Benzimidazole-Derived B2 as a Fluorescent Probe for Bacterial Outer Membrane Vesicle (OMV) Labeling: Integrating DFT, Molecular Dynamics, Flow Cytometry, and Confocal Microscopy
Abstract
1. Introduction
2. Results
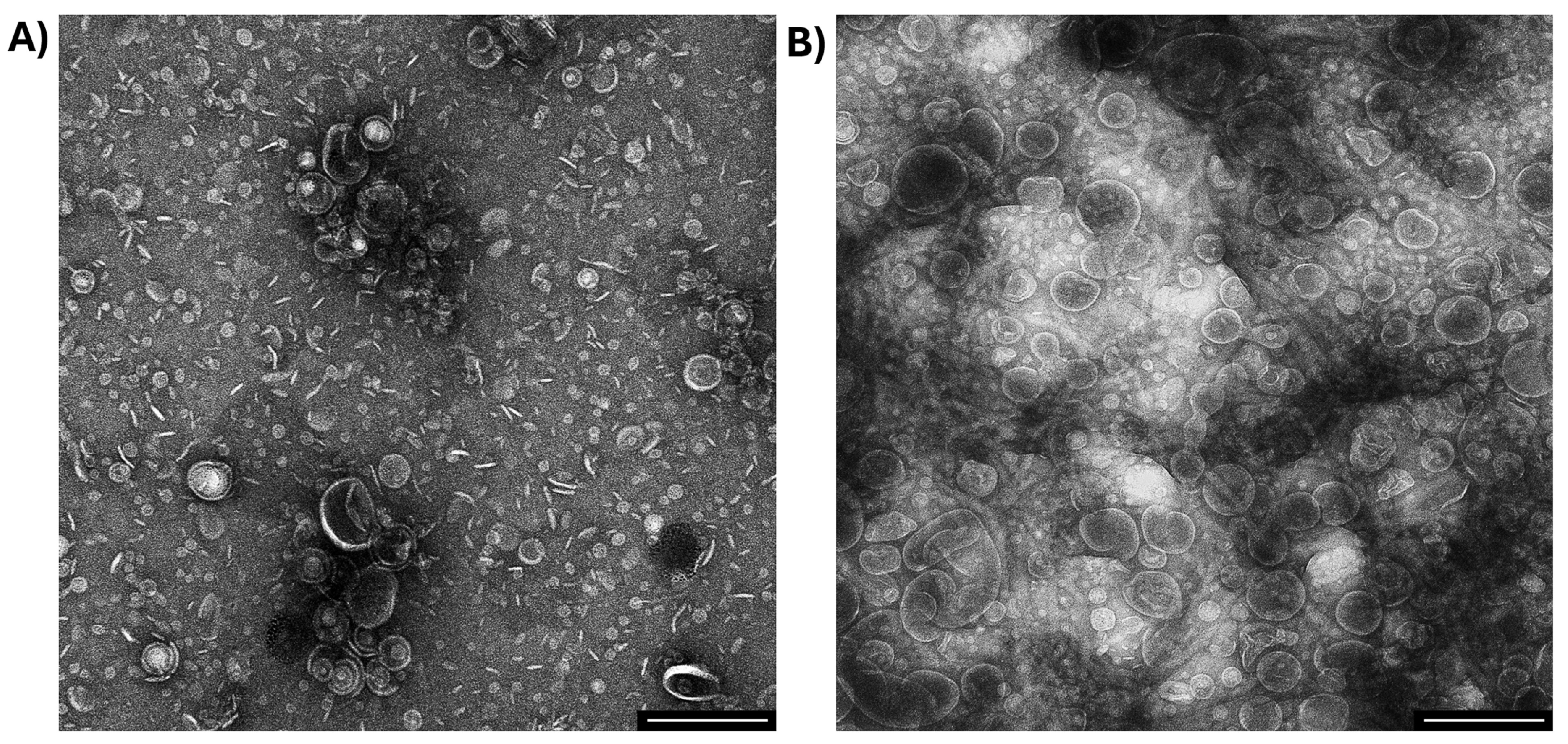
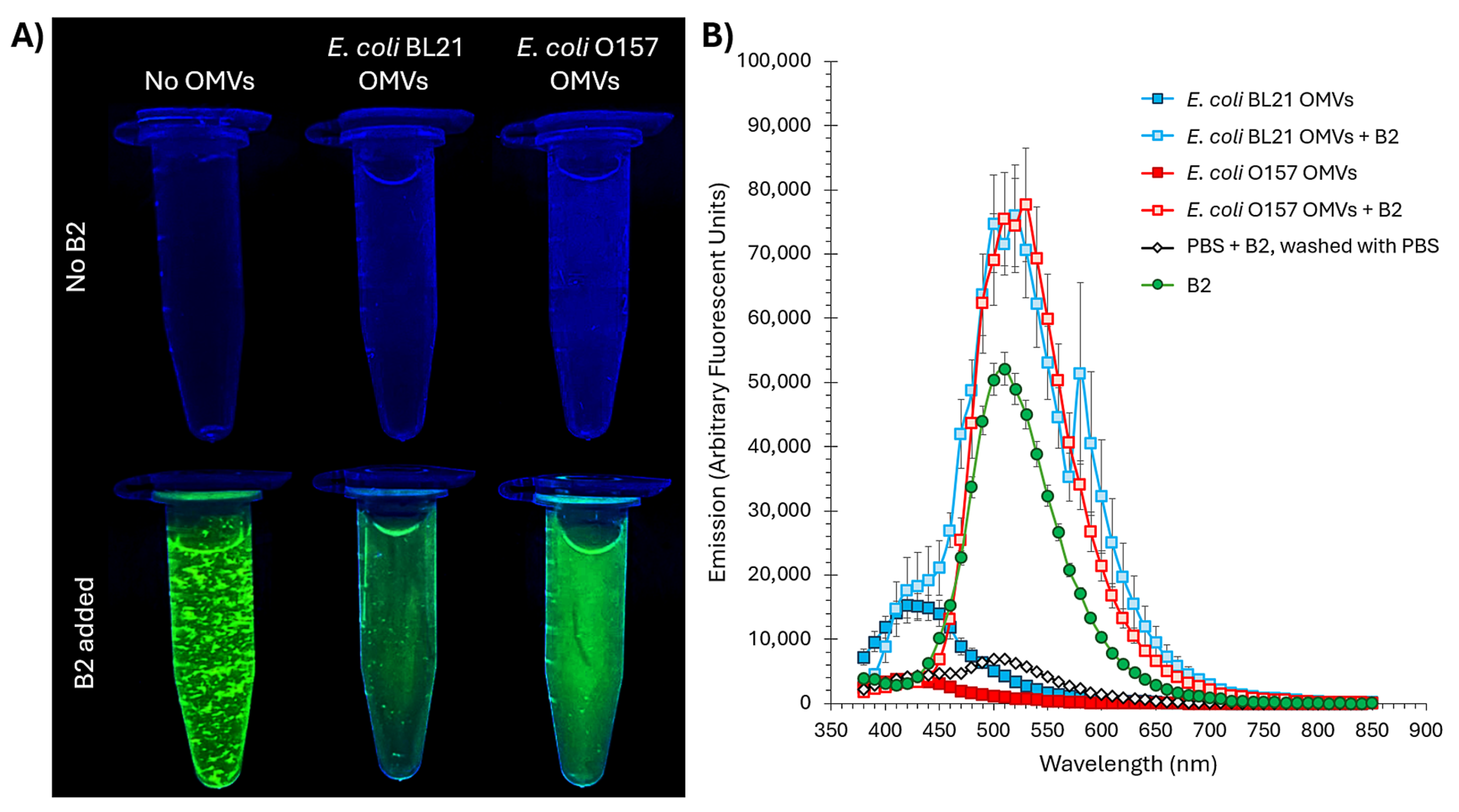
2.1. B2 Interacts with Outer Membrane Vesicles (OMVs)
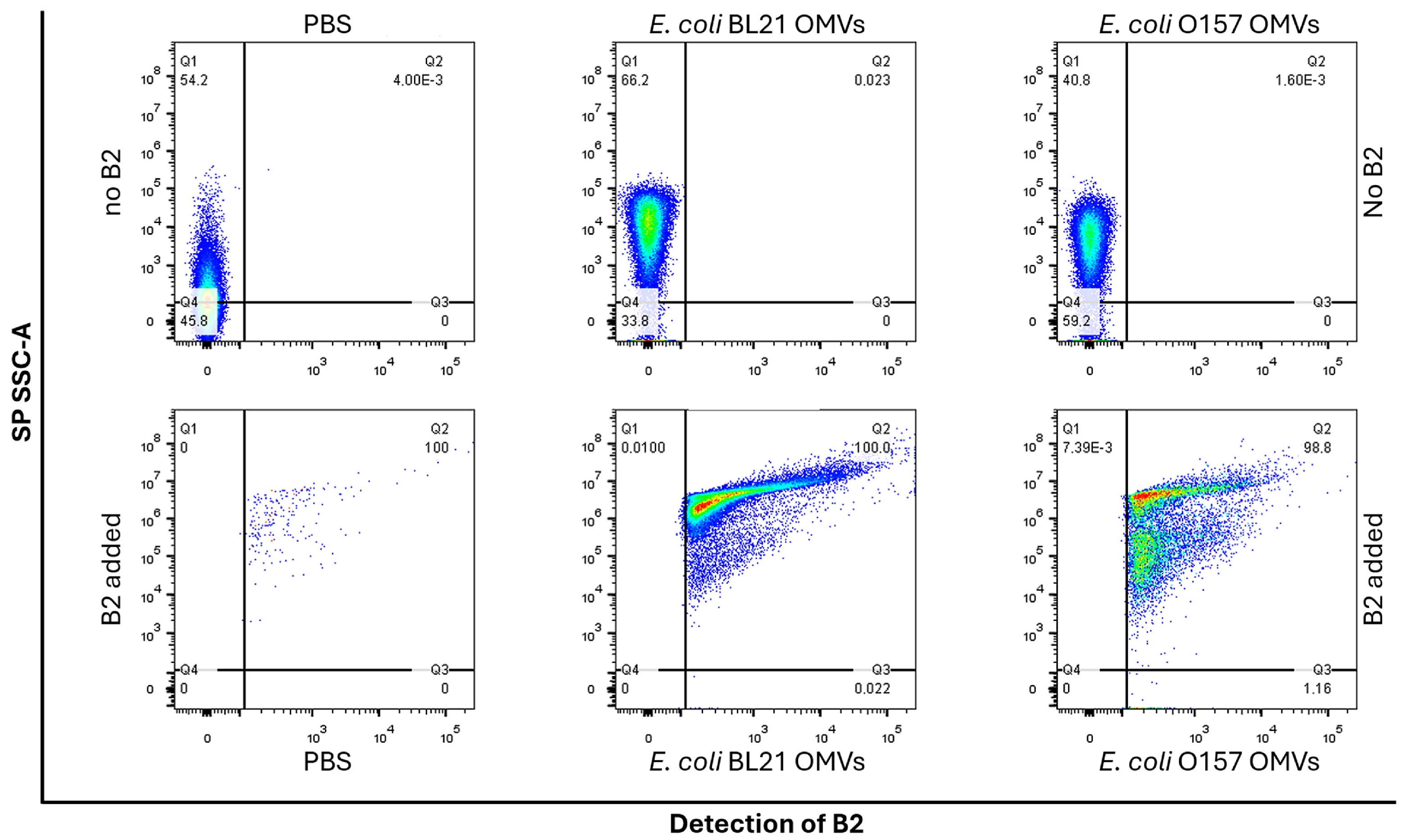
2.2. B2 Enables Reliable and Specific Detection of OMVs via Flow Cytometry
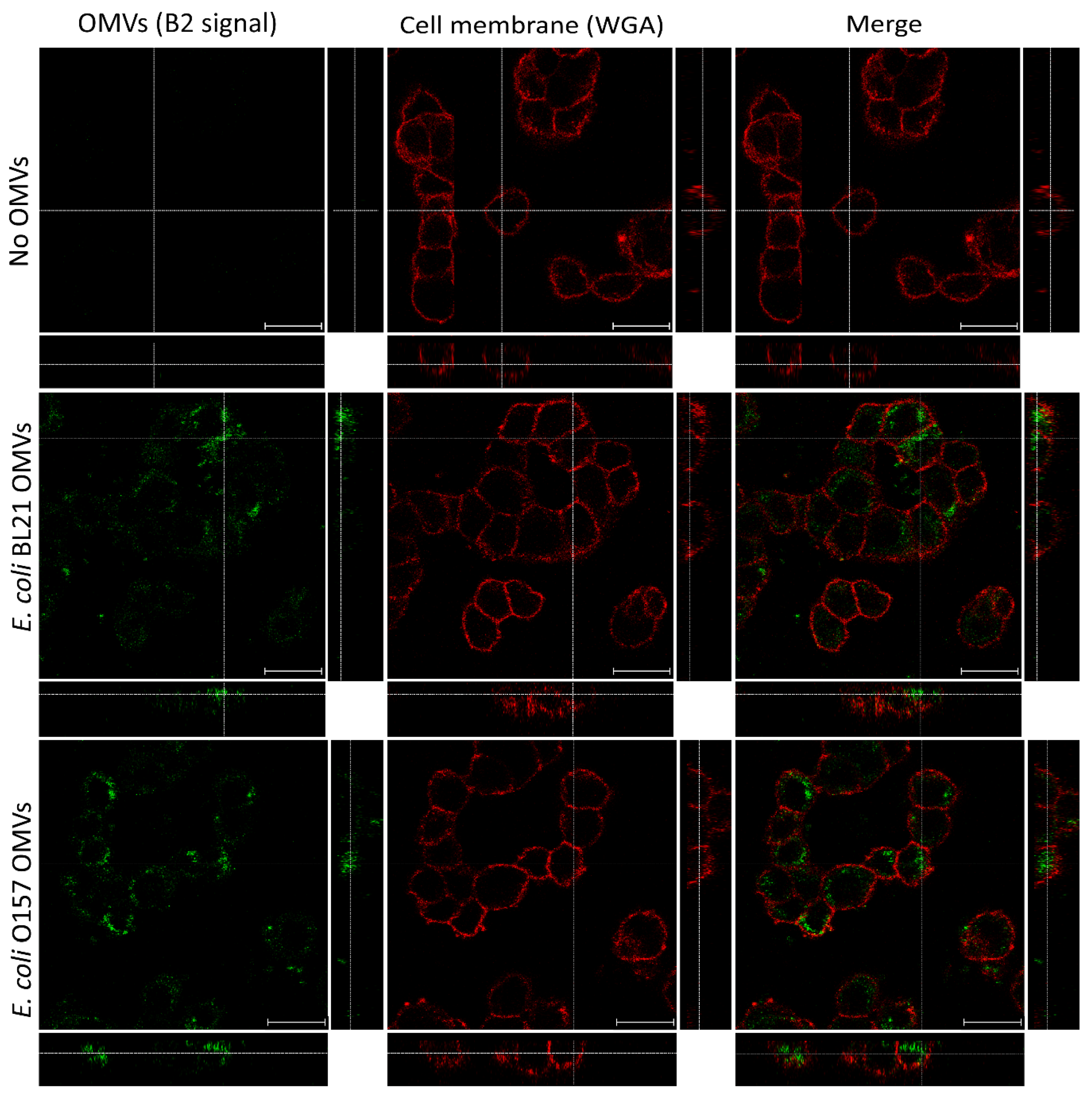
2.3. B2 Enables Precise Visualization of OMV Internalization in HT-29 Cells
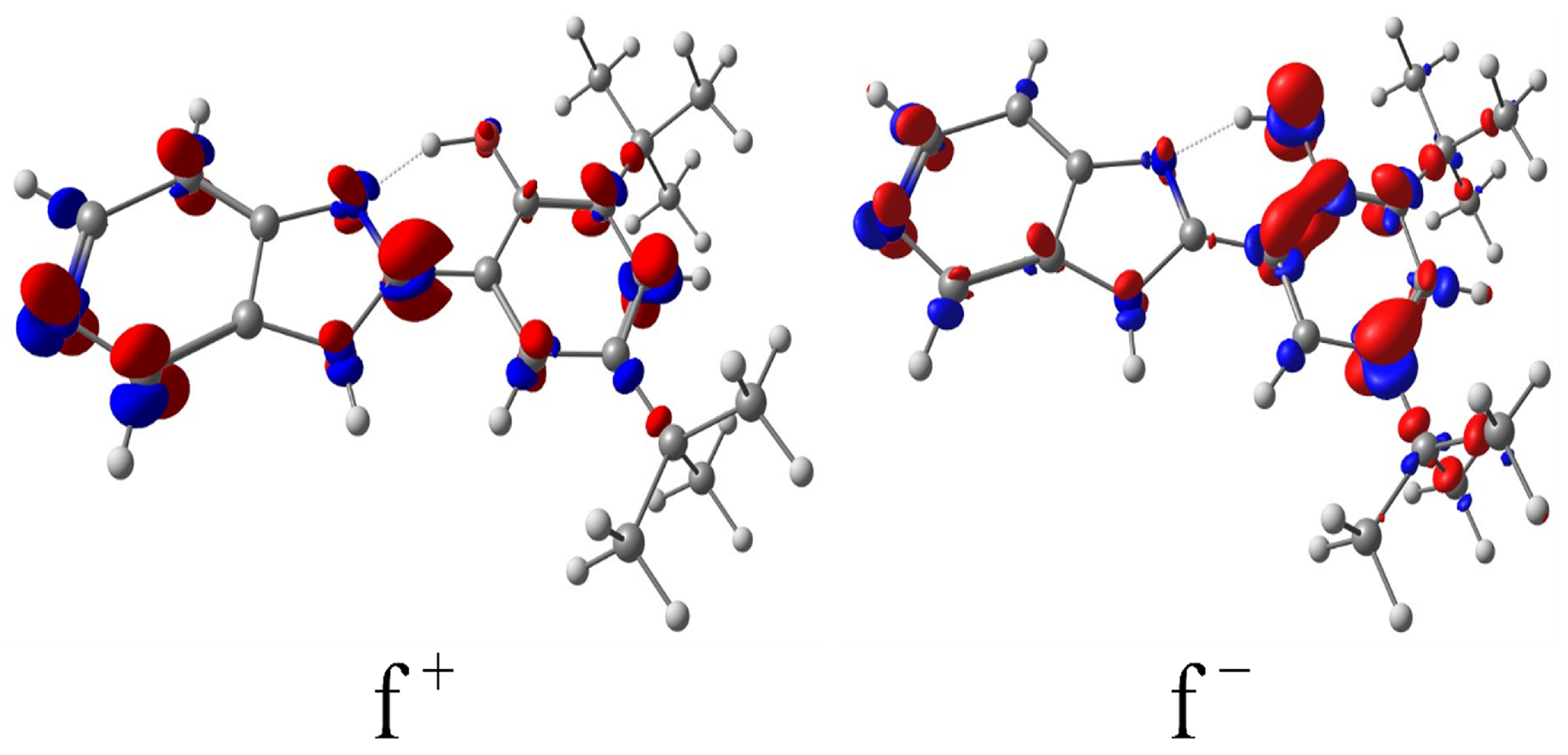
2.4. Quantum Chemistry Analysis: Density Functional Theory (DFT) Fukui Function
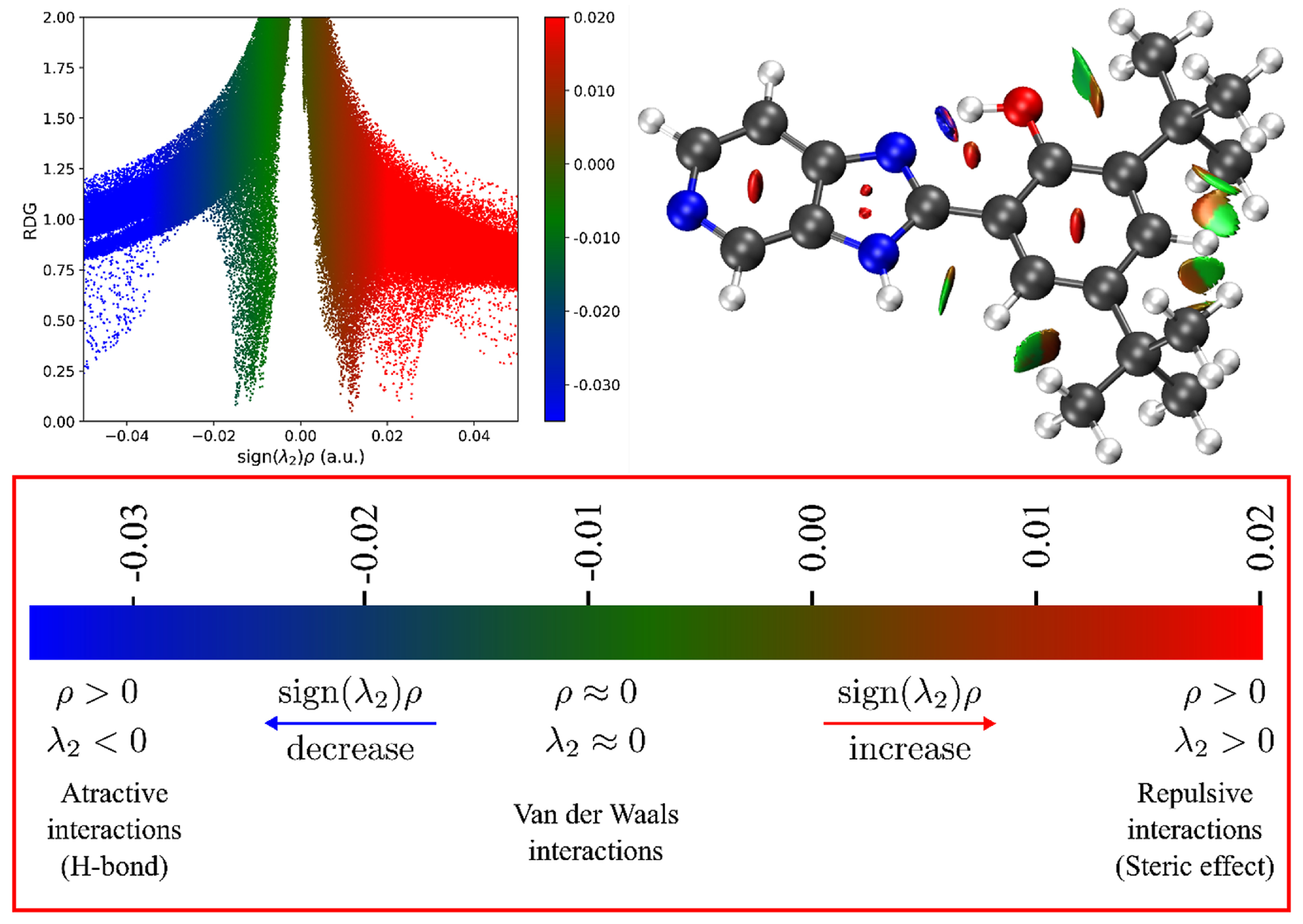
2.5. Quantum Chemistry Analysis: Non-Covalent Interaction Index (NCI)
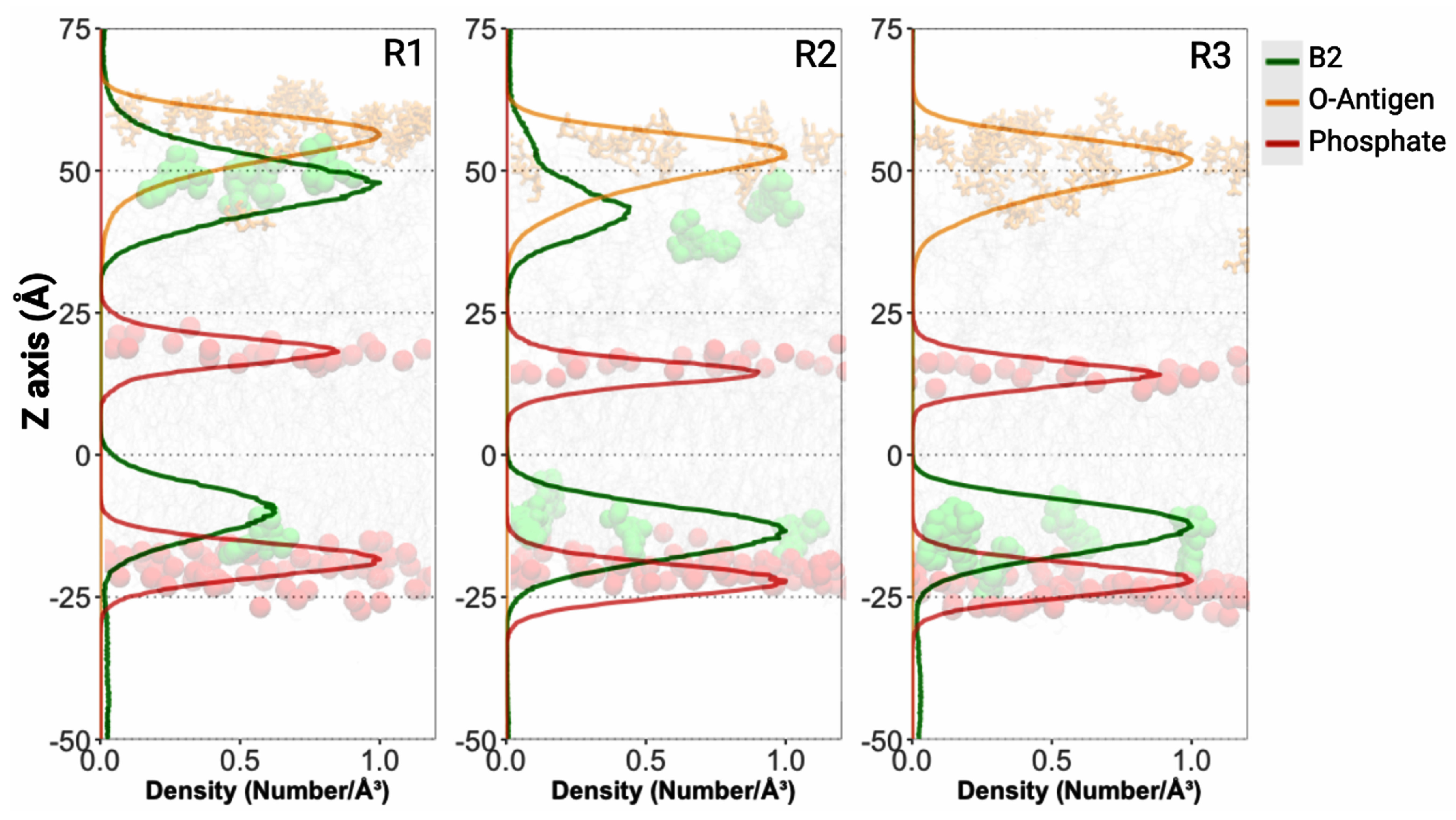
2.6. B2–OMV Interactions Characterized Through Molecular Dynamics Simulations
3. Discussion
4. Materials and Methods
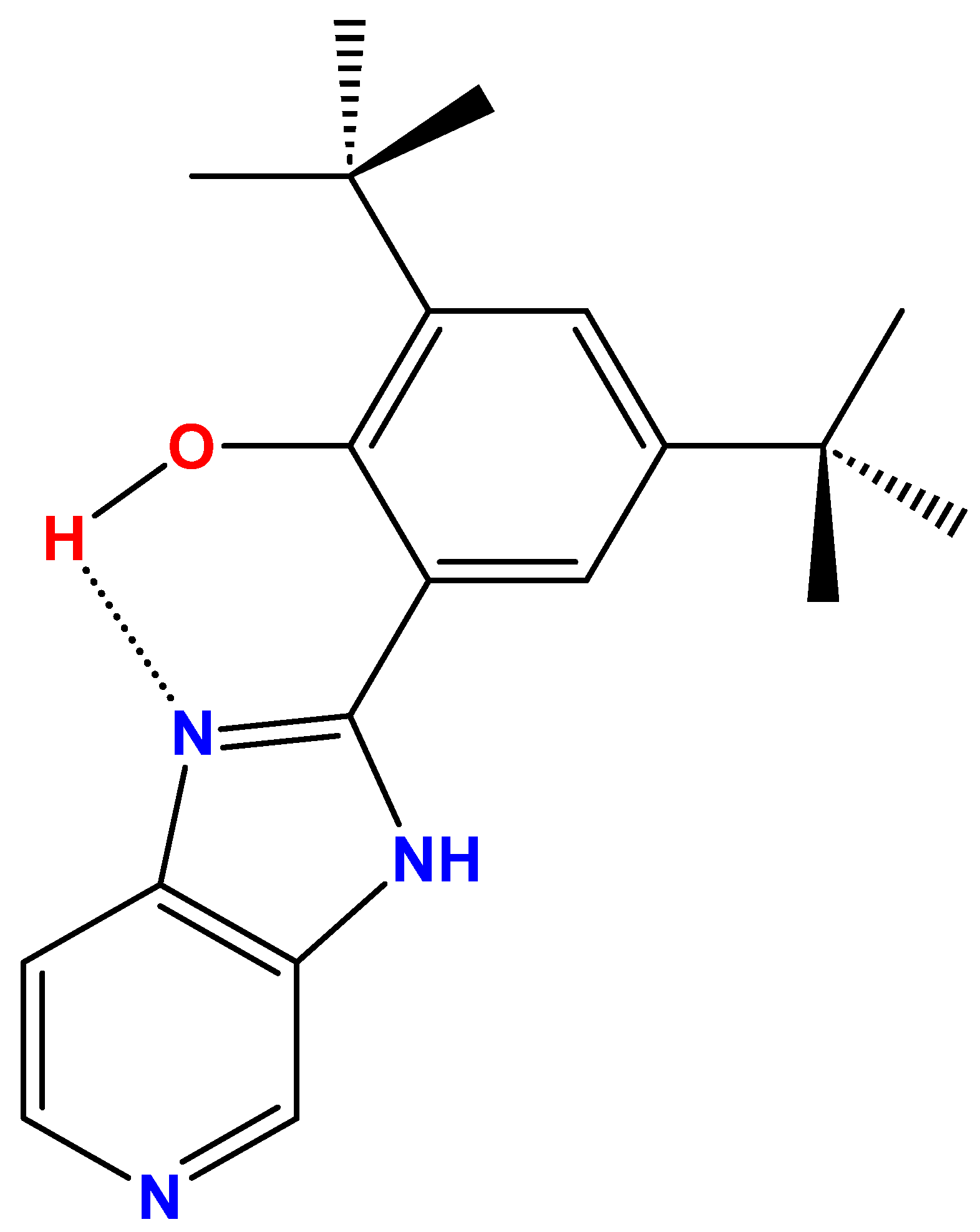
4.1. Synthesis and Characterization of B2
4.2. Bacterial Strains, Media, and Culture Conditions
4.3. OMV Isolation and Quantification
4.4. Transmission Electron Microscopy (TEM) of OMVs
4.5. OMV Fluorescent Labeling with B2
4.6. Flow Cytometry Analysis for Escherichia coli OMVs Stained with B2
4.7. Confocal Microscopy Analysis of HT-29 Cells with Escherichia coli OMVs Stained with B2
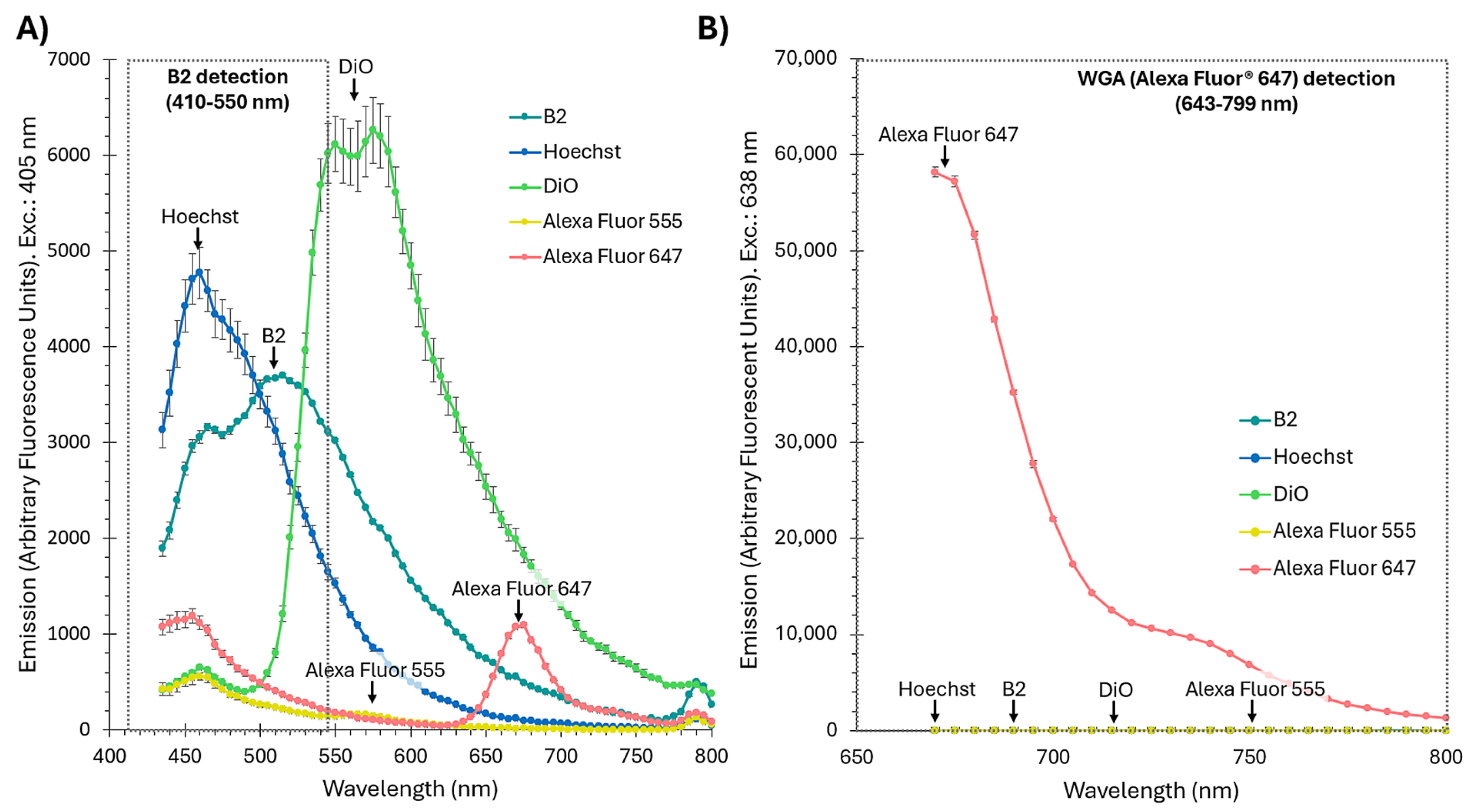
4.8. Fluorophore Emission Spectra Measurement
4.9. Quantum Chemical Studies
4.10. Molecular Dynamics Simulations of B2 Interacting with a Model OMV Membrane
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sartorio, M.G.; Pardue, E.J.; Feldman, M.F.; Haurat, M.F. Bacterial Outer Membrane Vesicles: From Discovery to Applications. Annu. Rev. Microbiol. 2021, 75, 609–630. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Zhao, G.; Wang, Z.; Zhang, X.; Wu, M.X.; Lu, M. Bacterial Membrane Vesicles: Physiological Roles, Infection Immunology, and Applications. Adv. Sci. 2023, 10, e2301357. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Nieh, M.P.; Chen, W.; Lei, Y. Outer membrane vesicles (OMVs) enabled bio-applications: A critical review. Biotechnol. Bioeng. 2022, 119, 34–47. [Google Scholar] [CrossRef]
- Magana, G.; Harvey, C.; Taggart, C.C.; Rodgers, A.M. Bacterial Outer Membrane Vesicles: Role in Pathogenesis and Host-Cell Interactions. Antibiotics 2023, 13, 32. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Cheng, X.; Feng, B.; Fan, D.; Liu, X.; Xie, R.; Luo, T.; Wegner, S.V.; Ma, D.; Chen, F.; et al. Engineering Versatile Bacteria-Derived Outer Membrane Vesicles: An Adaptable Platform for Advancing Cancer Immunotherapy. Adv. Sci. 2024, 11, e2400049. [Google Scholar] [CrossRef]
- Pathirana, R.D.; Kaparakis-Liaskos, M. Bacterial membrane vesicles: Biogenesis, immune regulation and pathogenesis. Cell. Microbiol. 2016, 18, 1518–1524. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.; Xiang, H.; Chen, Q.; Zhao, Y.; Gao, Q.; Huang, F.; Mao, L. A Review of Labeling Approaches Used in Small Extracellular Vesicles Tracing and Imaging. Int. J. Nanomed. 2023, 18, 4567–4588. [Google Scholar] [CrossRef]
- Puca, V.; Ercolino, E.; Celia, C.; Bologna, G.; Di Marzio, L.; Mincione, G.; Marchisio, M.; Miscia, S.; Muraro, R.; Lanuti, P.; et al. Detection and Quantification of eDNA-Associated Bacterial Membrane Vesicles by Flow Cytometry. Int. J. Mol. Sci. 2019, 20, 5307. [Google Scholar] [CrossRef]
- O’Donoghue, E.J.; Krachler, A.M. Mechanisms of outer membrane vesicle entry into host cells. Cell. Microbiol. 2016, 18, 1508–1517. [Google Scholar] [CrossRef]
- Verweij, F.J.; Balaj, L.; Boulanger, C.M.; Carter, D.R.F.; Compeer, E.B.; D’Angelo, G.; El Andaloussi, S.; Goetz, J.G.; Gross, J.C.; Hyenne, V.; et al. The power of imaging to understand extracellular vesicle biology in vivo. Nat. Methods 2021, 18, 1013–1026. [Google Scholar] [CrossRef]
- Goncalves, M.S. Fluorescent labeling of biomolecules with organic probes. Chem. Rev. 2009, 109, 190–212. [Google Scholar] [CrossRef]
- Xue, K.; Wang, L.; Liu, J. Bacterial outer membrane vesicles and their functionalization as vehicles for bioimaging, diagnosis and therapy. Mater. Adv. 2022, 3, 7185–7197. [Google Scholar] [CrossRef]
- Pužar Dominkuš, P.; Stenovec, M.; Sitar, S.; Lasič, E.; Zorec, R.; Plemenitaš, A.; Žagar, E.; Kreft, M.; Lenassi, M. PKH26 labeling of extracellular vesicles: Characterization and cellular internalization of contaminating PKH26 nanoparticles. Biochim. Biophys. Acta (BBA)-Biomembr. 2018, 1860, 1350–1361. [Google Scholar] [CrossRef]
- Liu, N.; Gujrati, V.; Werner, J.P.F.; Mishra, K.; Anzenhofer, P.; Stiel, A.C.; Mettenleiter, G.; Feuchtinger, A.; Walch, A.; Ntziachristos, V. Bacterial outer membrane vesicles as cationic dye carriers for optoacoustics-guided phototherapy of cancer. Cancer Nanotechnol. 2023, 14, 36. [Google Scholar] [CrossRef]
- Liao, Z.; Jaular, L.M.; Soueidi, E.; Jouve, M.; Muth, D.C.; Schøyen, T.H.; Seale, T.; Haughey, N.J.; Ostrowski, M.; Théry, C.; et al. Acetylcholinesterase is not a generic marker of extracellular vesicles. J. Extracell. Vesicles 2019, 8, 1628592. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, I.; Parra, F.; Rojas, D.; Silva, A.; Nevermann, J.; Otero, M.C.; Gil, F.; Calderón, I.L.; Fuentes, J.A. Hypervesiculation Meets Sec-Targeting: Enhancing Heterologous Protein Loading in Salmonella Typhi Outer Membrane Vesicles for Delivery and Immune Response. Int. J. Mol. Sci. 2025, 26, 4223. [Google Scholar] [CrossRef]
- Simonsen, J.B. Pitfalls associated with lipophilic fluorophore staining of extracellular vesicles for uptake studies. J. Extracell. Vesicles 2019, 8, 1582237. [Google Scholar] [CrossRef]
- Melling, G.E.; Conlon, R.; Pantazi, P.; Dellar, E.R.; Samuel, P.; Baena-Lopez, L.A.; Simpson, J.C.; Carter, D.R.F. Confocal microscopy analysis reveals that only a small proportion of extracellular vesicles are successfully labelled with commonly utilised staining methods. Sci. Rep. 2022, 12, 262. [Google Scholar] [CrossRef]
- Singh, A.N.; Nice, J.B.; Wu, M.; Brown, A.C.; Wittenberg, N.J. Multivariate Analysis of Individual Bacterial Outer Membrane Vesicles Using Fluorescence Microscopy. Chem. Biomed. Imaging 2024, 2, 352–361. [Google Scholar] [CrossRef]
- Baibek, A.; Konieczna, Z.; Ucuncu, M.; Alghamdi, Z.S.; Sharma, R.; Horrocks, M.H.; Bradley, M. A Long Fluorescence Lifetime Probe for Labeling of Gram-Negative Bacteria. Chem. Biomed. Imaging 2025, 3, 45–50. [Google Scholar] [CrossRef]
- Bayard, F.; Matile, S. Fluorescent Membrane Probes Obey the Israelachvili Rules. Helv. Chim. Acta 2024, 107, e202400062. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Sun, Y.; Xu, H.; Xu, Z.; Liang, X.; Yang, J.; Song, W.; Chen, M.; Fang, M. Evaluation of a biomarker (NO) dynamics in inflammatory zebrafish and periodontitis saliva samples via a fast-response and sensitive fluorescent probe. Bioorg. Chem. 2024, 143, 107014. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, J.; Rong, X.; Jiang, J.; Wang, Y.; Zhang, X.; Xu, Z.; Xu, K.; Wu, M.; Fang, M. Development of a hybrid rhodamine-hydrazine NIR fluorescent probe for sensitive detection and imaging of peroxynitrite in necrotizing enterocolitis model. Bioorg. Chem. 2024, 152, 107729. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, X.; Xi, D.; Zhang, Y.; Gao, X.; Xu, K.; Liu, H.; Fang, M. Precision Imaging of Biothiols in Live Cells and Treatment Evaluation during the Development of Liver Injury via a Near-Infrared Fluorescent Probe. Chem. Biomed. Imaging 2025, 3, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Carreno, A.; Gacitua, M.; Fuentes, J.A.; Paez-Hernandez, D.; Araneda, C.; Chavez, I.; Soto-Arriaza, M.; Manriquez, J.M.; Polanco, R.; Mora, G.C.; et al. Theoretical and experimental characterization of a novel pyridine benzimidazole: Suitability for fluorescence staining in cells and antimicrobial properties. New J. Chem. 2016, 40, 2362–2375. [Google Scholar] [CrossRef]
- Llancalahuen, F.M.; Fuentes, J.A.; Carreno, A.; Zuniga, C.; Paez-Hernandez, D.; Gacitua, M.; Polanco, R.; Preite, M.D.; Arratia-Perez, R.; Otero, C. New Properties of a Bioinspired Pyridine Benzimidazole Compound as a Novel Differential Staining Agent for Endoplasmic Reticulum and Golgi Apparatus in Fluorescence Live Cell Imaging. Front. Chem. 2018, 6, 345. [Google Scholar] [CrossRef]
- Carreno, A.; Gacitua, M.; Solis-Cespedes, E.; Paez-Hernandez, D.; Swords, W.B.; Meyer, G.J.; Preite, M.D.; Chavez, I.; Vega, A.; Fuentes, J.A. New Cationic fac-[Re(CO)3(deeb)B2]+ Complex, Where B2 Is a Benzimidazole Derivative, as a Potential New Luminescent Dye for Proteins Separated by SDS-PAGE. Front. Chem. 2021, 9, 647816. [Google Scholar] [CrossRef]
- Berrios, P.; Fuentes, J.A.; Salas, D.; Carreno, A.; Aldea, P.; Fernandez, F.; Trombert, A.N. Inhibitory effect of biofilm-forming Lactobacillus kunkeei strains against virulent Pseudomonas aeruginosa in vitro and in honeycomb moth (Galleria mellonella) infection model. Benef. Microbes 2018, 9, 257–268. [Google Scholar] [CrossRef]
- Akmayan, I.; Ozturk, A.B.; Ozbek, T. Recombinant proteins production in Escherichia coli BL21 for vaccine applications: A cost estimation of potential industrial-scale production scenarios. Prep. Biochem. Biotechnol. 2024, 54, 932–945. [Google Scholar] [CrossRef]
- Bruballa, A.C.; Shiromizu, C.M.; Bernal, A.M.; Pineda, G.E.; Sabbione, F.; Trevani, A.S.; Bentancor, L.V.; Ramos, M.V.; Fernandez-Brando, R.J.; Munoz, M.J.; et al. Role of Shiga Toxins in Cytotoxicity and Immunomodulatory Effects of Escherichia coli O157:H7 during Host-Bacterial Interactions in vitro. Toxins 2020, 12, 48. [Google Scholar] [CrossRef]
- Nevermann, J.; Silva, A.; Otero, C.; Oyarzun, D.P.; Barrera, B.; Gil, F.; Calderon, I.L.; Fuentes, J.A. Identification of Genes Involved in Biogenesis of Outer Membrane Vesicles (OMVs) in Salmonella enterica Serovar Typhi. Front. Microbiol. 2019, 10, 104. [Google Scholar] [CrossRef]
- Marchant, P.; Carreno, A.; Vivanco, E.; Silva, A.; Nevermann, J.; Otero, C.; Araya, E.; Gil, F.; Calderon, I.L.; Fuentes, J.A. “One for All”: Functional Transfer of OMV-Mediated Polymyxin B Resistance From Salmonella enterica sv. Typhi DtolR and DdegS to Susceptible Bacteria. Front. Microbiol. 2021, 12, 672467. [Google Scholar] [CrossRef] [PubMed]
- Marchant, P.; Vivanco, E.; Silva, A.; Nevermann, J.; Fuentes, I.; Barrera, B.; Otero, C.; Calderon, I.L.; Gil, F.; Fuentes, J.A. b-lactam-induced OMV release promotes polymyxin tolerance in Salmonella enterica sv. Typhi. Front. Microbiol. 2024, 15, 1389663. [Google Scholar] [CrossRef]
- Gupta, R.; Patey, G.N. Aggregation in dilute aqueous tert-butyl alcohol solutions: Insights from large-scale simulations. J. Chem. Phys. 2012, 137, 034509. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Shao, J.; Abdelmohsen, L.K.E.A.; van Hest, J.C.M. Amphiphilic AIEgen-polymer aggregates: Design, self-assembly and biomedical applications. Aggregate 2021, 3, e128. [Google Scholar] [CrossRef]
- de Rond, L.; van der Pol, E.; Bloemen, P.R.; Van Den Broeck, T.; Monheim, L.; Nieuwland, R.; van Leeuwen, T.G.; Coumans, F.A.W. A Systematic Approach to Improve Scatter Sensitivity of a Flow Cytometer for Detection of Extracellular Vesicles. Cytom. A 2020, 97, 582–591. [Google Scholar] [CrossRef]
- Chattopadhyay, P.K.; Gaylord, B.; Palmer, A.; Jiang, N.; Raven, M.A.; Lewis, G.; Reuter, M.A.; Nur-ur Rahman, A.K.; Price, D.A.; Betts, M.R.; et al. Brilliant violet fluorophores: A new class of ultrabright fluorescent compounds for immunofluorescence experiments. Cytom. A 2012, 81, 456–466. [Google Scholar] [CrossRef]
- Lee, E.Y.; Bang, J.Y.; Park, G.W.; Choi, D.S.; Kang, J.S.; Kim, H.J.; Park, K.S.; Lee, J.O.; Kim, Y.K.; Kwon, K.H.; et al. Global proteomic profiling of native outer membrane vesicles derived from Escherichia coli. Proteomics 2007, 7, 3143–3153. [Google Scholar] [CrossRef]
- Kulp, A.J.; Sun, B.; Ai, T.; Manning, A.J.; Orench-Rivera, N.; Schmid, A.K.; Kuehn, M.J. Genome-Wide Assessment of Outer Membrane Vesicle Production in Escherichia coli. PLoS ONE 2015, 10, e0139200. [Google Scholar] [CrossRef]
- Tian, Y.; Huang, S.; Zhou, G.; Fei, X.; Li, Y.A.; Li, Q.; Wang, S.; Shi, H. Evaluation of immunogenicity and protective efficacy of outer membrane vesicles from Salmonella Typhimurium and Salmonella Choleraesuis. Vet. Microbiol. 2024, 294, 110131. [Google Scholar] [CrossRef]
- Ling, Z.; Dayong, C.; Denggao, Y.; Yiting, W.; Liaoqiong, F.; Zhibiao, W. Escherichia Coli Outer Membrane Vesicles Induced DNA Double-Strand Breaks in Intestinal Epithelial Caco-2 Cells. Med. Sci. Monit. Basic. Res. 2019, 25, 45–52. [Google Scholar] [CrossRef]
- Canas, M.A.; Fabrega, M.J.; Gimenez, R.; Badia, J.; Baldoma, L. Outer Membrane Vesicles From Probiotic and Commensal Escherichia coli Activate NOD1-Mediated Immune Responses in Intestinal Epithelial Cells. Front. Microbiol. 2018, 9, 498. [Google Scholar] [CrossRef] [PubMed]
- Pasqua, M.; Zennaro, A.; Trirocco, R.; Fanelli, G.; Micheli, G.; Grossi, M.; Colonna, B.; Prosseda, G. Modulation of OMV Production by the Lysis Module of the DLP12 Defective Prophage of Escherichia coli K12. Microorganisms 2021, 9, 369. [Google Scholar] [CrossRef]
- Bielaszewska, M.; Greune, L.; Bauwens, A.; Dersch, P.; Mellmann, A.; Ruter, C. Virulence Factor Cargo and Host Cell Interactions of Shiga Toxin-Producing Escherichia coli Outer Membrane Vesicles. Methods Mol. Biol. 2021, 2291, 177–205. [Google Scholar] [CrossRef]
- Zamora, P.P.; Bieger, K.; Cuchillo, A.; Tello, A.; Muena, J.P. Theoretical determination of a reaction intermediate: Fukui function analysis, dual reactivity descriptor and activation energy. J. Mol. Struct. 2021, 1227, 129369. [Google Scholar] [CrossRef]
- Fontanini, R.E.; Flores-Moreno, R.; Zuniga-Gutierrez, B.A.; Kaya, S.; Katin, K.P.; Maslov, M.M.; Kochaev, A. Semiempirical Approach to the Fukui Function Analysis of Uric Acid under Different pH Conditions. J. Phys. Chem. A 2023, 127, 8228–8237. [Google Scholar] [CrossRef] [PubMed]
- Pucci, R.; Angilella, G.G.N. Density functional theory, chemical reactivity, and the Fukui functions. Found. Chem. 2022, 24, 59–71. [Google Scholar] [CrossRef]
- Ohkatsu, Y.; Ishikawa, S.-i.; Tobita, E. Consideration on the effect of ortho-substituents of phenols by semiempirical molecular orbital method MOPAC. Polym. Degrad. Stab. 2000, 67, 541–545. [Google Scholar] [CrossRef]
- Kajiyama, T.; Ohkatsu, Y. Effect of para-substituents of phenolic antioxidants. Polym. Degrad. Stab. 2001, 71, 445–452. [Google Scholar] [CrossRef]
- Novoa, T.; Laplaza, R.; Peccati, F.; Fuster, F.; Contreras-Garcia, J. The NCIWEB Server: A Novel Implementation of the Noncovalent Interactions Index for Biomolecular Systems. J. Chem. Inf. Model. 2023, 63, 4483–4489. [Google Scholar] [CrossRef]
- Denayer, M.; Vekeman, J.; Tielens, F.; De Proft, F. Towards a predictive model for polymer solubility using the noncovalent interaction index: Polyethylene as a case study. Phys. Chem. Chem. Phys. 2021, 23, 25374–25387. [Google Scholar] [CrossRef]
- Contreras-García, J.; Boto, R.A.; Izquierdo-Ruiz, F.; Reva, I.; Woller, T.; Alonso, M. A benchmark for the non-covalent interaction (NCI) index or… is it really all in the geometry? Theor. Chem. Acc. 2016, 135, 242. [Google Scholar] [CrossRef]
- Carreno, A.; Paez-Hernandez, D.; Cantero-Lopez, P.; Zuniga, C.; Nevermann, J.; Ramirez-Osorio, A.; Gacitua, M.; Oyarzun, P.; Saez-Cortez, F.; Polanco, R.; et al. Structural Characterization, DFT Calculation, NCI, Scan-Rate Analysis and Antifungal Activity against Botrytis cinerea of (E)-2-{[(2-Aminopyridin-2-yl)imino]-methyl}-4,6-di-tert-butylphenol (Pyridine Schiff Base). Molecules 2020, 25, 2741. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Li, Q.; Chen, X.; Onyango, S.; Xie, J.; Nie, S. Bacterial extracellular vesicles: Vital contributors to physiology from bacteria to host. Microbiol. Res. 2024, 284, 127733. [Google Scholar] [CrossRef]
- Qing, G.; Gong, N.; Chen, X.; Chen, J.; Zhang, H.; Wang, Y.; Wang, R.; Zhang, S.; Zhang, Z.; Zhao, X.; et al. Natural and engineered bacterial outer membrane vesicles. Biophys. Rep. 2019, 5, 184–198. [Google Scholar] [CrossRef]
- Lei, E.K.; Azmat, A.; Henry, K.A.; Hussack, G. Outer membrane vesicles as a platform for the discovery of antibodies to bacterial pathogens. Appl. Microbiol. Biotechnol. 2024, 108, 232. [Google Scholar] [CrossRef] [PubMed]
- Juodeikis, R.; Carding, S.R. Outer Membrane Vesicles: Biogenesis, Functions, and Issues. Microbiol. Mol. Biol. Rev. MMBR 2022, 86, e0003222. [Google Scholar] [CrossRef]
- Wang, F.; Ho, P.Y.; Kam, C.; Yang, Q.; Liu, J.; Wang, W.; Zhao, E.; Chen, S. An AIE-active probe for efficient detection and high-throughput identification of outer membrane vesicles. Aggregate 2023, 4, e312. [Google Scholar] [CrossRef]
- Kim, C.J.; Kuczler, M.D.; Dong, L.; Kim, J.; Amend, S.R.; Cho, Y.K.; Pienta, K.J. Extracellular Vesicle Uptake Assay via Confocal Microscope Imaging Analysis. J. Vis. Exp. 2022, 180, e62836. [Google Scholar] [CrossRef]
- Gerritzen, M.J.H.; Martens, D.E.; Wijffels, R.H.; Stork, M. High throughput nanoparticle tracking analysis for monitoring outer membrane vesicle production. J. Extracell. Vesicles 2017, 6, 1333883. [Google Scholar] [CrossRef]
- Kameli, N.; Borman, R.; Lpez-Iglesias, C.; Savelkoul, P.; Stassen, F.R.M. Characterization of Feces-Derived Bacterial Membrane Vesicles and the Impact of Their Origin on the Inflammatory Response. Front. Cell Infect. Microbiol. 2021, 11, 667987. [Google Scholar] [CrossRef]
- Yang, J.; Xu, Z.; Yu, L.; Wang, B.; Hu, R.; Tang, J.; Lv, J.; Xiao, H.; Tan, X.; Wang, G.; et al. Organic Fluorophores with Large Stokes Shift for the Visualization of Rapid Protein and Nucleic Acid Assays. Angew. Chem. Int. Ed. Engl. 2024, 63, e202318800. [Google Scholar] [CrossRef] [PubMed]
- Sednev, M.V.; Belov, V.N.; Hell, S.W. Fluorescent dyes with large Stokes shifts for super-resolution optical microscopy of biological objects: A review. Methods Appl. Fluoresc. 2015, 3, 042004. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, G.; Ke, G.; Ren, T.B.; Yuan, L. Organic Fluorophores with Large Stokes Shift for Bioimaging and Biosensing. ChemPhotoChem 2024, 8, e202300277. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, Z.; Xia, F.; Wen, Z.; Chen, R.; Zhu, D.; Wang, M.; Zhuge, X.; Dai, J. Reductions in bacterial viability stimulate the production of Extra-intestinal Pathogenic Escherichia coli (ExPEC) cytoplasm-carrying Extracellular Vesicles (EVs). PLoS Pathog. 2022, 18, e1010908. [Google Scholar] [CrossRef] [PubMed]
- Brameyer, S.; Plener, L.; Muller, A.; Klingl, A.; Wanner, G.; Jung, K. Outer Membrane Vesicles Facilitate Trafficking of the Hydrophobic Signaling Molecule CAI-1 between Vibrio harveyi Cells. J. Bacteriol. 2018, 200, 10–1128. [Google Scholar] [CrossRef]
- Richter, M.; Vader, P.; Fuhrmann, G. Approaches to surface engineering of extracellular vesicles. Adv. Drug Deliv. Rev. 2021, 173, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Chi, W.; Sun, P.P. Restriction of photoinduced electron transfer as a mechanism for the aggregation-induced emission of a trityl-functionalised maleimide fluorophore. Phys. Chem. Chem. Phys. 2023, 25, 4193–4200. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J.; Du, L.; Ma, C.; Leung, N.L.C.; Niu, Y.; Qin, A.; Sun, J.; Peng, Q.; Sung, H.H.Y.; et al. Drawing a clear mechanistic picture for the aggregation-induced emission process. Mater. Chem. Front. 2019, 3, 1143–1150. [Google Scholar] [CrossRef]
- Jiang, G.; Liu, H.; Liu, H.; Ke, G.; Ren, T.B.; Xiong, B.; Zhang, X.B.; Yuan, L. Chemical Approaches to Optimize the Properties of Organic Fluorophores for Imaging and Sensing. Angew. Chem. 2023, 136, e202315217. [Google Scholar] [CrossRef]
- Qi, J.; Hu, X.; Dong, X.; Lu, Y.; Lu, H.; Zhao, W.; Wu, W. Towards more accurate bioimaging of drug nanocarriers: Turning aggregation-caused quenching into a useful tool. Adv. Drug Deliv. Rev. 2019, 143, 206–225. [Google Scholar] [CrossRef]
- Arraud, N.; Gounou, C.; Turpin, D.; Brisson, A.R. Fluorescence triggering: A general strategy for enumerating and phenotyping extracellular vesicles by flow cytometry. Cytom. A 2016, 89, 184–195. [Google Scholar] [CrossRef]
- de Rond, L.; van der Pol, E.; Hau, C.M.; Varga, Z.; Sturk, A.; van Leeuwen, T.G.; Nieuwland, R.; Coumans, F.A.W. Comparison of Generic Fluorescent Markers for Detection of Extracellular Vesicles by Flow Cytometry. Clin. Chem. 2018, 64, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Arkesteijn, G.J.A.; Bremer, M.; Cimorelli, M.; Dignat-George, F.; Giebel, B.; Gorgens, A.; Hendrix, A.; Kuiper, M.; Lacroix, R.; et al. A compendium of single extracellular vesicle flow cytometry. J. Extracell. Vesicles 2023, 12, e12299. [Google Scholar] [CrossRef]
- Gankema, A.A.F.; Li, B.; Nieuwland, R.; Pol, E.V. Automated fluorescence gating and size determination reduce variation in measured concentration of extracellular vesicles by flow cytometry. Cytom. A 2022, 101, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Tertel, T.; Schoppet, M.; Stambouli, O.; Al-Jipouri, A.; James, P.F.; Giebel, B. Imaging flow cytometry challenges the usefulness of classically used extracellular vesicle labeling dyes and qualifies the novel dye Exoria for the labeling of mesenchymal stromal cell-extracellular vesicle preparations. Cytotherapy 2022, 24, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Kuerban, K.; Gao, X.; Zhang, H.; Liu, J.; Dong, M.; Wu, L.; Ye, R.; Feng, M.; Ye, L. Doxorubicin-loaded bacterial outer-membrane vesicles exert enhanced anti-tumor efficacy in non-small-cell lung cancer. Acta Pharm. Sin. B 2020, 10, 1534–1548. [Google Scholar] [CrossRef]
- Cecil, J.D.; O’Brien-Simpson, N.M.; Lenzo, J.C.; Holden, J.A.; Singleton, W.; Perez-Gonzalez, A.; Mansell, A.; Reynolds, E.C. Outer Membrane Vesicles Prime and Activate Macrophage Inflammasomes and Cytokine Secretion In Vitro and In Vivo. Front. Immunol. 2017, 8, 1017. [Google Scholar] [CrossRef]
- Zhuang, W.R.; Wang, Y.; Lei, Y.; Zuo, L.; Jiang, A.; Wu, G.; Nie, W.; Huang, L.L.; Xie, H.Y. Phytochemical Engineered Bacterial Outer Membrane Vesicles for Photodynamic Effects Promoted Immunotherapy. Nano Lett. 2022, 22, 4491–4500. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, R.; Cheng, K.; Zhang, K.; Wang, Y.; Zhang, Y.; Li, Y.; Liu, G.; Xu, J.; Xu, J.; et al. Bacterial Outer Membrane Vesicles Presenting Programmed Death 1 for Improved Cancer Immunotherapy via Immune Activation and Checkpoint Inhibition. ACS Nano 2020, 14, 16698–16711. [Google Scholar] [CrossRef]
- Bielaszewska, M.; Ruter, C.; Bauwens, A.; Greune, L.; Jarosch, K.A.; Steil, D.; Zhang, W.; He, X.; Lloubes, R.; Fruth, A.; et al. Host cell interactions of outer membrane vesicle-associated virulence factors of enterohemorrhagic Escherichia coli O157: Intracellular delivery, trafficking and mechanisms of cell injury. PLoS Pathog. 2017, 13, e1006159. [Google Scholar] [CrossRef] [PubMed]
- Maddipatla, R.; Tankam, P. Development of high-speed, integrated high-resolution optical coherence microscopy and dual-channel fluorescence microscopy for the simultaneous co-registration of reflectance and fluorescence signals. Opt. Lasers Eng. 2022, 149, 106823. [Google Scholar] [CrossRef]
- Song, D.; Ma, S. ChemInform Abstract: Recent Development of Benzimidazole-Containing Antibacterial Agents. ChemInform 2016, 47, 22. [Google Scholar] [CrossRef]
- Ates-Alagoz, Z. Antimicrobial Activities of 1-H-Benzimidazole-based Molecules. Curr. Top. Med. Chem. 2016, 16, 2953–2962. [Google Scholar] [CrossRef]
- Arumugam, N.; Abdul Rahim, A.S.; Osman, H.; Rosli, M.M.; Fun, H.K. Ethyl 1-tert-butyl-2-(4-hydr-oxy-3-methoxy-phen-yl)-1H-benzimidazole-5-carboxyl-ate. Acta Crystallogr. Sect. E Struct. Rep. Online 2010, 66 Pt 5, o1051–o1052. [Google Scholar] [CrossRef]
- Abonia, R.; Rengifo, E.; Cobo, J.; Low, J.N.; Glidewell, C. Hydrogen-bonded chains in 3-tert-butyl-5-[(4-methoxybenzyl)amino]-1-phenyl-1H-pyrazole and tetramolecular hydrogen-bonded aggregates in 5-[(benzotriazol-1-ylmethyl)(4-methoxybenzyl)amino]-3-tert-butyl-1-phenyl-1H-pyrazole. Acta Crystallogr. C 2007, 63, o29–o32. [Google Scholar] [CrossRef]
- Rossos, G.; Hadjikakou, S.K.; Kourkoumelis, N. Molecular Dynamics Simulation of 2-Benzimidazolyl-Urea with DPPC Lipid Membrane and Comparison with a Copper(II) Complex Derivative. Membranes 2021, 11, 743. [Google Scholar] [CrossRef]
- Benisvy, L.; Bill, E.; Blake, A.J.; Collison, D.; Davies, E.S.; Garner, C.D.; Guindy, C.I.; McInnes, E.J.; McArdle, G.; McMaster, J.; et al. Phenolate and phenoxyl radical complexes of Co(II) and Co(III). Dalton Trans. 2004, 21, 3647–3653. [Google Scholar] [CrossRef]
- Benisvy, L.; Blake, A.J.; Collison, D.; Davies, E.S.; Garner, C.D.; McInnes, E.J.; McMaster, J.; Whittaker, G.; Wilson, C. A phenoxyl radical complex of copper(II). Chem. Commun. 2001, 18, 1824–1825. [Google Scholar] [CrossRef]
- Bielaszewska, M.; Ruter, C.; Kunsmann, L.; Greune, L.; Bauwens, A.; Zhang, W.; Kuczius, T.; Kim, K.S.; Mellmann, A.; Schmidt, M.A.; et al. Enterohemorrhagic Escherichia coli hemolysin employs outer membrane vesicles to target mitochondria and cause endothelial and epithelial apoptosis. PLoS Pathog. 2013, 9, e1003797. [Google Scholar] [CrossRef]
- Turner, L.; Bitto, N.J.; Steer, D.L.; Lo, C.; D’Costa, K.; Ramm, G.; Shambrook, M.; Hill, A.F.; Ferrero, R.L.; Kaparakis-Liaskos, M. Helicobacter pylori Outer Membrane Vesicle Size Determines Their Mechanisms of Host Cell Entry and Protein Content. Front. Immunol. 2018, 9, 1466. [Google Scholar] [CrossRef]
- Canas, M.A.; Gimenez, R.; Fabrega, M.J.; Toloza, L.; Baldoma, L.; Badia, J. Outer Membrane Vesicles from the Probiotic Escherichia coli Nissle 1917 and the Commensal ECOR12 Enter Intestinal Epithelial Cells via Clathrin-Dependent Endocytosis and Elicit Differential Effects on DNA Damage. PLoS ONE 2016, 11, e0160374. [Google Scholar] [CrossRef]
- Marzoog, T.R.; Jabir, M.S.; Ibraheem, S.; Jawad, S.F.; Hamzah, S.S.; Sulaiman, G.M.; Mohammed, H.A.; Khan, R.A. Bacterial extracellular vesicles induced oxidative stress and mitophagy through mTOR pathways in colon cancer cells, HT-29: Implications for bioactivity. Biochim. Biophys. Acta Mol. Cell Res. 2023, 1870, 119486. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A Gen. Phys. 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Meelua, W.; Linnolahti, M.; Jitonnom, J. Mechanism of cationic ring-opening polymerisation of epsilon-caprolactone using metallocene/borate catalytic systems: A DFT and NCI study on chain initiation, propagation and termination. RSC Adv. 2024, 14, 11715–11727. [Google Scholar] [CrossRef] [PubMed]
- Hosseinpoor, S.; Pourayoubi, M.; Abrishami, M.; Sobati, M.; Karimi Ahmadabad, F.; Sabbaghi, F.; Nečas, M.; Dušek, M.; Kučeráková, M.; Kaur, M. Competing and directing interactions in new phosphoramide/thiophosphoramide structures: Energy considerations and evidence for CH···HC contacts and aliphatic–aromatic stacking. CrystEngComm 2023, 25, 2557–2569. [Google Scholar] [CrossRef]
- Feng, S.; Park, S.; Choi, Y.K.; Im, W. CHARMM-GUI Membrane Builder: Past, Current, and Future Developments and Applications. J. Chem. Theory Comput. 2023, 19, 2161–2185. [Google Scholar] [CrossRef]
- Pogozheva, I.D.; Armstrong, G.A.; Kong, L.; Hartnagel, T.J.; Carpino, C.A.; Gee, S.E.; Picarello, D.M.; Rubin, A.S.; Lee, J.; Park, S.; et al. Comparative Molecular Dynamics Simulation Studies of Realistic Eukaryotic, Prokaryotic, and Archaeal Membranes. J. Chem. Inf. Model. 2022, 62, 1036–1051. [Google Scholar] [CrossRef]
- Case, D.A.; Aktulga, H.M.; Belfon, K.; Ben-Shalom, I.Y.; Berryman, J.T.; Brozell, S.R.; Cerutti, D.S.; Cheatham, T.E.; Cisneros, G.A.I.; Cruzeiro, V.W.D.; et al. Amber 2024; Universidad de California: San Diego, CA, USA, 2024. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parra, F.; Carreño, A.; Ancede-Gallardo, E.; Majluf, D.; Soto, J.A.; Sepúlveda, R.V.; Aguayo, D.; Otero, M.C.; Calderón, I.L.; Gil, F.; et al. Benzimidazole-Derived B2 as a Fluorescent Probe for Bacterial Outer Membrane Vesicle (OMV) Labeling: Integrating DFT, Molecular Dynamics, Flow Cytometry, and Confocal Microscopy. Int. J. Mol. Sci. 2025, 26, 4682. https://doi.org/10.3390/ijms26104682
Parra F, Carreño A, Ancede-Gallardo E, Majluf D, Soto JA, Sepúlveda RV, Aguayo D, Otero MC, Calderón IL, Gil F, et al. Benzimidazole-Derived B2 as a Fluorescent Probe for Bacterial Outer Membrane Vesicle (OMV) Labeling: Integrating DFT, Molecular Dynamics, Flow Cytometry, and Confocal Microscopy. International Journal of Molecular Sciences. 2025; 26(10):4682. https://doi.org/10.3390/ijms26104682
Chicago/Turabian StyleParra, Francisco, Alexander Carreño, Evys Ancede-Gallardo, Diana Majluf, Jorge A. Soto, Romina V. Sepúlveda, Daniel Aguayo, María Carolina Otero, Iván L. Calderón, Fernando Gil, and et al. 2025. "Benzimidazole-Derived B2 as a Fluorescent Probe for Bacterial Outer Membrane Vesicle (OMV) Labeling: Integrating DFT, Molecular Dynamics, Flow Cytometry, and Confocal Microscopy" International Journal of Molecular Sciences 26, no. 10: 4682. https://doi.org/10.3390/ijms26104682
APA StyleParra, F., Carreño, A., Ancede-Gallardo, E., Majluf, D., Soto, J. A., Sepúlveda, R. V., Aguayo, D., Otero, M. C., Calderón, I. L., Gil, F., & Fuentes, J. A. (2025). Benzimidazole-Derived B2 as a Fluorescent Probe for Bacterial Outer Membrane Vesicle (OMV) Labeling: Integrating DFT, Molecular Dynamics, Flow Cytometry, and Confocal Microscopy. International Journal of Molecular Sciences, 26(10), 4682. https://doi.org/10.3390/ijms26104682








