Sodium-Glucose Cotransporter-2 Inhibitors in Liver Cirrhosis: A Systematic Review of Their Role in Ascites Management, Slowing Disease Progression, and Safety
Abstract
1. Introduction
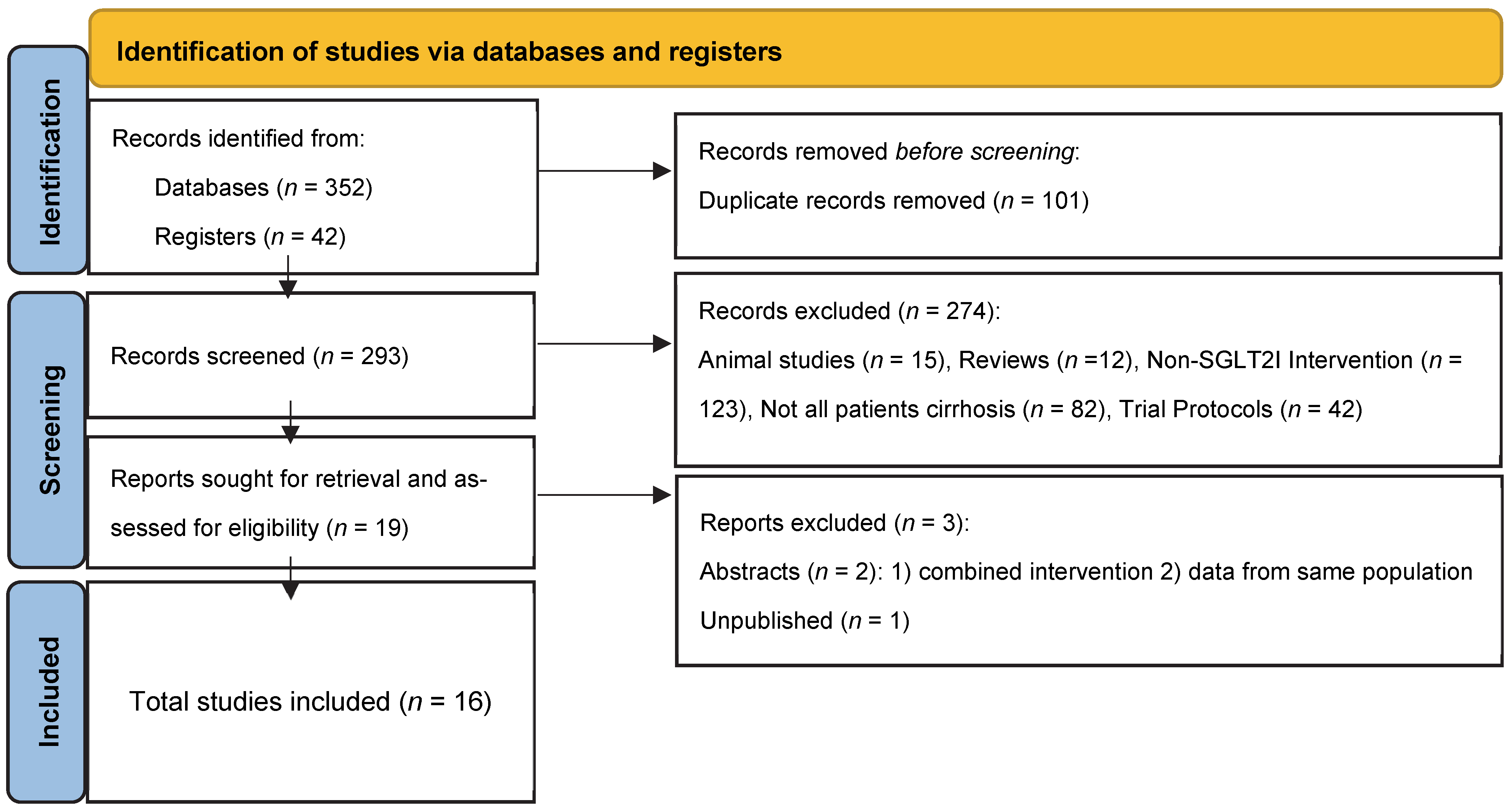
2. Materials and Methods
3. Results
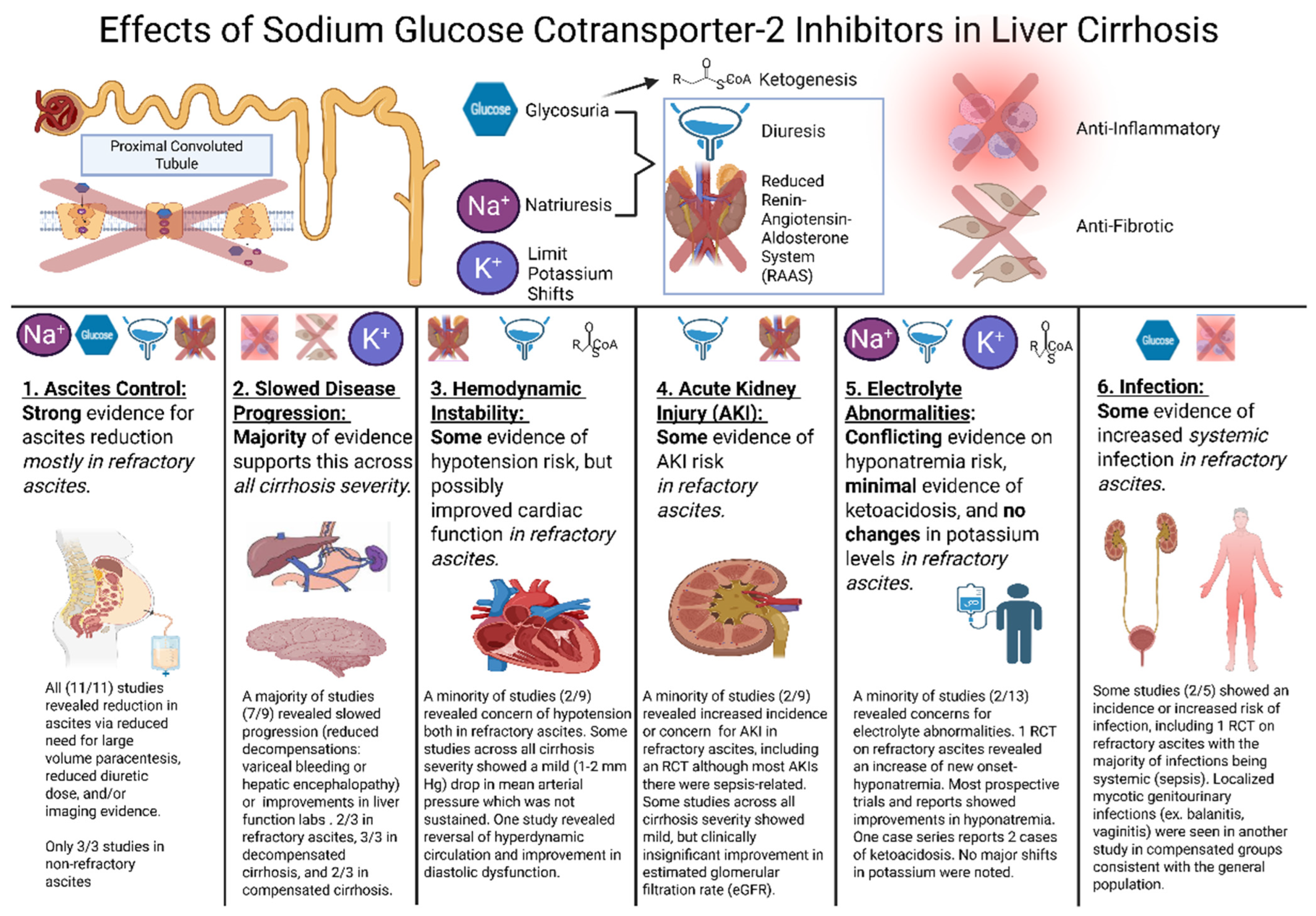
Summary of Results
4. Discussion
4.1. Overview
4.2. Ascites Control
4.3. Slowed Disease Progression
4.4. Hemodynamic Effects
4.5. Acute Kidney Injury Concerns
4.6. Electrolyte and Acid-Base Derangements
4.7. Infection Risk
4.8. Potential Clinical Applicability
4.9. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AKI | Acute Kidney Injury |
| C | Canagliflozin |
| CAD | Coronary artery disease |
| CART | Cell-free and Concentrated Ascites Reinfusion Therapy |
| CCM | Cirrhotic cardiomyopathy |
| CHF | Congestive Heart Failure |
| CI | Confidence Interval |
| CKD | Chronic Kidney Disease |
| CP | Child–Turcotte–Pugh Score |
| CR | Case report |
| CS | Case series |
| CSPH | Clinically significant portal hypertension |
| D | Dapagliflozin |
| DKA | Diabetic ketoacidosis |
| DPP4i | Dipeptidyl Peptidase-4 Inhibitors |
| E | Empagliflozin |
| E/e’ | ratio of early diastolic mitral inflow velocity to mitral annular velocity |
| eGFR | Estimated glomerular filtration rate |
| EtOH | Ethanol |
| EV | Esophageal varices |
| FDA | Federal Drug Administration |
| FIB-4 | Fibrosis-4 (FIB-4) Index for Liver Fibrosis |
| GIB | Gastrointestinal bleed |
| Hba1c | Hemoglobin A1c |
| HCC | Hepatocellular carcinoma |
| HE | Hepatic Encephalopathy |
| HR | Hazard Ratio |
| HRS-AKI | Hepatorenal syndrome–acute kidney injury |
| INR | International normalized ratio |
| LAVI | Left atrial volume index |
| LSM | Liver stiffness measures |
| LVEF | Left ventricular ejection fraction |
| LVP | Large Volume Paracentesis |
| MAP | Mean Arterial Pressure |
| MASLD | Metabolic-Associated Steatotic Liver Disease |
| MELDNa | Model for End-Stage Liver Disease with Sodium |
| MTF | Metformin |
| NAFLD | Non-Alcoholic Fatty Liver Disease |
| NIHQAT | National Institute of Health Quality Assessment Tool |
| N/A | Not applicable |
| NARHD | Non-ascites related hepatic decompensations |
| NR | Not reported |
| O | Other (medications) |
| OLT | Orthotopic liver transplantation |
| PC | Prospective cohort |
| PICOT | Population characteristics, interventions, controls, primary or significant outcomes, and time periods |
| PRISMA | Preferred Reporting Items for Systematic reviews and Meta-Analyses |
| PT | Pilot Trial |
| PVT | Portal Vein Thrombosis |
| RAAS | Renin–Angiotensin–Aldosterone System |
| RC | Retrospective cohort |
| RCR | Retrospective chart review |
| RCT | Randomized Controlled Trial |
| RoB | Risk of Bias |
| ROBINS-I | Risk Of Bias in Non-randomized Studies of Interventions |
| SGLT2I | Sodium-glucose cotransporter-2 inhibitors |
| SoCD | Standard of care diuretics |
| T1DM | Type 1 Diabetes Mellitus |
| T2DM | Type 2 Diabetes Mellitus |
| TIPS | Transjugular Intrahepatic Portosystemic Shunt |
| UTI | Urinary Tract Infection |
| VCTE | Vibration-controlled transient elastography |
References
- Padda, I.S.; Mahtani, A.U.; Parmar, M. Sodium-Glucose Transport2 (SGLT2) Inhibitors. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Teo, Y.H.; Teo, Y.N.; Syn, N.L.; Kow, C.S.; Yoong, C.S.Y.; Tan, B.Y.Q.; Yeo, T.C.; Lee, C.H.; Lin, W.; Sia, C.H. Effects of Sodium/Glucose Cotransporter 2 (SGLT2) Inhibitors on Cardiovascular and Metabolic Outcomes in Patients Without Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized-Controlled Trials. J. Am. Heart Assoc. 2021, 10, e019463. [Google Scholar] [CrossRef]
- Baigent, C.; Emberson, J.; Haynes, R.; Herrington, W.G.; Judge, P.; Landray, M.J.; Mayne, K.J.; Ng, S.Y.; Preiss, D.; Roddick, A.J.; et al. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: Collaborative meta-analysis of large placebo-controlled trials. Lancet 2022, 400, 1788–1801. [Google Scholar] [CrossRef]
- Adhikari, R.; Jha, K.; Dardari, Z.; Heyward, J.; Blumenthal, R.S.; Eckel, R.H.; Alexander, G.C.; Blaha, M.J. National Trends in Use of Sodium-Glucose Cotransporter-2 Inhibitors and Glucagon-like Peptide-1 Receptor Agonists by Cardiologists and Other Specialties, 2015 to 2020. J. Am. Heart Assoc. 2022, 11, e023811. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Yuan, Y.; Zheng, C.; Liu, S.; Weng, H. Effects of sodium-glucose co-transporter 2 inhibitors on liver fibrosis in non-alcoholic fatty liver disease patients with type 2 diabetes mellitus: An updated meta-analysis of randomized controlled trials. J. Diabetes Complicat. 2023, 37, 108558. [Google Scholar] [CrossRef]
- Wei, Q.; Xu, X.; Guo, L.; Li, J.; Li, L. Effect of SGLT2 Inhibitors on Type 2 Diabetes Mellitus with Non-Alcoholic Fatty Liver Disease: A Meta-Analysis of Randomized Controlled Trials. Front. Endocrinol. 2021, 12, 635556. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.Q.; Terrault, N.A.; Tacke, F.; Gluud, L.L.; Arrese, M.; Bugianesi, E.; Loomba, R. Global epidemiology of cirrhosis—Aetiology, trends and predictions. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 388–398. [Google Scholar] [CrossRef]
- Tyagi, K.; Madan, S.; Prakash Bhatt, S.; Ansari, I.A.; Dutta, K.; Misra, A. Sodium-glucose cotransporter-2 inhibitors in patients with type 2 diabetes mellitus and moderate to severe hepatic fibrosis: A retrospective study. Clin. Nutr. ESPEN 2023, 57, 305–310. [Google Scholar] [CrossRef]
- Lee, H.W.; Kim, K.H.; Ahn, S.H.; Lee, H.C.; Choi, J. The associations between fibrosis changes and liver-related events in patients with metabolic dysfunction-associated steatotic liver disease. Liver Int. 2024, 44, 1448–1455. [Google Scholar] [CrossRef]
- Siafarikas, C.; Kapelios, C.J.; Papatheodoridi, M.; Vlachogiannakos, J.; Tentolouris, N.; Papatheodoridis, G. Sodium-glucose linked transporter 2 inhibitors in liver cirrhosis: Beyond their antidiabetic use. Liver Int. 2024, 44, 884–893. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Fujishima, Y.; Wakasugi, D.; Io, F.; Sato, Y.; Uchida, S.; Kitajima, Y. Effects of SGLT2 inhibitors on the onset of esophageal varices and extrahepatic cancer in type 2 diabetic patients with suspected MASLD: A nationwide database study in Japan. J. Gastroenterol. 2024, 59, 1120–1132. [Google Scholar] [CrossRef]
- Asada, S.; Kaji, K.; Nishimura, N.; Koizumi, A.; Matsuda, T.; Tanaka, M.; Yorioka, N.; Sato, S.; Kitagawa, K.; Namisaki, T.; et al. Tofogliflozin Delays Portal Hypertension and Hepatic Fibrosis by Inhibiting Sinusoidal Capillarization in Cirrhotic Rats. Cells 2024, 13, 538. [Google Scholar] [CrossRef]
- Garcia-Pagan, J.C.; Gluud, L.L.; Mandorfer, M.; Schattenberg, J.M.; De Gottardi, A.; Berzigotti, A.; Fortea, J.I.; Albillos, A.; Alvarado-Tapias, E.; Berning, M.; et al. Effects of Zibotentan and Dapagliflozin on patients with compensated cirrhosis: A randomized placebo controlled exploratory pilot study. In Proceedings of the European Association for the Study of the Liver, Milan, Italy, 5–8 June 2024. [Google Scholar]
- Chung, S.W.; Moon, H.-S.; Shin, H.; Han, H.; Park, S.; Cho, H.; Park, J.; Hur, M.H.; Park, M.K.; Won, S.-H.; et al. Inhibition of sodium-glucose cotransporter-2 and liver-related complications in individuals with diabetes: A Mendelian randomization and population-based cohort study. Hepatology 2024, 80, 633–648. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, Y.; Honda, A.; Yokose, S.; Nagata, M.; Miyamoto, J. Weaning from concentrated ascites reinfusion therapy for refractory ascites by SGLT2 inhibitor. Clin. Kidney J. 2022, 15, 831–833. [Google Scholar] [CrossRef]
- Kalambokis, G.N.; Tsiakas, I.; Filippas-Ntekuan, S.; Christaki, M.; Despotis, G.; Milionis, H. Empagliflozin eliminates refractory ascites and hepatic hydrothorax in a patient with primary biliary cirrhosis. Off. J. Am. Coll. Gastroenterol.|ACG 2021, 116, 618–619. [Google Scholar] [CrossRef]
- Montalvo-Gordon, I.; Chi-Cervera, L.A.; García-Tsao, G. Sodium-glucose cotransporter 2 inhibitors ameliorate ascites and peripheral edema in patients with cirrhosis and diabetes. Hepatology 2020, 72, 1880–1882. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Honda, A.; Yokose, S.; Nagata, M.; Miyamoto, J. The Effects of SGLT2 Inhibitors on Liver Cirrhosis Patients with Refractory Ascites: A Literature Review. J. Clin. Med. 2023, 12, 2253. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liu, X.; Gao, Y.; Duan, M.; Hou, B.; Chen, Y. Pharmacological Interventions for Cirrhotic Ascites: From Challenges to Emerging Therapeutic Horizons. Gut Liver 2024, 18, 934–948. [Google Scholar] [CrossRef] [PubMed]
- Aithal, G.P.; Palaniyappan, N.; China, L.; Harmala, S.; Macken, L.; Ryan, J.M.; Wilkes, E.A.; Moore, K.; Leithead, J.A.; Hayes, P.C.; et al. Guidelines on the management of ascites in cirrhosis. Gut 2021, 70, 9–29. [Google Scholar] [CrossRef]
- Mansour, D.; McPherson, S. Management of decompensated cirrhosis. Clin. Med. 2018, 18, s60–s65. [Google Scholar] [CrossRef]
- Engelmann, C.; Claria, J.; Szabo, G.; Bosch, J.; Bernardi, M. Pathophysiology of decompensated cirrhosis: Portal hypertension, circulatory dysfunction, inflammation, metabolism and mitochondrial dysfunction. J. Hepatol. 2021, 75 (Suppl. S1), S49–S66. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.R.; Kamath, P.S. Ascites and hepatorenal syndrome: Pathophysiology and management. Mayo Clin. Proc. 1996, 71, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.V.; Carrillo-Perez, D.L.; Rosado-Canto, R.; Garcia-Juarez, I.; Torre, A.; Kershenobich, D.; Carrillo-Maravilla, E. Electrolyte and Acid-Base Disturbances in End-Stage Liver Disease: A Physiopathological Approach. Dig. Dis. Sci. 2017, 62, 1855–1871. [Google Scholar] [CrossRef]
- Liu, J.; Li, L.; Li, S.; Jia, P.; Deng, K.; Chen, W.; Sun, X. Effects of SGLT2 inhibitors on UTIs and genital infections in type 2 diabetes mellitus: A systematic review and meta-analysis. Sci. Rep. 2017, 7, 2824. [Google Scholar] [CrossRef] [PubMed]
- Puckrin, R.; Saltiel, M.P.; Reynier, P.; Azoulay, L.; Yu, O.H.Y.; Filion, K.B. SGLT-2 inhibitors and the risk of infections: A systematic review and meta-analysis of randomized controlled trials. Acta Diabetol. 2018, 55, 503–514. [Google Scholar] [CrossRef]
- Albillos, A.; Lario, M.; Alvarez-Mon, M. Cirrhosis-associated immune dysfunction: Distinctive features and clinical relevance. J. Hepatol. 2014, 61, 1385–1396. [Google Scholar] [CrossRef]
- Ata, F.; Yousaf, Z.; Khan, A.A.; Razok, A.; Akram, J.; Ali, E.A.H.; Abdalhadi, A.; Ibrahim, D.A.; Al Mohanadi, D.; Danjuma, M.I. SGLT-2 inhibitors associated euglycemic and hyperglycemic DKA in a multicentric cohort. Sci. Rep. 2021, 11, 10293. [Google Scholar] [CrossRef]
- Al-Hindi, B.; Mohammed, M.A.; Mangantig, E.; Martini, N.D. Prevalence of sodium-glucose transporter 2 inhibitor-associated diabetic ketoacidosis in real-world data: A systematic review and meta-analysis. J. Am. Pharm. Assoc. 2024, 64, 9–26.e6. [Google Scholar] [CrossRef]
- Mansour, M.M.; Obeidat, A.E.; Darweesh, M.; Mahfouz, R.; Kuwada, S.; Pyrsopoulos, N.T. The Impact of Cirrhosis on Outcomes of Patients Admitted with Diabetic Ketoacidosis: A Nationwide Study. Cureus 2022, 14, e25870. [Google Scholar] [CrossRef]
- Chao, H.Y.; Kornelius, E. Two alcoholic liver cirrhosis patients developed diabetic ketoacidosis after SGLT2 inhibitors-prescription. J. Formos. Med. Assoc. 2020, 119, 1886–1887. [Google Scholar] [CrossRef]
- Chandra, S.; Ravula, S.; Errabelli, P.; Spencer, H.; Singh, M. No Good Deed: Acidosis in Chronic Kidney and Liver Disease. J. Ren. Nutr. 2023, 33, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Bakosh, M.F.; Ghazy, R.M.; Ellakany, W.I.; Kamal, A. Empagliflozin as a novel therapy for cirrhotic refractory ascites: A randomized controlled study. Egypt. Liver J. 2024, 14, 76. [Google Scholar] [CrossRef]
- Singh, V.; De, A.; Aggrawal, R.; Singh, A.; Charak, S.; Bhagat, N. Safety and Efficacy of Dapagliflozin in Recurrent Ascites: A Pilot Study. Dig. Dis. Sci. 2025, 70, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Saffo, S.; Kaplan, D.E.; Mahmud, N.; Serper, M.; John, B.V.; Ross, J.S.; Taddei, T. Impact of SGLT2 inhibitors in comparison with DPP4 inhibitors on ascites and death in veterans with cirrhosis on metformin. Diabetes Obes. Metab. 2021, 23, 2402–2408. [Google Scholar] [CrossRef] [PubMed]
- Huynh, D.J.; Renelus, B.D.; Jamorabo, D.S. Reduced mortality and morbidity associated with metformin and SGLT2 inhibitor therapy in patients with type 2 diabetes mellitus and cirrhosis. BMC Gastroenterol. 2023, 23, 450. [Google Scholar] [CrossRef]
- Ayoub, M.; Faris, C.; Rajamanuri, M.; Chela, H.; Anwar, N.; Daglilar, E.; Chumbe, J.T. The use of sodium-glucose cotransporter-2 Inhibitors (SGLT2-I) in addition to furosemide and spironolactone in patients with decompensated cirrhosis: An international analysis. In Proceedings of the Hepatology, Milan, Italy, 5–8 June 2024; p. S475. [Google Scholar]
- El-Din, Z.S.; Afify, M.; Zayed, E.; Elsabaawy, D.; Tharwa, E.S.; Elsharawy, A.; Abdelsameea, E.; Rady, M.A. Dapagliflozin as an oral antihyperglycemic agent in the management of diabetes mellitus in patients with liver cirrhosis. World J. Exp. Med. 2024, 14, 95272. [Google Scholar] [CrossRef]
- Saffo, S.; Garcia-Tsao, G.; Taddei, T. SGLT2 inhibitors in patients with cirrhosis and diabetes mellitus: A tertiary center cohort study and insights about a potential therapeutic target for portal hypertension. J. Diabetes 2020, 13, 265–269. [Google Scholar] [CrossRef]
- Sharma, P.; Anikhindi, A.; Ashish, K.; Anil, A. Sodium Glucose Co-Transporter-2 (Sglt) Inhibitors in Patients with Compensated Cirrhosis And Diabetes: A Prospective Study. Int. J. Gastroenterol. Hepatol. 2023, 4, 2716. [Google Scholar]
- Shen, I.; Stojanova, J.; Yeo, M.; Olsen, N.; Lockart, I.; Wang, M.; Roggeveld, J.; Heerspink, H.J.L.; Greenfield, J.R.; Day, R.; et al. A potential novel treatment for cirrhosis-related ascites: Empagliflozin is safe and tolerable in advanced chronic liver disease. Br. J. Clin. Pharmacol. 2024, 90, 2529–2538. [Google Scholar] [CrossRef]
- Kalambokis, G.; Tsiakas, I.; Filippas-Ntekouan, S.; Christaki, M.; Milionis, H. Empagliflozin controls cirrhotic refractory ascites along with improvement of natriuresis and circulatory, cardiac, and renal function: A pilot study. Eur. J. Intern. Med. 2024, 130, 162–164. [Google Scholar] [CrossRef] [PubMed]
- Seidita, A.; Mandreucci, F.; Pistone, M.; Calderone, S.; Giuliano, A.; Chiavetta, M.; Giannitrapani, L.; Citarrella, R.; Soresi, M.; Licata, A.; et al. Sodium-Glucose Cotransporter 2 Inhibition in Patients with Liver Cirrhosis and Diabetes: A Possible Role in Ascites Control? Ital. J. Med. 2024, 18. [Google Scholar] [CrossRef]
- Hu, K.; Goel, A.; Tarlow, B.; Cheng, X.; Kim, S.; Kim, W.R.; Kwo, P. Empagliflozin in Diuretic-Refractory Ascites (DRAin-Em): Results of a Single-Center Feasibility Study. J. Gen. Intern. Med. 2024, 40, 1680–1682. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernan, M.A.; Reeves, B.C.; Savovic, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- National Heart Lung and Blood Institute. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 14 February 2025).
- Lensen, S. When to pool data in a meta-analysis (and when not to)? Fertil. Steril. 2023, 119, 902–903. [Google Scholar] [CrossRef]
- Abu-Hammour, M.-N.; Vignarajah, A.; Vigneswaramoorthy, N.; Chiang, D. Association of Sodium Glucose Co-Transporter-2 Inhibitors and Serious Liver Events in Patients with Cirrhosis on Diuretics: A Global Cohort Study. Am. J. Gastroenterol. 2024, 119, S1218. [Google Scholar] [CrossRef]
- Shi, R.; Gao, Y.; Qin, W.; Dong, Y.; Zhou, L.; Qi, C.; Xing, Y.; Wang, C.; Huang, S.; Zhao, Y.; et al. Empagliflozin for the Treatment of Liver Cirrhosis with Refractory Ascites: A Pilot-Controlled Study. Preprint 2024. [Google Scholar] [CrossRef]
- Balcar, L.; Tonon, M.; Semmler, G.; Calvino, V.; Hartl, L.; Incicco, S.; Jachs, M.; Bauer, D.; Hofer, B.S.; Gambino, C.G.; et al. Risk of further decompensation/mortality in patients with cirrhosis and ascites as the first single decompensation event. JHEP Rep. 2022, 4, 100513. [Google Scholar] [CrossRef]
- Stachteas, P.; Nasoufidou, A.; Patoulias, D.; Karakasis, P.; Karagiannidis, E.; Mourtzos, M.A.; Samaras, A.; Apostolidou, X.; Fragakis, N. The Role of Sodium-Glucose Co-Transporter-2 Inhibitors on Diuretic Resistance in Heart Failure. Int. J. Mol. Sci. 2024, 25, 3122. [Google Scholar] [CrossRef]
- Patil, V.; Jain, M.; Venkataraman, J. Paracentesis-induced acute kidney injury in decompensated cirrhosis-prevalence and predictors. Clin. Exp. Hepatol. 2019, 5, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, C.A., Jr.; Chen, Y.L.; Messina, E.J. Inhibition of NO synthesis or endothelium removal reveals a vasoconstrictor effect of insulin on isolated arterioles. Am. J. Physiol. 1999, 276, H815–H820. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, E.; Cushman, W.C. Diabetes and hypertension: The bad companions. Lancet 2012, 380, 601–610. [Google Scholar] [CrossRef]
- Xia, C.; Han, Y.; Yin, C.; Geng, R.; Liu, Z.; Du, Y.; Yu, M. Relationship between sodium–glucose cotransporter-2 inhibitors and muscle atrophy in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Front. Endocrinol. 2023, 14, 1220516. [Google Scholar] [CrossRef] [PubMed]
- Shabani, E.; Heidari, S.M.; Roozitalab, M.; Zarei, H.; Mirshekari, A.; Ebrahimi, S.; Doshantapeh, A.G.; Vatankhah, S.A.; Dehghani-Ghorbi, M. Systematic review and meta-analysis of the association between the use of SGLT2 inhibitors and hepatocellular carcinoma. Immunopathol Persa 2025, 11, e43765. [Google Scholar] [CrossRef]
- Yumusak, O.; Doulberis, M. Update on cirrhotic cardiomyopathy: From etiopathogenesis to treatment. Ann. Gastroenterol. 2024, 37, 381–391. [Google Scholar] [CrossRef]
- Nassif, M.E.; Windsor, S.L.; Borlaug, B.A.; Kitzman, D.W.; Shah, S.J.; Tang, F.; Khariton, Y.; Malik, A.O.; Khumri, T.; Umpierrez, G.; et al. The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: A multicenter randomized trial. Nat. Med. 2021, 27, 1954–1960. [Google Scholar] [CrossRef]
- Faluk, M.; Wardhere, A.; Thakker, R.; Khan, F.A. SGLT2 inhibitors in heart failure with preserved ejection fraction. Curr. Probl. Cardiol. 2024, 49, 102388. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Rocca, H.-P.B.L.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Francoz, C.; Durand, F.; Kahn, J.A.; Genyk, Y.S.; Nadim, M.K. Hepatorenal Syndrome. Clin. J. Am. Soc. Nephrol. 2019, 14, 774–781. [Google Scholar] [CrossRef]
- Covington, E.W.; Slaten, K.; Harnden, A. Analysis of SGLT2 Inhibitor Therapy and Other Potential Risk Factors for the Development of Bacteremia in Patients with Urosepsis. J. Pharm. Technol. 2022, 38, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.J.; Mousa, O.Y.; Syed, K.; John, S. Acute on Chronic Liver Failure. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Siqueira, F.; Kelly, T.; Saab, S. Refractory Ascites: Pathogenesis, Clinical Impact, and Management. Gastroenterol. Hepatol. 2009, 5, 647–656. [Google Scholar]
- Verma, A.; Patel, A.B.; Waikar, S.S. SGLT2 Inhibitor: Not a Traditional Diuretic for Heart Failure. Cell Metab. 2020, 32, 13–14. [Google Scholar] [CrossRef] [PubMed]
- Dhoop, S.; Shehada, M.; Sawaf, B.; Patel, M.; Roberts, L.; Smith, W.-L.; Hart, B. The Efficacy and Safety of Sodium-Glucose Cotransporter Protein 2 Inhibitors (SGLT2Is) in Liver Cirrhosis: A Systematic Review and Meta-Analysis. medRxiv 2024. [Google Scholar] [CrossRef]
| Author Year | Design | SGLT2I (n) | Control (n) | % Decomp | % MASLD | MELD Na | Covariates | Inclusion: | Exclusion: | SGLT2I Effect on Primary Outcome | End Point (Weeks) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | |||||||||||
| Saffo 2021 [37] | RC | NR 423 | DPP4i 423 | 0 | 47 | 9.3 | 9.3 | Age, Gender, MELD-Na, FIB-4, EV, CAD, HbA1c, medications EtOH use. | Compensated T2DM FIB-4 > 1.45 | Prior diagnosis of ascites, History of TIPS, OLT | Ascites incidence insignificant decrease (HR: 0.68, CI: 0.37–1.25, =0.22) | 144 |
| Huynh 2023 [38] | RC | E 1403 | MTF 1403 | 0 | 100 | 6.3 | 7.4 | Age, Gender, Race/Ethnicity, Cirrhosis Etiology, FIB-4, EV MELD-Na, CAD, Hba1c, medications | Compensated T2DM | Prior decompensations Biliary Cirrhosis, T1DM | All-cause mortality significantly decreased (HR 0.57, CI: 0.41–0.81) | 260 |
| p < 0.01 | ||||||||||||
| Singh 2024 [35] | RCT | D 20 | SoCD 20 | 100 | 20 | 22.0 | 22.0 | Age, Gender, Cirrhosis Etiology, MELD-Na, Ascites Grade | Refractory ascites within 1 year | eGFR < 60, PVT, HCC, GIB, HE, Hypoglycemia, Hyponatremia, AKI, infection in past month | Significantly higher complete or partial control of ascites (70% vs. 35%, p = 0.04) | 26 |
| Bakosh 2024 [34] | RCT | E 21 | SoCD 21 | 100 | 0 | 19.0 | 14.0 | Age, Gender, Cirrhosis Etiology, MELD-Na/CP, Diabetes, Weight | Refractory ascites for >3 months | EtOH use, Hypotension, DKA | Significantly lower LVP need (42.9% vs. 100%, p < 0.01) | 12 |
| p = 0.306 | ||||||||||||
| Ayoub 2024 [39] | RC | NR 8038 | SoCD 4019 | 100 | NR | NR | Patient characteristics and comorbidities | Decompensated receiving SoCD | Alcoholic cirrhosis | Significantly lower rate of new decompensation at 1 month (28% vs. 39.2%, p < 0.001), 3 months (38% vs. 49.6%, p < 0.001), and 6 months (43.6% vs. 55.2%, p < 0.001) in the group receiving SGLT2I. | 26 | |
| Seif El-Din 2024 [40] | PC | D 200 | Insulin 100 | 73 | 24 | 14.4 | 17.5 | Age, Gender, Cirrhosis Etiology CP | T2DM | Renal Impairment, Active Decompensation., Non-Insulin/SGLT2I DM treatments, T1DM | No primary outcome reported | 12 |
| p = NR | ||||||||||||
| Author Year | Design | SGLT2I (n) | MASLD (%) | Decompensated (%) | Initial Condition | Clinically Significant Findings | End Point (Weeks) |
|---|---|---|---|---|---|---|---|
| Montalvo-Gordon 2020 [18] | CS | E (3) | 100 | 100 | Refractory ascites, with SoCD not tolerated, HE | Mean 7.5 kg weight loss associated with improved ascites control, normalization of hyponatremia | 24 |
| Saffo 2020 [41] | RCR | E (33) C (17) D (13) O (15) | 50 | 19 | Mainly compensated | 10.2% developed NARHDs, 17% ascites, 3.8% liver mortality, 9.0% adverse events (85% mycotic genital infection), 1 patient had surgery for balanitis, no AKI or electrolyte disturbances | 109 |
| Chao 2020 [32] | CS | E(1) D(1) | 0 | 0 | Child Pugh A with chronic alcoholism for >10 years, Hba1c-8.4–8.5 | Euglycemic DKA developed 3 days and 3 weeks after SGLT2I initiation | 3 |
| Kalambokis 2021 [17] | CR | E (1) | 0 | 100 | Hepatic Hydothorax not tolerating SoCD with recent HRS-AKI, Hyponatremia | Resolution of ascites and hyponatremia, no further clinical decompensations | 16 |
| Miyamoto 2021 [16] | CR | E (1) | 100 | 100 | 6 rounds of CART for refractory ascites | Maintained ascites control off SoCD | 12 |
| Sharma 2023 [42] | PT | D (20) | 85 | 0 | All compensated patients with CSPH, mean CP~6. | 3.4 kg weight loss | 24 |
| Shen 2024 [43] | PT | E (10) | 10 | 50 | Refractory ascites, with SoCD not tolerated (71%) or not working (29%) | Tolerated in decompensated cirrhosis with adverse events similar to heart failure and CKD data | 4 |
| Kalambokis 2024 [44] | PT | E (14) | NR | 100 | Refractory ascites, 50% had Na < 130, CP > 12 | 7 kg weight loss, marked increase in natriuresis, reduction in hyperdynamic circulation, reduction of RAAS activity. | 12 |
| Seidita 2024 [45] | CS (4) | D (4) | 75 | 100 | Severe abdominal ascites | 75% ascites resolution and 2–3 pt. reduction in CP, normalization of mild hyponatremia | 24 |
| Hu 2024 [46] | PT | E (8) | 50 | 100 | Refractory ascites | Furosemide reduced from 80 mg to 40 mg | 12 |
| Cochrane Risk of Bias 2.0 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Randomization | Intervention Deviation | Missing Data | Outcome Measurement | Outcome Reporting | Total | Notes | ||||
| Bakosh 2024 [34] | Low | Some | Some | Low | Low | Some Risk | Patients not blinded. 10% (n = 2) of SGLT2I group withdrew to AKI. | ||||
| Singh 2024 [35] | Low | Low | Low | Low | Low | Low Risk | Low risk | ||||
| NIH Quality Assessment Tool for Case Series Studies | |||||||||||
| Studies | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Notes | |
| Seidita 2024 [45] | Yes | Yes | Yes | Partially | Yes | Yes | Yes | No | Yes | Major limitations are small sample size and high drop-out rate in addition to limited statistical analysis and lack of comparison group. Heterogeneity in cirrhosis etiology and baseline characteristics. | |
| Saffo 2020 [41] | Yes | Yes | Yes | Partially | Yes | Yes | Yes | Yes | Yes | Retrospective, single center design, and lack of comparison group. Heterogeneity in etiology, disease severity, and treatments. | |
| Chao 2020 [32] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | N/A | Yes | Small sample size, single center design, and lack of comparison group which limits generalizability | |
| Hu 2024 [46] | Yes | Yes | Yes | Partially | Yes | Yes | Yes | Yes | Yes | Small sample size, single center design, lack of comparison group, and relatively short follow-up period which limits generalizability. Heterogeneity in etiology and comorbidities | |
| Sharma 2023 [42] | Yes | Yes | Yes | Partially | Yes | Yes | Yes | Yes | Yes | Single center design, lack of comparison group, and high screen failure rate. This study only included compensated patients which may introduce selection bias. Heterogeneity in etiology and treatments which limits comparisons between patients. | |
| Montalvo-Gordon 2020 [18] | Yes | Yes | Yes | Partially | Yes | Yes | Yes | Yes | Yes | Small sample size (n = 2) and heterogeneity in etiology. | |
| Shen 2024 [43] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Small sample size (n = 10), relatively short follow-up, single-center study | |
| Kalambokis 2024 [44] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Small sample size (n = 14). Single-center study with no control group and open-label design. | |
| ROBINS-I V2 for Observational Studies | |||||||||||
| Studies | Confounding | Selection Bias | Classification | Deviation | Missing Data | Measurement of Outcome | Selection Reporting | Overall | Notes | ||
| Huynh 2023 [38] | Moderate | Low | Low | Low | Low | Low | Low | Moderate | MELDNa higher in control group. | ||
| Saffo 2021 [37] | Low | Low | Low | Low | Low | Low | Low | Low | - | ||
| Seif El-Din 2024 [40] | High | Low | Low | Low | Low | Low | High | High | Suboptimal control group. No primary or secondary outcomes declared prospectively. | ||
| Ayoub 2024 [39] | Unclear | Moderate | High | Low | Low | Low | Low | High | Unable to classify indication for SGLT2I initiation and prevalence of refractory ascites. propensity-matched baseline characteristics not available. | ||
| Outcome | Associated with SGLT2I | No Difference (No Incidence in Single-Arm) | Associated with Control (or Opposite Effect in Single Arm) |
|---|---|---|---|
| Ascites Reduction | Refractory: Singh 2024 [35], Bakosh 2024 [34], Montalvo-Gordon 2020 [18], Kalambokis 2021 [17], Miyamoto 2021 [16], Shen [43], Kalambokis 2024 [44], Hu 2024 [46] Decompensated: Seif El-Din 2024 [40], Ayoub 2024 [39], Seidita 2024 [45] | ||
| Slowed Disease Progression | Refractory: Bakosh 2024 [34], Kalambokis 2021 [17] Decompensated: Seidita 2024 [45], Seif El Din 2024 [40], Ayoub 2024 [39] Compensated: Huynh 2023 [38], Sharma 2023 [42] | Refractory: Singh 2024 [35] Compensated: Saffo 2021 [37] | |
| Hemodynamic Instability | Refractory: Shen 2024 [43], Hu 2024 [46] | Refractory: Singh 2024 [35], Bakosh 2024 [34], Miyamoto 2021 [16], Kalambokis 2021 [17] Decompensated: Seif El-Din 2024 [40] Compensated: Sharma 2023 [42] | Refractory: Kalambokis 2024 [44] |
| AKI/HRS Risk | Refractory: Singh 2024 [35], Hu 2024 [46] | Refractory: Bakosh 2024 [34], Kalambokis 2021 [17], Miyamoto 2021 [16], Shen 2024 [43], Kalambokis 2024 [44] Compensated: Saffo 2020 [41], Sharma 2023 [42] | |
| Electrolyte/Acid Base abnormalities (Hyponatremia, Hypokalemia, Ketoacidosis) | Refractory: Bakosh 2024 [34], Chao 2020 [32] | Refractory: Singh 2024 [35], Hu 2024 [46] Decompensated: Seif El Din 2024 [40] Compensated: Saffo 2020 [41] | Refractory: Montalvo-Gordon 2021 [18], Kalambokis 2021 [17], Miyamoto 2021 [17], Kalambokis 2024 [44], Shen [43] Decompensated: Seidita 2024 [45] Compensated: Sharma 2023 [42] |
| Infection Risk (all infections) | Refractory: Singh 2024 [35] Compensated: Saffo 2020 [41] | Refractory: Bakosh 2024 [34], Kalambokis 2024 [44] | Decompensated: Seif-El Din 2024 [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhoop, S.; Ghazaleh, S.; Roberts, L.; Shehada, M.; Patel, M.; Smith, W.-L.; Rabeeah, S.; Sawaf, B.; Vadehra, P.; Hart, B.; et al. Sodium-Glucose Cotransporter-2 Inhibitors in Liver Cirrhosis: A Systematic Review of Their Role in Ascites Management, Slowing Disease Progression, and Safety. Int. J. Mol. Sci. 2025, 26, 4781. https://doi.org/10.3390/ijms26104781
Dhoop S, Ghazaleh S, Roberts L, Shehada M, Patel M, Smith W-L, Rabeeah S, Sawaf B, Vadehra P, Hart B, et al. Sodium-Glucose Cotransporter-2 Inhibitors in Liver Cirrhosis: A Systematic Review of Their Role in Ascites Management, Slowing Disease Progression, and Safety. International Journal of Molecular Sciences. 2025; 26(10):4781. https://doi.org/10.3390/ijms26104781
Chicago/Turabian StyleDhoop, Sudheer, Sami Ghazaleh, Luke Roberts, Mohammed Shehada, Manthanbhai Patel, Wade-Lee Smith, Sana Rabeeah, Bisher Sawaf, Priya Vadehra, Benjamin Hart, and et al. 2025. "Sodium-Glucose Cotransporter-2 Inhibitors in Liver Cirrhosis: A Systematic Review of Their Role in Ascites Management, Slowing Disease Progression, and Safety" International Journal of Molecular Sciences 26, no. 10: 4781. https://doi.org/10.3390/ijms26104781
APA StyleDhoop, S., Ghazaleh, S., Roberts, L., Shehada, M., Patel, M., Smith, W.-L., Rabeeah, S., Sawaf, B., Vadehra, P., Hart, B., & Hassan, M. (2025). Sodium-Glucose Cotransporter-2 Inhibitors in Liver Cirrhosis: A Systematic Review of Their Role in Ascites Management, Slowing Disease Progression, and Safety. International Journal of Molecular Sciences, 26(10), 4781. https://doi.org/10.3390/ijms26104781






