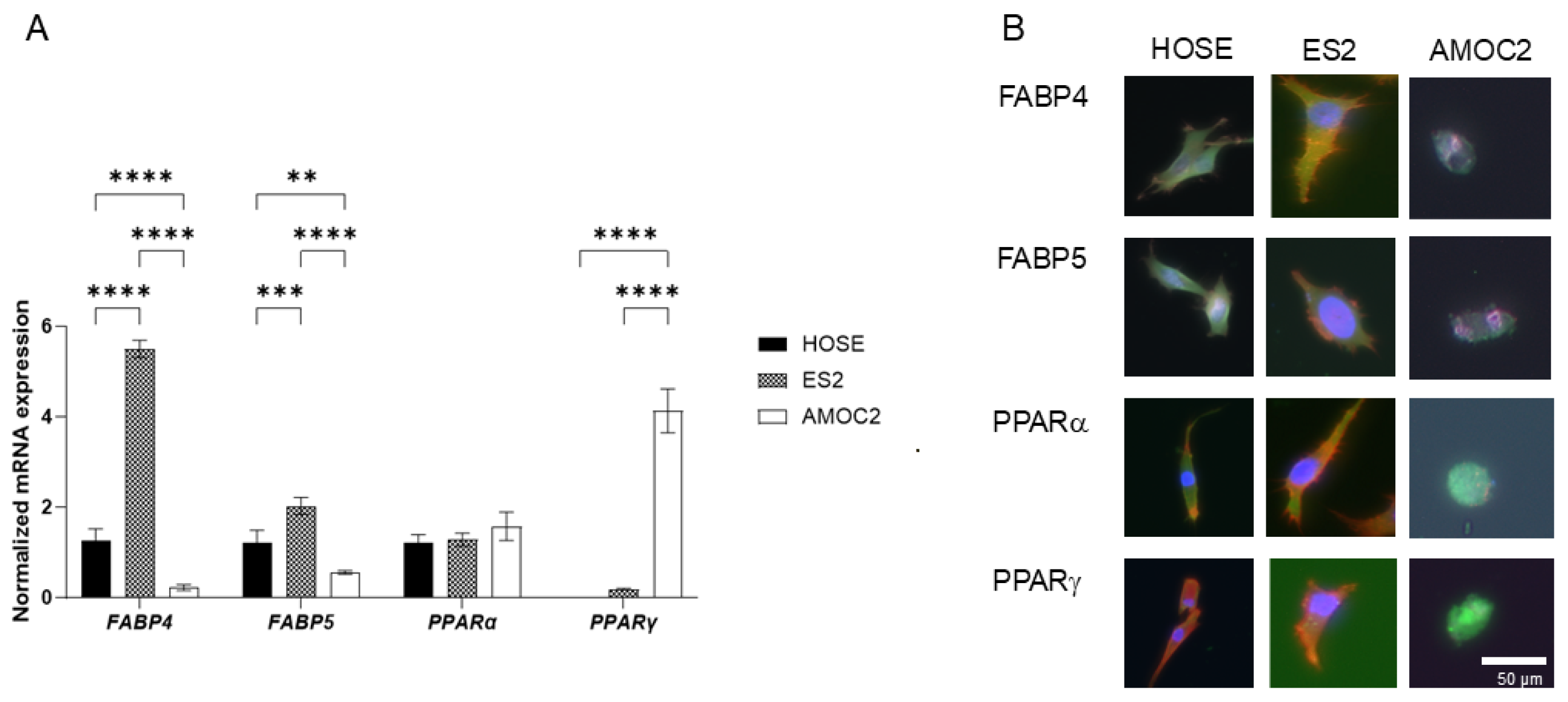
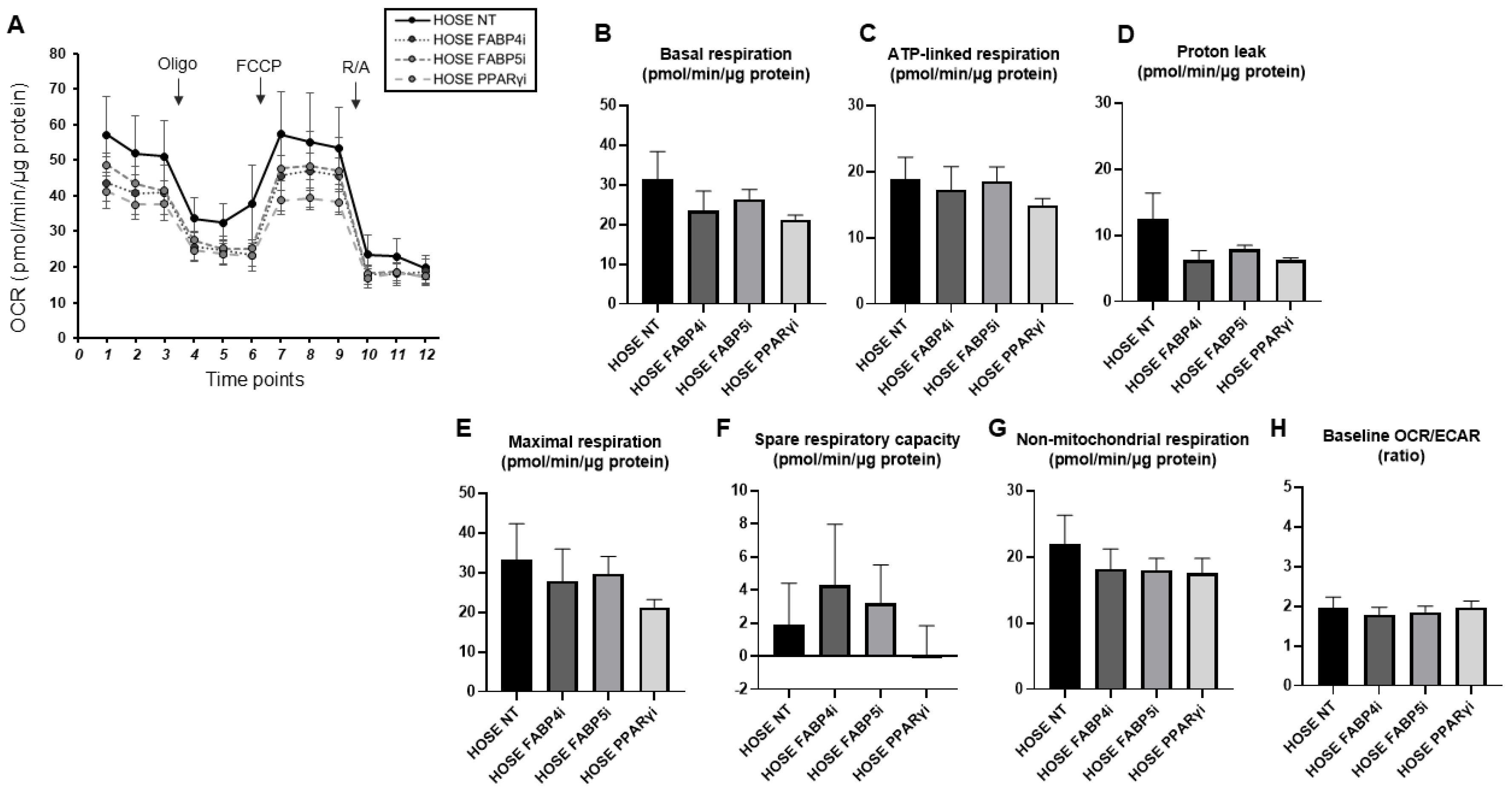
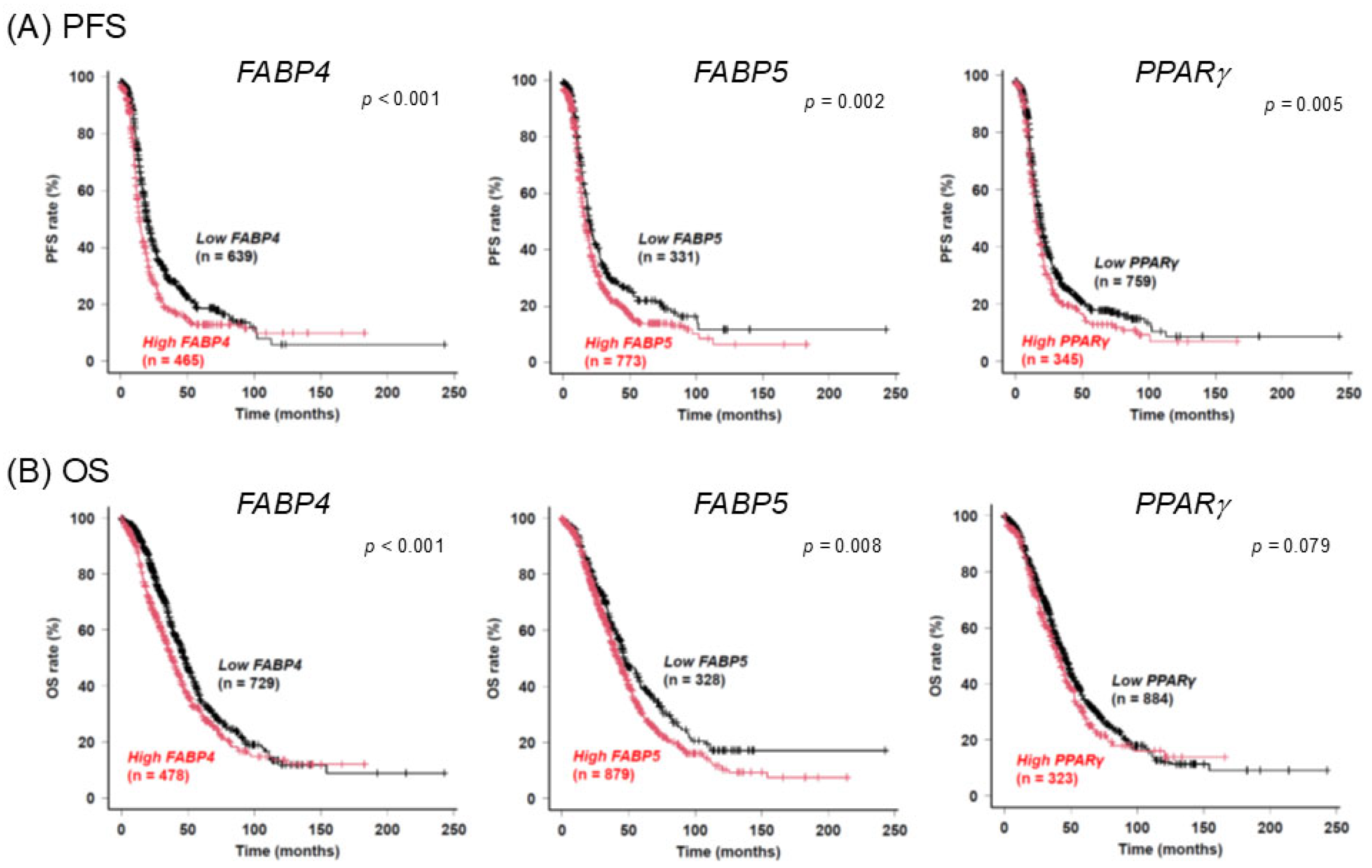
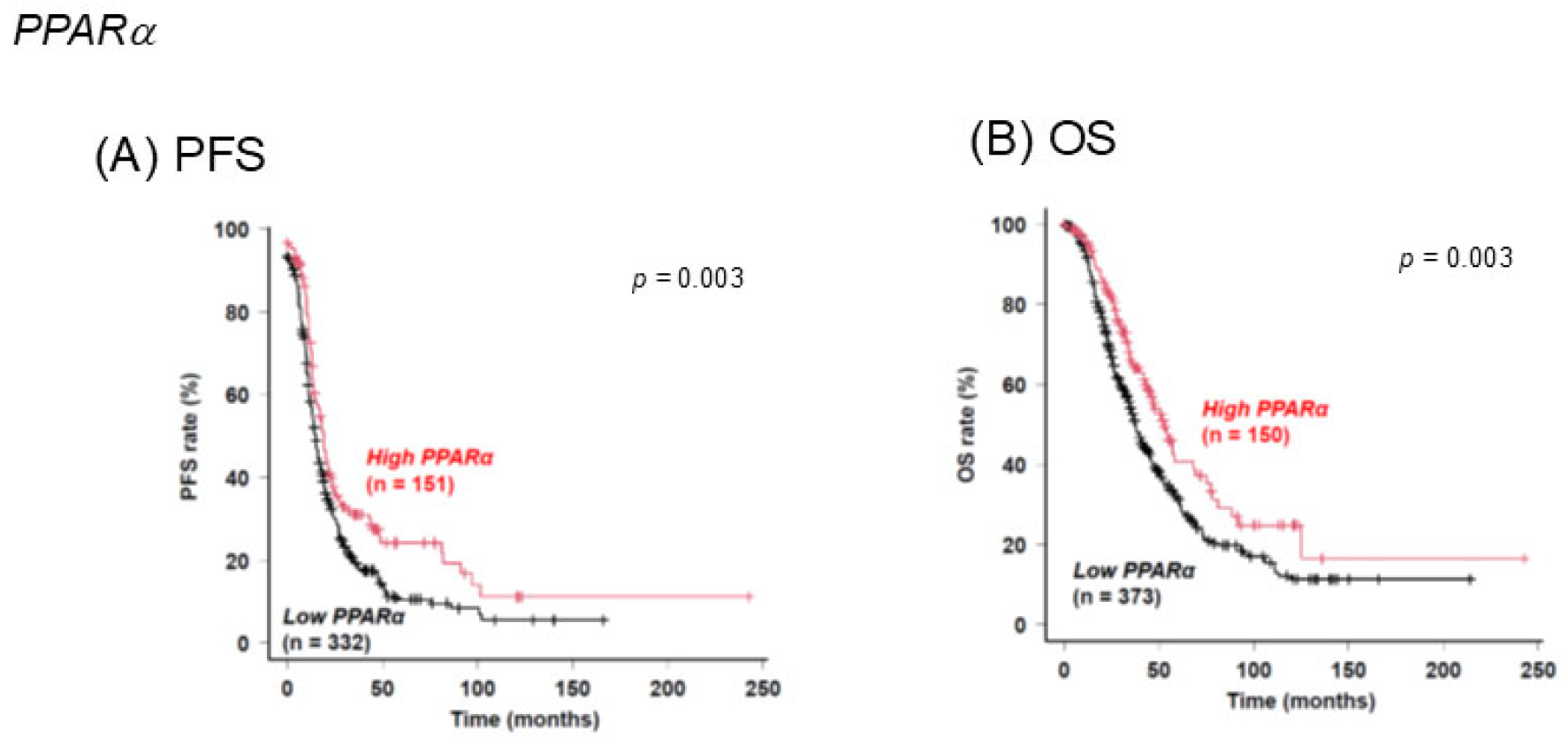
2. Results
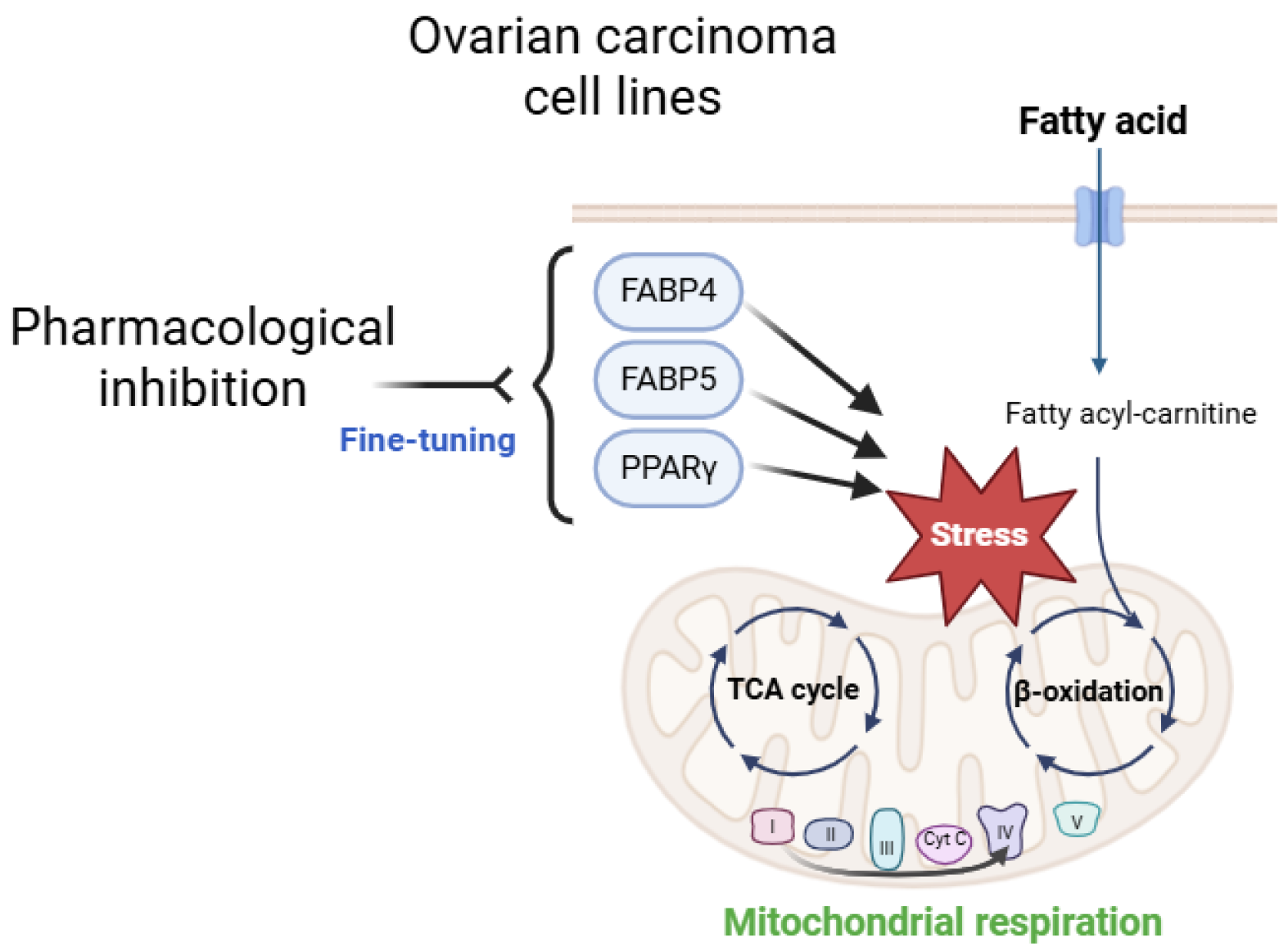
3. Discussion
4. Materials and Methods
4.1. Two-Dimensional and Three-Dimensional Cell Cultures of Ovarian Surface Epithelium Cell and Ovarian Carcinoma Cell Lines AMOC-2 and ES2
4.2. Analysis of Morphological Aspects of Spheroids Derived from HOSE, AMOC-2, and ES2 Cells
4.3. Immunocytochemistry
4.4. Real-Time Cellular Metabolic Function Analysis of HOSE, AMOC-2, and ES2 Cell Lines
4.5. Databases and Gene Expression Data Analysis
4.6. Other Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Chávarri-Guerra, Y.; González-Ochoa, E.; De-la-Mora-Molina, H.; Soto-Perez-de-Celis, E. Systemic therapy for non-serous ovarian carcinoma. Chin. Clin. Oncol. 2020, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef]
- Park, H.K.; Ruterbusch, J.J.; Cote, M.L. Recent Trends in Ovarian Cancer Incidence and Relative Survival in the United States by Race/Ethnicity and Histologic Subtypes. Cancer Epidemiol. Biomark. Prev. 2017, 26, 1511–1518. [Google Scholar] [CrossRef]
- del Carmen, M.G.; Birrer, M.; Schorge, J.O. Clear cell carcinoma of the ovary: A review of the literature. Gynecol. Oncol. 2012, 126, 481–490. [Google Scholar] [CrossRef]
- Hayashi, T.; Konishi, I. Molecular Histopathology for Establishing Diagnostic Method and Clinical Therapy for Ovarian Carcinoma. J. Clin. Med. Res. 2023, 15, 68–75. [Google Scholar] [CrossRef]
- Chan, J.K.; Teoh, D.; Hu, J.M.; Shin, J.Y.; Osann, K.; Kapp, D.S. Do clear cell ovarian carcinomas have poorer prognosis compared to other epithelial cell types? A study of 1411 clear cell ovarian cancers. Gynecol. Oncol. 2008, 109, 370–376. [Google Scholar] [CrossRef]
- Le Page, C.; Rahimi, K.; Köbel, M.; Tonin, P.N.; Meunier, L.; Portelance, L.; Bernard, M.; Nelson, B.H.; Bernardini, M.Q.; Bartlett, J.M.S.; et al. Characteristics and outcome of the COEUR Canadian validation cohort for ovarian cancer biomarkers. BMC Cancer 2018, 18, 347. [Google Scholar] [CrossRef]
- La Vecchia, C. Ovarian cancer: Epidemiology and risk factors. Eur. J. Cancer Prev. 2017, 26, 55–62. [Google Scholar] [CrossRef]
- Avgerinos, K.I.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metab. Clin. Exp. 2019, 92, 121–135. [Google Scholar] [CrossRef]
- Kolb, R.; Sutterwala, F.S.; Zhang, W. Obesity and cancer: Inflammation bridges the two. Curr. Opin. Pharmacol. 2016, 29, 77–89. [Google Scholar] [CrossRef]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K. Body Fatness and Cancer—Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Metzinger, M.N.; Lewellen, K.A.; Cripps, S.N.; Carey, K.D.; Harper, E.I.; Shi, Z.; Tarwater, L.; Grisoli, A.; Lee, E.; et al. Obesity Contributes to Ovarian Cancer Metastatic Success through Increased Lipogenesis, Enhanced Vascularity, and Decreased Infiltration of M1 Macrophages. Cancer Res. 2015, 75, 5046–5057. [Google Scholar] [CrossRef]
- Ham, S.; Choi, J.H.; Shin, S.G.; Lee, E.J. High visceral fat-to-muscle ratio is an independent factor that predicts worse overall survival in patients with primary epithelial ovarian, fallopian tube, and peritoneal cancer. J. Ovarian Res. 2023, 16, 19. [Google Scholar] [CrossRef]
- Biffi, G.; Tuveson, D.A. Diversity and Biology of Cancer-Associated Fibroblasts. Physiol. Rev. 2021, 101, 147–176. [Google Scholar] [CrossRef]
- Chen, Y.; McAndrews, K.M.; Kalluri, R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat. Rev. Clin. Oncol. 2021, 18, 792–804. [Google Scholar] [CrossRef]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef]
- Choi, S.Y.; Kang, S.H.; Oh, S.Y.; Lee, K.Y.; Lee, H.J.; Gum, S.; Kwon, T.G.; Kim, J.W.; Lee, S.T.; Hong, Y.J.; et al. Differential Angiogenic Potential of 3-Dimension Spheroid of HNSCC Cells in Mouse Xenograft. Int. J. Mol. Sci. 2021, 22, 8245. [Google Scholar] [CrossRef]
- Wróblewski, M.; Szewczyk-Golec, K.; Hołyńska-Iwan, I.; Wróblewska, J.; Woźniak, A. Characteristics of Selected Adipokines in Ascites and Blood of Ovarian Cancer Patients. Cancers 2021, 13, 4702. [Google Scholar] [CrossRef]
- Kim, B.; Jung, J. Impact of Obesity and Lysosomal Dysfunction on Chemoresistance in Ovarian Cancer. Biomedicines 2024, 12, 604. [Google Scholar] [CrossRef]
- Simão, V.A.; Floriano, J.F.; Cesário, R.C.; Tonon, K.D.S.; de Oliveira, L.R.C.; Delella, F.K.; Almeida, F.; Santos, L.D.D.; Seiva, F.R.F.; de Campos Zuccari, D.A.P.; et al. Extracellular Signaling Molecules from Adipose-Derived Stem Cells and Ovarian Cancer Cells Induce a Hybrid Epithelial-Mesenchymal Phenotype in a Bidirectional Interaction. Cells 2025, 14, 374. [Google Scholar] [CrossRef]
- Mukherjee, A.; Chiang, C.Y.; Daifotis, H.A.; Nieman, K.M.; Fahrmann, J.F.; Lastra, R.R.; Romero, I.L.; Fiehn, O.; Lengyel, E. Adipocyte-Induced FABP4 Expression in Ovarian Cancer Cells Promotes Metastasis and Mediates Carboplatin Resistance. Cancer Res. 2020, 80, 1748–1761. [Google Scholar] [CrossRef]
- Stewart, J.; Cunningham, N.; Banerjee, S. New therapies for clear cell ovarian carcinoma. Int. J. Gynecol. Cancer 2023, 33, 385–393. [Google Scholar] [CrossRef]
- Bogacka, I.; Kurzynska, A.; Bogacki, M.; Chojnowska, K. Peroxisome proliferator-activated receptors in the regulation of female reproductive functions. Folia Histochem. Cytobiol. 2015, 53, 189–200. [Google Scholar] [CrossRef]
- Komar, C.M. Peroxisome proliferator-activated receptors (PPARs) and ovarian function—Implications for regulating steroidogenesis, differentiation, and tissue remodeling. Reprod. Biol. Endocrinol. RBE 2005, 3, 41. [Google Scholar] [CrossRef]
- Prat, M.; Coulson, K.; Blot, C.; Jacquemin, G.; Romano, M.; Renoud, M.L.; AlaEddine, M.; Le Naour, A.; Authier, H.; Rahabi, M.C.; et al. PPARγ activation modulates the balance of peritoneal macrophage populations to suppress ovarian tumor growth and tumor-induced immunosuppression. J. Immunother. Cancer 2023, 11, e007031. [Google Scholar] [CrossRef]
- George Warren, W.; Osborn, M.; Yates, A.; Wright, K.; O’Sullivan, S.E. The emerging role of fatty acid binding protein 5 (FABP5) in cancers. Drug Discov. Today 2023, 28, 103628. [Google Scholar] [CrossRef]
- Habanjar, O.; Diab-Assaf, M.; Caldefie-Chezet, F.; Delort, L. 3D Cell Culture Systems: Tumor Application, Advantages, and Disadvantages. Int. J. Mol. Sci. 2021, 22, 12200. [Google Scholar] [CrossRef]
- Nath, S.; Devi, G.R. Three-dimensional culture systems in cancer research: Focus on tumor spheroid model. Pharmacol. Ther. 2016, 163, 94–108. [Google Scholar] [CrossRef]
- Ohguro, H.; Watanabe, M.; Sato, T.; Hikage, F.; Furuhashi, M.; Okura, M.; Hida, T.; Uhara, H. 3D Spheroid Configurations Are Possible Indictors for Evaluating the Pathophysiology of Melanoma Cell Lines. Cells 2023, 12, 759. [Google Scholar] [CrossRef]
- Miyamoto, S.; Nishikiori, N.; Sato, T.; Watanabe, M.; Umetsu, A.; Tsugeno, Y.; Hikage, F.; Sasaya, T.; Kato, H.; Ogi, K.; et al. Three-Dimensional Spheroid Configurations and Cellular Metabolic Properties of Oral Squamous Carcinomas Are Possible Pharmacological and Pathological Indicators. Cancers 2023, 15, 2793. [Google Scholar] [CrossRef]
- Hikage, F.; Atkins, S.; Kahana, A.; Smith, T.J.; Chun, T.H. HIF2A-LOX Pathway Promotes Fibrotic Tissue Remodeling in Thyroid-Associated Orbitopathy. Endocrinology 2019, 160, 20–35. [Google Scholar] [CrossRef]
- Watanabe, M.; Ida, Y.; Furuhashi, M.; Tsugeno, Y.; Ohguro, H.; Hikage, F. Screening of the Drug-Induced Effects of Prostaglandin EP2 and FP Agonists on 3D Cultures of Dexamethasone-Treated Human Trabecular Meshwork Cells. Biomedicines 2021, 9, 930. [Google Scholar] [CrossRef]
- Cao, Z.; Zhang, S. An integrative and comparative study of pan-cancer transcriptomes reveals distinct cancer common and specific signatures. Sci. Rep. 2016, 6, 33398. [Google Scholar] [CrossRef]
- Weinstein, J.N.; Collisson, E.A.; Mills, G.B.; Shaw, K.R.; Ozenberger, B.A.; Ellrott, K.; Shmulevich, I.; Sander, C.; Stuart, J.M. The Cancer Genome Atlas Pan-Cancer analysis project. Nat. Genet. 2013, 45, 1113–1120. [Google Scholar] [CrossRef]
- Butelmann, T.; Gu, Y.; Li, A.; Tribukait-Riemenschneider, F.; Hoffmann, J.; Molazem, A.; Jaeger, E.; Pellegrini, D.; Forget, A.; Shastri, V.P. 3D Printed Solutions for Spheroid Engineering and Cancer Research. Int. J. Mol. Sci. 2022, 23, 8188. [Google Scholar] [CrossRef]
- Zhao, L.; Xiu, J.; Liu, Y.; Zhang, T.; Pan, W.; Zheng, X.; Zhang, X. A 3D Printed Hanging Drop Dripper for Tumor Spheroids Analysis Without Recovery. Sci. Rep. 2019, 9, 19717. [Google Scholar] [CrossRef]
- Amaral, R.L.F.; Miranda, M.; Marcato, P.D.; Swiech, K. Comparative Analysis of 3D Bladder Tumor Spheroids Obtained by Forced Floating and Hanging Drop Methods for Drug Screening. Front. Physiol. 2017, 8, 605. [Google Scholar] [CrossRef]
- Shi, H.; Rath, E.M.; Lin, R.C.Y.; Sarun, K.H.; Clarke, C.J.; McCaughan, B.C.; Ke, H.; Linton, A.; Lee, K.; Klebe, S.; et al. 3-Dimensional mesothelioma spheroids provide closer to natural pathophysiological tumor microenvironment for drug response studies. Front. Oncol. 2022, 12, 973576. [Google Scholar] [CrossRef]
- Ganguli, A.; Mostafa, A.; Saavedra, C.; Kim, Y.; Le, P.; Faramarzi, V.; Feathers, R.W.; Berger, J.; Ramos-Cruz, K.P.; Adeniba, O.; et al. Three-dimensional microscale hanging drop arrays with geometric control for drug screening and live tissue imaging. Sci. Adv. 2021, 7, eabc1323. [Google Scholar] [CrossRef] [PubMed]
- Ohguro, H.; Watanabe, M.; Sato, T.; Nishikiori, N.; Umetsu, A.; Higashide, M.; Yano, T.; Suzuki, H.; Miyazaki, A.; Takada, K.; et al. Application of Single Cell Type-Derived Spheroids Generated by Using a Hanging Drop Culture Technique in Various In Vitro Disease Models: A Narrow Review. Cells 2024, 13, 1549. [Google Scholar] [CrossRef] [PubMed]
- Nishikiori, N.; Takada, K.; Sato, T.; Miyamoto, S.; Watanabe, M.; Hirakawa, Y.; Sekiguchi, S.; Furuhashi, M.; Yorozu, A.; Takano, K.; et al. Physical Properties and Cellular Metabolic Characteristics of 3D Spheroids Are Possible Definitive Indices for the Biological Nature of Cancer-Associated Fibroblasts. Cells 2023, 12, 2160. [Google Scholar] [CrossRef]
- Kimura, I.; Ichimura, A.; Ohue-Kitano, R.; Igarashi, M. Free Fatty Acid Receptors in Health and Disease. Physiol. Rev. 2020, 100, 171–210. [Google Scholar] [CrossRef]
- Currie, E.; Schulze, A.; Zechner, R.; Walther, T.C.; Farese, R.V., Jr. Cellular fatty acid metabolism and cancer. Cell Metab. 2013, 18, 153–161. [Google Scholar] [CrossRef]
- Yoon, H.; Lee, S. Fatty Acid Metabolism in Ovarian Cancer: Therapeutic Implications. Int. J. Mol. Sci. 2022, 23, 2170. [Google Scholar] [CrossRef]
- Kado, T.; Kusakari, N.; Tamaki, T.; Murota, K.; Tsujiuchi, T.; Fukushima, N. Oleic acid stimulates cell proliferation and BRD4-L-MYC-dependent glucose transporter transcription through PPARα activation in ovarian cancer cells. Biochem. Biophys. Res. Commun. 2023, 657, 24–34. [Google Scholar] [CrossRef]
- Murali, R.; Balasubramaniam, V.; Srinivas, S.; Sundaram, S.; Venkatraman, G.; Warrier, S.; Dharmarajan, A.; Gandhirajan, R.K. Deregulated Metabolic Pathways in Ovarian Cancer: Cause and Consequence. Metabolites 2023, 13, 560. [Google Scholar] [CrossRef]
- Anglesio, M.S.; Carey, M.S.; Köbel, M.; Mackay, H.; Huntsman, D.G. Clear cell carcinoma of the ovary: A report from the first Ovarian Clear Cell Symposium, June 24th, 2010. Gynecol. Oncol. 2011, 121, 407–415. [Google Scholar] [CrossRef]
- Wiegand, K.C.; Shah, S.P.; Al-Agha, O.M.; Zhao, Y.; Tse, K.; Zeng, T.; Senz, J.; McConechy, M.K.; Anglesio, M.S.; Kalloger, S.E.; et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N. Engl. J. Med. 2010, 363, 1532–1543. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, G. Endometriosis-associated Ovarian Clear Cell Carcinoma: A Special Entity? J. Cancer 2021, 12, 6773–6786. [Google Scholar] [CrossRef] [PubMed]
- Eyster, K.M.; Klinkova, O.; Kennedy, V.; Hansen, K.A. Whole genome deoxyribonucleic acid microarray analysis of gene expression in ectopic versus eutopic endometrium. Fertil. Steril. 2007, 88, 1505–1533. [Google Scholar] [CrossRef] [PubMed]
- Kubo, K.; Kamada, Y.; Hasegawa, T.; Sakamoto, A.; Nakatsuka, M.; Matsumoto, T.; Masuyama, H. Inflammation of the adipose tissue in the retroperitoneal cavity adjacent to pelvic endometriosis. J. Obstet. Gynaecol. Res. 2021, 47, 3598–3606. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Chen, O.; Yao, F.; Cui, L. miR-455 targets FABP4 to protect human endometrial stromal cells from cytotoxicity induced by hydrogen peroxide. Mol. Med. Rep. 2019, 20, 4781–4790. [Google Scholar] [CrossRef]
- Banner, C.D.; Göttlicher, M.; Widmark, E.; Sjövall, J.; Rafter, J.J.; Gustafsson, J.A. A systematic analytical chemistry/cell assay approach to isolate activators of orphan nuclear receptors from biological extracts: Characterization of peroxisome proliferator-activated receptor activators in plasma. J. Lipid Res. 1993, 34, 1583–1591. [Google Scholar] [CrossRef]
- Ivan, C.; Hu, W.; Bottsford-Miller, J.; Zand, B.; Dalton, H.J.; Liu, T.; Huang, J.; Nick, A.M.; Lopez-Berestein, G.; Coleman, R.L.; et al. Epigenetic analysis of the Notch superfamily in high-grade serous ovarian cancer. Gynecol. Oncol. 2013, 128, 506–511. [Google Scholar] [CrossRef]
- Chen, F.; Liu, N. A 10-gene expression signature of Notch pathway predicts recurrence in ovarian carcinoma. Oncol. Lett. 2015, 10, 1704–1708. [Google Scholar] [CrossRef]
- Lee, D.; Nakai, A.; Miwa, Y.; Negishi, K.; Tomita, Y.; Kurihara, T. Pemafibrate prevents choroidal neovascularization in a mouse model of neovascular age-related macular degeneration. PeerJ 2023, 11, e14611. [Google Scholar] [CrossRef]
- Ichioka, H.; Hirohashi, Y.; Sato, T.; Furuhashi, M.; Watanabe, M.; Ida, Y.; Hikage, F.; Torigoe, T.; Ohguro, H. G-Protein-Coupled Receptors Mediate Modulations of Cell Viability and Drug Sensitivity by Aberrantly Expressed Recoverin 3 within A549 Cells. Int. J. Mol. Sci. 2023, 24, 771. [Google Scholar] [CrossRef]
- Watanabe, M.; Sato, T.; Tsugeno, Y.; Umetsu, A.; Suzuki, S.; Furuhashi, M.; Ida, Y.; Hikage, F.; Ohguro, H. Human Trabecular Meshwork (HTM) Cells Treated with TGF-β2 or Dexamethasone Respond to Compression Stress in Different Manners. Biomedicines 2022, 10, 1338. [Google Scholar] [CrossRef]
- Tsugeno, Y.; Sato, T.; Watanabe, M.; Furuhashi, M.; Umetsu, A.; Ida, Y.; Hikage, F.; Ohguro, H. Benzalkonium Chloride, Even at Low Concentrations, Deteriorates Intracellular Metabolic Capacity in Human Conjunctival Fibroblasts. Biomedicines 2022, 10, 2315. [Google Scholar] [CrossRef] [PubMed]
- Endo, K.; Sato, T.; Umetsu, A.; Watanabe, M.; Hikage, F.; Ida, Y.; Ohguro, H.; Furuhashi, M. 3D culture induction of adipogenic differentiation in 3T3-L1 preadipocytes exhibits adipocyte-specific molecular expression patterns and metabolic functions. Heliyon 2023, 9, e20713. [Google Scholar] [CrossRef] [PubMed]
- Kolendowski, B.; Valdes, Y.R.; Hirte, H.; Itamochi, H.; Lee, W.; Carey, M.; Shepherd, T.G.; DiMattia, G.E. Characterization of Mutational Status, Spheroid Formation, and Drug Response of a New Genomically-Stable Human Ovarian Clear Cell Carcinoma Cell Line, 105C. Cells 2020, 9, 2408. [Google Scholar] [CrossRef]
- Fukumoto, T.; Magno, E.; Zhang, R. SWI/SNF Complexes in Ovarian Cancer: Mechanistic Insights and Therapeutic Implications. Mol. Cancer Res. 2018, 16, 1819–1825. [Google Scholar] [CrossRef]
- Zhang, X.; Shetty, M.; Clemente, V.; Linder, S.; Bazzaro, M. Targeting Mitochondrial Metabolism in Clear Cell Carcinoma of the Ovaries. Int. J. Mol. Sci. 2021, 22, 4750. [Google Scholar] [CrossRef]
- Yabushita, H.; Ueno, N.; Sawaguchi, K.; Higuchi, K.; Noguchi, M.; Ishihara, M. Establishment and characterization of a new human cell-line (AMOC-2) derived from a serous adenocarcinoma of ovary. Nihon Sanka Fujinka Gakkai Zasshi 1989, 41, 888–894. [Google Scholar]
- Sasaki, R.; Narisawa-Saito, M.; Yugawa, T.; Fujita, M.; Tashiro, H.; Katabuchi, H.; Kiyono, T. Oncogenic transformation of human ovarian surface epithelial cells with defined cellular oncogenes. Carcinogenesis 2009, 30, 423–431. [Google Scholar] [CrossRef]
- Ohguro, H.; Ida, Y.; Hikage, F.; Umetsu, A.; Ichioka, H.; Watanabe, M.; Furuhashi, M. STAT3 Is the Master Regulator for the Forming of 3D Spheroids of 3T3-L1 Preadipocytes. Cells 2022, 11, 300. [Google Scholar] [CrossRef]
- Akama, T.; Leung, B.M.; Labuz, J.; Takayama, S.; Chun, T.H. Designing 3-D Adipospheres for Quantitative Metabolic Study. Methods Mol. Biol. 2017, 1566, 177–183. [Google Scholar]
- Lánczky, A.; Győrffy, B. Web-Based Survival Analysis Tool Tailored for Medical Research (KMplot): Development and Implementation. J. Med. Internet Res. 2021, 23, e27633. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, M.; Matsuura, M.; Sato, T.; Usami, M.; Saito, T.; Furuhashi, M.; Takada, K.; Ohguro, H. Fatty Acid Metabolism Regulators Have Pivotal Roles in the Pathogenesis of Ovarian Carcinoma. Int. J. Mol. Sci. 2025, 26, 4794. https://doi.org/10.3390/ijms26104794
Watanabe M, Matsuura M, Sato T, Usami M, Saito T, Furuhashi M, Takada K, Ohguro H. Fatty Acid Metabolism Regulators Have Pivotal Roles in the Pathogenesis of Ovarian Carcinoma. International Journal of Molecular Sciences. 2025; 26(10):4794. https://doi.org/10.3390/ijms26104794
Chicago/Turabian StyleWatanabe, Megumi, Motoki Matsuura, Tatsuya Sato, Makoto Usami, Tsuyoshi Saito, Masato Furuhashi, Kohichi Takada, and Hiroshi Ohguro. 2025. "Fatty Acid Metabolism Regulators Have Pivotal Roles in the Pathogenesis of Ovarian Carcinoma" International Journal of Molecular Sciences 26, no. 10: 4794. https://doi.org/10.3390/ijms26104794
APA StyleWatanabe, M., Matsuura, M., Sato, T., Usami, M., Saito, T., Furuhashi, M., Takada, K., & Ohguro, H. (2025). Fatty Acid Metabolism Regulators Have Pivotal Roles in the Pathogenesis of Ovarian Carcinoma. International Journal of Molecular Sciences, 26(10), 4794. https://doi.org/10.3390/ijms26104794






