A Novel Squalenoylated Temozolomide Nanoparticle with Long Circulating Properties Reverses Drug Resistance in Glioblastoma
Abstract
1. Introduction
2. Results
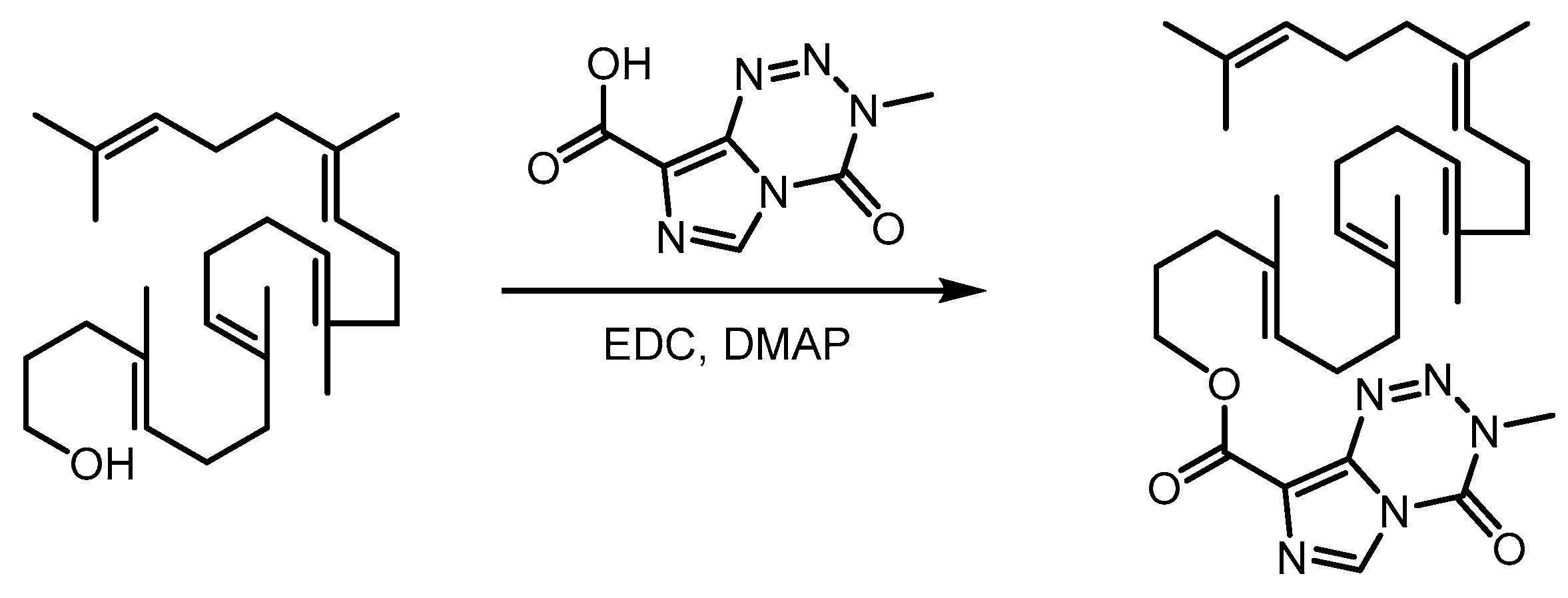
2.1. Synthesis of Squalene-Temozolomide Conjugate
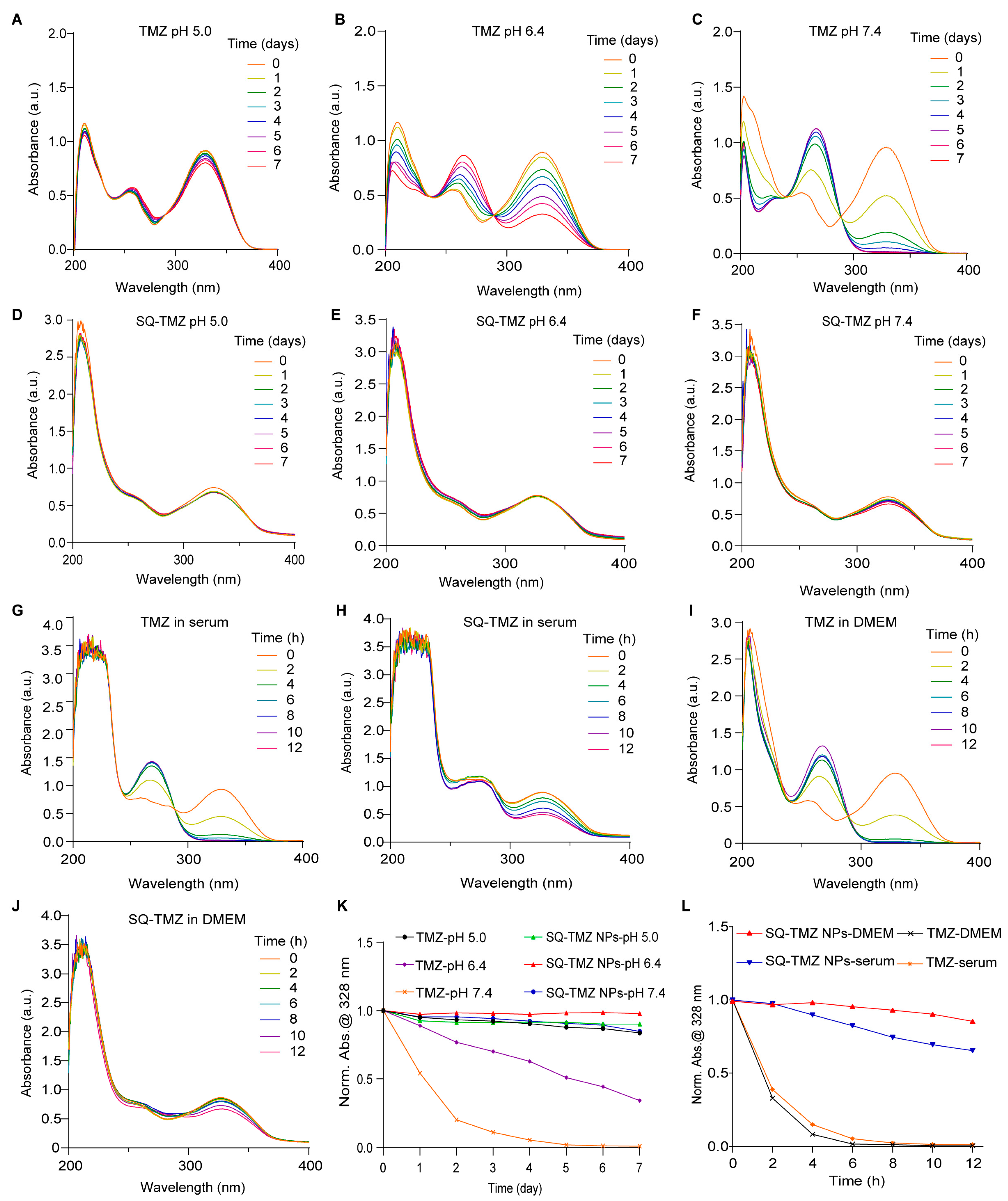
2.2. Characterization and Stability of SQ-TMZ NPs
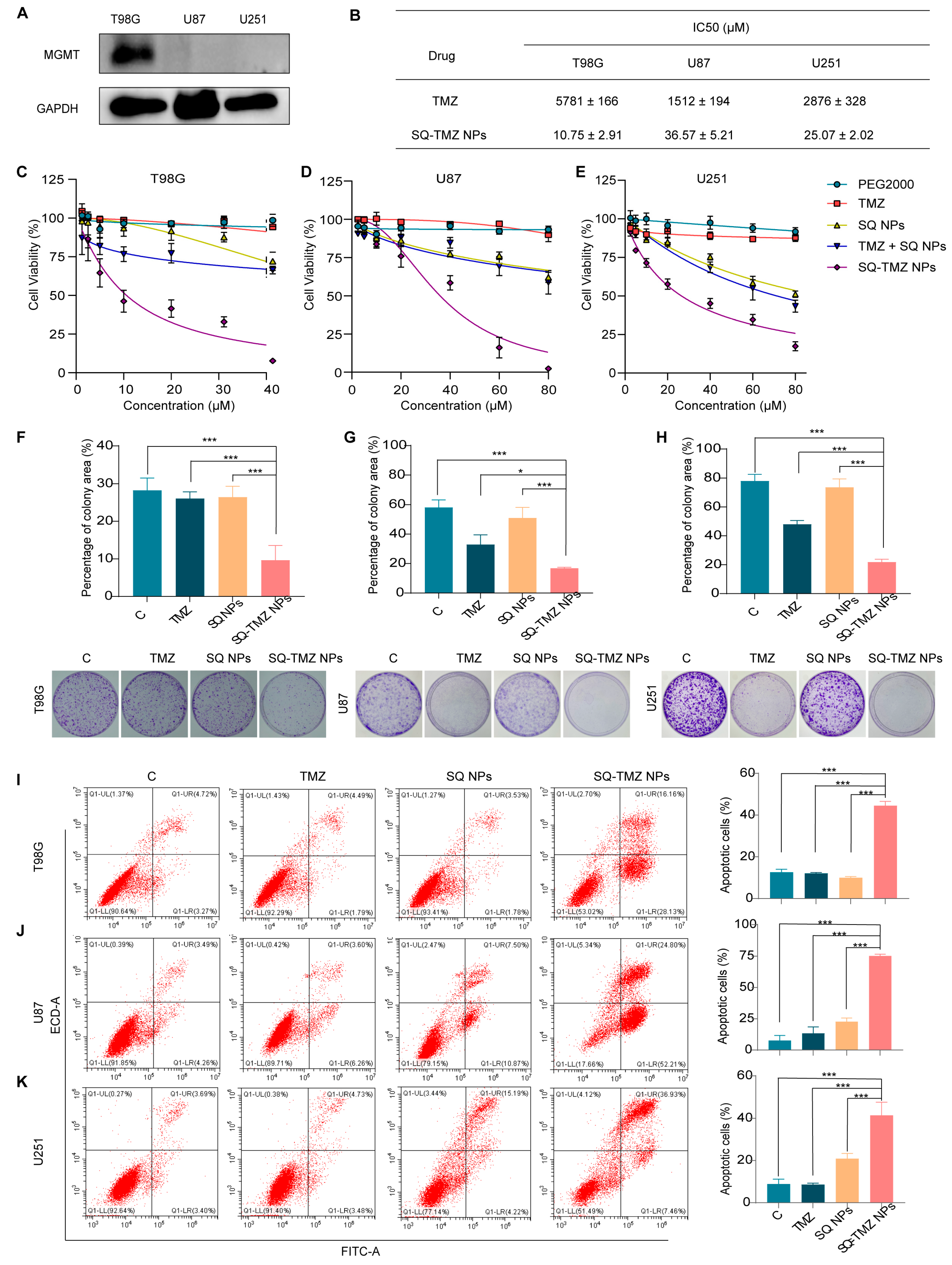
2.3. Cytotoxicity and Resistance Reversal in GBM Cells
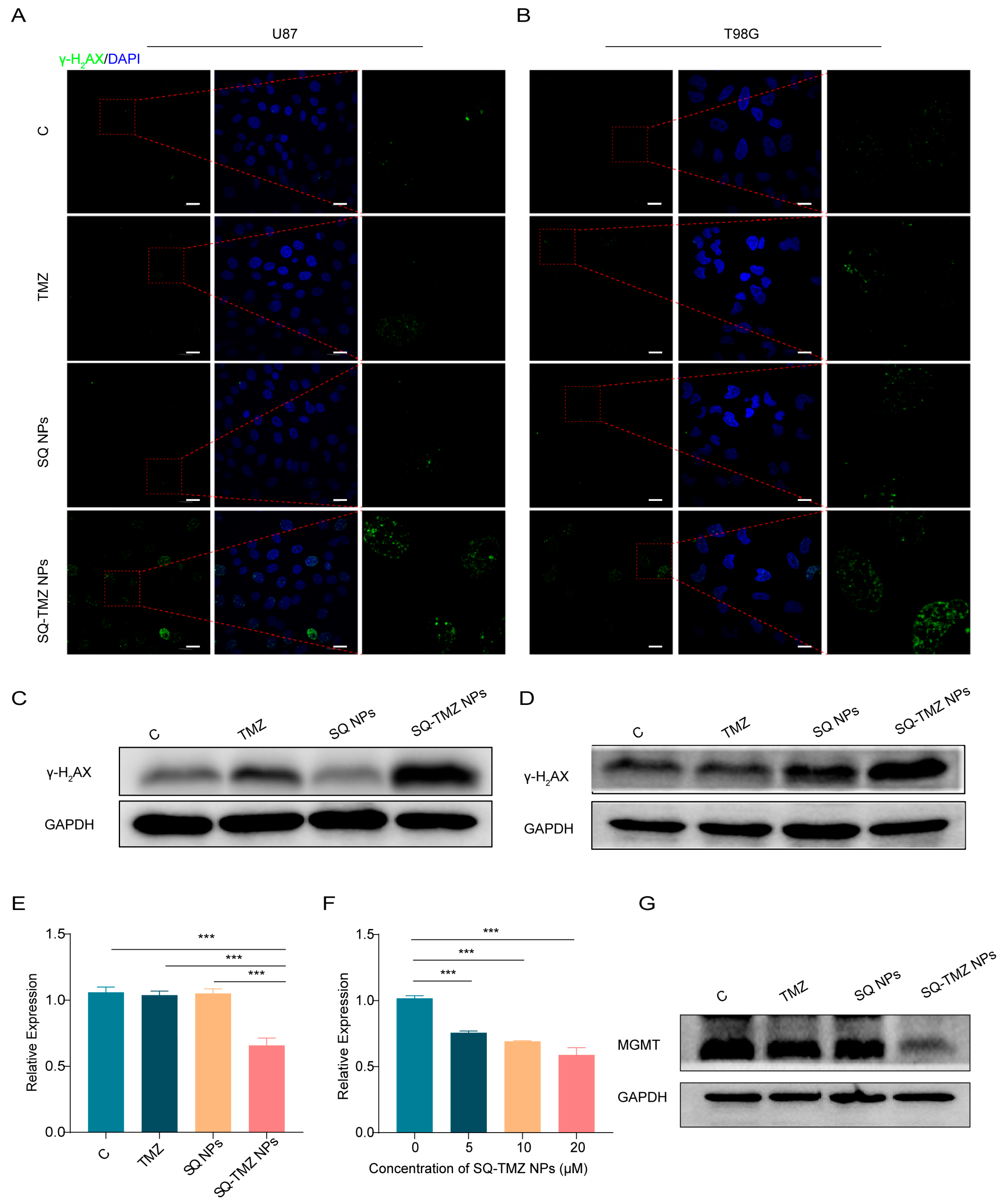
2.4. SQ-TMZ NPs Induce ROS Accumulation and Enhance DNA Damage to Overcome TMZ Resistance in GBM Cells
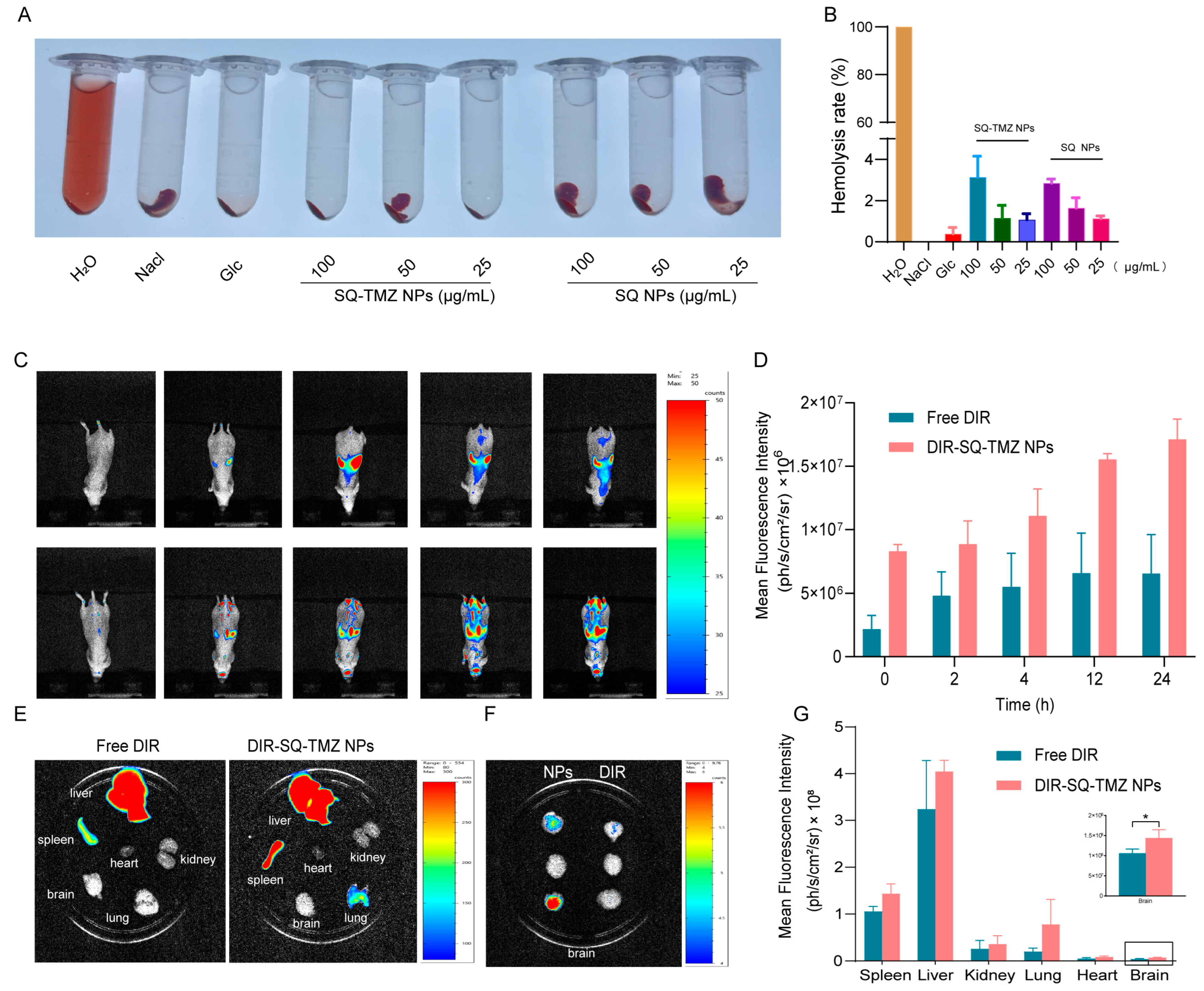
2.5. SQ-TMZ NPs Exhibit Favorable Blood Compatibility and Efficient Blood–Brain Barrier Penetration
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Squalene-Temozolomide (SQ-TMZ) Copolymer
4.3. Preparation of SQ-TMZ Nanoparticles (SQ-TMZ NPs)
4.4. Characterization of SQ-TMZ Nanoparticles (SQ-TMZ NPs)
4.5. Cytotoxicity of SQ-TMZ NPs
4.6. Colony Formation Assay
4.7. Apoptosis of SQ-TMZ NPs
4.8. Western Blot
4.9. Quantitative RT-PCR Analysis of MGMT mRNA Expression
4.10. Immunofluorescence Staining
4.11. Analysis of Intracellular ROS Levels
4.12. Brain Targeting of SQ-TMZ NPs
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BBB | Blood–brain barrier |
| BER | Base excision repair |
| DCFH-DA | 2′,7′-Dichlorodihydrofluorescein diacetate |
| EtOH | Ethanol |
| HR-MS | High-resolution mass spectrometry |
| IC50 | Half maximal inhibitory concentration |
| IF | Immunofluorescence |
| GBM | Glioblastoma multiforme |
| MMR | Mismatch repair |
| MGMT | O6-methylguanine-DNA methyltransferase |
| NMR | Nuclear magnetic resonance spectroscopy |
| NPs | Nanoparticles |
| PDI | polydispersity index |
| ROS | reactive oxygen species |
| SQ | Squalene |
| TEM | Transmission electron microscopy |
| THF | Tetrahydrofuran |
| TMZ | Temozolomide |
| WB | Western blot |
References
- Gomes, I.; Oliveira, R.J.d.S.; Girol, A.P. Signaling pathways in glioblastoma. Crit. Rev. Oncol./Hematol. 2025, 209, 104647. [Google Scholar] [CrossRef] [PubMed]
- Bai, P.; Wang, P.; Ren, T.; Tang, Q.; Lin, Z.; Zhang, N.; Zhao, L.; Zhong, R.; Sun, G. Natural small molecule thymoquinone increases the chemosensitivity of glioblastoma to temozolomide through inhibiting Wnt/β-catenin signaling pathway to downregulate MGMT expression: In vitro and in vivo validation. Biochem. Pharmacol. 2025, 236, 116886. [Google Scholar] [CrossRef] [PubMed]
- Ke, G.; Hu, P.; Xiong, H.; Zhang, J.; Xu, H.; Xiao, C.; Liu, Y.; Cao, M.; Zheng, Q. Enhancing temozolomide efficacy in GBM: The synergistic role of chuanxiong rhizoma essential oil. Phytomedicine 2025, 140, 156575. [Google Scholar] [CrossRef]
- Singh, N.; Miner, A.; Hennis, L.; Mittal, S. Mechanisms of temozolomide resistance in glioblastoma-a comprehensive review. Cancer Drug Resist. 2021, 4, 17–43. [Google Scholar] [CrossRef]
- Wen, J.; Wu, X.; Shu, Z.; Wu, D.; Yin, Z.; Chen, M.; Luo, K.; Liu, K.; Shen, Y.; Le, Y.; et al. Clusterin-mediated polarization of M2 macrophages: A mechanism of temozolomide resistance in glioblastoma stem cells. Stem Cell Res. Ther. 2025, 16, 146. [Google Scholar] [CrossRef]
- Hwang, T.; Sitko, L.K.; Khoirunnisa, R.; Navarro-Aguad, F.; Samuel, D.M.; Park, H.; Cheon, B.; Mutsnaini, L.; Lee, J.; Otlu, B.; et al. Comprehensive whole-genome sequencing reveals origins of mutational signatures associated with aging, mismatch repair deficiency and temozolomide chemotherapy. Nucleic Acids Res. 2025, 53, gkae1122. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; Stupp, R.; Reifenberger, G.; Brandes, A.A.; van den Bent, M.J.; Wick, W.; Hegi, M.E. MGMT promoter methylation in malignant gliomas: Ready for personalized medicine? Nat. Rev. Neurol. 2010, 6, 39–51. [Google Scholar] [CrossRef]
- Lan, Y.; Li, X.; Liu, B.; Lu, J.; Zuo, B.; Wang, Y.; Cao, S.; Fu, X.; Yue, Q.; Luo, X.; et al. Framework nucleic acid-based nanoparticles enhance temozolomide sensitivity in glioblastoma. Drug Resist. Updates 2024, 76, 101122. [Google Scholar] [CrossRef]
- Han, X.; Abdallah, M.O.E.; Breuer, P.; Stahl, F.; Bakhit, Y.; Potthoff, A.-L.; Pregler, B.E.F.; Schneider, M.; Waha, A.; Wüllner, U.; et al. Downregulation of MGMT expression by targeted editing of DNA methylation enhances temozolomide sensitivity in glioblastoma. Neoplasia 2023, 44, 100929. [Google Scholar] [CrossRef]
- Kitange, G.J.; Carlson, B.L.; Schroeder, M.A.; Grogan, P.T.; Lamont, J.D.; Decker, P.A.; Wu, W.; James, C.D.; Sarkaria, J.N. Induction of MGMT expression is associated with temozolomide resistance in glioblastoma xenografts. Neuro-Oncology 2009, 11, 281–291. [Google Scholar] [CrossRef]
- Skinner, M.; Ward, S.M.; Emrick, T.A.-O. Versatile Synthesis of Polymer-Temozolomide Conjugates. ACS Macro Lett. 2017, 6, 215–218. [Google Scholar] [CrossRef]
- Jatyan, R.; Singh, P.; Sahel, D.K.; Karthik, Y.G.; Mittal, A.; Chitkara, D. Polymeric and small molecule-conjugates of temozolomide as improved therapeutic agents for glioblastoma multiforme. J. Control. Release 2022, 350, 494–513. [Google Scholar] [CrossRef]
- Hazam, H.; Prades, L.; Cailleau, C.; Mougin, J.; Feng, J.; Benhamou, D.; Gobeaux, F.; Hamdi, L.; Couvreur, P.; Sitbon, P.; et al. A nanomedicine approach for the treatment of long-lasting pain. J. Control. Release 2024, 373, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Dormont, F.A.-O.; Brusini, R.; Cailleau, C.A.-O.; Reynaud, F.A.-O.; Peramo, A.; Gendron, A.A.-O.; Mougin, J.A.-O.X.; Gaudin, F.; Varna, M.; Couvreur, P.A.-O. Squalene-based multidrug nanoparticles for improved mitigation of uncontrolled inflammation in rodents. Sci. Adv. 2020, 6, eaaz5466. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.A.-O.X.; Lepetre-Mouelhi, S.A.-O.; Gautier, A.A.-O.; Mura, S.A.-O.; Cailleau, C.A.-O.; Coudore, F.; Hamon, M.A.-O.; Couvreur, P.A.-O. A new painkiller nanomedicine to bypass the blood-brain barrier and the use of morphine. Sci. Adv. 2019, 5, eaau5148. [Google Scholar] [CrossRef]
- Jezierzański, M.; Nafalska, N.; Stopyra, M.; Furgoł, T.; Miciak, M.A.-O.; Kabut, J.; Gisterek-Grocholska, I.A.-O. Temozolomide (TMZ) in the Treatment of Glioblastoma Multiforme-A Literature Review and Clinical Outcomes. Curr. Ocology 2024, 31, 3994–4002. [Google Scholar] [CrossRef] [PubMed]
- Yasaswi, P.S.; Shetty, K.; Yadav, K.S. Temozolomide nano enabled medicine: Promises made by the nanocarriers in glioblastoma therapy. J. Control. Release 2021, 336, 549–571. [Google Scholar] [CrossRef]
- Guo, H.; Mi, P.A.-O. Polymer-drug and polymer-protein conjugated nanocarriers: Design, drug delivery, imaging, therapy, and clinical applications. Wiley Interdiscip. Rev. Nanomed. NanobioTechnol. 2024, 16, e1988. [Google Scholar] [CrossRef]
- Du, K.; Li, X.; Feng, F.A.-O. Polymer-Drug Conjugates Codeliver a Temozolomide Intermediate and Nitric Oxide for Enhanced Chemotherapy against Glioblastoma Multiforme. ACS Appl. Bio Mater. J. 2024, 7, 1810–1819. [Google Scholar] [CrossRef]
- Sharma, S.; Roy, R.; Vartak, A.; Sen, E.; Sk, U.H. Synthesis and characterization of a novel Naphthalimide-Selenium based Temozolomide drug conjugate in glioma cells. Bioorganic Chem. 2024, 154, 107998. [Google Scholar] [CrossRef]
- Ostermann, S.; Csajka, C.; Buclin, T.; Leyvraz, S.; Lejeune, F.; Decosterd, L.A.; Stupp, R. Plasma and Cerebrospinal Fluid Population Pharmacokinetics of Temozolomide in Malignant Glioma Patients. Clin. Cancer Res. 2004, 10, 3728–3736. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Kievit, F.M.; Chiarelli, P.A.; Stephen, Z.R.; Lin, G.; Silber, J.R.; Ellenbogen, R.G.; Zhang, M. siRNA nanoparticle suppresses drug-resistant gene and prolongs survival in an orthotopic glioblastoma xenograft mouse model. Adv. Funct. Mater. 2021, 31, 2007166. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Liu, Y.; Fan, M.; Wei, S.; Ismail, M.; Zheng, M. PEG length effect of peptide-functional liposome for blood brain barrier (BBB) penetration and brain targeting. J. Control. Release 2024, 372, 85–94. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, J.; Wen, C.; Zhang, X.; Zhu, X.; Ma, M.; Zhao, X.; Sui, X. A Novel Squalenoylated Temozolomide Nanoparticle with Long Circulating Properties Reverses Drug Resistance in Glioblastoma. Int. J. Mol. Sci. 2025, 26, 4723. https://doi.org/10.3390/ijms26104723
Feng J, Wen C, Zhang X, Zhu X, Ma M, Zhao X, Sui X. A Novel Squalenoylated Temozolomide Nanoparticle with Long Circulating Properties Reverses Drug Resistance in Glioblastoma. International Journal of Molecular Sciences. 2025; 26(10):4723. https://doi.org/10.3390/ijms26104723
Chicago/Turabian StyleFeng, Jiao, Chengyong Wen, Xiao Zhang, Xiaolong Zhu, Mengmeng Ma, Xiaohong Zhao, and Xinbing Sui. 2025. "A Novel Squalenoylated Temozolomide Nanoparticle with Long Circulating Properties Reverses Drug Resistance in Glioblastoma" International Journal of Molecular Sciences 26, no. 10: 4723. https://doi.org/10.3390/ijms26104723
APA StyleFeng, J., Wen, C., Zhang, X., Zhu, X., Ma, M., Zhao, X., & Sui, X. (2025). A Novel Squalenoylated Temozolomide Nanoparticle with Long Circulating Properties Reverses Drug Resistance in Glioblastoma. International Journal of Molecular Sciences, 26(10), 4723. https://doi.org/10.3390/ijms26104723




