Some Properties of the C. elegans Multicopper Oxidase F21D5.3, an Ortholog of Human Ceruloplasmin
Abstract
1. Introduction
2. Results
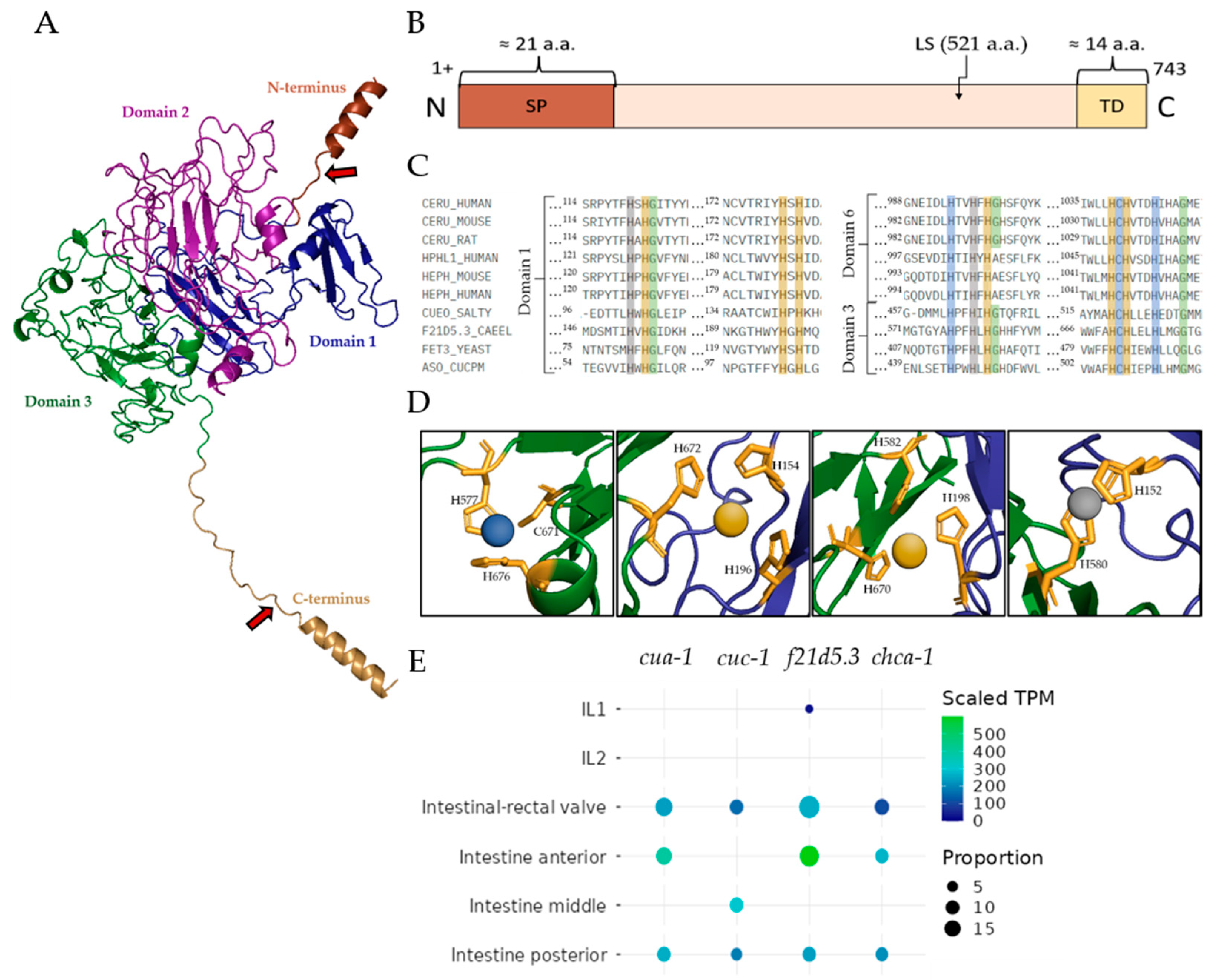
2.1. Description of the Putative Cp-like Protein F21D5.3
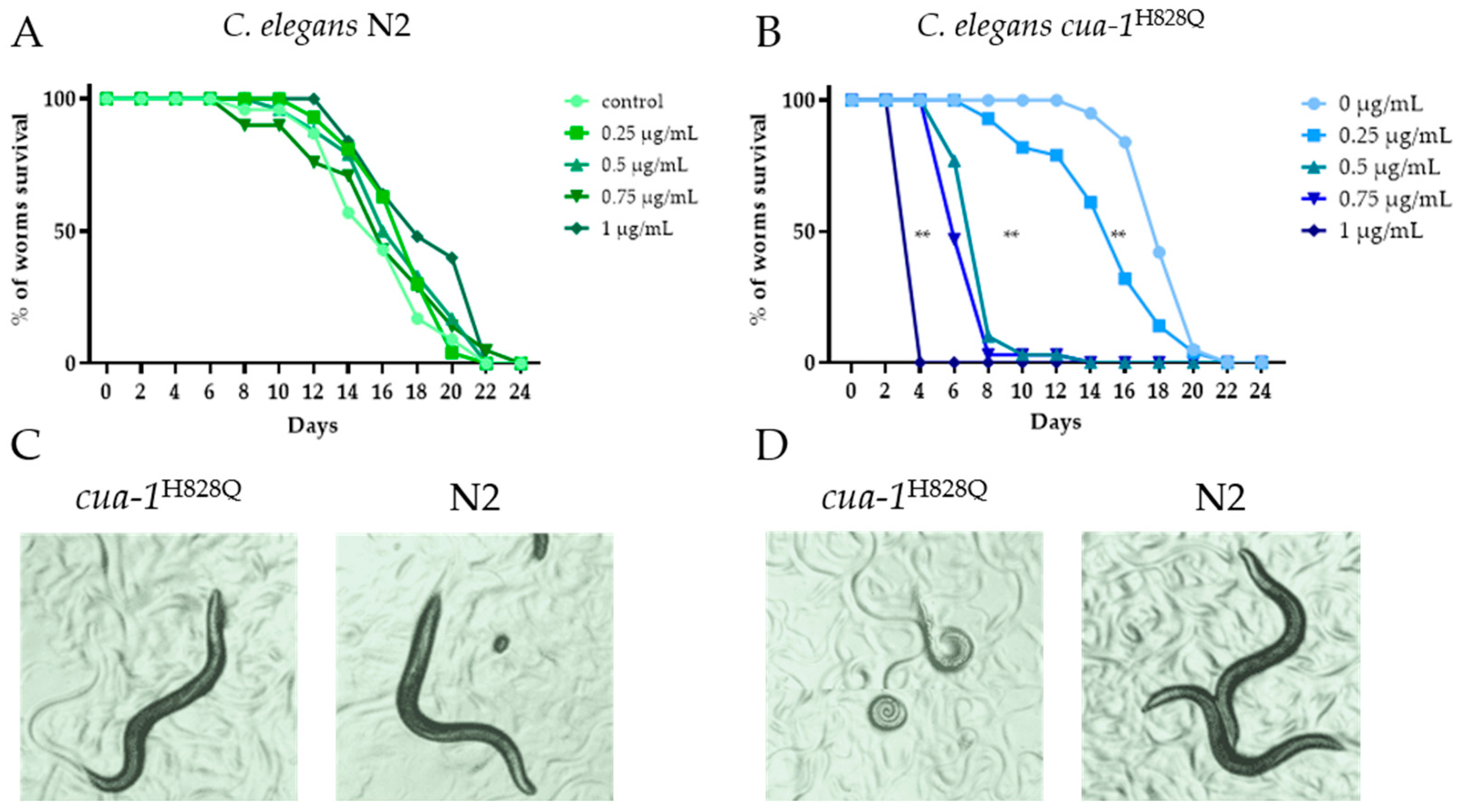
2.2. Effect of Silver Nanoparticle Treatment on the Worm Lifespan
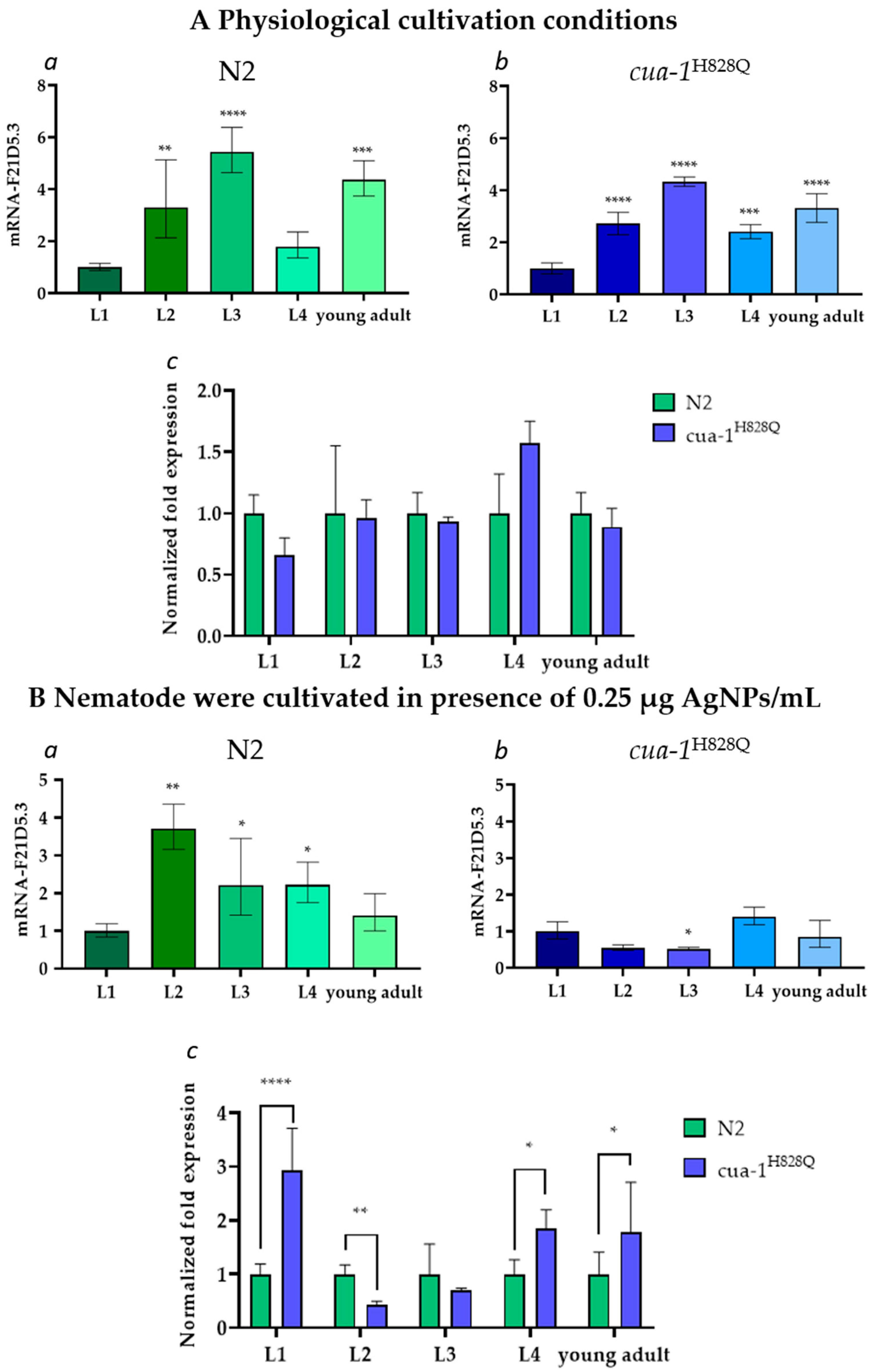
2.3. The F21D5.3 Gene Expression During the Life Cycle of N2 and cua-1H828Q Strains
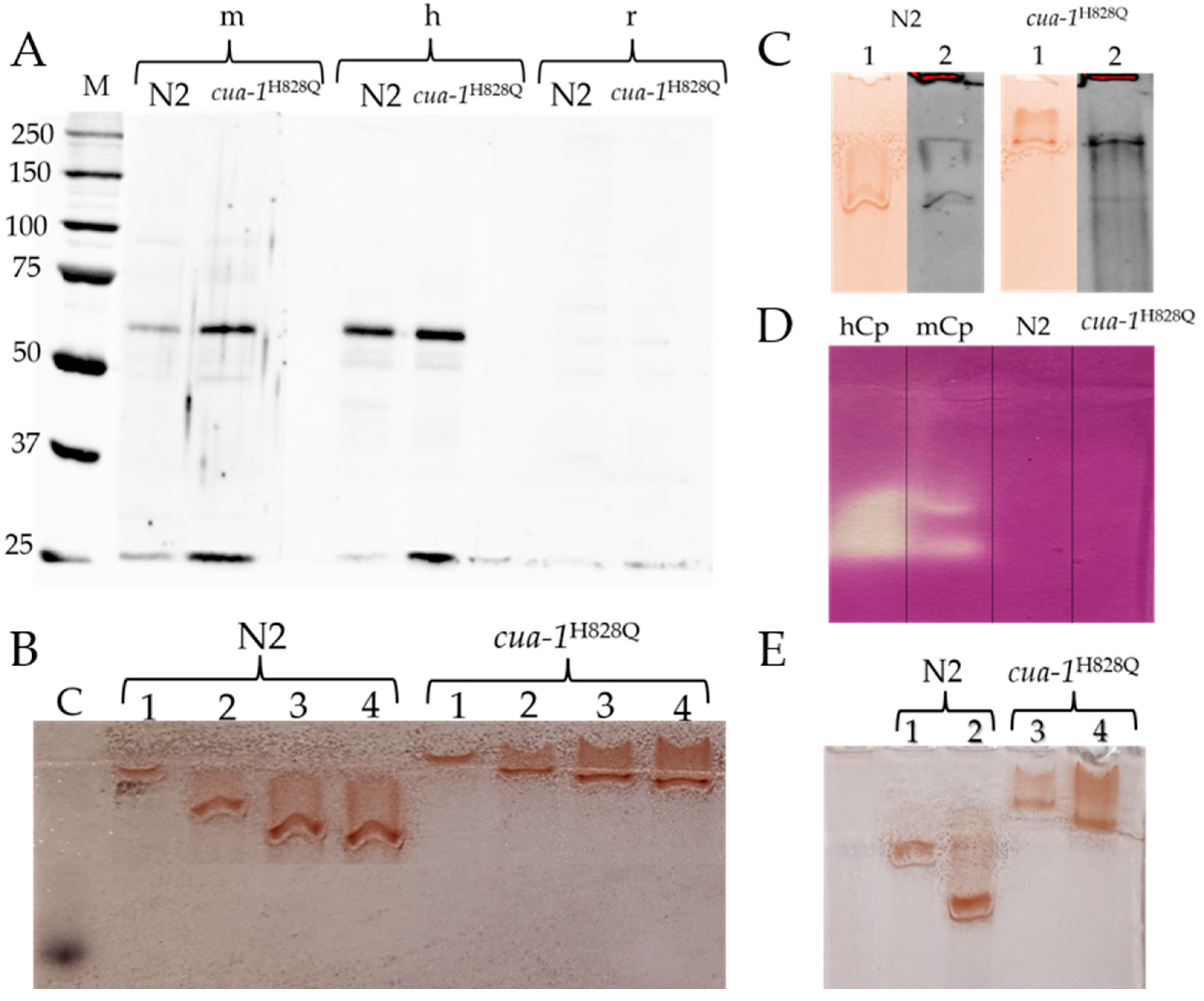
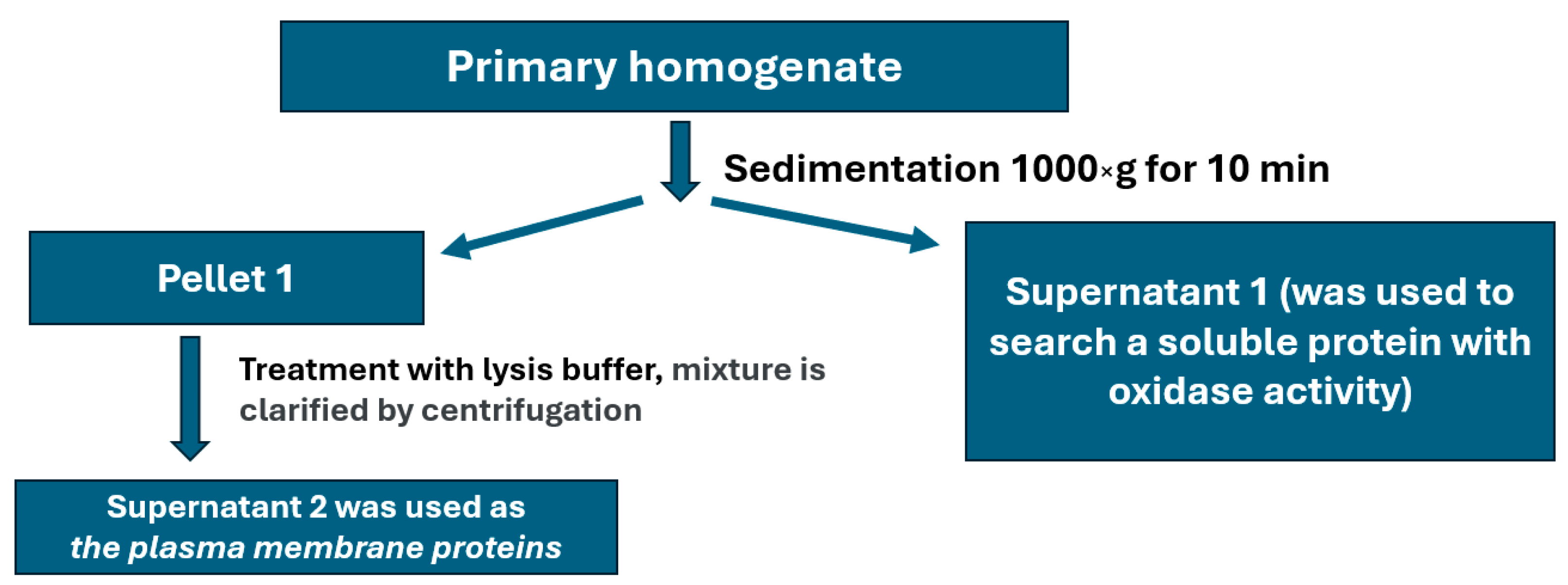
2.4. The Search for Antigenic, Oxidase, and Ferroxidase Activities in the Plasma Membrane Proteins of N2 and cua-1H828Q Strains
3. Discussion
4. Materials and Methods
4.1. Worm Strains and Cultivation Conditions
4.2. Synchronization of Nematodes for Liquid Conditions
4.3. Worm Cultivation in Liquid Culture
4.4. Silver Nanoparticles (AgNPs) Characteristics
4.5. Treatment of Worms with AgNPs
4.6. Lifespan
4.7. Isolation of Plasma Membrane Fraction
4.8. Gene Expression Analyses
4.9. Western Blotting (WB)
4.10. Determination of Oxidase and Ferroxidase Activity
4.11. Atomic Absorption Spectroscopy
4.12. In Silico Analysis
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Philipp, T.M.; Gernoth, L.; Will, A.; Schwarz, M.; Ohse, V.A.; Kipp, A.P.; Steinbrenner, H.; Klotz, L.-O. Selenium-binding protein 1 (SELENBP1) is a copper-dependent thiol oxidase. Redox Biol. 2023, 65, 102807. [Google Scholar] [CrossRef] [PubMed]
- Lutsenko, S.; Roy, S.; Tsvetkov, P. Mammalian copper homeostasis: Physiological roles and molecular mechanisms. Physiol. Rev. 2025, 105, 441–491. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Nepovimova, E.; Kuca, K.; Valko, M. Heavy metals: Toxicity and human health effects. Arch. Toxicol. 2025, 99, 153–209. [Google Scholar] [CrossRef] [PubMed]
- Han, J. Copper trafficking systems in cells: Insights into coordination chemistry and toxicity. Dalton Trans. 2023, 52, 15277–15296. [Google Scholar] [CrossRef]
- Vogt, S.; Ralle, M. Opportunities in multidimensional trace metal imaging: Taking copper-associated disease research to the next level. Anal. Bioanal. Chem. 2013, 405, 1809–1820. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jiang, Y.; Shi, H.; Peng, Y.; Fan, X.; Li, C. The molecular mechanisms of copper metabolism and its roles in human diseases. Pflugers Arch. 2020, 472, 1415–1429. [Google Scholar] [CrossRef]
- Abdeen, A.H.; Trist, B.G.; Double, K.L. Empirical evidence for biometal dysregulation in Parkinson’s disease from a systematic review and Bradford Hill analysis. NPJ Parkinsons Dis. 2022, 8, 83. [Google Scholar] [CrossRef]
- Li, Y.; Han, Y.; Shu, Q.; Kan, Y.-K.; Wang, Z. Cuproptosis and copper as potential mechanisms and intervention targets in Alzheimer’s disease. Biomed. Pharmacother. 2025, 183, 117814. [Google Scholar] [CrossRef]
- Xue, Q.; Kang, R.; Klionsky, D.J.; Tang, D.; Liu, J.; Chen, X. Copper metabolism in cell death and autophagy. Autophagy 2023, 19, 2175–2195. [Google Scholar] [CrossRef]
- Eljazzar, S.; Abu-Hijleh, H.; Alkhatib, D.; Sokary, S.; Ismail, S.; Al-Jayyousi, G.F.; Tayyem, R. The Role of Copper Intake in the Development and Management of Type 2 Diabetes: A Systematic Review. Nutrients 2023, 15, 1655. [Google Scholar] [CrossRef]
- Incecik, F.; Bisgin, A.; Yılmaz, M. MEDNIK syndrome with a frame shift causing mutation in AP1S1 gene and literature review of the clinical features. Metab. Brain Dis. 2018, 33, 2065–2068. [Google Scholar] [CrossRef]
- Blades, B.; Ayton, S.; Hung, Y.H.; Bush, A.I.; La Fontaine, S. Copper and lipid metabolism: A reciprocal relationship. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129979. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Harada, M. Wilson disease and hepatocellular carcinoma. Intern. Med. 2004, 43, 1012–1013. [Google Scholar] [CrossRef]
- Petruzzelli, R.; Catalano, F.; Crispino, R.; Polishchuk, E.V.; Elia, M.; Masone, A.; Lavigna, G.; Grasso, A.; Battipaglia, M.; Sepe, L.V.; et al. Prion protein promotes copper toxicity in Wilson disease. Nat. Commun. 2025, 16, 1468. [Google Scholar] [CrossRef]
- Polishchuk, E.V.; Merolla, A.; Lichtmannegger, J.; Romano, A.; Indrieri, A.; Ilyechova, E.Y.; Concilli, M.; De Cegli, R.; Crispino, R.; Mariniello, M.; et al. Activation of Autophagy, Observed in Liver Tissues from Patients with Wilson Disease and From ATP7B-Deficient Animals, Protects Hepatocytes from Copper-Induced Apoptosis. Gastroenterology 2019, 156, 1173–1189.e5. [Google Scholar] [CrossRef] [PubMed]
- Vonk, W.I.; Wijmenga, C.; van de Sluis, B. Relevance of animal models for understanding mammalian copper homeostasis. Am. J. Clin. Nutr. 2008, 88, 840S–845S. [Google Scholar] [CrossRef] [PubMed]
- Zischka, H.; Lichtmannegger, J. Pathological mitochondrial copper overload in livers of Wilson’s disease patients and related animal models. Ann. N. Y. Acad. Sci. 2014, 1315, 6–15. [Google Scholar] [CrossRef]
- Reed, E.; Lutsenko, S.; Bandmann, O. Animal models of Wilson disease. J. Neurochem. 2018, 146, 356–373. [Google Scholar] [CrossRef]
- Philipp, T.M.; Gong, W.; Köhnlein, K.; Ohse, V.A.; Müller, F.I.; Priebs, J.; Steinbrenner, H.; Klotz, L.O. SEMO-1, a novel methanethiol oxidase in Caenorhabditis elegans, is a pro-aging factor conferring selective stress resistance. Biofactors 2022, 48, 699–706. [Google Scholar] [CrossRef]
- Ohse, V.A.; Klotz, L.O.; Priebs, J. Copper Homeostasis in the Model Organism C. elegans. Cells 2024, 13, 727. [Google Scholar] [CrossRef]
- Członkowska, A.; Litwin, T.; Dusek, P.; Ferenci, P.; Lutsenko, S.; Medici, V.; Rybakowski, J.K.; Weiss, K.H.; Schilsky, M.L. Wilson disease. Nat. Rev. Dis. Primers 2018, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Chun, H.; Sharma, A.K.; Lee, J.; Chan, J.; Jia, S.; Kim, B.-E. The Intestinal Copper Exporter CUA-1 Is Required for Systemic Copper Homeostasis in Caenorhabditis elegans. J. Biol. Chem. 2017, 292, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Catalano, F.; O’Brien, T.J.; Mekhova, A.A.; Sepe, L.V.; Elia, M.; De Cegli, R.; Gallotta, I.; Santonicola, P.; Zampi, G.; Ilyechova, E.Y.; et al. A new Caenorhabditis elegans model to study copper toxicity in Wilson disease. Traffic 2024, 25, e12920. [Google Scholar] [CrossRef]
- Ovchinnikova, E.V.; Garbuz, M.M.; Ovchinnikova, A.A.; Kumeiko, V.V. Epidemiology of Wilson’s Disease and Pathogenic Variants of the ATP7B Gene Leading to Diversified Protein Disfunctions. Int. J. Mol. Sci. 2024, 25, 2402. [Google Scholar] [CrossRef]
- Vasin, A.; Klotchenko, S.; Puchkova, L. Phylogenetic analysis of six-domain multi-copper blue proteins. PLoS Curr. 2013, 5, ecurrents.tol.574bcb0f133fe52835911abc4e296141. [Google Scholar] [CrossRef]
- Skvortsov, A.N.; Ilyechova, E.Y.; Puchkova, L.V. Chemical background of silver nanoparticles interfering with mammalian copper metabolism. J. Hazard Mater. 2023, 451, 131093. [Google Scholar] [CrossRef]
- Jimenez-Arroyo, N.; Rudino-Pinera, E. Crystal Structure of Laccase from Thermus Thermophilus HB27 Complexed with Ag, Crystal of the Holoenzyme Soaked for 30 m in 5 mM AgNO3 at 278 K; PDB: Upton, NY, USA, 2016; (Deposited: 2015-01-19, Released: 2016-02-17); Available online: https://www.rcsb.org/structure/5afa (accessed on 25 December 2024).
- Orlov, I.A.; Sankova, T.P.; Babich, P.S.; Sosnin, I.M.; Ilyechova, E.Y.; A Kirilenko, D.; Brunkov, P.N.; Ataev, G.L.; E Romanov, A.; Puchkova, L.V. New silver nanoparticles induce apoptosis-like process in E. coli and interfere with mammalian copper metabolism. Int. J. Nanomed. 2016, 11, 6561–6574. [Google Scholar] [CrossRef]
- Ilyechova, E.Y.; Saveliev, A.N.; Skvortsov, A.N.; Babich, P.S.; Zatulovskaia, Y.A.; Pliss, M.G.; Korzhevskii, D.E.; Tsymbalenko, N.V.; Puchkova, L.V. The effects of silver ions on copper metabolism in rats. Metallomics 2014, 6, 1970–1987. [Google Scholar] [CrossRef]
- Sankova, T.P.; Sosnin, I.M.; Karpenko, M.N.; Ilyechova, E.Y.; Orlov, Y.A.; Polyakov, D.S.; Skomorokhova, E.A.; Sukhanova, A.S.; Rozhkova, N.A.; Babich, P.S.; et al. On the biological activity of silver nanoparticles. Mater. Phys. Mech. 2015, 24, 289–296. [Google Scholar]
- Skomorokhova, E.A.; Sankova, T.P.; Orlov, I.A.; Savelev, A.N.; Magazenkova, D.N.; Pliss, M.G.; Skvortsov, A.N.; Sosnin, I.M.; A Kirilenko, D.; Grishchuk, I.V.; et al. Size-Dependent Bioactivity of Silver Nanoparticles: Antibacterial Properties, Influence on Copper Status in Mice, and Whole-Body Turnover. Nanotechnol. Sci. Appl. 2020, 13, 137–157. [Google Scholar] [CrossRef] [PubMed]
- Babich, P.S.; Skvortsov, A.N.; Rusconi, P.; Tsymbalenko, N.V.; Mutanen, M.; Puchkova, L.V.; Broggini, M. Non-hepatic tumors change the activity of genes encoding copper trafficking proteins in the liver. Cancer Biol Ther. 2013, 14, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Xu, C.; Nagarajan, S.; Liu, Z.; O Hemphill, W.; Shi, R.; Uversky, V.N.; A Caldwell, G.; A Caldwell, K.; Witt, S.N. Alpha-synuclein inhibits Snx3-retromer-mediated retrograde recycling of iron transporters in S. cerevisiae and C. elegans models of Parkinson’s disease. Hum. Mol. Genet. 2018, 27, 1514–1532. [Google Scholar] [CrossRef] [PubMed]
- Weishaupt, A.K.; Lamann, K.; Tallarek, E.; Pezacki, A.T.; Matier, C.D.; Schwerdtle, T.; Aschner, M.; Chang, C.J.; Stürzenbaum, S.R.; Bornhorst, J. Dysfunction in atox-1 and ceruloplasmin alters labile Cu levels and consequently Cu homeostasis in C. elegans. Front. Mol. Biosci. 2024, 11, 1354627. [Google Scholar] [CrossRef]
- Magazenkova, D.N.; Skomorokhova, E.A.; Farroukh, M.A.; Zharkova, M.S.; Jassem, Z.M.; Rekina, V.E.; Shamova, O.V.; Puchkova, L.V.; Ilyechova, E.Y. Influence of Silver Nanoparticles on the Growth of Ascitic and Solid Ehrlich Adenocarcinoma: Focus on Copper Metabolism. Pharmaceutics 2013, 15, 1099. [Google Scholar] [CrossRef]
- Sokolov, A.V.; Kostevich, V.A.; Romanico, D.N.; Zakharova, E.T.; Vasilyev, V.B. Two-Stage Method for Purification of Ceruloplas min Based on Its Interaction with Neomycin. Biochemistry 2012, 77, 631–638. [Google Scholar]
- Owen, J.; Smith, H. Detection of ceruloplasmin after zone electrophoresis. Clin. Chim. Acta 1961, 6, 441–444. [Google Scholar] [CrossRef]
- Chen, H.; Attieh, Z.K.; Su, T.; Syed, B.A.; Gao, H.; Alaeddine, R.M.; Fox, T.C.; Usta, J.; Naylor, C.E.; Evans, R.W.; et al. Hephaestin is a ferroxidase that maintains partial activity in sex-linked anemia mice. Blood 2004, 103, 3933–3939. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Madeira, F.; Pearce, M.; Tivey, A.R.N.; Basutkar, P.; Lee, J.; Edbali, O.; Madhusoodanan, N.; Kolesnikov, A.; Lopez, R. Search and sequence analysis tools services from EMBL-EBI in 2022. Nucleic Acids Res. 2022, 50, W276–W279. [Google Scholar] [CrossRef]
- Packer, J.S.; Zhu, Q.; Huynh, C.; Sivaramakrishnan, P.; Preston, E.; Dueck, H.; Stefanik, D.; Tan, K.; Trapnell, C.; Kim, J.; et al. A lineage-resolved molecular atlas of C. elegans embryogenesis at single-cell resolution. Science 2019, 365, eaax1971. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samuseva, P.D.; Mekhova-Caramalac, A.A.; Catalano, F.; Shchukina, A.D.; Baikina, S.A.; Magazenkova, D.N.; Puchkova, L.V.; Ilyechova, E.Y. Some Properties of the C. elegans Multicopper Oxidase F21D5.3, an Ortholog of Human Ceruloplasmin. Int. J. Mol. Sci. 2025, 26, 4776. https://doi.org/10.3390/ijms26104776
Samuseva PD, Mekhova-Caramalac AA, Catalano F, Shchukina AD, Baikina SA, Magazenkova DN, Puchkova LV, Ilyechova EY. Some Properties of the C. elegans Multicopper Oxidase F21D5.3, an Ortholog of Human Ceruloplasmin. International Journal of Molecular Sciences. 2025; 26(10):4776. https://doi.org/10.3390/ijms26104776
Chicago/Turabian StyleSamuseva, Polina D., Aleksandra A. Mekhova-Caramalac, Federico Catalano, Anna D. Shchukina, Sofia A. Baikina, Daria N. Magazenkova, Ludmila V. Puchkova, and Ekaterina Yu. Ilyechova. 2025. "Some Properties of the C. elegans Multicopper Oxidase F21D5.3, an Ortholog of Human Ceruloplasmin" International Journal of Molecular Sciences 26, no. 10: 4776. https://doi.org/10.3390/ijms26104776
APA StyleSamuseva, P. D., Mekhova-Caramalac, A. A., Catalano, F., Shchukina, A. D., Baikina, S. A., Magazenkova, D. N., Puchkova, L. V., & Ilyechova, E. Y. (2025). Some Properties of the C. elegans Multicopper Oxidase F21D5.3, an Ortholog of Human Ceruloplasmin. International Journal of Molecular Sciences, 26(10), 4776. https://doi.org/10.3390/ijms26104776






