Unraveling the Metabolic Pathways Between Metabolic-Associated Fatty Liver Disease (MAFLD) and Sarcopenia
Abstract
1. Introduction
2. Literature Search
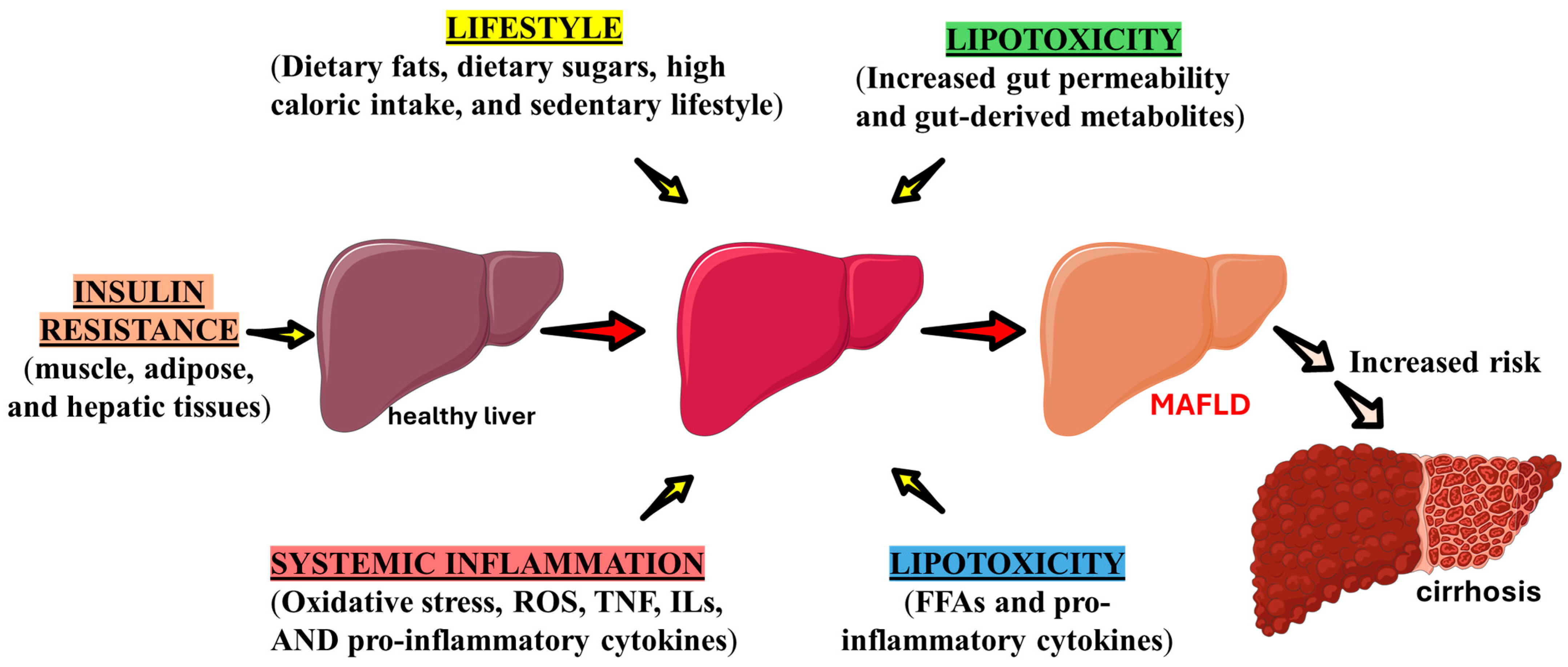
3. MAFLD, Inflammatory Processes, Oxidative Stress, and Mitochondrial Dysfunction
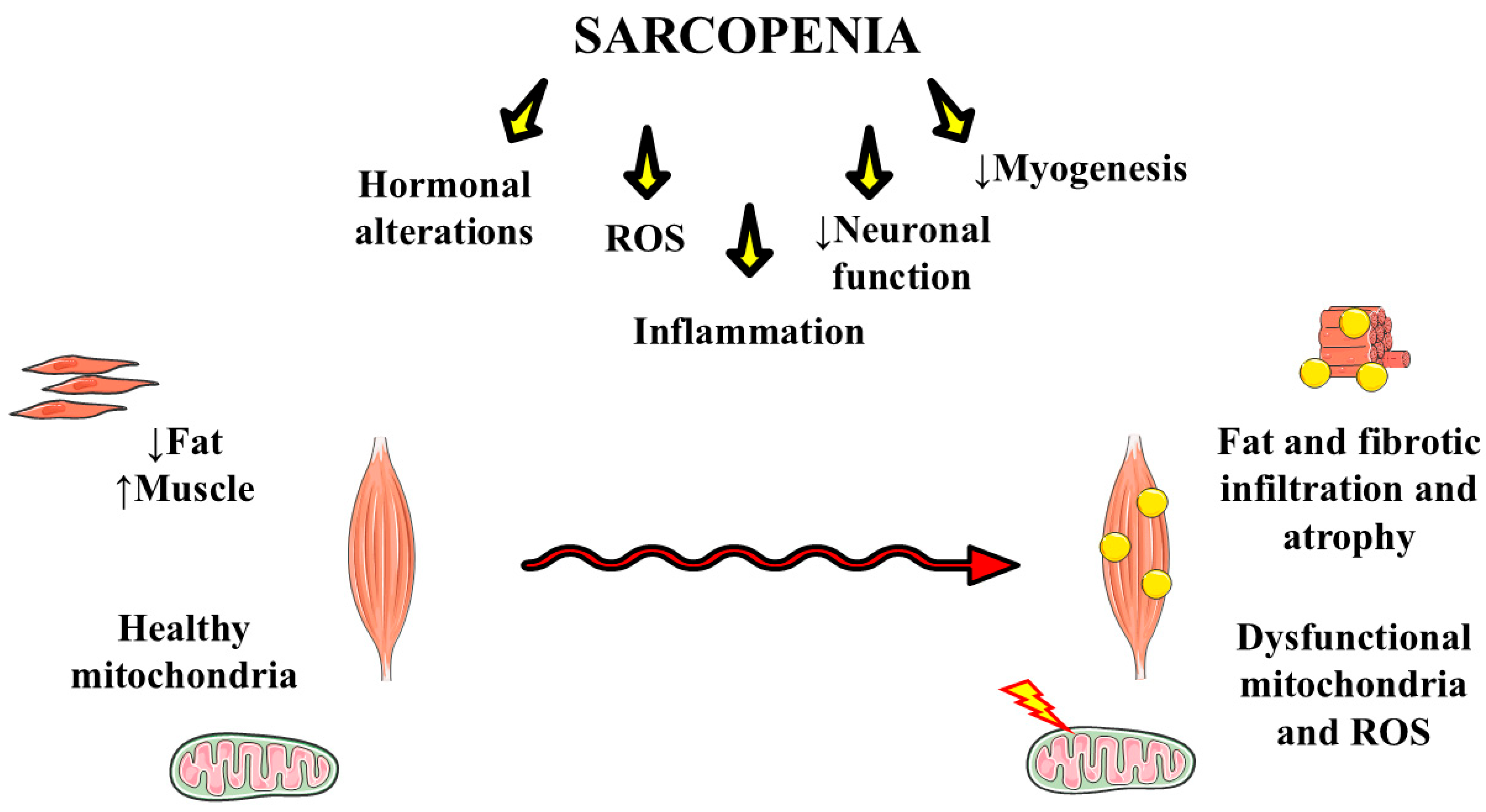
4. Sarcopenia
Sarcopenia, Oxidative, and Inflammation Processes
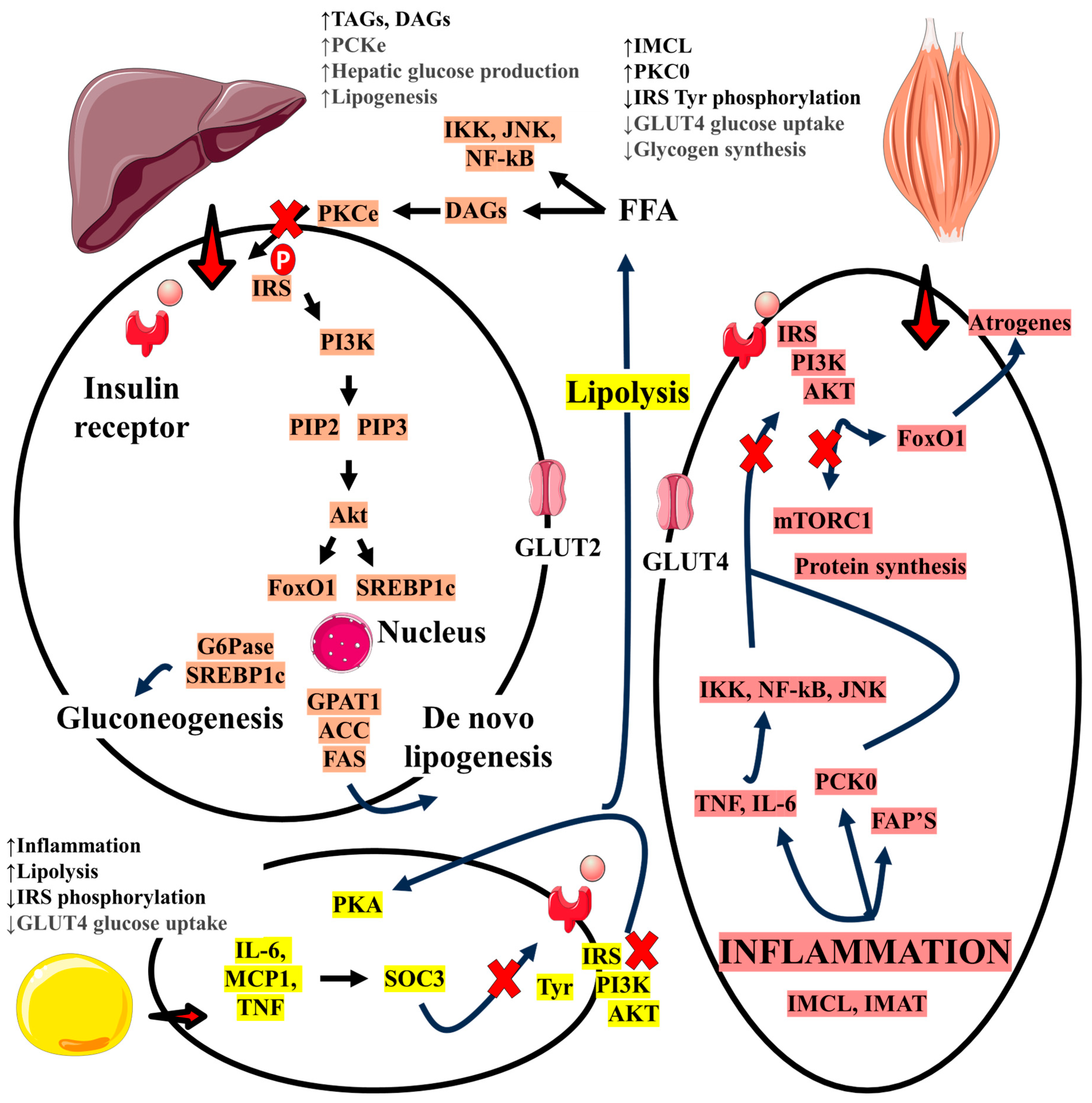
5. Role of Insulin Resistance, MAFLD, and the Relationship with Sarcopenia
6. Relationship Between MAFLD and Sarcopenia
7. The Role of Myokines and Hepatokines in Sarcopenia and MAFLD
8. Prevention of MAFLD and Sarcopenia
9. Conclusions
10. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Demori, I.; Grasselli, E. The Role of the Stress Response in Metabolic Dysfunction-Associated Fatty Liver Disease: A Psychoneuroendocrineimmunology-Based Perspective. Nutrients 2023, 15, 795. [Google Scholar] [CrossRef] [PubMed]
- Ohno, R.; Kaneko, H.; Suzuki, Y.; Okada, A.; Matsuoka, S.; Ueno, K.; Fujiu, K.; Michihata, N.; Jo, T.; Takeda, N.; et al. Association of Metabolic Dysfunction-Associated Fatty Liver Disease With Risk of HF and AF. JACC Asia 2023, 3, 908–921. [Google Scholar] [CrossRef] [PubMed]
- Lian, R.-T.; Zhuang, A.-Q.; Yue, J.; Chen, Y.; Ma, K.-F.; Wu, Y.-H. Availability, Functionality, and Safety as well as Quality Control of Hepatocytes as Seeding Cells in Liver Regenerative Medicine: State of the Art and Challenges. Curr. Stem Cell Res. Ther. 2023, 18, 1090–1105. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Luo, P.; Cao, Y.; Peng, D.; Huo, S.; Guo, J.; Wang, M.; Shi, W.; Zhang, C.; Li, S.; et al. The role of STAT3/VAV3 in glucolipid metabolism during the development of HFD-induced MAFLD. Int. J. Biol. Sci. 2024, 20, 2027–2043. [Google Scholar] [CrossRef]
- Zhang, H.; Targher, G.; Byrne, C.D.; Kim, S.U.; Wong, V.W.; Valenti, L.; Glickman, M.; Ponce, J.; Mantzoros, C.S.; Crespo, J.; et al. A global survey on the use of the international classification of diseases codes for metabolic dysfunction-associated fatty liver disease. Hepatol. Int. 2024, 18, 1178–1201. [Google Scholar] [CrossRef]
- Viswanath, A.; Fouda, S.; Fernandez, C.J.; Pappachan, J.M. Metabolic-associated fatty liver disease and sarcopenia: A double whammy. World J. Hepatol. 2024, 16, 152–163. [Google Scholar] [CrossRef]
- Zhao, Q.; Yin, Y.; Deng, Y. Metabolic associated fatty liver disease and sarcopenia additively increase mortality: A real-world study. Nutr. Diabetes 2023, 13, 21. [Google Scholar] [CrossRef]
- Tsutsumi, T.; Nakano, D.; Hashida, R.; Sano, T.; Kawaguchi, M.; Amano, K.; Kawaguchi, T. The Inter-Organ Crosstalk Reveals an Inevitable Link between MAFLD and Extrahepatic Diseases. Nutrients 2023, 15, 1123. [Google Scholar] [CrossRef]
- De Cól, J.P.; de Lima, E.P.; Pompeu, F.M.; Cressoni Araújo, A.; de Alvares Goulart, R.; Bechara, M.D.; Laurindo, L.F.; Méndez-Sánchez, N.; Barbalho, S.M. Underlying Mechanisms behind the Brain-Gut-Liver Axis and Metabolic-Associated Fatty Liver Disease (MAFLD): An Update. Int. J. Mol. Sci. 2024, 25, 3694. [Google Scholar] [CrossRef]
- Karimi, M.; Akhgarjand, C.; Houjaghani, H.; Nejad, M.M.; Sohrabpour, A.A.; Poustchi, H.; Mohammadi, H.; Chamari, M.; Imani, H. The Effect of Intermittent Fasting Diet in Comparison With Low-Calorie Diet on Inflammation, Lipid Profile, Glycemic Index, Liver Fibrosis in Patients With Metabolic-Associated Fatty Liver Disease (MAFLD): A Randomized Controlled Trial. Clin. Ther. 2025, 47, e9–e16. [Google Scholar] [CrossRef]
- Gofton, C.; Upendran, Y.; Zheng, M.-H.; George, J. MAFLD: How is it different from NAFLD? Clin. Mol. Hepatol. 2023, 29, S17–S31. [Google Scholar] [CrossRef] [PubMed]
- Pipitone, R.M.; Ciccioli, C.; Infantino, G.; La Mantia, C.; Parisi, S.; Tulone, A.; Pennisi, G.; Grimaudo, S.; Petta, S. MAFLD: A multisystem disease. Ther. Adv. Endocrinol. Metab. 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P. NAFLD, MAFLD, and beyond: One or several acronyms for better comprehension and patient care. Intern. Emerg. Med. 2023, 18, 993–1006. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Duseja, A.; al Mahtab, M.; Anirvan, P.; Acharya, S.K.; Akbar, S.M.F.; Butt, A.S.; Dassanayake, A.; De, A.; Dhakal, G.; et al. INASL-SAASL Consensus Statements on NAFLD Name Change to MAFLD. J. Clin. Exp. Hepatol. 2023, 13, 518–522. [Google Scholar] [CrossRef]
- Zeng, X.-F.; Varady, K.A.; Wang, X.-D.; Targher, G.; Byrne, C.D.; Tayyem, R.; Latella, G.; Bergheim, I.; Valenzuela, R.; George, J.; et al. The role of dietary modification in the prevention and management of metabolic dysfunction-associated fatty liver disease: An international multidisciplinary expert consensus. Metab. Clin. Exp. 2024, 161, 156028. [Google Scholar] [CrossRef]
- Duan, H.; Song, S.; Li, R.; Hu, S.; Zhuang, S.; Liu, S.; Li, X.; Gao, W. Strategy for treating MAFLD: Electroacupuncture alleviates hepatic steatosis and fibrosis by enhancing AMPK mediated glycolipid metabolism and autophagy in T2DM rats. Diabetol. Metab. Syndr. 2024, 16, 218. [Google Scholar] [CrossRef]
- Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421. [CrossRef]
- Badmus, O.O.; Hillhouse, S.A.; Anderson, C.D.; Hinds, T.D.; Stec, D.E. Molecular mechanisms of metabolic associated fatty liver disease (MAFLD): Functional analysis of lipid metabolism pathways. Clin. Sci. 2022, 136, 1347–1366. [Google Scholar] [CrossRef]
- Sakurai, Y.; Kubota, N.; Yamauchi, T.; Kadowaki, T. Role of Insulin Resistance in MAFLD. Int. J. Mol. Sci. 2021, 22, 4156. [Google Scholar] [CrossRef]
- Gao, J.; Cang, X.; Liu, L.; Lin, J.; Zhu, S.; Liu, L.; Liu, X.; Zhu, J.; Xu, C. Farrerol alleviates insulin resistance and hepatic steatosis of metabolic associated fatty liver disease by targeting PTPN1. J. Cell. Mol. Med. 2024, 28, e70096. [Google Scholar] [CrossRef]
- Joo, S.K.; Kim, W. Interaction between sarcopenia and nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2023, 29, S68–S78. [Google Scholar] [CrossRef] [PubMed]
- Mellen, R.H.; Girotto, O.S.; Marques, E.B.; Laurindo, L.F.; Grippa, P.C.; Mendes, C.G.; Garcia, L.N.H.; Bechara, M.D.; Barbalho, S.M.; Sinatora, R.V.; et al. Insights into Pathogenesis, Nutritional and Drug Approach in Sarcopenia: A Systematic Review. Biomedicines 2023, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Minniti, G.; Pescinini-Salzedas, L.M.; Minniti, G.; Laurindo, L.F.; Barbalho, S.M.; Vargas Sinatora, R.; Sloan, L.A.; Haber, R.S.A.; Araújo, A.C.; Quesada, K.; et al. Organokines, Sarcopenia, and Metabolic Repercussions: The Vicious Cycle and the Interplay with Exercise. Int. J. Mol. Sci. 2022, 23, 13452. [Google Scholar] [CrossRef]
- Tian, M.; Xu, H.; Wang, H.; Wang, H.; Dai, Z.; Ding, C.; Guo, H.; Jin, X. Pretreatment Computed Tomography-Defined Sarcopenia, Treatment-Associated Muscle Loss, and Survival in Patients With Cervical Cancer: A Systematic Review and Meta-Analysis. Nutr. Rev. 2024, 83, 797–808. [Google Scholar] [CrossRef]
- Zhao, Z.-H.; Zou, J.; Huang, X.; Fan, Y.-C.; Wang, K. Assessing causal relationships between sarcopenia and nonalcoholic fatty liver disease: A bidirectional Mendelian randomization study. Front. Nutr. 2022, 9, 971913. [Google Scholar] [CrossRef]
- Martini, C.A.N.; Weigert, C.S.; Stiegemaier, A.C.B.; Ferreira, A.P.R.B.; Gonçalves, E.L.; Valle, S.F. Use of the SARC-F Score as an Aid in Fragility Fractures Prevention. Rev. Bras. Ortop. 2022, 58, 157–163. [Google Scholar] [CrossRef]
- Petermann-Rocha, F.; Gray, S.R.; Forrest, E.; Welsh, P.; Sattar, N.; Celis-Morales, C.; Ho, F.K.; Pell, J.P. Associations of muscle mass and grip strength with severe NAFLD: A prospective study of 333,295 UK Biobank participants. J. Hepatol. 2022, 76, 1021–1029. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Vachliotis, I.D.; Mantzoros, C.S. Sarcopenia, sarcopenic obesity and nonalcoholic fatty liver disease. Metab. Clin. Exp. 2023, 147, 155676. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Flato, U.A.P.; Tofano, R.J.; Goulart, R.A.; Guiguer, E.L.; Detregiachi, C.R.P.; Buchaim, D.V.; Araújo, A.C.; Buchaim, R.L.; Reina, F.T.R.; et al. Physical Exercise and Myokines: Relationships with Sarcopenia and Cardiovascular Complications. Int. J. Mol. Sci. 2020, 21, 3607. [Google Scholar] [CrossRef]
- Duarte, M.P.; Almeida, L.S.; Neri, S.G.; Oliveira, J.S.; Wilkinson, T.J.; Ribeiro, H.S.; Lima, R.M. Prevalence of sarcopenia in patients with chronic kidney disease: A global systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2024, 15, 501–512. [Google Scholar] [CrossRef]
- Evans, W.J.; Guralnik, J.; Cawthon, P.; Appleby, J.; Landi, F.; Clarke, L.; Vellas, B.; Ferrucci, L.; Roubenoff, R. Sarcopenia: No consensus, no diagnostic criteria, and no approved indication—How did we get here? GeroScience 2024, 46, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Cao, Y.; Ji, G.; Zhang, L. Lean nonalcoholic fatty liver disease and sarcopenia. Front. Endocrinol. 2023, 14, 1217249. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Mejía, M.M.; Qi, X.; Abenavoli, L.; Barranco-Fragoso, B.; Barbalho, S.M.; Méndez-Sánchez, N. NAFLD-MASLD-MAFLD continuum: A swinging pendulum? Ann. Hepatol. 2024, 29, 101526. [Google Scholar] [CrossRef]
- Nakamura, A.; Yoshimura, T.; Ichikawa, T. Liver Disease-Related Sarcopenia: A Predictor of Poor Prognosis by Accelerating Hepatic Decompensation in Advanced Chronic Liver Disease. Cureus 2023, 15, e49078. [Google Scholar] [CrossRef]
- Feng, Z.; Zhao, F.; Wang, Z.; Tang, X.; Xie, Y.; Qiu, L. The relationship between sarcopenia and metabolic dysfunction-associated fatty liver disease among the young and middle-aged populations. BMC Gastroenterol. 2024, 24, 111. [Google Scholar] [CrossRef]
- Musio, A.; Perazza, F.; Leoni, L.; Stefanini, B.; Dajti, E.; Menozzi, R.; Petroni, M.L.; Colecchia, A.; Ravaioli, F. Osteosarcopenia in NAFLD/MAFLD: An Underappreciated Clinical Problem in Chronic Liver Disease. Int. J. Mol. Sci. 2023, 24, 7517. [Google Scholar] [CrossRef]
- Hao, X.-Y.; Zhang, K.; Huang, X.-Y.; Yang, F.; Sun, S.-Y. Muscle strength and non-alcoholic fatty liver disease/metabolic-associated fatty liver disease. World J. Gastroenterol. 2024, 30, 636–643. [Google Scholar] [CrossRef]
- Zhou, D.; Shi, Y.; Zhang, D.; Zuo, J.; Zeng, C.; Mamtawla, G.; Huang, L.; Gao, X.; Zhang, L.; Wang, X. Liver-secreted FGF21 induces sarcopenia by inhibiting satellite cell myogenesis via klotho beta in decompensated cirrhosis. Redox Biol. 2024, 76, 103333. [Google Scholar] [CrossRef]
- Zhu, X.; Zeng, C.; Yu, B. White adipose tissue in metabolic associated fatty liver disease. Clin. Res. Hepatol. Gastroenterol. 2024, 48, 102336. [Google Scholar] [CrossRef]
- Fernández-Mincone, T.; Contreras-Briceño, F.; Espinosa-Ramírez, M.; García-Valdés, P.; López-Fuenzalida, A.; Riquelme, A.; Arab, J.P.; Cabrera, D.; Arrese, M.; Barrera, F. Nonalcoholic fatty liver disease and sarcopenia: Pathophysiological connections and therapeutic implications. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 1141–1157. [Google Scholar] [CrossRef]
- Luo, W.; Zhou, Y.; Tang, Q.; Wang, Y.; Liu, Y.; Ai, L. Downhill running and caloric restriction attenuate insulin resistance associated skeletal muscle atrophy via the promotion of M2-like macrophages through TRIB3-AKT pathway. Free Radic. Biol. Med. 2024, 210, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Ferenc, K.; Jarmakiewicz-Czaja, S.; Filip, R. What Does Sarcopenia Have to Do with Nonalcoholic Fatty Liver Disease? Life 2023, 14, 37. [Google Scholar] [CrossRef]
- Fouda, S.; Jeeyavudeen, M.S.; Pappachan, J.M.; Jayanthi, V. Pathobiology of Metabolic-Associated Fatty Liver Disease. Endocrinol. Metab. Clin. N. Am. 2023, 52, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Mantri, A.; Köhlmoos, A.; Schelski, D.S.; Seel, W.; Stoffel-Wagner, B.; Krawitz, P.; Stehle, P.; Holst, J.J.; Weber, B.; Koban, L.; et al. Impact of Synbiotic Intake on Liver Metabolism in Metabolically Healthy Participants and Its Potential Preventive Effect on Metabolic-Dysfunction-Associated Fatty Liver Disease (MAFLD): A Randomized, Placebo-Controlled, Double-Blinded Clinical Trial. Nutrients 2024, 16, 1300. [Google Scholar] [CrossRef]
- Seo, J.Y.; Cho, E.J.; Kim, M.J.; Kwak, M.; Yang, J.I.; Chung, S.J.; Yim, J.Y.; Yoon, J.W.; Chung, G.E. The relationship between metabolic dysfunction-associated fatty liver disease and low muscle mass in an asymptomatic Korean population. J. Cachexia Sarcopenia Muscle 2022, 13, 2953–2960. [Google Scholar] [CrossRef]
- Malik, A.; Javaid, S.; Malik, M.I.; Qureshi, S. Relationship between sarcopenia and metabolic dysfunction-associated steatotic liver disease (MASLD): A systematic review and meta-analysis. Ann. Hepatol. 2024, 29, 101544. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Q.; Zhao, W.; Ye, B.; Li, S.; Zhang, Z.; Ju, J.; He, J.; Xia, M.; Xiong, T.; et al. Associations of traditional healthy lifestyle and sleep quality with metabolic dysfunction-associated fatty liver disease: Two population-based studies. Nutr. Diabetes 2024, 14, 79. [Google Scholar] [CrossRef]
- Wang, X.; Lyu, L.; Li, W.; Xu, L.; Zhang, H.; Wang, S.; Liu, Y.; Ping, F.; Li, Y. Impact of rosuvastatin on metabolic syndrome patients with moderate to severe metabolic associated fatty liver disease without overt diabetes: A randomized clinical trial. Diabetes Metab. Syndr. 2024, 18, 103126. [Google Scholar] [CrossRef]
- Liu, Z.-C.; Fu, H.-J.; Li, N.-C.; Deng, F.-J.; Gan, Y.-K.; Ye, Y.-J.; Huang, B.-H.; Liu, C.; Chen, J.-H.; Li, X.-F. Systematic pharmacology and experimental validation to elucidate the inflammation-associated mechanism of Huanglian Wendan (HLWD) decoction in the treatment of MAFLD associated with atherosclerosis. J. Ethnopharmacol. 2024, 337, 118841. [Google Scholar] [CrossRef]
- Boccatonda, A.; Andreetto, L.; D’ardes, D.; Cocco, G.; Rossi, I.; Vicari, S.; Schiavone, C.; Cipollone, F.; Guagnano, M.T. From NAFLD to MAFLD: Definition, Pathophysiological Basis and Cardiovascular Implications. Biomedicines 2023, 11, 883. [Google Scholar] [CrossRef]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Xu, J.; Wang, X.; Fan, Z.; Li, H. Macrophage-derived exosomal miR-155 regulating hepatocyte pyroptosis in MAFLD. Heliyon 2024, 10, e35197. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.-C.; Yue, R.; Xiang, W.-W.; He, Y.-D.; Liu, X.; Wang, F.-J. Effect of Modified Xiaoyao Powder on intestinal barrier and intestinal flora in mice with metabolic associated fatty liver disease based on “gut-liver axis”. Zhongguo Zhong Yao Za Zhi = China J. Chin. Mater. Medica 2024, 49, 4499–4509. [Google Scholar] [CrossRef]
- Semmler, G.; Balcar, L.; Wernly, S.; Datz, L.; Semmler, M.; Rosenstatter, L.; Stickel, F.; Aigner, E.; Wernly, B.; Datz, C. No association of NAFLD-related polymorphisms in PNPLA3 and TM6SF2 with all-cause and cardiovascular mortality in an Austrian population study. Wien. Klin. Wochenschr. 2024, 136, 251–257. [Google Scholar] [CrossRef]
- Stachowicz, A.; Czepiel, K.; Wiśniewska, A.; Stachyra, K.; Ulatowska-Białas, M.; Kuśnierz-Cabala, B.; Surmiak, M.; Majka, G.; Kuś, K.; Wood, M.E.; et al. Mitochondria-targeted hydrogen sulfide donor reduces fatty liver and obesity in mice fed a high fat diet by inhibiting de novo lipogenesis and inflammation via mTOR/SREBP-1 and NF-κB signaling pathways. Pharmacol. Res. 2024, 209, 107428. [Google Scholar] [CrossRef]
- Biao, Y.; Li, D.; Zhang, Y.; Gao, J.; Xiao, Y.; Yu, Z.; Li, L. Wulingsan Alleviates MAFLD by Activating Autophagy via Regulating the AMPK/mTOR/ULK1 Signaling Pathway. Can. J. Gastroenterol. Hepatol. 2024, 2024, 9777866. [Google Scholar] [CrossRef]
- Alisi, A.; McCaughan, G.; Grønbæk, H. Role of gut microbiota and immune cells in metabolic-associated fatty liver disease: Clinical impact. Hepatol. Int. 2024, 18, 861–872. [Google Scholar] [CrossRef]
- Cheng, Z.; Chu, H.; Seki, E.; Lin, R.; Yang, L. Hepatocyte programmed cell death: The trigger for inflammation and fibrosis in metabolic dysfunction-associated steatohepatitis. Front. Cell Dev. Biol. 2024, 12, 1431921. [Google Scholar] [CrossRef]
- Malladi, N.; Lahamge, D.; Somwanshi, B.S.; Tiwari, V.; Deshmukh, K.; Balani, J.K.; Chakraborty, S.; Alam, J.; Banerjee, S.K. Paricalcitol attenuates oxidative stress and inflammatory response in the liver of NAFLD rats by regulating FOXO3a and NFκB acetylation. Cell. Signal. 2024, 121, 111299. [Google Scholar] [CrossRef]
- Orhan, S.; Turkmen, R.; Demirel, H.H.; Akosman, M.S.; Turkmen, T.; Fırat, F. Chlorogenic acid mitigates potassium dichromate-induced acute hepato-nephrotoxicity by attenuating the NF-κB signalling pathway. Mol. Biol. Rep. 2024, 51, 798. [Google Scholar] [CrossRef]
- Hao, J.; Jin, X.; Li, Z.; Zhu, Y.; Wang, L.; Jiang, X.; Wang, D.; Qi, L.; Jia, D.; Gao, B. Anti-Obesity Activity of Sanghuangporus vaninii by Inhibiting Inflammation in Mice Fed a High-Fat Diet. Nutrients 2024, 16, 2159. [Google Scholar] [CrossRef] [PubMed]
- Abouzed, D.E.E.; Ezelarab, H.A.; Selim, H.M.R.M.; Elsayed, M.M.; El Hamd, M.A.; Aboelez, M.O. Multimodal modulation of hepatic ischemia/reperfusion-induced injury by phytochemical agents: A mechanistic evaluation of hepatoprotective potential and safety profiles. Int. Immunopharmacol. 2024, 138, 112445. [Google Scholar] [CrossRef]
- Caliri, A.W.; Tommasi, S.; Besaratinia, A. Relationships among smoking, oxidative stress, inflammation, macromolecular damage, and cancer. Mutat. Res. Rev. Mutat. Res. 2021, 787, 108365. [Google Scholar] [CrossRef] [PubMed]
- Shepard, C.R. TLR9 in MAFLD and NASH: At the Intersection of Inflammation and Metabolism. Front. Endocrinol. 2021, 11, 613639. [Google Scholar] [CrossRef] [PubMed]
- Filipovic, B.; Marjanovic-Haljilji, M.; Mijac, D.; Lukic, S.; Kapor, S.; Kapor, S.; Starcevic, A.; Popovic, D.; Djokovic, A. Molecular Aspects of MAFLD-New Insights on Pathogenesis and Treatment. Curr. Issues Mol. Biol. 2023, 45, 9132–9148. [Google Scholar] [CrossRef]
- Angelico, F.; Alcantara-Payawal, D.; Rani, R.A.; Mustafa, N.; Thongtang, N.; Chaiteerakij, R.; Bunchorntavakul, C.; Sukonthasarn, A. Review and expert opinion on MAFLD, oxidative stress and multifunctional management. Drugs Context 2024, 13, 2023-9-3. [Google Scholar] [CrossRef]
- Anastasopoulos, N.-A.; Charchanti, A.V.; Barbouti, A.; Mastoridou, E.M.; Goussia, A.C.; Karampa, A.D.; Christodoulou, D.; Glantzounis, G.K. The Role of Oxidative Stress and Cellular Senescence in the Pathogenesis of Metabolic Associated Fatty Liver Disease and Related Hepatocellular Carcinoma. Antioxidants 2023, 12, 1269. [Google Scholar] [CrossRef]
- Gu, X.; Chu, Q.; Ma, X.; Wang, J.; Chen, C.; Guan, J.; Ren, Y.; Wu, S.; Zhu, H. New insights into iNKT cells and their roles in liver diseases. Front. Immunol. 2022, 13, 1035950. [Google Scholar] [CrossRef]
- Akkız, H.; Gieseler, R.K.; Canbay, A. Liver Fibrosis: From Basic Science towards Clinical Progress, Focusing on the Central Role of Hepatic Stellate Cells. Int. J. Mol. Sci. 2024, 25, 7873. [Google Scholar] [CrossRef]
- Nassir, F. NAFLD: Mechanisms, Treatments, and Biomarkers. Biomolecules 2022, 12, 824. [Google Scholar] [CrossRef]
- Chan, M.M.; Daemen, S.; Beals, J.W.; Terekhova, M.; Yang, B.Q.; Fu, C.F.; He, L.; Park, A.C.; Smith, G.I.; Razani, B.; et al. Steatosis drives monocyte-derived macrophage accumulation in human metabolic dysfunction-associated fatty liver disease. JHEP Rep. Innov. Hepatol. 2023, 5, 100877. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Chen, Z.; Li, Y.; Wang, X.; Yang, C.; Li, Y.; Zhu, Y.; Lu, Y.; Liu, Q.; Xu, N.; et al. Transketolase promotes MAFLD by limiting inosine-induced mitochondrial activity. Cell Metab. 2024, 36, 1013–1029.e1015. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Schmidt, H.M.; Wood, K.C.; Straub, A.C. CoenzymeQ in cellular redox regulation and clinical heart failure. Free Radic. Biol. Med. 2021, 167, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Guo, Y.; Su, W.; Zhang, H.; Wei, X.; Ma, X.; Gong, S.; Qu, G.; Zhang, L.; Xu, H.; et al. The mitochondria-targeted antioxidant MitoQ ameliorates inorganic arsenic-induced DCs/Th1/Th2/Th17/Treg differentiation partially by activating PINK1-mediated mitophagy in murine liver. Ecotoxicol. Environ. Saf. 2024, 277, 116350. [Google Scholar] [CrossRef]
- Shi, H.; Li, Q.-Y.; Li, H.; Wang, H.-Y.; Fan, C.-X.; Dong, Q.-Y.; Pan, B.-C.; Ji, Z.-L.; Li, J.-Y. ROS-induced oxidative stress is a major contributor to sperm cryoinjury. Hum. Reprod. 2024, 39, 310–325. [Google Scholar] [CrossRef]
- Miah, R.; Nina, S.; Murate, T.; Kataoka, N.; Matsutani, M.; Ano, Y.; Matsushita, K.; Yakushi, T. Dissection and Reconstitution Provide Insights into Electron Transport in the Membrane-Bound Aldehyde Dehydrogenase Complex of Gluconacetobacter diazotrophicus. J. Bacteriol. 2022, 204, e0055821. [Google Scholar] [CrossRef]
- Fedotcheva, N.; Olenin, A.; Beloborodova, N. Influence of Microbial Metabolites on the Nonspecific Permeability of Mitochondrial Membranes under Conditions of Acidosis and Loading with Calcium and Iron Ions. Biomedicines 2021, 9, 558. [Google Scholar] [CrossRef]
- Li, Y.; Wu, J.; Yang, M.; Wei, L.; Wu, H.; Wang, Q.; Shi, H. Physiological evidence of mitochondrial permeability transition pore opening caused by lipid deposition leading to hepatic steatosis in db/db mice. Free Radic. Biol. Med. 2021, 162, 523–532. [Google Scholar] [CrossRef]
- Fromenty, B.; Robin, M.A.; Igoudjil, A.; Mansouri, A.; Pessayre, D. The ins and outs of mitochondrial dysfunction in NASH. Diabetes Metab. 2004, 30, 121–138. [Google Scholar] [CrossRef]
- Zaib, S.; Hayyat, A.; Ali, N.; Gul, A.; Naveed, M.; Khan, I. Role of Mitochondrial Membrane Potential and Lactate Dehydrogenase A in Apoptosis. Anti-Cancer Agents Med. Chem. 2022, 22, 2048–2062. [Google Scholar] [CrossRef]
- Li, Q.; Guo, P.; Wang, S.; Su, L.; Yu, W.; Guo, J.; Hu, L.; Zhang, H.; Pan, J.; Tang, Z.; et al. Drp1 Aggravates Copper Nanoparticle-Induced ER-Phagy by Disturbing Mitochondria-Associated Membranes in Chicken Hepatocytes. J. Agric. Food Chem. 2024, 72, 16506–16518. [Google Scholar] [CrossRef] [PubMed]
- Sami Alkafaas, S.; Obeid, O.K.; Ali Radwan, M.; Elsalahaty, M.I.; Samy ElKafas, S.; Hafez, W.; Janković, N.; Hessien, M. Novel insight into mitochondrial dynamin-related protein-1 as a new chemo-sensitizing target in resistant cancer cells. Bioorganic Chem. 2024, 150, 107574. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Krantz, S.; Qin, X.; Li, S.; Gunasekara, H.; Kim, Y.-M.; Zimnicka, A.; Bae, M.; Ma, K.; Toth, P.T.; et al. Caveolin-1 controls mitochondrial damage and ROS production by regulating fission—Fusion dynamics and mitophagy. Redox Biol. 2022, 52, 102304. [Google Scholar] [CrossRef] [PubMed]
- Chevrollier, A.; Boursier, J.; Desquiret-Dumas, V. Food perception induces fast fragmentation of hepatic mitochondria. Trends Endocrinol. Metab. 2024, 35, 680–682. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.C. Mitochondrial Dynamics and Its Involvement in Disease. Annu. Rev. Pathol. 2020, 15, 235–259. [Google Scholar] [CrossRef]
- Ariyoshi, K.; Nishiyama, K.; Kato, Y.; Mi, X.; Ito, T.; Azuma, Y.-T.; Nishimura, A.; Nishida, M. Inhibition of Drp1–Filamin Protein Complex Prevents Hepatic Lipid Droplet Accumulation by Increasing Mitochondria–Lipid Droplet Contact. Int. J. Mol. Sci. 2024, 25, 5446. [Google Scholar] [CrossRef]
- Henschke, S.; Nolte, H.; Magoley, J.; Kleele, T.; Brandt, C.; Hausen, A.C.; Wunderlich, C.M.; Bauder, C.A.; Aschauer, P.; Manley, S.; et al. Food perception promotes phosphorylation of MFFS131 and mitochondrial fragmentation in liver. Science 2024, 384, 438–446. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, S.; Wu, J.; Wang, Y. Mitochondrial metabolic dysfunction and non-alcoholic fatty liver disease: New insights from pathogenic mechanisms to clinically targeted therapy. J. Transl. Med. 2023, 21, 510. [Google Scholar] [CrossRef]
- Zhang, J.; Li, W.-J.; Chen, S.-Q.; Chen, Z.; Zhang, C.; Ying, R.; Liu, H.-B.; Chen, L.-W.; Tang, Y.-H.; Lu, Z.-Q.; et al. Mutual promotion of mitochondrial fission and oxidative stress contributes to mitochondrial-DNA-mediated inflammation and epithelial-mesenchymal transition in paraquat-induced pulmonary fibrosis. World J. Emerg. Med. 2023, 14, 209–216. [Google Scholar] [CrossRef]
- He, J.; Qian, Y.-C.; Yin, Y.-C.; Kang, J.-R.; Pan, T.-R. Polydatin: A potential NAFLD therapeutic drug that regulates mitochondrial autophagy through SIRT3-FOXO3-BNIP3 and PINK1-PRKN mechanisms—A network pharmacology and experimental investigation. Chem.-Biol. Interact. 2024, 398, 111110. [Google Scholar] [CrossRef]
- Dai, L.; Jiang, R.; Zhan, Z.; Zhang, L.; Qian, Y.; Xu, X.; Yang, W.; Zhang, Z. Machine learning-based algorithm identifies key mitochondria-related genes in non-alcoholic steatohepatitis. Lipids Health Dis. 2024, 23, 137. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Matsui, T.; Shinozawa, T. Triclocarban induces lipid droplet accumulation and oxidative stress responses by inhibiting mitochondrial fatty acid oxidation in HepaRG cells. Toxicol. Lett. 2024, 396, 11–18. [Google Scholar] [CrossRef]
- Gnoni, A.; Di Chiara Stanca, B.; Giannotti, L.; Gnoni, G.V.; Siculella, L.; Damiano, F. Quercetin Reduces Lipid Accumulation in a Cell Model of NAFLD by Inhibiting De Novo Fatty Acid Synthesis through the Acetyl-CoA Carboxylase 1/AMPK/PP2A Axis. Int. J. Mol. Sci. 2022, 23, 1044. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, D.K.; Heilmann, R.M.; Paital, B.; Patel, A.; Yadav, V.K.; Wong, D.; Jergens, A.E. Oxidative stress, hormones, and effects of natural antioxidants on intestinal inflammation in inflammatory bowel disease. Front. Endocrinol. 2023, 14, 1217165. [Google Scholar] [CrossRef]
- Sabouny, R.; Shutt, T.E. Reciprocal Regulation of Mitochondrial Fission and Fusion. Trends Biochem. Sci. 2020, 45, 564–577. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Cai, J.; Zhang, X.-J.; Zhang, P.; She, Z.-G.; Chen, S.; Li, H. NAFLD as a continuous driver in the whole spectrum of vascular disease. J. Mol. Cell. Cardiol. 2022, 163, 118–132. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, P.; Lu, S.; Ma, W. METTL3 inhibitor STM2457 improves metabolic dysfunction-associated fatty liver disease by regulating mitochondrial function in mice. Nan Fang Yi Ke Da Xue Xue Bao = J. South. Med. Univ. 2023, 43, 1689–1696. [Google Scholar] [CrossRef]
- Chen, J.-Y.; Yang, Y.-J.; Meng, X.-Y.; Lin, R.-H.; Tian, X.-Y.; Zhang, Y.; Lai, W.-F.; Yang, C.; Ma, X.-Q.; Huang, M.-Q. Oxysophoridine inhibits oxidative stress and inflammation in hepatic fibrosis via regulating Nrf2 and NF-κB pathways. Phytomed. Int. J. Phytother. Phytopharm. 2024, 132, 155585. [Google Scholar] [CrossRef]
- Arconzo, M.; Piccinin, E.; Pasculli, E.; Cariello, M.; Loiseau, N.; Bertrand-Michel, J.; Guillou, H.; Matrella, M.L.; Villani, G.; Moschetta, A. Hepatic-specific Pgc-1α ablation drives fibrosis in a MASH model. Liver Int. 2024, 44, 2738–2752. [Google Scholar] [CrossRef]
- Ramanathan, R.; Ali, A.H.; Ibdah, J.A. Mitochondrial dysfunction plays central role in nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2022, 23, 7280. [Google Scholar] [CrossRef]
- Li, W.; Cai, Z.; Schindler, F.; Afjehi-Sadat, L.; Montsch, B.; Heffeter, P.; Heiss, E.H.; Weckwerth, W. Elevated PINK1/Parkin-Dependent Mitophagy and Boosted Mitochondrial Function Mediate Protection of HepG2 Cells from Excess Palmitic Acid by Hesperetin. J. Agric. Food Chem. 2024, 72, 13039–13053. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, A.; Yoshikawa, S.; Ikeda, Y.; Taniguchi, K.; Sawamura, H.; Morikawa, S.; Nakashima, M.; Asai, T.; Matsuda, S. Tactics with Prebiotics for the Treatment of Metabolic Dysfunction-Associated Fatty Liver Disease via the Improvement of Mitophagy. Int. J. Mol. Sci. 2023, 24, 5465. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.-P.; Li, Y.-Q.; Li, Y.-J.; Zi, L.; Tao, Y.-X.; Hao, J.-J.; Zhang, M.; Gu, W.; Zhang, F.; Yu, J.; et al. In vivo identification of the pharmacodynamic ingredients of Polygonum cuspidatum for remedying the mitochondria to alleviate metabolic dysfunction-associated fatty liver disease. Biomed. Pharmacother. 2022, 156, 113849. [Google Scholar] [CrossRef]
- Gu, M.; Zhang, Y.; Lin, Z.; Hu, X.; Zhu, Y.; Xiao, W.; Jia, X.; Chen, W.; Lu, G.; Gong, W. Decrease in UCP1 by sustained high lipid promotes NK cell necroptosis to exacerbate nonalcoholic liver fibrosis. Cell Death Dis. 2024, 15, 518. [Google Scholar] [CrossRef]
- Andres, M.; Hennuyer, N.; Zibar, K.; Bicharel-Leconte, M.; Duplan, I.; Enée, E.; Vallez, E.; Herledan, A.; Loyens, A.; Staels, B.; et al. Insulin-degrading enzyme inhibition increases the unfolded protein response and favours lipid accumulation in the liver. Br. J. Pharmacol. 2024, 181, 3610–3626. [Google Scholar] [CrossRef]
- Peyman, M.; Babin-Ebell, A.; Rodríguez-Rodríguez, R.; Rigon, M.; Aguilar-Recarte, D.; Villarroya, J.; Planavila, A.; Villarroya, F.; Palomer, X.; Barroso, E.; et al. SIRT1 regulates hepatic vldlr levels. Cell Commun. Signal. CCS 2024, 22, 297. [Google Scholar] [CrossRef]
- Yu, Z.; Fan, X.; Zhao, X.; He, T.; Li, X.; Du, H.; Zhao, M.; Zhu, R.; Li, M.; Zhang, Z.; et al. Polystyrene Nanoplastics Induce Lipid Metabolism Disorder by Activating the PERK-ATF4 Signaling Pathway in Mice. ACS Appl. Mater. Interfaces 2024, 16, 34524–34537. [Google Scholar] [CrossRef]
- Wang, L.; Xie, Z.; Wu, M.; Chen, Y.; Wang, X.; Li, X.; Liu, F. The role of taurine through endoplasmic reticulum in physiology and pathology. Biochem. Pharmacol. 2024, 226, 116386. [Google Scholar] [CrossRef]
- Makio, T.; Simmen, T. The Discovery of Mitochondria-Endoplasmic Reticulum Contact Sites (MERCs) as Mitochondria-Associated Membranes (MAMs). Contact 2024, 7, 25152564241261228. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, Y.; Wang, D.; Huang, Z.; Xiao, X.; Zheng, Q.; Li, S.; Long, D.; Feng, L. Mitochondrial Dysfunction in Metabolic Dysfunction Fatty Liver Disease (MAFLD). Int. J. Mol. Sci. 2023, 24, 17514. [Google Scholar] [CrossRef]
- Seidita, A.; Cusimano, A.; Giuliano, A.; Meli, M.; Carroccio, A.; Soresi, M.; Giannitrapani, L. Oxidative Stress as a Target for Non-Pharmacological Intervention in MAFLD: Could There Be a Role for EVOO? Antioxidants 2024, 13, 731. [Google Scholar] [CrossRef] [PubMed]
- Sayer, A.A.; Cruz-Jentoft, A. Sarcopenia definition, diagnosis and treatment: Consensus is growing. Age Ageing 2022, 51, afac220. [Google Scholar] [CrossRef] [PubMed]
- Benz, E.; Pinel, A.; Guillet, C.; Capel, F.; Pereira, B.; De Antonio, M.; Pouget, M.; Cruz-Jentoft, A.J.; Eglseer, D.; Topinkova, E.; et al. Sarcopenia and Sarcopenic Obesity and Mortality Among Older People. JAMA Netw. Open 2024, 7, e243604. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.-R.; Lee, S.; Song, S.-K. A Review of Sarcopenia Pathophysiology, Diagnosis, Treatment and Future Direction. J. Korean Med. Sci. 2022, 37, e146. [Google Scholar] [CrossRef]
- Lian, R.; Liu, Q.; Jiang, G.; Zhang, X.; Tang, H.; Lu, J.; Yang, M. Blood biomarkers for sarcopenia: A systematic review and meta-analysis of diagnostic test accuracy studies. Ageing Res. Rev. 2024, 93, 102148. [Google Scholar] [CrossRef]
- Goisser, S.; Kob, R.; Sieber, C.C.; Bauer, J.M. Diagnosis and therapy of sarcopenia-an update. Der Internist 2019, 60, 141–148. [Google Scholar] [CrossRef]
- Affourtit, C.; Carré, J.E. Mitochondrial involvement in sarcopenia. Acta Physiol. 2024, 240, e14107. [Google Scholar] [CrossRef]
- Jimenez-Gutierrez, G.E.; Martínez-Gómez, L.E.; Martínez-Armenta, C.; Pineda, C.; Martínez-Nava, G.A.; Lopez-Reyes, A. Molecular Mechanisms of Inflammation in Sarcopenia: Diagnosis and Therapeutic Update. Cells 2022, 11, 2359. [Google Scholar] [CrossRef]
- Najm, A.; Niculescu, A.-G.; Grumezescu, A.M.; Beuran, M. Emerging Therapeutic Strategies in Sarcopenia: An Updated Review on Pathogenesis and Treatment Advances. Int. J. Mol. Sci. 2024, 25, 4300. [Google Scholar] [CrossRef]
- Bhasin, S.; Travison, T.G.; Manini, T.M.; Patel, S.; Pencina, K.M.; Fielding, R.A.; Magaziner, J.M.; Newman, A.B.; Kiel, D.P.; Cooper, C.; et al. Sarcopenia Definition: The Position Statements of the Sarcopenia Definition and Outcomes Consortium. J. Am. Geriatr. Soc. 2020, 68, 1410–1418. [Google Scholar] [CrossRef]
- Tagliafico, A.S.; Bignotti, B.; Torri, L.; Rossi, F. Sarcopenia: How to measure, when and why. La Radiol. Medica 2022, 127, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Ben Kirk, B.; Cawthon, P.M.; Arai, H.; A Ávila-Funes, J.; Barazzoni, R.; Bhasin, S.; Binder, E.F.; Bruyere, O.; Cederholm, T.; Chen, L.-K.; et al. The Conceptual Definition of Sarcopenia: Delphi Consensus from the Global Leadership Initiative in Sarcopenia (GLIS). Age Ageing 2024, 53, afae052. [Google Scholar] [CrossRef]
- Liu, D.; Wang, S.; Liu, S.; Wang, Q.; Che, X.; Wu, G. Frontiers in sarcopenia: Advancements in diagnostics, molecular mechanisms, and therapeutic strategies. Mol. Asp. Med. 2024, 97, 101270. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Feng, J.; Yu, Y.; Ling, M.; Wang, X. Advances in sarcopenia: Mechanisms, therapeutic targets, and intervention strategies. Arch. Pharmacal Res. 2024, 47, 301–324. [Google Scholar] [CrossRef]
- Wang, H.; Huang, W.Y.; Zhao, Y. Efficacy of Exercise on Muscle Function and Physical Performance in Older Adults with Sarcopenia: An Updated Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 8212. [Google Scholar] [CrossRef]
- Hou, Y.; Xiang, J.; Wang, B.; Duan, S.; Song, R.; Zhou, W.; Tan, S.; He, B. Pathogenesis and comprehensive treatment strategies of sarcopenia in elderly patients with type 2 diabetes mellitus. Front. Endocrinol. 2024, 14, 1263650. [Google Scholar] [CrossRef]
- Qiu, C.; Yang, X.; Yu, P. Sarcopenia: Pathophysiology and Treatment Strategies. Endocr. Metab. Immune Disord. Drug Targets 2024, 24, 31–38. [Google Scholar] [CrossRef]
- Byrne, T.; Cooke, J.; Bambrick, P.; McNeela, E.; Harrison, M. Circulating inflammatory biomarker responses in intervention trials in frail and sarcopenic older adults: A systematic review and meta-analysis. Exp. Gerontol. 2023, 177, 112199. [Google Scholar] [CrossRef]
- Santos, C.A.F.; Amirato, G.R.; Paixão, V.; Almeida, E.B.; Amaral, J.B.D.; Monteiro, F.R.; Roseira, T.; Juliano, Y.; Novo, N.F.; Rossi, M.; et al. Association among inflammaging, body composition, physical activity, and physical function tests in physically active women. Front. Med. 2023, 10, 1206989. [Google Scholar] [CrossRef]
- Tylutka, A.; Walas, Ł.; Zembron-Lacny, A. Level of IL-6, TNF, and IL-1β and age-related diseases: A systematic review and meta-analysis. Front. Immunol. 2024, 15, 1330386. [Google Scholar] [CrossRef]
- Bagnato, C.; Magna, A.; Mereu, E.; Bernardini, S.; Bartimoccia, S.; Marti, R.; Lazzerini, P.E.; D’amico, A.; Ettorre, E.; Desideri, G.; et al. Impact of Hospitalization on Sarcopenia, NADPH-Oxidase 2, Oxidative Stress, and Low-Grade Endotoxemia in Elderly Patients. Antioxidants 2025, 14, 304. [Google Scholar] [CrossRef] [PubMed]
- Klimova, A.V.; Sokolova, A.V.; Dragunov, D.O.; Kulagina, N.P.; Shmigol, T.A.; Negrebetsky, V.V.; Golubev, Y.V.; Arutyunov, G.P. [The Effect of Metformin on Short-Chain Fatty Acid Levels in Patients with Chronic Heart Failure, Prediabetes, and Sarcopenia]. Kardiologiia 2025, 65, 46–51. [Google Scholar] [CrossRef]
- Nishikawa, H.; Fukunishi, S.; Asai, A.; Yokohama, K.; Nishiguchi, S.; Higuchi, K. Pathophysiology and mechanisms of primary sarcopenia (Review). Int. J. Mol. Med. 2021, 48, 156. [Google Scholar] [CrossRef]
- Inojosa, A.C.; Ribeiro, A.V.H.; Araújo, T.F.; Xavier, M.E.; Rêgo, D.; Bandeira, F. Body Composition, Sarcopenia, and Serum Myokines in Acromegaly: A Narrative Review. J. Bone Metab. 2024, 31, 182–195. [Google Scholar] [CrossRef]
- He, Y.; Duan, W.; Xu, P.; Lin, T.; Xiang, Q.; Dong, B.; Ge, N.; Yue, J. Exploring the impact of interleukins on sarcopenia development: A systematic review and meta-analysis. Exp. Gerontol. 2024, 193, 112480. [Google Scholar] [CrossRef]
- Morawin, B.; Tylutka, A.; Bielewicz, F.; Zembron-Lacny, A. Diagnostics of inflammaging in relation to sarcopenia. Front. Public Health 2023, 11, 1162385. [Google Scholar] [CrossRef]
- Oh, H.-J.; Jin, H.; Lee, B.-Y. Hesperidin Ameliorates Sarcopenia through the Regulation of Inflammaging and the AKT/mTOR/FoxO3a Signaling Pathway in 22–26-Month-Old Mice. Cells 2023, 12, 2015. [Google Scholar] [CrossRef]
- Salaffi, F.; Di Matteo, A.; Farah, S.; Di Carlo, M. Inflammaging and Frailty in Immune-Mediated Rheumatic Diseases: How to Address and Score the Issue. Clin. Rev. Allergy Immunol. 2023, 64, 206–221. [Google Scholar] [CrossRef]
- Damluji, A.A.; Alfaraidhy, M.; AlHajri, N.; Rohant, N.N.; Kumar, M.; Al Malouf, C.; Bahrainy, S.; Kwak, M.J.; Batchelor, W.B.; Forman, D.E.; et al. Sarcopenia and Cardiovascular Diseases. Circulation 2023, 147, 1534–1553. [Google Scholar] [CrossRef]
- Bilski, J.; Pierzchalski, P.; Szczepanik, M.; Bonior, J.; Zoladz, J.A. Multifactorial Mechanism of Sarcopenia and Sarcopenic Obesity. Role of Physical Exercise, Microbiota and Myokines. Cells 2022, 11, 160. [Google Scholar] [CrossRef]
- Vinokurov, A.Y.; Bazhenov, P.A.; Pogonyalova, M.Y.; Seryogina, E.S.; Vetrova, E.A.; Andreeva, L.; Abramov, A.Y.; Angelova, P.R. Enhancement of Energy Metabolism in Skeletal Myocytes Protects Against Age-Related Sarcopenia. J. Cell. Mol. Med. 2025, 29, e70588. [Google Scholar] [CrossRef] [PubMed]
- Dupont, J.; Vercauteren, L.; Amini, N.; Lapauw, L.; De Schaepdryver, M.; Poesen, K.; Dedeyne, L.; Verschueren, S.; Tournoy, J.; Koppo, K.; et al. Are inflammatory markers associated with sarcopenia-related traits in older adults with sarcopenia?—A cross-sectional analysis of the ENHANce study. Exp. Gerontol. 2023, 178, 112196. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.; Louissaint, J.; Parikh, N.S.; Long, M.T.; Tapper, E.B. Cognitive Function, Sarcopenia, and Inflammation Are Strongly Associated with Frailty: A Framingham Cohort Study. Am. J. Med. 2021, 134, 1530–1538. [Google Scholar] [CrossRef] [PubMed]
- Prud’homme, G.J.; Wang, Q. Anti-Inflammatory Role of the Klotho Protein and Relevance to Aging. Cells 2024, 13, 1413. [Google Scholar] [CrossRef]
- Xu, X.; Wen, Z. The mediating role of inflammaging between mitochondrial dysfunction and sarcopenia in aging: A review. Am. J. Clin. Exp. Immunol. 2023, 12, 109. [Google Scholar]
- Cilenti, L.; Mahar, R.; Di Gregorio, J.; Ambivero, C.T.; Merritt, M.E.; Zervos, A.S. Regulation of Metabolism by Mitochondrial MUL1 E3 Ubiquitin Ligase. Front. Cell Dev. Biol. 2022, 10, 904728. [Google Scholar] [CrossRef]
- Kalykaki, M.; Rubio-Tomás, T.; Tavernarakis, N. The role of mitochondria in cytokine and chemokine signalling during ageing. Mech. Ageing Dev. 2024, 222, 111993. [Google Scholar] [CrossRef]
- Guo, A.; Huang, K.; Lu, Q.; Tao, B.; Li, K.; Jiang, D. TRIM16 facilitates SIRT-1-dependent regulation of antioxidant response to alleviate age-related sarcopenia. J. Cachexia Sarcopenia Muscle 2024, 15, 2056–2070. [Google Scholar] [CrossRef]
- Kim, M.-J.; Sinam, I.S.; Siddique, Z.; Jeon, J.-H.; Lee, I.-K. The Link between Mitochondrial Dysfunction and Sarcopenia: An Update Focusing on the Role of Pyruvate Dehydrogenase Kinase 4. Diabetes Metab. J. 2023, 47, 153–163. [Google Scholar] [CrossRef]
- Xu, Z.; Fu, T.; Guo, Q.; Zhou, D.; Sun, W.; Zhou, Z.; Chen, X.; Zhang, J.; Liu, L.; Xiao, L.; et al. Disuse-associated loss of the protease LONP1 in muscle impairs mitochondrial function and causes reduced skeletal muscle mass and strength. Nat. Commun. 2022, 13, 894. [Google Scholar] [CrossRef]
- Imi, Y.; Ogawa, W.; Hosooka, T. Insulin resistance in adipose tissue and metabolic diseases. Diabetol. Int. 2022, 14, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhu, G.; Fu, G.; Zha, W.; Li, H. Metabolic Syndrome Ameliorated by 4-Methylesculetin by Reducing Hepatic Lipid Accumulation. Int. J. Mol. Sci. 2022, 23, 10465. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, Z.; Wang, Y.; Wu, X.; Sun, Y.; Lou, H.; Xu, J.; Yao, J.; Cong, D. Hidden pathway: The role of extracellular matrix in type 2 diabetes mellitus-related sarcopenia. Front. Endocrinol. 2025, 16, 1560396. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Baghoomian, A.; Zhang, Z.; Cui, Y.; Whang, E.C.; Li, X.; Fraga, J.; Spellman, R.A.; Dong, T.S.; Li, W.; et al. Hepatic lipopolysaccharide binding protein partially uncouples inflammation from fibrosis in MAFLD. J. Clin. Investig. 2024, 134, e179752. [Google Scholar] [CrossRef]
- Rajendran, R.; Suman, S.; Divakaran, S.J.; Swatikrishna, S.; Tripathi, P.; Jain, R.; Sagar, K.; Rajakumari, S. Sesaminol alters phospholipid metabolism and alleviates obesity-induced NAFLD. FASEB J. 2024, 38, e23835. [Google Scholar] [CrossRef]
- Liu, Z.-J.; Zhu, C.-F. Causal relationship between insulin resistance and sarcopenia. Diabetol. Metab. Syndr. 2023, 15, 46. [Google Scholar] [CrossRef]
- Yang, L.; Liu, D.; Jiang, S.; Li, H.; Chen, L.; Wu, Y.; Essien, A.E.; Opoku, M.; Naranmandakh, S.; Liu, S.; et al. SIRT1 signaling pathways in sarcopenia: Novel mechanisms and potential therapeutic targets. Biomed. Pharmacother. 2024, 177, 116917. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, Y.; Zheng, C. Type 2 diabetes mellitus related sarcopenia: A type of muscle loss distinct from sarcopenia and disuse muscle atrophy. Front. Endocrinol. 2024, 15, 1375610. [Google Scholar] [CrossRef]
- Li, C.; Yu, K.; Shyh-Chang, N.; Jiang, Z.; Liu, T.; Ma, S.; Luo, L.; Guang, L.; Liang, K.; Ma, W.; et al. Pathogenesis of sarcopenia and the relationship with fat mass: Descriptive review. J. Cachexia Sarcopenia Muscle 2022, 13, 781–794. [Google Scholar] [CrossRef]
- Cespiati, A.; Meroni, M.; Lombardi, R.; Oberti, G.; Dongiovanni, P.; Fracanzani, A.L. Impact of Sarcopenia and Myosteatosis in Non-Cirrhotic Stages of Liver Diseases: Similarities and Differences across Aetiologies and Possible Therapeutic Strategies. Biomedicines 2022, 10, 182. [Google Scholar] [CrossRef]
- Reichelt, S.; Merle, U.; Klauss, M.; Kahlert, C.; Lurje, G.; Mehrabi, A.; Czigany, Z. Shining a spotlight on sarcopenia and myosteatosis in liver disease and liver transplantation: Potentially modifiable risk factors with major clinical impact. Liver Int. 2024, 44, 1483–1512. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Huang, X.; Dong, M.; Wen, S.; Zhou, L.; Yuan, X. The Association Between Sarcopenia and Diabetes: From Pathophysiology Mechanism to Therapeutic Strategy. Diabetes Metab. Syndr. Obesity Targets Ther. 2023, 16, 1541–1554. [Google Scholar] [CrossRef] [PubMed]
- Al Saedi, A.; Debruin, D.A.; Hayes, A.; Hamrick, M. Lipid metabolism in sarcopenia. Bone 2022, 164, 116539. [Google Scholar] [CrossRef]
- Sun, X.; Liu, Z.; Chen, F.; Du, T. Sarcopenia modifies the associations of nonalcoholic fatty liver disease with all-cause and cardiovascular mortality among older adults. Sci. Rep. 2021, 11, 15647. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, Z.; Zhou, C.; Ye, Z.; He, P.; Zhang, Y.; Li, H.; Liu, C.; Qin, X. Predicted fat mass and lean mass in relation to all-cause and cause-specific mortality. J. Cachexia Sarcopenia Muscle 2022, 13, 1064–1075. [Google Scholar] [CrossRef]
- Moon, J.H.; Koo, B.K.; Kim, W. Non-alcoholic fatty liver disease and sarcopenia additively increase mortality: A Korean nationwide survey. J. Cachexia Sarcopenia Muscle 2021, 12, 964–972. [Google Scholar] [CrossRef]
- Sinn, D.H.; Kang, D.; Kang, M.; Guallar, E.; Hong, Y.S.; Lee, K.H.; Park, J.; Cho, J.; Gwak, G. Nonalcoholic fatty liver disease and accelerated loss of skeletal muscle mass: A longitudinal cohort study. Hepatology 2022, 76, 1746–1754. [Google Scholar] [CrossRef]
- Fujii, H.; Kawada, N.; Japan Study Group of NAFLD (JSG-NAFLD). The Role of Insulin Resistance and Diabetes in Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2020, 21, 3863. [Google Scholar] [CrossRef]
- Lee, G.B.; Huh, Y.; Lee, S.H.; Han, B.; Kim, Y.-H.; Kim, D.-H.; Kim, S.M.; Choi, Y.S.; Cho, K.H.; Nam, G.E. Association of low muscle strength with metabolic dysfunction-associated fatty liver disease: A nationwide study. World J. Gastroenterol. 2023, 29, 5962–5973. [Google Scholar] [CrossRef]
- Fox, R.; Stenning, K.; Slee, A.; Macnaughtan, J.; Davies, N. Sarcopenia in liver cirrhosis: Prevalence, pathophysiology and therapeutic strategies. Anal. Biochem. 2022, 647, 114581. [Google Scholar] [CrossRef]
- Ferguson, D.; Finck, B.N. Emerging therapeutic approaches for the treatment of NAFLD and type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2021, 17, 484–495. [Google Scholar] [CrossRef]
- Liu, Z.; Jin, P.; Liu, Y.; Zhang, Z.; Wu, X.; Weng, M.; Cao, S.; Wang, Y.; Zeng, C.; Yang, R.; et al. A comprehensive approach to lifestyle intervention based on a calorie-restricted diet ameliorates liver fat in overweight/obese patients with NAFLD: A multicenter randomized controlled trial in China. Nutr. J. 2024, 23, 64. [Google Scholar] [CrossRef] [PubMed]
- Li, A.A.; Kim, D.; Ahmed, A. Association of Sarcopenia and NAFLD: An Overview. Clin. Liver Dis. 2020, 16, 73–76. [Google Scholar] [CrossRef]
- Koo, B.K.; Kim, D.; Joo, S.K.; Kim, J.H.; Chang, M.S.; Kim, B.G.; Lee, K.L.; Kim, W. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J. Hepatol. 2017, 66, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Petta, S.; Ciminnisi, S.; Di Marco, V.; Cabibi, D.; Cammà, C.; Licata, A.; Marchesini, G.; Craxì, A. Sarcopenia is associated with severe liver fibrosis in patients with non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2017, 45, 510–518. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Jung, K.S.; Kim, S.U.; Yoon, H.-J.; Yun, Y.J.; Lee, B.-W.; Kang, E.S.; Han, K.-H.; Lee, H.C.; Cha, B.-S. Sarcopaenia is associated with NAFLD independently of obesity and insulin resistance: Nationwide surveys (KNHANES 2008–2011). J. Hepatol. 2015, 63, 486–493. [Google Scholar] [CrossRef]
- Peng, T.-C.; Wu, L.-W.; Chen, W.-L.; Liaw, F.-Y.; Chang, Y.-W.; Kao, T.-W. Nonalcoholic fatty liver disease and sarcopenia in a Western population (NHANES III): The importance of sarcopenia definition. Clin. Nutr. 2019, 38, 422–428. [Google Scholar] [CrossRef]
- Wang, Y.-M.; Zhu, K.-F.; Zhou, W.-J.; Zhang, Q.; Deng, D.-F.; Yang, Y.-C.; Lu, W.-W.; Xu, J.; Yang, Y.-M. Sarcopenia is associated with the presence of nonalcoholic fatty liver disease in Zhejiang Province, China: A cross-sectional observational study. BMC Geriatr. 2021, 21, 55. [Google Scholar] [CrossRef]
- Seo, D.; Lee, Y.-H.; Park, S.; Choi, Y.; Huh, B.; Lee, E.; Huh, K.; Kim, S.; Cha, B.-S. Sarcopenia is associated with non-alcoholic fatty liver disease in men with type 2 diabetes. Diabetes Metab. 2020, 46, 362–369. [Google Scholar] [CrossRef]
- Chung, G.E.; Kim, M.J.; Yim, J.Y.; Kim, J.S.; Yoon, J.W. Sarcopenia Is Significantly Associated with Presence and Severity of Nonalcoholic Fatty Liver Disease. J. Obes. Metab. Syndr. 2019, 28, 129–138. [Google Scholar] [CrossRef]
- Hong, K.S.; Kim, M.C.; Ahn, J.H. Sarcopenia Is an Independent Risk Factor for NAFLD in COPD: A Nationwide Survey (KNHANES 2008–2011). Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.; Joo, S.K.; Koo, B.K.; Lin, H.; Kim, W. Muscle alterations are independently associated with significant fibrosis in patients with nonalcoholic fatty liver disease. Liver Int. 2021, 41, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Gan, D.; Wang, L.; Jia, M.; Ru, Y.; Ma, Y.; Zheng, W.; Zhao, X.; Yang, F.; Wang, T.; Mu, Y.; et al. Low muscle mass and low muscle strength associate with nonalcoholic fatty liver disease. Clin. Nutr. 2020, 39, 1124–1130. [Google Scholar] [CrossRef] [PubMed]
- Golabi, P.; Gerber, L.; Paik, J.M.; Deshpande, R.; de Avila, L.; Younossi, Z.M. Contribution of sarcopenia and physical inactivity to mortality in people with non-alcoholic fatty liver disease. JHEP Rep. Innov. Hepatol. 2020, 2, 100171. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, C.W.; Park, C.-H.; Choi, J.Y.; Han, K.; Merchant, A.T.; Park, Y.-M. Low skeletal muscle mass is associated with non-alcoholic fatty liver disease in Korean adults: The Fifth Korea National Health and Nutrition Examination Survey. Hepatobiliary Pancreat. Dis. Int. 2016, 15, 39–47. [Google Scholar] [CrossRef]
- Kwon, Y.; Jeong, S.J. Relative Skeletal Muscle Mass Is an Important Factor in Non-Alcoholic Fatty Liver Disease in Non-Obese Children and Adolescents. J. Clin. Med. 2020, 9, 3355. [Google Scholar] [CrossRef]
- Pacifico, L.; Perla, F.M.; Andreoli, G.; Grieco, R.; Pierimarchi, P.; Chiesa, C. Nonalcoholic Fatty Liver Disease Is Associated With Low Skeletal Muscle Mass in Overweight/Obese Youths. Front. Pediatr. 2020, 8, 158. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Kim, S.U.; Song, K.J.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Lee, B.-W.; Kang, E.S.; Cha, B.-S.; Han, K.-H. Sarcopenia is associated with significant liver fibrosis independently of obesity and insulin resistance in nonalcoholic fatty liver disease: Nationwide surveys (KNHANES 2008–2011). Hepatology 2016, 63, 776–786. [Google Scholar] [CrossRef]
- Bhanji, R.A.; Narayanan, P.; Moynagh, M.R.; Takahashi, N.; Angirekula, M.; Kennedy, C.C.; Mara, K.C.; Dierkhising, R.A.; Watt, K.D. Differing Impact of Sarcopenia and Frailty in Nonalcoholic Steatohepatitis and Alcoholic Liver Disease. Liver Transplant. 2019, 25, 14–24. [Google Scholar] [CrossRef]
- Su, X.; Xu, J.; Zheng, C. The relationship between non-alcoholic fatty liver and skeletal muscle mass to visceral fat area ratio in women with type 2 diabetes. BMC Endocr. Disord. 2019, 19, 76. [Google Scholar] [CrossRef]
- Febbraio, M.A.; Pedersen, B.K. Who would have thought—Myokines two decades on. Nat. Rev. Endocrinol. 2020, 16, 619–620. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.; Li, K.; Xiao, Q. Sarcopenic obesity: Myokines as potential diagnostic biomarkers and therapeutic targets? Exp. Gerontol. 2020, 139, 111022. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.H.; Moon, K.M.; Min, K.-W. Exercise-Induced Myokines can Explain the Importance of Physical Activity in the Elderly: An Overview. Healthcare 2020, 8, 378. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, F.; Yang, W.; Gao, D.; Yang, L.; Yu, C.; Chen, C.; Li, X.; Zhang, J.S. FGF1 ameliorates obesity-associated hepatic steatosis by reversing IGFBP2 hypermethylation. FASEB J. 2023, 37, e22881. [Google Scholar] [CrossRef]
- Nasso, R.; D’errico, A.; Motti, M.L.; Masullo, M.; Arcone, R. Dietary Protein and Physical Exercise for the Treatment of Sarcopenia. Clin. Pract. 2024, 14, 1451–1467. [Google Scholar] [CrossRef]
- Yoshida, T.; Delafontaine, P. Mechanisms of IGF-1-Mediated Regulation of Skeletal Muscle Hypertrophy and Atrophy. Cells 2020, 9, 1970. [Google Scholar] [CrossRef]
- Ahmad, S.S.; Ahmad, K.; Lee, E.J.; Lee, Y.-H.; Choi, I. Implications of Insulin-Like Growth Factor-1 in Skeletal Muscle and Various Diseases. Cells 2020, 9, 1773. [Google Scholar] [CrossRef]
- Eilers, W.; Chambers, D.; Cleasby, M.; Foster, K. Local myostatin inhibition improves skeletal muscle glucose uptake in insulin-resistant high-fat diet-fed mice. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E163–E174. [Google Scholar] [CrossRef]
- Oguz, A.; Sahin, M.; Tuzun, D.; Kurutas, E.B.; Ulgen, C.; Bozkus, O.; Gul, K. Irisin is a predictor of sarcopenic obesity in type 2 diabetes mellitus: A cross-sectional study. Medicine 2021, 100, e26529. [Google Scholar] [CrossRef]
- Baht, G.S.; Bareja, A.; Lee, D.E.; Rao, R.R.; Huang, R.; Huebner, J.L.; Bartlett, D.B.; Hart, C.R.; Gibson, J.R.; Lanza, I.R.; et al. Meteorin-like facilitates skeletal muscle repair through a Stat3/IGF-1 mechanism. Nat. Metab. 2020, 2, 278–289. [Google Scholar] [CrossRef]
- Rozanska, O.; Uruska, A.; Zozulinska-Ziolkiewicz, D. Brain-Derived Neurotrophic Factor and Diabetes. Int. J. Mol. Sci. 2020, 21, 841. [Google Scholar] [CrossRef] [PubMed]
- Domin, R.; Dadej, D.; Pytka, M.; Zybek-Kocik, A.; Ruchała, M.; Guzik, P. Effect of Various Exercise Regimens on Selected Exercise-Induced Cytokines in Healthy People. Int. J. Environ. Res. Public Health 2021, 18, 1261. [Google Scholar] [CrossRef]
- Stefan, N.; Schick, F.; Birkenfeld, A.L.; Häring, H.-U.; White, M.F. The role of hepatokines in NAFLD. Cell Metab. 2023, 35, 236–252. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y. Editorial: The roles and mechanisms of hepatokines, adipokines and myokines in the development of non-alcoholic fatty liver disease (NAFLD). Front. Endocrinol. 2022, 13, 1074842. [Google Scholar] [CrossRef]
- dos Santos, J.P.M.; de Maio, M.C.; Lemes, M.A.; Laurindo, L.F.; Haber, J.F.d.S.; Bechara, M.D.; Prado, P.S.D.; Rauen, E.C.; Costa, F.; Pereira, B.C.d.A.; et al. Non-Alcoholic Steatohepatitis (NASH) and Organokines: What Is Now and What Will Be in the Future. Int. J. Mol. Sci. 2022, 23, 498. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Dos Santos, A.; Zanuso, B.D.O.; Miola, V.; Barbalho, S.; Bueno, P.S.; Flato, U.; Detregiachi, C.; Buchaim, D.; Buchaim, R.; Tofano, R.; et al. Adipokines, Myokines, and Hepatokines: Crosstalk and Metabolic Repercussions. Int. J. Mol. Sci. 2021, 22, 2639. [Google Scholar] [CrossRef]
- Kirk, B.; Feehan, J.; Lombardi, G.; Duque, G. Muscle, Bone, and Fat Crosstalk: The Biological Role of Myokines, Osteokines, and Adipokines. Curr. Osteoporos. Rep. 2020, 18, 388–400. [Google Scholar] [CrossRef]
- An, X.; Liu, J.; Li, Y.; Dou, Z.; Li, N.; Suo, Y.; Ma, Y.; Sun, M.; Tian, Z.; Xu, L. Chemerin/CMKLR1 ameliorates nonalcoholic steatohepatitis by promoting autophagy and alleviating oxidative stress through the JAK2-STAT3 pathway. Peptides 2021, 135, 170422. [Google Scholar] [CrossRef]
- Didyk, O.K.; Chernyavskyi, V.V.; Shypulin, V.P.; Tishchenko, V.V. Effectiveness of rifaximin and probiotics for the correction of intestinal permeability in patients with metabolic-associated fatty liver disease in combination with type 2 diabetes mellitus. Wiadomosci Lek. 2024, 77, 732–738. [Google Scholar] [CrossRef]
- Czaja-Stolc, S.; Chatrenet, A.; Potrykus, M.; Ruszkowski, J.; Torreggiani, M.; Lichodziejewska-Niemierko, M.; Dębska-Ślizień, A.; Piccoli, G.B.; Małgorzewicz, S. Adipokines and Myokines as Markers of Malnutrition and Sarcopenia in Patients Receiving Kidney Replacement Therapy: An Observational, Cross-Sectional Study. Nutrients 2024, 16, 2480. [Google Scholar] [CrossRef]
- Kawao, N.; Nishikawa, A.; Matsumura, D.; Yamada, A.; Ohira, T.; Mizukami, Y.; Kaji, H. Roles of Insulin-Like Growth Factor-1 in Muscle Wasting and Osteopenia in Mice with Hyponatremia. Calcif. Tissue Int. 2025, 116, 61. [Google Scholar] [CrossRef] [PubMed]
- Barbalho, S.M.; Prado Neto, E.V.; De Alvares Goulart, R.; Bechara, M.D.; Baisi Chagas, E.F.; Audi, M.; Guissoni Campos, L.M.; Landgraf Guiger, E.; Buchaim, R.L.; Buchaim, D.V.; et al. Myokines: A descriptive review. J. Sports Med. Phys. Fit. 2020, 60, 1583–1590. [Google Scholar] [CrossRef]
- Al Hashmi, K.; Giglio, R.V.; Pantea Stoian, A.; Patti, A.M.; Al Waili, K.; Al Rasadi, K.; Ciaccio, M.; Rizzo, M. Metabolic dysfunction-associated fatty liver disease: Current therapeutic strategies. Front. Nutr. 2024, 11, 1355732. [Google Scholar] [CrossRef]
- Rivera, F.B.; Adizas, A.; Cubarrubias, D.; Bantayan, N.R.; Choi, S.; Carado, G.P.; Yu, M.G.; Lerma, E.; Vijayaraghavan, K. The Roles of Non-Pharmacologic and Emerging Pharmacologic Management of Non-alcoholic Fatty Liver Disease and Sarcopenia: A Narrative Review. J. ASEAN Fed. Endocr. Soc. 2024, 39, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wu, Y.; Zheng, J.; Wang, M.; Goh, G.B.; Lin, S. The prognostic role of diet quality in patients with MAFLD and physical activity: Data from NHANES. Nutr. Diabetes 2024, 14, 4. [Google Scholar] [CrossRef]
- Cheng, C.; England, E. Nutrition: Macronutrients. FP Essent. 2024, 539, 7–12. [Google Scholar] [PubMed]
- Lampignano, L.; Tatoli, R.; Donghia, R.; Bortone, I.; Castellana, F.; Zupo, R.; Lozupone, M.; Panza, F.; Conte, C.; Sardone, R. Nutritional patterns as machine learning predictors of liver health in a population of elderly subjects. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 2233–2241. [Google Scholar] [CrossRef]
- Beygi, M.; Ahi, S.; Zolghadri, S.; Stanek, A. Management of Metabolic-Associated Fatty Liver Disease/Metabolic Dysfunction-Associated Steatotic Liver Disease: From Medication Therapy to Nutritional Interventions. Nutrients 2024, 16, 2220. [Google Scholar] [CrossRef]
- Elhoseeny, M.M.; Rageh, F.; Rezk, S.M.; Othman, A.A.A. Frequency and risk factors of metabolic associated fatty liver disease among medical students in Egypt. Sci. Rep. 2025, 15, 13470. [Google Scholar] [CrossRef]
- González-Rodríguez, L.G.; Borrás Olivares, I.; Ghazi, Y.; Lozano Estevan, M.C.; Ortega, R.M. Diet to maintain adequate muscle and bone health. Nutr. Hosp. 2024, 41, 12–15. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Okada, H.; Nakajima, H.; Kitagawa, N.; Okamura, T.; Majima, S.; Senmaru, T.; Ushigome, E.; Nakanishi, N.; Nakahata, Y.; et al. Effects of weight loss on metabolic dysfunction-associated fatty liver disease in Japanese people: Non-alcoholic fatty liver disease in the Gifu area, longitudinal analysis study. Hepatol. Res. 2024, 54, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Song, X.; Chen, Y.; Chen, X.; Yu, C. Relationship between relative skeletal muscle mass and nonalcoholic fatty liver disease: A systematic review and meta-analysis. Hepatol. Int. 2020, 14, 115–126. [Google Scholar] [CrossRef]
- Liu, Z.; Fang, T. Association between dietary carbohydrate to fiber ratio and metabolic dysfunction associated fatty liver disease in adults: Evidence from the NHANES 2017–2020. J. Health Popul. Nutr. 2024, 43, 43. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhou, J.; Ding, Y.; Tu, C. Dietary inflammatory index is associated with metabolic dysfunction-associated fatty liver disease among United States adults. Front. Nutr. 2024, 11, 1340453. [Google Scholar] [CrossRef]
- Boushey, C.; Ard, J.; Bazzano, L.; Heymsfield, S.; Mayer-Davis, E.; Sabaté, J.; Snetselaar, L.; Van Horn, L.; Schneeman, B.; English, L.K.; et al. USDA Nutrition Evidence Systematic Reviews. In Dietary Patterns and Growth, Size, Body Composition, and/or Risk of Overweight or Obesity: A Systematic Review; USDA Nutrition Evidence Systematic Review: Alexandria, VA, USA, 2020. [Google Scholar]
- Merli, M.; Berzigotti, A.; Zelber-Sagi, S.; Dasarathy, S.; Montagnese, S.; Genton, L.; Plauth, M.; Parés, A. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J. Hepatol. 2019, 70, 172–193. [Google Scholar] [CrossRef]
- Lai, J.C.; Tandon, P.; Bernal, W.; Tapper, E.B.; Ekong, U.; Dasarathy, S.; Carey, E.J. Malnutrition, frailty, and sarcopenia in patients with cirrhosis: 2021 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2021, 74, 1611–1644. [Google Scholar] [CrossRef]
- Iqbal, U.; Jadeja, R.N.; Khara, H.S.; Khurana, S. A Comprehensive Review Evaluating the Impact of Protein Source (Vegetarian vs. Meat Based) in Hepatic Encephalopathy. Nutrients 2021, 13, 370. [Google Scholar] [CrossRef]
- Cronin, O.; Barton, W.; Skuse, P.; Penney, N.C.; Garcia-Perez, I.; Murphy, E.F.; Woods, T.; Nugent, H.; Fanning, A.; Melgar, S.; et al. A Prospective metagenomic and metabolomic analysis of the impact of exercise and/or whey protein supplementation on the gut microbiome of sedentary adults. mSystems 2018, 3. [Google Scholar] [CrossRef]
- Han, E.; Kim, M.K.; Im, S.-S.; Kim, H.S.; Kwon, T.K.; Jang, B.K. High Sodium Intake, as Assessed by Urinary Sodium Excretion, Is Associated with Nonalcoholic Fatty Liver Disease or Sarcopenia. Gut Liver 2023, 17, 456–465. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Barazzoni, R.; Busetto, L.; Campmans-Kuijpers, M.; Cardinale, V.; Chermesh, I.; Eshraghian, A.; Kani, H.T.; Khannoussi, W.; Lacaze, L.; et al. European guideline on obesity care in patients with gastrointestinal and liver diseases—Joint ESPEN/UEG guideline. Clin. Nutr. 2022, 41, 2364–2405. [Google Scholar] [CrossRef]
- Yan, J.-H.; Guan, B.-J.; Gao, H.-Y.; Peng, X.-E. Omega-3 polyunsaturated fatty acid supplementation and non-alcoholic fatty liver disease: A meta-analysis of randomized controlled trials. Medicine 2018, 97, e12271. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, M.V.; Siddiqui, M.S.; Forsgren, M.F.; Sanyal, A.J. Harnessing Muscle-Liver Crosstalk to Treat Nonalcoholic Steatohepatitis. Front. Endocrinol. 2020, 11, 592373. [Google Scholar] [CrossRef] [PubMed]
- Stockton, K.A.; Mengersen, K.; Paratz, J.D.; Kandiah, D.; Bennell, K.L. Effect of vitamin D supplementation on muscle strength: A systematic review and meta-analysis. Osteoporos. Int. 2011, 22, 859–871. [Google Scholar] [CrossRef]
- Jaffar, H.M.; Al-Asmari, F.; Khan, F.A.; Rahim, M.A.; Zongo, E. Silymarin: Unveiling its pharmacological spectrum and therapeutic potential in liver diseases—A comprehensive narrative review. Food Sci. Nutr. 2024, 12, 3097–3111. [Google Scholar] [CrossRef]
- Santangeli, E.; Abbati, C.; Chen, R.; Di Carlo, A.; Leoni, S.; Piscaglia, F.; Ferri, S. Pathophysiological-Based Nutritional Interventions in Cirrhotic Patients with Sarcopenic Obesity: A State-of-the-Art Narrative Review. Nutrients 2024, 16, 427. [Google Scholar] [CrossRef]
- Lombardo, M.; Aiello, G.; Fratantonio, D.; Karav, S.; Baldelli, S. Functional Role of Extracellular Vesicles in Skeletal Muscle Physiology and Sarcopenia: The Importance of Physical Exercise and Nutrition. Nutrients 2024, 16, 3097. [Google Scholar] [CrossRef]
- Zhou, X.-D.; Cai, J.; Targher, G.; Byrne, C.D.; Shapiro, M.D.; Sung, K.-C.; Somers, V.K.; Chahal, C.A.A.; George, J.; Chen, L.-L.; et al. Metabolic dysfunction-associated fatty liver disease and implications for cardiovascular risk and disease prevention. Cardiovasc. Diabetol. 2022, 21, 270. [Google Scholar] [CrossRef]
- Barouki, R.; Samson, M.; Blanc, E.B.; Colombo, M.; Zucman-Rossi, J.; Lazaridis, K.N.; Miller, G.W.; Coumoul, X. The exposome and liver disease—How environmental factors affect liver health. J. Hepatol. 2023, 79, 492–505. [Google Scholar] [CrossRef]
- Alghamdi, W.; Mosli, M.; Alqahtani, S.A. Gut microbiota in MAFLD: Therapeutic and diagnostic implications. Ther. Adv. Endocrinol. Metab. 2024, 15. [Google Scholar] [CrossRef]
- Petroni, M.L.; Caletti, M.T.; Grave, R.D.; Bazzocchi, A.; Gómez, M.P.A.; Marchesini, G. Prevention and Treatment of Sarcopenic Obesity in Women. Nutrients 2019, 11, 1302. [Google Scholar] [CrossRef]
- Meza-Valderrama, D.; Sánchez-Rodríguez, D.; Peña, Y.C.; Ramírez-Fuentes, C.; Muñoz-Redondo, E.; Morgado-Pérez, A.; Ortíz-Agurto, N.; Finis-Gallardo, P.; Marco, E. Resistance Training and Nutritional Supplementation in Older Adults with Sarcopenia after Acute Disease: A Feasibility Study. Nutrients 2024, 16, 3053. [Google Scholar] [CrossRef] [PubMed]
| Myokine | Role in MAFLD | Role in Sarcopenia |
|---|---|---|
| IGF-1 | Controls the anabolic and catabolic processes, in addition to muscular growth and regeneration. | |
| GDF-8 | Regulates the development and growth of muscle mass by inhibiting the protein synthesis of skeletal muscle. | |
| Irisin | Related to the loss of adipose tissue and improvement in insulin sensitivity after physical exercise. | |
| METRNL | Able to convert white adipose tissue into brown adipose tissue, decreasing insulin resistance. | |
| BDNF | Acts on myogenesis and activation of satellite cells in the skeletal muscle. | |
| FGF-21 | Regulates the metabolic activity. | Regulates the metabolic activity. |
| BAIBA | Suppresses inflammation, which is linked to the loss of adipose tissue. | Suppresses inflammation. |
| Apelin | It has regenerative and anti-inflammatory action and is indicated as a pharmacological agent and biomarker of sarcopenia. | |
| Decorin | Stimulates muscle growth by inhibiting myostatin. | |
| IL-6 | Can stimulate both anti-inflammatory and pro-inflammatory responses. | Can stimulate both anti-inflammatory and pro-inflammatory responses. |
| IL-7 | Assists in the process of myogenesis and also stimulates the differentiation of satellite cells. | |
| IL-15 | Participates in the regulation of metabolism. | Participates in the regulation of metabolism. |
| Hepatokine | Role in MAFLD | Role in sarcopenia |
| ANGPTL 4 | Promotes lower fat absorption by inhibiting pancreatic lipases, including obese individuals who have lower levels, which encourages the accumulation of fat. | |
| LECT2 | Acts as a chemotactic to neutrophils and is closely linked to oxidative stress and weight gain, encouraging the increase in inflammatory cytokines and damaging insulin signaling. | Acts as a chemotactic to neutrophils and is closely linked to oxidative stress and weight gain, encouraging the increase in inflammatory cytokines and damaging insulin signaling. |
| SHBG | Acts as a protective factor against MAFLD by inhibiting the lipogenesis process. | |
| Chemerin | Participates in the regulation of lipolysis, glucose absorption, and adipocyte differentiation. | |
| Fetuin-A | Induces insulin resistance by inhibiting insulin receptors and their signaling. | Induces insulin resistance by inhibiting insulin receptors and their signaling. |
| FGF-21 | Acts as a regulator of metabolic processes involving lipids and glucose. FGF-21 is considered a new marker of MAFLD. | |
| IL-6 | It may have pro-oncologic and pro-inflammatory actions. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Losasso, M.R.; Parussolo, M.L.C.; Oliveira Silva, A.; Direito, R.; Quesada, K.; Penteado Detregiachi, C.R.; Bechara, M.D.; Méndez-Sánchez, N.; Abenavoli, L.; Araújo, A.C.; et al. Unraveling the Metabolic Pathways Between Metabolic-Associated Fatty Liver Disease (MAFLD) and Sarcopenia. Int. J. Mol. Sci. 2025, 26, 4673. https://doi.org/10.3390/ijms26104673
Losasso MR, Parussolo MLC, Oliveira Silva A, Direito R, Quesada K, Penteado Detregiachi CR, Bechara MD, Méndez-Sánchez N, Abenavoli L, Araújo AC, et al. Unraveling the Metabolic Pathways Between Metabolic-Associated Fatty Liver Disease (MAFLD) and Sarcopenia. International Journal of Molecular Sciences. 2025; 26(10):4673. https://doi.org/10.3390/ijms26104673
Chicago/Turabian StyleLosasso, Marina Ribas, Maria Luiza Cesto Parussolo, Antony Oliveira Silva, Rosa Direito, Karina Quesada, Claudia Rucco Penteado Detregiachi, Marcelo Dib Bechara, Nahum Méndez-Sánchez, Ludovico Abenavoli, Adriano Cressoni Araújo, and et al. 2025. "Unraveling the Metabolic Pathways Between Metabolic-Associated Fatty Liver Disease (MAFLD) and Sarcopenia" International Journal of Molecular Sciences 26, no. 10: 4673. https://doi.org/10.3390/ijms26104673
APA StyleLosasso, M. R., Parussolo, M. L. C., Oliveira Silva, A., Direito, R., Quesada, K., Penteado Detregiachi, C. R., Bechara, M. D., Méndez-Sánchez, N., Abenavoli, L., Araújo, A. C., de Alvares Goulart, R., Guiger, E. L., Fornari Laurindo, L., & Maria Barbalho, S. (2025). Unraveling the Metabolic Pathways Between Metabolic-Associated Fatty Liver Disease (MAFLD) and Sarcopenia. International Journal of Molecular Sciences, 26(10), 4673. https://doi.org/10.3390/ijms26104673














