Effects of Cnidium officinale, Pueraria lobata Ohwi, and Leonurus japonicus Extract on Vascular Endothelial Dysfunctions in Ovariectomized Rats and Molecular Mechanisms
Abstract
1. Introduction
2. Results
2.1. E-Screening of CPL Extracts Using MCF-7 Cells
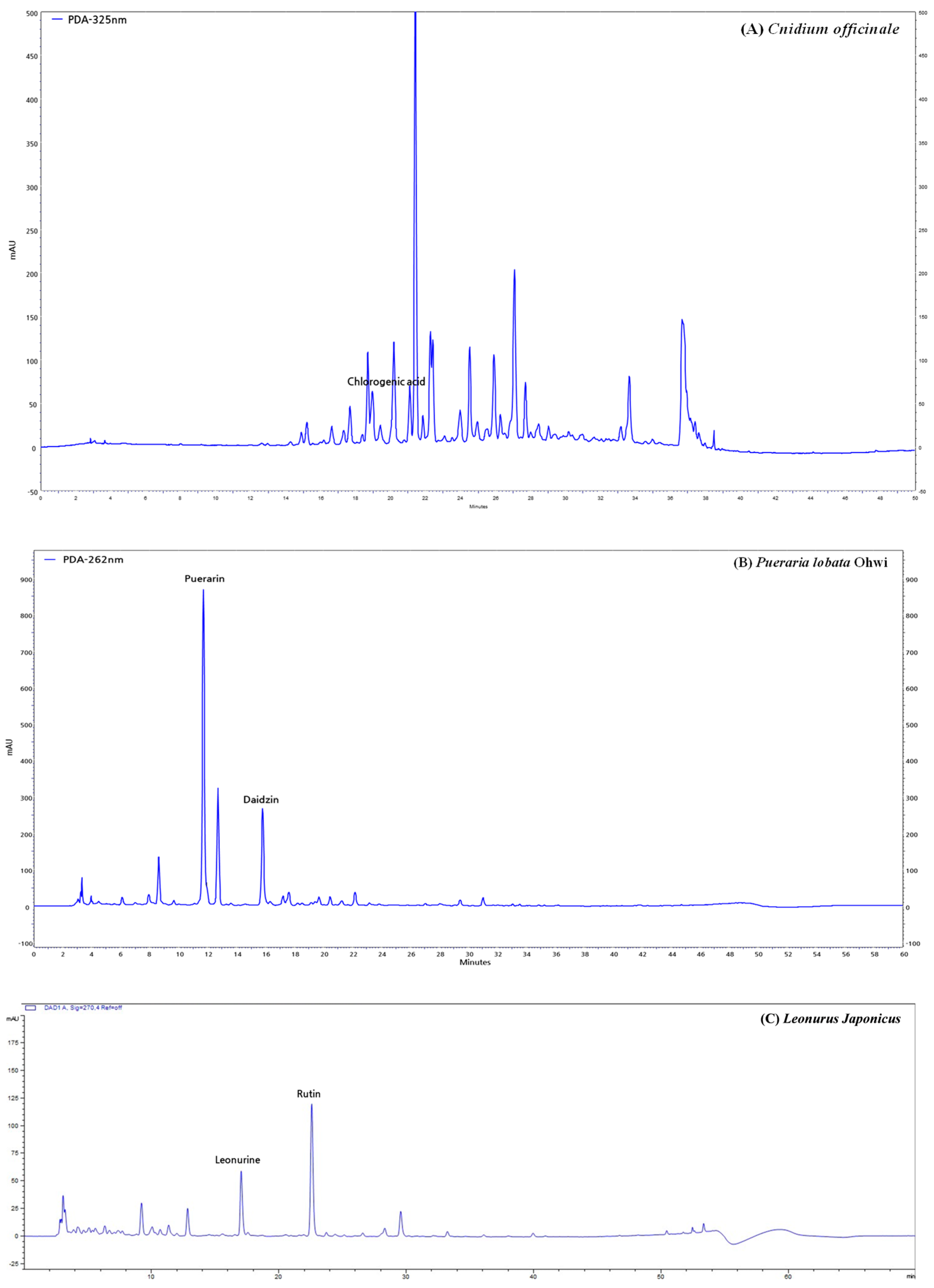
2.2. High-Performance Liquid Chromatography (HPLC) Analysis of CPL Extracts
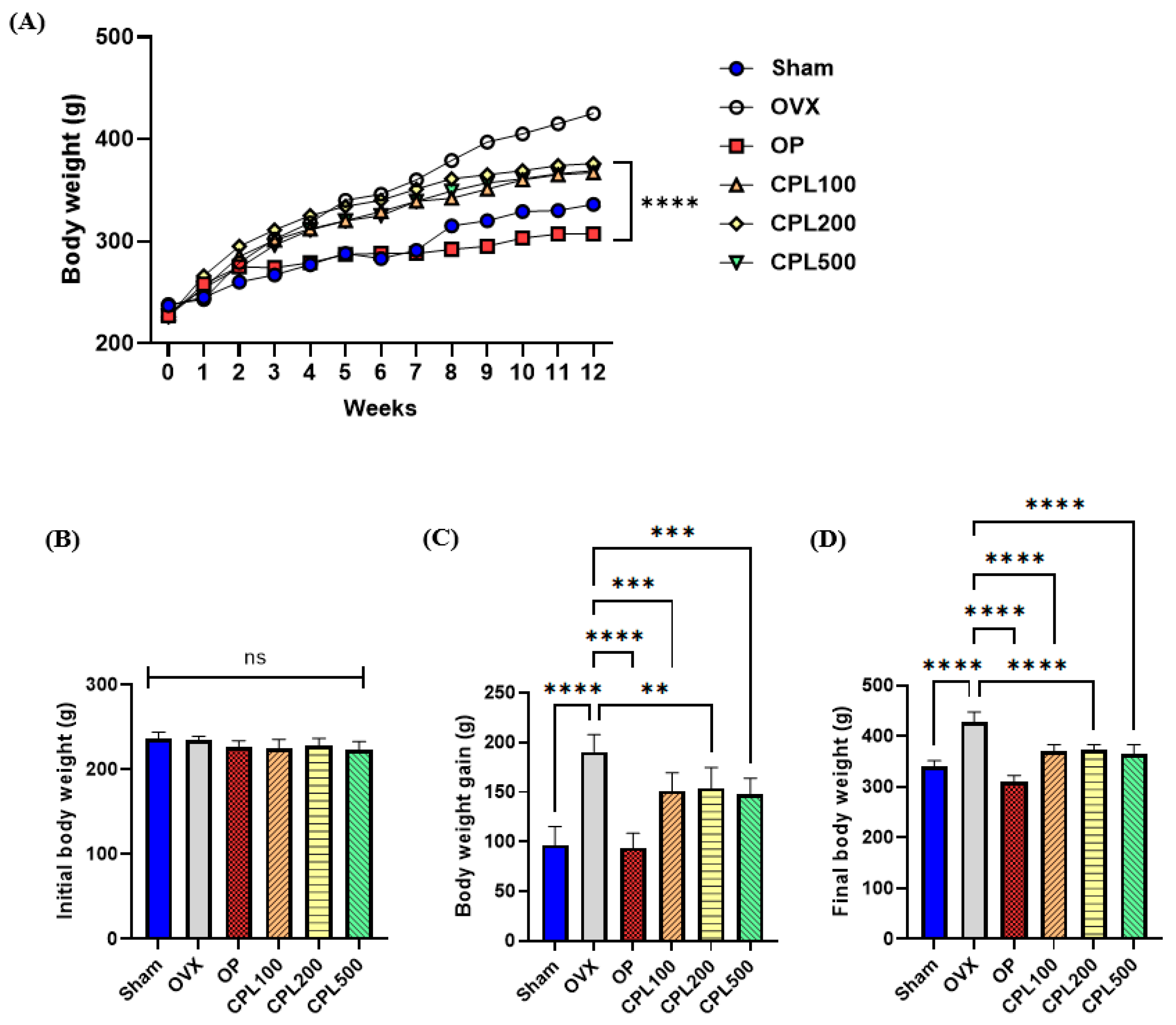
2.3. Body Weight and Body Weight Gain
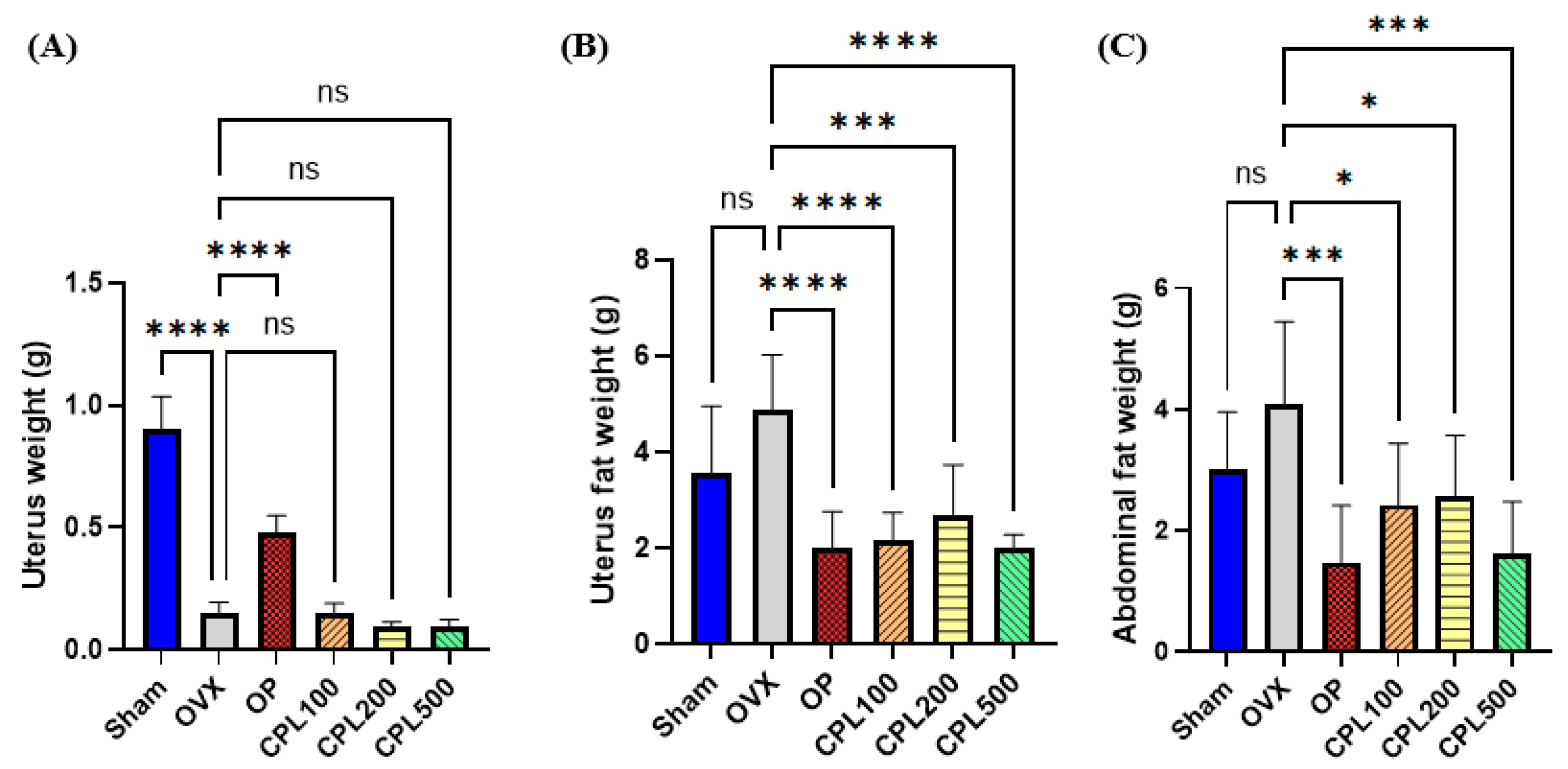
2.4. Organ and Fat Weight
2.5. Tail Temperatures
2.6. Serum Lipid Profiles
2.7. Serum Hepatic Function Profiles
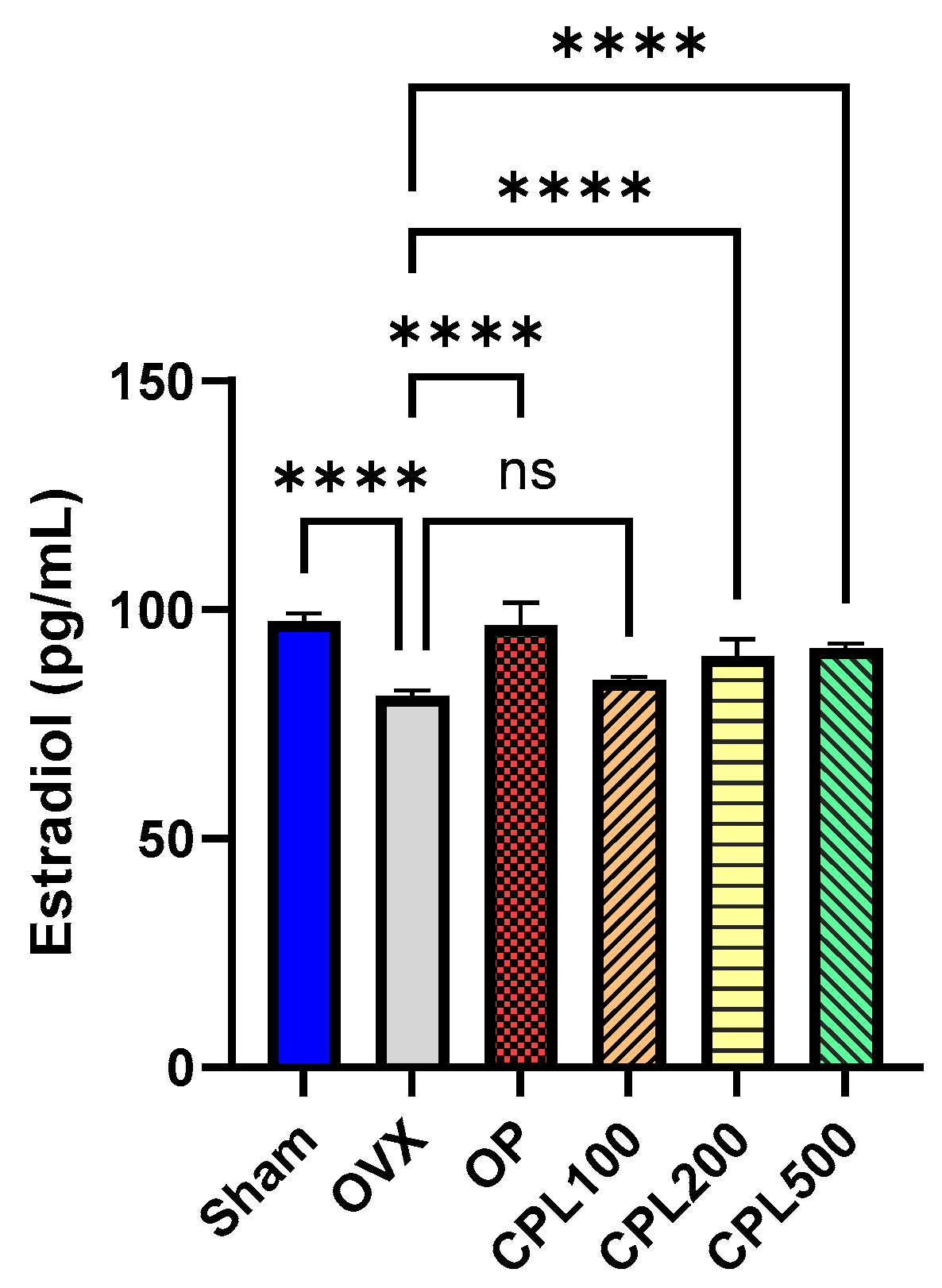
2.8. Serum Estradiol (E2) Levels
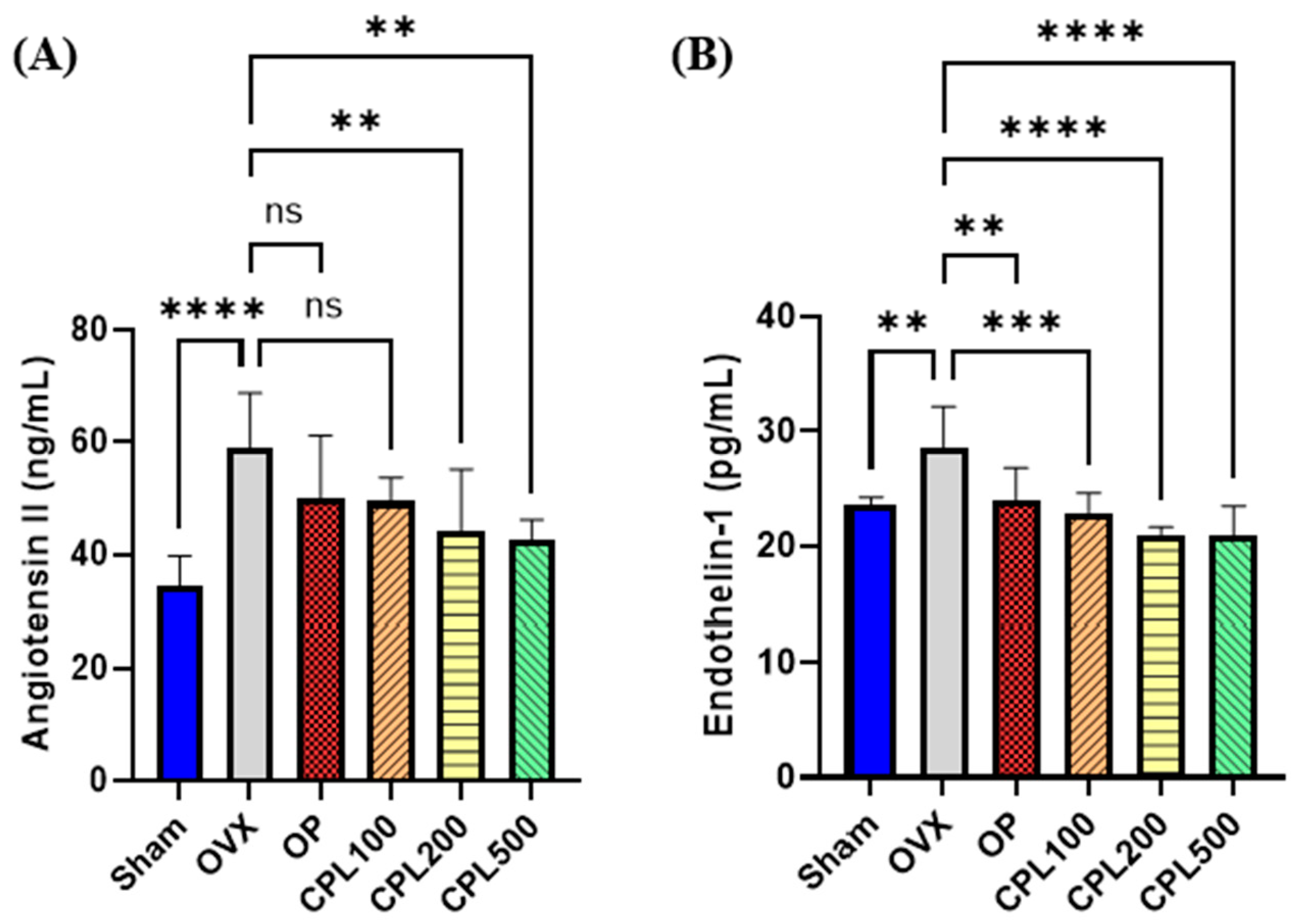
2.9. Serum Vasoactive Substances
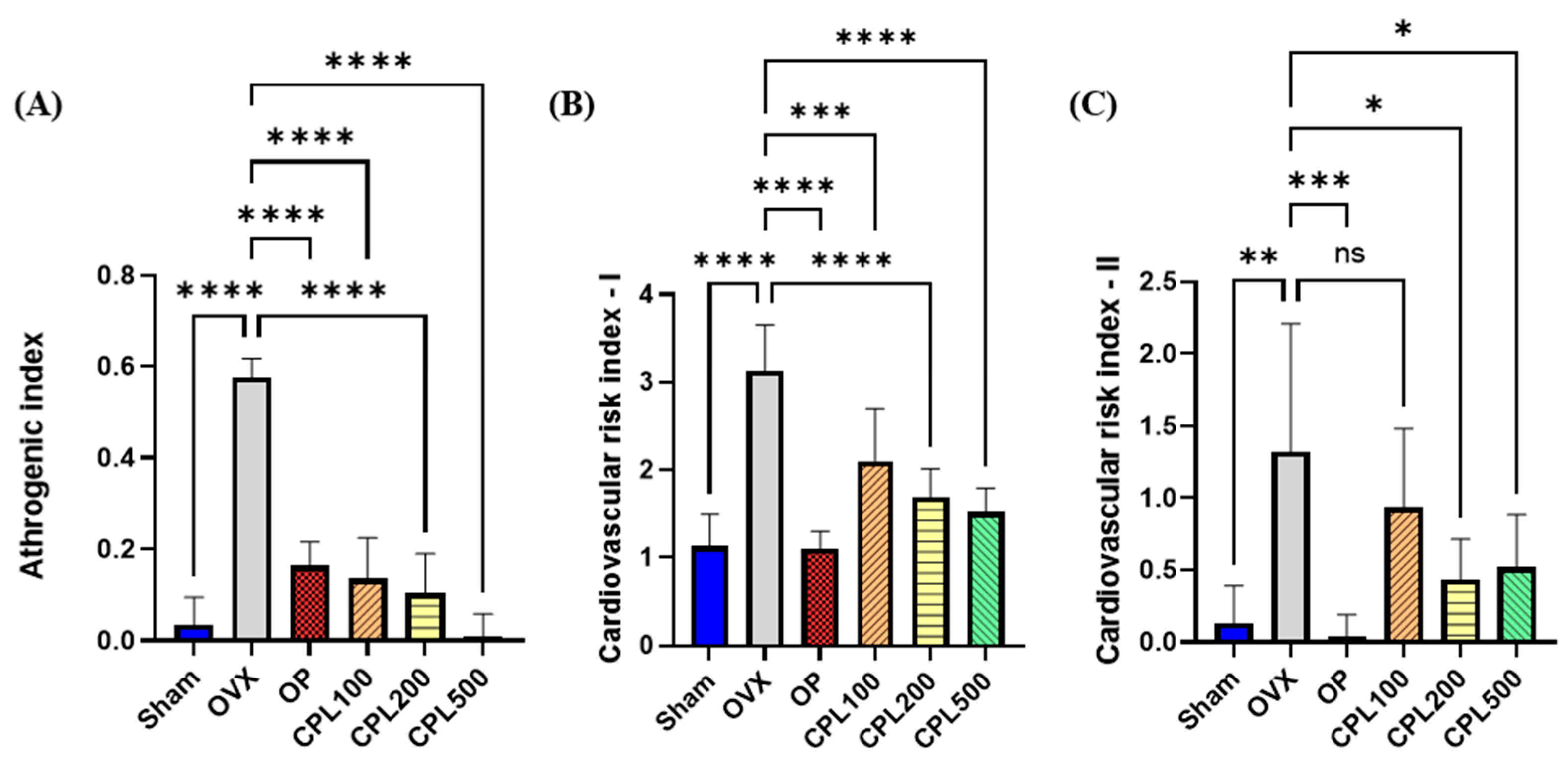
2.10. Serum Cardiovascular Risk Indices
2.11. Endometrium Thickness
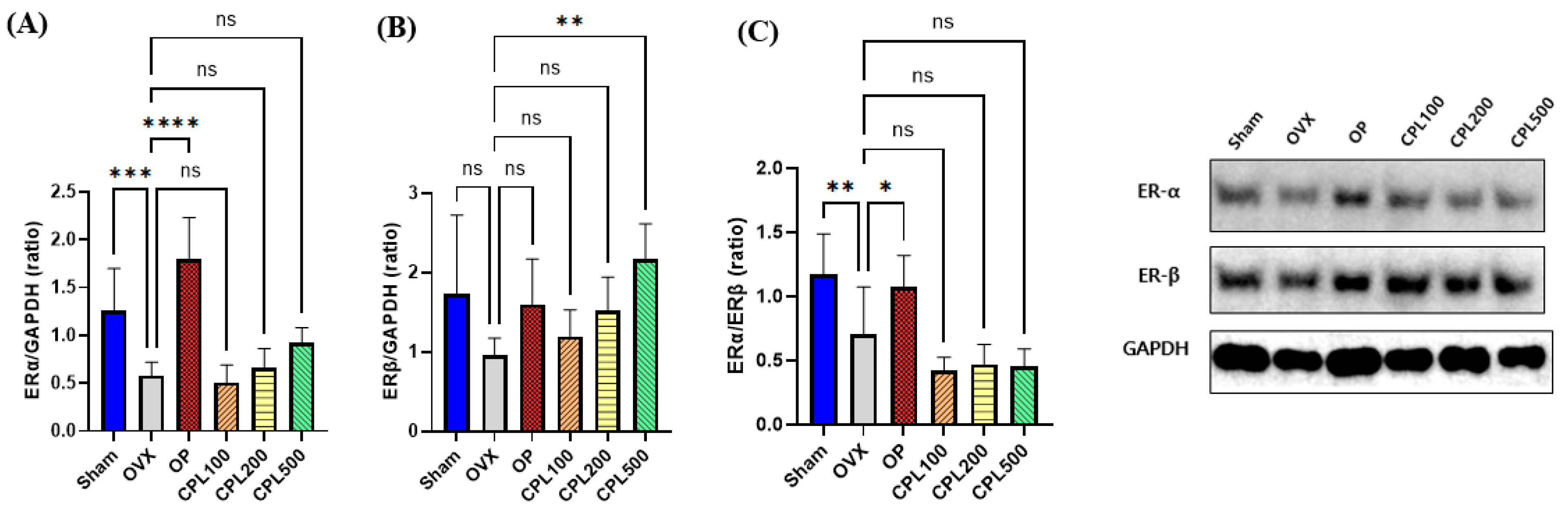
2.12. Protein Expression Levels and Their Estrogenic Effect in the Uterus
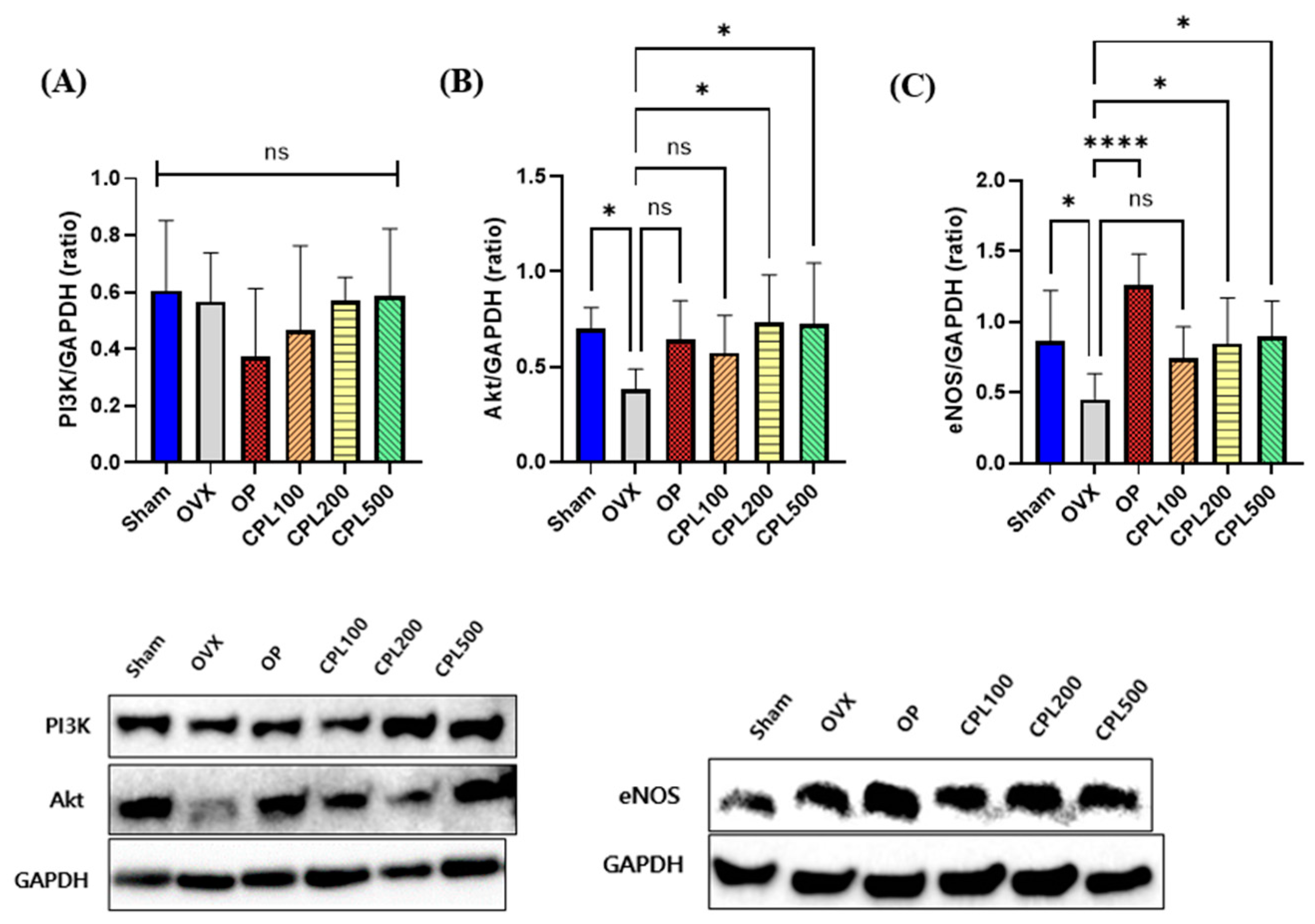
2.13. Protein Expression Levels and Their Vasodilation Effect in the Liver
2.14. Cell Adhesion Molecule Levels in HUVECs
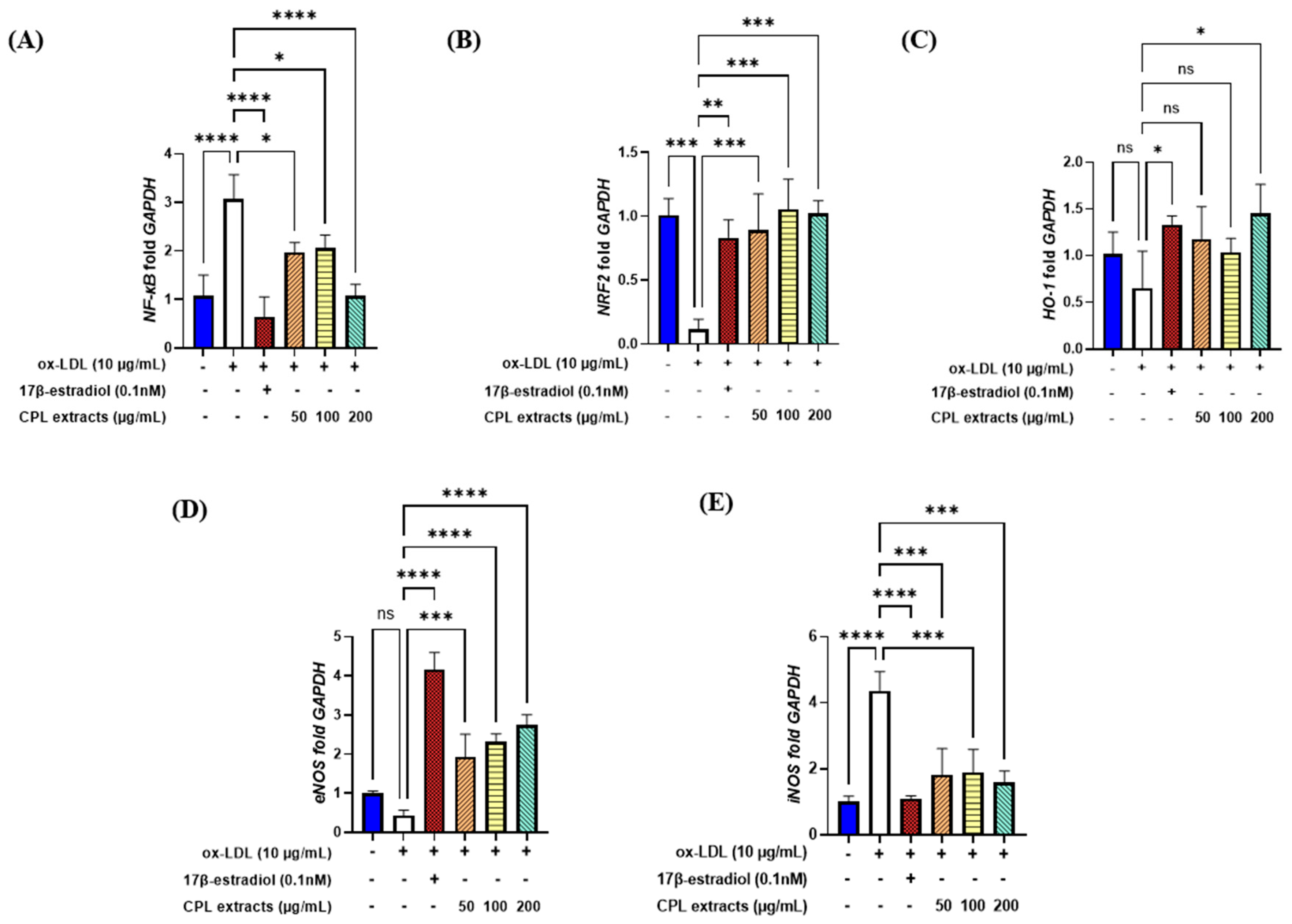
2.15. Gene Expression Levels in HUVECs
3. Discussion
4. Materials and Methods
4.1. Preparation of CPL Extracts
4.2. Animals
4.3. Ovariectomy Procedure and Treatment
4.4. Body Weight Measurement
4.5. Serum Biochemical Analysis
4.6. Cardiovascular Index
4.7. Tail Temperature Measurements
4.8. Uterine Histological Analysis
4.9. Western Blotting
4.10. Cell Proliferation Assay
4.11. Soluble Vascular Cell Adhesion Molecule-1 and Soluble Intercellular Adhesion Molecule-1 Assay (sVCAM-1 and sICAM-1 Assay)
4.12. mRNA Expression Analysis
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ilankoon, I.M.P.S.; Samarasinghe, K.; Elgán, C. Menopause is a natural stage of aging: A qualitative study. BMC Women’s Health 2021, 21, 47. [Google Scholar] [CrossRef]
- Stanhewicz, A.E.; Wenner, M.M.; Stachenfeld, N.S. Sex differences in endothelial function important to vascular health and overall cardiovascular disease risk across the lifespan. Am. J. Physiol.-Heart Circ. Physiol. 2018, 315, H1569–H1588. [Google Scholar] [CrossRef]
- Erdélyi, A.; Pálfi, E.; Tűű, L.; Nas, K.; Szűcs, Z.; Török, M.; Jakab, A.; Várbíró, S. The importance of nutrition in menopause and perimenopause—A review. Nutrients 2023, 16, 27. [Google Scholar] [CrossRef]
- Archer, D.F. Endometrial bleeding during hormone therapy: The effect of progestogens. Maturitas 2007, 57, 71–76. [Google Scholar] [CrossRef]
- Lee, Y.M.; Lee, Y.R.; Kim, J.S.; Kim, Y.H.; Kim, J. Cinidium officinale and its bioactive compound, butylidenephthalide, inhibit laser-induced choroidal neovascularization in a rat model. Molecules 2015, 20, 20699–20708. [Google Scholar] [CrossRef]
- Hu, M.; Yu, Z.; Wang, J.; Fan, W.; Liu, Y.; Li, J.; Xiao, H.; Li, Y.; Peng, W.; Wu, C. Traditional Uses, Origins, Chemistry and Pharmacology of Bombyx batryticatus: A review. Molecules 2017, 22, 1779. [Google Scholar] [CrossRef]
- Bae, K.-E.; Choi, Y.-W.; Kim, S.-T.; Kim, Y.-K. Components of Rhizome Extract of Cnidium officinale Makino and Their In vitro Biological Effects. Molecules 2011, 16, 8833–8847. [Google Scholar] [CrossRef]
- Liu, J.; Shi, Y.C.; Lee, D.Y.W. Applications of Pueraria lobata in treating diabetics and reducing alcohol drinking. Chin. Herb. Med. 2019, 11, 141–149. [Google Scholar] [CrossRef]
- Lim, D.W.; Lee, C.; Kim, I.-H.; Kim, Y.T. Anti-Inflammatory Effects of Total Isoflavones from Pueraria lobata on Cerebral Ischemia in Rats. Molecules 2013, 18, 10404–10412. [Google Scholar] [CrossRef]
- Bae, I.; Choi, C.; Youn, D.; Kim, H.; Kim, K.; Yoon, Y.; Jeong, H. Effects of LEONURI HERBA extract on the cerebral blood flow and cytokines in cerebral ischemic rats. J. Physiol. Pathol. Korean Med. 2007, 21, 611–616. [Google Scholar]
- Liu, S.; Sun, C.; Tang, H.; Peng, C.; Peng, F. Leonurine: A comprehensive review of pharmacokinetics, pharmacodynamics, and toxicology. Front. Pharmacol. 2024, 15, 1428406. [Google Scholar] [CrossRef]
- Jin, H.J.; Park, S.M.; Kim, E.O.; Kim, S.C. Hepatoprotective effect of Ikwiseungyang-tang via Nrf2 activation. Herb. Formula Sci. 2021, 29, 167–179. [Google Scholar] [CrossRef]
- Mehta, J.; Kling, J.M.; Manson, J.E. Risks, benefits, and treatment modalities of menopausal hormone therapy: Current concepts. Front. Endocrinol. 2021, 12, 564781. [Google Scholar] [CrossRef]
- Mannino, C.A.; South, S.M.; Quinones-Jenab, V.; Inturrisi, C.E. Estradiol replacement in ovariectomized rats is antihyperalgesic in the formalin test. J. Pain 2007, 8, 334–342. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, N. Chandra, Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Ferretti, M.; Cavani, F.; Manni, P.; Carnevale, G.; Bertoni, L.; Zavatti, M.; Palumbo, C. Ferutinin dose-dependent effects on uterus and mammary gland in ovariectomized rats. Histol. Histopathol. 2014, 29, 1027–1037. [Google Scholar] [CrossRef]
- Paterni, I.; Granchi, C.; Katzenellenbogen, J.A.; Minutolo, F. Estrogen receptors alpha (ERα) and beta (ERβ): Subtype-selective ligands and clinical potential. Steroids 2014, 90, 13–29. [Google Scholar] [CrossRef]
- Ko, S.H.; Kim, H.S. Menopause-associated lipid metabolic disorders and foods beneficial for postmenopausal women. Nutrients 2020, 12, 202. [Google Scholar] [CrossRef]
- Ryczkowska, K.; Adach, W.; Janikowski, K.; Banach, M.; Bielecka-Dabrowa, A. Menopause and women’s cardiovascular health: Is it really an obvious relationship? Arch. Med. Sci. 2023, 19, 458–466. [Google Scholar] [CrossRef]
- Maleki, N.; Ravesh, R.K.; Salehiyeh, S.; Faiz, A.F.; Ebrahimi, M.; Sharbati, A.; Panji, M.; Khiyavi, H.A.; Safizadeh, F.; Abbasi, M.; et al. Comparative effects of estrogen and silibinin on cardiovascular risk biomarkers in ovariectomized rats. Gene 2022, 823, 146365. [Google Scholar] [CrossRef]
- Freedman, R.R. Menopausal hot flashes: Mechanisms, endocrinology, treatment. J. Steroid Biochem. Mol. Biol. 2014, 142, 115–120. [Google Scholar] [CrossRef]
- Kumar, S.; Lata, K.; Mukhopadhyay, S.; Mukherjee, T.K. Role of estrogen receptors in pro-oxidative and anti-oxidative actions of estrogens: A perspective. Biochim. Biophys. Acta 2010, 1800, 1127–1135. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.J.; Han, Z.H.; Li, Y.Q.; Xue, J.H.; Gao, D.F.; Wu, X.S.; Wang, C.X. PI3K/AKT signaling pathway plays a role in enhancement of eNOS activity by recombinant human angiotensin converting enzyme 2 in human umbilical vein endothelial cells. Int. J. Clin. Exp. Pathol. 2014, 7, 8112–8117. [Google Scholar]
- Zhong, J.C.; Yu, X.Y.; Lin, Q.X.; Li, X.H.; Huang, X.Z.; Xiao, D.Z.; Lin, S.G. Enhanced angiotensin converting enzyme 2 regulates the insulin/Akt signalling pathway by blockade of macrophage migration inhibitory factor expression. Br. J. Pharmacol. 2008, 153, 66–74. [Google Scholar] [CrossRef]
- Michell, B.J.; Griffiths, J.E.; Mitchelhill, K.I.; Rodriguez-Crespo, I.; Tiganis, T.; Bozinovski, S.; de Montellano, P.R.; Kemp, B.E.; Pearson, R.B. The Akt kinase signals directly to endothelial nitric oxide synthase. Curr. Biol. 1999, 9, 845–848. [Google Scholar] [CrossRef]
- Lee, K.Y.; Kim, J.H.; Kim, E.Y.; Yeom, M.; Jung, H.S.; Sohn, Y. Water extract of Cnidii rhizoma suppresses RANKL-induced osteoclastogenesis in RAW 264.7 cell by inhibiting NFATc1/c-Fos signaling and prevents ovariectomized bone loss in SD-rat. BMC Complement Altern Med. 2019, 19, 207. [Google Scholar] [CrossRef]
- Bhargava, P.; Lee, C.H. Role and function of macrophages in the metabolic syndrome. Biochem. J. 2012, 442, 253–262. [Google Scholar] [CrossRef]
- Zhang, M.; An, C.; Gao, Y.; Leak, R.K.; Chen, J.; Zhang, F. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog. Neurobiol. 2013, 100, 30–47. [Google Scholar] [CrossRef]
- Kim, Y.M.; Pae, H.O.; Park, J.E.; Lee, Y.C.; Woo, J.M.; Kim, N.H.; Choi, Y.K.; Lee, B.S.; Kim, S.R.; Chung, H.T. Heme oxygenase in the regulation of vascular biology: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011, 14, 137–167. [Google Scholar] [CrossRef]
- Pae, H.O.; Son, Y.; Kim, N.H.; Jeong, H.J.; Chang, K.C.; Chung, H.T. Role of heme oxygenase in preserving vascular bioactive NO. Nitric Oxide. 2010, 23, 251–257. [Google Scholar] [CrossRef]
- Oh, J.; Hong, S.; Ko, S.H.; Kim, H.S. Evaluation of antioxidant effects of pumpkin (Cucurbita pepo L.) seed extract on aging- and menopause-related diseases using Saos-2 cells and ovariectomized rats. Antioxidants 2024, 13, 241. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Haglund, O.; Luostarinen, R.; Wallin, R.; Wibell, L.; Saldeen, T. The effects of fish oil on triglycerides, cholesterol, fibrinogen and malondialdehyde in humans supplemented with vitamin E. J. Nutr. 1991, 121, 165–169. [Google Scholar] [CrossRef]
- Lee, Y.M.; Kim, J.B.; Bae, J.H.; Lee, J.S.; Kim, P.S.; Jang, H.H.; Kim, H.R. Estrogen-like activity of aqueous extract from Agrimonia pilosa Ledeb. in MCF-7 cells. BMC Complement. Altern. Med. 2012, 12, 260. [Google Scholar] [CrossRef]
- Gong, L.; Lei, Y.; Liu, Y.; Tan, F.; Li, S.; Wang, X.; Xu, M.; Cai, W.; Du, B.; Xu, F.; et al. Vaccarin prevents ox-LDL-induced HUVEC EndMT, inflammation and apoptosis by suppressing ROS/p38 MAPK signaling. Am. J. Transl. Res. 2019, 11, 2140–2154. [Google Scholar]
- Yang, J.; Yoo, H.; Kim, Y.; Seol, I. The effect of Lonicera japonica Thunberg on inflammatory factor expression associated with atherosclerosis. J. Int. Korean Med. 2021, 42, 25–39. [Google Scholar] [CrossRef]
- Rosano, G.M.C.; Vitale, C.; Marazzi, G.; Volterrani, M. Menopause and cardiovascular disease: The evidence. Climacteric 2007, 10, 19–24. [Google Scholar] [CrossRef]
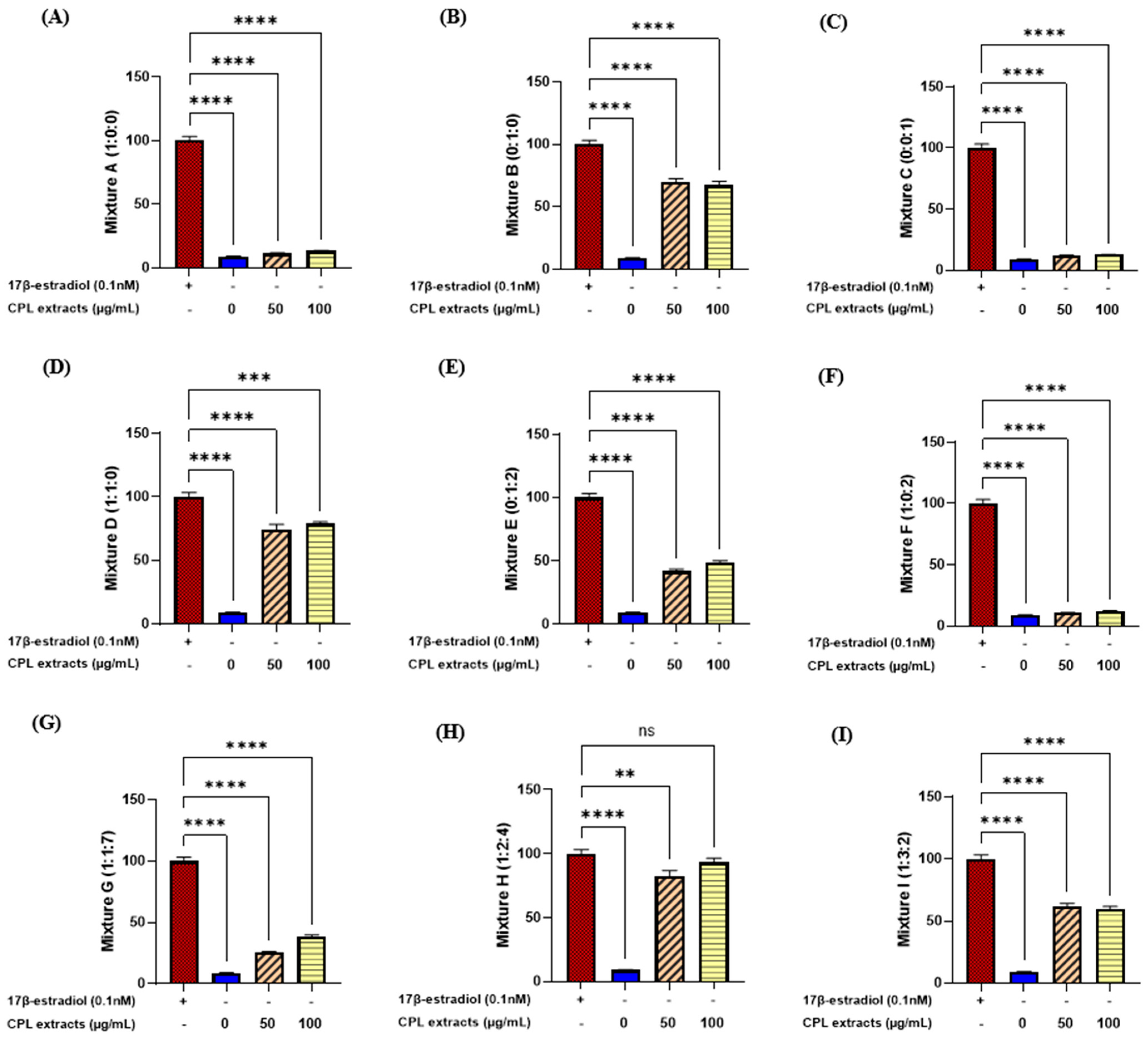





| Mixture | Cnidii rhizoma | Puerariae radix | Leonuri herba |
|---|---|---|---|
| A | 1 | 0 | 0 |
| B | 0 | 1 | 0 |
| C | 0 | 0 | 1 |
| D | 1 | 1 | 0 |
| E | 0 | 1 | 2 |
| F | 1 | 0 | 2 |
| G | 1 | 1 | 7 |
| H | 1 | 2 | 4 |
| I | 1 | 3 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, J.; Kim, M.; Kim, J.; Jang, J.; Noh, D.; Kim, H.-S. Effects of Cnidium officinale, Pueraria lobata Ohwi, and Leonurus japonicus Extract on Vascular Endothelial Dysfunctions in Ovariectomized Rats and Molecular Mechanisms. Int. J. Mol. Sci. 2025, 26, 4708. https://doi.org/10.3390/ijms26104708
Oh J, Kim M, Kim J, Jang J, Noh D, Kim H-S. Effects of Cnidium officinale, Pueraria lobata Ohwi, and Leonurus japonicus Extract on Vascular Endothelial Dysfunctions in Ovariectomized Rats and Molecular Mechanisms. International Journal of Molecular Sciences. 2025; 26(10):4708. https://doi.org/10.3390/ijms26104708
Chicago/Turabian StyleOh, Joohee, Minseo Kim, Jinsoo Kim, Jiwon Jang, Dongjin Noh, and Hyun-Sook Kim. 2025. "Effects of Cnidium officinale, Pueraria lobata Ohwi, and Leonurus japonicus Extract on Vascular Endothelial Dysfunctions in Ovariectomized Rats and Molecular Mechanisms" International Journal of Molecular Sciences 26, no. 10: 4708. https://doi.org/10.3390/ijms26104708
APA StyleOh, J., Kim, M., Kim, J., Jang, J., Noh, D., & Kim, H.-S. (2025). Effects of Cnidium officinale, Pueraria lobata Ohwi, and Leonurus japonicus Extract on Vascular Endothelial Dysfunctions in Ovariectomized Rats and Molecular Mechanisms. International Journal of Molecular Sciences, 26(10), 4708. https://doi.org/10.3390/ijms26104708







