Serum Antibody Titres Against Porphyromonas gingivalis Fim A in Patients with Periodontitis with and Without Diabetes Mellitus Type 2
Abstract
1. Introduction
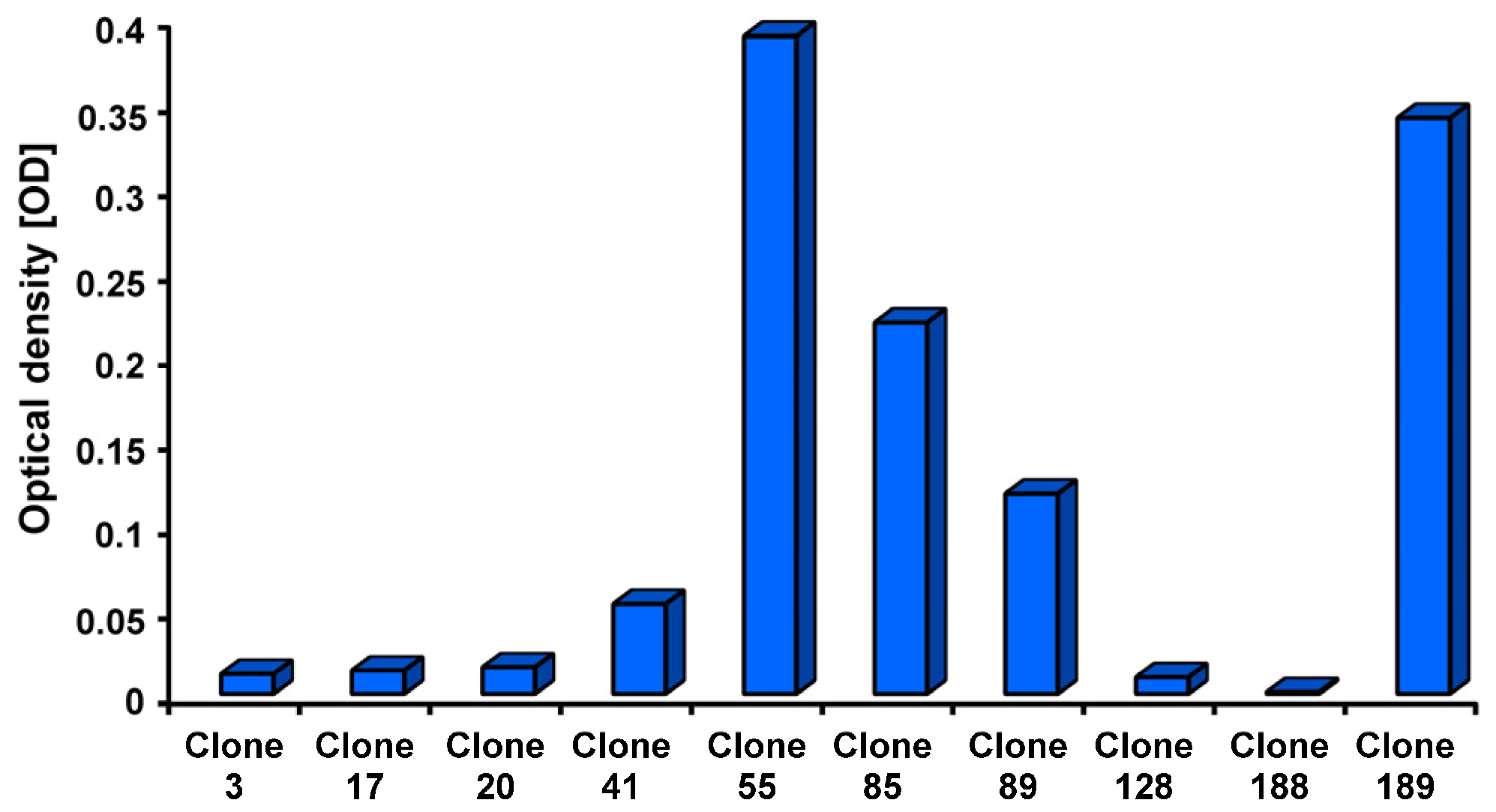
2. Results
3. Materials and Methods
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Page, R.C.; Kornman, K.S. The pathogenesis of human periodontitis: An introduction. Periodontology 2000 1997, 14, 9–11. [Google Scholar] [CrossRef]
- Meyle, J.; Chapple, I. Molecular aspects of the pathogenesis of periodontitis. Periodontology 2000 2015, 69, 7–17. [Google Scholar] [CrossRef]
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef]
- Loe, H.; Theilade, E.; Jensen, S.B. Experimental Gingivitis in Man. J. Periodontol. (1930) 1965, 36, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Darveau, R.P.; Curtis, M.A. The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 2012, 10, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Igboin, C.O.; Griffen, A.L.; Leys, E.J. Porphyromonas gingivalis strain diversity. J. Clin. Microbiol. 2009, 47, 3073–3081. [Google Scholar] [CrossRef] [PubMed]
- Enersen, M.; Nakano, K.; Amano, A. Porphyromonas gingivalis fimbriae. J. Oral. Microbiol. 2013, 5, 20265. [Google Scholar] [CrossRef]
- Holt, S.C.; Kesavalu, L.; Walker, S.; Genco, C.A. Virulence factors of Porphyromonas gingivalis. Periodontology 2000 1999, 20, 168–238. [Google Scholar] [CrossRef]
- Xu, Z.; He, Y.; Zeng, X.; Zeng, X.; Huang, J.; Lin, X.; Chen, J. Enhanced Human Gingival Fibroblast Response and Reduced Porphyromonas gingivalis Adhesion with Titania Nanotubes. Biomed. Res. Int. 2020, 2020, 5651780. [Google Scholar] [CrossRef]
- Harokopakis, E.; Hajishengallis, G. Integrin activation by bacterial fimbriae through a pathway involving CD14, Toll-like receptor 2, and phosphatidylinositol-3-kinase. Eur. J. Immunol. 2005, 35, 1201–1210. [Google Scholar] [CrossRef]
- Amano, A.; Nakagawa, I.; Okahashi, N.; Hamada, N. Variations of Porphyromonas gingivalis fimbriae in relation to microbial pathogenesis. J. Periodontal Res. 2004, 39, 136–142. [Google Scholar] [CrossRef]
- Khalid, M.; Petroianu, G.; Adem, A. Advanced Glycation End Products and Diabetes Mellitus: Mechanisms and Perspectives. Biomolecules 2022, 12, 542. [Google Scholar] [CrossRef] [PubMed]
- Piche, J.E.; Swan, R.H.; Hallmon, W.W. The glycosylated hemoglobin assay for diabetes: Its value to the periodontist. Two case reports. J. Periodontol. 1989, 60, 640–642. [Google Scholar] [CrossRef] [PubMed]
- Kerner, W.; Bruckel, J.; German Diabetes, A. Definition, classification and diagnosis of diabetes mellitus. Exp. Clin. Endocrinol. Diabetes 2014, 122, 384–386. [Google Scholar] [CrossRef]
- Ramasamy, R.; Yan, S.F.; Schmidt, A.M. Receptor for AGE (RAGE): Signaling mechanisms in the pathogenesis of diabetes and its complications. Ann. N. Y. Acad. Sci. 2011, 1243, 88–102. [Google Scholar] [CrossRef]
- Baeza, M.; Morales, A.; Cisterna, C.; Cavalla, F.; Jara, G.; Isamitt, Y.; Pino, P.; Gamonal, J. Effect of periodontal treatment in patients with periodontitis and diabetes: Systematic review and meta-analysis. J. Appl. Oral. Sci. 2020, 28, e20190248. [Google Scholar] [CrossRef]
- Kocher, T.; Konig, J.; Borgnakke, W.S.; Pink, C.; Meisel, P. Periodontal complications of hyperglycemia/diabetes mellitus: Epidemiologic complexity and clinical challenge. Periodontology 2000 2018, 78, 59–97. [Google Scholar] [CrossRef]
- Oberti, L.; Gabrione, F.; Nardone, M.; Di Girolamo, M. Two-way relationship between diabetes and periodontal disease: A reality or a paradigm? J. Biol. Regul. Homeost. Agents 2019, 33, 153–159. [Google Scholar] [PubMed]
- Sanz, M.; Ceriello, A.; Buysschaert, M.; Chapple, I.; Demmer, R.T.; Graziani, F.; Herrera, D.; Jepsen, S.; Lione, L.; Madianos, P.; et al. Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology. J. Clin. Periodontol. 2018, 45, 138–149. [Google Scholar] [CrossRef]
- Choi, Y.H.; McKeown, R.E.; Mayer-Davis, E.J.; Liese, A.D.; Song, K.B.; Merchant, A.T. Serum C-reactive protein and immunoglobulin G antibodies to periodontal pathogens may be effect modifiers of periodontitis and hyperglycemia. J. Periodontol. 2014, 85, 1172–1181. [Google Scholar] [CrossRef]
- Dye, B.A.; Herrera-Abreu, M.; Lerche-Sehm, J.; Vlachojannis, C.; Pikdoken, L.; Pretzl, B.; Schwartz, A.; Papapanou, P.N. Serum antibodies to periodontal bacteria as diagnostic markers of periodontitis. J. Periodontol. 2009, 80, 634–647. [Google Scholar] [CrossRef] [PubMed]
- Ingalagi, P.; Bhat, K.G.; Kulkarni, R.D.; Kotrashetti, V.S.; Kumbar, V.; Kugaji, M. Detection and comparison of prevalence of Porphyromonas gingivalis through culture and Real Time-polymerase chain reaction in subgingival plaque samples of chronic periodontitis and healthy individuals. J. Oral. Maxillofac. Pathol. 2022, 26, 288. [Google Scholar] [CrossRef] [PubMed]
- Tuominen, H.; Rautava, J. Oral Microbiota and Cancer Development. Pathobiology 2021, 88, 116–126. [Google Scholar] [CrossRef]
- Groeger, S.; Hudel, M.; Zechel, S.; Chakraborty, T.; Lochnit, G.; Meyle, J.; Domann, E. Generation and functional characterization of recombinant Porphyromonas gingivalis W83 FimA. J. Biotechnol. 2021, 340, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Levene, H. Robust tests for equality of variances. In Contributions to Probability and Statistics: Essays in Honor of Harold Hotelling; Ingram Olkin, H.H., Ed.; Stanford University Press: Redwood City, CA, USA, 1960; pp. 278–292. [Google Scholar]
- Dunn, O.J. Multiple comparisons among means. J. Acoust. Soc. Am. 1961, 56, 54–64. [Google Scholar] [CrossRef]
- Holm, S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 1979, 6, 65–70. [Google Scholar]
- Hornbeck, P.V. Enzyme-Linked Immunosorbent Assays. Curr. Protoc. Immunol. 2015, 110, 2.1.1–2.1.23. [Google Scholar] [CrossRef]
- Amano, A.; Kuboniwa, M.; Nakagawa, I.; Akiyama, S.; Morisaki, I.; Hamada, S. Prevalence of specific genotypes of Porphyromonas gingivalis fimA and periodontal health status. J. Dent. Res. 2000, 79, 1664–1668. [Google Scholar] [CrossRef]
- Kugaji, M.; Muddapur, U.; Bhat, K.; Joshi, V.; Manubolu, M.; Pathakoti, K.; Peram, M.R.; Kumbar, V. Variation in the Occurrence of fimA Genotypes of Porphyromonas gingivalis in Periodontal Health and Disease. Int. J. Environ. Res. Public. Health 2020, 17, 1826. [Google Scholar] [CrossRef]
- Stanker, L.H.; Hnasko, R.M. A Double-Sandwich ELISA for Identification of Monoclonal Antibodies Suitable for Sandwich Immunoassays. Methods Mol. Biol. 2015, 1318, 69–78. [Google Scholar]
- Bennani, M.; Range, H.; Meuric, V.; Mora, F.; Bouchard, P.; Carra, M.C. Shared detection of Porphyromonas gingivalis in cohabiting family members: A systematic review and meta-analysis. J. Oral. Microbiol. 2020, 12, 1687398. [Google Scholar] [CrossRef] [PubMed]
- Sims, T.J.; Lernmark, A.; Mancl, L.A.; Schifferle, R.E.; Page, R.C.; Persson, G.R. Serum IgG to heat shock proteins and Porphyromonas gingivalis antigens in diabetic patients with periodontitis. J. Clin. Periodontol. 2002, 29, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Sims, T.J.; Schifferle, R.E.; Ali, R.W.; Skaug, N.; Page, R.C. Immunoglobulin G response of periodontitis patients to Porphyromonas gingivalis capsular carbohydrate and lipopolysaccharide antigens. Oral. Microbiol. Immunol. 2001, 16, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Montevecchi, M.; Valeriani, L.; Gatto, M.R.; D’Alessandro, G.; Piana, G. Subgingival pathogens in chronic periodontitis patients affected by type 2 diabetes mellitus: A retrospective case-control study. J. Periodontal Implant. Sci. 2021, 51, 409–421. [Google Scholar] [CrossRef]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef]
- Sathe, A.; Cusick, J.K. Biochemistry, Immunoglobulin M. In StatPearls; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Deshpande, S.S. Enzyme Immunoassays: From Concept to Product Develepment; Springer: New York, NY, USA, 1996. [Google Scholar]


| Cohort | Number | Female | Male | Mean Age (years) | SD Age [years] | Variance Age [years2] |
|---|---|---|---|---|---|---|
| C | 13 | 5 | 8 | 44 | 9 | 86.3 |
| P | 26 | 14 | 12 | 51 | 12 | 164.8 |
| P + D | 15 | 7 | 8 | 47 | 9 | 93.6 |
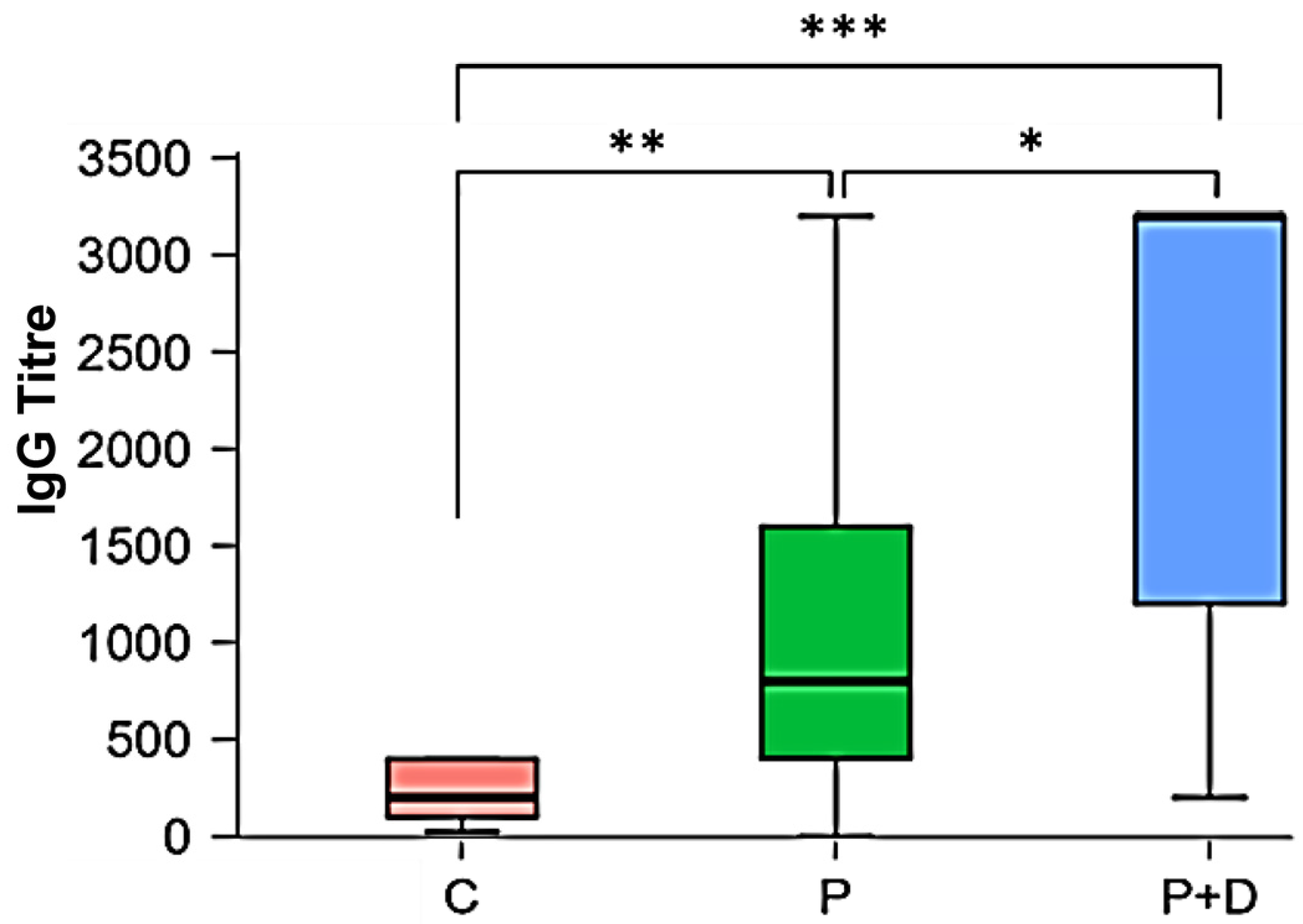
| Descriptive Statistics: IgG Titres | |||
|---|---|---|---|
| Diagnosis | C | P | P + D |
| Number | 13 | 26 | 15 |
| Mode | 1:200; 1:400 | 1:1600 | 1:3200 |
| Median | 1:200 | 1:800 | 1:3200 |
| Shapiro–Wilk | 0.848 | 0.825 | 0.762 |
| p-value Shapiro–Wilk | 0.027 | <0.001 | 0.001 |
| IgG Titres | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis | No Titre | 1:25 | 1:50 | 1:100 | 1:200 | 1:400 | 1:800 | 1:1600 | 1:3200 | Total | |
| C | Number | 0 | 1 | 1 | 3 | 4 | 4 | 0 | 0 | 0 | 13 |
| % | 0 | 7.69 | 7.69 | 23.08 | 30.77 | 30.77 | 0 | 0 | 0 | 100 | |
| P | Number | 1 | 0 | 1 | 0 | 4 | 6 | 5 | 7 | 2 | 26 |
| % | 3.85 | 0 | 3.85 | 0 | 15.39 | 23.08 | 19.23 | 26.92 | 7.69 | 100 | |
| P + D | Number | 0 | 0 | 0 | 0 | 2 | 1 | 1 | 3 | 8 | 15 |
| % | 0 | 0 | 0 | 0 | 13.33 | 6.67 | 6.67 | 20 | 53.33 | 100 | |
| Kruskal–Wallis Test | Levine Test | |||
|---|---|---|---|---|
| Factor | Statistics | df | p | p |
| diagnosis | 20.951 | 2 | <0.001 *** | <0.001 *** |
| Dunn’s Post Hoc Test with Bonferroni–Holm Correction | |
|---|---|
| Comparison | p |
| C − P | 0.008 ** |
| C − P + D | <0.001 *** |
| P − P + D | 0.02 * |
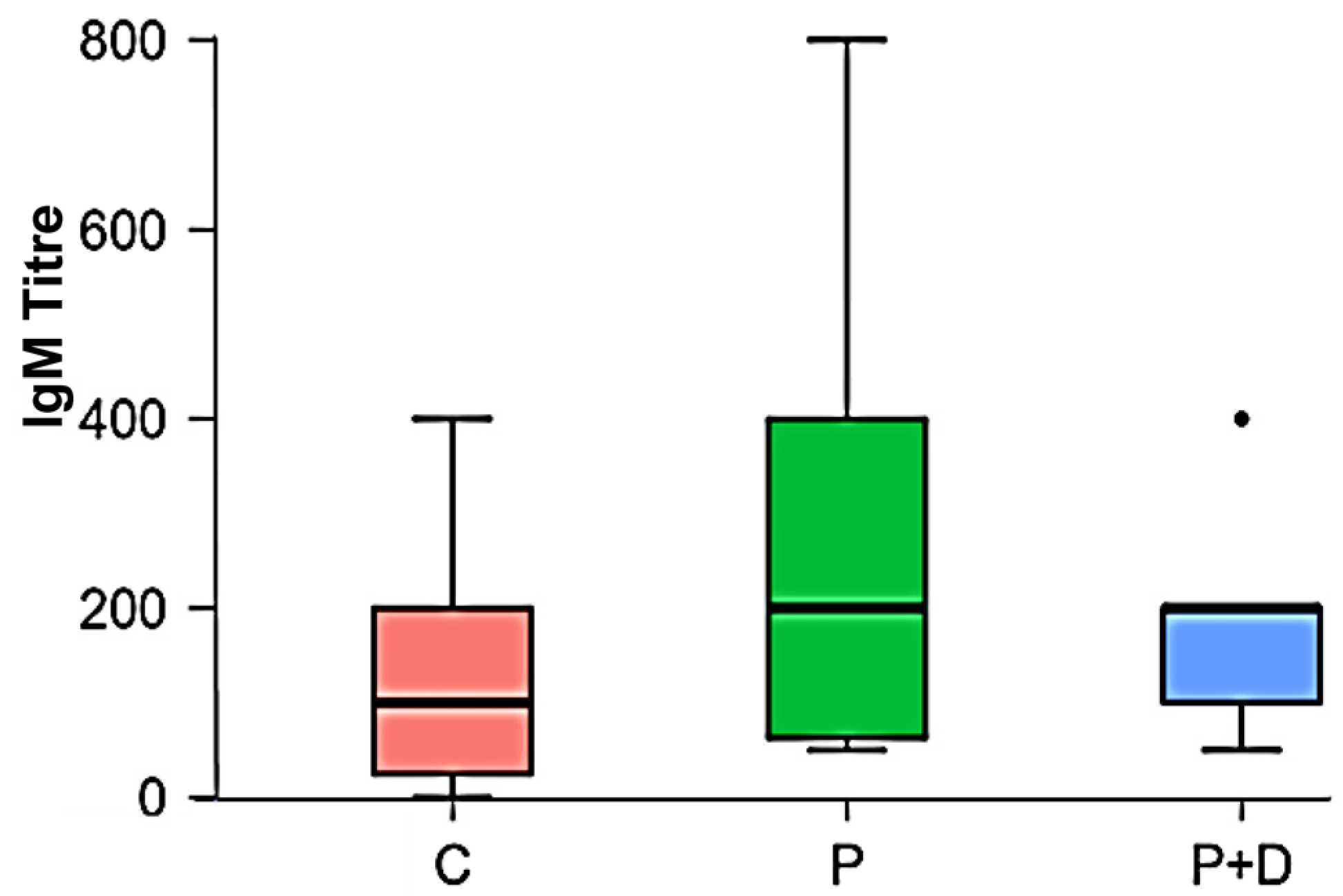
| Descriptive Statistics: IgM Titres | |||
|---|---|---|---|
| Diagnosis | C | P | P + D |
| Number | 13 | 26 | 15 |
| Mode | 1:200 | 1:50; 1:400 | 1:200 |
| Median | 1:100 | 1:200 | 1:200 |
| Shapiro–Wilk | 0.862 | 0.803 | 0.819 |
| p-value Shapiro–Wilk | 0.041 | <0.001 | 0.007 |
| Kruskal–Wallis Test | |||
|---|---|---|---|
| Factor | Statistics | df | p |
| Diagnosis | 2.504 | 2 | 0.286 |
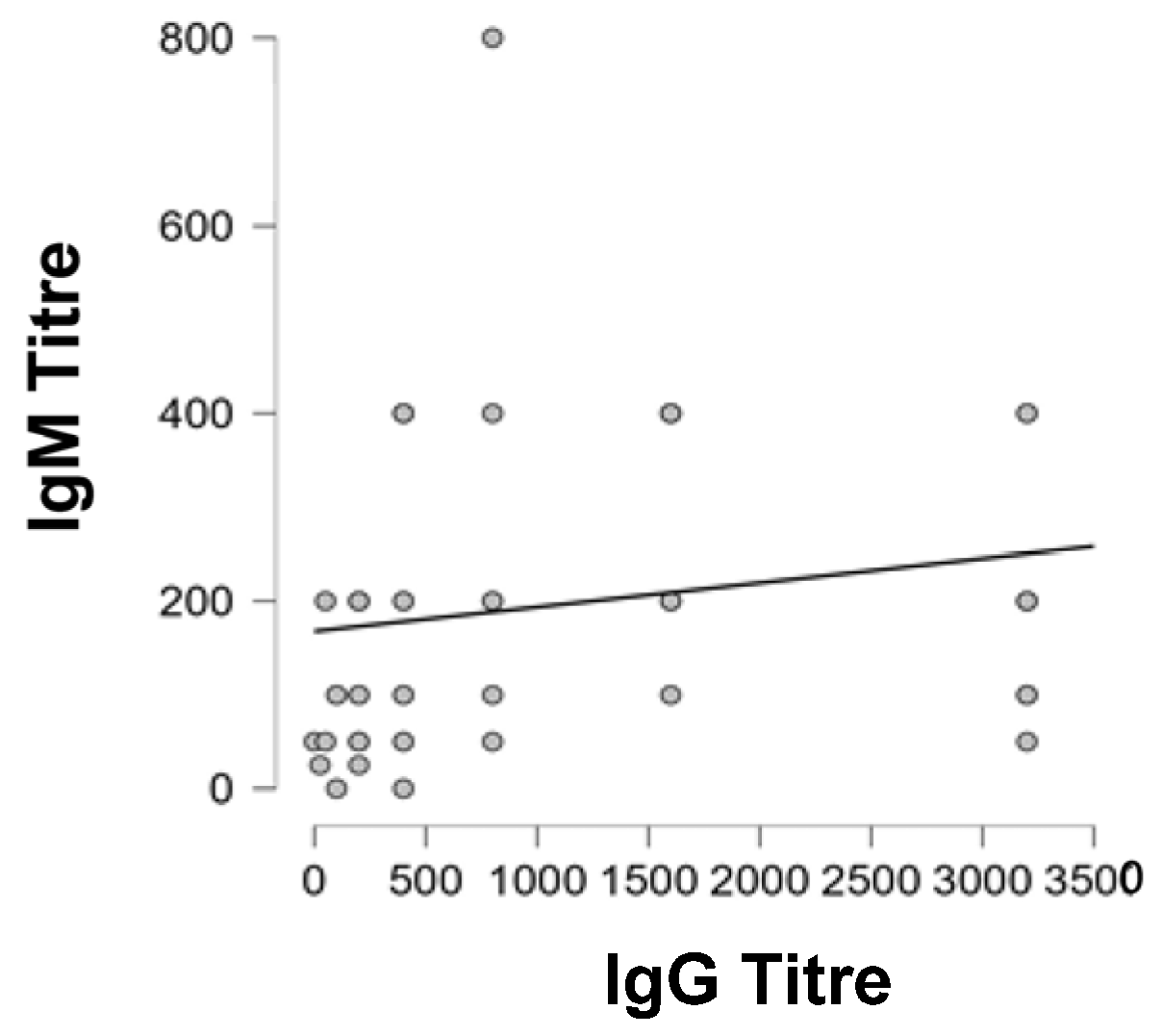
| Spearman’s Correlations | |||
|---|---|---|---|
| Comparison | Spearman’s Rho | p | |
| IgG Titre | IgM Titre | 0.421 | 0.002 ** |
| Inclusion Criteria | Exclusion Criteria | ||
|---|---|---|---|
| Periodontitis Patients | Periodontitis Plus Diabetes Mellitus Type 2 Patients | Orally Healthy Controls | Whole Cohort |
| Chronic periodontitis: periodontal screening index (PSI) ≥ 4 | Chronic periodontitis: periodontal screening index (PSI) ≥ 4 | Orally healthy individuals: periodontal screening index (PSI) < 1 | Pregnancy |
| Smoking | |||
| BMI < 18.5 kg/m2 | |||
| Persistent alcohol, drug or medication abuse | |||
| Age 18–70 years | Diabetes mellitus type 2 with HbA1c-value ≥ 8.5% Age 18–70 years | Age 18–70 years | Severe cardiovascular diseases (coronary heart disease, cerebral vascular disease, peripheral vascular disease, heart valve disease, heart failure) |
| All genders | All genders | All genders | Allergies to supportive medications/antiseptics and dental materials (e.g., gloves or chlorhexidine) |
| Patients with at least 12 natural teeth (without subgingival inlay, tooth crown or caries) | Patients with at least 12 natural teeth (without subgingival inlay, tooth crown or caries) | Individuals with at least 12 natural teeth (without subgingival inlay, tooth crown or caries) | Other serious diseases (including cancer, liver diseases, lung diseases, chronic infectious diseases like HIV, hepatitis, rheumatological diseases, haematological diseases, severe psychiatric illness |
| Systemic enteral or parenteral medication such as daily vitamins or nutritional supplements and certain calcium canal blockers (e.g., nifedipine) besides antidiabetic/insulin replacement | |||
| Allergies to supportive medications/antiseptics and dental materials (e.g., gloves or chlorhexidine) | |||
| Serious dental diseases (severe caries and/or pulp diseases requiring extensive dental, surgical or prosthetic treatment, diseases requiring systemic medication, systemic, topical or inhaled steroid treatment for more than 30 consecutive days within 6 weeks before the initial examination) | |||
| Any periodontal treatment within 6 months prior to study entry | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Groeger, S.; Mueller, N.; Herrmann, J.M.; Meyle, J. Serum Antibody Titres Against Porphyromonas gingivalis Fim A in Patients with Periodontitis with and Without Diabetes Mellitus Type 2. Int. J. Mol. Sci. 2025, 26, 4726. https://doi.org/10.3390/ijms26104726
Groeger S, Mueller N, Herrmann JM, Meyle J. Serum Antibody Titres Against Porphyromonas gingivalis Fim A in Patients with Periodontitis with and Without Diabetes Mellitus Type 2. International Journal of Molecular Sciences. 2025; 26(10):4726. https://doi.org/10.3390/ijms26104726
Chicago/Turabian StyleGroeger, Sabine, Nathalie Mueller, Jens Martin Herrmann, and Joerg Meyle. 2025. "Serum Antibody Titres Against Porphyromonas gingivalis Fim A in Patients with Periodontitis with and Without Diabetes Mellitus Type 2" International Journal of Molecular Sciences 26, no. 10: 4726. https://doi.org/10.3390/ijms26104726
APA StyleGroeger, S., Mueller, N., Herrmann, J. M., & Meyle, J. (2025). Serum Antibody Titres Against Porphyromonas gingivalis Fim A in Patients with Periodontitis with and Without Diabetes Mellitus Type 2. International Journal of Molecular Sciences, 26(10), 4726. https://doi.org/10.3390/ijms26104726







