Biochemical and Biomechanical Properties of Scaffold-Free Hyaline Cartilage Generated Under Dynamic Conditions
Abstract
1. Introduction
2. Results
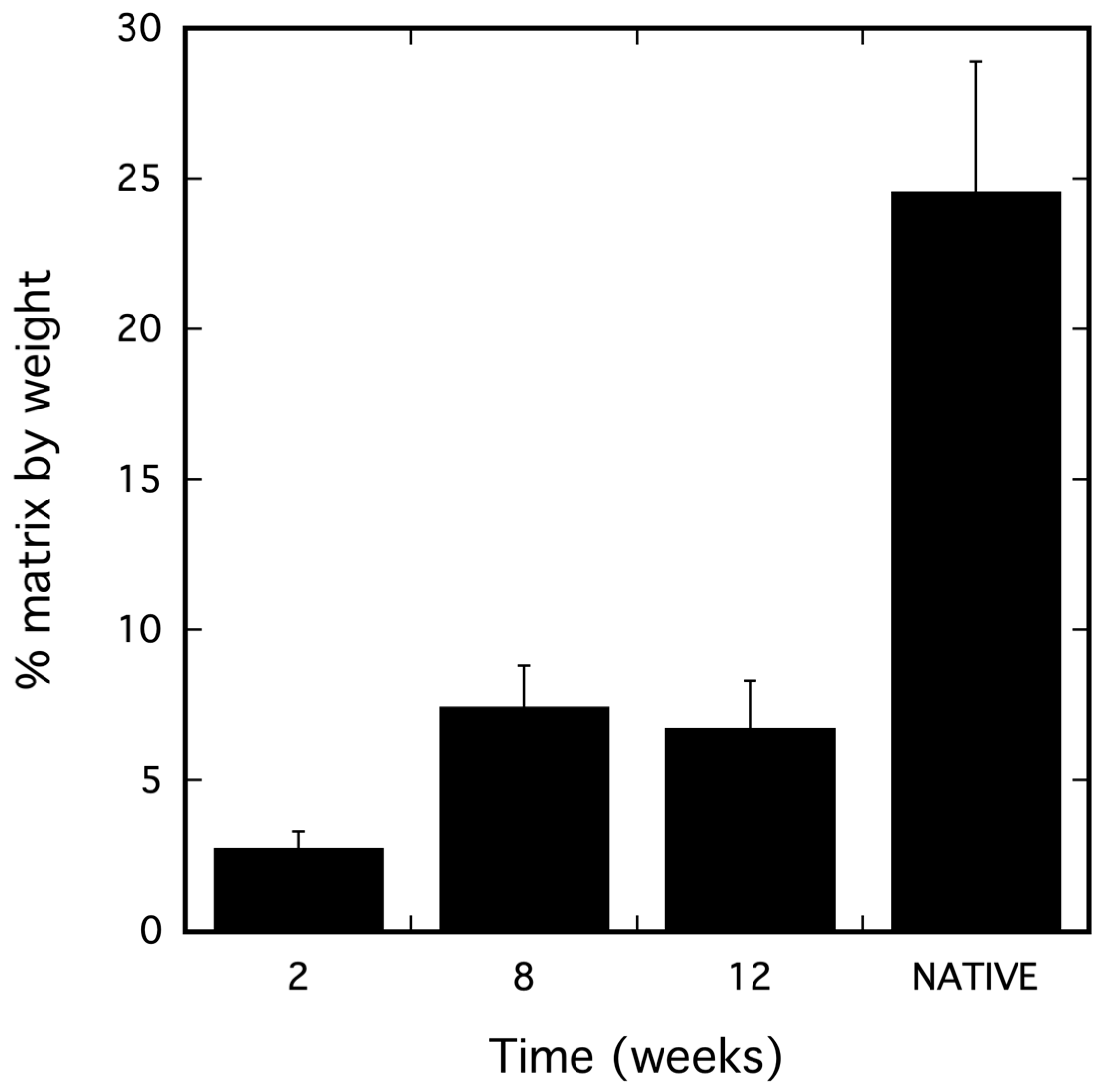
2.1. dSRC Composition
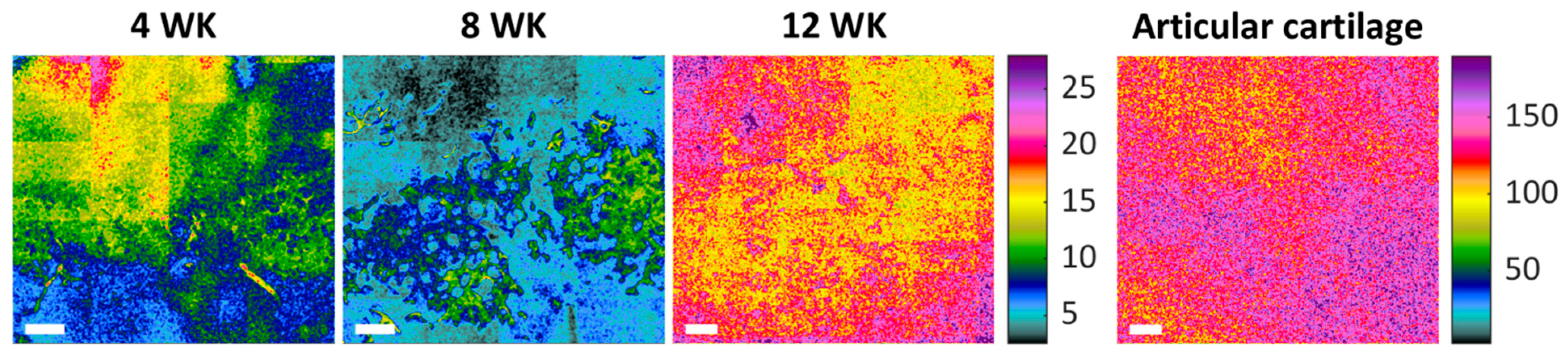
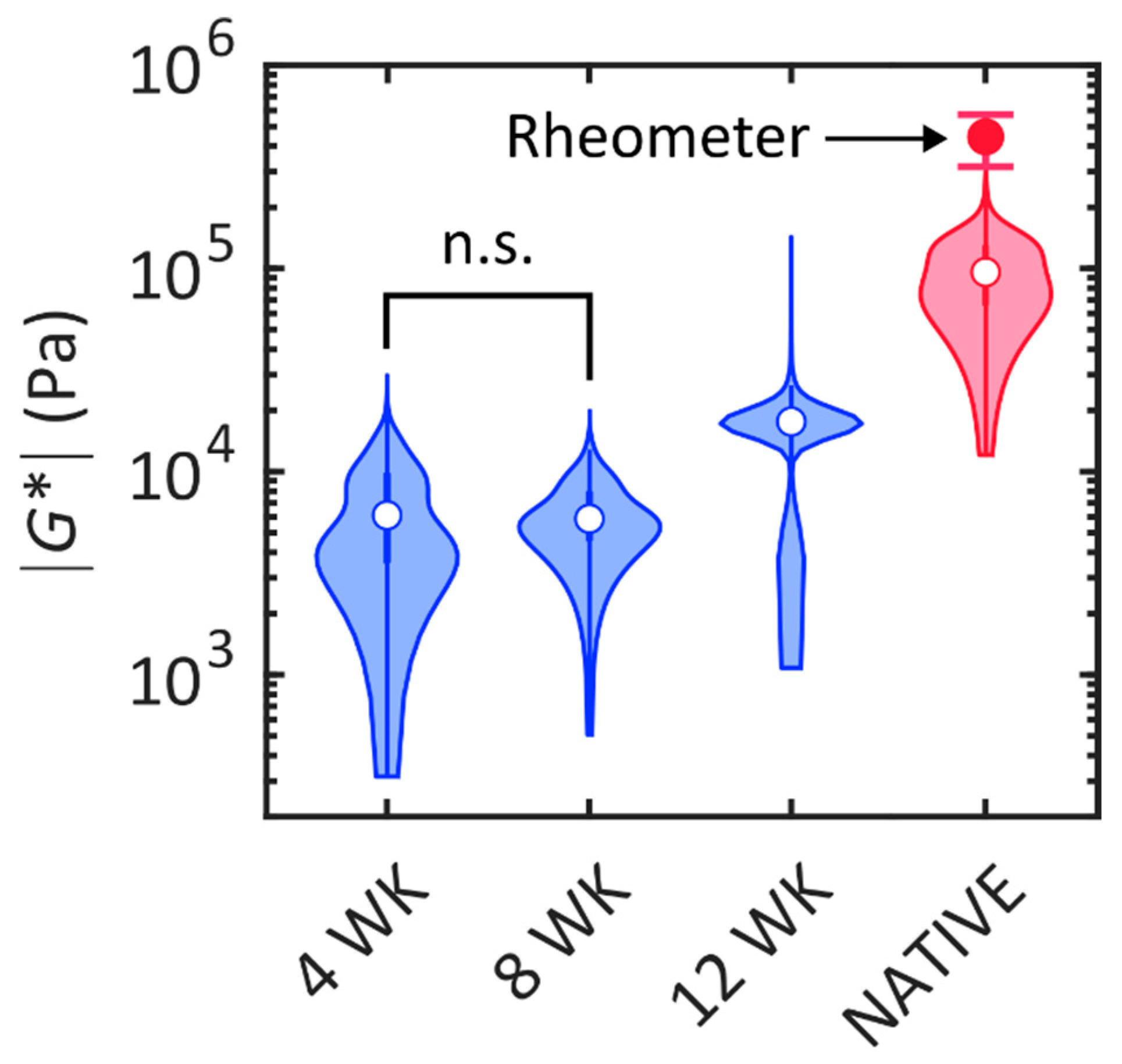
2.2. Biomechanical Analysis
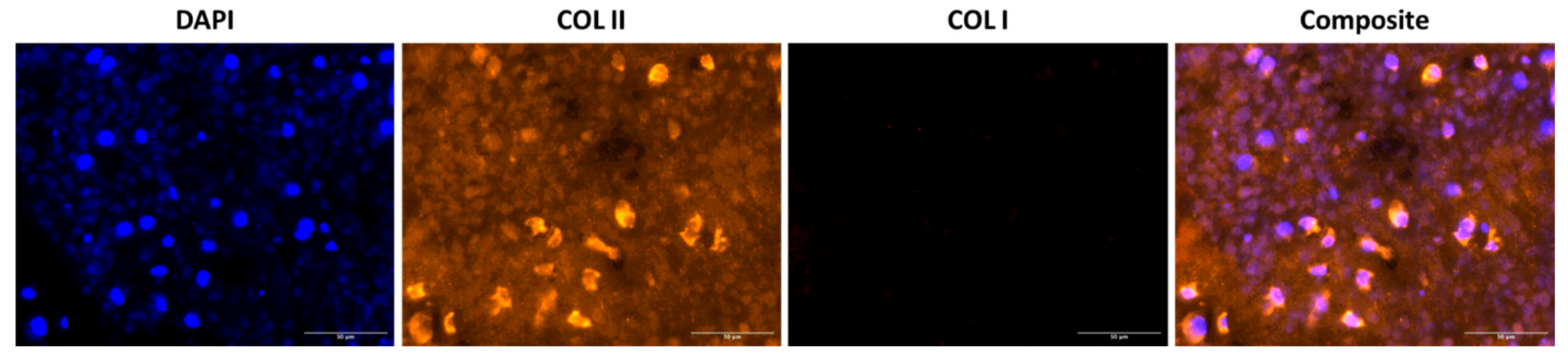
2.3. Histological Analysis of dSRC Maturation
3. Discussion
4. Materials and Methods
4.1. Chondrocyte Isolation and dSRC Formation
4.2. Biochemical Analysis
4.3. Laser Speckle rHEologicAl micRoscopy (SHEAR)
4.4. Shear Rheometry of Native Cartilage
4.5. Histology
4.6. Immunohistochemistry
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACI | Autologous chondrocyte implantation |
| CMOS | Complementary metal-oxide semiconductor |
| DAPI | 4′,6-Diamidino-2-phenylindole dihydrochloride |
| DMAB | 4-(dimethylamino)benzaldehyde |
| dSRC | Dynamic self-regenerating cartilage |
| |G*| | Shear modulus |
| GAG | Glycosaminoglycan |
| H&E | Hematoxylin and eosin |
| HeNe | Helium neon |
| OA | Osteoarthritis |
| PEG | Polyethylene glycol |
| sGAG | Sulfonated glycosaminoglycan |
| SD | Standard deviation |
| SHEAR | Speckle rHEologicAl micRoscopy |
References
- Hinckel, B.B.; Thomas, D.; Vellios, E.E.; Hancock, K.J.; Calcei, J.G.; Sherman, S.L.; Eliasberg, C.D.; Fernandes, T.L.; Farr, J.; Lattermann, C.; et al. Algorithm for Treatment of Focal Cartilage Defects of the Knee: Classic and New Procedures. Cartilage 2021, 13 (Suppl. 1), 473S–495S. [Google Scholar] [CrossRef] [PubMed]
- Vincent, T.L.; Wann, A.K.T. Mechanoadaptation: Articular cartilage through thick and thin. J. Physiol. 2019, 597, 1271–1281. [Google Scholar] [CrossRef]
- Watt, F.E.; Corp, N.; Kingsbury, S.R.; Frobell, R.; Englund, M.; Felson, D.T.; Levesque, M.; Majumdar, S.; Wilson, C.; Beard, D.J.; et al. Towards prevention of post-traumatic osteoarthritis: Report from an international expert working group on considerations for the design and conduct of interventional studies following acute knee injury. Osteoarthr. Cartil. 2019, 27, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wu, Y.; Li, G.; Lin, Q.; Zhang, W.; Liu, H.; Su, J. Articular cartilage repair biomaterials: Strategies and applications. Mater. Today Bio 2024, 24, 100948. [Google Scholar] [CrossRef]
- Weishorn, J.; Wiegand, J.; Zietzschmann, S.; Koch, K.A.; Rehnitz, C.; Renkawitz, T.; Walker, T.; Bangert, Y. Factors Influencing Long-term Outcomes After Matrix-Induced Autologous Chondrocyte Implantation: Long-term Results at 10 Years. Am. J. Sports Med. 2024, 52, 2782–2791. [Google Scholar] [CrossRef]
- Kwon, H.; Brown, W.E.; Lee, C.A.; Wang, D.; Paschos, N.; Hu, J.C.; Athanasiou, K.A. Surgical and tissue engineering strategies for articular cartilage and meniscus repair. Nat. Rev. Rheumatol. 2019, 15, 550–570. [Google Scholar] [CrossRef]
- Zhao, Z.; Fan, C.; Chen, F.; Sun, Y.; Xia, Y.; Ji, A.; Wang, D.A. Progress in Articular Cartilage Tissue Engineering: A Review on Therapeutic Cells and Macromolecular Scaffolds. Macromol. Biosci. 2020, 20, e1900278. [Google Scholar] [CrossRef]
- Niemeyer, P.; Hanus, M.; Belickas, J.; Laszlo, T.; Gudas, R.; Fiodorovas, M.; Cebatorius, A.; Pastucha, M.; Izadpanah, K.; Prokes, J.; et al. Treatment of Large Cartilage Defects in the Knee by Hydrogel-Based Autologous Chondrocyte Implantation: A 5-Year Follow-Up of a Prospective, Multicenter, Single-Arm Phase III Trial. Cartilage 2025, 13, 19476035251334737. [Google Scholar] [CrossRef] [PubMed]
- Tseng, T.H.; Chen, C.P.; Jiang, C.C.; Weng, P.W.; Chan, Y.S.; Hsu, H.C.; Chiang, H. Biphasic cartilage repair implant versus microfracture in the treatment of focal chondral and osteochondral lesions of the knee: A prospective, multi-center, randomized clinical trial. J. Orthop. Traumatol. 2024, 25, 62. [Google Scholar] [CrossRef]
- van den Bosch, M.H.J. Inflammation in osteoarthritis: Is it time to dampen the alarm(in) in this debilitating disease? Clin. Exp. Immunol. 2019, 195, 153–166. [Google Scholar] [CrossRef]
- Santhamoorthy, M.; Kim, S.C. A Review of the Development of Biopolymer Hydrogel-Based Scaffold Materials for Drug Delivery and Tissue Engineering Applications. Gels 2025, 11, 178. [Google Scholar] [CrossRef] [PubMed]
- Li, C.S.; Xu, Y.; Li, J.; Qin, S.H.; Huang, S.W.; Chen, X.M.; Luo, Y.; Gao, C.T.; Xiao, J.H. Ultramodern natural and synthetic polymer hydrogel scaffolds for articular cartilage repair and regeneration. Biomed. Eng. Online 2025, 24, 13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ye, Q.; Zhu, Z.; Shi, S.; Xu, C.; Xie, R.; Li, Y. Hyaluronic Acid-Based Dynamic Hydrogels for Cartilage Repair and Regeneration. Gels 2024, 10, 703. [Google Scholar] [CrossRef]
- Irawan, V.; Sung, T.C.; Higuchi, A.; Ikoma, T. Collagen Scaffolds in Cartilage Tissue Engineering and Relevant Approaches for Future Development. Tissue Eng. Regen. Med. 2018, 15, 673–697. [Google Scholar] [CrossRef]
- Tang, Y.; Huang, J.; Damiri, F.; Xiao, Z.; Liao, X.; Zhang, W.; Chen, Y.; Berrada, M.; Song, Z.; Liu, Y. The preparation of silk fibroin-based hydrogels and their applications in cartilage repair. Int. J. Biol. Macromol. 2025, 310, 143610. [Google Scholar] [CrossRef]
- Fugazzola, M.C.; De Ruijter, M.; Veraa, S.; Plomp, S.; van Buul, W.; Hermsen, G.; van Weeren, R. A hybrid repair strategy for full-thickness cartilage defects: Long-term experimental study in eight horses. J. Orthop. Res. 2025, 43, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Xu, Y.; Han, Y.; Cui, J.; Jing, Z.; Li, D.; Liu, J.; Xiao, C.; Li, D.; Cai, B. Collagen-Based Hydrogels for Cartilage Regeneration. Orthop. Surg. 2023, 15, 3026–3045. [Google Scholar] [CrossRef]
- Zhao, X.; Papadopoulos, A.; Ibusuki, S.; Bichara, D.A.; Saris, D.B.; Malda, J.; Anseth, K.S.; Gill, T.J.; Randolph, M.A. Articular cartilage generation applying PEG-LA-DM/PEGDM copolymer hydrogels. BMC Musculoskelet. Disord. 2016, 17, 245. [Google Scholar] [CrossRef]
- Hu, J.C.; Athanasiou, K.A. A self-assembling process in articular cartilage tissue engineering. Tissue Eng. 2006, 12, 969–979. [Google Scholar] [CrossRef]
- Meppelink, A.M.; Zhao, X.; Griffin, D.J.; Erali, R.; Gill, T.J.; Bonassar, L.J.; Redmond, R.W.; Randolph, M.A. Hyaline Articular Matrix Formed by Dynamic Self-Regenerating Cartilage and Hydrogels. Tissue Eng. Part A 2016, 22, 962–970. [Google Scholar] [CrossRef]
- Fan, Y.; Guastaldi, F.P.S.; Runyan, G.; Wang, Y.; Farinelli, W.A.; Randolph, M.A.; Redmond, R.W. Laser Ablation Facilitates Implantation of Dynamic Self-Regenerating Cartilage for Articular Cartilage Regeneration. J. Funct. Biomater. 2024, 15, 148. [Google Scholar] [CrossRef]
- Hajjarian, Z.; Nadkarni, S.K. Tutorial on laser speckle rheology: Technology, applications, and opportunities. J. Biomed. Opt. 2020, 25, 1–19. [Google Scholar] [CrossRef]
- Leartprapun, N.; Zeng, Z.; Hajjarian, Z.; Bossuyt, V.; Nadkarni, S.K. Laser speckle rheological microscopy reveals wideband viscoelastic spectra of biological tissues. Sci. Adv. 2024, 10, eadl1586. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.M.; Wise, B.C.; Bonnevie, E.D.; Mauck, R.L. A Systematic Review and Guide to Mechanical Testing for Articular Cartilage Tissue Engineering. Tissue Eng. Part C Methods 2019, 25, 593–608. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.H.; Aroush, D.R.; Asnacios, A.; Chen, W.C.; Dokukin, M.E.; Doss, B.L.; Durand-Smet, P.; Ekpenyong, A.; Guck, J.; Guz, N.V.; et al. A comparison of methods to assess cell mechanical properties. Nat. Methods 2018, 15, 491–498. [Google Scholar] [CrossRef]
- Hajjarian, Z.; Nia, H.T.; Ahn, S.; Grodzinsky, A.J.; Jain, R.K.; Nadkarni, S.K. Laser Speckle Rheology for evaluating the viscoelastic properties of hydrogel scaffolds. Sci. Rep. 2016, 6, 37949. [Google Scholar] [CrossRef] [PubMed]
- Metzler, N.F.; Kondo, M.; Matsukura, K.; Ford, A.J.; Grainger, D.W.; Okano, T. Differentiated and Untreated Juvenile Chondrocyte Sheets Regenerate Cartilage Similarly In Vivo. Tissue Eng. Part. A 2025, 31, 184–194. [Google Scholar] [CrossRef]
- Mumme, M.; Wixmerten, A.; Ivkovic, A.; Peretti, G.M.; Yilmaz, T.; Reppenhagen, S.; Pullig, O.; Miot, S.; Izadpanah, K.; Jakob, M.; et al. Clinical relevance of engineered cartilage maturation in a randomized multicenter trial for articular cartilage repair. Sci. Transl. Med. 2025, 17, eads0848. [Google Scholar] [CrossRef]
- Wang, Z.; Iwashita, T.; Porcar, L.; Wang, Y.; Liu, Y.; Sanchez-Diaz, L.E.; Wu, B.; Huang, G.R.; Egami, T.; Chen, W.R. Local elasticity in nonlinear rheology of interacting colloidal glasses revealed by neutron scattering and rheometry. Phys. Chem. Chem. Phys. 2018, 21, 38–45. [Google Scholar] [CrossRef]
- Wyse Jackson, T.; Michel, J.; Lwin, P.; Fortier, L.A.; Das, M.; Bonassar, L.J.; Cohen, I. Structural origins of cartilage shear mechanics. Sci. Adv. 2022, 8, eabk2805. [Google Scholar] [CrossRef]
- Hajjarian, Z.; Brachtel, E.F.; Tshikudi, D.M.; Nadkarni, S.K. Mapping Mechanical Properties of the Tumor Microenvironment by Laser Speckle Rheological Microscopy. Cancer Res. 2021, 81, 4874–4885. [Google Scholar] [CrossRef] [PubMed]
- Cissell, D.D.; Link, J.M.; Hu, J.C.; Athanasiou, K.A. A Modified Hydroxyproline Assay Based on Hydrochloric Acid in Ehrlich’s Solution Accurately Measures Tissue Collagen Content. Tissue Eng. Part C Methods 2017, 23, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Mason, T.G. Estimating the viscoelastic moduli of complex fluids using the generalized Stokes–Einstein equation. Rheol. Acta 2000, 39, 371–378. [Google Scholar] [CrossRef]
- Dasgupta, B.R.; Tee, S.Y.; Crocker, J.C.; Frisken, B.J.; Weitz, D.A. Microrheology of polyethylene oxide using diffusing wave spectroscopy and single scattering. Phys. Rev. E 2002, 65, 051505. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guastaldi, F.P.S.; Kostyra, D.M.; Leartprapun, N.; Nadkarni, S.; Randolph, M.A.; Redmond, R.W. Biochemical and Biomechanical Properties of Scaffold-Free Hyaline Cartilage Generated Under Dynamic Conditions. Int. J. Mol. Sci. 2025, 26, 4719. https://doi.org/10.3390/ijms26104719
Guastaldi FPS, Kostyra DM, Leartprapun N, Nadkarni S, Randolph MA, Redmond RW. Biochemical and Biomechanical Properties of Scaffold-Free Hyaline Cartilage Generated Under Dynamic Conditions. International Journal of Molecular Sciences. 2025; 26(10):4719. https://doi.org/10.3390/ijms26104719
Chicago/Turabian StyleGuastaldi, Fernando P. S., David M. Kostyra, Nichaluk Leartprapun, Seemantini Nadkarni, Mark A. Randolph, and Robert W. Redmond. 2025. "Biochemical and Biomechanical Properties of Scaffold-Free Hyaline Cartilage Generated Under Dynamic Conditions" International Journal of Molecular Sciences 26, no. 10: 4719. https://doi.org/10.3390/ijms26104719
APA StyleGuastaldi, F. P. S., Kostyra, D. M., Leartprapun, N., Nadkarni, S., Randolph, M. A., & Redmond, R. W. (2025). Biochemical and Biomechanical Properties of Scaffold-Free Hyaline Cartilage Generated Under Dynamic Conditions. International Journal of Molecular Sciences, 26(10), 4719. https://doi.org/10.3390/ijms26104719







