IGFBP-2 and IGF-II: Key Components of the Neural Stem Cell Niche? Implications for Glioblastoma Pathogenesis
Abstract
1. Introduction
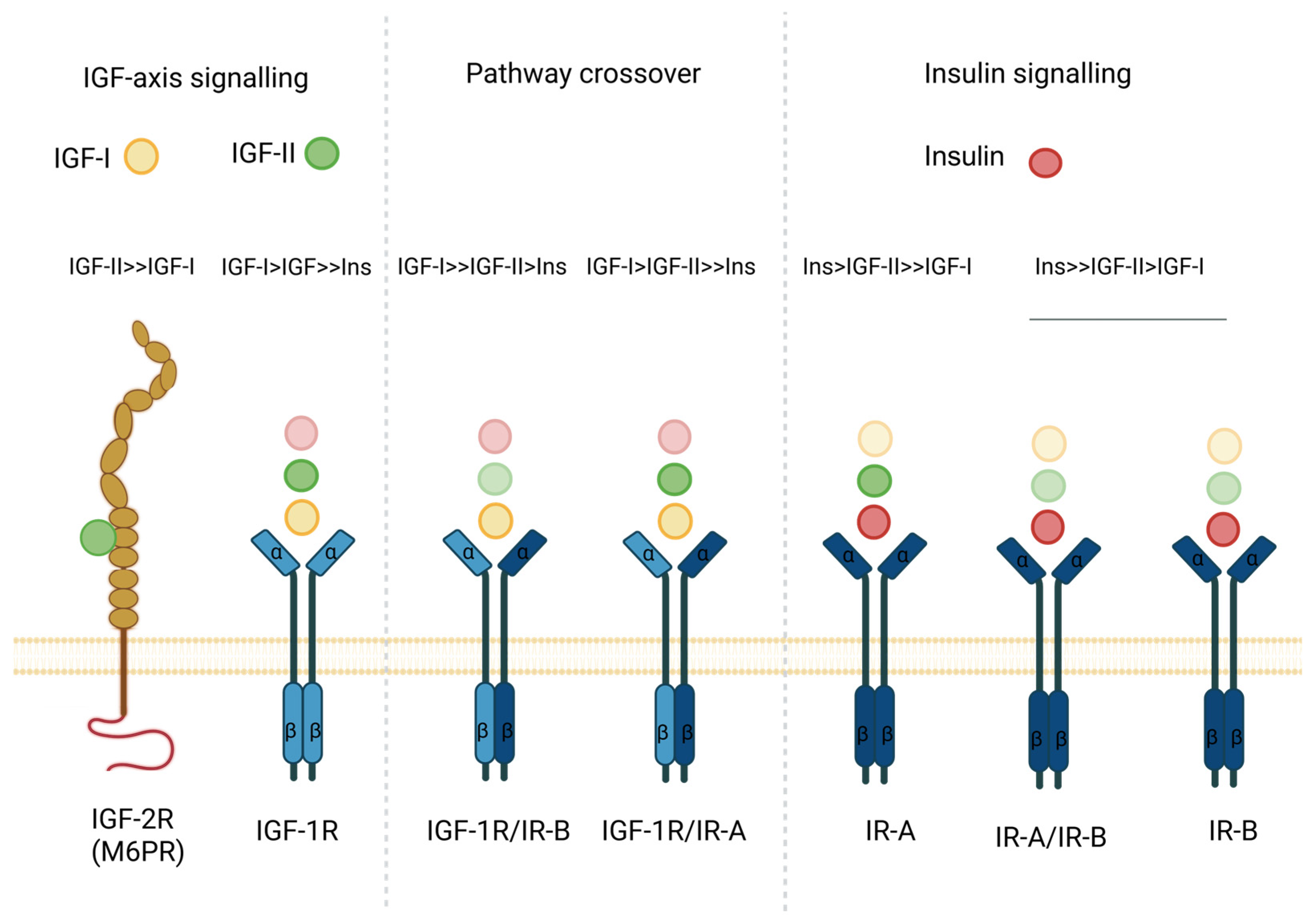
2. IGF and Insulin Axis Components
3. IGFBP-2, IGF-II and the Adult Human Brain
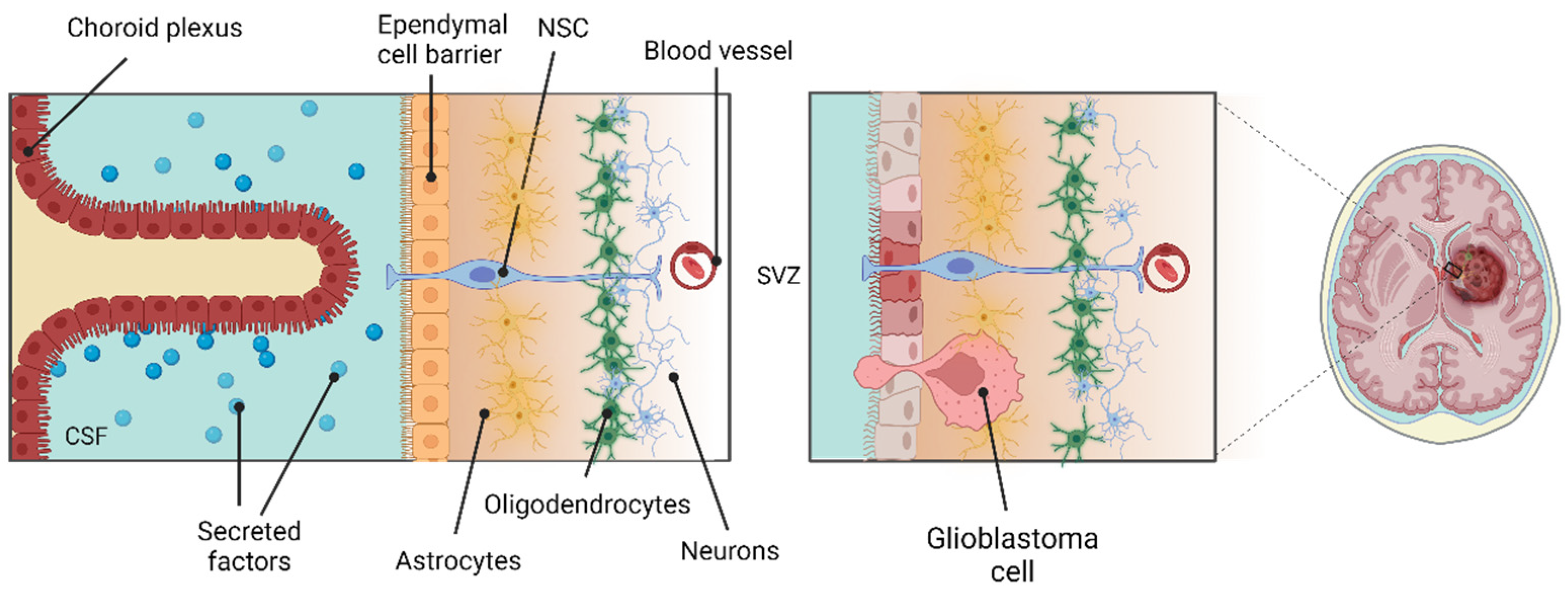
3.1. Neural Stem Cells Are Retained in the Adult Human Brain
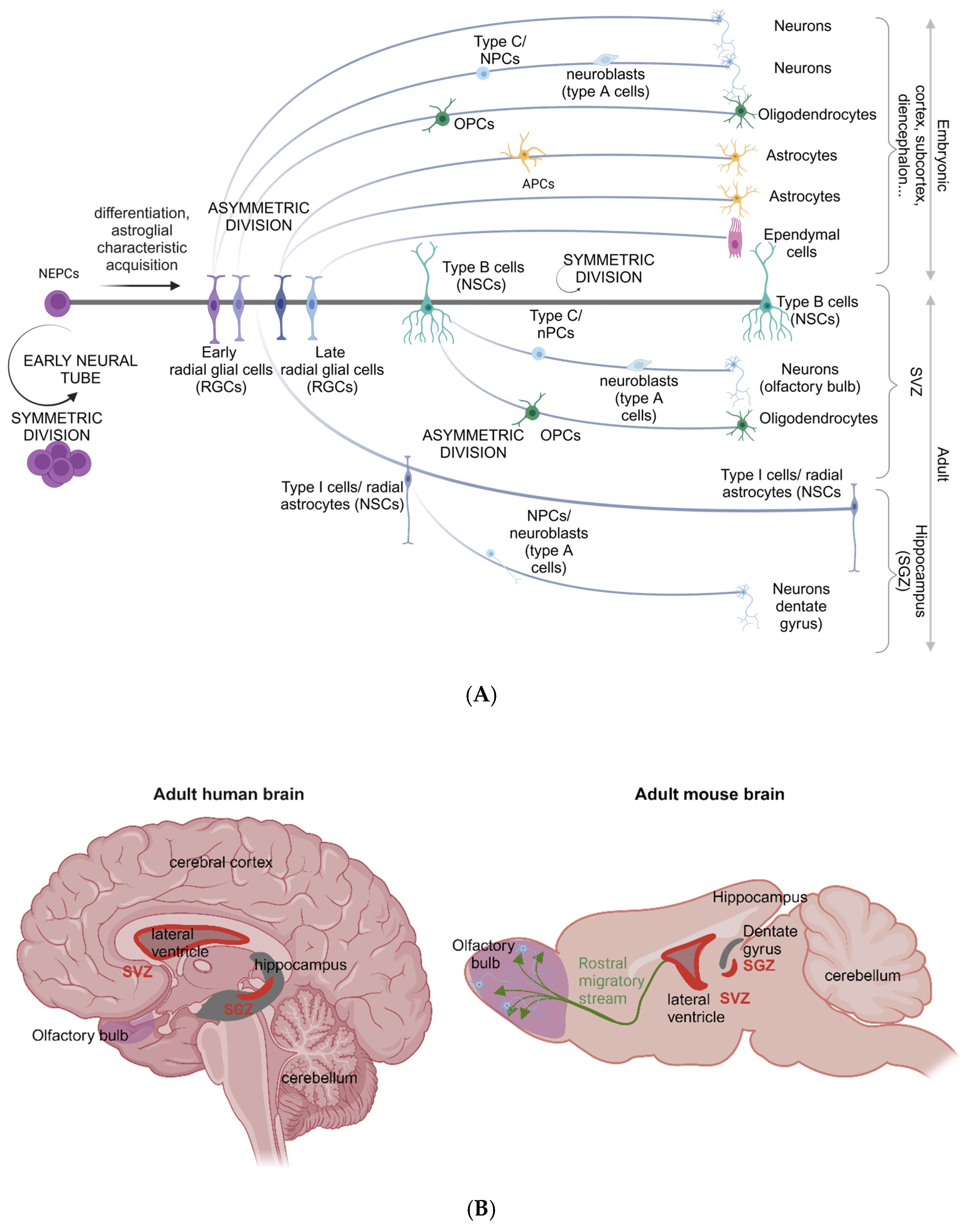
3.2. The Neural Stem Cell Secretome: IGFBP-2 and IGF-II
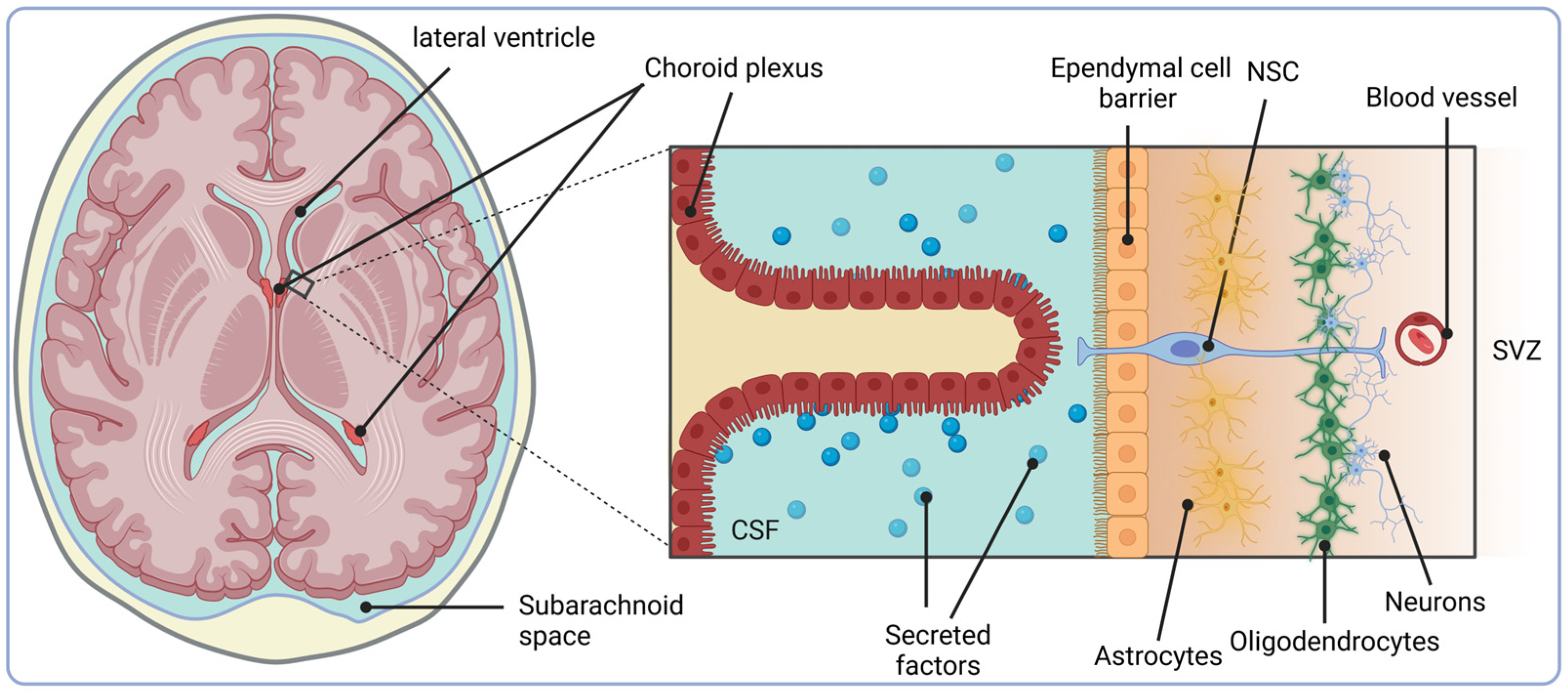
3.3. The SVZ Niche, IGFBP-2 and IGF-II
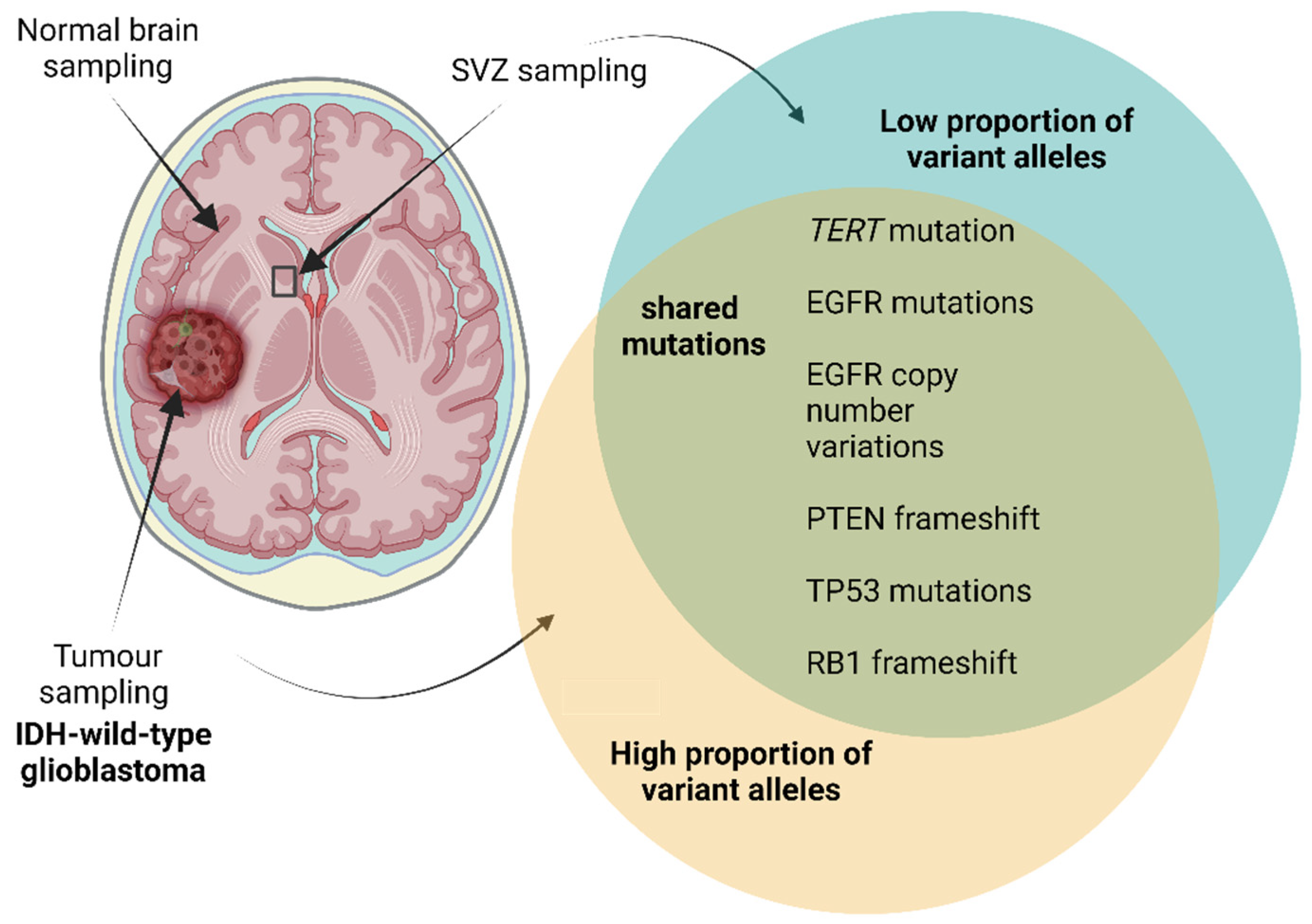
4. Glioblastoma: Identifying the Cell of Origin
Evidence for Neural Stem Cells as the Glioblastoma Cell of Origin
5. IGFBP-2, IGF-II and Glioblastoma
5.1. IGFBP-2 and Glioblastoma
5.2. IGF-II and Glioblastoma
6. Discussion
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| APC | Astrocyte progenitor cell |
| APOE | Apolipoprotein E |
| BBB | Blood–brain barrier |
| BMP | Bone morphogenic protein |
| CDK4 | Cyclin-dependent kinase 4 |
| CHI3L1 | Chitinase-3-like protein 1 |
| ChP | Choroid plexus |
| CM | Conditioned media |
| CNS | Central nervous system |
| CSC | Cancer stem cell |
| CSF | Cerebral spinal fluid |
| ECM | Extracellular matrix |
| EGF | Epidermal growth factor |
| EGFR | Epidermal growth factor receptor |
| ERK | Extracellular signal-related kinase |
| ESC | Embryonic stem cell |
| FABP7 | Fatty acid binding protein 7 |
| FAK | Focal adhesion kinase |
| FBS | Foetal bovine serum |
| FGF | Fibroblast growth factor |
| GFAP | Glial fibrillary acidic protein |
| GSC | Glioma stem cell |
| IDH | Isocitrate dehydrogenase |
| IGF | Insulin-like growth factor |
| IGF-1R | Insulin-like growth factor I receptor |
| IGF-2R | Insulin-like growth factor II receptor |
| IGFBP | Insulin-like growth factor binding protein |
| IHC | Immunohistochemistry |
| ILK | Integrin linked kinase |
| IQGAP1 | IQ motif containing GTPase-activating protein 1 |
| IR | Insulin receptor |
| IRS | Insulin receptor substrate |
| ISH | In situ hybridisation |
| JNK | JUN NH2 terminal kinase |
| LC-MS/MS | Liquid chromatography with tandem mass spectrometry |
| LGALS3 | Galectin 3 |
| M6P | Mannose-6-phosphate |
| MAPK | Mitogen-activated protein kinase |
| MDM2 | Mouse double minute 2 homolog |
| MEK | Mitogen-activated protein kinase |
| MMP | Matrix metalloproteinase |
| mRNA | Messenger ribonucleic acid |
| NF1 | Neurofibromatosis type 1 |
| NPC | Neural precursor cell |
| NSC | Neural stem cell |
| NSPC | Neural stem/progenitor cell |
| OLIG2 | Oligodendrocyte transcription factor 2 |
| OPC | Oligodendrocyte precursor cell |
| OS | Overall survival |
| PDGFR | Platelet-derived growth factor receptor |
| PFS | Progression free survival |
| PI3K | Phosphatidylinositol-3-kinase |
| PTEN | Phosphatase and tensin homolog |
| qPCR | Quantitative polymerase chain reaction |
| RB | Retinoblastoma protein |
| Rembrandt | Repository for Molecular Brain Neoplasia Data |
| RGC | Radial glial cell |
| RGD | Arginine–glycine–aspartate |
| RNA | Ribonucleic acid |
| ROS | Reactive oxygen species |
| Shh | Sonic hedgehog |
| SOX2 | Sex determining region Y-box 2 |
| STAT3 | Signal transducer and activator of transcription 3 |
| SVZ | Subventricular zone |
| SGZ | Subgranular zone |
| TERT | Telomerase reverse transcriptase |
| TGF | Transforming growth factor |
| TIMP1 | Tissue inhibitor matrix metalloproteinase 1 |
| TMZ | Temozolomide |
| TP53 | Tumour protein p53 |
| VEGF | Vascular endothelial growth factor |
| WHO | World Health Organisation |
References
- Brodbelt, A.; Greenberg, D.; Winters, T.; Williams, M.; Vernon, S.; Collins, V.P. Glioblastoma in England: 2007–2011. Eur. J. Cancer 2015, 51, 533–542. [Google Scholar] [CrossRef] [PubMed]
- David, N.; Louis, A.P. Pieter Wesseling, Daniel J Brat, Ian A Cree, Dominique Figarella-Branger, Cynthia Hawkins, H K Ng, Stefan M Pfister, Guido Reifenberger, Riccardo Soffietti, Andreas von Deimling, David W Ellison. In WHO Classification of Central Nervous System Tumour, 5th ed.; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro-Oncol. 2019, 21 (Suppl. S5), v1–v100. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qi, D.; Li, J.; Quarles, C.C.; Fonkem, E.; Wu, E. Assessment and prediction of glioblastoma therapy response: Challenges and opportunities. Brain 2023, 146, 1281–1298. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gerber, N.K.; Goenka, A.; Turcan, S.; Reyngold, M.; Makarov, V.; Kannan, K.; Beal, K.; Omuro, A.; Yamada, Y.; Gutin, P.; et al. Transcriptional diversity of long-term glioblastoma survivors. Neuro-Oncol. 2014, 16, 1186–1195. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kalya, M.; Kel, A.; Wlochowitz, D.; Wingender, E.; Beißbarth, T. IGFBP2 Is a Potential Master Regulator Driving the Dysregulated Gene Network Responsible for Short Survival in Glioblastoma Multiforme. Front. Genet. 2021, 12, 670240. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, F.; Li, Y.; Zhang, K.; Li, Y.; He, P.; Liu, Y.; Yuan, H.; Lu, H.; Liu, J.; Che, S.; et al. FBLN4 as candidate gene associated with long-term and short-term survival with primary glioblastoma. Onco Targets Ther. 2017, 10, 387–395. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lindström, M.S. Expanding the scope of candidate prognostic marker IGFBP2 in glioblastoma. Biosci. Rep. 2019, 39, BSR20190770. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yuan, Q.; Cai, H.-Q.; Zhong, Y.; Zhang, M.-J.; Cheng, Z.-J.; Hao, J.-J.; Wang, M.-R.; Wan, J.-H. Overexpression of IGFBP2 mRNA predicts poor survival in patients with glioblastoma. Biosci. Rep. 2019, 39, BSR20190045. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Holmes, K.M.; Annala, M.; Chua, C.Y.X.; Dunlap, S.M.; Liu, Y.; Hugen, N.; Moore, L.M.; Cogdell, D.; Hu, L.; Nykter, M.; et al. Insulin-like growth factor-binding protein 2-driven glioma progression is prevented by blocking a clinically significant integrin, integrin-linked kinase, and NF-κB network. Proc. Natl. Acad. Sci. USA 2012, 109, 3475–3480. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Y.; Li, F.; Yang, Y.T.; Xu, X.D.; Chen, J.S.; Chen, T.L.; Chen, H.J.; Zhu, Y.B.; Lin, J.Y.; Li, Y.; et al. IGFBP2 promotes vasculogenic mimicry formation via regulating CD144 and MMP2 expression in glioma. Oncogene 2019, 38, 1815–1831. [Google Scholar] [CrossRef] [PubMed]
- Mendes, K.N.; Wang, G.K.; Fuller, G.N.; Zhang, W. JNK mediates insulin-like growth factor binding protein 2/integrin alpha5-dependent glioma cell migration. Int. J. Oncol. 2010, 37, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.K.; Hu, L.; Fuller, G.N.; Zhang, W. An interaction between insulin-like growth factor-binding protein 2 (IGFBP2) and integrin alpha5 is essential for IGFBP2-induced cell mobility. J. Biol. Chem. 2006, 281, 14085–14091. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Li, Z.; Master, L.M.; Master, Z.W.; Wu, A. Exogenous IGFBP-2 promotes proliferation, invasion, and chemoresistance to temozolomide in glioma cells via the integrin β1-ERK pathway. Br. J. Cancer 2014, 111, 1400–1409. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Loras, A.; Gonzalez-Bonet, L.G.; Gutierrez-Arroyo, J.L.; Martinez-Cadenas, C.; Marques-Torrejon, M.A. Neural Stem Cells as Potential Glioblastoma Cells of Origin. Life 2023, 13, 905. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pollard, S.M.; Yoshikawa, K.; Clarke, I.D.; Danovi, D.; Stricker, S.; Russell, R.; Bayani, J.; Head, R.; Lee, M.; Bernstein, M.; et al. Glioma stem cell lines expanded in adherent culture have tumor-specific phenotypes and are suitable for chemical and genetic screens. Cell Stem Cell 2009, 4, 568–580. [Google Scholar] [CrossRef] [PubMed]
- Bracko, O.; Singer, T.; Aigner, S.; Knobloch, M.; Winner, B.; Ray, J.; Clemenson, G.D.; Suh, H.; Couillard-Despres, S.; Aigner, L.; et al. Gene expression profiling of neural stem cells and their neuronal progeny reveals IGF2 as a regulator of adult hippocampal neurogenesis. J. Neurosci. 2012, 32, 3376–3387. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferrón, S.R.; Radford, E.J.; Domingo-Muelas, A.; Kleine, I.; Ramme, A.; Gray, D.; Sandovici, I.; Constancia, M.; Ward, A.; Menheniott, T.R.; et al. Differential genomic imprinting regulates paracrine and autocrine roles of IGF2 in mouse adult neurogenesis. Nat. Commun. 2015, 6, 8265. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Massagué, J.; Czech, M.P. The subunit structures of two distinct receptors for insulin-like growth factors I and II and their relationship to the insulin receptor. J. Biol. Chem. 1982, 257, 5038–5045. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Jones, E.Y.; Forbes, B.E. Interactions of IGF-II with the IGF2R/cation-independent mannose-6-phosphate receptor mechanism and biological outcomes. Vitam. Horm. 2009, 80, 699–719. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Fung, M.R.; Barlow, D.P.; Wagner, E.F. Regulation of embryonic growth and lysosomal targeting by the imprinted Igf2/Mpr gene. Nature 1994, 372, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Allard, J.B.; Duan, C. IGF-Binding Proteins: Why Do They Exist and Why Are There So Many? Front. Endocrinol. 2018, 9, 117. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Elgin, R.G.; Busby, W.H.; Clemmons, D.R. An insulin-like growth factor (IGF) binding protein enhances the biologic response to IGF-I. Proc. Natl. Acad. Sci. USA 1987, 84, 3254–3258. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guler, H.P.; Zapf, J.; Schmid, C.; Froesch, E.R. Insulin-like growth factors I and II in healthy man. Estimations of half-lives and production rates. Acta Endocrinol. 1989, 121, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Massoner, P.; Ladurner-Rennau, M.; Eder, I.E.; Klocker, H. Insulin-like growth factors and insulin control a multifunctional signalling network of significant importance in cancer. Br. J. Cancer 2010, 103, 1479–1484. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rosenfeld, R.G.; Pham, H.; Conover, C.A.; Hintz, R.L.; Baxter, R.C. Structural and immunological comparison of insulin-like growth factor binding proteins of cerebrospinal and amniotic fluids. J. Clin. Endocrinol. Metab. 1989, 68, 638–646. [Google Scholar] [CrossRef] [PubMed]
- LeRoith, D.; Holly, J.M.P.; Forbes, B.E. Insulin-like growth factors: Ligands, binding proteins, and receptors. Mol. Metab. 2021, 52, 101245. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Allan, G.J.; Flint, D.J.; Patel, K. Insulin-like growth factor axis during embryonic development. Reproduction 2001, 122, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, C.; Kar, S. The insulin-like growth factor-II/mannose-6-phosphate receptor: Structure, distribution and function in the central nervous system. Brain Res. Brain Res. Rev. 2004, 44, 117–140. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Tocheny, C.E.; Shaw, L.M. The Insulin-like Growth Factor Signaling Pathway in Breast Cancer: An Elusive Therapeutic Target. Life 2022, 12, 1992. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hintz, R.L.; Liu, F. Demonstration of specific plasma protein binding sites for somatomedin. J. Clin. Endocrinol. Metab. 1977, 45, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Andress, D.L.; Birnbaum, R.S. A novel human insulin-like growth factor binding protein secreted by osteoblast-like cells. Biochem. Biophys. Res. Commun. 1991, 176, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Baxter, R.C.; Martin, J.L.; Tyler, M.I.; Howden, M.E. Growth hormone-dependent insulin-like growth factor (IGF) binding protein from human plasma differs from other human IGF binding proteins. Biochem. Biophys. Res. Commun. 1986, 139, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Bautista, C.M.; Wergedal, J.; Baylink, D.J. Isolation of an inhibitory insulin-like growth factor (IGF) binding protein from bone cell-conditioned medium: A potential local regulator of IGF action. Proc. Natl. Acad. Sci. USA 1989, 86, 8338–8342. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moses, A.C.; Nissley, S.P.; Passamani, J.; White, R.M. Further characterization of growth hormone-dependent somatomedin-binding proteins in rat serum and demonstration of somatomedin-binding proteins produced by rat liver cells in culture. Endocrinology 1979, 104, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Mottola, C.; MacDonald, R.G.; Brackett, J.L.; Mole, J.E.; Anderson, J.K.; Czech, M.P. Purification and amino-terminal sequence of an insulin-like growth factor-binding protein secreted by rat liver BRL-3A cells. J. Biol. Chem. 1986, 261, 11180–11188. [Google Scholar] [CrossRef] [PubMed]
- Shimasaki, S.; Gao, L.; Shimonaka, M.; Ling, N. Isolation and molecular cloning of insulin-like growth factor-binding protein-6. Mol. Endocrinol. 1991, 5, 938–948. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shimasaki, S.; Shimonaka, M.; Zhang, H.P.; Ling, N. Identification of five different insulin-like growth factor binding proteins (IGFBPs) from adult rat serum and molecular cloning of a novel IGFBP-5 in rat and human. J. Biol. Chem. 1991, 266, 10646–10653. [Google Scholar] [CrossRef] [PubMed]
- Shimonaka, M.; Schroeder, R.; Shimasaki, S.; Ling, N. Identification of a novel binding protein for insulin-like growth factors in adult rat serum. Biochem. Biophys. Res. Commun. 1989, 165, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Baxter, R.C. Characterization of the acid-labile subunit of the growth hormone-dependent insulin-like growth factor binding protein complex. J. Clin. Endocrinol. Metab. 1988, 67, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Firth, S.M.; Clemmons, D.R.; Baxter, R.C. Mutagenesis of basic amino acids in the carboxyl-terminal region of insulin-like growth factor binding protein-5 affects acid-labile subunit binding. Endocrinology 2001, 142, 2147. [Google Scholar] [CrossRef] [PubMed]
- Twigg, S.M.; Kiefer, M.C.; Zapf, J.; Baxter, R.C. Insulin-like growth factor-binding protein 5 complexes with the acid-labile subunit. Role of the carboxyl-terminal domain. J. Biol. Chem. 1998, 273, 28791–28798. [Google Scholar] [CrossRef] [PubMed]
- Cerro, J.A.; Grewal, A.; Wood, T.L.; Pintar, J.E. Tissue-specific expression of the insulin-like growth factor binding protein (IGFBP) mRNAs in mouse and rat development. Regul. Pept. 1993, 48, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Conover, C.A.; Oxvig, C.; Overgaard, M.T.; Christiansen, M.; Giudice, L.C. Evidence that the insulin-like growth factor binding protein-4 protease in human ovarian follicular fluid is pregnancy associated plasma protein-A. J. Clin. Endocrinol. Metab. 1999, 84, 4742–4745. [Google Scholar] [CrossRef] [PubMed]
- Laursen, L.S.; Overgaard, M.T.; Søe, R.; Boldt, H.B.; Sottrup-Jensen, L.; Giudice, L.C.; Conover, C.A.; Oxvig, C. Pregnancy-associated plasma protein-A (PAPP-A) cleaves insulin-like growth factor binding protein (IGFBP)-5 independent of IGF: Implications for the mechanism of IGFBP-4 proteolysis by PAPP-A. FEBS Lett. 2001, 504, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Monget, P.; Mazerbourg, S.; Delpuech, T.; Maurel, M.-C.; Manière, S.; Zapf, J.; Lalmanach, G.; Oxvig, C.; Overgaard, M.T. Pregnancy-associated plasma protein-A is involved in insulin-like growth factor binding protein-2 (IGFBP-2) proteolytic degradation in bovine and porcine preovulatory follicles: Identification of cleavage site and characterization of IGFBP-2 degradation. Biol. Reprod. 2003, 68, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Rorive, S.; Berton, A.; D’Haene, N.; Takacs, C.N.; Debeir, O.; Decaestecker, C.; Salmon, I. Matrix metalloproteinase-9 interplays with the IGFBP2-IGFII complex to promote cell growth and motility in astrocytomas. Glia 2008, 56, 1679–1690. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.I.; Gockerman, A.; Busby, W.H.; Camacho-Hubner, C.; Clemmons, D.R. Extracellular matrix contains insulin-like growth factor binding protein-5: Potentiation of the effects of IGF-I. J. Cell Biol. 1993, 121, 679–687. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bondy, C.A.; Werner, H.; Roberts, C.T.; LeRoith, D. Cellular pattern of insulin-like growth factor-I (IGF-I) and type I IGF receptor gene expression in early organogenesis: Comparison with IGF-II gene expression. Mol. Endocrinol. 1990, 4, 1386–1398. [Google Scholar] [CrossRef] [PubMed]
- Rotwein, P.; Burgess, S.K.; Milbrandt, J.D.; Krause, J.E. Differential expression of insulin-like growth factor genes in rat central nervous system. Proc. Natl. Acad. Sci. USA 1988, 85, 265–269. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pera, E.M.; Wessely, O.; Li, S.Y.; De Robertis, E.M. Neural and head induction by insulin-like growth factor signals. Dev. Cell 2001, 1, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Richard-Parpaillon, L.; Héligon, C.; Chesnel, F.; Boujard, D.; Philpott, A. The IGF pathway regulates head formation by inhibiting Wnt signaling in Xenopus. Dev. Biol. 2002, 244, 407–417. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ayer-le Lievre, C.; Ståhlbom, P.A.; Sara, V.R. Expression of IGF-I and -II mRNA in the brain and craniofacial region of the rat fetus. Development 1991, 111, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Bach, M.A.; Shen-Orr, Z.; Lowe, W.L.; Roberts, C.T.; LeRoith, D. Insulin-like growth factor I mRNA levels are developmentally regulated in specific regions of the rat brain. Brain Res. Mol. Brain Res. 1991, 10, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Bondy, C.; Lee, W.H. Correlation between insulin-like growth factor (IGF)-binding protein 5 and IGF-I gene expression during brain development. J. Neurosci. 1993, 13, 5092–5104. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Freude, S.; Leeser, U.; Müller, M.; Hettich, M.M.; Udelhoven, M.; Schilbach, K.; Tobe, K.; Kadowaki, T.; Köhler, C.; Schröder, H.; et al. IRS-2 branch of IGF-1 receptor signaling is essential for appropriate timing of myelination. J. Neurochem. 2008, 107, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.C.; Werther, G.A. Des (1-3) IGF-I potently enhances differentiated cell growth in olfactory bulb organ culture. Growth Factors 1994, 11, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Shinar, Y.; McMorris, F.A. Developing oligodendroglia express mRNA for insulin-like growth factor-I, a regulator of oligodendrocyte development. J. Neurosci. Res. 1995, 42, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, W.P.; Li, X.S.; Williams, M. Expression of IGF-1 mRNA in the murine subventricular zone during postnatal development. Brain Res. Mol. Brain Res. 1992, 12, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, W.P.; Li, X.S.; Williams, M.; Benkovic, S. Localization of insulin-like growth factor-1 mRNA in murine central nervous system during postnatal development. Dev. Biol. 1991, 147, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Ye, P.; Umayahara, Y.; Ritter, D.; Bunting, T.; Auman, H.; Rotwein, P.; D’ercole, A.J. Regulation of insulin-like growth factor I (IGF-I) gene expression in brain of transgenic mice expressing an IGF-I-luciferase fusion gene. Endocrinology 1997, 138, 5466–5475. [Google Scholar] [CrossRef] [PubMed]
- Logan, A.; Gonzalez, A.M.; Hill, D.J.; Berry, M.; Gregson, N.A.; Baird, A. Coordinated pattern of expression and localization of insulin-like growth factor-II (IGF-II) and IGF-binding protein-2 in the adult rat brain. Endocrinology 1994, 135, 2255–2264. [Google Scholar] [CrossRef] [PubMed]
- Charalambous, M.; Menheniott, T.R.; Bennett, W.R.; Kelly, S.M.; Dell, G.; Dandolo, L.; Ward, A. An enhancer element at the Igf2/H19 locus drives gene expression in both imprinted and non-imprinted tissues. Dev. Biol. 2004, 271, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Hynes, M.A.; Brooks, P.J.; Van Wyk, J.J.; Lund, P.K. Insulin-like growth factor II messenger ribonucleic acids are synthesized in the choroid plexus of the rat brain. Mol. Endocrinol. 1988, 2, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Stylianopoulou, F.; Efstratiadis, A.; Herbert, J.; Pintar, J. Pattern of the insulin-like growth factor II gene expression during rat embryogenesis. Development 1988, 103, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Daughaday, W.H.; Trivedi, B. Measurement of derivatives of proinsulin-like growth factor-II in serum by a radioimmunoassay directed against the E-domain in normal subjects and patients with nonislet cell tumor hypoglycemia. J. Clin. Endocrinol. Metab. 1992, 75, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Scalia, P.; Marino, I.R.; Asero, S.; Pandini, G.; Grimberg, A.; El-Deiry, W.S.; Williams, S.J. Autocrine IGF-II-Associated Cancers: From a Rare Paraneoplastic Event to a Hallmark in Malignancy. Biomedicines 2023, 12, 40. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Haselbacher, G.; Humbel, R. Evidence for two species of insulin-like growth factor II (IGF II and “big” IGF II) in human spinal fluid. Endocrinology 1982, 110, 1822–1824. [Google Scholar] [CrossRef] [PubMed]
- Haselbacher, G.K.; Schwab, M.E.; Pasi, A.; Humbel, R.E. Insulin-like growth factor II (IGF II) in human brain: Regional distribution of IGF II and of higher molecular mass forms. Proc. Natl. Acad. Sci. USA 1985, 82, 2153–2157. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, W.H.; Wang, G.M.; Seaman, L.B.; Vannucci, S.J. Coordinate IGF-I and IGFBP5 gene expression in perinatal rat brain after hypoxia-ischemia. J. Cereb. Blood Flow Metab. 1996, 16, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Walter, H.J.; Berry, M.; Hill, D.J.; Logan, A. Spatial and temporal changes in the insulin-like growth factor (IGF) axis indicate autocrine/paracrine actions of IGF-I within wounds of the rat brain. Endocrinology 1997, 138, 3024–3034. [Google Scholar] [CrossRef] [PubMed]
- Brar, A.K.; Chernausek, S.D. Localization of insulin-like growth factor binding protein-4 expression in the developing and adult rat brain: Analysis by in situ hybridization. J. Neurosci. Res. 1993, 35, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Delhanty, P.J.; Hill, D.J.; Shimasaki, S.; Han, V.K. Insulin-like growth factor binding protein-4, -5 and -6 mRNAs in the human fetus: Localization to sites of growth and differentiation? Growth Regul. 1993, 3, 8–11. [Google Scholar] [PubMed]
- Stenvers, K.L.; Zimmermann, E.M.; Gallagher, M.; Lund, P.K. Expression of insulin-like growth factor binding protein-4 and -5 mRNAs in adult rat forebrain. J. Comp. Neurol. 1994, 339, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Michels, K.M.; Bondy, C.A. Localization of insulin-like growth factor binding protein-2 messenger RNA during postnatal brain development: Correlation with insulin-like growth factors I and II. Neuroscience 1993, 53, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Ocrant, I.; Fay, C.T.; Parmelee, J.T. Characterization of insulin-like growth factor binding proteins produced in the rat central nervous system. Endocrinology 1990, 127, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Wood, T.L.; Brown, A.L.; Rechler, M.M.; Pintar, J.E. The expression pattern of an insulin-like growth factor (IGF)-binding protein gene is distinct from IGF-II in the midgestational rat embryo. Mol. Endocrinol. 1990, 4, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, R.R.; Bondy, C.A. Insulin-like growth factors cross the blood-brain barrier. Endocrinology 1994, 135, 1753–1761. [Google Scholar] [CrossRef] [PubMed]
- Altman, J. Autoradiographic investigation of cell proliferation in the brains of rats and cats. Anat. Rec. 1963, 145, 573–591. [Google Scholar] [CrossRef] [PubMed]
- Altman, J.; Das, G.D. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 1965, 124, 319–335. [Google Scholar] [CrossRef] [PubMed]
- de Carlos, J.A.; López-Mascaraque, L.; Valverde, F. Dynamics of cell migration from the lateral ganglionic eminence in the rat. J. Neurosci. 1996, 16, 6146–6156. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fuentealba, L.C.; Rompani, S.B.; Parraguez, J.I.; Obernier, K.; Romero, R.; Cepko, C.L.; Alvarez-Buylla, A. Embryonic Origin of Postnatal Neural Stem Cells. Cell 2015, 161, 1644–1655. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Merkle, F.T.; Tramontin, A.D.; García-Verdugo, J.M.; Alvarez-Buylla, A. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc. Natl. Acad. Sci. USA 2004, 101, 17528–17532. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yuzwa, S.A.; Borrett, M.J.; Innes, B.T.; Voronova, A.; Ketela, T.; Kaplan, D.R.; Bader, G.D.; Miller, F.D. Developmental Emergence of Adult Neural Stem Cells as Revealed by Single-Cell Transcriptional Profiling. Cell Rep. 2017, 21, 3970–3986. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, P.S.; Perfilieva, E.; Björk-Eriksson, T.; Alborn, A.-M.; Nordborg, C.; Peterson, D.A.; Gage, F.H. Neurogenesis in the adult human hippocampus. Nat. Med. 1998, 4, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Doetsch, F.; García-Verdugo, J.M.; Alvarez-Buylla, A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J. Neurosci. 1997, 17, 5046–5061. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hu, X.-L.; Chen, G.; Zhang, S.; Zheng, J.; Wu, J.; Bai, Q.-R.; Wang, Y.; Wang, H.; Feng, H.; Li, J.; et al. Persistent Expression of VCAM1 in Radial Glial Cells Is Required for the Embryonic Origin of Postnatal Neural Stem Cells. Neuron 2017, 95, 309–325.e6. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-K.; Belz, T.; Bock, D.; Takacs, A.; Wu, H.; Lichter, P.; Chai, M.; Schütz, G. The nuclear receptor tailless is required for neurogenesis in the adult subventricular zone. Genes Dev. 2008, 22, 2473–2478. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Menn, B.; Garcia-Verdugo, J.M.; Yaschine, C.; Gonzalez-Perez, O.; Rowitch, D.; Alvarez-Buylla, A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J. Neurosci. 2006, 26, 7907–7918. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fukuda, S.; Kato, F.; Tozuka, Y.; Yamaguchi, M.; Miyamoto, Y.; Hisatsune, T. Two distinct subpopulations of nestin-positive cells in adult mouse dentate gyrus. J. Neurosci. 2003, 23, 9357–9366. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Garcia, A.D.; Doan, N.B.; Imura, T.; Bush, T.G.; Sofroniew, M.V. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat. Neurosci. 2004, 7, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Steiner, B.; Kronenberg, G.; Jessberger, S.; Brandt, M.D.; Reuter, K.; Kempermann, G. Differential regulation of gliogenesis in the context of adult hippocampal neurogenesis in mice. Glia 2004, 46, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Suh, H.; Consiglio, A.; Ray, J.; Sawai, T.; D’Amour, K.A.; Gage, F.H. In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell Stem Cell 2007, 1, 515–528. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Merkle, F.T.; Mirzadeh, Z.; Alvarez-Buylla, A. Mosaic organization of neural stem cells in the adult brain. Science 2007, 317, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Saxe, M.D.; Gallina, I.S.; Gage, F.H. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J. Neurosci. 2009, 29, 13532–13542. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, C.; Teng, E.M.; Summers, R.G.; Ming, G.L.; Gage, F.H. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J. Neurosci. 2006, 26, 3–11. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Smith, A.W.; Parashar, B.; Wernicke, A.G. Subventricular zone-associated glioblastoma: A call for translational research to guide clinical decision making. Neurogenesis 2016, 3, e1225548. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sanai, N.; Tramontin, A.D.; Quiñones-Hinojosa, A.; Barbaro, N.M.; Gupta, N.; Kunwar, S.; Lawton, M.T.; McDermott, M.W.; Parsa, A.T.; García-Verdugo, J.M.; et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature 2004, 427, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Sanai, N.; Nguyen, T.; Ihrie, R.A.; Mirzadeh, Z.; Tsai, H.-H.; Wong, M.; Gupta, N.; Berger, M.S.; Huang, E.; Garcia-Verdugo, J.-M.; et al. Corridors of migrating neurons in the human brain and their decline during infancy. Nature 2011, 478, 382–386. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kriegstein, A.; Alvarez-Buylla, A. The glial nature of embryonic and adult neural stem cells. Annu. Rev. Neurosci. 2009, 32, 149–184. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, C.; Deng, W.; Gage, F.H. Mechanisms and functional implications of adult neurogenesis. Cell 2008, 132, 645–660. [Google Scholar] [CrossRef] [PubMed]
- Passarelli, J.P.; Nimjee, S.M.; Townsend, K.L. Stroke and Neurogenesis: Bridging Clinical Observations to New Mechanistic Insights from Animal Models. Transl. Stroke Res. 2024, 15, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Hu, J.; Ralls, S.; Kitamura, T.; Loh, Y.P.; Yang, Y.; Mukouyama, Y.-S.; Ahn, S. The molecular profiles of neural stem cell niche in the adult subventricular zone. PLoS ONE 2012, 7, e50501. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matarredona, E.R.; Talaverón, R.; Pastor, A.M. Interactions Between Neural Progenitor Cells and Microglia in the Subventricular Zone: Physiological Implications in the Neurogenic Niche and After Implantation in the Injured Brain. Front. Cell Neurosci. 2018, 12, 268. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dause, T.J.; Denninger, J.K.; Smith, B.M.; Kirby, E.D. The neural stem cell secretome across neurodevelopment. Exp. Neurol. 2022, 355, 114142. [Google Scholar] [CrossRef] [PubMed]
- Morizur, L.; Chicheportiche, A.; Gauthier, L.R.; Daynac, M.; Boussin, F.D.; Mouthon, M.A. Distinct Molecular Signatures of Quiescent and Activated Adult Neural Stem Cells Reveal Specific Interactions with Their Microenvironment. Stem Cell Rep. 2018, 11, 565–577. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vogel, A.D.; Upadhya, R.; Shetty, A.K. Neural stem cell derived extracellular vesicles: Attributes and prospects for treating neurodegenerative disorders. eBioMedicine 2018, 38, 273–282. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Komada, M.; Saitsu, H.; Kinboshi, M.; Miura, T.; Shiota, K.; Ishibashi, M. Hedgehog signaling is involved in development of the neocortex. Development 2008, 135, 2717–2727. [Google Scholar] [CrossRef] [PubMed]
- Daynac, M.; Tirou, L.; Faure, H.; Mouthon, M.-A.; Gauthier, L.R.; Hahn, H.; Boussin, F.D.; Ruat, M. Hedgehog Controls Quiescence and Activation of Neural Stem Cells in the Adult Ventricular-Subventricular Zone. Stem Cell Rep. 2016, 7, 735–748. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Červenka, J.; Tylečková, J.; Skalníková, H.K.; Kepková, K.V.; Poliakh, I.; Valeková, I.; Pfeiferová, L.; Kolář, M.; Vaškovičová, M.; Pánková, T.; et al. Proteomic Characterization of Human Neural Stem Cells and Their Secretome During. Front. Cell Neurosci. 2020, 14, 612560. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Muffley, L.A.; Pan, S.C.; Smith, A.N.; Ga, M.; Hocking, A.M.; Gibran, N.S. Differentiation state determines neural effects on microvascular endothelial cells. Exp. Cell Res. 2012, 318, 2085–2093. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Tham, M.; Ramasamy, S.; Gan, H.T.; Ramachandran, A.; Poonepalli, A.; Yu, Y.H.; Ahmed, S. CSPG is a secreted factor that stimulates neural stem cell survival possibly by enhanced EGFR signaling. PLoS ONE 2010, 5, e15341. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dulken, B.W.; Leeman, D.S.; Boutet, S.C.; Hebestreit, K.; Brunet, A. Single-Cell Transcriptomic Analysis Defines Heterogeneity and Transcriptional Dynamics in the Adult Neural Stem Cell Lineage. Cell Rep. 2017, 18, 777–790. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Denninger, J.K.; Chen, X.; Turkoglu, A.M.; Sarchet, P.; Volk, A.R.; Rieskamp, J.D.; Yan, P.; Kirby, E.D. Defining the adult hippocampal neural stem cell secretome: In vivo versus in vitro transcriptomic differences and their correlation to secreted protein levels. Brain Res. 2020, 1735, 146717. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dause, T.J.; Denninger, J.K.; Osap, R.; Walters, A.E.; Rieskamp, J.D.; Kirby, E.D. Autocrine VEGF drives neural stem cell proximity to the adult hippocampus vascular niche. Life Sci. Alliance 2024, 7, e202402659. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kirby, E.D.; Kuwahara, A.A.; Messer, R.L.; Wyss-Coray, T. Adult hippocampal neural stem and progenitor cells regulate the neurogenic niche by secreting VEGF. Proc. Natl. Acad. Sci. USA 2015, 112, 4128–4133. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lun, M.P.; Monuki, E.S.; Lehtinen, M.K. Development and functions of the choroid plexus-cerebrospinal fluid system. Nat. Rev. Neurosci. 2015, 16, 445–457. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Walter, H.J.; Berry, M.; Hill, D.J.; Cwyfan-Hughes, S.; Holly, J.M.; Logan, A. Distinct sites of insulin-like growth factor (IGF)-II expression and localization in lesioned rat brain: Possible roles of IGF binding proteins (IGFBPs) in the mediation of IGF-II activity. Endocrinology 1999, 140, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Spector, R.; Robert Snodgrass, S.; Johanson, C.E. A balanced view of the cerebrospinal fluid composition and functions: Focus on adult humans. Exp. Neurol. 2015, 273, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Lehtinen, M.K.; Zappaterra, M.W.; Chen, X.; Yang, Y.J.; Hill, A.D.; Lun, M.; Maynard, T.; Gonzalez, D.; Kim, S.; Ye, P.; et al. The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron 2011, 69, 893–905. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mirzadeh, Z.; Merkle, F.T.; Soriano-Navarro, M.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell 2008, 3, 265–278. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Silva-Vargas, V.; Maldonado-Soto, A.R.; Mizrak, D.; Codega, P.; Doetsch, F. Age-Dependent Niche Signals from the Choroid Plexus Regulate Adult Neural Stem Cells. Cell Stem Cell 2016, 19, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Mashayekhi, F.; Draper, C.E.; Bannister, C.M.; Pourghasem, M.; Owen-Lynch, P.J.; Miyan, J.A. Deficient cortical development in the hydrocephalic Texas (H-Tx) rat: A role for CSF. Brain 2002, 125 Pt 8, 1859–1874. [Google Scholar] [CrossRef] [PubMed]
- Owen-Lynch, P.J.; Draper, C.E.; Mashayekhi, F.; Bannister, C.M.; Miyan, J.A. Defective cell cycle control underlies abnormal cortical development in the hydrocephalic Texas rat. Brain 2003, 126 Pt 3, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Sawamoto, K.; Wichterle, H.; Gonzalez-Perez, O.; Cholfin, J.A.; Yamada, M.; Spassky, N.; Murcia, N.S.; Garcia-Verdugo, J.M.; Marin, O.; Rubenstein, J.L.R.; et al. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science 2006, 311, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, A.N.; Levison, S.W.; Wood, T.L. Insulin and IGF receptor signalling in neural-stem-cell homeostasis. Nat. Rev. Endocrinol. 2015, 11, 161–170. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lehtinen, M.K.; Walsh, C.A. Neurogenesis at the brain-cerebrospinal fluid interface. Annu. Rev. Cell Dev. Biol. 2011, 27, 653–679. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dziegielewska, K.; Evans, C.; Lai, P.; Lorscheider, F.; Malinowska, D.; Møllgrd, K.; Saunders, N. Proteins in cerebrospinal fluid and plasma of fetal rats during development. Dev. Biol. 1981, 83, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Marques, F.; Sousa, J.C.; Coppola, G.; Gao, F.; Puga, R.; Brentani, H.; Geschwind, D.H.; Sousa, N.; Correia-Neves, M.; Palha, J.A. Transcriptome signature of the adult mouse choroid plexus. Fluids Barriers CNS 2011, 8, 10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Parada, C.; Gato, A.; Bueno, D. Mammalian embryonic cerebrospinal fluid proteome has greater apolipoprotein and enzyme pattern complexity than the avian proteome. J. Proteome Res. 2005, 4, 2420–2428. [Google Scholar] [CrossRef] [PubMed]
- Zappaterra, M.D.; Lisgo, S.N.; Lindsay, S.; Gygi, S.P.; Walsh, C.A.; Ballif, B.A. A comparative proteomic analysis of human and rat embryonic cerebrospinal fluid. J. Proteome Res. 2007, 6, 3537–3548. [Google Scholar] [CrossRef] [PubMed]
- Grove, E.A.; Tole, S.; Limon, J.; Yip, L.; Ragsdale, C.W. The hem of the embryonic cerebral cortex is defined by the expression of multiple Wnt genes and is compromised in Gli3-deficient mice. Development 1998, 125, 2315–2325. [Google Scholar] [CrossRef] [PubMed]
- Haskell, G.T.; LaMantia, A.S. Retinoic acid signaling identifies a distinct precursor population in the developing and adult forebrain. J. Neurosci. 2005, 25, 7636–7647. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hébert, J.M.; Mishina, Y.; McConnell, S.K. BMP signaling is required locally to pattern the dorsal telencephalic midline. Neuron 2002, 35, 1029–1041. [Google Scholar] [CrossRef] [PubMed]
- Shimogori, T.; Banuchi, V.; Ng, H.Y.; Strauss, J.B.; Grove, E.A. Embryonic signaling centers expressing BMP, WNT and FGF proteins interact to pattern the cerebral cortex. Development 2004, 131, 5639–5647. [Google Scholar] [CrossRef] [PubMed]
- Lun, M.P.; Johnson, M.B.; Broadbelt, K.G.; Watanabe, M.; Kang, Y.-J.; Chau, K.F.; Springel, M.W.; Malesz, A.; Sousa, A.M.; Pletikos, M.; et al. Spatially heterogeneous choroid plexus transcriptomes encode positional identity and contribute to regional CSF production. J. Neurosci. 2015, 35, 4903–4916. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hunter, N.L.; Dymecki, S.M. Molecularly and temporally separable lineages form the hindbrain roof plate and contribute differentially to the choroid plexus. Development 2007, 134, 3449–3460. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bartke, A.; Chandrashekar, V.; Dominici, F.; Turyn, D.; Kinney, B.; Steger, R.; Kopchick, J. Insulin-like growth factor 1 (IGF-1) and aging: Controversies and new insights. Biogerontology 2003, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, A.N.; Schneider, J.S.; Qin, M.; Tyler, W.A.; Pintar, J.E.; Fraidenraich, D.; Wood, T.L.; Levison, S.W. IGF-II promotes stemness of neural restricted precursors. Stem Cells 2012, 30, 1265–1276. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, R.L.; Kassem, N.A.; Sadeghi, M.; Preston, J.E. Insulin-like growth factor-II uptake into choroid plexus and brain of young and old sheep. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Ureña, A.; Lázaro-Carot, L.; Jiménez-Villalba, E.; Montalbán-Loro, R.; Mateos-White, I.; Duart-Abadía, P.; Martínez-Gurrea, I.; Nakayama, K.I.; Fariñas, I.; Kirstein, M.; et al. IGF2 interacts with the imprinted gene Cdkn1c to promote terminal differentiation of neural stem cells. Development 2023, 150, dev200563. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deng, Y.; Wang, L.; Ge, L.; Duan, D.; Zhuo, Y.; Yuan, T.; Yan, W.; Huang, P.; Teng, X.; Lu, M. Effects of IGFBP-2 on proliferation and differentiation in neural stem cell line C17.2. J. Neurorestoratology 2017, 5, 143–153. [Google Scholar] [CrossRef]
- Shahin, W.S.; Ebed, S.O.; Tyler, S.R.; Miljkovic, B.; Choi, S.H.; Zhang, Y.; Zhou, W.; Evans, I.A.; Yeaman, C.; Engelhardt, J.F. Redox-dependent Igfbp2 signaling controls Brca1 DNA damage response to govern neural stem cell fate. Nat. Commun. 2023, 14, 444. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iqbal, M.A.; Eftekharpour, E. Regulatory Role of Redox Balance in Determination of Neural Precursor Cell Fate. Stem Cells Int. 2017, 2017, 9209127. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nickenig, G.; Baudler, S.; Müller, C.; Werner, C.; Werner, N.; Welzel, H.; Strehlow, K.; Böhm, M. Redox-sensitive vascular smooth muscle cell proliferation is mediated by GKLF and Id3 in vitro and in vivo. FASEB J. 2002, 16, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Shubham, S.; Mandal, K.; Trivedi, V.; Chauhan, R.; Naseera, S. Survival and prognostic factors for glioblastoma multiforme: Retrospective single-institutional study. Indian J. Cancer 2017, 54, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Peeters, M.C.M.; Dirven, L.; Koekkoek, J.A.F.; Gortmaker, E.G.; Fritz, L.; Vos, M.J.; Taphoorn, M.J.B. Prediagnostic symptoms and signs of adult glioma: The patients’ view. J. Neurooncol. 2020, 146, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Ohgaki, H.; Kleihues, P. Genetic pathways to primary and secondary glioblastoma. Am. J. Pathol. 2007, 170, 1445–1453. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007, 114, 97–109. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bonnet, D.; Dick, J.E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997, 3, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Furth, J.; Kahn, C.M.; Breedis, C. The Transmission of Leukemia of Mice with a Single Cell. Cancer Res. 1937, 31, 276–282. [Google Scholar]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Collins, A.T.; Berry, P.A.; Hyde, C.; Stower, M.J.; Maitland, N.J. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005, 65, 10946–10951. [Google Scholar] [CrossRef] [PubMed]
- Hemmati, H.D.; Nakano, I.; Lazareff, J.A.; Masterman-Smith, M.; Geschwind, D.H.; Bronner-Fraser, M.; Kornblum, H.I. Cancerous stem cells can arise from pediatric brain tumors. Proc. Natl. Acad. Sci. USA 2003, 100, 15178–15183. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, C.; Heidt, D.G.; Dalerba, P.; Burant, C.F.; Zhang, L.; Adsay, V.; Wicha, M.; Clarke, M.F.; Simeone, D.M. Identification of pancreatic cancer stem cells. Cancer Res. 2007, 67, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, C.A.; Pollett, A.; Gallinger, S.; Dick, J.E. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 2007, 445, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Ricci-Vitiani, L.; Lombardi, D.G.; Pilozzi, E.; Biffoni, M.; Todaro, M.; Peschle, C.; De Maria, R. Identification and expansion of human colon-cancer-initiating cells. Nature 2007, 445, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Clarke, I.D.; Terasaki, M.; Bonn, V.E.; Hawkins, C.; Squire, J.; Dirks, P.B. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003, 63, 5821–5828. [Google Scholar] [PubMed]
- Singh, S.K.; Hawkins, C.; Clarke, I.D.; Squire, J.A.; Bayani, J.; Hide, T.; Henkelman, R.M.; Cusimano, M.D.; Dirks, P.B. Identification of human brain tumour initiating cells. Nature 2004, 432, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Bachoo, R.M.; Maher, E.A.; Ligon, K.L.; Sharpless, N.E.; Chan, S.S.; You, M.J.; Tang, Y.; DeFrances, J.; Stover, E.; Weissleder, R.; et al. Epidermal growth factor receptor and Ink4a/Arf: Convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell 2002, 1, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Sage, J.C.; Miller, M.R.; Verhaak, R.G.; Hippenmeyer, S.; Vogel, H.; Foreman, O.; Bronson, R.T.; Nishiyama, A.; Luo, L.; et al. Mosaic analysis with double markers reveals tumor cell of origin in glioma. Cell 2011, 146, 209–221. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sutter, R.; Yadirgi, G.; Marino, S. Neural stem cells, tumour stem cells and brain tumours: Dangerous relationships? Biochim. Biophys. Acta 2007, 1776, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kotliarova, S.; Kotliarov, Y.; Li, A.; Su, Q.; Donin, N.M.; Pastorino, S.; Purow, B.W.; Christopher, N.; Zhang, W.; et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell 2006, 9, 391–403. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, G.; Bozek, D.A.; Rajakulendran, N.; Monteiro, V.; Ahmadi, M.; Steinhart, Z.; Kushida, M.M.; Yu, H.; Coutinho, F.J.; Cavalli, F.M.G.; et al. Genome-Wide CRISPR-Cas9 Screens Expose Genetic Vulnerabilities and Mechanisms of Temozolomide Sensitivity in Glioblastoma Stem Cells. Cell Rep. 2019, 27, 971–986.e9. [Google Scholar] [CrossRef] [PubMed]
- Suvà, M.L.; Rheinbay, E.; Gillespie, S.M.; Patel, A.P.; Wakimoto, H.; Rabkin, S.D.; Riggi, N.; Chi, A.S.; Cahill, D.P.; Nahed, B.V.; et al. Reconstructing and reprogramming the tumor-propagating potential of glioblastoma stem-like cells. Cell 2014, 157, 580–594. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Llaguno, S.A.; Chen, J.; Kwon, C.-H.; Jackson, E.L.; Li, Y.; Burns, D.K.; Alvarez-Buylla, A.; Parada, L.F. Malignant astrocytomas originate from neural stem/progenitor cells in a somatic tumor suppressor mouse model. Cancer Cell 2009, 15, 45–56. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Llaguno, S.R.A.; Wang, Z.; Sun, D.; Chen, J.; Xu, J.; Kim, E.; Hatanpaa, K.J.; Raisanen, J.M.; Burns, D.K.; Johnson, J.E.; et al. Adult Lineage-Restricted CNS Progenitors Specify Distinct Glioblastoma Subtypes. Cancer Cell 2015, 28, 429–440. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chow, L.M.; Endersby, R.; Zhu, X.; Rankin, S.; Qu, C.; Zhang, J.; Broniscer, A.; Ellison, D.W.; Baker, S.J. Cooperativity within and among Pten, p53, and Rb pathways induces high-grade astrocytoma in adult brain. Cancer Cell 2011, 19, 305–316. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Galvao, R.P.; Kasina, A.; McNeill, R.S.; Harbin, J.E.; Foreman, O.; Verhaak, R.G.W.; Nishiyama, A.; Miller, C.R.; Zong, H. Transformation of quiescent adult oligodendrocyte precursor cells into malignant glioma through a multistep reactivation process. Proc. Natl. Acad. Sci. USA 2014, 111, E4214–E4223. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Llaguno, S.A.; Sun, D.; Pedraza, A.M.; Vera, E.; Wang, Z.; Burns, D.K.; Parada, L.F. Cell-of-origin susceptibility to glioblastoma formation declines with neural lineage restriction. Nat. Neurosci. 2019, 22, 545–555. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Assanah, M.; Lochhead, R.; Ogden, A.; Bruce, J.; Goldman, J.; Canoll, P. Glial progenitors in adult white matter are driven to form malignant gliomas by platelet-derived growth factor-expressing retroviruses. J. Neurosci. 2006, 26, 6781–6790. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dawson, M.R.; Polito, A.; Levine, J.M.; Reynolds, R. NG2-expressing glial progenitor cells: An abundant and widespread population of cycling cells in the adult rat CNS. Mol. Cell Neurosci. 2003, 24, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Dimou, L.; Simon, C.; Kirchhoff, F.; Takebayashi, H.; Götz, M. Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J. Neurosci. 2008, 28, 10434–10442. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ligon, K.L.; Huillard, E.; Mehta, S.; Kesari, S.; Liu, H.; Alberta, J.A.; Bachoo, R.M.; Kane, M.; Louis, D.N.; DePinho, R.A.; et al. Olig2-regulated lineage-restricted pathway controls replication competence in neural stem cells and malignant glioma. Neuron 2007, 53, 503–517. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jackson, E.L.; Garcia-Verdugo, J.M.; Gil-Perotin, S.; Roy, M.; Quinones-Hinojosa, A.; VandenBerg, S.; Alvarez-Buylla, A. PDGFR alpha-positive B cells are neural stem cells in the adult SVZ that form glioma-like growths in response to increased PDGF signaling. Neuron 2006, 51, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Ligon, K.L.; Alberta, J.A.; Kho, A.T.; Weiss, J.; Kwaan, M.R.; Nutt, C.L.; Louis, D.N.; Stiles, C.D.; Rowitch, D.H. The oligodendroglial lineage marker OLIG2 is universally expressed in diffuse gliomas. J. Neuropathol. Exp. Neurol. 2004, 63, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.R.; Park, J.K.; Noll, E.; Chan, J.A.; Alberta, J.; Yuk, D.; Alzamora, M.G.; Louis, D.N.; Stiles, C.D.; Rowitch, D.H.; et al. Oligodendrocyte lineage genes (OLIG) as molecular markers for human glial brain tumors. Proc. Natl. Acad. Sci. USA 2001, 98, 10851–10856. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, J.H.; Lee, J.E.; Kahng, J.Y.; Kim, S.H.; Park, J.S.; Yoon, S.J.; Um, J.-Y.; Kim, W.K.; Lee, J.-K.; Park, J.; et al. Human glioblastoma arises from subventricular zone cells with low-level driver mutations. Nature 2018, 560, 243–247. [Google Scholar] [CrossRef] [PubMed]
- The Telomeres Mendelian Randomization Collaboration; Haycock, P.C.; Burgess, S.; Nounu, A.; Zheng, J.; Okoli, G.N.; Bowden, J.; Wade, K.H.; Timpson, N.J.; Evans, D.M.; et al. Association Between Telomere Length and Risk of Cancer and Non-Neoplastic Diseases: A Mendelian Randomization Study. JAMA Oncol. 2017, 3, 636–651. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Howell, A.E.; Robinson, J.W.; Wootton, R.E.; McAleenan, A.; Tsavachidis, S.; Ostrom, Q.T.; Bondy, M.; Armstrong, G.; Relton, C.; Haycock, P.; et al. Testing for causality between systematically identified risk factors and glioma: A Mendelian randomization study. BMC Cancer 2020, 20, 508. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fontán-Lozano, Á.; Morcuende, S.; Davis-López de Carrizosa, M.A.; Benítez-Temiño, B.; Mejías, R.; Matarredona, E.R. To Become or Not to Become Tumorigenic: Subventricular Zone. Front. Oncol. 2020, 10, 602217. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carrano, A.; Zarco, N.; Phillipps, J.; Lara-Velazquez, M.; Suarez-Meade, P.; Norton, E.S.; Chaichana, K.L.; Quiñones-Hinojosa, A.; Asmann, Y.W.; Guerrero-Cázares, H. Human Cerebrospinal Fluid Modulates Pathways Promoting Glioblastoma Malignancy. Front. Oncol. 2021, 11, 624145. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lim, D.A.; Cha, S.; Mayo, M.C.; Chen, M.-H.; Keles, E.; VandenBerg, S.; Berger, M.S. Relationship of glioblastoma multiforme to neural stem cell regions predicts invasive and multifocal tumor phenotype. Neuro-Oncol. 2007, 9, 424–429. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Norton, E.S.; Whaley, L.A.; Ulloa-Navas, M.J.; García-Tárraga, P.; Meneses, K.M.; Lara-Velazquez, M.; Zarco, N.; Carrano, A.; Quiñones-Hinojosa, A.; García-Verdugo, J.M.; et al. Glioblastoma disrupts the ependymal wall and extracellular matrix structures of the subventricular zone. Fluids Barriers CNS 2022, 19, 58. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Adeberg, S.; Bostel, T.; König, L.; Welzel, T.; Debus, J.; Combs, S.E. A comparison of long-term survivors and short-term survivors with glioblastoma, subventricular zone involvement: A predictive factor for survival? Radiat. Oncol. 2014, 9, 95. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chaichana, K.L.; McGirt, M.J.; Frazier, J.; Attenello, F.; Guerrero-Cazares, H.; Quinones-Hinojosa, A. Relationship of glioblastoma multiforme to the lateral ventricles predicts survival following tumor resection. J. Neurooncol. 2008, 89, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Jafri, N.F.; Clarke, J.L.; Weinberg, V.; Barani, I.J.; Cha, S. Relationship of glioblastoma multiforme to the subventricular zone is associated with survival. Neuro-Oncol. 2013, 15, 91–96. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Young, G.S.; Macklin, E.A.; Setayesh, K.; Lawson, J.D.; Wen, P.Y.; Norden, A.D.; Drappatz, J.; Kesari, S. Longitudinal MRI evidence for decreased survival among periventricular glioblastoma. J. Neurooncol. 2011, 104, 261–269. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mummareddy, N.; Salwi, S.R.; Kumar, N.G.; Zhao, Z.; Ye, F.; Le, C.H.; Mobley, B.C.; Thompson, R.C.; Chambless, L.B.; Mistry, A.M. Prognostic relevance of CSF and peri-tumoral edema volumes in glioblastoma. J. Clin. Neurosci. 2021, 84, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Guerrero-Cazares, H.; Ye, X.; Ford, E.; McNutt, T.; Kleinberg, L.; Lim, M.; Chaichana, K.; Quinones-Hinojosa, A.; Redmond, K. Increased subventricular zone radiation dose correlates with survival in glioblastoma patients after gross total resection. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 616–622. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sonoda, Y.; Saito, R.; Kanamori, M.; Kumabe, T.; Uenohara, H.; Tominaga, T. The association of subventricular zone involvement at recurrence with survival after repeat surgery in patients with recurrent glioblastoma. Neurol. Med.-Chir. 2014, 54, 302–309. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alonso, M.I.; Martín, C.; Carnicero, E.; Bueno, D.; Gato, A. Cerebrospinal fluid control of neurogenesis induced by retinoic acid during early brain development. Dev. Dyn. 2011, 240, 1650–1659. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.T.; Lehtinen, M.K.; Sive, H. Zebrafish cerebrospinal fluid mediates cell survival through a retinoid signaling pathway. Dev. Neurobiol. 2016, 76, 75–92. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, X.; Liu, J.; Ketova, T.; Fleming, J.T.; Grover, V.K.; Cooper, M.K.; Litingtung, Y.; Chiang, C. Transventricular delivery of Sonic hedgehog is essential to cerebellar ventricular zone development. Proc. Natl. Acad. Sci. USA 2010, 107, 8422–8427. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kaiser, K.; Gyllborg, D.; Procházka, J.; Salašová, A.; Kompaníková, P.; Molina, F.L.; Laguna-Goya, R.; Radaszkiewicz, T.; Harnoš, J.; Procházková, M.; et al. WNT5A is transported via lipoprotein particles in the cerebrospinal fluid to regulate hindbrain morphogenesis. Nat. Commun. 2019, 10, 1498. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martín, C.; Bueno, D.; Alonso, M.; Moro, J.; Callejo, S.; Parada, C.; Martín, P.; Carnicero, E.; Gato, A. FGF2 plays a key role in embryonic cerebrospinal fluid trophic properties over chick embryo neuroepithelial stem cells. Dev. Biol. 2006, 297, 402–416. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, L.K.; Dufresne, M.; Joppé, S.E.; Petryszyn, S.; Aumont, A.; Calon, F.; Barnabé-Heider, F.; Furtos, A.; Parent, M.; Chaurand, P.; et al. Aberrant Lipid Metabolism in the Forebrain Niche Suppresses Adult Neural Stem Cell Proliferation in an Animal Model of Alzheimer’s Disease. Cell Stem Cell 2015, 17, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Muthusamy, N.; Sommerville, L.J.; Moeser, A.J.; Stumpo, D.J.; Sannes, P.; Adler, K.; Blackshear, P.J.; Weimer, J.M.; Ghashghaei, H.T. MARCKS-dependent mucin clearance and lipid metabolism in ependymal cells are required for maintenance of forebrain homeostasis during aging. Aging Cell 2015, 14, 764–773. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Banizs, B.; Pike, M.M.; Millican, C.L.; Ferguson, W.B.; Komlosi, P.; Sheetz, J.; Bell, P.D.; Schwiebert, E.M.; Yoder, B.K. Dysfunctional cilia lead to altered ependyma and choroid plexus function, and result in the formation of hydrocephalus. Development 2005, 132, 5329–5339. [Google Scholar] [CrossRef] [PubMed]
- Olstad, E.W.; Ringers, C.; Hansen, J.N.; Wens, A.; Brandt, C.; Wachten, D.; Yaksi, E.; Jurisch-Yaksi, N. Ciliary Beating Compartmentalizes Cerebrospinal Fluid Flow in the Brain and Regulates Ventricular Development. Curr. Biol. 2019, 29, 229–241.e6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Siyahhan, B.; Knobloch, V.; de Zélicourt, D.; Asgari, M.; Daners, M.S.; Poulikakos, D.; Kurtcuoglu, V. Flow induced by ependymal cilia dominates near-wall cerebrospinal fluid dynamics in the lateral ventricles. J. R. Soc. Interface 2014, 11, 20131189. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rickman, D.S.; Bobek, M.P.; Misek, D.E.; Kuick, R.; Blaivas, M.; Kurnit, D.M.; Taylor, J.; Hanash, S.M. Distinctive molecular profiles of high-grade and low-grade gliomas based on oligonucleotide microarray analysis. Cancer Res. 2001, 61, 6885–6891. [Google Scholar] [PubMed]
- Freije, W.A.; Castro-Vargas, F.E.; Fang, Z.; Horvath, S.; Cloughesy, T.; Liau, L.M.; Mischel, P.S.; Nelson, S.F. Gene expression profiling of gliomas strongly predicts survival. Cancer Res. 2004, 64, 6503–6510. [Google Scholar] [CrossRef] [PubMed]
- Nigro, J.M.; Misra, A.; Zhang, L.; Smirnov, I.; Colman, H.; Griffin, C.; Ozburn, N.; Chen, M.; Pan, E.; Koul, D.; et al. Integrated array-comparative genomic hybridization and expression array profiles identify clinically relevant molecular subtypes of glioblastoma. Cancer Res. 2005, 65, 1678–1686. [Google Scholar] [CrossRef] [PubMed]
- Nutt, C.L.; Mani, D.R.; Betensky, R.A.; Tamayo, P.; Cairncross, J.G.; Ladd, C.; Pohl, U.; Hartmann, C.; E McLaughlin, M.; Batchelor, T.T.; et al. Gene expression-based classification of malignant gliomas correlates better with survival than histological classification. Cancer Res. 2003, 63, 1602–1607. [Google Scholar] [PubMed]
- Phillips, H.S.; Kharbanda, S.; Chen, R.; Forrest, W.F.; Soriano, R.H.; Wu, T.D.; Misra, A.; Nigro, J.M.; Colman, H.; Soroceanu, L.; et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 2006, 9, 157–173. [Google Scholar] [CrossRef] [PubMed]
- McDonald, K.L.; O’Sullivan, M.G.; Parkinson, J.F.; Shaw, J.M.; Payne, C.A.; Brewer, J.M.; Young, L.; Reader, D.J.; Wheeler, H.T.; Cook, R.J.; et al. IQGAP1 and IGFBP2: Valuable biomarkers for determining prognosis in glioma patients. J. Neuropathol. Exp. Neurol. 2007, 66, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Colman, H.; Zhang, L.; Sulman, E.P.; McDonald, J.M.; Shooshtari, N.L.; Rivera, A.; Popoff, S.; Nutt, C.L.; Louis, D.N.; Cairncross, J.G.; et al. A multigene predictor of outcome in glioblastoma. Neuro-Oncol. 2010, 12, 49–57. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ceccarelli, M.; Barthel, F.P.; Malta, T.M.; Sabedot, T.S.; Salama, S.R.; Murray, B.A.; Morozova, O.; Newton, Y.; Radenbaugh, A.; Pagnotta, S.M.; et al. Molecular Profiling Reveals Biologically Discrete Subsets and Pathways of Progression in Diffuse Glioma. Cell 2016, 164, 550–563. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Prasad, B.; Tian, Y.; Li, X. Large-Scale Analysis Reveals Gene Signature for Survival Prediction in Primary Glioblastoma. Mol. Neurobiol. 2020, 57, 5235–5246. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yin, W.; Tang, G.; Zhou, Q.; Cao, Y.; Li, H.; Fu, X.; Wu, Z.; Jiang, X. Expression Profile Analysis Identifies a Novel Five-Gene Signature to Improve Prognosis Prediction of Glioblastoma. Front. Genet. 2019, 10, 419. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, Z.; Du, M.; Lu, L. A Novel 16-Genes Signature Scoring System as Prognostic Model to Evaluate Survival Risk in Patients with Glioblastoma. Biomedicines 2022, 10, 317. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Y.; Shen, S.; Yan, Z.; Yan, L.; Ding, H.; Wang, A.; Xu, Q.; Sun, L.; Yuan, Y. Expression characteristics and their functional role of IGFBP gene family in pan-cancer. BMC Cancer 2023, 23, 371. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fukushima, T.; Tezuka, T.; Shimomura, T.; Nakano, S.; Kataoka, H. Silencing of insulin-like growth factor-binding protein-2 in human glioblastoma cells reduces both invasiveness and expression of progression-associated gene CD24. J. Biol. Chem. 2007, 282, 18634–18644. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.S.; Gokulnath, P.; Bashir, M.; Shwetha, S.D.; Jaiswal, J.; Shastry, A.H.; Arimappamagan, A.; Santosh, V.; Kondaiah, P. Insulin-like growth factor binding protein-2 regulates β-catenin signaling pathway in glioma cells and contributes to poor patient prognosis. Neuro-Oncol. 2016, 18, 1487–1497. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sehgal, P.; Kumar, N.; Kumar, V.R.P.; Patil, S.; Bhattacharya, A.; Kumar, M.V.; Mukherjee, G.; Kondaiah, P. Regulation of protumorigenic pathways by insulin like growth factor binding protein2 and its association along with β-catenin in breast cancer lymph node metastasis. Mol. Cancer 2013, 12, 63. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, X.; Zhang, Y.; Zhang, P.; Wei, M.; Tian, T.; Guan, Y.; Han, C.; Wei, W.; Ma, Y. IGFBP2 drives epithelial-mesenchymal transition in hepatocellular carcinoma via activating the Wnt/β-catenin pathway. Infect. Agent. Cancer 2023, 18, 73. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hsieh, D.; Hsieh, A.; Stea, B.; Ellsworth, R. IGFBP2 promotes glioma tumor stem cell expansion and survival. Biochem. Biophys. Res. Commun. 2010, 397, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Robilliard, L.D.; Yu, J.; Anchan, A.; Finlay, G.; Angel, C.E.; Graham, E.S. Comprehensive Assessment of Secreted Immuno-Modulatory Cytokines by Serum-Differentiated and Stem-like Glioblastoma Cells Reveals Distinct Differences between Glioblastoma Phenotypes. Int. J. Mol. Sci. 2022, 23, 14164. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Berezovsky, A.D.; Poisson, L.M.; Cherba, D.; Webb, C.P.; Transou, A.D.; Lemke, N.W.; Hong, X.; Hasselbach, L.A.; Irtenkauf, S.M.; Mikkelsen, T.; et al. Sox2 promotes malignancy in glioblastoma by regulating plasticity and astrocytic differentiation. Neoplasia 2014, 16, 193–206.e25. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Kunhiraman, H.; McSwain, L.; Shahab, S.W.; Gershon, T.R.; MacDonald, T.J.; Kenney, A.M. IGFBP2 promotes proliferation and cell migration through STAT3 signaling in Sonic hedgehog medulloblastoma. Acta Neuropathol. Commun. 2023, 11, 62. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vanner, R.J.; Remke, M.; Gallo, M.; Selvadurai, H.J.; Coutinho, F.; Lee, L.; Kushida, M.; Head, R.; Morrissy, S.; Zhu, X.; et al. Quiescent sox2(+) cells drive hierarchical growth and relapse in sonic hedgehog subgroup medulloblastoma. Cancer Cell 2014, 26, 33–47. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Friend, K.E.; Khandwala, H.M.; Flyvbjerg, A.; Hill, H.; Li, J.; McCutcheon, I.E. Growth hormone and insulin-like growth factor-I: Effects on the growth of glioma cell lines. Growth Horm. IGF Res. 2001, 11, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Schlenska-Lange, A.; Knüpfer, H.; Lange, T.J.; Kiess, W.; Knüpfer, M. Cell proliferation and migration in glioblastoma multiforme cell lines are influenced by insulin-like growth factor I in vitro. Anticancer Res. 2008, 28, 1055–1060. [Google Scholar] [PubMed]
- Zamykal, M.; Martens, T.; Matschke, J.; Günther, H.S.; Kathagen, A.; Schulte, A.; Peters, R.; Westphal, M.; Lamszus, K. Inhibition of intracerebral glioblastoma growth by targeting the insulin-like growth factor 1 receptor involves different context-dependent mechanisms. Neuro-Oncol. 2015, 17, 1076–1085. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Antoniades, H.N.; Galanopoulos, T.; Neville-Golden, J.; Maxwell, M. Expression of insulin-like growth factors I and II and their receptor mRNAs in primary human astrocytomas and meningiomas; in vivo studies using in situ hybridization and immunocytochemistry. Int. J. Cancer 1992, 50, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Hirano, H.; Lopes, M.B.S.; Laws, E.R.; Asakura, T.; Goto, M.; Carpenter, J.E.; Karns, L.R.; VandenBerg, S.R. Insulin-like growth factor-1 content and pattern of expression correlates with histopathologic grade in diffusely infiltrating astrocytomas. Neuro-Oncol. 1999, 1, 109–119. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Merrill, M.J.; Edwards, N.A. Insulin-like growth factor-I receptors in human glial tumors. J. Clin. Endocrinol. Metab. 1990, 71, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Soroceanu, L.; Kharbanda, S.; Chen, R.; Soriano, R.H.; Aldape, K.; Misra, A.; Zha, J.; Forrest, W.F.; Nigro, J.M.; Modrusan, Z.; et al. Identification of IGF2 signaling through phosphoinositide-3-kinase regulatory subunit 3 as a growth-promoting axis in glioblastoma. Proc. Natl. Acad. Sci. USA 2007, 104, 3466–3471. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Suvasini, R.; Shruti, B.; Thota, B.; Shinde, S.V.; Friedmann-Morvinski, D.; Nawaz, Z.; Prasanna, K.V.; Thennarasu, K.; Hegde, A.S.; Arivazhagan, A.; et al. Insulin growth factor-2 binding protein 3 (IGF2BP3) is a glioblastoma-specific marker that activates phosphatidylinositol 3-kinase/mitogen-activated protein kinase (PI3K/MAPK) pathways by modulating IGF-2. J. Biol. Chem. 2011, 286, 25882–25890. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sandberg-Nordqvist, A.C.; Ståhlbom, P.A.; Reinecke, M.; Collins, V.P.; von Holst, H.; Sara, V. Characterization of insulin-like growth factor 1 in human primary brain tumors. Cancer Res. 1993, 53, 2475–2478. [Google Scholar] [PubMed]
- Sandberg, A.C.; Engberg, C.; Lake, M.; von Holst, H.; Sara, V.R. The expression of insulin-like growth factor I and insulin-like growth factor II genes in the human fetal and adult brain and in glioma. Neurosci. Lett. 1988, 93, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Dmitrenko, V.V.; Kavsan, V.M.; Boyko, O.I.; Rymar, V.I.; Stepanenko, A.A.; Balynska, O.V.; Mausheva, T.A.; Rozumenko, V.D.; Zozulya, Y.P. Expression of genes belonging to the IGF-system in glial tumors. Tsitol Genet. 2011, 45, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Kavsan, V.; Shostak, K.; Dmitrenko, V.; Zozulya, Y.; Rozumenko, V.; Demotes-Mainard, J. Characterization of genes with increased expression in human glioblastomas. Tsitol Genet. 2005, 39, 37–49. [Google Scholar] [PubMed]
- Maris, C.; D’Haene, N.; Trépant, A.-L.; Le Mercier, M.; Sauvage, S.; Allard, J.; Rorive, S.; Demetter, P.; Decaestecker, C.; Salmon, I. IGF-IR: A new prognostic biomarker for human glioblastoma. Br. J. Cancer 2015, 113, 729–737. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mohlin, S.; Hamidian, A.; Påhlman, S. HIF2A and IGF2 expression correlates in human neuroblastoma cells and normal immature sympathetic neuroblasts. Neoplasia 2013, 15, 328–334. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mu, Q.; Wang, L.; Yu, F.; Gao, H.; Lei, T.; Li, P.; Liu, P.; Zheng, X.; Hu, X.; Chen, Y.; et al. Imp2 regulates GBM progression by activating IGF2/PI3K/Akt pathway. Cancer Biol. Ther. 2015, 16, 623–633. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bendall, S.C.; Stewart, M.H.; Menendez, P.; George, D.; Vijayaragavan, K.; Werbowetski-Ogilvie, T.; Ramos-Mejia, V.; Rouleau, A.; Yang, J.; Bossé, M.; et al. IGF and FGF cooperatively establish the regulatory stem cell niche of pluripotent human cells in vitro. Nature 2007, 448, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.P.; Tirosh, I.; Trombetta, J.J.; Shalek, A.K.; Gillespie, S.M.; Wakimoto, H.; Cahill, D.P.; Nahed, B.V.; Curry, W.T.; Martuza, R.L.; et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 2014, 344, 1396–1401. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alcantara Llaguno, S.R.; Parada, L.F. Cell of origin of glioma: Biological and clinical implications. Br. J. Cancer 2016, 115, 1445–1450. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, J.; Li, Y.; Yu, T.-S.; McKay, R.M.; Burns, D.K.; Kernie, S.G.; Parada, L.F. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature 2012, 488, 522–526. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferguson, K.M.; Blin, C.; Alfazema, N.; Gangoso, E.; Pollard, S.M.; Marques-Torrejon, M.A. Lrig1 regulates the balance between proliferation and quiescence in glioblastoma stem cells. Front. Cell Dev. Biol. 2022, 10, 983097. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marqués-Torrejón, M.Á.; Williams, C.A.C.; Southgate, B.; Alfazema, N.; Clements, M.P.; Garcia-Diaz, C.; Blin, C.; Arranz-Emparan, N.; Fraser, J.; Gammoh, N.; et al. LRIG1 is a gatekeeper to exit from quiescence in adult neural stem cells. Nat. Commun. 2021, 12, 2594. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Elmlinger, M.W.; Deininger, M.H.; Schuett, B.S.; Meyermann, R.; Duffner, F.; Grote, E.H.; Ranke, M.B. In vivo expression of insulin-like growth factor-binding protein-2 in human gliomas increases with the tumor grade. Endocrinology 2001, 142, 1652–1658. [Google Scholar] [CrossRef] [PubMed]
- Fuller, G.N.; Rhee, C.H.; Hess, K.R.; Caskey, L.S.; Wang, R.; Bruner, J.M.; Yung, W.K.; Zhang, W. Reactivation of insulin-like growth factor binding protein 2 expression in glioblastoma multiforme: A revelation by parallel gene expression profiling. Cancer Res. 1999, 59, 4228–4232. [Google Scholar] [PubMed]
- Tseng, L.Y.-H.; Brown, A.L.; Yang, Y.W.-H.; Romanus, J.A.; Orlowski, C.C.; Taylor, T.; Rechler, M.M. The fetal rat binding protein for insulin-like growth factors is expressed in the choroid plexus and cerebrospinal fluid of adult rats. Mol. Endocrinol. 1989, 3, 1559–1568. [Google Scholar] [CrossRef] [PubMed]
- Wood, T.L.; Streck, R.D.; Pintar, J.E. Expression of the IGFBP-2 gene in post-implantation rat embryos. Development 1992, 114, 59–66. [Google Scholar] [CrossRef] [PubMed]
- van Straaten, H.W.; Hekking, J.W.; Wiertz-Hoessels, E.J.; Thors, F.; Drukker, J. Effect of the notochord on the differentiation of a floor plate area in the neural tube of the chick embryo. Anat. Embryol. 1988, 177, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Placzek, M.; Tanaka, H.; Dodd, J.; Jessell, T.M. Control of cell pattern in the developing nervous system: Polarizing activity of the floor plate and notochord. Cell 1991, 64, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Song, C.; Liu, Y.; Zhang, J.; Wei Song, S. IGFBP2 promotes neural stem cell maintenance and proliferation differentially associated with glioblastoma subtypes. Brain Res. 2019, 1704, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.P.; Chin, S.S.; Fliegner, K.H.; Liem, R.K. Alpha-internexin, a novel neuronal intermediate filament protein, precedes the low molecular weight neurofilament protein (NF-L) in the developing rat brain. J. Neurosci. 1990, 10, 2735–2748. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Burgdorf, J.S.; Yoon, S.; Dos Santos, M.; Lammert, C.R.; Moskal, J.R.; Penzes, P. An IGFBP2-derived peptide promotes neuroplasticity and rescues deficits in a mouse model of Phelan-McDermid syndrome. Mol. Psychiatry 2023, 28, 1101–1111. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Holmin, S.; Mathiesen, T.; Langmoen, I.A.; Sandberg Nordqvist, A.C. Depolarization induces insulin-like growth factor binding protein-2 expression in vivo via NMDA receptor stimulation. Growth Horm. IGF Res. 2001, 11, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Lu, X.; Huang, Q.; Tang, J.; Weng, J.; Yang, Z.; Lv, M.; Xu, X.; Xia, F.; Zhang, M.; et al. IGFBP2 Plays an Essential Role in Cognitive Development during Early Life. Adv. Sci. 2019, 6, 1901152. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van Doorn, J. Insulin-like growth factor-II and bioactive proteins containing a part of the E-domain of pro-insulin-like growth factor-II. Biofactors 2020, 46, 563–578. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Greenall, S.A.; Bentley, J.D.; Pearce, L.A.; Scoble, J.A.; Sparrow, L.G.; Bartone, N.A.; Xiao, X.; Baxter, R.C.; Cosgrove, L.J.; Adams, T.E. Biochemical characterization of individual human glycosylated pro-insulin-like growth factor (IGF)-II and big-IGF-II isoforms associated with cancer. J. Biol. Chem. 2013, 288, 59–68. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- PotalPotalitsyn, P.; Mrázková, L.; Selicharová, I.; Tencerová, M.; Ferenčáková, M.; Chrudinová, M.; Turnovská, T.; Brzozowski, A.M.; Marek, A.; Kaminský, J.; et al. Non-glycosylated IGF2 prohormones are more mitogenic than native IGF2. Commun. Biol. 2023, 6, 863. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Frasca, F.; Pandini, G.; Scalia, P.; Sciacca, L.; Mineo, R.; Costantino, A.; Goldfine, I.D.; Belfiore, A.; Vigneri, R. Insulin receptor isoform A, a newly recognized, high-affinity insulin-like growth factor II receptor in fetal and cancer cells. Mol. Cell Biol. 1999, 19, 3278–3288. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, J.; Morehouse, C.; Streicher, K.; Higgs, B.W.; Gao, J.; Czapiga, M.; Boutrin, A.; Zhu, W.; Brohawn, P.; Chang, Y.; et al. Altered expression of insulin receptor isoforms in breast cancer. PLoS ONE 2011, 6, e26177. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vella, V.; Milluzzo, A.; Scalisi, N.M.; Vigneri, P.; Sciacca, L. Insulin Receptor Isoforms in Cancer. Int. J. Mol. Sci. 2018, 19, 3615. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Benabou, E.; Salamé, Z.; Wendum, D.; Lequoy, M.; Tahraoui, S.; Merabtene, F.; Chrétien, Y.; Scatton, O.; Rosmorduc, O.; Fouassier, L.; et al. Insulin receptor isoform A favors tumor progression in human hepatocellular carcinoma by increasing stem/progenitor cell features. Cancer Lett. 2019, 450, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Quetglas, I.; Pinyol, R.; Dauch, D.; Torrecilla, S.; Tovar, V.; Moeini, A.; Alsinet, C.; Portela, A.; Rodriguez-Carunchio, L.; Solé, M.; et al. IGF2 Is Up-regulated by Epigenetic Mechanisms in Hepatocellular Carcinomas and Is an Actionable Oncogene Product in Experimental Models. Gastroenterology 2016, 151, 1192–1205. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Kwon, Y.; Joo, J.Y.; Kim, D.G.; Yoon, J.H. Secretomics to Discover Regulators in Diseases. Int. J. Mol. Sci. 2019, 20, 3893. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martin, R.M.; Holly, J.M.; Davey Smith, G.; Gunnell, D. Associations of adiposity from childhood into adulthood with insulin resistance and the insulin-like growth factor system: 65-year follow-up of the Boyd Orr Cohort. J. Clin. Endocrinol. Metab. 2006, 91, 3287–3295. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ziegler, A.N.; Feng, Q.; Chidambaram, S.; Testai, J.M.; Kumari, E.; Rothbard, D.E.; Constancia, M.; Sandovici, I.; Cominski, T.; Pang, K.; et al. Insulin-like Growth Factor II: An Essential Adult Stem Cell Niche Constituent in Brain and Intestine. Stem Cell Rep. 2019, 12, 816–830. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harland, A.J.; Perks, C.M. IGFBP-2 and IGF-II: Key Components of the Neural Stem Cell Niche? Implications for Glioblastoma Pathogenesis. Int. J. Mol. Sci. 2025, 26, 4749. https://doi.org/10.3390/ijms26104749
Harland AJ, Perks CM. IGFBP-2 and IGF-II: Key Components of the Neural Stem Cell Niche? Implications for Glioblastoma Pathogenesis. International Journal of Molecular Sciences. 2025; 26(10):4749. https://doi.org/10.3390/ijms26104749
Chicago/Turabian StyleHarland, Abigail J., and Claire M. Perks. 2025. "IGFBP-2 and IGF-II: Key Components of the Neural Stem Cell Niche? Implications for Glioblastoma Pathogenesis" International Journal of Molecular Sciences 26, no. 10: 4749. https://doi.org/10.3390/ijms26104749
APA StyleHarland, A. J., & Perks, C. M. (2025). IGFBP-2 and IGF-II: Key Components of the Neural Stem Cell Niche? Implications for Glioblastoma Pathogenesis. International Journal of Molecular Sciences, 26(10), 4749. https://doi.org/10.3390/ijms26104749






