Spinach Extract Reduces Kidney Damage in Diabetic Rats by Impairing the AGEs/RAGE Axis
Abstract
1. Introduction
2. Results
2.1. Flavonoid Characterization in SME
2.2. Effect of SME on STZ-Induced Changes in Body Weight, Glucose and Creatinine Levels, and Renal Pathological Features
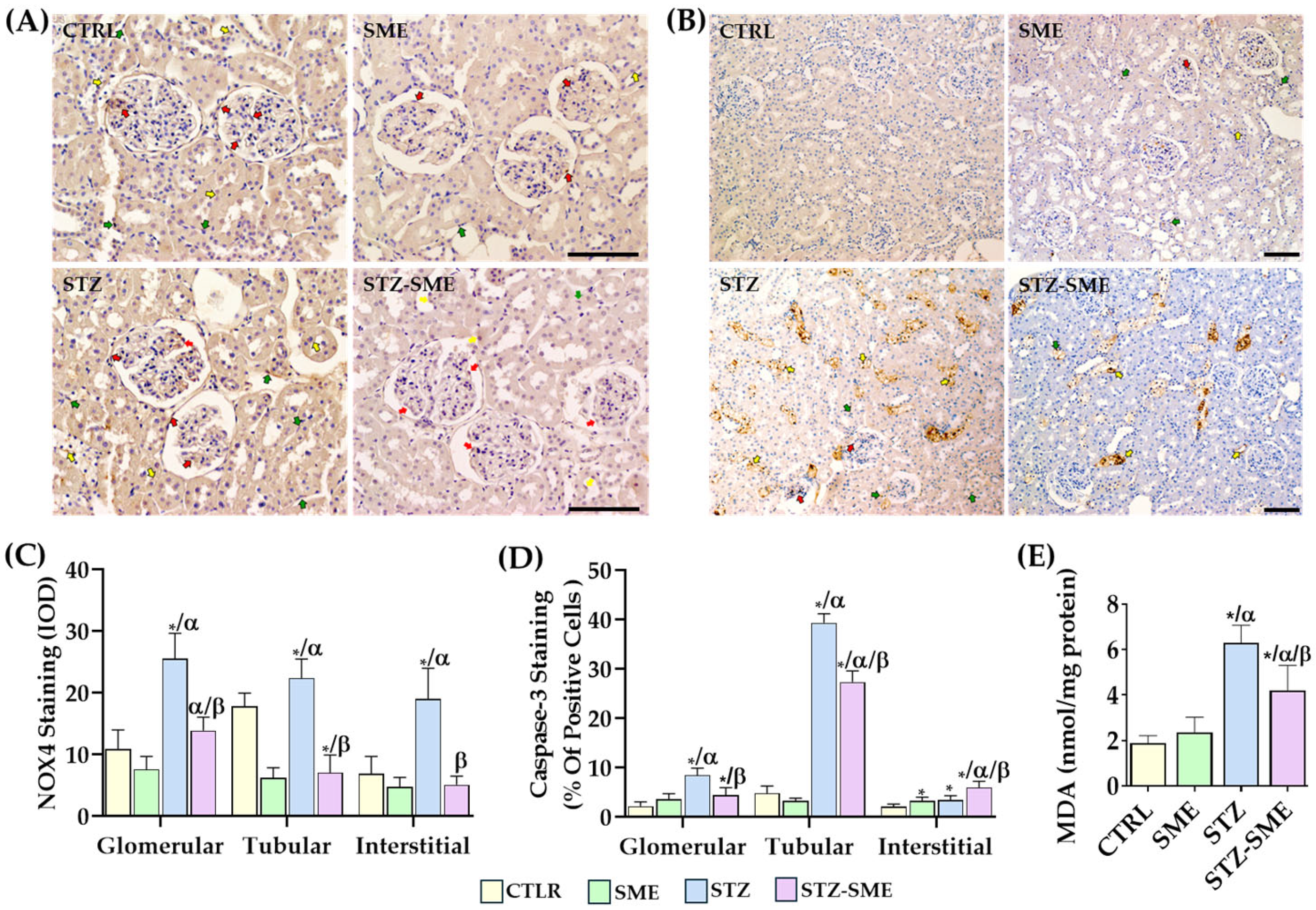
2.3. Effect of SME on STZ-Induced Nephrotoxicity
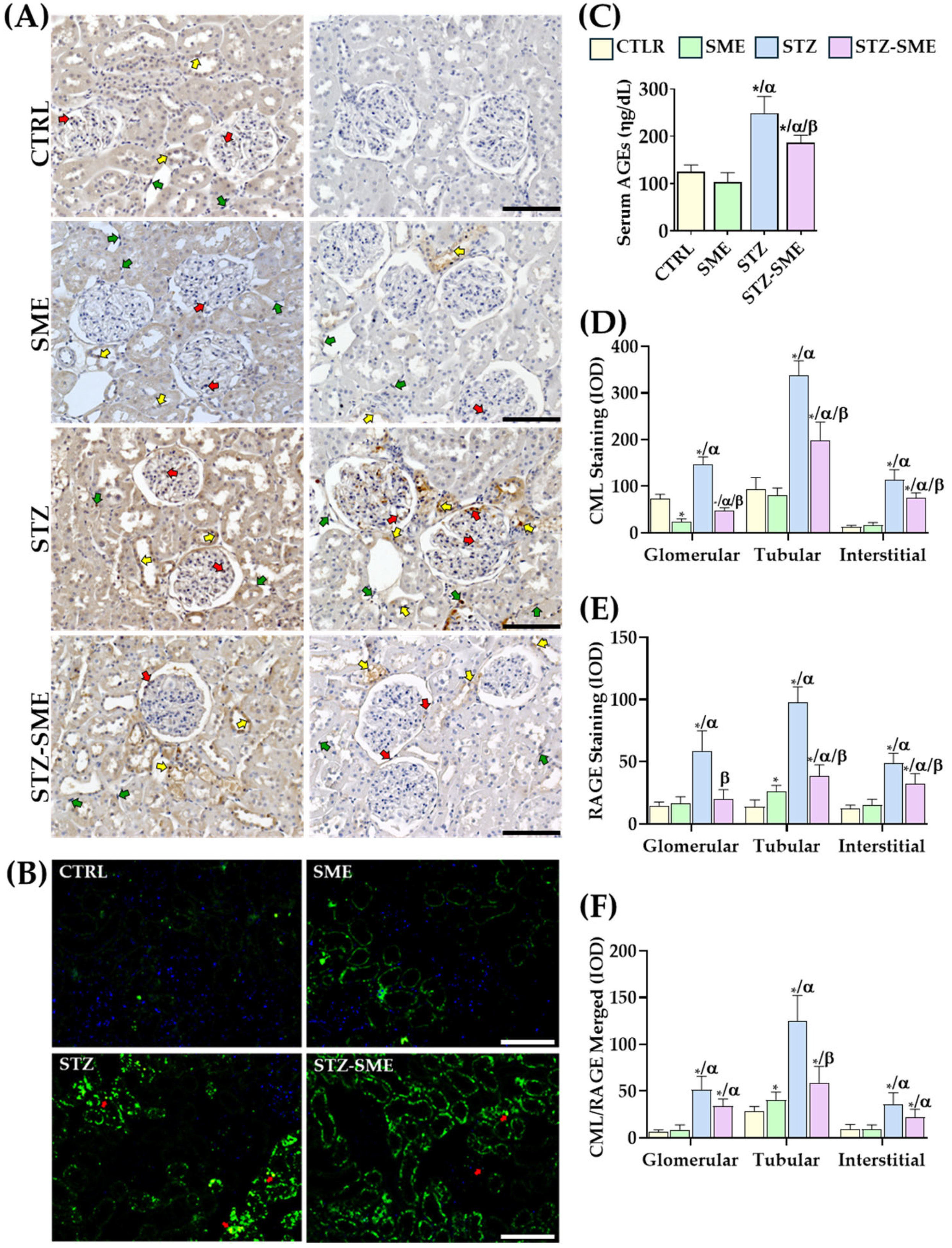
2.4. The Effect of SME on the Accumulation of CML and the Expression of RAGE
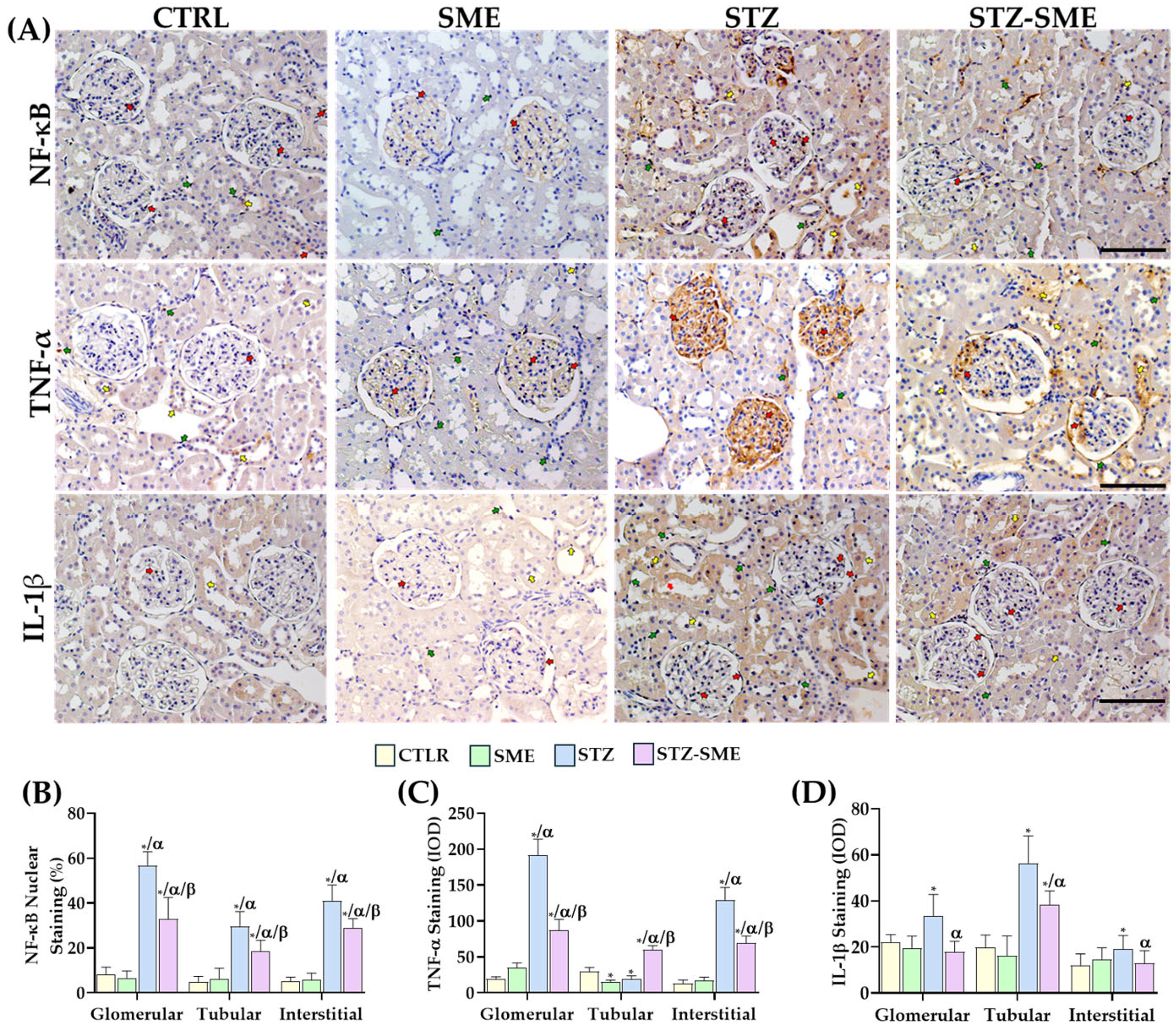
2.5. The Effect of SME on the Activation of the Inflammatory Response
2.6. Effect of SME on the Expression of Fibrotic Factors in the Kidney Cortex
3. Discussion
4. Materials and Methods
4.1. Flavonoid Characterization
4.2. Experimental Conditions—Animals
4.3. Experimental Design of a Diabetes-Induced Kidney Damage Model in Rats and Treatments
4.4. Lipid Peroxidation
4.5. Detection of Total AGEs in Serum
4.6. Histology and Immunohistochemical (IHC) Staining
4.7. Immunofluorescence for CML and RAGE in Kidney Tissue
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akhtar, M.; Taha, N.M.; Nauman, A.; Mujeeb, I.B.; Al-Nabet, A.D.M.H. Diabetic Kidney Disease: Past and Present. Adv. Anat. Pathol. 2020, 27, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.Y.; Yoo, T.H. Pathophysiologic Mechanisms and Potential Biomarkers in Diabetic Kidney Disease. Diabetes Metab. J. 2022, 46, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Lin, J.H.; Hammes, H.P.; Zhang, C. Cellular Phenotypic Transitions in Diabetic Nephropathy: An Update. Front. Pharmacol. 2022, 13, 1038073. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jin, M.; Cheng, C.K.; Li, Q. Tubular Injury in Diabetic Kidney Disease: Molecular Mechanisms and Potential Therapeutic Perspectives. Front. Endocrinol. 2023, 14, 1238927. [Google Scholar] [CrossRef]
- Yamagishi, S.; Fukami, K.; Ueda, S.; Okuda, S. Molecular Mechanisms of Diabetic Nephropathy and Its Therapeutic Intervention. Curr. Drug Targets 2007, 8, 952–959. [Google Scholar] [CrossRef]
- Samsu, N. Diabetic Nephropathy: Challenges in Pathogenesis, Diagnosis, and Treatment. BioMed Res. Int. 2021, 2021, 1497449. [Google Scholar] [CrossRef]
- Fotheringham, A.K.; Gallo, L.A.; Borg, D.J.; Forbes, J.M. Advanced Glycation End Products (AGEs) and Chronic Kidney Disease: Does the Modern Diet AGE the Kidney? Nutrients 2022, 14, 2675. [Google Scholar] [CrossRef]
- Miyata, T.; Ueda, Y.; Horie, K.; Nangaku, M.; Tanaka, S.; Van Ypersele De Strihou, C.; Kurokawa, K. Renal Catabolism of Advanced Glycation End Products: The Fate of Pentosidine. Kidney Int. 1998, 53, 416–422. [Google Scholar] [CrossRef]
- Vlassara, H.; Uribarri, J.; Cai, W.; Striker, G. Advanced Glycation End Product Homeostasis: Exogenous Oxidants and Innate Defenses. Ann. N. Y. Acad. Sci. 2008, 1126, 46–52. [Google Scholar] [CrossRef]
- Pasupulati, A.K.; Nagati, V.; Paturi, A.S.V.; Reddy, G.B. Non-Enzymatic Glycation and Diabetic Kidney Disease. Vitam. Horm. 2024, 125, 251–285. [Google Scholar] [CrossRef]
- Tanji, N.; Markowitz, G.S.; Fu, C.; Kislinger, T.; Taguchi, A.; Pischetsrieder, M.; Stern, D.; Schmidt, A.M.; D’Agati, V.D. Expression of Advanced Glycation End Products and Their Cellular Receptor RAGE in Diabetic Nephropathy and Nondiabetic Renal Disease. J. Am. Soc. Nephrol. 2000, 11, 1656–1666. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; He, J.C.; Cai, W.; Liu, H.I.; Zhu, L.; Vlassara, H. Advanced Glycation Endproduct (AGE) Receptor 1 Is a Negative Regulator of the Inflammatory Response to AGE in Mesangial Cells. Proc. Natl. Acad. Sci. USA 2004, 101, 11767–11772. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.Y.; Lu, C.H.; Wu, C.H.; Li, K.J.; Kuo, Y.M.; Hsieh, S.C.; Yu, C.L. The Development of Maillard Reaction, and Advanced Glycation End Product (AGE)-Receptor for AGE (RAGE) Signaling Inhibitors as Novel Therapeutic Strategies for Patients with AGE-Related Diseases. Molecules 2020, 25, 5591. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Huang, X.R.; Zhu, H.J.; Oldfield, M.; Cooper, M.; Truong, L.D.; Johnson, R.J.; Lan, H.Y. Advanced Glycation End Products Activate Smad Signaling via TGF-Beta-Dependent and Independent Mechanisms: Implications for Diabetic Renal and Vascular Disease. FASEB J. 2004, 18, 176–178. [Google Scholar] [CrossRef]
- Oldfield, M.D.; Bach, L.A.; Forbes, J.M.; Nikolic-Paterson, D.; McRobert, A.; Thallas, V.; Atkins, R.C.; Osicka, T.; Jerums, G.; Cooper, M.E. Advanced Glycation End Products Cause Epithelial-Myofibroblast Transdifferentiation via the Receptor for Advanced Glycation End Products (RAGE). J. Clin. Investig. 2001, 108, 1853–1863. [Google Scholar] [CrossRef]
- Li, J.H.; Wang, W.; Huang, X.R.; Oldfield, M.; Schmidt, A.M.; Cooper, M.E.; Lan, H.Y. Advanced Glycation End Products Induce Tubular Epithelial-Myofibroblast Transition through the RAGE-ERK1/2 MAP Kinase Signaling Pathway. Am. J. Pathol. 2004, 164, 1389–1397. [Google Scholar] [CrossRef]
- Abdelkader, H.; Mustafa, W.W.; Alqahtani, A.M.; Alsharani, S.; Al Fatease, A.; Alany, R.G. Glycation-Induced Age-Related Illnesses, Antiglycation and Drug Delivery Strategies. J. Pharm. Pharmacol. 2022, 74, 1546–1567. [Google Scholar] [CrossRef]
- Pandjaitan, N.; Howard, L.R.; Morelock, T.; Gil, M.I. Antioxidant Capacity and Phenolic Content of Spinach as Affected by Genetics and Maturation. J. Agric. Food Chem. 2005, 53, 8618–8623. [Google Scholar] [CrossRef]
- Jaime, L.; Vázquez, E.; Fornari, T.; López-Hazas, M.d.C.; García-Risco, M.R.; Santoyo, S.; Reglero, G. Extraction of Functional Ingredients from Spinach (Spinacia oleracea L.) Using Liquid Solvent and Supercritical CO2 Extraction. J. Sci. Food Agric. 2015, 95, 722–729. [Google Scholar] [CrossRef]
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef]
- Roberts, J.L.; Moreau, R. Functional Properties of Spinach (Spinacia oleracea L.) Phytochemicals and Bioactives. Food Funct. 2016, 7, 3337–3353. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, M.N.I.; Mitsuhashi, S.; Sigetomi, K.; Ubukata, M. Quercetin Inhibits Advanced Glycation End Product Formation via Chelating Metal Ions, Trapping Methylglyoxal, and Trapping Reactive Oxygen Species. Biosci. Biotechnol. Biochem. 2017, 81, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kim, H.J.; Sparrow, J.R. Quercetin and Cyanidin-3-Glucoside Protect against Photooxidation and Photodegradation of A2E in Retinal Pigment Epithelial Cells. Exp. Eye Res. 2017, 160, 45–55. [Google Scholar] [CrossRef]
- Bodiga, V.L.; Eda, S.R.; Veduruvalasa, V.D.; Mididodla, L.D.; Parise, P.K.; Kodamanchili, S.; Jallepalli, S.; Inapurapu, S.P.; Neerukonda, M.; Vemuri, P.K.; et al. Attenuation of Non-Enzymatic Thermal Glycation of Bovine Serum Albumin (BSA) Using β-Carotene. Int. J. Biol. Macromol. 2013, 56, 41–48. [Google Scholar] [CrossRef]
- Bautista-Pérez, R.; Cano-Martínez, A.; Gutiérrez-Velázquez, E.; Martínez-Rosas, M.; Pérez-Gutiérrez, R.M.; Jiménez-Gómez, F.; Flores-Estrada, J. Spinach Methanolic Extract Attenuates the Retinal Degeneration in Diabetic Rats. Antioxidants 2021, 10, 717. [Google Scholar] [CrossRef]
- Perez Gutierrez, R.M.; Velazquez, E.G. Glucopyranoside Flavonoids Isolated from Leaves of Spinacia oleracea (Spinach) Inhibit the Formation of Advanced Glycation End Products (AGEs) and Aldose Reductase Activity (RLAR). Biomed. Pharmacother. 2020, 128, 110299. [Google Scholar] [CrossRef]
- Son, Y.G.; Jung, J.; Lee, D.K.; Park, S.W.; Kim, J.Y.; Kim, H.J. Validation of an Analytical Method of 3′,4′,5-Trihydroxy-3-Methoxy-6,7-Methylenedioxyflavone 4′-Glucuronide for Standardization of Spinacia oleracea. Molecules 2024, 29, 2494. [Google Scholar] [CrossRef]
- Hashemzaei, M.; Tabrizian, K.; Alizadeh, Z.; Pasandideh, S.; Rezaee, R.; Mamoulakis, C.; Tsatsakis, A.; Skaperda, Z.; Kouretas, D.; Shahraki, J. Resveratrol, Curcumin and Gallic Acid Attenuate Glyoxal-Induced Damage to Rat Renal Cells. Toxicol. Rep. 2020, 7, 1571–1577. [Google Scholar] [CrossRef]
- Liu, D.; Chen, J.; Xie, Y.; Mei, X.; Xu, C.; Liu, J.; Cao, X. Investigating the Molecular Mechanisms of Glyoxal-Induced Cytotoxicity in Human Embryonic Kidney Cells: Insights from Network Toxicology and Cell Biology Experiments. Environ. Toxicol. 2022, 37, 2269–2280. [Google Scholar] [CrossRef]
- Nahdi, A.M.T.A.; John, A.; Raza, H. Elucidation of Molecular Mechanisms of Streptozotocin-Induced Oxidative Stress, Apoptosis, and Mitochondrial Dysfunction in Rin-5F Pancreatic β-Cells. Oxidative Med. Cell. Longev. 2017, 2017, 7054272. [Google Scholar] [CrossRef]
- Nakai, K.; Umehara, M.; Minamida, A.; Yamauchi-Sawada, H.; Sunahara, Y.; Matoba, Y.; Okuno-Ozeki, N.; Nakamura, I.; Nakata, T.; Yagi-Tomita, A.; et al. Streptozotocin Induces Renal Proximal Tubular Injury through P53 Signaling Activation. Sci. Rep. 2023, 13, 8705. [Google Scholar] [CrossRef] [PubMed]
- Naz, R.; Saqib, F.; Awadallah, S.; Wahid, M.; Latif, M.F.; Iqbal, I.; Mubarak, M.S. Food Polyphenols and Type II Diabetes Mellitus: Pharmacology and Mechanisms. Molecules 2023, 28, 3996. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Alam, M.B.; Lee, S.H.; Kim, Y.J.; Lee, S.; An, H.; Choi, H.J.; Son, H.U.; Park, C.H.; Kim, H.H.; et al. Spatholobus Suberectus Exhibits Antidiabetic Activity In Vitro and In Vivo through Activation of AKT-AMPK Pathway. Evid.-Based Complement. Alternat. Med. 2017, 2017, 6091923. [Google Scholar] [CrossRef] [PubMed]
- Do, M.H.; Hur, J.; Choi, J.; Kim, Y.; Park, H.Y.; Ha, S.K. Spatholobus Suberectus Ameliorates Diabetes-Induced Renal Damage by Suppressing Advanced Glycation End Products in Db/Db Mice. Int. J. Mol. Sci. 2018, 19, 2774. [Google Scholar] [CrossRef]
- Yuan, Y.; Sun, H.; Sun, Z. Advanced Glycation End Products (AGEs) Increase Renal Lipid Accumulation: A Pathogenic Factor of Diabetic Nephropathy (DN). Lipids Health Dis. 2017, 16, 126. [Google Scholar] [CrossRef]
- Nerlich, A.G.; Schleicher, E.D. N(Epsilon)-(Carboxymethyl)Lysine in Atherosclerotic Vascular Lesions as a Marker for Local Oxidative Stress. Atherosclerosis 1999, 144, 41–47. [Google Scholar] [CrossRef]
- Teissier, T.; Quersin, V.; Gnemmi, V.; Daroux, M.; Howsam, M.; Delguste, F.; Lemoine, C.; Fradin, C.; Schmidt, A.; Cauffiez, C.; et al. Knockout of Receptor for Advanced Glycation End-products Attenuates Age-related Renal Lesions. Aging Cell 2019, 18, e12850. [Google Scholar] [CrossRef]
- Rabbani, N.; Thornalley, P.J. Advanced Glycation End Products in the Pathogenesis of Chronic Kidney Disease. Kidney Int. 2018, 93, 803–813. [Google Scholar] [CrossRef]
- Wagner, Z.; Wittmann, I.; Mazak, I.; Schinzel, R.; Heidland, A.; Kientsch-Engel, R.; Nagy, J. Nε-(Carboxymethyl)Lysine Levels in Patients with Type 2 Diabetes: Role of Renal Function. Am. J. Kidney Dis. 2001, 38, 785–791. [Google Scholar] [CrossRef]
- Maessen, D.E.M.; Stehouwer, C.D.A.; Schalkwijk, C.G. The Role of Methylglyoxal and the Glyoxalase System in Diabetes and Other Age-Related Diseases. Clin. Sci. 2015, 128, 839–861. [Google Scholar] [CrossRef]
- Vistoli, G.; De Maddis, D.; Cipak, A.; Zarkovic, N.; Carini, M.; Aldini, G. Advanced Glycoxidation and Lipoxidation End Products (AGEs and ALEs): An Overview of Their Mechanisms of Formation. Free Radic. Res. 2013, 47 (Suppl. S1), 3–27. [Google Scholar] [CrossRef] [PubMed]
- Asano, M.; Fujita, Y.; Ueda, Y.; Suzuki, D.; Miyata, T.; Sakai, H.; Saito, A. Renal Proximal Tubular Metabolism of Protein-Linked Pentosidine, an Advanced Glycation End Product. Nephron 2002, 91, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; Van Ypersele de Strihou, C.; Imasawa, T.; Yoshino, A.; Ueda, Y.; Ogura, H.; Kominami, K.; Onogi, H.; Inagi, R.; Nangaku, M.; et al. Glyoxalase I Deficiency Is Associated with an Unusual Level of Advanced Glycation End Products in a Hemodialysis Patient. Kidney Int. 2001, 60, 2351–2359. [Google Scholar] [CrossRef] [PubMed]
- Curran, C.S.; Kopp, J.B. RAGE Pathway Activation and Function in Chronic Kidney Disease and COVID-19. Front. Med. 2022, 9, 970423. [Google Scholar] [CrossRef]
- Kierdorf, K.; Fritz, G. RAGE Regulation and Signaling in Inflammation and Beyond. J. Leukoc. Biol. 2013, 94, 55–68. [Google Scholar] [CrossRef]
- Sun, H.; Yuan, Y.; Sun, Z. Update on Mechanisms of Renal Tubule Injury Caused by Advanced Glycation End Products. Biomed Res. Int. 2016, 2016, 5475120. [Google Scholar] [CrossRef]
- He, C.-J.; Zheng, F.; Stitt, A.; Striker, L.; Hattori, M.; Vlassara, H. Differential Expression of Renal AGE-Receptor Genes in NOD Mice: Possible Role in Nonobese Diabetic Renal Disease. Kidney Int. 2000, 58, 1931–1940. [Google Scholar] [CrossRef]
- Liu, J.; Huang, K.; Cai, G.Y.; Chen, X.M.; Yang, J.R.; Lin, L.R.; Yang, J.; Huo, B.G.; Zhan, J.; He, Y.N. Receptor for Advanced Glycation End-Products Promotes Premature Senescence of Proximal Tubular Epithelial Cells via Activation of Endoplasmic Reticulum Stress-Dependent P21 Signaling. Cell. Signal. 2014, 26, 110–121. [Google Scholar] [CrossRef]
- Cheng, M.; Liu, H.; Zhang, D.; Liu, Y.; Wang, C.; Liu, F.; Chen, J. HMGB1 Enhances the AGE-Induced Expression of CTGF and TGF-β via RAGE-Dependent Signaling in Renal Tubular Epithelial Cells. Am. J. Nephrol. 2015, 41, 257–266. [Google Scholar] [CrossRef]
- Penfold, S.A.; Coughlan, M.T.; Patel, S.K.; Srivastava, P.M.; Sourris, K.C.; Steer, D.; Webster, D.E.; Thomas, M.C.; MacIsaac, R.J.; Jerums, G.; et al. Circulating High-Molecular-Weight RAGE Ligands Activate Pathways Implicated in the Development of Diabetic Nephropathy. Kidney Int. 2010, 78, 287–295. [Google Scholar] [CrossRef]
- You, X.L.; Zhao, M.L.; Liu, Y.R.; Tang, Z.S.; Zhao, Y.T.; Yan-Liu; Song, Z.X. Hypericum perforatum L. Protects against Renal Function Decline in Ovariectomy Rat Model by Regulating Expressions of NOS3 and AKT1 in AGE-RAGE Pathway. Phytomedicine 2024, 123, 155160. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Jung, D.H.; Lee, I.S.; Pyun, B.J.; Kim, J.S. Osteomeles Schwerinae Extracts Inhibits the Binding to Receptors of Advanced Glycation End Products and TGF-Β1 Expression in Mesangial Cells under Diabetic Conditions. Phytomedicine 2016, 23, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Jung, D.H.; Lee, I.S.; Choi, S.J.; Yu, S.Y.; Ku, S.K.; Kim, M.H.; Kim, J.S. Effects of Allium victorialis Leaf Extracts and Its Single Compounds on Aldose Reductase, Advanced Glycation End Products and TGF-Β1 Expression in Mesangial Cells. BMC Complement. Altern. Med. 2013, 13, 251. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xiao, Y.; Gao, H.; Li, B.; Xu, L.; Cheng, M.; Jiang, B.; Ma, Y. Grape Seed Proanthocyanidins Ameliorate Diabetic Nephropathy via Modulation of Levels of AGE, RAGE and CTGF. Nephron Exp. Nephrol. 2009, 111, e31–e41. [Google Scholar] [CrossRef]
- Hou, B.; Qiang, G.; Zhao, Y.; Yang, X.; Chen, X.; Yan, Y.; Wang, X.; Liu, C.; Zhang, L.; Du, G. Salvianolic Acid A Protects Against Diabetic Nephropathy through Ameliorating Glomerular Endothelial Dysfunction via Inhibiting AGE-RAGE Signaling. Cell. Physiol. Biochem. 2017, 44, 2378–2394. [Google Scholar] [CrossRef]
- Qiu, Y.Y.; Tang, L.Q.; Wei, W. Berberine Exerts Renoprotective Effects by Regulating the AGEs-RAGE Signaling Pathway in Mesangial Cells during Diabetic Nephropathy. Mol. Cell. Endocrinol. 2017, 443, 89–105. [Google Scholar] [CrossRef]
- Mou, X.; Zhou, D.Y.; Zhou, D.; Liu, K.; Chen, L.J.; Liu, W.H. A Bioinformatics and Network Pharmacology Approach to the Mechanisms of Action of Shenxiao Decoction for the Treatment of Diabetic Nephropathy. Phytomedicine 2020, 69, 153192. [Google Scholar] [CrossRef]
- Niu, H.; Fan, L.; Zhao, L.; Yao, R.; He, X.; Lu, B.; Pang, Z. The Therapeutic Mechanism of PuRenDan for the Treatment of Diabetic Nephropathy: Network Pharmacology and Experimental Verification. J. Ethnopharmacol. 2022, 293, 115283. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J.; Jiang, M.; Fu, Y.; Zhu, Y.; Jiao, N.; Liu, L.; Du, Q.; Wu, H.; Xu, H.; et al. Loganin and Catalpol Exert Cooperative Ameliorating Effects on Podocyte Apoptosis upon Diabetic Nephropathy by Targeting AGEs-RAGE Signaling. Life Sci. 2020, 252, 117653. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, R.; Liu, H.; Li, L.; Chen, B.; Jia, Q.; Wang, L.; Ma, R.; Tian, S.; Wang, M.; et al. Aqueous Extract of Mori Folium Exerts Bone Protective Effect Through Regulation of Calcium and Redox Homeostasis via PTH/VDR/CaBP and AGEs/RAGE/Nox4/NF-ΚB Signaling in Diabetic Rats. Front. Pharmacol. 2018, 9, 1239. [Google Scholar] [CrossRef]
- Park, C.H.; Yokozawa, T.; Noh, J.S. Oligonol, a Low-Molecular-Weight Polyphenol Derived from Lychee Fruit, Attenuates Diabetes-Induced Renal Damage through the Advanced Glycation End Product-Related Pathway in Db/Db Mice. J. Nutr. 2014, 144, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Jha, J.C.; Gray, S.P.; Barit, D.; Okabe, J.; El-Osta, A.; Namikoshi, T.; Thallas-Bonke, V.; Wingler, K.; Szyndralewiez, C.; Heitz, F.; et al. Genetic Targeting or Pharmacologic Inhibition of NADPH Oxidase Nox4 Provides Renoprotection in Long-Term Diabetic Nephropathy. J. Am. Soc. Nephrol. 2014, 25, 1237–1254. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, E.R.D.; Guerra, G.C.B.; de Souza Araújo, D.F.; de Araújo, A.A.; Fernandes, J.M.; de Araújo Júnior, R.F.; da Silva, V.C.; de Carvalho, T.G.; de Santis Ferreira, L.; Zucolotto, S.M. Gastroprotective and Antioxidant Activity of Kalanchoe brasiliensis and Kalanchoe pinnata Leaf Juices against Indomethacin and Ethanol-Induced Gastric Lesions in Rats. Int. J. Mol. Sci. 2018, 19, 1265. [Google Scholar] [CrossRef] [PubMed]
- Fukami, K.; Ueda, S.; Yamagishi, S.I.; Kato, S.; Inagaki, Y.; Takeuchi, M.; Motomiya, Y.; Bucala, R.; Iida, S.; Tamaki, K.; et al. AGEs Activate Mesangial TGF-Beta-Smad Signaling via an Angiotensin II Type I Receptor Interaction. Kidney Int. 2004, 66, 2137–2147. [Google Scholar] [CrossRef]
- Flores-Estrada, J.; Cano-Martínez, A.; Vargas-González, Á.; Castrejón-Téllez, V.; Cornejo-Garrido, J.; Martínez-Rosas, M.; Guarner-Lans, V.; Rubio-Ruíz, M.E. Hepatoprotective Mechanisms Induced by Spinach Methanolic Extract in Rats with Hyperglycemia-An Immunohistochemical Analysis. Antioxidants 2023, 12, 2013. [Google Scholar] [CrossRef]
- Putt, D.A.; Zhong, Q.; Lash, L.H. Adaptive Changes in Renal Mitochondrial Redox Status in Diabetic Nephropathy. Toxicol. Appl. Pharmacol. 2012, 258, 188–198. [Google Scholar] [CrossRef]
- Mitsuhashi, T.; Nakayama, H.; Itoh, T.; Kuwajima, S.; Aoki, S.; Atsumi, T.; Koike, T. Immunochemical Detection of Advanced Glycation End Products in Renal Cortex from STZ-Induced Diabetic Rat. Diabetes 1993, 42, 826–832. [Google Scholar] [CrossRef]
- Jozi, F.; Kheiripour, N.; Taheri, M.A.; Ardjmand, A.; Ghavipanjeh, G.; Nasehi, Z.; Shahaboddin, M.E. L-Lysine Ameliorates Diabetic Nephropathy in Rats with Streptozotocin-Induced Diabetes Mellitus. BioMed Res. Int. 2022, 2022, 4547312. [Google Scholar] [CrossRef]

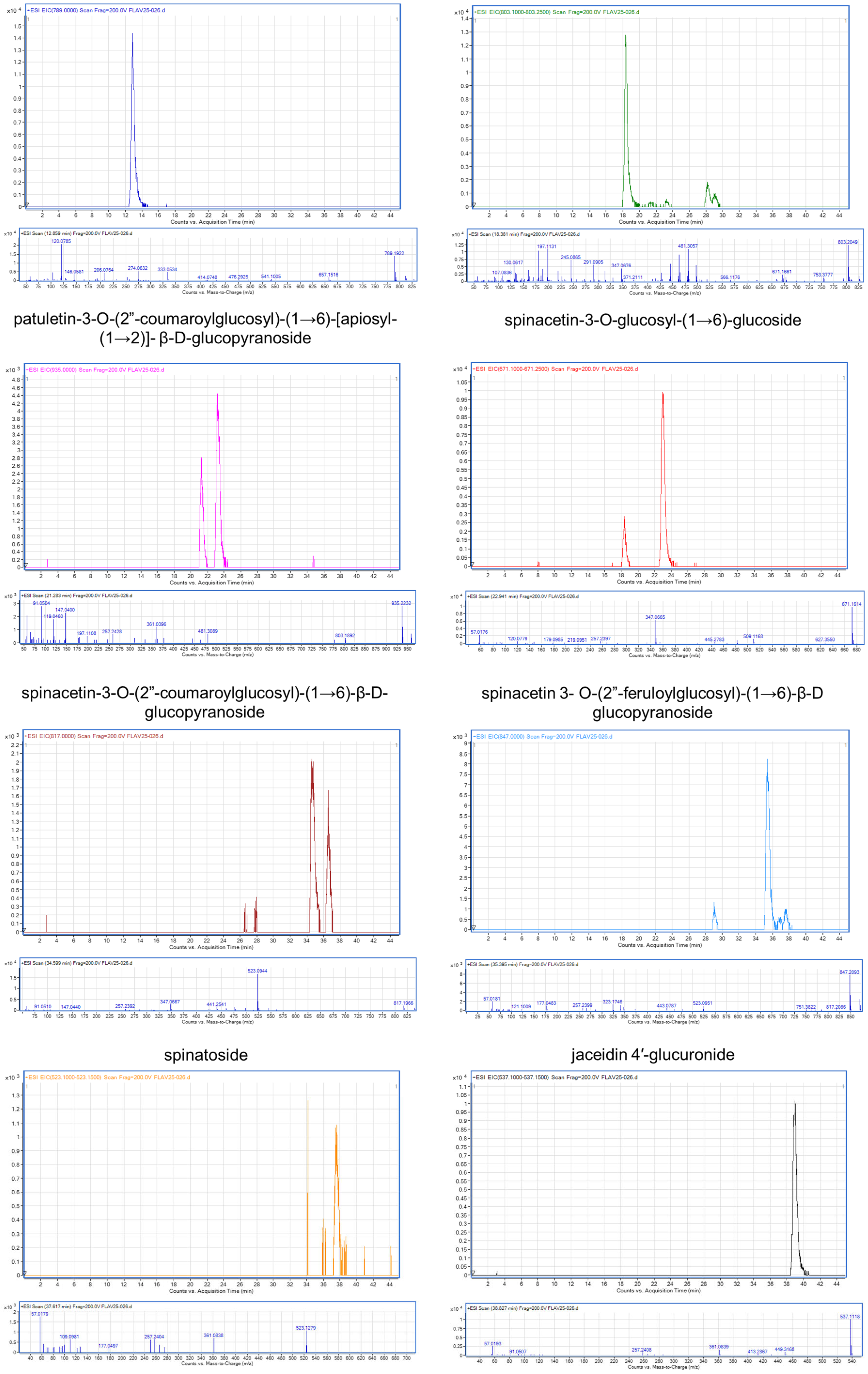


| Peak | Retention Time (tR; min) | Ion (m/z) | Compound | References |
|---|---|---|---|---|
| 1 | 12.85 | 789 | patuletin-3-O-β-d-glucopyranosyl-(1→6)-[β-d-apiofuranosyl-(1→2)]-β-d-glucopyranoside | [26] |
| 2 | 18.38 | 803.25 | spinacetin 3-O-glucosyl-(1→6)- [apiosyl(1→2)]-glucoside | [26,27] |
| 3 | 21.28 | 935 | patuletin-3-O-(2”-coumaroylglucosyl)-(1→6)-[apiosyl-(1→2)]-β-D-glucopyranoside | [26] |
| 4 | 22.94 | 671.25 | spinacetin-3-O-glucosyl-(1→6)-glucoside | [27] |
| 5 | 34.59 | 817 | spinacetin-3-O-(2”-coumaroylglucosyl)-(1→6)-β-D-glucopyranoside | [26] |
| 6 | 35.39 | 847 | spinacetin 3-O-(2”-feruloylglucosyl)-(1→6)-β-D glucopyranoside | [26] |
| 7 | 37.61 | 523.15 | spinatoside | [27] |
| 8 | 38.82 | 537.15 | jaceidin 4′-glucuronide | [27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Estrada, J.; Cano-Martínez, A.; Ibarra-Lara, L.; Jiménez, A.; Palacios-Reyes, C.; García, L.J.P.; Ortiz-López, M.G.; Rodríguez-Peña, O.N.; Hernández-Portilla, L.B. Spinach Extract Reduces Kidney Damage in Diabetic Rats by Impairing the AGEs/RAGE Axis. Int. J. Mol. Sci. 2025, 26, 4730. https://doi.org/10.3390/ijms26104730
Flores-Estrada J, Cano-Martínez A, Ibarra-Lara L, Jiménez A, Palacios-Reyes C, García LJP, Ortiz-López MG, Rodríguez-Peña ON, Hernández-Portilla LB. Spinach Extract Reduces Kidney Damage in Diabetic Rats by Impairing the AGEs/RAGE Axis. International Journal of Molecular Sciences. 2025; 26(10):4730. https://doi.org/10.3390/ijms26104730
Chicago/Turabian StyleFlores-Estrada, Javier, Agustina Cano-Martínez, Luz Ibarra-Lara, Adriana Jiménez, Carmen Palacios-Reyes, Luis J. Pinto García, María G. Ortiz-López, Olga Nelly Rodríguez-Peña, and Luis Barbo Hernández-Portilla. 2025. "Spinach Extract Reduces Kidney Damage in Diabetic Rats by Impairing the AGEs/RAGE Axis" International Journal of Molecular Sciences 26, no. 10: 4730. https://doi.org/10.3390/ijms26104730
APA StyleFlores-Estrada, J., Cano-Martínez, A., Ibarra-Lara, L., Jiménez, A., Palacios-Reyes, C., García, L. J. P., Ortiz-López, M. G., Rodríguez-Peña, O. N., & Hernández-Portilla, L. B. (2025). Spinach Extract Reduces Kidney Damage in Diabetic Rats by Impairing the AGEs/RAGE Axis. International Journal of Molecular Sciences, 26(10), 4730. https://doi.org/10.3390/ijms26104730







